Abstract
Purpose
To determine the relationship between the American College of Cardiology/American Heart Association (ACC/AHA) risk score and plaque phenotype of the coronary and carotid arteries assessed directly using CT angiography and MRI.
Materials and Methods
Asymptomatic subjects eligible for statin therapy by risk score were enrolled in a prospective study of disease burden using coronary artery calcium (CAC) scoring, coronary CT angiography, and MRI of the carotid arteries. Quartiles were calculated for noncalcified plaque, CAC, and average carotid wall volume and were compared with ACC/AHA risk quartiles.
Results
Two hundred three subjects were studied (60% men; mean age, 65 years). There were weak correlations between risk and carotid wall volume (Kendall tau = 0.29), noncalcified plaque (tau = 0.16), and CAC (tau = 0.33). ACC/AHA risk alone misclassified plaque extent compared with measurement by carotid wall volume, CAC, and noncalcified plaque in 22.1%, 24.1%, and 29.6% of subjects, respectively. On average, 13% of the subjects were underclassified, and 12.5% were overclassified.
Conclusion
Approximately 25% of subjects had large discrepancies between ACC/AHA risk and plaque burden at imaging. These results suggest that clinical risk score models alone do not fully reflect the amount of atherosclerotic disease present.
© RSNA, 2020
See also the commentary by Truong and Villines in this issue.
Summary
A quarter of subjects had an atherosclerotic phenotype at imaging that did not correlate with their American College of Cardiology/American Heart Association risk score; coronary CT angiography and carotid MRI may reveal presence of atherosclerosis in asymptomatic individuals even if not expected based on risk scores alone.
Key Points
■ Approximately 25% of subjects were determined to have large discrepancies between American College of Cardiology/American Heart Association risk and actual plaque burden at imaging, with approximately equal halves under- and overclassified.
■ Clinical risk score models alone do not fully reflect the amount of atherosclerotic disease present.
■ These findings encourage further research on the role of atherosclerotic phenotype imaging in cardiovascular risk prediction.
Introduction
Cardiovascular disease is the leading cause of death in men and women worldwide, accounting for approximately 31% of all deaths (1). Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are the primary preventive treatment of coronary heart disease and are recommended for asymptomatic subjects based on a predicted risk greater than 7.5% for cardiovascular events (2). This risk may be predicted using the Pooled Cohort Equations, but other risk scores are appropriate if they apply to the patient’s demographics. Currently, the most commonly used score in the United States is the American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Equation Risk Score Estimator (3). Risk estimators use clinical factors such as age, sex, race, blood pressure, serum cholesterol levels, tobacco use, and diabetes to determine the individual patient’s risk for future cardiovascular events, rather than direct assessment of the extent of atherosclerotic disease in the coronary and carotid arteries. The 2019 ACC/AHA guideline acknowledges the limitations of risk scores and states that imaging using coronary artery calcium (CAC) score may be useful in certain intermediate-risk patients (2). Of note, in contrast to a risk score that attempts to predict cardiovascular events based on statistical assumptions, an imaging test detects actual presence and extent of atherosclerotic disease. Actual risk for future cardiovascular events is heavily correlated with presence and extent of disease and may be additionally modified by clinical risk factors. New antiatherosclerotic therapies emerging on the market promise large reductions of low-density lipoprotein levels but at high costs, also creating the need for more precise identification of at-risk subjects.
CAC and coronary CT angiography are noninvasive modalities to image atherosclerosis that have been studied as prognostic indicators of coronary artery disease (CAD) in subjects with subclinical disease (4–7). The strong predictive value of imaging becomes apparent in large-scale analyses (44 052 patients) in which individuals without any clinical risk factors but a CAC greater than or equal to 400 experienced a significantly higher event rate than subjects with three or more risk factors but a CAC of 0. Thus, imaging presence of significant disease “overpowered” three or more risk factors (8).
Previous studies have examined the relationship of atherosclerotic disease in the coronary and carotid arteries using coronary CT angiography and carotid US, indicating low-to-moderate correlation of disease (9,10). Studies have also shown that carotid MRI may be a superior noninvasive modality to measure carotid wall thickness and characterize composition and high-risk features of plaque (11,12). However, prior studies have not compared coronary and carotid disease burden as determined by coronary CT angiography and carotid MRI with the most recent ACC/AHA risk scores in asymptomatic individuals.
The purpose of this study was to determine the relationship between ACC/AHA risk and plaque phenotype directly assessed with CT angiography and MRI of the coronary and carotid arteries in asymptomatic subjects. Of note, CT angiography and carotid MRI are not currently indicated for asymptomatic individuals. We hypothesized that assessment of coronary vasculature with CT angiography and CAC and the carotid arteries with MRI would show that a subset of asymptomatic subjects has significantly more or less coronary and/or carotid plaque than their ACC/AHA risk score suggests. Such subjects would potentially either be overtreated (no disease is present) or undertreated (marked disease burden) in relationship to their actual disease burden.
