Abstract
Glioblastoma multiforme (GBM) is a highly aggressive and devastating brain tumor characterized by poor prognosis and high rates of recurrence. Numerous therapeutic strategies and delivery systems are developed to prolong the survival time. They exhibit enhanced therapeutic effects in animal models, whereas few of them is applied in clinical trials. Taking into account the drug-resistance and high recurrence of GBM, combined-therapeutic strategies are exploited to maximize therapeutic efficacy. The combined therapies demonstrate superior results than those of single therapies against GBM. The co-therapeutic agents, the timing of therapeutic strategies and the delivery systems greatly affect the overall outcomes. Herein, the current advances in combined therapies for glioblastoma via systemic administration are exhibited in this review. And we will discuss the pros and cons of these combined-therapeutic strategies via nanotechnology, and provide the guidance for developing rational delivery systems to optimize treatments against GBM and other malignancies in central nervous system.
Keywords: glioblastoma, combined therapy, nanotechnology, drug delivery
Introduction
Glioblastoma multiforme (GBM) is a primary malignant brain tumor of the central nervous system (CNS) with poor prognosis and high mortality 1, 2. The median survival rate is around 12-18 months, and the long-term survival time is less than 5 years 3, 4. The current clinical treatments for GBM consist of tumor resection, radiotherapy and chemotherapy. Maximal tumor resection improves the overall survival in GBM. But it is difficult to distinguish tumor from normal brain tissues. Several intraoperative technologies, such as 5-aminolevulinic acid (ALA) and fluorescein (FLCN) guided resection, facilitate the identification of tumor and simultaneous preservation of neurologic function 5, 6. They significantly improve the gross total resection with standard surgery from 36% to over 64% in high-grade gliomas (HGG). However, the infiltrative nature of GBM raises the challenge to realize a complete resection. Furthermore, the extent of resection must be balanced with the brain function 7. The introduction of Stupp regimen and modified Stupp extends the median survival of the patients, whereas the overall survival is still poor 8-10.
Besides the standard therapy, the therapeutic strategies like gene therapy, immunotherapy, phototherapy and thermotherapy, have been applied for anti-glioma treatments 11-15. They exhibit enhanced therapeutic effects in animal models, but few of them is applied in clinical trials. Since the GBM is a complex disease with intricate mechanisms in their growth, progression and invasion processes, accumulating evidences indicate that single therapeutic strategy tends to result in drug resistances and tumor cell tolerance, which finally leads to tumor recurrence and metastasis 16, 17. Therefore, combined-therapeutic strategies with various mechanisms of agents should be able to overcome these problems.
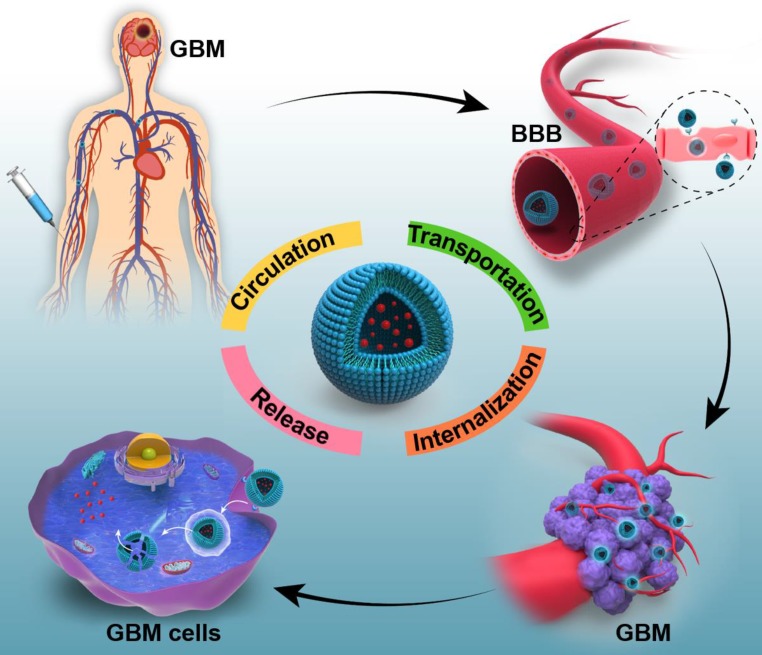
However, the current therapeutic agents have some critical problems, such as short half-life in circulation, hard transportation to the diseased areas and difficulty of controlled releasing the diverse drugs in the corresponding sites. These problems result in insufficient accumulation of therapeutic drugs in the tumor cells, and prevent adequate destruction of malignant tumors 18, 19. Therefore, efficient drug delivery will be very critical and important for effective therapy 20. For a typical cancer treatment, a five-step CAPIR cascade in drug delivery process is presented by Shen's group 21. It includes circulation in the blood, accumulation in the tumor, penetration into tumor tissue, internalization into tumor cells and intracellular drug release. High efficiency at every step is very important to ensure the high therapeutic efficiency of the whole treatment. Furthermore, in view of the location of glioblastoma, the specific blood-brain barrier (BBB) also constructs a major and critical obstacle for the drug delivery 22, 23. It is estimated that over 98% of the small molecular drugs and almost 100% of large molecular drugs cannot cross the BBB 24, 25. Given this, the challenges of drug delivery in the GBM treatment should be composed of the following steps: long circulation in the blood, effective transportation across the BBB, efficient internalization into glioma cells and controlled drug release in the cells (Figure 1). All of these steps are the key factors to ensure sufficient therapeutic agents accumulating in GBM cells.
