Abstract
Study Objectives:
Asthma, chronic obstructive pulmonary disease (COPD), and obstructive sleep apnea (OSA) are very prevalent disorders. Their coexistence in the same individual has an unclear effect on natural history and long-term outcomes.
Methods:
The OLDOSA (Obstructive Lung Disease and Obstructive Sleep Apnea) cohort enrolled 4,980 veterans with an acute hospitalization and in whom asthma, COPD, OSA, overlapping conditions, or none of these disorders at baseline had been diagnosed. Pulmonary function, polysomnography, positive airway pressure (PAP) recommendations and adherence, and vital status were collected and analyzed. Various proportional hazards models were built for patients with OSA to test the effect of PAP therapy on survival.
Results:
Ten-year all-cause cumulative mortality rate was 52.8%; median time to death was 2.7 years. In nonoverlapping asthma, OSA and COPD, mortality rates were 54.2%, 60.4%, and 63.0%, respectively. The overlap syndromes had the following mortality: COPD-OSA 53.2%, asthma-COPD 62.1%, asthma-OSA 63.5%, and triple overlap asthma-COPD-OSA 67.8%. In patients with OSA not on PAP therapy, after adjustment for age, comorbidities, and lung function, risk of death was 1.34 (1.05–1.71) times higher than those undergoing treatment. Similarly, in patients with OSA nonadherent to PAP therapy the adjusted risk of death was 1.78 (1.13–2.82) times higher versus those using it at least 70% of nights and more than 4 hours nightly.
Conclusions:
In this large longitudinal cohort of hospitalized veterans with high comorbid burden, asthma, COPD, OSA and their overlap syndromes had very high long-term mortality. In patients with OSA, PAP initiation and superior therapeutic adherence were associated with significantly better survival.
Citation:
Ioachimescu OC, Janocko NJ, Ciavatta M-M, Howard M, Warnock MV. Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort study: 10-year assessment. J Clin Sleep Med. 2020;16(2):267–277.
Keywords: asthma, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, positive airway pressure, mortality, obstructive lung disease, overlap syndrome
BRIEF SUMMARY
Current Knowledge/Study Rationale: Long-term effect on natural history and outcomes of coexistent or overlapping conditions of asthma, chronic obstructive pulmonary disease (COPD), and obstructive sleep apnea (OSA) is currently unknown. Furthermore, lung function and/or comorbid burden and use of positive airway pressure (PAP) therapy could affect these outcomes.
Study Impact: This observational, point-of-care study presents the 10-year analysis of a large cohort of veterans hospitalized at baseline, with or without the aforementioned conditions. We found that the cohort had elevated comorbidity scores and very high long-term cumulative mortality, especially for the triple overlap syndrome. In patients with OSA, PAP initiation and superior therapeutic adherence was associated with lower mortality, independently of sex, race, age, comorbidities, body mass, and lung function.
INTRODUCTION
Obstructive lung and airway disorders such as chronic obstructive pulmonary disease (COPD), asthma, and obstructive sleep apnea (OSA) are very common in the general population. Because of their high prevalence, concurrent effects of smoking or other pathogenic factors, and strong interactions between upper and lower airway pathophysiology, these illnesses could coexist in the form of various overlap syndromes.1–6
The term “overlap syndrome” was first used in 1985, when it was posited that the COPD-OSA association had more deleterious effects than either disease alone.7 Indeed, during the next decades, the overlap syndrome has been shown to have higher mortality than either condition alone,8–10 and several nonrandomized studies found that PAP therapy could mitigate the outcome differences between COPD and overlap syndrome.8,11,12 The term ‘alternative overlap syndrome’, delineating the asthma-OSA coexistence, has been proposed more recently for better diagnostic clarity, improved endophenotypic classification and outcome stratification, and for medical nomenclature standardization.5,6 Several small studies investigated the OSA-asthma association,13,14 whereas a recent analysis of a large hospitalized patient dataset (n = 179,789) confirmed significant adverse effects of either OSA or obesity on asthma. The presence of OSA was associated with longer hospitalization, need for invasive respiratory therapy, higher hospital charges, and health care utilization.15 Asthma-COPD overlap (ACO) or ACO syndrome has been defined as persistent airflow limitation with features of both asthma and COPD.3,16 Prevalence of ACO varies widely, depending on population and nosologic definitions.17 Patients with ACO seem to carry higher comorbid burden (eg, diabetes, hypertension, coronary artery disease),18 to be more symptomatic and to experience more frequent and worse exacerbations versus either COPD or asthma groups.19 Currently, the prevalence and the outcome implications of these overlaps, including the triple overlap in the general population, are unknown.
The Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) study was designed as a longitudinal, point-of-care, observational investigation with the aim to better characterize natural histories and long-term outcomes of patients with asthma, COPD, OSA, and their overlap syndromes. This report presents a 10-year survival analysis of the OLDOSA cohort, constituted by all patients hospitalized during the calendar year 2008 at the Atlanta VA Medical Center, with or without the aforementioned diagnoses. The primary endpoint was all-cause mortality. Our hypotheses were that coexistence of at least two of these disorders (overlap syndromes or lung function corollaries of these conditions) would affect adversely and independently from other comorbidities long-term survival, and that adherence to positive airway pressure (PAP) therapy in those with OSA would be associated with lower mortality.
METHODS
Study population
All patients enrolled at the Atlanta Veterans Affairs (VA) during the calendar year 2008, that is, those who had at least one medical visit that year, constituted the base cohort (53,123 patients). Our interest was to search among these individuals for diagnoses of asthma, COPD, OSA, or overlap syndromes, and to study comparatively their effect on long-term outcomes. Since January 2009, we extracted electronically at several specified times diagnostic and follow-up data for the base cohort. As we previously reported, among these veterans, chart diagnoses of asthma, OSA, and COPD were found to be very common, in 8%, 14% and 19% of patients, respectively; either diagnosis was present in 33% of the entire base cohort.20 The diagnosis of OLDOSA syndrome,6 that is, OSA associated with either asthma or COPD, was present in 5% of the cohort.20 However, due to the size of the base cohort, the accuracy of these chart diagnoses could not be validated, hence we decided to constitute the OLDOSA cohort, a subgroup of the base cohort.
The OLDOSA cohort (n = 4,980) included all patients hospitalized at least once between January 1, 2008 and December 31, 2008 at the Atlanta VA Medical Center, in any of its inpatient units. The presence of the aforementioned diagnoses was verified for the patients from the OLDOSA cohort. The date of inclusion was day 1 of the (first) hospitalization during calendar year 2008. Last day of follow-up was represented by the date of death (where applicable), last visit in any VA or non-VA clinic before the respective electronic extraction dates, that is, September 15, 2016, March 20, 2018, July 12, 2018, and December 22, 2018. The burden of associated conditions potentially affecting outcomes was assessed by the use of a validated instrument, Charlson Comorbidity Index (CCI).21,22 Procedures followed for study data collection, and the standards used for polysomnography are shown in the supplemental material.21–25,33,34 For patients with OSA, auto-adjustable or fixed PAP therapy by (oro)nasal mask was offered and instituted, per clinician recommendations, and patient preferences. A PAP coordinator provided initial setup and education on operation and care for device and accessories. Adherence to PAP therapy was assessed by targeted history, from in-person or telephone encounters, and objectively by PAP card or cloud-based downloads. We used standard criteria for good PAP adherence, that is, mean usage of ≥ 4 h/night, ≥ 70% of nights.
Emory University Institution Review Board and Atlanta VA Research and Development Committee approved the project (IRB#49576/R&D#0002). Several preliminary analyses were performed and presented before at American Thoracic Society and American Federation of Medical Research meetings.20,26 The study did not have any ongoing, dedicated funding.