Materials and Methods
This study was approved by our institutional review board in accordance with the Health Insurance Portability and Accountability Act, and written informed consent was obtained from all participants. We prospectively enrolled 242 asymptomatic men and women over the age of 55 years as part of the Randomized Trial of Imaging Versus Risk Factor-Based Therapy for Plaque Regression (ClinicalTrials.gov NCT01212900). A subset of 106 subjects with follow-up data at carotid MRI was previously reported (no CT angiography data or no baseline comparisons between modalities) (13). The main inclusion criteria of this trial were age of 55 years or older and qualification for lipid-lowering therapy based on Adult Treatment Panel III guidelines (14). Main exclusion criteria were contraindication for statin therapy, use of nonstatin lipid-lowering therapy, ineligibility for MRI scan, and liver failure. Clinical information and blood analysis results were collected at baseline. Ten-year atherosclerotic cardiovascular disease risk was calculated via the 2013 ACC/AHA Guidelines on the Assessment of Cardiovascular Risk (3).
Image Acquisition
Coronary CT angiography.—CT angiography was performed with a 320–detector row volumetric scanner. Baseline calcium scoring was performed with noncontrast CT and the Agatston method (120 kV, 140 mA, 3-mm collimation at 3-mm slice increments) (15). Angiography was performed with a 350-msec gantry rotation, a 100-kV tube voltage, and scanner-adapted tube current (300–580 mA). Intravenous iodinated contrast material (Isovue-370; Bracco Diagnostics, Singen, Germany) was administered based on weight: 50 mL for subjects up to 59 kg, 60 mL for subjects 60–100 kg, and 70 mL for subjects weighing more than 100 kg.
Carotid MRI.—All subjects also underwent carotid MRI examinations using a 3-T scanner (Magnetom Verio; Siemens) and a four-channel carotid artery phased-array coil. Isotropic three-dimensional time-of-flight noncontrast MR angiography was used to locate the carotid bifurcation and the internal carotid artery.
Axial T1- and T2-weighted black-blood sequences were performed prior to contrast material administration to determine internal carotid artery wall thickness. Slices were taken at the proximal 10 mm of the internal carotid artery (five slices with 2-mm slice thickness, no slice gap). Gadopentetate dimeglumine (Magnevist; Bayer) was administered at 0.1 mmol/kg, and postcontrast T1-weighted black-blood sequences were acquired using the same slice positions and acquisition parameters as precontrast images, but the inversion times were reduced by 100–200 msec to compensate for the T1 shortening effect of blood after administration of gadolinium-based contrast material.
Image Analysis
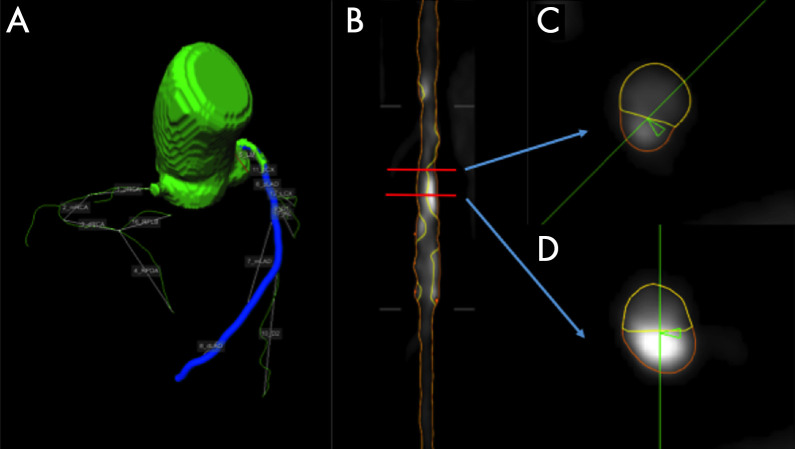
CT angiography.—Analysis of CT angiographic images for calcified, noncalcified, and total plaque was performed using a QAngioCT workstation (Research Edition, version 2.0.5; Medis Medical Imaging Systems, Leiden, the Netherlands). QAngioCT performed longitudinal contouring of the inner lumen and outer wall automatically, and manual adjustments were made as needed (Fig 1). Clinical information was not available to the reader. Results of automated contouring of the inner lumen and outer wall were also reviewed on transverse reconstructed cross sections of the artery on a section-by-section basis at 0.5-mm increments. Thresholds for plaque characterization were adaptively corrected based on lumen attenuation, reducing the impact of different contrast agent concentrations in the lumen on plaque measurements.
Figure 1:
An example analysis of left anterior descending coronary artery in a 71-year-old man. A, Coronary artery segmentation in a coronary tree model. B, A curved multiplanar view of the left anterior descending artery, with lumen wall in yellow and external vessel wall in orange. C, A cross-sectional view of a noncalcified plaque. D, A cross-sectional view of a calcified plaque.
In addition to these quantitative analyses, all CT angiograms were reviewed for presence or absence of any (calcified and/or noncalcified) coronary plaque by an experienced cardiologist (V.S.).
