Abstract
Background
Existing methods for preparing influenza vaccines pose the greatest challenge against highly pandemic avian influenza H7N9 outbreak in the poultry and humans. Exploring a new strategy for manufacturing and delivering a safe and effective H7N9 vaccine is needed urgently.
Results
An alternative approach is to develop an influenza H7N9 oral vaccine based on yeast display technology in a timely manner. Hemagglutinin (HA) of A/Anhui/1/2013 (AH-H7N9) is used as a model antigen and characterized its expression on the surface of Saccharomyces cerevisiae (S.cerevisiae) EBY 100. Mice administrated orally with S.cerevisiae EBY100/pYD5-HA produced significant titers of IgG antibody as well as significant amounts of cytokines IFN-γ and IL-4. Importantly, S.cerevisiae EBY100/pYD5-HA could provide effective immune protection against homologous A/Anhui/1/2013 (AH-H7N9) virus challenge.
Conclusions
Our findings suggest that platform based on yeast surface technology provides an alternative approach to prepare a promising influenza H7N9 oral vaccine candidate that can significantly shorten the preparedness period and result in effective protection against influenza A pandemic.
Keywords: S.cerevisiae EBY100/pYD5-HA, Yeast display technology, Influenza A pandemic
Background
The highly pathogenic H7N9 virus has severely affected the poultry industry and posed a serious threat to human health [1]. The most effective way to curtail pandemics is by mass vaccination [2]. Currently, there are two types of licensed vaccines against seasonal influenza in the US: subunit (split) inactivated vaccines and live attenuated influenza vaccine (LAIV) [3, 4]. Both vaccines rely on embryonated chicken eggs as substrates for production. The process of constructing a new vaccine strain based on newly circulating viruses is quite lengthy. It involves in ovo (in chicken eggs) or in vitro (in cell culture using reverse genetics techniques) reassortment between the internal genes of a donor virus such as A/PR/8/34 with the hemagglutinin (HA) and neuraminidase (NA) of the new influenza strain [5]. The candidate vaccine strains must be further selected based on their high growth capability in eggs and high yield of HA content before they can be used for production of vaccines. In this case, manufacturing problems experienced in recent years illustrate that the current methods of production are fragile in ensuing an adequate and timely supply of influenza vaccine [6]. More importantly, the egg-based technology may not be suitable to respond to a pandemic crisis. Also, due to the high pathogenicity of H7N9 strains, the conventional production would require biosafety level 3 containment facilities and take several months following the identification of new potential strains. Therefore, a strategy that can rapidly produce new influenza vaccines is needed as a priority for pandemic preparedness.
Saccharomyces cerevisiae (S.cerevisiae), a nonpathogenic yeast, is an ideal organism to express viral or tumor antigens, and is the most common host for cell surface display [7, 8]. Recently, influenza H5N1 HA has been expressed on the surface of S.cerevisiae by C-terminal display expression plasmid pYD1 [9]. Although detailed information is provided that the HA-presented on the surface of S.cerevisiae has immunogenicity in animal models, intramuscularly or intraperitoneally route would bring serious inflammation since the diameter of yeast is around 10 μm which could not be absorbed completely. As a new platform based on S.cerevisiae N-terminal surface display technology for H7N9 vaccine development, little is known regarding the protective immunity of S.cerevisiae—based vaccine by oral administration route.
To address this question, we chose HA of A/Anhui/1/2013 (AH-H7N9) as a model antigen which was regarded as the strong immunogenicity for candidate vaccine target [10], and constructed S.cerevisiae EBY100/pYD5-HA. Further, we investigated the immunogenicity of oral administration with S.cerevisiae EBY100/pYD5-HA in mice. Our data demonstrate that oral vaccination with S.cerevisiae EBY100/pYD5-HA in the absence of mucosal adjuvant can elicit significantly humoral and cellular immune responses, as well as significant HI titers. Most importantly, S.cerevisiae EBY100/pYD5-HA would be able to provide effective immune protection against homologous H7N9 virus infection. These findings clearly support that influenza oral vaccine based on S.cerevisiae surface display technology is likely to play an important role in preventing and controlling H7N9 outbreaks and thus may provide a feasible foundation for developing safe and effective vaccines against other avian influenza viruses.
Methods
Plasmids, yeast and culture conditions
The HA gene (1632 bp) of A/Anhui/1/2013 (AH-H7N9) was PCR-amplified from pCDNA3.1/H7N9/HA using the following primers: HA-F: CTAGCTAGCAATGCAGACAAAATC (Nhe I); HA-R: CCGGAATTCTATACAAATAGTGCACC (EcoRI) and subcloned into the yeast display plasmid, pYD5, which was kindly provided by Dr. Z Wang [11] and allowed the NH2 terminus of the displayed protein of interest to be free. The shuttle plasmid pYD5-HA was transformed into competent Escherichia coli DH5α (New England Biolabs, Beverly, MA) and then electroporated into competent S.cerevisiae EBY100 (Invitrogen, San Diego, CA). Recombinant yeast transformants were grown on selective plate which contained 0.67% yeast nitrogen base (YNB) without amino acids, 2% dextrose, 0.01% leucine, 2% agar and 1 M sorbitol at 30 °C for 3 days. Single positive clone S.cerevisiae EBY100/pYD5-HA was selected and cultured in 3 mL of YNB-CAA (20 g/L dextrose, 6.7 g/L yeast nitrogen base without amino acids, 13.61 g/L Na2HPO4, 7.48 g/L NaH2PO4 and 5 g/L casamino acids) overnight at 30 °C with shaking. Inducible expression of S.cerevisiae EBY100/pYD5-HA was performed in YNB-CAA medium where dextrose was replaced by 20 g/L of galactose at 20 °C for 3 days with shaking. Meanwhile, S.cerevisiae EBY100 containing empty pYD5 was used as a negative control for the following tests.