Study variables
Baseline demographic data included date of birth, self-identified sex, and race. Baseline and follow-up anthropometric parameters of height and weight (with calculated body mass index, BMI), as well as dates of first hospitalization, first and last sleep and PAP clinic visit were extracted. Spirometry data (before January 1, 2008 for baseline assessment and after December 31, 2008 for follow-up) were recorded, as performed in the local Pulmonary Function Laboratory, and in accordance with the contemporaneous American Thoracic Society guidelines and recommendations.27,28
Statistical analysis
Descriptive statistics of available variables were performed for the OLDOSA cohort (n = 4,980). Categorical variables were summarized as frequencies or group percentages. Continuous variables were characterized as mean ± standard deviation or as median (25th to 75th interquartile range, IQR), as appropriate for normal and nonnormal distributions, respectively. The t test and analysis of variance were used to compare mean values, whereas categorical variables were compared using χ2 test. Kaplan-Meier survival plots were built to illustrate long-term survival curves for various subcategories. Log-rank tests were used to compare survival times between different groups. In patients with OSA, proportional hazards models were used to estimate the association between PAP therapy and risk of death in both unadjusted and adjusted models for the following parameters: age, sex, race, CCI, BMI, and forced expiratory volume in 1 second (FEV1). Among patients with OSA started on PAP therapy, similarly unadjusted and adjusted models were used to estimate the association between PAP adherence and risk of death. In all models, follow-up time was censored based on the date of the last clinic visit (including the last CPAP clinic visit). Complete case analyses were used in most models; when data are available and appropriate, we verified the validity of the models by conditional mean imputation. A value of P = .05 was set as the level of statistical significance. Statistical analyses were performed using SAS9.4 and JMP14Pro (SAS Institute, Cary, North Carolina).
RESULTS
Patient characteristics
The OLDOSA cohort was composed of 93.8% men and 6.2% women, as expected in a military veteran population. By race, 57% were self-described as white/Caucasian, and 41% black/African-American. At enrollment, median age was 61 years (IQR 54–69). Median baseline BMI was 29 kg/m2 (IQR 24–34). Median baseline prebronchodilator FEV1 was 2.1 L (IQR 1.6–2.7), or 68% predicted (IQR 52–83). Baseline median FEV1/ FVC ratio was 0.68 (IQR 0.58–0.76).
Disease categorization
In the OLDOSA cohort (n = 4,980), asthma, OSA, and COPD were found in 7%, 15% and 29% of patients, respectively (Figure 1). Either disease was present in 39% of the patients. The OLDOSA syndrome, that is, OSA plus either asthma or COPD6 was present in 8% of the cohort. As expected in a group of patients selected by an event of hospitalization (OLDOSA cohort), the prevalence of these disorders was higher than initially estimated in the base cohort.20
Figure 1. Study diagram.
Proportional Venn diagram of the OLDOSA Cohort (n = 4,980). Patient population outside the circles and inside the rectangle is represented by patients with no OSA, COPD, or asthma. Asthma, COPD, and OSA “only” = nonintersecting or nonoverlapping disorders. ACO = asthma and COPD (no sleep apnea), AOS = asthma and OSA (no COPD), COPD = chronic obstructive pulmonary disease, OSA = obstructive sleep apnea, OS = COPD and OSA (no asthma), TO = triple overlap of asthma, COPD and OSA.
During the 10-year follow-up, 702 additional cases of OSA were added (incident disease), leading to a total of 1,469 patients (29.5% of the cohort), perhaps reflecting improved awareness and better access to sleep medicine services over time. These were as follows: 275, 345, 23, and 59 additional cases of OSA in the baseline groups without any disease, and with COPD, asthma or ACO, respectively. In addition, between the initial electronic extraction in January 2009 and the last individual chart review in 2018, among the 4,980 patients, 101 new diagnoses of asthma and additional 430 new diagnoses of COPD were made.
In the entire OLDOSA cohort, median CCI was 5 (IQR 3–7, n = 2,117). The CCI was significantly higher in the triple overlap (Table 1); those without any of the aforementioned disorders had similar severity scores. The average FEV1 loss was less than 68 mL/y of follow-up and was comparable among the groups.
Table 1.
Main characteristics at enrollment and mortality status at 10 years of the Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort by primary conditions and their overlap syndromes.
PAP usage and adherence
At 10-year review, among 1,469 patients with OSA, 933 patients (63.5%) had been started on PAP therapy. Approximately 536 patients were not initiated on PAP therapy due to mild disease, patient refusal, appointment no-shows, or death. One visit to PAP clinic with a PAP coordinator or any order for any specific equipment or supplies placed a patient with OSA in the PAP therapy category. As of December 2018, only 134 patients met the standard criteria for good adherence. When adjudicating patients who did not order or pick up any supplies or did not have any follow-up visits in neither sleep nor PAP clinics as nonadherent (missing data imputation using assumption of nonuse), percentage of patients with OSA started on PAP therapy who met the adherence criteria in the last 2 years of follow-up was only 134/1,469 (9%). However, 34/134 adherent patients and 363/591 nonadherent patients were already deceased as of December 2018; considering this, the rate of good adherence among survivors was 100/328 (30%).