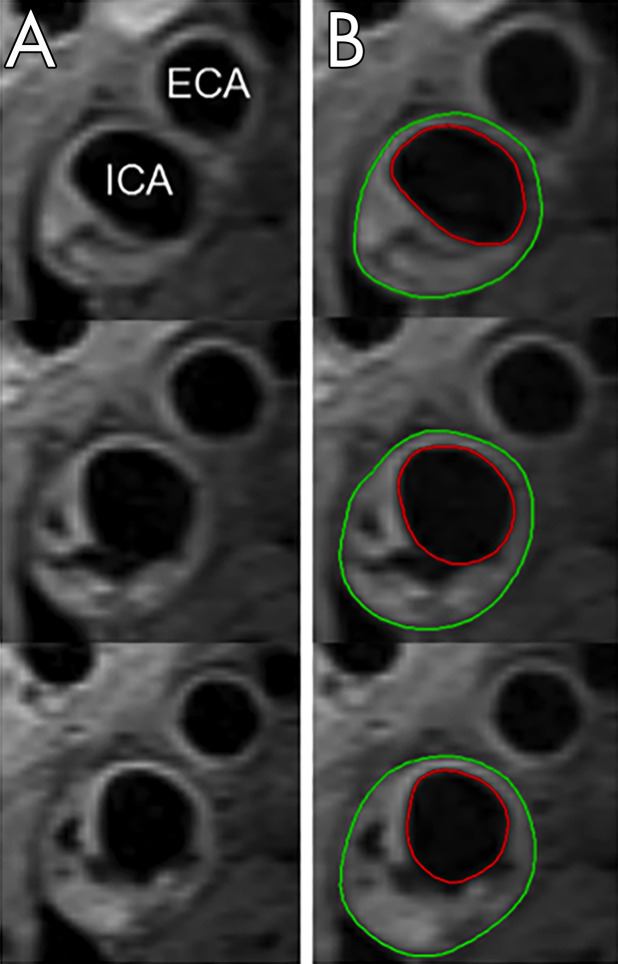
MRI analysis.—MRI analysis for average wall carotid volume was performed using QPlaque 1.0.16 (Medis). Clinical information was not available to the reader. Images were aligned using the carotid bifurcation as a landmark, and the lumen and outer wall of the internal carotid artery were traced in five continuous axial slices (Fig 2). T1-weighted precontrast images were the first choice for wall volume measurements. If image artifact was present, T2-weighted or postcontrast images were used. Interscan reproducibility for carotid wall volume measurement at 3 T was previously assessed and is excellent (coefficient of variance 5.7%) (16).
Figure 2:
Assessing carotid wall volume at MRI in a 72-year-old patient. A, Axial slices at the bifurcation of the right common carotid artery into the internal carotid and external carotid artery show wall thickening and plaque formation in the internal carotid artery wall. B, The lumen and outer wall of the internal carotid artery were traced in five continuous axial slices (three shown), and the average wall volume was calculated. Red circle indicates the lumen, green circle the outer wall. ECA = external carotid artery, ICA = internal carotid artery.
Statistical Analysis
Statistical analysis was performed with R version 3.0.3 (https://www.r-project.org). Baseline patient summary statistics for continuous variables were reported as means with standard deviations. The log-transformed CAC score ([1 + absolute CAC score]) was used for calcium score analysis because of the skewed distribution of the calcium score. Noncalcified plaque at CT angiography was used for analysis because previous studies have shown an improved risk stratification in asymptomatic subjects over the use of the CAC score alone (5, 25). In addition, noncalcified plaque is modifiable by drug treatment.
Because there were no clinically known severity grades for several parameters, and to avoid issues with nonnormality and outliers, each patient’s noncalcified coronary plaque, coronary calcium score, average carotid wall volume, and ACC/AHA risk was sorted into four quartiles (with quartile 1 being the lowest 25% of the study sample and quartile 4 being the highest 25%) and compared with risk quartile in a table and graphical format. Baseline characteristics for subjects with a risk score misclassification two or more degrees away from their corresponding noncalcified coronary plaque, coronary calcium score, and/or average carotid wall volume quartile were summarized with means with standard deviations (and median for coronary calcium score).
