Abstract
Study Objectives:
Sleep-wake disturbances are frequent among patients hospitalized for traumatic injuries but remain poorly documented because of the lack of tools validated for hospitalized patients. This study aimed to validate actigraphy for nighttime sleep monitoring of hospitalized patients with severe traumatic injuries, using ambulatory polysomnography (PSG).
Methods:
We tested 17 patients (30.4 ± 14.7 years, 16.6 ± 8.2 days postinjury) who had severe orthopedic injuries and/or spinal cord injury, with or without traumatic brain injury. When medically stable, patients wore an actigraph on a nonparalyzed arm and underwent ambulatory PSG at the bedside. Data were converted to 1-minute epochs. The following parameters were calculated for the nighttime period: total sleep time, total wake time, sleep efficiency, and number of awakenings. Epoch-by-epoch concordance between actigraphy and PSG was analyzed to derive sensitivity, specificity, and accuracy. PSG sleep parameters were compared to those obtained from four actigraphy scoring algorithms by Bland-Altman plots.
Results:
Sensitivity to detect sleep was ≥ 92% and accuracy was > 85% for all four actigraphy algorithms used, whereas specificity varied from 48% to 60%. The low-activity wake threshold (20 activity counts per epoch) was most closely associated with PSG on all sleep parameters. This scoring algorithm also had the highest specificity (59.9%) and strong sensitivity (92.8%).
Conclusions:
Actigraphy is valid for monitoring nighttime sleep and wakefulness in patients hospitalized with traumatic injuries, with sensitivity, specificity and accuracy comparable to actigraphic recordings in healthy individuals. A scoring algorithm using a low wake threshold is best suited for this population and setting.
Citation:
Bigué JL, Duclos C, Dumont M, et al. Validity of actigraphy for nighttime sleep monitoring in hospitalized patients with traumatic injuries. J Clin Sleep Med. 2020;16(2):185–192.
Keywords: actigraphy, acute care, polysomnography, sleep, traumatic brain injury
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-wake disturbances are frequent among hospitalized patients with acute traumatic injuries, and actigraphy is the only method that enables the objective assessment of sleep quality over several days. This study aimed to validate actigraphy for nighttime sleep monitoring of hospitalized patients with severe traumatic injuries, using ambulatory polysomnography, and to identify the optimal scoring method for this population and setting.
Study Impact: Results confirm that actigraphy is a valid tool for monitoring nighttime sleep and wakefulness in patients hospitalized with acute traumatic injuries. Though objective monitoring of sleep and wakefulness in acute care is currently not a standard clinical procedure, actigraphy could enable the medical staff to identify and treat early sleep-wake disturbances, which could optimize recovery.
INTRODUCTION
Sleep and wakefulness disturbances are frequently observed in patients hospitalized with acute traumatic injuries.1,2 Typically, these patients have an absence of a consolidated 24-hour sleep-wake cycle, fragmented periods of sleep and wakefulness, and low sleep efficiency.1,3,4 These sleep-wake disturbances are probably disabling enough to impede early rehabilitation interventions. A greater consolidation of the 24-hour sleep-wake cycle, with a higher concentration of sleep time during the night, is associated with better outcomes among patients with traumatic brain injury (TBI), including a shorter length of stay in rehabilitation and better functional and cognitive recovery.1,2,5–7 Improving evaluation of nighttime sleep quality in patients hospitalized for traumatic injury may support early treatment of sleep-wake disturbances, leading to earlier and better recovery.
Currently, objective monitoring of sleep and wakefulness in acute care is not a standard clinical procedure. Ideally, sleep quality would be monitored for several consecutive days, as sleep quality may vary from one night to another. In addition, the trend in improvement over many days is also informative of the patient’s state of recovery.2 Prolonged monitoring would also enable the medical team to assess the effects of interventions aimed at improving nighttime sleep and daytime wakefulness. Polysomnography (PSG) is the gold-standard procedure to measure sleep and can identify sleep stages and detect abnormal events during sleep such as sleep apneas. However, it is not suitable for monitoring over several consecutive days, because it is difficult to tolerate the electrodes for a long period. PSG is also time consuming (material installation and sleep scoring), requires specialized staff, and is not well tolerated by patients who are disoriented and/or uncollaborative. For example, in a recent study, on the 59 hospitalized trauma patients eligible for a 24-hour PSG study, PSG was possible in only 7 patients.4 We therefore need to validate other tools that can easily assess sleep quality for a prolonged period of time in hospitalized patients.