Detection of HA protein expression
1 OD600nm of S.cerevisiae EBY100/pYD5-HA pellets (1 OD600nm ≈ 107 cells) was collected at 72 h post-induction, and washed three times with 500 µL of sterile phosphate-buffered saline (PBS) for Western blotting, immunofluorescence and flow cytometric assay.
For Western blot analysis, 1 OD600nm of S.cerevisiae EBY100/pYD5-HA pellets were re-suspended with 50 µl of 6× loading buffer and boiled for 10 min. Treated samples were resolved using SDS–polyacrylamide gel electrophoresis and then electrophoretically transferred to nitrocellulose membrane (Bio-rad, Hercules, California, USA). After blocking with 5% non-fat milk at room temperature for 2 h, the blot was probed with a monoclonal mouse anti-HA antibody (1: 500 diluted) (kindly provided by NIH Biodefense and Emerging Infections Research Resources Repository, Manassas, VA, USA), and incubated overnight at 4 °C. The membrane was followed by 1: 5000 diluted horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Sigma-Aldrich Corporation, St. Louis, MO, USA) at room temperature for 1 h and developed with the West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA) and imaged using Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Furthermore, 1 OD600 of S.cerevisiae EBY100/pYD5-HA pellets were treated by PNGase F kit (New England Biolabs, Beverly, MA, USA) for detecting N-glycosylation of HA protein.
For immunofluorescence assay and flow cytometric analysis, 1 OD600nm of S.cerevisiae EBY 100 pellets was incubated with a monoclonal mouse anti-HA antibody (1: 500 diluted) at 4 °C for 1 h, and followed by FITC-conjugated goat anti-mouse IgG (1: 5000 diluted) at 4 °C for 30 min and re-suspended with 500 µL of sterile PBS. Finally, 5 µL of S.cerevisiae EBY100/pYD5-HA pellets were used for immunofluorescence assay (Olympus IX70, Japan), and 300 µL of S.cerevisiae EBY100/pYD5-HA cells were analyzed by flow cytometry analysis (BD FacsCalibur, BD Bioscience, San Jose, CA, USA), respectively.
Quantification of S.cerevisiae EBY100/pYD5-HA expressing HA protein by indirect ELISA
S.cerevisiae EBY100/pYD5-HA expressing HA protein was assayed using a modified, previously published ELISA protocol [12]. Briefly, 10 OD600nm of S.cerevisiae EBY100/pYD5-HA cells (1 OD600nm ≈ 107 cells) were re-suspended in 100 μL of a monoclonal mouse anti-HA antibody (0, 5, 10, 25, 50, 75, 100, 125 and 150 μg/mL) (kindly provided by NIH Biodefense and Emerging Infections Research Resources Repository, Manassas, VA, USA) in PBS containing 1% BSA and incubated at room temperature for 1 h. Then, cells were washed 3 times in PBS. Goat anti-mouse IgG antibody conjugated with horseradish peroxidase (1 mg/ml) (Sigma-Aldrich Corporation, St. Louis, MO, USA) was added and the mixture was incubated at room temperature for 1 h. After washing in the same way, the cells were re-suspended in 100 μL of PBS and subjected to protein surface detection by incubating in 100 μl of HRP substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma-Aldrich Corporation, St. Louis, MO, USA) in the dark for 30 min followed by addition of 100 μl of 2 mol/L H2SO4 to stop the reaction. The yeast cell suspension was spun down and the supernatant OD450 was measured using a microplate reader. S.cerevisiae EBY100/pYD5 was used a negative control.
Vaccine, animals, immunization and virus challenge
S.cerevisiae EBY100/pYD5-HA cells were harvested at 72 h post-induction and treated by heat-inactivation at 60 °C for 1 h. The final concentration of S.cerevisiae EBY100/pYD5-HA was adjusted to 1.0 OD600nm/µL using sterile PBS and stored at 4 °C until use.
Eight-week-old female BALB/c mice (Jackson Laboratories, ME, USA) were used for all studies and housed in the specific pathogen-free (SPF) facilities. Mice (n = 10/group) were vaccinated orally with 150 OD600nm of S.cerevisiae EBY100/pYD5-HA on day 1 for prime immunization and boosted on day 14. For comparison, 150 OD600nm of S.cerevisiae EBY 100/pYD5 or 150 μL of PBS was used as a negative control. Mice weights were recorded at pre-immunization and post-immunization.