The Kendall rank correlation coefficient (Kendall tau) was used to calculate the correlation between the risk scores and plaque phenotype.
Results
Study Sample Characteristics
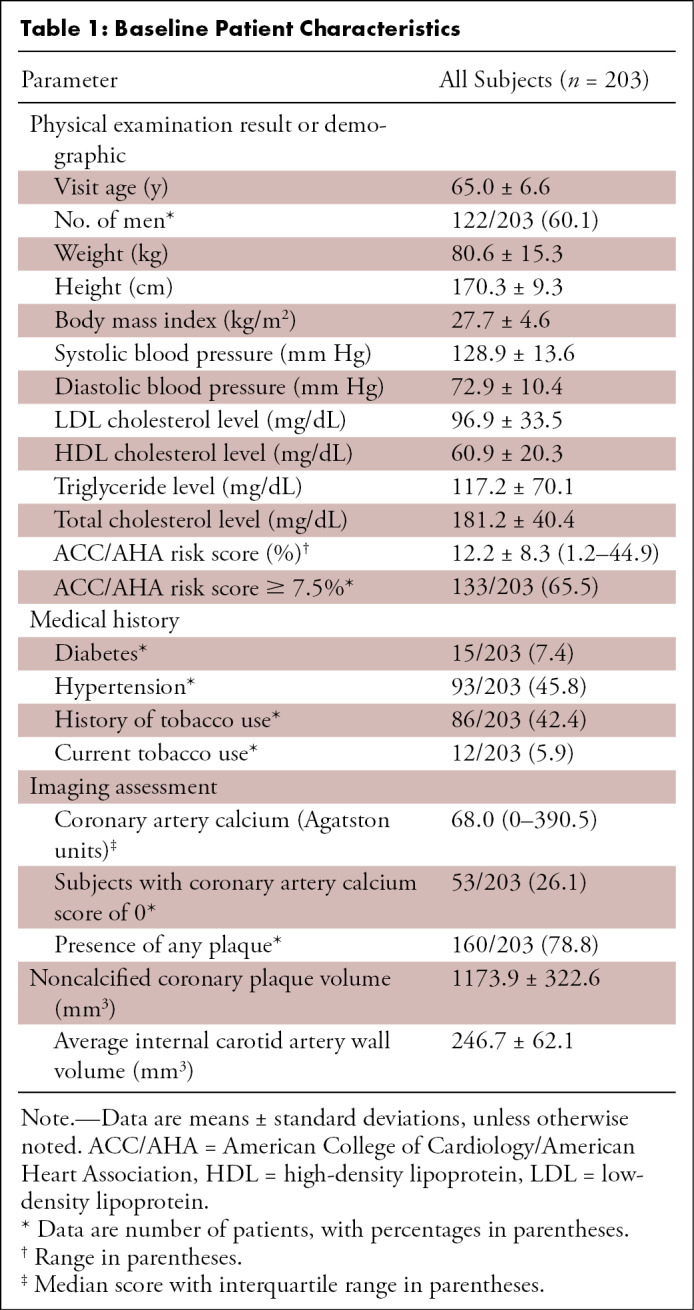
Of the 242 subjects enrolled, 203 underwent all imaging studies and were included in this analysis. Baseline participant characteristics are shown in Table 1. The study sample was predominantly male (60%). Average age was 65 years old. Relatively few participants had diabetes (8%). History of smoking was reported in 43% of subjects, but only a small portion (6%) was currently smoking. Most of the participants were overweight, and the mean body mass index was 27.7 kg/m2 (overweight designated as body mass index of 25–29.9 kg/m2). Hypertension was present in nearly half of the participants (46%), but blood pressure was reasonably well controlled (median systolic blood pressure was 129 mm Hg, and median diastolic blood pressure was 73 mm Hg). The study sample had a median ACC/AHA risk score of 12%, indicating moderate to high risk.
Table 1:
Baseline Patient Characteristics
Coronary and Carotid Disease at Imaging Compared with Clinical Risk Score
Subjects were independently sorted into ranked quartiles according to their ACC/AHA risk, noncalcified coronary plaque, CAC, and average carotid wall volume. The percentage of the cohort in each quartile for noncalcified coronary plaque, CAC, average carotid wall volume, and presence of coronary plaque was compared with the corresponding ACC/AHA risk quartile (Fig 3).
Figure 3a:
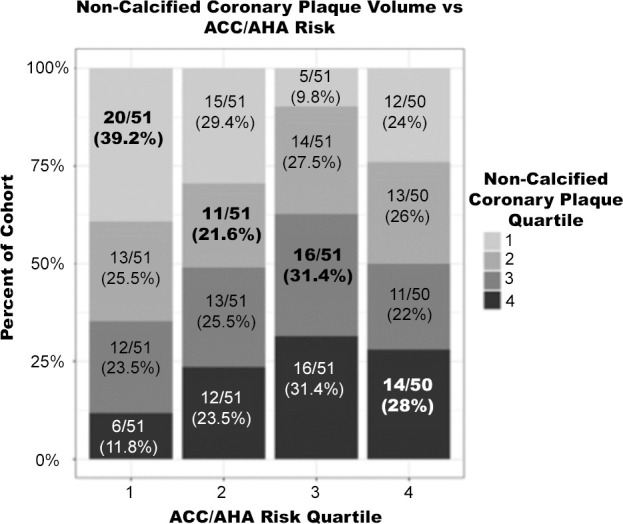
Percentage of cohort per imaging quartile risk compared with American College of Cardiology/American Heart Association (ACC/AHA) risk quartile. Bolded values represent matched quartiles. (a) Noncalcified coronary plaque volume versus ACC/AHA risk. (b) Average carotid wall volume versus ACC/AHA risk. (c) Coronary artery calcium score versus ACC/AHA risk.
Figure 3b:
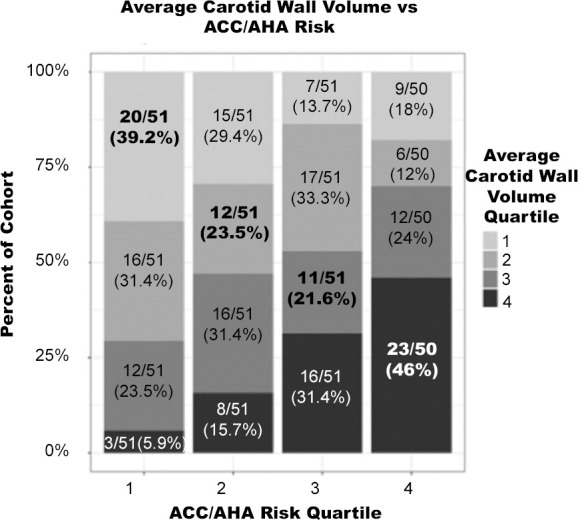
Percentage of cohort per imaging quartile risk compared with American College of Cardiology/American Heart Association (ACC/AHA) risk quartile. Bolded values represent matched quartiles. (a) Noncalcified coronary plaque volume versus ACC/AHA risk. (b) Average carotid wall volume versus ACC/AHA risk. (c) Coronary artery calcium score versus ACC/AHA risk.