Actigraphy is a noninvasive, inexpensive technique that allows the measurement of rest-activity patterns and is recognized as an indirect measure of sleep quality.8 It consists of a wrist-worn device with an accelerometer that monitors motion in all directions. The quantity of movements during a specific period of time (epoch) is then calculated and enables sleep-wake scoring. From this scoring, sleep characteristics can be derived from actigraphy such as sleep onset latency, total sleep time, number and duration of awakenings, and sleep efficiency.9
Actigraphy thus appears to be a suitable option for the assessment of sleep within a hospitalized population and has been successfully used in acute settings and rehabilitation centers.1–3,10–12 With the aim of validating actigraphy with PSG in a rehabilitation center, Kamper and colleagues recently tested patients with mild to severe TBI for 1 night, 139 ± 254 days postinjury.12 Actigraphy showed moderate to strong correlations with PSG for nighttime total sleep time (r = .78, P < .01) and sleep efficiency (r = .66, P < .01). However, epoch-by-epoch concordance was not assessed for this study. No study has yet validated actigraphy in an acute trauma care setting.
Several algorithms have been developed to optimize validity of actigraphy among healthy individuals. Some algorithms use a predetermined threshold of motor activity per minute to score a 1-minute epoch as wake. Low thresholds are more specific (ie, better able to detect wake), whereas high thresholds are more sensitive (ie, better able to detect sleep).13–15 Other algorithms use variables derived from statistical analyses in order to build a regression equation that identifies a specific epoch as sleep or wakefulness,13,16 but these methods have led to similar sensitivity (range: 94% to 99%) and specificity (range: 34% to 53%) than those observed with threshold methods.
The aim of the current study was to validate nighttime sleep actigraphy in hospitalized patients with severe traumatic injuries (brain, orthopedic, and/or spinal cord injuries) using ambulatory PSG. A second objective was to identify the actigraphy scoring algorithm that provides optimal sensitivity and specificity for this population and setting. We hypothesized that low threshold would have a better total agreement with PSG, because of the better ability of detecting wake episodes, particularly in a bedridden population, where movements are more limited and wakefulness harder to detect. A third objective was to explore differences in the validity of actigraphy between patients with TBI, who are known to have more severe sleep-wake disturbances,17 and patients with orthopedic and/or spinal cord injury, without TBI (non-TBI).
METHODS
Participants
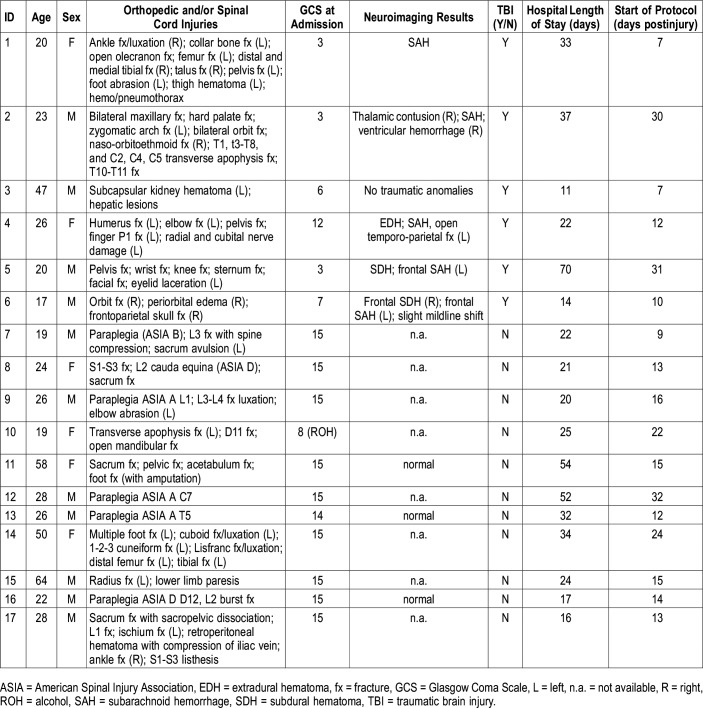
The study included 17 patients (11 men) aged 30.4 ± 14.7 years (range: 17 to 64 years old) who had experienced severe orthopedic injuries and/or a spinal cord injury (Table 1). Of these, six also experienced moderate to severe TBI and constituted the TBI subgroup. All patients were hospitalized at Hôpital du Sacré-Coeur de Montréal, a level 1 trauma center. Severe orthopedic injury was defined as a complex traumatic injury, such as multiple fractures with or without damage to peripheral nerves or to the vascular system, which necessitates intervention by a specialized multidisciplinary team. TBI was defined as an alteration in brain function or other evidence of brain pathology caused by an external force,18 and TBI severity was established with the Glasgow Coma Scale at admission to the emergency department.19 Patients were excluded if they were quadriplegic. None had a history of psychiatric conditions, drug abuse, neurological disorders, or a diagnosis of sleep disorder before their injury. The hospital ethics committee approved the study. Patient consent was obtained from the family and subsequently from the patients, when they were cognitively able to provide consent for themselves.