As of September 2016, among the 238 patients with OSA on PAP alive and not lost to follow-up, PAP therapy was applied for a median of 84.5% (IQR 27–98) of nights, with a median of 5.6 hours (IQR 3.5–7.5); 62% of patients used PAP for ≥ 4 h/night.
As of December 2018, PAP therapy was used by 210 patients with OSA who were still alive and not lost to follow-up, for a median of 54% of the nights (IQR 4–94), with a median of 5.0 hours (IQR 1.2–7.0); 54% of them used PAP for ≥ 4 h/night. When incident disease during the period of follow-up was included, 45/321 of patients (14%) with OSA and 89/404 with OSA-COPD overlap syndrome (22%) met good compliance criteria.
Mortality
During follow-up, 2,630 deaths were registered, accounting for a 52.8% cumulative all-cause mortality rate. Median time to death was 2.7 years (IQR 0.8–5.7, range 0–10.8). As of December 2018, survivors were followed for a median of 9.8 years (IQR 7.5–10.2, range 0–10.9).
Mortality rate was higher in men (62.8%) versus women (34.4%) (P < .001). Cumulative mortality rate was 64.8% in white/Caucasians, and 55.9% in black/African-Americans (P = .0005). Ten-year cumulative mortality rates were very high in patients with history of nonmetastatic solid tumors (61.3%), type 2 diabetes mellitus with end-organ damage (64.4%), myocardial infarction (70.3%), congestive heart failure (76.0%), moderate-severe chronic kidney or liver disease (75.0%) and 76.3%, respectively), and metastatic solid tumors (97.9%). Although cumulative mortality rate in patients with COPD was high (63.0%), the rates stratified by objective lung function impairment for those with mild, moderate, severe, and very severe airflow limitation were 46.8, 71.6, 75.0, and 93.5%, respectively. Airflow limitation severity was categorized by standard FEV1 percent predicted criteria as mild (≥ 80%), moderate (50% to 79%), severe (30% to 49%) and very severe (< 30%).28
Kaplan-Meier survival curves for patients with asthma, COPD, OSA, different overlap syndromes, or none of these conditions are shown in Figure 2. Mortality rates in patients with nonoverlapping baseline diagnoses of asthma, OSA, and COPD were 54.2%, 60.4%, and 63.0%, respectively (versus 47.6% in those with none of the conditions). The overlap syndromes with the diagnoses present since the first visit in 2008 had the following mortality rates: ACO (no OSA) 62.1%, COPD-OSA overlap (no OSA) 53.2%, asthma-OSA overlap (no COPD) 63.5%, and triple asthma-COPD-OSA overlap 67.8% (P < .0001, Table 2).
Figure 2. Survival plots by conditions.
Kaplan-Meier survival plot by condition (P < .0001, log rank test). Triple overlap (asthma + COPD + OSA) had the worst survival. Asthma (green); COPD, chronic obstructive pulmonary disease (red); OSA, obstructive sleep apnea (blue); none, none of the three conditions (dotted black); COPD + OSA (no asthma; brown); Asthma + COPD (no OSA; pink); asthma + OSA (no COPD; light green); asthma + COPD + OSA (purple).
Table 2.
Survival analyses by positive airway pressure therapy initiation in patients with obstructive sleep apnea (n = 1,469).
The unadjusted hazard ratio of death for COPD was 1.35 (1.24–1.46, P < .0001); for asthma was 1.27 (1.11–1.46, P = .0005); and for OSA was 1.18 (1.07–1.30, P = .0015) versus those without that specific condition.