For the virus challenge experiments, all vaccinated mice were anesthetized and intranasally inoculated with 50 μL of 10 × 50% lethal dose (LD50) A/Anhui/1/2013 (AH-H7N9) virus on day 35 after the initial immunization. Mice were monitored for survival and body weight change for 14 days. At day 3 post infection, mice (n = 3/group) were humanely euthanized, lungs were collected and washed with 1.0 mL of sterile PBS. A TCID50 assay was determined virus titers from clarified homogenates [9]. All animal immunizations complied with the Guidelines for Use and Care of Experimental Animal s and were approved by the Animal Committee of the Institute of Southwest Jiaotong University.
All virus challenge experiments with A/Anhui/1/2013 (AH-H7N9) were performed under enhanced biosafety level-3(BLS3)-plus containment facilities.
Enzyme-linked immunosorbent assay (ELISA)
Sera were isolated from blood samples collected at day 13 and 28 after the initial immunization, and stored at − 20 °C until use. Avidin–biotin system (ABS)-enzyme-linked immunosorbent assay (ELISA) was conducted to measure HA-specific IgG titers, as described previously [13]. Briefly, 96-well microplates (Costar, Corning, NY, USA) were coated with 2 μg/mL of recombinant HA protein, and incubated overnight at 4 °C. Plates were washed three times with TBS containing 0.05% Tween 20 (TBST) and then blocked with TBST containing 1% bovine serum albumin (BSA) at room temperature for 2 h. twofold diluted sera samples were added and incubated at 37 °C for 1 h. Bound antibodies were detected using biotinylated goat anti-mouse IgG (1: 5000 diluted) and followed by 1: 1000 diluted alkaline phosphatase-conjugated streptavidin (R&D Systems, USA). Finally, plates were developed using a pNPP phosphatase substrate (MP Biomedicals, Santa Ana, CA, USA), and the reaction was allowed to develop at room temperature for 25 min and then stopped by adding 50 μL of 2 mol/L NaOH to each well. Optical density (OD) was measured at 405 nm. The IgG titer was determined to be the lowest serum dilution with an OD greater than twice the mean OD of naïve serum plus 2 standard deviations.
ELISpot assay
Capture antibodies mouse IFN-γ and IL-4 cytokines (3 µg/mL in coating buffer, R&D Systems, USA) were coated on the Multiscreen 96-well filtration plates (Millipore, Billerica, MA, USA), respectively. Spleen cells (n = 3/group) were harvested at 2 weeks post prime-boost immunization. Freshly isolated splenocytes (1 × 106 cells) were cultured on the plate with 100 µL of RPMI 1640 media with 10% FBS, and then stimulated with or without 10 μg/mL of HA-specific peptide (PKGRGLFGAIAGFIENGWEGL) for 36 h in a humidified 37 °C CO2 incubator. After incubation, the plates were washed with sterile PBS and further incubated with biotinylated anti-mouse IFN-γ and IL-4 antibodies (1:5000 diluted). After three additional washes, alkaline phosphatase (AP) conjugated streptavidin (1: 1000) was added to each well and incubated at room temperature for 3 h. The plates were washed and developed with BCIP/NBT Chromogen (R&D Systems, USA). The number of IFN-γ and IL-4 secreting cells was counted using an ImmunoSpot ELISpot plate reader (Cellular Technology Ltd, Shaker Heights, OH, USA).
Hemagglutination inhibition (HI) assay
HI assay was performed to assess specific response to HA of H7N9 virus, as described previously [13]. Briefly, sera were treated by Receptor destroying enzyme (RDE) (Denka-Seiken, Tokyo, Japan) at 37 °C overnight, and then followed by heat-activation at 56 °C for 45 min. Two fold serial diluted samples (25 μL) in V-bottom 96-well plates were mixed with the equal volume of 4 HA units each well and incubated at room temperature for 30 min. 50 μL of 0.5% (v/v) chicken red blood cells (cRBCs) was added to each well, and hemagglutination was assessed visually after 40 min. The HI titer was expressed as the reciprocal of the highest dilution of the samples and the significant HI titer was greater than 16.
Statistical analysis
A two-tailed Student’s t-test and one-way ANOVA with post hoc analysis were used when comparing two different groups. A p value less than 0.05 was considered to be significant.