Figure 3c:
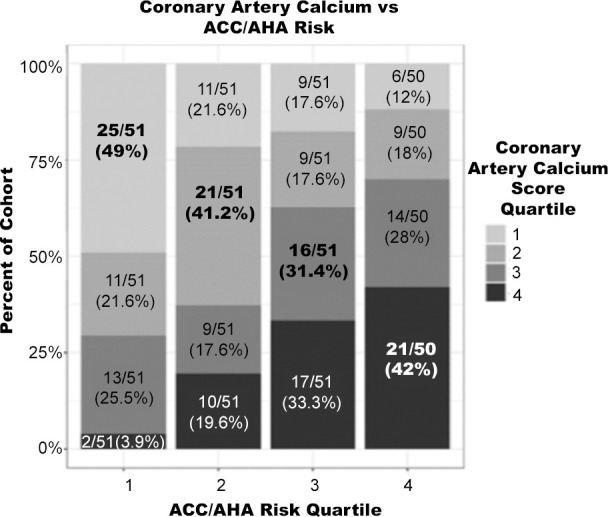
Percentage of cohort per imaging quartile risk compared with American College of Cardiology/American Heart Association (ACC/AHA) risk quartile. Bolded values represent matched quartiles. (a) Noncalcified coronary plaque volume versus ACC/AHA risk. (b) Average carotid wall volume versus ACC/AHA risk. (c) Coronary artery calcium score versus ACC/AHA risk.
There were moderate-to-low correlations between ACC/AHA risk score and average carotid wall volume (Kendall tau = 0.29), CAC score (Kendall tau = 0.33), and presence of noncalcified coronary plaque (Kendall tau = 0.22). Subjects in the third average carotid wall volume quartile were most poorly matched to their corresponding ACC/AHA risk quartile, with 21.6% matched. Subjects in the highest average carotid wall volume quartile were best matched at 46%. Across all four quartiles, carotid wall volume was correctly matched to ACC/AHA risk in an average of 32.6% of subjects.
Correlation between ACC/AHA risk score and noncalcified coronary plaque (Kendall tau = 0.16), and correlation between average carotid wall volume and calcium score (Kendall tau = 0.15) were weak. Subjects in the second noncalcified coronary plaque volume quartile were most poorly matched to their corresponding ACC/AHA risk quartile, with 21.6% matched. Subjects in the lowest noncalcified coronary plaque volume quartile were the best matched, with 39.2% correctly matched. Averaged across all four quartiles, noncalcified coronary plaque volume was correctly matched to ACC/AHA risk in 30.1% of subjects. Clinical risk correlated slightly worse with CAD (Kendall tau = 0.16) assessed with imaging compared with carotid artery disease (Kendall tau = 0.29).
Imaging Correlations
Quartiles comparing coronary and carotid artery disease (as assessed with coronary CT angiography and carotid MRI, respectively) are seen in Figure 4. On average, 30.6% of subjects had the same quartile of coronary and carotid disease, with the greatest correlation in subjects having either very low or very high disease in both vascular beds.
Figure 4:
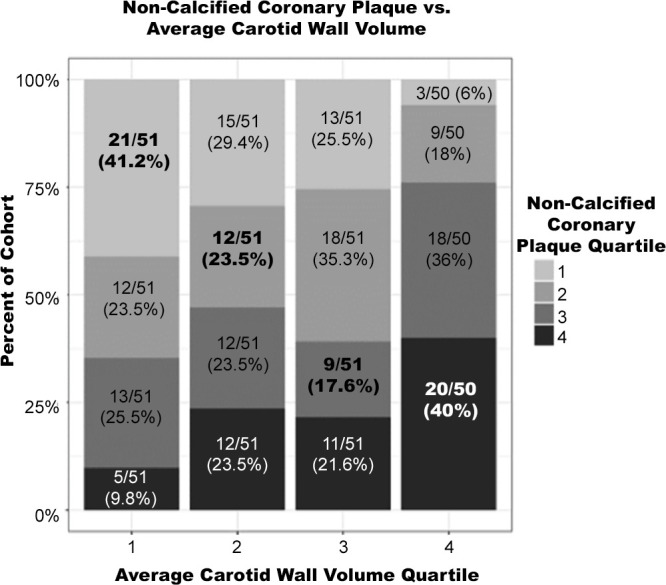
Noncalcified coronary plaque volume versus average carotid wall volume quartiles. Subjects with very low and very high average carotid wall volumes (first and fourth quartiles) were most aligned with their respective noncalcified coronary plaque volume quartiles. Subjects with average carotid wall volumes in the third quartile were most mismatched with noncalcified coronary plaque volume.
Underclassified and Overclassified Clinical Risk Score
Misclassified risk was defined as a subject having an imaging-assessed measurement fall two or more quartiles away from their clinical risk quartile. Clinical risk was misclassified when compared with average carotid wall volume, CAC, and noncalcified coronary plaque in 22.1%, 24.1%, and 29.6% of subjects, respectively (Fig 5). Misclassified subjects were further analyzed for risk underclassification (defined as plaque quartile two or more quartiles higher than their corresponding ACC/AHA risk quartile) and overclassification (defined as plaque quartile two or more quartiles lower than their corresponding ACC/AHA risk quartile). By these measures, 13% of the subjects were underclassified, and 12.5% were overclassified. Noncalcified coronary plaque volume was most discrepant with ACC/AHA risk. Only 30.1% of subjects were correctly classified, whereas 14.8% of subjects were underclassified and 14.8% were overclassified. ACC/AHA risk compared with average carotid wall volume found that 32.5% of subjects were correctly classified, 11.3% were underclassified, and 10.8% overclassified compared with ACC/AHA risk. CAC score correlated to ACC/AHA risk the best at 40.8% of subjects correctly classified, 12.3% underclassified, and 11.8% overclassified.