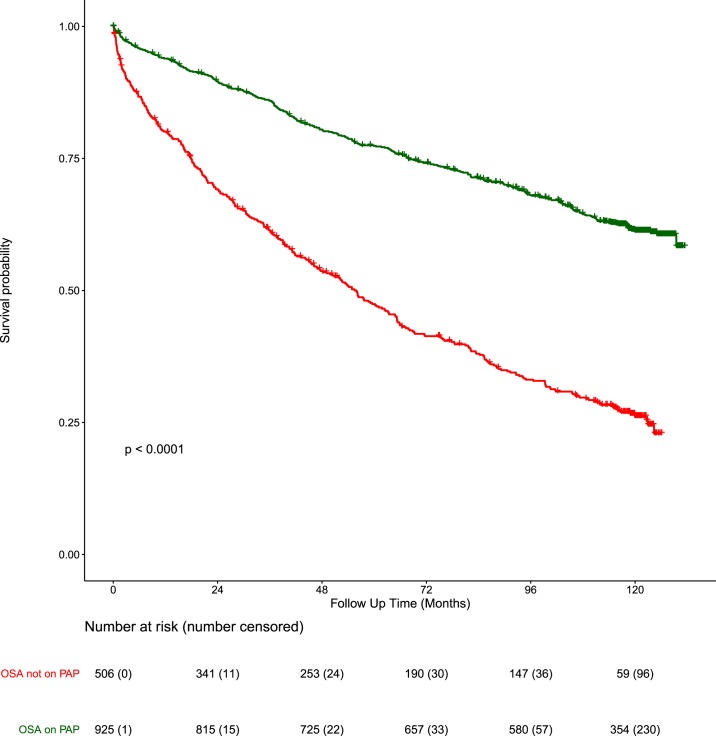
Figure 3 illustrates a Kaplan-Meier survival plot by OSA and PAP therapy. Among all patients with OSA, we found that those not started on PAP had an unadjusted hazard ratio of death of 2.86 (95% confidence interval [95% CI] 2.46–3.33, P < .0001, Table 2, model 1a) times higher versus those on treatment. In the model adjusted for FEV1, age, and comorbidities, hazard ratio was 1.34 (95% CI 1.05–1.71, P < .0001, Table 2, model 1c) higher versus those started on PAP therapy. Age-adjusted CCI22 did not improve significantly the performance of the aforementioned models (similar Akaike Information Criterion values).
Figure 3. Survival plots by PAP therapy.
Kaplan-Meier survival plots of patients with OSA on PAP therapy (green), OSA not on PAP therapy (red), P < .0001, log-rank test. OSA = obstructive sleep apnea, PAP = positive airway pressure.
The cumulative mortality rate of patients with OSA started on PAP who met good adherence criteria was 25.4% versus 61.4% in those who did not. (Figure 4, P < .0001). Similar results were found in our adjusted proportional hazards models in patients with OSA started on PAP therapy, but with suboptimal adherence. The unadjusted risk of death in those with poor adherence was 1.83 (95% CI 1.28–2.60, P = .00019, Table 3, model 2a) times higher versus those who met good adherence criteria. After adjusting for FEV1, age, BMI, and comorbidities, the hazard ratio was 1.78 (95% CI 1.13–2.82), P = .0134, Table 3, model 2c) for poor versus good adherence groups. For all models, we also included variables hypothesized to influence outcomes such as sex and race (Table 2, model 1b; Table 3, model 2b). Mortality differences remained in various subgroups (OSA, OSA-COPD, OSA-asthma and OSA-asthma-COPD, Table 1) based on PAP therapy initiation and PAP adherence.
Figure 4. Survival plots by PAP adherence.
Kaplan-Meier survival plots in patients with OSA, PAP adherent (bright green) and PAP nonadherent (dark green), P = .00071, log rank test. OSA = obstructive sleep apnea, PAP = positive airway pressure.
Table 3.
Survival analyses by positive airway pressure therapeutic adherence in patients with obstructive sleep apnea started on positive airway pressure therapy (n = 933).
DISCUSSION
In the base cohort, we have shown that asthma, COPD, and OSA are very common diagnoses among veterans, and disproportionately more frequent in inpatient versus outpatient settings, particularly for COPD.20,26 However, these analyses included noncurated diagnoses of COPD, asthma, or OSA, as size of the base cohort did not allow accuracy validation. As such, a subset of the base cohort, called OLDOSA cohort, was constituted with the goals to assess long-term outcomes of patients hospitalized in our system, and to stratify outcomes by validated diagnoses and their overlaps. In summary, we found a rate of reclassification below 10%.