Figure 1.
The schematic illustration exhibits the challenges of drug delivery via systemic administration for glioblastoma therapy. They generally include: (1) long circulation in the blood; (2) effective transportation across the BBB; (3) efficient internalization into GBM cells and (4) controlled drug release in the tumor cells.
To overcome these physiological barriers in GBM treatments, nanotechnology has been explored to solve these problems and potentiate the therapeutic effects. These delivery systems can be classified as liposomes, polymer nanoparticles, lipopolymer nanoparticles, dendrimer nanoparticles, hybrid nanoparticles and so on 26-29. Firstly, the diversity of these biomaterials can enable loading of various therapeutic agents simultaneously. Secondly, these nanodelivery systems can be modified with specific targeting, which shows the guidance to BBB and the GBM cells. The reported brain tumor targeting moieties include transferrin receptor (TfR), angiopep-2 peptide, TAT peptide, RGD peptide, chlorotoxin and so on 30-32. The modified nanodelivery systems can transport across the BBB by receptor-mediated endocytosis, adsorptive-mediated endocytosis and carrier-mediated transport 33. These targeting modifications greatly improve the brain tumor targeting and reduce the side effects to normal tissues. Thirdly, stimuli-sensitive responses are introduced into delivery systems to ensure the maximal drug retention at the desired sites, such as pH, ROS, enzyme, light and thermal responses 34. These strategies with nanotechnology prompt the drug accumulation in brain tumors. Intensive advances have exhibited the effective and promising outcomes for glioblastoma therapy these decades, but few of them is applied in clinical trials until now. Herein, in this review, we will focus on the combined-therapeutic strategies with diverse delivery systems, investigate the current combined strategies with improved therapeutic effects, and discuss the future guidance for glioblastoma treatments and other CNS diseases' therapies.
Combined therapies against glioblastoma
The complexity of glioblastoma multiforme motivates researchers to develop various therapeutic strategies. Comparing with single therapy, combined therapies have emerged new issues to concern. The choice of the therapeutic strategy for combination is the first key factor. It contains the selection of combined methods and the therapeutic agents, and synergism of these strategies. The delivery systems based on the therapeutic agents are the second important issue to concern. These systems of combined therapies are required to load the multiple therapeutic agents and deliver them to the corresponding targeted sites for synergistic treatments. Herein, various combined-therapeutic strategies for anti-glioma treatments are presented in the following sections. And the merits of the current delivery systems are discussed, which should inspire and guide to develop optimized delivery platforms for further clinical applications.
Combined chemotherapies
Chemotherapy was the most common treatment for glioblastoma. Single chemotherapy facilitated the drug resistance of the tumor after a certain period, which greatly hindered the successful treatment of GBM. Combination with diverse mechanisms of anticancer drugs should improve the GBM therapy, and some efforts had entered into clinical trials 35-38.