Table 1.
Demographic and clinical characteristics of patients.
Protocol
The study began when patients had reached medical stability, were no longer intubated, had no elevated intracranial pressure, no fever or signs of infection, and no hemodynamic instability. For the eight patients who were continuously sedated at the beginning of their intensive care unit stay, continuous sedation had stopped 12.4 ± 7.8 days (range: 3 to 25) prior to the beginning of the protocol. Ten patients received analgesic agents during the study (seven patients received hydromorphone, one patient received morphine, one patient received oxycodone, and five patients received pregabalin; some patients received multiple analgesic agents during the PSG recording) and three patients received psychostimulants (amantadine, methylphenidate, and lisdexamfetamine).
PSG recordings were carried out at the bedside with the Siesta ambulatory system (Compumedics Limited, Charlotte, North Carolina, United States) for an average 20 hours. Only nighttime sleep periods were used for the concordance analysis with actigraphy, as some patients were not able to tolerate the PSG material for the 24 hours. Assessing nighttime sleep periods allowed us to compare our results with previous studies in healthy participants. Nighttime sleep periods were defined as the first sleep period beginning between 8:00 pm and 10:00 pm. If a patient was already sleeping at 8:00 pm, the nighttime sleep period began at the start of the sleep period that was already ongoing at 8:00 pm. The end of the sleep period was marked by a period of awakening longer than 15 consecutive minutes, which began between 6:00 am and 9:00 am. PSG comprised four electroencephalographic electrodes (F4, C3, C4, O2) with an ear lobe (A2) reference, as well as two electrooculographic and three chin electromyographic electrodes. It also included oronasal thermistance and oxygen saturation measure for 14 patients who were able to tolerate the material. A technician stayed next to the patient’s room during the recording to observe the PSG recording and replace electrodes if needed.
Each patient wore an actigraph (Actiwatch-L or Actiwatch-Spectrum, Philips Healthcare, Andover, Massachusetts, United States) on a nonparalyzed arm throughout the duration of PSG recording. The actigraph recorded wrist movements per 1-minute epochs with an accelerometer of a 0.05-g sensitivity.
Data analysis
For PSG recording, sleep scoring was carried out with commercial software (Harmonie, Stellate Systems, Montreal, Canada) using standard criteria and 30-second epochs.20 The 30-second epochs were then rescored into 1-minute epochs: to score sleep, both 30-second epochs needed to be scored as sleep, otherwise it was scored as wakefulness. For actigraphy, the movements of each 1-minute epoch were automatically converted into an electric signal and digitally integrated to provide an activity count per epoch. Actigraphy data were downloaded into Actiware 5.0 (MiniMitter Philips Healthcare, Andover, Massachusetts), the dedicated software. A visual inspection of raw data recorded by actigraphy was performed to ensure there were no malfunctions or artifacts. Visual inspection of both PSG and actigraphy recordings was carried out to ensure that there were no temporal gaps between these two measures.