In this large longitudinal study of US veterans constituting the OLDOSA cohort (94% men, 41% African Americans), we confirm that asthma, COPD, and OSA are very prevalent disorders. The OLDOSA cohort had very high long-term all-cause mortality, especially if afflicted by overlapping conditions. Patients with OSA started on PAP and those with superior therapeutic adherence had significantly lower mortality. The global long-term adherence to PAP therapy in this cohort of veterans was very low.
The 10-year all-cause, cumulative mortality rate in the OLDOSA cohort was 52.8%. Mortality was higher in older participants, in men, in those self-identified as white/Caucasian, in individuals with larger comorbid burden, as expressed by higher CCI (especially in those with history of congestive heart failure, type 2 diabetes, myocardial infarction, chronic liver and kidney disease) or in patients with lower baseline FEV1, in patients with OSA not on PAP, and in those nonadherent to PAP therapy. Patients with overlap syndromes seemed to have distinct clinical courses, where the triple overlap fared the worst.
Compared to the study by Marin et al8 on patients with COPD and COPD-OSA overlap syndrome followed for an average of 9.4 years, our study found mortality rates to be much higher (52.8% versus 32.7%), leading to a shorter mean duration of observation, at least in part due to older age (61 ± 13 in OLDOSA versus 57 ± 8) and much sicker patients (mean CCI was 5.3 ± 3 versus 1.2 ± 1 in the Spanish study). Indeed, in the OLDOSA cohort we found a median time to death of 2.7 (IQR 0.8–5.7) years, in line with the fact that prior, recent hospitalizations may herald higher short-term mortality. In patients with OSA, we reconfirmed a significant association between lower mortality and PAP treatment initiation or superior PAP therapeutic adherence. The triple overlap asthma-COPD-OSA had the highest mortality (67.8%). It is yet unclear if the triple overlap is not in fact a reflection of diagnostic difficulties encountered by clinicians in complex patients when using traditional nosologic characterizations, or if the higher disease burden “buries” the lines of well-defined conditions. In this study, we did not analyze subsequent hospitalizations, COPD exacerbations, or actual causes of death. In another study, on 1,112 patients with COPD and 2,284 patients with OSA (227 having COPD-OSA overlap),12 the mean CCI was 1.5, 1.7, and 2.0 in OSA, COPD, and overlap syndrome, respectively. That study also found a beneficial effect of PAP therapy used ≥ 4 h/night. Our study found that the combination of ≥ 4 h/night and ≥ 70% of nights (standard good adherence criteria) was associated with significantly better survival. The potential benefits of PAP therapy on survival noted in our study may be explained by the fact that most randomized studies that failed to show such a relationship were shorter term, perhaps not reaching the period of follow-up when the survival curves start to diverge, had few fatal events, did not account for or had poor PAP adherence.29,30
Several findings of our investigation deserve comments. First and perhaps not unexpectedly, individuals without any of the mentioned disorders had lower 10-year all-cause mortality rate, whereas the overlap syndromes (especially the triple overlap) experienced worse outcomes, even after adjustment for age and comorbidities. This may be due to the additive negative consequences of different comorbidities (with the inherent roles of the lung function impairments, body habitus, and/or the use of PAP therapy). Alternatively, the coexistence of multiple diagnoses (especially COPD plus asthma) in the same individuals may be a reflection of more complicated, sicker patients, with phenotypes-endotypes that are harder to characterize and possibly to treat. For example, smokers with a concurrent diagnosis of asthma that does not have the typical bronchodilative response to inhaled beta agonist, with coexistent obesity and OSA may be a disease category harder to define and less responsive to usual therapies, as usual clinical experience would suggest.
Second, patients with OSA started on PAP therapy had lower mortality versus those not started on treatment (Figure 3), independent of comorbidities, FEV1, sex, race, and age. As large-scale, long-term validation of real-life PAP usage in OSA is scarce, this 10-year analysis of the OLDOSA cohort confirms prior associations between PAP use and better survival. Reasons for not starting PAP therapy in patients with OSA and still alive before PAP clinic appointment, such as patient refusal or no-show, likely reflect overall lack of “health consciousness” or poorer global adherence to medical appointments, screening measurements or treatment recommendations, with understandable effect on long-term outcomes.