Results
Expression of HA protein on the surface of S.cerevisiae EBY 100
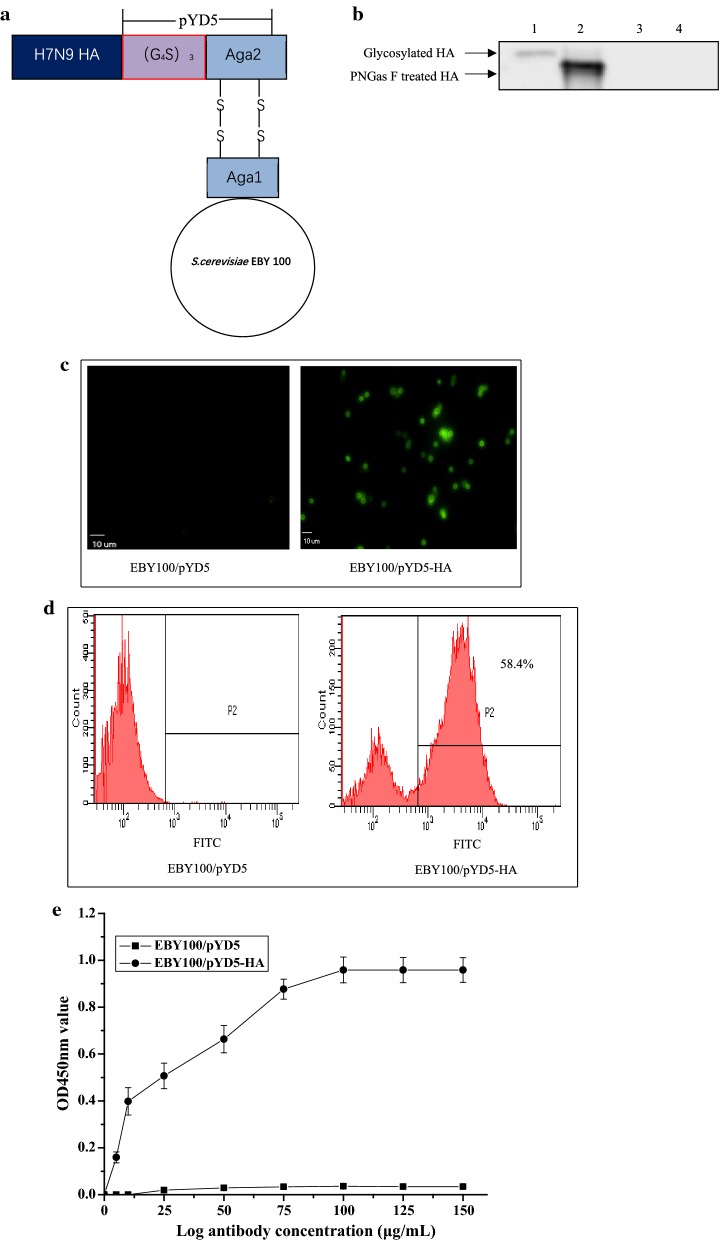
To determine the functional display of HA on yeast surface, the HA gene without signal peptide was subcloned into a display plasmid pYD5 led to the fusion of HA to N—terminus of Aga2, the second subunit of a-agglutinin receptor (Fig. 1a). The C-terminus of Aga2 entails both a secretion signal peptide and a binding site for Aga1, another subunit of the yeast a-agglutinin. The Aga2-HA was bound to Aga1 through two disulfide bonds, resulting in the HA display on yeast wall. The Aga1 gene was pre-integrated into the chromosome of the yeast.
Fig. 1.
Construction of yeast display vector and analysis of the expression of HA protein. a Schematic diagram of S.cerevisiae EBY100/pYD5-HA. The HA-Aga2 fusion protein binds to Aga1 through two disulfide bonds after its secretion from the S.cerevisiae. Aga1 is the first subunit of the yeast a-agglutinin receptor. A GS linker is inserted between Aga2 and HA to stabilize the fusion protein expression. b Western blotting analysis. Lane 1: HA glycoprotein. Lane 2: Deglycosylation of HA protein. Lane 3: S.cerevisiae EBY100/pYD5 control. Lane 4: Deglycosylation of S.cerevisiae EBY100/pYD5. c Immunofluorescence microscope. S.cerevisiae EBY100/pYD5 (left) and S.cerevisiae EBY100/pYD5-HA (right) (magnification 400×). d Flow cytometric analysis. S.cerevisiae EBY100/pYD5 (left) and S.cerevisiae EBY100/pYD5-HA (right). e Quantification of S.cerevisiae EBY100/pYD5-HA expressing HA protein by indirect ELISA. The values were obtained from three independent experiments. Bar = mean ± SD
Expression of HA protein was confirmed by Western blotting (Fig. 1b). As we expected, a specific band was observed at expected size for HA glycoprotein (approximately 65 kDa) (Fig. 1b, Lane 1).
On the other hand, to determine whether HA protein has a functional N-glycosylation, S.cerevisiae EBY 100/pYD5-HA cells were treated by PNGase F kit for detection of deglycosylation. As shown in Fig. 1b Lane 2, an expected size was indicated that HA protein had N-glycosylation modification. As a result, no specific bands were observed in S.cerevisiae EBY 100/pYD5 control cells. Overall, these findings indicate HA protein possess the post translational process of N-glycosylation.
Further, we performed immunofluorescence assay and flow cytometric analysis. As compared to S.cerevisiae EBY100/pYD5 controls, strong fluorescence derived from S.cerevisiae EBY100/pYD5-HA was observed (Fig. 1c, d). Taken together, these results demonstrate that HA protein display on the surface of S.cerevisiae EBY100 can be recognized by a monoclonal mouse anti-HA antibody that showing high binding specificity.