Actigraphy sleep-wake scoring algorithms
Four algorithms were used to score each 1-minute epoch of actigraphy data as sleep or wakefulness: two threshold methods, and two regression equation-based methods.
Threshold methods
The two threshold algorithms were provided by the actigraph manufacturer (Actiware 5.0). These algorithms, which have previously been validated among patients with sleep disorders,14 classify an epoch as sleep or wakefulness by considering the activity count of the analyzed epoch while taking into account the epochs prior to and following the analyzed epoch. As an example, a 1-minute epoch is scored as follows:
Where A represents the sum of activity counts computed by the algorithm and En represents the sum of activity counts for the analyzed epoch E0, the preceding epochs (E−2 and E−1) and the subsequent ones (E1 and E2). If A exceeds the threshold, the epoch is scored as wake; otherwise, if A is equal or inferior to the threshold, the epoch is scored as sleep. In this study, only thresholds of 20 (Act20, low threshold) and 40 (Act40, medium threshold) were used because of their superior sensitivity/specificity ratio.13,14
Regression equation-based methods
The third algorithm was developed by Lötjönen et al,21 based on a method developed by Sadeh et al.22 For this method, the activity count was converted into four variables: the mean activity in a window of 7 epochs including the analyzed epoch and those around it; the standard deviation of the activity in a window of 8 epochs including the analyzed epoch and those around it; the number of activity counts above 10 in a window of 11 epochs around the scored epoch; and the natural logarithm of the activity in the analyzed epoch. The regression equation used by Lötjönen et al21 is:
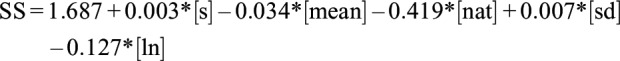 |
Where SS, representing the sleep score, is either positive or negative and accordingly indicates sleep or wake; s represents the activity value of the analyzed epoch; mean represents the mean activity in a window of 7 epoch including and around the analyzed epoch; nat represents the number of activity counts above 10 in a window of 11 epochs including and around the analyzed epoch; sd represents the standard deviation of the activity in a window of 8 epochs around the analyzed epoch; and ln represents the natural logarithm of the activity in the analyzed epoch. In this equation, the activity value of the analyzed epoch is the independent variable and the PSG sleep/wake classification is the dependent one. The third method (Lötjonen et al) consisted in directly applying this function to our data.
The fourth method was once again derived from Lötjönen et al,21 but was modified by Paquet et al.13 Though the same variables as Lötjönen et al21 were used, the variable “mean” was not considered significant and was thus excluded. The equation is as follows:
Sleep parameters
Sleep parameters were calculated with the same definition for the PSG and the four actigraphy scoring algorithms. These four sleep parameters were total sleep time, total wake time, sleep efficiency, and the number of awakenings. Total sleep time was the sum of all 1-minute epochs identified as sleep in the sleep period. Sleep efficiency was defined as the percentage of sleep time during the sleep period: (total sleep time / sleep period duration) × 100. Total wake time was the sum of all 1-minute epochs identified as wake in the sleep period. Number of awakenings was the number of periods of one or more consecutive minutes of wakefulness.
Statistical analyses
Epoch-by-epoch agreement between actigraphy and PSG
We carried out an epoch-by-epoch analysis, which compared the minute-by-minute data of both actigraphy and PSG, for each of the four actigraphy scoring algorithms (Act20, Act40, Lötjonen et al, and Paquet et al), in order to assess sensitivity, specificity, and accuracy. Sensitivity was defined as the proportion of all epochs scored as sleep by the PSG that are also scored as sleep by actigraphy. Specificity was defined as the proportion of all epochs scored as wake by the PSG that are also scored as wake by actigraphy. Accuracy was defined as the proportion of all epochs that are correctly identified by actigraphy. To compare the algorithms, analysis of variance (ANOVA) with one repeated measure (four algorithms) was performed separately on sensitivity, specificity, and accuracy. When significant differences were found, the post hoc Tukey Honest Significant Difference test, a simple effect analysis, was used to compare the means.