Third, patients with OSA and good adherence to PAP therapy had significantly better survival than patients not meeting adherence criteria (Figure 4), indicating that treatment ≥ 70% of nights and ≥ 4 h/night is associated with better survival. What the study cannot explain is if PAP initiation or good therapeutic adherence are the primary “protective” factors or they are simply surrogate markers of superior adherence with other medical recommendations and treatments. When analyzed by disease category, good PAP adherence was the highest (22%) in those with COPD-OSA overlap syndrome versus 14% in those with OSA alone. This may explain, perhaps in addition to slightly better lung function both at baseline (Table 1) and at follow-up (data not shown), why COPD-OSA overlap syndrome did not have significantly worse 10-year all-cause mortality than the individual conditions.
Finally, smoking exposure and intensity were not collected due to concerns of recording variability, unreconcilable discrepancies, documentation bias, and over-adjustment for confounders or mediators.31 However, we estimated the effects of smoking both indirectly (diagnoses of cardiac, cerebral or peripheral vascular disease and pulmonary function testing—baseline FEV1 and delta FEV1), and directly, as smoking status from medical notes was used in the adjudication of COPD and asthma diagnoses, together with existing pulmonary function tests and imaging studies.
The main strengths of this point-of-care study are: (1) this is a large cohort of patients, in which African Americans were well represented; (2) a near-closed system (minimal loss to follow-up); (3) with adjudicated diagnoses, and (4) represents the first large, long-term longitudinal cohort of asthma-COPD-OSA overlap syndromes. Limitations of this study are represented by nonrandomized, point-of-care, observational design (issue of missingness); controlled for presence of comorbidities in only a (random) subgroup of patients; and in a single center at the VA, with significant sex imbalance (external validity issue). Further, the cohort selection was based on a likely relevant event, that is, hospitalization (potential selection bias by enrolling “sicker” participants, with expectedly worse outcomes). We were unable to control for other covariates (such as socioeconomic status or physical fitness) and for OSA severity. Nevertheless, the latter carries in itself several potential sources of bias: (1) not all OLDOSA cohort members had sleep studies, and some of them were done outside VA (albeit a minority, this may increase the magnitude of missing data); (2) apnea-hypopnea index and long-term mortality rates do not correlate well; (3) hypopnea criteria evolved over time and differ between laboratories (eg, flow criteria, oxygen desaturation threshold or association with arousals); and (4) apnea-hypopnea index does have significant night-to-night variability. We found that the long-term PAP adherence was poor, yet this has been insufficiently studied in decade-long observational studies.32
CONCLUSIONS
We found that asthma, COPD, OSA, and their overlap syndromes are very prevalent among military veterans. The overall 10-year cumulative mortality rate in this cohort of hospitalized patients with and without asthma, COPD, or OSA was very high. Patients with COPD and OSA had significantly worse outcomes than other conditions, whereas the presence of all three disorders (triple overlap) had the highest mortality. PAP therapy initiation and superior adherence to treatment for OSA was associated with lower mortality, a finding that was independent of sex, race, age, comorbidities, body mass, and lung function.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Atlanta VA Medical Center. The content of this manuscript does not represent the views of the Department of Veterans Affairs or the Government of the USA. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the following individuals for their contribution and help with the study or manuscript: Prof. Eugene Huang, PhD (Dept. of Biostatistics and Bioinformatics, Emory University); Christine Jasien (Atlanta VA Research Service Line, data extraction); Tiffany Elliott and Amy Anderson (Atlanta VA Research Service Line, study coordination and regulatory affairs).
ABBREVIATIONS
- ACO
asthma-COPD overlap
- CCI
Charlson Comorbidity Index
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- IQR
interquartile range
- OSA
obstructive sleep apnea
- OLDOSA
Obstructive Lung Disease and Obstructive Sleep Apnea
- PAP
positive airway pressure
- VA
Veterans Affairs
REFERENCES
- 1.Khatri SB, Ioachimescu OC. The intersection of obstructive lung disease and sleep apnea. Cleve Clin J Med. 2016;83(2):127–140. doi: 10.3949/ccjm.83a.14104. [DOI] [PubMed] [Google Scholar]
- 2.Owens RL, Macrea MM, Teodorescu M. The overlaps of asthma or COPD with OSA: A focused review. Respirology. 2017;22(6):1073–1083. doi: 10.1111/resp.13107. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma (GINA) Diagnosis and Initial Treatment of Asthma, COPD and Asthma - COPD Overlap (a joint project of GINA and GOLD). ginasthma.org. Updated April 2017. Accessed 2018.