Quantification of HA protein on the yeast surface by indirect ELISA
When increasing concentrations of monoclonal anti-HA antibody was used against 10 OD600nm of S.cerevisiae EBY100/pYD5-HA cells/mL expressing HA, it was found that yeast displayed HA at approximately 100 μg on the cell surface (Fig. 1e). When the concentration of antibodies was increased beyond this point, the optical densities were more or less stable suggesting that expression of HA proteins on the cell surface was at its saturation limit at 100 μg/mL. In other words, 10 OD600nm of S.cerevisiae EBY100/pYD5-HA had 100 μg of HA protein displayed on the yeast surface, which was 1 OD600nm of S.cerevisiae EBY100/pYD5-HA had 10 μg of HA protein. This yeast-ELISA method was a high-throughput one to detect surface proteins without the need for protein extraction and purification [14].
Mice weights were recorded at pre-immunization and post-immunization, as shown in Table 1. These data indicate that mice vaccinated orally with recombinant yeast have no significant weight changes for prime-boost immunization.
Table 1.
Mice weighs change at pre- and post-immunization (n = 10/group)
| Group | Dose | Prime | Boost | ||
|---|---|---|---|---|---|
| Pre-immunization | Post-immunization | Pre-immunization | Post-immunization | ||
| PBS | 150 μL | 20.52 ± 1.21* | 20.52 ± 1.21* | 20.61 ± 1.21* | 20.61 ± 1.21* |
| EBY100/pYD5 | 150 OD600nm | 20.58 ± 1.11* | 20.52 ± 1.11* | 20.54 ± 1.11* | 20.52 ± 1.11* |
| EBY100-pYD-HA | 150 OD600nm | 20.56 ± 1.12* | 20.50 ± 1.11* | 20.51 ± 1.11* | 20.50 ± 1.11* |
The data are presented as mean ± standard deviation (SD)
Asterisk indicates statistical significance compared to S.cerevisiae EBY100/pYD5 and PBS groups (p < 0.05)
Antibody response and HI assay
The antibody responses in the sera of mice vaccinated orally with S.cerevisiae EBY100/pYD5-HA were evaluated by ELISA. Mice that received S.cerevisiae EBY100/pYD5-HA produced a low and detectable level of HA- specific IgG antibody after the prime immunization, and greatly increased to a high level after boost immunization (Fig. 2a). In contrast, no HA-specific antibodies were observed in PBS or S.cerevisiae EBY100/pYD5 group (Fig. 2a). Collectively, these results highlight a prime-boost immunization regimen with S.cerevisiae EBY100/pYD5-HA can induce strong humoral immune responses in a mouse model.
Fig. 2.
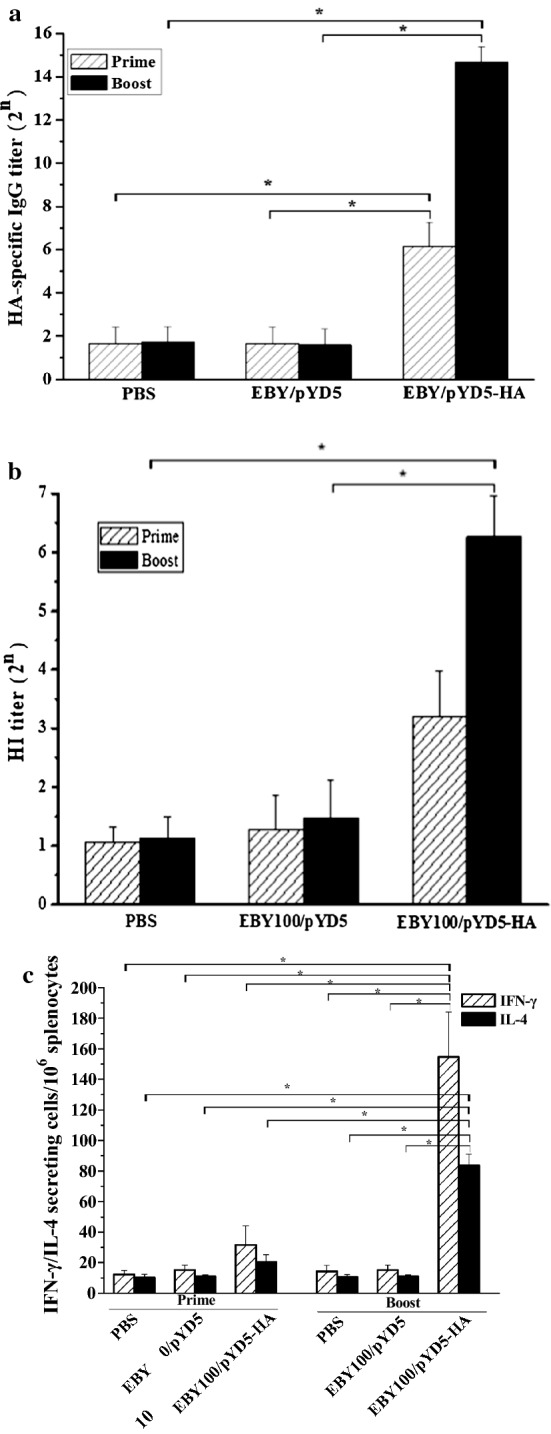
Antibody response detected by ELISA and Hemagglutination inhibition (HI) assay. a HA - specific IgG titers. b HI titers. c The cellular immune responses were assayed by ELISpot assay. Splenocytes derived from vaccination (n = 3/group) were incubated on the IFN-γ or IL-4 capture antibody coated with stimulation of HA peptide. IFN-γ and IL-4 spots were counted. The data are presented as mean ± standard deviation (SD). Asterisk indicates statistical significance compared to S.cerevisiae EBY100/pYD5 and PBS groups (p < 0.05)
Further, we assessed the functional significance of the antibody responses induced by S.cerevisiae EBY100/pYD5-HA, hemagglutinin inhibition (HI) titers were determined by measuring the ability of sera to inhibit agglutination of chicken erythrocytes. As compared to the control groups (PBS or S.cerevisiae EBY100/pYD5), significant HI titers were observed at higher levels after boost immunization (Fig. 2b). Therefore, unadjuvanted S.cerevisiae EBY100/pYD5-HA was immunogenic and could induce higher levels of HI titers which might have a correlation with immune protection.