Agreement between actigraphy and PSG on sleep parameters
In order to compare the different sleep assessment methods (PSG, Act20, Act40, Lötjonen et al, and Paquet et al), repeated-measures ANOVAs were performed on the four sleep parameters (total sleep time, total wake time, sleep efficiency, and number of awakenings). Subsequently, if any significant differences were found, the post hoc Dunnett test was performed to compare actigraphy algorithms to PSG, which acted as control.
To assess agreement between PSG and actigraphy, sleep parameters obtained from PSG were compared to those obtained from the four actigraphy scoring algorithms by Bland-Altman plots. For each sleep parameter, a graph was made with the average of (on x axes) and the difference between (on y axes) PSG and actigraphy estimates of each patient. The mean differences (bias) and the standard deviation of the differences were calculated. Mean difference represented the difference between PSG and actigraphy. A positive bias indicates an underestimation of the parameters by the actigraphy, whereas a negative bias indicates an overestimation. The standard deviation of the mean differences provides an estimation of the bias variation between the two measures. The Bland-Altman analyses were carried out with the software GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, California, United States).
Comparing the efficacy of actigraphy between patients with and without TBI
Lastly, subgroups of patients with TBI and those without TBI were compared to explore whether actigraphy performs differently in the assessment of sleep period. Using an independent sample t test, the sensitivity, specificity, and accuracy obtained with each algorithm were compared between groups.
RESULTS
PSG and actigraphy were recorded simultaneously, 16.6 ± 8.2 days postinjury, while patients were hospitalized in a regular neurologic or orthopedic unit. Overall, sleep period duration varied between 454 and 671 minutes (mean: 569.2 ± 73.5 minutes). In these sleep periods, participants had an average of 466.1 ± 63.1 minutes of sleep and an average of 101.0 ± 47.4 minutes of wakefulness.
Epoch-by-epoch agreement between actigraphy and PSG
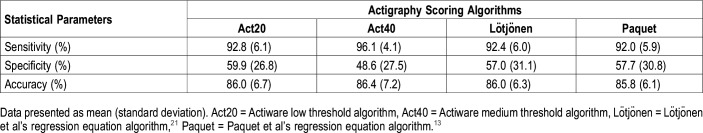
Table 2 presents sensitivity, specificity, and accuracy values obtained from epoch-by-epoch comparisons between the four actigraphy scoring algorithms and PSG. Sensitivity was at least 92% for all algorithms. Repeated-measures ANOVA showed a significant scoring algorithm effect for sensitivity (F3,48 = 21.8; P < .001). The post hoc analysis revealed that the sensitivity of Act40 was significantly higher than that of the other three algorithms (P < .01). A significant scoring algorithm effect was also found for specificity (F3,48 = 5.2: P < .003), which ranged from 48.6% to 59.9%, and Act40 had significantly lower specificity compared to the other three algorithms. Accuracy was higher than 85% for all four actigraphy scoring algorithms, and no significant differences were found between the algorithms.
Table 2.
Sensitivity, specificity, and accuracy of epoch-by-epoch comparison with polysomnography of four actigraphy scoring algorithms (n = 17).
Agreement between actigraphy and PSG on sleep parameters
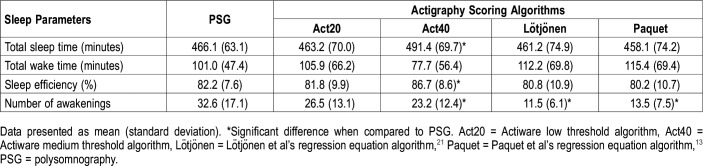
Table 3 shows sleep parameters derived from PSG and actigraphy. A significant scoring algorithm effect was found for total sleep time (F4,64 = 4.5; P = .003) and post hoc analysis showed that Act40 overestimated this sleep parameter compared to PSG. A significant scoring algorithm effect was also obtained for sleep efficiency (F4,64 = 5.7; P < .001), and again, Act40 overestimated sleep efficiency compared to PSG values. A significant scoring algorithm effect was also found for the number of awakenings (F4,64 = 19.6; P < .001) where Act40, Lötjonen et al, and Paquet et al algorithms underestimated the number of awakenings when compared to PSG. However, no significant differences were found for total wake time.
Table 3.
Sleep parameters scored by polysomnography and estimated by the four actigraphy scoring algorithms (n = 17).