Figure 5a:
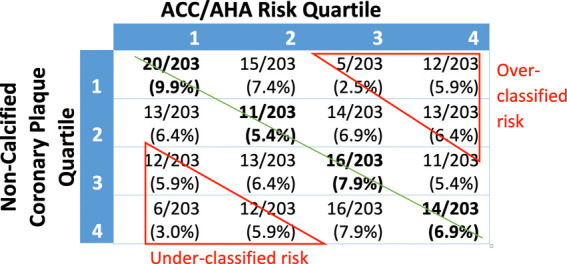
Imaging quartile versus risk quartile by percentage of total study subjects. Green line represents line of identity where American College of Cardiology/American Heart Association (ACC/AHA) risk quartile and imaging quartile are the same. Overclassified risk and underclassified risk were defined as being two or more quartiles away from the line of identity. (a) ACC/AHA risk compared with noncalcified coronary plaque volume: 14.8% were underclassified, 14.8% were overclassified, and 30.1% were correctly classified. (b) ACC/AHA risk compared with average carotid wall volume: 11.3% were underclassified, 10.8% were overclassified, and 32.5% were correctly classified. (c) ACC/AHA risk compared with coronary artery calcium score: 12.3% were underclassified, 11.8% were overclassified, and 40.8% were correctly classified.
Figure 5b:
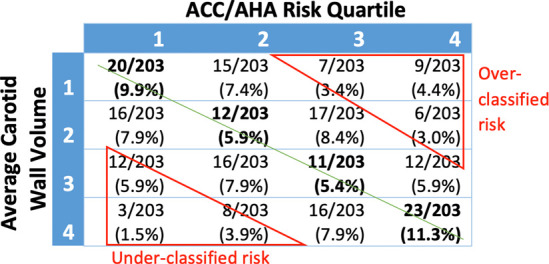
Imaging quartile versus risk quartile by percentage of total study subjects. Green line represents line of identity where American College of Cardiology/American Heart Association (ACC/AHA) risk quartile and imaging quartile are the same. Overclassified risk and underclassified risk were defined as being two or more quartiles away from the line of identity. (a) ACC/AHA risk compared with noncalcified coronary plaque volume: 14.8% were underclassified, 14.8% were overclassified, and 30.1% were correctly classified. (b) ACC/AHA risk compared with average carotid wall volume: 11.3% were underclassified, 10.8% were overclassified, and 32.5% were correctly classified. (c) ACC/AHA risk compared with coronary artery calcium score: 12.3% were underclassified, 11.8% were overclassified, and 40.8% were correctly classified.
Figure 5c:
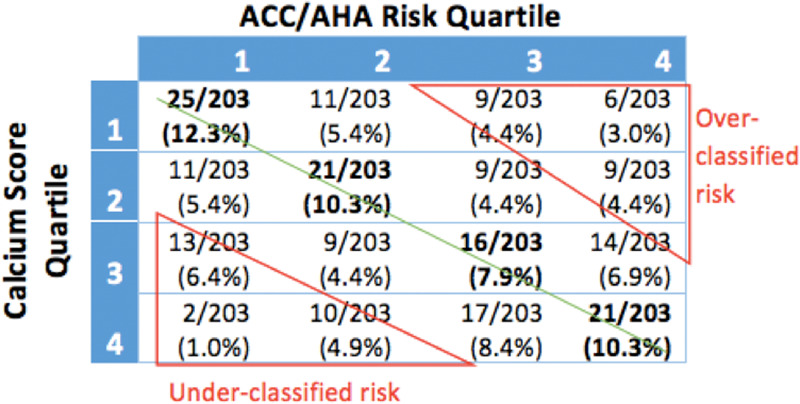
Imaging quartile versus risk quartile by percentage of total study subjects. Green line represents line of identity where American College of Cardiology/American Heart Association (ACC/AHA) risk quartile and imaging quartile are the same. Overclassified risk and underclassified risk were defined as being two or more quartiles away from the line of identity. (a) ACC/AHA risk compared with noncalcified coronary plaque volume: 14.8% were underclassified, 14.8% were overclassified, and 30.1% were correctly classified. (b) ACC/AHA risk compared with average carotid wall volume: 11.3% were underclassified, 10.8% were overclassified, and 32.5% were correctly classified. (c) ACC/AHA risk compared with coronary artery calcium score: 12.3% were underclassified, 11.8% were overclassified, and 40.8% were correctly classified.