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. www.goldcopd.com. Updated 2019.
- 5.Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med. 2014;62(4):665–675. doi: 10.2310/JIM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 6.Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology. 2013;18(3):421–431. doi: 10.1111/resp.12062. [DOI] [PubMed] [Google Scholar]
- 7.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661. [PubMed] [Google Scholar]
- 8.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 9.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 10.Kendzerska T, Leung RS, Aaron SD, Ayas N, Sandoz JS, Gershon AS. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome) Ann Am Thorac Soc. 2019;16(1):71–81. doi: 10.1513/AnnalsATS.201802-136OC. [DOI] [PubMed] [Google Scholar]
- 11.Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung. 2014;192(2):251–258. doi: 10.1007/s00408-014-9555-z. [DOI] [PubMed] [Google Scholar]
- 12.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9(8):767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract. 2015;3(4):566–575.e1. doi: 10.1016/j.jaip.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FP, Liu T, Wang G, Mao H. Asthma or asthma-COPD overlap syndrome? Respirology. 2017;22(3):612. doi: 10.1111/resp.12993. [DOI] [PubMed] [Google Scholar]
- 15.Becerra MB, Becerra BJ, Teodorescu M. Healthcare burden of obstructive sleep apnea and obesity among asthma hospitalizations: Results from the U.S.-based Nationwide Inpatient Sample. Respir Med. 2016;117:230–236. doi: 10.1016/j.rmed.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Terminology, definitions and classification of chronic pulmonary emphysema and related conditions: a report of the conclusions of a CIBA guest symposium. Thorax. 1959;14:286–299. [Google Scholar]
- 17.Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70(7):683–691. doi: 10.1136/thoraxjnl-2014-206740. [DOI] [PubMed] [Google Scholar]
- 18.Tommola M, Ilmarinen P, Tuomisto LE, et al. Differences between asthma-COPD overlap syndrome and adult-onset asthma. Eur Respir J. 2017;49(5):1602383. doi: 10.1183/13993003.02383-2016. [DOI] [PubMed] [Google Scholar]
- 19.Slats A, Taube C. Asthma and chronic obstructive pulmonary disease overlap: asthmatic chronic obstructive pulmonary disease or chronic obstructive asthma? Ther Adv Respir Dis. 2016;10(1):57–71. doi: 10.1177/1753465815617082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioachimescu OC, Tanukonda V, Anderson AL, Ciavatta MM, McCarver K. Asthma, chronic obstructive pulmonary disease, obstructive sleep apnea and overlap syndromes in veteran patients - prevalence and outcomes. Am J Respir Crit Care Med. 2014;189:A5846. [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 24.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ICD-9-CM. International Classification of Diseases, 9th edition, Clinical Modification: update. Official authorized addendum, effective October 1, 1986. J Am Med Rec Assoc. 1986;57(9_suppl):1–32. [PubMed] [Google Scholar]
- 26.Janocko NJ, Ciavatta MM, Ioachimescu OC. Obstructive sleep apnea, asthma and chronic obstructive pulmonary disease – long-term outcomes in a cohort of 1,314 veterans. J Investig Med. 2017;65:396–568. [Google Scholar]
- 27.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 29.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 30.Lisan Q, Van Sloten T, Marques Vidal P, Haba Rubio J, Heinzer R, Empana JP. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the Sleep Heart Health Study. JAMA Otolaryngol Head Neck Surg. 2019;145(6):509. doi: 10.1001/jamaoto.2019.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 32.Ioachimescu OC, Anthony J, Constantin T, Ciavatta MM, McCarver K, Sweeney ME. VAMONOS (Veterans Affairs’ Metabolism, Obstructed and Non-Obstructed Sleep) study: effects of CPAP therapy on glucose metabolism in patients with obstructive sleep apnea. J Clin Sleep Med. 2017;13(3):455–466. doi: 10.5664/jcsm.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–523. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 34.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20(6):406–422. [PubMed] [Google Scholar]