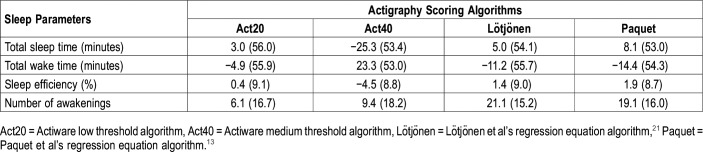
The Bland-Altman method was used to compare sleep parameters obtained with actigraphy to those obtained with PSG. Table 4 depicts the difference between PSG and the four actigraphy algorithms. We observed that Act20, Lötjonen et al, and Paquet et al underestimated total sleep time, whereas Act40 overestimated it. Regarding the total wake time, we observed an overestimation by Act20, Lötjonen et al, and Paquet et al, but an underestimation for Act40. Sleep efficiency was underestimated by an average of 1.2% with Act20, Lötjonen et al, and Paquet et al, but was overestimated by Act40. Finally, all the algorithms underestimated the number of awakenings. For each sleep parameter, we observed a high standard deviation, which suggests high interindividual variability among our algorithms.
Table 4.
Mean difference and standard deviation of mean difference obtained from Bland-Altman plots between polysomnography and actigraphy scoring algorithms (n = 17).
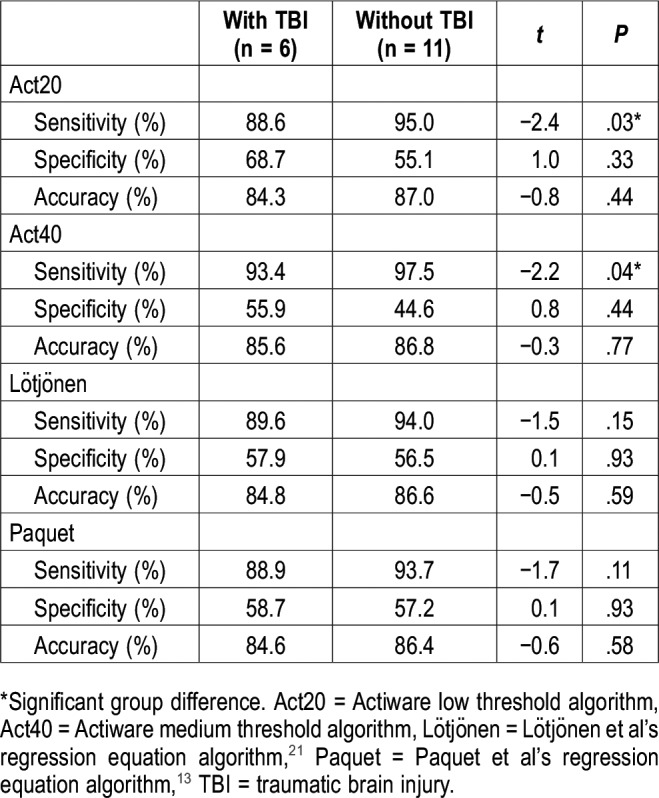
Group differences between patients with TBI and without TBI on actigraphy
Table 5 shows the results of the independent-sample t tests. Group differences were found for the sensitivity of Act20 (t15 = −2.4; P < .03) and Act40 (t15 = −2.2; P < .04), where actigraphy showed higher sensitivity in patients without TBI than in patients with TBI. No other group differences were found.
Table 5.
Comparison of actigraphy parameters among patients with and without TBI.
DISCUSSION
This study validated the use of actigraphy for the assessment of nighttime sleep and wakefulness among hospitalized patients with severe traumatic injuries. Our main result was that actigraphy in hospitalized trauma patients has an excellent sensitivity (above 92%) and high epoch-by-epoch accuracy (above 85%) when compared with ambulatory PSG. The sensitivity and accuracy obtained in this study are similar to those reported in studies among healthy individuals under normal conditions.13,16,23,24 Specificity, which is the ability of actigraphy to detect wakefulness, was rather low (below 60%) but nevertheless similar or even higher than what has been observed in previous studies among healthy control patients.13,16,23 Consequently, we conclude that actigraphy is a valid tool for monitoring nighttime sleep and wakefulness in patients hospitalized with acute severe traumatic injuries.