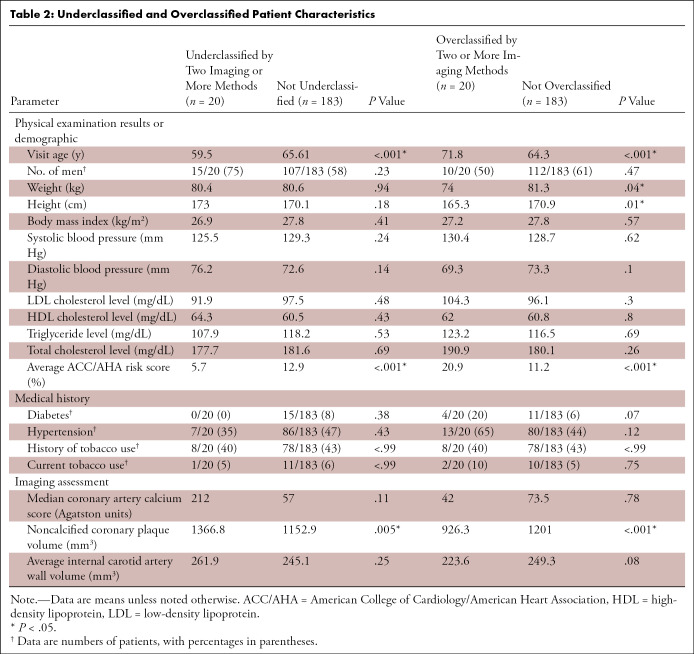
Subanalysis of Misclassified Patient Characteristics
Subgroup analysis of severely misclassified subjects (defined as under- or overclassification with two or more imaging modalities) was also done. Twenty subjects (9.9%) had either underclassified risk or overclassified risk with two or more imaging methods. Underclassified subjects were significantly younger and, as expected, had greater noncalcified coronary plaque volume and lower ACC/AHA risk. Overclassified subjects were significantly older, of lower body height, weighed less, and had lower noncalcified coronary plaque volume and higher ACC/AHA risk (Table 2).
Table 2:
Underclassified and Overclassified Patient Characteristics
Discussion
The 2013 and 2019 ACC/AHA guidelines for primary prevention of cardiovascular disease increased the number of asymptomatic subjects eligible for antiatherosclerotic therapy (17). Current guidelines encapsulate current and future risk over 10 years, whereas imaging defines the current atherosclerotic phenotype actually present. In this study of asymptomatic subjects eligible for antiatherosclerotic therapy, we hypothesized that greater risk would be associated with greater atherosclerotic disease burden at imaging. In general, about 75% of subjects showed some agreement between disease risk and disease burden, whereas 25% had a large discrepancy in risk category. The risk score showed slightly worse correlation to CAD (Kendall tau = 0.16 for noncalcified plaque, 0.22 for any plaque) than to carotid disease (Kendall tau = 0.29); the extent of CAC and carotid wall disease were poorly correlated (Kendall tau = 0.15).
The use of CAC score and CT angiography as noninvasive modalities to image atherosclerosis and as indicators of CAD have been widely described in the literature (4–7). CAC has been shown to improve coronary heart disease risk prediction in asymptomatic individuals when added to clinical risk models based on the Framingham risk score (7,18). Zavodni et al showed that vulnerable carotid plaque features and morphology at MRI are associated with cardiovascular events in asymptomatic subjects, which suggests that this modality could be used to reclassify baseline risk in this patient population (19).
Unlike previous studies that compared carotid wall thickness measured by heavily operator-dependent two-dimensional US imaging to CAD diagnosed by using coronary CT angiography (9,10), the current study compared MRI carotid wall volume to CAC and CT angiography three-dimensional noncalcified plaque volume, which, to our knowledge, has not been reported in the past.
In the current study, clinical risk was greater than two quartiles away from the imaging quartile in 22.1%, 24.1%, and 29.6% (for average carotid wall volume, CAC, and noncalcified coronary plaque, respectively) of subjects. Age was a significant factor in subjects with severely misclassified clinical risk (as defined by under- or overclassification with two or more imaging modalities). Younger subjects tended to be more frequently underclassified, and older subjects tended to be more frequently overclassified by using risk scores.
The mismatch between clinical risk and noncalcified plaque is especially of note as it is a target for high-intensity statins and has previously been linked to cardiovascular risk factors such as low-density lipoprotein cholesterol level, systolic blood pressure, and diabetes and it appears to be modifiable by drug therapy (20). Correlation between ACC/AHA risk score and noncalcified coronary plaque was weak (Kendall tau = 0.16), highlighting the additional diagnostic information noncalcified coronary plaque measurement may provide distinct from clinical risk score. These results are also consistent with previous studies correlating coronary atherosclerosis with subclinical carotid plaque (21).
The current study demonstrated that clinical risk score substantially over- or underestimates plaque in a significant portion of asymptomatic subjects. Although this study did not analyze actual treatment decision, preventative treatment is usually based on risk score. Previous studies have shown that clinical risk score is a largely effective method for determining whether an antiatherosclerotic treatment is needed when averaged over a large population (22,23). However, the results of this study show that a risk score alone may be insufficient for determining the individual patient’s cardiovascular risk. This view is also reflected in the 2019 ACC/AHA guidelines, which affirms the use of imaging, calcium scoring specifically, in certain subgroups and within a patient-physician discussion (2). Asymptomatic subjects may have an ACC/AHA risk score of less than or equal to 7.5% and not be prescribed statin therapy but in actuality have atherosclerosis that may be better identified with imaging. Improved selection of patients who may benefit from newer treatment options is needed, as adjunctive therapies to statins have greater adverse effect profiles and newer antiatherosclerotic medications such as proprotein convertase subtilisin/kexin type 9, or PCSK9, inhibitors cost upward of $14 530 annually (24). Clinicians should consider supplementing a clinical risk score with direct assessment of vasculature with imaging in selected cases.