Cellular immune responses induced by S.cerevisiae EBY100/pYD5-HA
To investigate the cellular immune responses induced by S.cerevisiae EBY100/pYD5-HA, we determined IFN-γ and IL-4 secreting splenocytes by ELISpot. Splenocytes were isolated at 2 weeks post prime-boost immunization. After stimulation with HA specific peptide, higher levels of IFN-γ and IL-4 secreting cells were observed in the S.cerevisiae EBY100/pYD5-HA group than those in the control groups that received PBS or S.cerevisiae EBY100/pYD5 (Fig. 2c). However, the levels of IFN-γ secreting splenocytes were significant higher than IL-4 levels in the mice vaccinated orally with S.cerevisiae EBY100/pYD5-HA (Fig. 2c). Taken together, S.cerevisiae EBY100/pYD5-HA could induce Th1 and Th2 type immune responses with preferences of the Th1 type immune responses as evidenced by higher levels of IFN-γ production.
Protective immunity induced by S.cerevisiae EBY100/pYD5-HA
To determine whether S.cerevisiae EBY100/pYD5-HA could elicit protection against lethal homologous H7N9 virus infection, immunized mice were intranasally challenged with 50 μL 10 × LD50 of A/Anhui/1/2013 (AH-H7N9) virus at 2 weeks after the final immunization, and their health status was monitored for 14 days. As shown in Fig. 3, the control groups that received PBS or S.cerevisiae EBY100/pYD5 showed clinical signs of severe disease and significant body weight loss staring on day 3 after virus infection, and died or reached the humane euthanasia endpoint on 6 days post challenge. In contrast, all mice vaccinated with S.cerevisiae EBY100/pYD5-HA were 100% protected from lethal challenge and no significant body weight loss was observed (Fig. 3a, b).
Fig. 3.

Immune protection conferred by S.cerevisiae EBY100/pYD5-HA against lethal H7N9 virus challenge. Mice were intranasally challenged with a lethal dose (10 × LD50) of A/Anhui/1/2013 (AH-H7N9) virus at 2 weeks after the final immunization (n = 10/group). a Weight change as a percentage. Bars indicate SDs. b Lung viral titers were determined by a plaque assay at day 3 after challenge (n = 3 of 10 challenged mice). c Survival rate. Asterisk indicates significant difference compared to S.cerevisiae EBY100/pYD5 and PBS groups (p < 0.05)
We also determined virus titers in the lungs of the challenged mice (n = 3) on day 3 post infection. The lung titers of mice vaccinated with S.cerevisiae EBY100/pYD5-HA were approximately 600 fold lower than the naïve control (PBS) and the negative control (S.cerevisiae EBY100/pYD5). Thus, our results show that oral immunization with S.cerevisiae EBY100/pYD5-HA could decrease virus shedding in the lungs.
Taken together, S.cerevisiae EBY100/pYD5-HA can provide effective protection against H7N9 virus infection. These results further support that this platform based on yeast surface display technology can provide a feasible strategy for developing safe and effective influenza oral vaccines.
Discussion
Highly pathogenic avian influenza (HPAI) H7N9 virus remains global concerns. Current strategies for developing and manufacturing these vaccines are time consuming and somewhat limited in the ability to generate vaccines quickly [15]. Furthermore, conventional influenza vaccines utilizing the HA and NA of influenza viruses have safety and production issues [16]. To address these questions, we hypothesized that a new platform for manufacturing influenza vaccines based on yeast surface display technology could provide potential of protective immunity. In the present study, HA was chosen as a model antigen for influenza vaccine development because of that HA of influenza H7N9 virus has strong immunogenicity and neutralizing activity [1, 6, 17]. Thus, we generated HA protein of A/Anhui/1/2013 (AH-H7N9) virus was displayed on the surface of S.cerevisiae EBY100 (Fig. 1a). Further, we tested its immunogenicity in mice by oral administration without the presence of mucosal adjuvant. Notably, HA presented on the surface of S.cerevisiae EBY100 could not only elicit strongly humoral immune response, as well as significant cellular immune response, but also confer effective protection against H7N9 virus. Therefore, this study provides the first evidence supporting an alternative strategy for developing influenza pandemic oral vaccines based on yeast surface display technology.