Our second objective was to compare four actigraphy scoring algorithms in order to identify the optimal algorithm for this clinical population. Although a medium automatic wake threshold (Act40) had a better ability to detect sleep when compared to the low wake threshold (Act20) and the two regression equation methods (Lötjönen et al and Paquet et al), it was significantly worse in detecting wake. As predicted, the sleep parameters identified by Act20 showed a greater similarity with PSG results in terms of total sleep time, total wake time, sleep efficiency, and number of awakenings, when compared to other algorithms. Act20 also had the highest specificity (59.9%) and an excellent sensitivity (92.8%). Given that Act20 is the algorithm with the lowest wake threshold value, it is not surprising that this algorithm is more suitable for bed-ridden patients and/or those with acute traumatic injuries, whose mobility may be restricted. Ultimately, given some variation in the ability to detect sleep (sensitivity) or wake (specificity) between the algorithms, the choice of algorithm should be made in regard to the research question, that is, whether the main objective of the study is detecting sleep or wakefulness.
The third objective of this study was to explore the effect of TBI on the validity of actigraphy. The only group differences that we found were for sensitivity, where actigraphy showed lower sensitivity using Act20 and Act40 in patients with TBI compared to those without TBI. This result is in line with the general weaknesses of automatic threshold scoring methods, as discussed by Paquet and colleagues,13 which seem more affected in their ability to identify sleep when sleep is highly fragmented, as is the case in patients with TBI.4 Though the generalization of this result is limited considering our small sample, it points to the need for future validation studies among different patient populations in order to appraise the strengths and weaknesses of actigraphy in the hospital setting.
Limitations
Our study showed strong concordance between actigraphy and PSG for nighttime sleep in hospitalized patients with severe traumatic injuries, even among bedridden patients with moderate to severe TBI. However, despite the overall validity of nighttime actigraphy in this population and setting, we found a high interindividual variability agreement between actigraphy and PSG, which has important implications when actigraphy is used as a clinical tool on an individual level. Nursing interventions, noise, medication, and pain may all have contributed to interindividual variability in actigraphy validity compared to PSG. Unfortunately, our sample size was not large enough to explore the effect of these factors. Combining actigraphy with a sleep diary can increase validity of actigraphy, but patients may not always be able to use a sleep diary accurately, because they may be confused, disoriented, or have orthopedic injuries that limit writing. A nursing sleep diary could also be an interesting alternative to complement actigraphy but requires thorough and continuous monitoring in order to be useful in this context.
The evaluation of sleep duration and quality over the entire 24-hour sleep-wake cycle provides very useful information, especially to assess total daily sleep time and/or the distribution of sleep and wakefulness over a 24-hour period. In the current study, however, although actigraphs were worn during both daytime and nighttime, most patients could not tolerate the PSG material for the entire 24 hours, and the quality of the PSG signal was, in some patients, poorer during the daytime period. Therefore, it was not possible to assess the validity of actigraphic recordings in the daytime. Actigraphy can be expected to have a lower specificity in the daytime compared to nighttime when patients have periods during which they rest in bed without sleeping. However, this hypothesis remains to be tested.
CONCLUSIONS
Though actigraphy had previously been shown to be valid and reliable for the assessment of sleep and sleep disorders among healthy and clinical populations,12–14,25,26 the current study is the first to validate actigraphy for nighttime sleep-wake monitoring in an acute care setting, among bedridden patients with severe traumatic injuries. Validating actigraphy among patients with acute traumatic injury and promoting objective sleep monitoring in this clinical setting could have important implications for optimizing recovery, especially among patients who are unable to properly communicate their sleep disturbances. Future studies using actigraphy among patients with acute traumatic injury should favor a low wake threshold value for a more accurate assessment of nocturnal sleep and wake. Given the small size of our sample, this study serves an exploratory purpose and aims to foster future validation studies with larger samples and covering the entire daily sleep-wake cycle.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Hôpital du Sacré-Coeur de Montréal, Montreal, Canada. This study was supported by the Canadian Institutes of Health Research (grant no. 115172) and by the Fonds pour la Recherche du Québec– Santé (grant no. 24742). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the patients and their families for their collaboration, as well as the staff of the Intensive Care Unit, Neurological Unit, and Orthopedic Unit for their help in monitoring patients during actigraphy recordings.