However, due to the physiological barriers of glioblastoma, some clinical trials on the combination therapies did not show survival advantages and failed to obtain the desirable outcomes 39-41. Hence, numerous delivery systems were developed to overcome these challenges and obtained better therapeutic effects 42, 43. For example, Guo et al. utilized a liposome (LP), which was composed of egg yolk phosphatidylcholine (EPC), cholesterol (Chol) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol) 2000] (DSPE-PEG2000), to separately encapsulate the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and doxorubicin (DOX) 44. The median survival time of the combination group (DOX-LP+TRAIL-LP) extended to 48 days, compared with the single groups TRAIL-LP (32 days) and DOX-LP (39 days). The combined chemotherapies showed improved therapeutic effects in the intracranial U87MG-bearing mice. The proportion of the drugs was easy to adjust by the detached drug load, but the synergism effects of these anticancer drugs in vivo might be hard to control.
To increase the drug accumulation in the brain tumors, active targeting was introduced into the nanosystems to enhance the drug concentration in tumor sites and reduce the systemic toxicity 45-47. Erica Locatelli et al. developed a targeted delivery nanoparticle (Ag/Ali@PNP-Cltx-99mTc) based on the poly(lactic-co-glycolic acid)-block-polyethylene glycol (PLGA-b-PEG) copolymer 48. The nanosystem conjugated with chlorotoxin (Cltx), which expressed the specific binding to matrix metalloproteinase-2 (MMP-2), a receptor overexpressed on the brain cancer cells. The in vivo biodistribution data exhibited that the targeted nanosystem Ag/Ali@PNP-Cltx-99mTc significantly increased drug concentration in tumors. The enhanced drug accumulation obviously reduced the tumor size in vivo. The current approved anticancer drug temozolomide (TMZ) had been reported to associate with the introduction of O6-methylguanine-DNA methyltransferase (MGMT), which increased TMZ-resistance in chemotherapy 49-54. Sang-Soo Kim et al. developed a cationic liposome (scL-p53) encapsulating the tumor suppressor gene p53 plasmid DNA to sensitize the cancer cells to TMZ 55. This nanocomplex was modified with an antitransferrin receptor (TfR) single chain antibody fragment (scFv) to target the tumor cells. The combination of scL-p53 with TMZ obviously down-modulated the MGMT expression by 95% for 24 h in vivo. The mice treated with cycle administration of TMZ finished at day 45, whereas 63.6% of the mice were still alive when treated with this nanosystem. These studies demonstrated that the active brain targeting significantly enhanced the drug concentration in tumors with the same doses in comparison with the non-targeted systems.
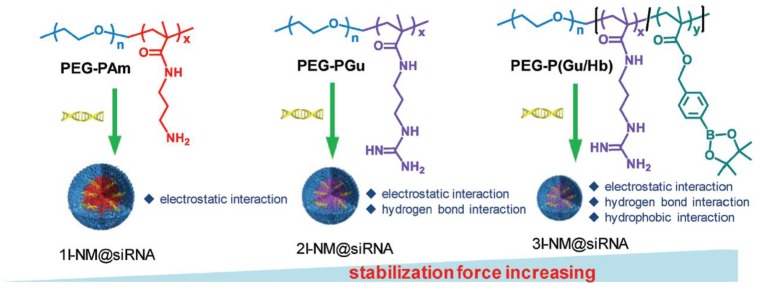
Active brain targeting ensured the guidance of drug accumulation in tumor tissues, and the effective release of therapeutic drugs in tumor cells was also critical for efficient treatments against GBM 56. Hence, plenty of stimuli-sensitive drug delivery systems were designed based on the microenvironment of GBM, including pH, ROS, enzyme, and so on 57-59. For example, the anticancer drug doxorubicin (DOX) could potentiate the antitumor effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which expressed by pORF-hTRAIL. Inspired by these, Jiang's group designed a targeting dendrigraft poly-L-lysine (DGL)-based nanosystem (DGDPT/pORF-hTRAIL) with pH-response to carry the chemotherapeutic drug DOX and the gene agent pORF-hTRAIL for anti-glioma therapy 60, 61. This nanosystem was modified with peptide HAIYPRH (T7), a transferrin receptor-specific peptide, for brain tumor cells targeting. The target significantly increased the drug accumulation in tumors in vivo. The median survival of DGDPT/pORF-hTRAIL was extended to 57 days, in comparison with 34 days for the free DOX. More importantly, the dose of the anticancer drug DOX in DGDPT/pORF-hTRAIL was less than 0.35 mg/kg mice, whereas the dose of free DOX was 5 mg/kg mice. This nanosystem greatly increased the pharmaceutical effect of the DOX and reduced the side toxicity to normal tissues. Also, Shi's group designed a nanoparticle (3I-NM@siRNA) for combined siRNA delivery, which was stabilized by electrostatics, hydrogen bonds and hydrophobic interactions (Figure 2) 62. The angiopep-2 targeting decorated 3I-NM@siRNA was prepared with poly(ethyleneglycol)-block-poly[(N-(3-methacrylamidopropyl)guanidinium-co-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzyl acrylate)] (PEG-b-P(Gu/Hb))/ Ang-poly(ethylene glycol)-block-poly(N-(3-methacryl amidopropyl) guanidinium) (Ang-PEG-b-PGu). The nanoparticles could trigger drug release under ROS environment that enriched in the cancer cells. Treatment with Ang-3I-NM@(siPLK1+siVEGFR2) significantly increased the median survival time to 36 days, which was longer than the mice treated with 3I-NM@(siPLK1+siVEGFR2) (18 days), Ang-3I-NM@(siPLK1) (24 days) and Ang-3I-NM@ (siVEGFR2) (26 days).