The cell surface display technology can be designed to express a target on the surface of cell through linkage with a genetically fused anchor protein. Compared to intracellular expression of viral proteins, the display of viral antigen on cell surface or wall can facilitate their recognition by host immune system, thereby enhancing their capability of eliciting protective immunity in the vaccinated hosts [18]. Basically, there have N-terminal (target protein-anchor protein fusion) and C-terminal fusion (anchor protein-target protein fusion) methods for S. cerevisiae surface displays [7]. In this study, the N-terminus of the anchor protein (Aga2p) is genetically fused to the C-terminus of HA protein via Glycine–Serine linker, resulting in free N-terminal display of HA (Fig. 1a). To elevate the HA display on yeast surface, the culture temperature was lowered from 30 °C to 20 °C after galactose-induction. Further, recombinant S.cerevisiae EBY100/pYD5-HA was confirmed for high display efficiency of HA protein (Fig. 1c, d) and quantification of HA protein on the yeast surface was determined by indirect ELISA (Fig. 1e) which would produce sufficient antigens for subsequent oral vaccination. These findings clearly indicate HA can be presented on the surface of S.cerevisiae EBY100 with stable and high expression efficiency which has great contributions to immune efficacy by oral administration route.
One of the reasons for interest in recombinant S.cerevisiae as a vaccine vehicle is its lacking of toxicity [8]. Besides being inherently nonpathogenic, this particular species of yeast can be heat-killed before administration and has been shown to be safe in humans in several clinical trials, with maximum tolerated dose not reached [19, 20]. Most importantly, recombinant S. cerevisiae has been shown to induce a strong host immune response to non-self-antigens [21–23]. In addition to the convenience of production, for purposes of vaccination, yeast has been shown to have natural adjuvant activity making the expressed proteins more immunogenic when administered along with yeast cell wall components [8]. Development of genetic systems to display foreign proteins on the surface of yeast via fusion to glycosylphosphatidylinositol-anchored (GPI) proteins has further simplified the purification of recombinant proteins by not requiring harsh treatments for cellular lysis or protein purification [24]. These characteristics make S. cerevisiae a potential tool for vaccine delivery.
Our team has constructed S.cerevisiae EBY100/pYD1-HA which C terminal of HA is free and then investigated its immunogenicity by conventional injection immune route (i.m. or i.p.) [9]. However, due to the diameter of S.cerevisiae is around 10 μm, oral administration is more safe and effective immune route than injectable route for yeast surface display system. Consequently, we firstly tested the immunogenicity of S.cerevisiae EBY100/pYD5-HA by oral vaccination.
Current influenza vaccines based on conventional manufacturing platform have failed to provide sufficient protection against infections of rapidly mutated influenza viruses. Thus, a novel platform based on surface display technology of S. cerevisiae can meet requirements of developing safe and effective influenza oral vaccines. The main goal of this study was to evaluate the immune efficacy and protective immunity based on S. cerevisiae surface display technology by oral administration route. HI antibody response is an important factor for evaluating immunogenicity against corresponding viruses. Significant HI titer was obtained in the S.cerevisiae EBY100/pYD5-HA (Fig. 2b). Regarding the T cell responses, mice vaccinated with S.cerevisiae EBY100/pYD5-HA were found to be more effective in generating T cells secreting IFN-γ indicating Th1 responses, as compared to T cells secreting IL-4 cytokine representing Th2 type responses, which was not a protective response (Fig. 2c). All mice that received S.cerevisiae EBY100/pYD5-HA group were completely protected from lethal challenge of H7N9 virus (Fig. 3c), and remained healthy and showed no signs of abnormal behavior after virus infection. Collectively, these findings reinforced how significant it is use S. cerevisiae display system for developing safe and effective influenza vaccine against H7N9 virus.
In conclusion, this study represents a new platform based on S.cerevisiae surface display technique for manufacturing influenza vaccine using HA as a model antigen for protection against highly pathogenic strains of avian influenza. Considering the current platforms for approved influenza vaccines, this platform will offer significant advantages for influenza oral vaccine development particularly if a pandemic occurs. Future studies will be needed to elucidate the mechanism of the cross-protective immunity induced by the proposed candidate vaccines, and investigate detailed comparative immunogenicity and protective of yeast vaccines in comparison with inactivated whole virus and attenuated live H7N9 vaccines.
Conclusions
This study describes the construction of a new yeast display platform for presentation of virus antigen that H7N9 HA is used as a model. Based on the new design method for virus antigen display on the surface of yeast, NH2 terminus of HA is free. Further, the high immunogenicity is investigated by oral administration route in mice. The obtained data strongly suggest that N-terminal yeast display platform can be considered for virus or bacteria oral vaccine development.
Acknowledgements
The authors gratefully acknowledge for Dr. Dominic Man-Kit Lam for technical assistance.