ABBREVIATIONS
- Act20
wake threshold of 20 activity counts per epoch
- Act40
wake threshold of 40 activity counts per epoch
- ANOVA
analysis of variance
- PSG
polysomnography
- TBI
traumatic brain injury
REFERENCES
- 1.Duclos C, Dumont M, Blais H, et al. Rest-activity cycle disturbances in the acute phase of moderate to severe traumatic brain injury. Neurorehabil Neural Repair. 2014;28(5):472–482. doi: 10.1177/1545968313517756. [DOI] [PubMed] [Google Scholar]
- 2.Duclos C, Dumont M, Arbour C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2017;88(3):268–275. doi: 10.1212/WNL.0000000000003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duclos C, Dumont M, Potvin MJ, et al. Evolution of severe sleep-wake cycle disturbances following traumatic brain injury: a case study in both acute and subacute phases post-injury. BMC Neurol. 2016;16(1):186. doi: 10.1186/s12883-016-0709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiseman-Hakes C, Duclos C, Blais H, et al. Sleep in the acute phase of severe traumatic brain injury: a snapshot of polysomnography. Neurorehabil Neural Repair. 2016;30(8):713–721. doi: 10.1177/1545968315619697. [DOI] [PubMed] [Google Scholar]
- 5.Makley MJ, Johnson-Greene L, Tarwater PM, et al. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabil Neural Repair. 2009;23(4):320–326. doi: 10.1177/1545968308325268. [DOI] [PubMed] [Google Scholar]
- 6.Nakase-Richardson R, Sherer M, Barnett SD, et al. Prospective evaluation of the nature, course, and impact of acute sleep abnormality after traumatic brain injury. Arch Phys Med Rehabil. 2013;94(5):875–882. doi: 10.1016/j.apmr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb EM, Towns S, Kamper JE, et al. The relationship between sleep-wake cycle disturbance and trajectory of cognitive recovery during acute traumatic brain injury. J Head Trauma Rehabil. 2016;31(2):108–116. doi: 10.1097/HTR.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 8.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 10.Chiu HY, Chen PY, Chen NH, Chuang LP, Tsai PS. Trajectories of sleep changes during the acute phase of traumatic brain injury: a 7-day actigraphy study. J Formos Med Assoc. 2013;112(9):545–553. doi: 10.1016/j.jfma.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Towns SJ, Zeitzer J, Kamper J, et al. Implementation of actigraphy in acute traumatic brain injury neurorehabilitation admissions: a Veterans Administration TBI model systems feasibility study. PM R. 2016;8(11):1046–1054. doi: 10.1016/j.pmrj.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kamper JE, Garofano J, Schwartz DJ, et al. Concordance of actigraphy with polysomnography in traumatic brain injury neurorehabilitation admissions. J Head Trauma Rehabil. 2016;31(2):117–125. doi: 10.1097/HTR.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 13.Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30(10):1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 15.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76(11):1058–1063. [PubMed] [Google Scholar]
- 16.de Souza L, Benedito-Silva AA, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Duclos C, Dumont M, Paquet J, et al. Sleep-wake disturbances in hospitalized patients with traumatic brain injury: association with brain trauma but not with an abnormal melatonin circadian rhythm. Sleep. doi: 10.1093/sleep/zsz191. 2019 Sep 28. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Lötjönen J, Korhonen I, Hirvonen K, Eskelinen S, Myllymaki M, Partinen M. Automatic sleep-wake and nap analysis with a new wrist worn online activity monitoring device vivago WristCare. Sleep. 2003;26(1):86–90. [PubMed] [Google Scholar]
- 22.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 23.Blood ML, Sack RL, Percy DC, Pen JC. A comparison of sleep detection by wrist actigraphy, behavioral response, and polysomnography. Sleep. 1997;20(6):388–395. [PubMed] [Google Scholar]
- 24.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30, viii.. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20(1):24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maglione JE, Liu L, Neikrug AB, et al. Actigraphy for the assessment of sleep measures in Parkinson’s disease. Sleep. 2013;36(8):1209–1217. doi: 10.5665/sleep.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]