Figure 2.
The schematic illustration of the formation of 1I-NM@siRNA, 2I-NM@siRNA and 3I-NM@siRNA utilizing different polymers to generate different numbers of stabilizing interactions. Adapted with permission from 62. Copyright 2019 WILEY‐VCH Verlag GmbH & Co.
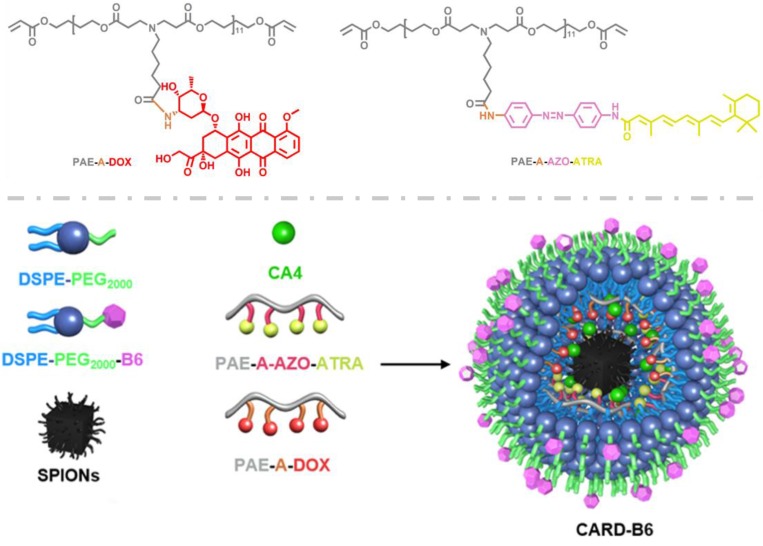
Numerous researches detected that the available drug therapies were dissatisfied because they failed to penetrate into depth of the tumor tissues and kill the brain cancer stem cells (BCSCs), which was the most responsible factor for tumor recurrence 63-65. Intensive studies had been arisen to inhibit the growth of cancer stem cells for effective treatments to glioblastoma 66. Lu et al. in our group designed a brain targeting and multifunctional nanoparticle (CARD-B6) to synergistically aim at both the glioblastoma cells and glioblastoma stem cells (GSCs) (Figure 3) 67. In this nanosystem, acid-sensitive poly(β-amino ester) (PAE) was utilized to load the antiangiogenic drug combretastain A4 (CA4), and triggered to release the cargo in the GBM microenvironment. The azobenzene (AZO) bond in the PAE, rapidly broken up in the hypoxic GSCs, was applied to conjugate with all-trans retinoic acid (ATRA), which could induce the GSCs differentiation into glioblastoma cells. The amide bond in the PAE was conjugated with anticancer drug doxorubicin (DOX) for controlled release in tumor cells. The designed CARD-B6 could spatiotemporally trigger to deliver these drugs to their corresponding target sites. And the survival time of the CARD-B6 treated mice significantly enhanced, with 60% survivals vs. 0% for all the other control groups.
Figure 3.
The structural composition and preparation route of the multifunctional nanoparticles CARD-B6. Adapted with permission from 67. Copyright 2017 WILEY‐VCH Verlag GmbH & Co.