Authors’ contributions
HL, BX, TG, QC and YR contributed to study design and data interpretation. HL was the principal investigator. All contributed to data analysis and results interpretation. HL wrote the manuscript and produced all figures. All authors read and approved the final manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (No. 2019YFN0134) and the Fundamental Research Funds for the Central Universities (No. 2682018CX73) to H. Lei.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal studies complied with the Guidelines for Use and Care of Experimental Animals and were approved by the Animal Committee of the Institute of Southwest Jiaotong University consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uyeki TM, Peiris M. Novel avian influenza A virus infections of humans. Infect Dis Clin North Am. 2019;33:907–932. doi: 10.1016/j.idc.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monto AS. Vaccines and antiviral drugs in pandemic preparedness. Emerg Infect Dis. 2006;12:55–60. doi: 10.3201/eid1201.051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 4.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–2522. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatta M, Zhong G, Chiba S, Lopes TJS, Neumann G, Kawaoka Y. Effectiveness of whole, inactivated, low pathogenicity influenza A(H7N9) vaccine against antigenically distinct, highly pathogenic H7N9 virus. Emerg Infect Dis. 2018;24:1910–1913. doi: 10.3201/eid2410.180403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutsakos M, Kedzierska K, Subbarao K. Immune responses to avian influenza viruses. J Immunol. 2019;202:382–391. doi: 10.4049/jimmunol.1801070. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Yamada R, Ogino C, Kondo A. Recent developments in yeast cell surface display toward extended applications in biotechnology. Appl Microbiol Biotechnol. 2012;95:577–591. doi: 10.1007/s00253-012-4175-0. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Kumar P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019;19:foz007. doi: 10.1093/femsyr/foz007. [DOI] [PubMed] [Google Scholar]
- 9.Lei H, Jin S, Karlsson E, Schultz-Cherry S, Ye K. Yeast surface-displayed H5N1 avian influenza vaccines. J Immunol Res. 2016;2016:4131324. doi: 10.1155/2016/4131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Samal SK. Innovation in newcastle disease virus vectored avian influenza vaccines. Viruses. 2019;11:300. doi: 10.3390/v11030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Mathias A, Stavrou S, Neville DM., Jr A new yeast display vector permitting free scFv a minotermini can augment ligand binding affinities. Protein Eng Des Sel. 2005;18:337–343. doi: 10.1093/protein/gzi036. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Shibasaki S, Ueda M, Tanaka A, Fukuda H, Kondo A. Development of novel whole-cell immunoadsorbents by yeast surface display of the IgG-binding domain. Appl Microbiol Biotechnol. 2001;57:500–505. doi: 10.1007/s002530100802. [DOI] [PubMed] [Google Scholar]
- 13.Lei H, Xu Y, Chen J, Wei X, Lam DM. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology. 2010;407:319–324. doi: 10.1016/j.virol.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Tang YQ, Han SY, Zheng H, Wu L, Ueda M, Wang XN, et al. Construction of cell surface-engineered yeasts displaying antigen to detect antibodies by immunofluorescence and yeast-ELISA. Appl Microbiol Biotechnol. 2008;79:1019–1026. doi: 10.1007/s00253-008-1509-z. [DOI] [PubMed] [Google Scholar]
- 15.de Vries RD, Herfst S, Richard M. Avian influenza A virus pandemic preparedness and vaccine development. Vaccines (Basel) 2018;6:E46. doi: 10.3390/vaccines6030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipatov AS, Webby RJ, Govorkova EA, Krauss S, Webster RG. Efficacy of H5 influenza vaccines produced by reverse genetics in a lethal mouse model. J Infect Dis. 2005;191:1216–1220. doi: 10.1086/428951. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng TT, Lai CC, Weng TC, Cyue MH, Tsai SY, Tseng YF, et al. The stability and immunogenicity of inactivated MDCK cell-derived influenza H7N9 viruses. Vaccine. 2019;37:7117–7122. doi: 10.1016/j.vaccine.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Shin KS, Pan JG, Kim CJ. Surface-displayed viral antigens on Salmonella carrier vaccine. Nat Biotechnol. 2000;18:645–648. doi: 10.1038/76494. [DOI] [PubMed] [Google Scholar]
- 19.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14:4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stubbs AC, Wilson CC. Recombinant yeast as a vaccine vector for the induction of cytotoxic T lymphocyte responses. Curr Opin Mol Ther. 2002;4:35–40. [PubMed] [Google Scholar]
- 21.Heintel T, Breinig F, Schmitt MJ, Meyerhans A. Extensive MHC class I-restricted CD8 T lymphocyte responses against various yeast genera in humans. FEMS Immunol Med Microbiol. 2003;39:279–286. doi: 10.1016/S0928-8244(03)00294-3. [DOI] [PubMed] [Google Scholar]
- 22.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 23.Wetzel D, Rolf T, Suckow M, Kranz A, Barbian A, Chan JA, et al. Establishment of a yeast-based VLP platform for antigen presentation. Microb Cell Fact. 2018;17:17. doi: 10.1186/s12934-018-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inokuma K, Kurono H, den Haan R, van Zyl WH, Hasunuma T, Kondo A. Novel strategy for anchorage position control of GPI-attached proteins in the yeast cell wall using different GPI-anchoring domains. Metab Eng. 2019;57:110–117. doi: 10.1016/j.ymben.2019.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.