One limitation of this study was the small sample size of 203 subjects who were recruited from a single center, which may not be generalizable. However, the smaller sample size allows for more accurate phenotyping compared with larger epidemiologic studies. Another limitation was the lack of follow-up cardiovascular event data to assess the long-term clinical outcome of under- and overclassified subjects. The application of our findings may also be restricted by the cost and limited access to carotid MRI in the greater patient population.
In conclusion, our results showed that roughly a quarter of all subjects were determined to have large discrepancies between ACC/AHA risk and actual plaque burden at imaging, with approximately equal halves under- and overclassified. These results suggest that clinical risk score models alone do not fully reflect the amount of atherosclerotic disease present and do encourage further research on the role of atherosclerotic phenotype imaging in cardiovascular risk prediction.
Acknowledgments
Acknowledgments
We thank the Clinical Center Radiology Department research nursing team for the excellent research study support.
Work supported by the National Institutes of Health Medical Scholars Research Program and the National Institutes of Health intramural program.
Disclosures of Conflicts of Interest: A.C. disclosed no relevant relationships. Y.C. disclosed no relevant relationships. E.B.T. disclosed no relevant relationships. M.A.A. disclosed no relevant relationships. C.T.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: employee of Merck & Co, Kiniksa. Other relationships: disclosed no relevant relationships. S.L. disclosed no relevant relationships. D.A.B. Activities related to the present article: receives research support from Siemens. Activities not related to the present article: receives travel support from Siemens. Other relationships: disclosed no relevant relationships. V.S. disclosed no relevant relationships.
Abbreviations:
- ACC/AHA
- American College of Cardiology/American Heart Association
- CAC
- coronary artery calcium
- CAD
- coronary artery disease
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385(9963):117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Cardiology and American Heart Association . ASCVD Risk Estimator. http://tools.acc.org/ASCVD-Risk-estimator. Published 2014. Accessed February 27, 2017. [Google Scholar]
- 4.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez K, Kwan AC, Lai S, et al. Coronary plaque burden at coronary CT angiography in asymptomatic men and women. Radiology 2015;277(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasir K, Clouse M. Role of nonenhanced multidetector CT coronary artery calcium testing in asymptomatic and symptomatic individuals. Radiology 2012;264(3):637–649. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010;303(16):1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging 2012;5(4):467–473. [DOI] [PubMed] [Google Scholar]
- 9.Cohen GI, Aboufakher R, Bess R, et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging 2013;6(11):1160–1167. [DOI] [PubMed] [Google Scholar]
- 10.La Grutta L, Marasà M, Toia P, et al. Integrated non-invasive approach to atherosclerosis with cardiac CT and carotid ultrasound in patients with suspected coronary artery disease. Radiol Med (Torino) 2017;122(1):16–21. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol 2014;34(7):1341–1345. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Moody AR, Roifman I, Bluemke DA, Zavodni AE. Advanced MRI for carotid plaque imaging. Int J Cardiovasc Imaging 2016;32(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandfort V, Lai S, Ahlman MA, et al. Obesity is associated with progression of atherosclerosis during statin treatment. J Am Heart Assoc 2016;5(7):e003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Yarnykh VL, Hatsukami TS, et al. Scan-rescan reproducibility of carotid atherosclerotic plaque morphology and tissue composition measurements using multicontrast MRI at 3T. J Magn Reson Imaging 2010;31(1):168–176. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370(15):1422–1431. [DOI] [PubMed] [Google Scholar]
- 18.Blaha MJ, Silverman MG, Budoff MJ. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: Clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ Cardiovasc Imaging 2014;7(2):398–408; discussion 408. [DOI] [PubMed] [Google Scholar]
- 19.Zavodni AE, Wasserman BA, McClelland RL, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014;271(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stegman B, Shao M, Nicholls SJ, et al. Coronary atheroma progression rates in men and women following high-intensity statin therapy: A pooled analysis of REVERSAL, ASTEROID and SATURN. Atherosclerosis 2016;254:78–84. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Jin H, Cheng W, Rao S, Lu X, Zeng M. Correlation of coronary atherosclerosis and subclinical plaque phenotype of carotid artery: a 320-row multidetector computed tomographic angiography study. J Comput Assist Tomogr 2013;37(5):701–706. [DOI] [PubMed] [Google Scholar]
- 22.Yeboah J, Sillau S, Delaney JC, et al. Implications of the new American College of Cardiology/American Heart Association cholesterol guidelines for primary atherosclerotic cardiovascular disease event prevention in a multi ethnic cohort: Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2015;169(3):387–395.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagidipati NJ, Navar AM, Mulder H, Sniderman AD, Peterson ED, Pencina MJ. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force recommendations vs the ACC/AHA guidelines. JAMA 2017;317(15):1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316(7):743–753. [DOI] [PubMed] [Google Scholar]
- 25.Halon DA, Barnett-Griness O, Rubinshtein R, et al. Plaque Morphology as Predictor of Late Plaque Events in Patients With Asymptomatic Type 2 Diabetes: A Long-Term Observational Study. JACC Cardiovasc Imaging 2019; 12(7 part 2)1353–1363. https://www.ncbi.nlm.nih.gov/pubmed/29778864 [DOI] [PubMed] [Google Scholar]