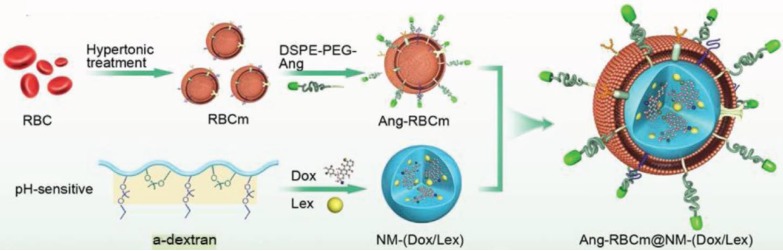
Recently, biomimetic nanosystems were exploited for drug delivery applications, including the utilization of red blood cell membranes, tumor cell membranes, platelet membranes and etc. 68-73. They had good biocompatibility and avoided immunogenicity. For instance, Shi's group designed a biomimetic nanomedicine (Ang-RBCm@NM-(Dox/Lex)) by functionalizing with red blood cell membranes (RBCms) (Figure 4) 74. This nanosystem was equipped with angiopep-2 at the surface of red blood cell membranes. The target had high affinity for the low density lipoprotein receptor-related protein (LRP) receptor, which was overexpressed on both the endothelial cells of the BBB and glioblastoma cells. A pH-sensitive a-dextran that coloaded the anticancer drug doxorubicin (Dox) and lexiscan (Lex), and encapsulated in the RBCm, could transiently open the BBB to enhance the permeability of the nanomedicine Ang-RBCm@NM-(Dox/Lex). The Ang-RBCm@NM-(Dox/Lex) exhibited a longer blood circulation time, with the half lifetime of 9.3 h, whereas the circulation time of NM-(Dox/Lex) without RBCm camouflage was only 2.4 h. And the biodistribution of Ang-RBCm@NM-(Dox/Lex) in the orthotopic brain tumor was about 3.5-fold higher than that of NM-(Dox/Lex). The biomimetic nanomedicine enhanced the median survival time to 38 days, in comparison with RBCm@NM-(Dox/Lex) (28 d), Ang-RBCm@NM-Dox (25 d), NM-(Dox/Lex) (22 d), free Dox (22 d) or PBS (18 d) treated groups.
Figure 4.
The schematic illustration of the formation of the biomimetic nanomedicine (Ang-RBCm@NM-(Dox/Lex)). Adapted with permission from 74. Copyright 2018 WILEY‐VCH Verlag GmbH & Co.
Chemotherapy was the most widely studied treatment for anti-glioma therapy. Mounting researches were reported from the non-targeting and non-sensitive delivery systems to the active targeting and stimuli-sensitive systems. Intelligent multifunctional delivery systems were designed in dependence on the specific characteristics of the GBM. They exhibited better drug accumulation and longer survival time for GBM treatments. However, GBM was a complex disease. The initiation, growth and procession of the GBM were not completely understood until now. Hence the therapeutic drugs might not involve in every critical point of the whole treatments, and the designed drug delivery systems based on existing knowledge of GBM could not completely eliminate the GBM. Moreover, most of the biomaterials utilized in these systems were not approved by FDA, which aggravated difficulties for the clinical translations.
Biomimetic drug delivery systems offered new opportunities to mimic the biological vehicles. The naturally derived cell membranes decorated the drug delivery systems, and brought longer circulation and better biocompatibility for these nanosystems. However, they also generated new problems, like the preparation operations and the cost of the biological products, the strictness production conditions for the clinical translations and applications.
Combined chemotherapy with radiotherapy
Radiotherapy was one of the clinical treatments for GBM, and amount of efforts had been made to increase the efficacy 4. However, the invasive tumor growth of the glioblastoma limited the radiotherapy's efficacy and induced tumor recurrence. Since the combination of radiotherapy with chemotherapy turned into the standard treatment strategy to inhibit GBM recurrence 75-78, the radiotherapy concomitant with free temozolomide or carmustine entered into clinical trials. But the regimens were still insufficient for the requirements 79.
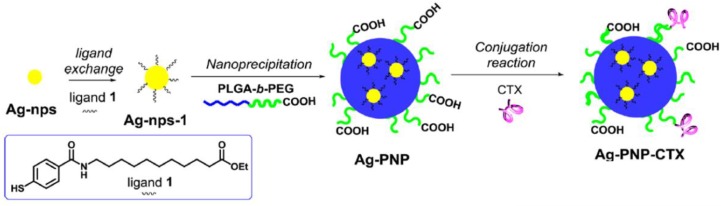
Hence, delivery systems with nanotechnology were exploited for the combined therapies to maximize the therapeutic effects. Shi et al. developed an integrin targeting 177Lu radiolabeled 3PRGD2 (a dimeric RGD peptide with 3 PEG4 linkers) for radiotherapy 80. The cellular uptake of 177Lu-3PRGD2 in U87MG tumors was about 6%ID/g for 1 h. The combination with the anti-angiogenic agent Endostar exhibited significant tumor inhibition in comparison with the control group. Later, Matteo Tamborini et al. reported that combining the chlorotoxin-nanovectors and radiation could exhibit a synergistic effect for in vivo GBM growth (Figure 5) 81. Chlorotoxin, a selective target and anticancer drug, was conjugated on poly(lactic-co-glycolic acid) (PLGA) nanoparticles (PNP). Low dose of radiation (2 Gy) rendered the brain extracellular matrix permeable and facilitated the accumulation of the nanovectors (Ag-PNP-CTX). The combination therapy significantly inhibited approximately 50% tumor growth in U87MG-bearing mice.
Figure 5.
Schematic procedure for the synthesis of chlorotoxin-nanovectors (Ag-PNP-CTX). Adapted with permission from 81. Copyright 2016 American Chemical Society.
As the clinical standard treatment, radiotherapy often resulted in the modest improvement for glioblastoma treatments without reducing the quality of life or cognition. From the available better treatments, radiotherapy with concomitant chemotherapy was the current standard of care. However, the reported clinical trials regarding the combination of radiotherapy and chemotherapy exhibited diverse results. For instance, the addition of bevacizumab to radiotherapy-temozolomide did not improve the survival against GBM in phase 3 study 79. And sorafenib combined with radiotherapy-temozolomide exhibited severe side effects in phase 1 study 82. These might be due to the mechanisms of these drugs or the insufficient drug concentrations in the tumors, which counteracted the therapeutic effects. Hence, the definition of tumor margins, the choice of the chemotherapeutic drugs and drug delivery systems were very important for the regimen.
Combined chemotherapy with phototherapy
Phototherapy was composed of the photothermal therapy (PTT) and the photodynamic therapy (PDT), which was a promising non-invasive strategy for cancer treatments. The former one utilized the photothermal agents to generate heat and kill tumor cells under light absorption. And the latter one produced cytotoxic reactive oxygen species, such as singlet oxygen (1O2), free radicals or peroxides, to induce cell death 83-85. Nanotechnology assisted the phototherapy into further applications since most of the photosensitizers and photothermal agents were hydrophobic and had low tumor selectivity 15, 86-89.
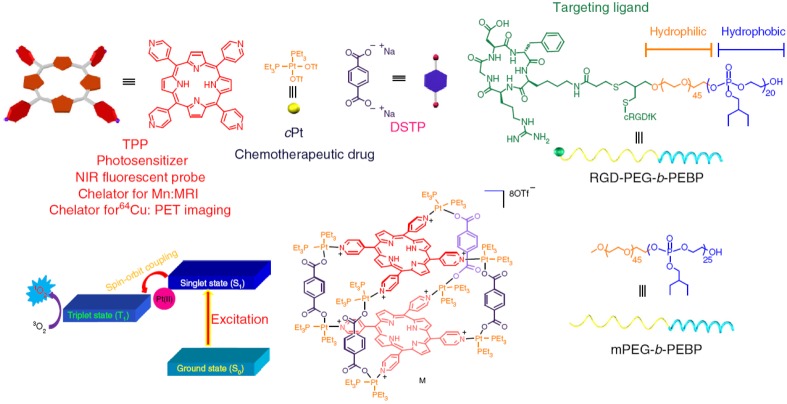
However, phototherapy alone could not kill the tumor cells entirely due to the uneven light distribution and hypoxic condition in the tumor tissues, and it was easy to induce local recurrence and distant metastasis, especially for glioblastoma 90, 91. Hence, combination of the phototherapy with chemotherapy could highlight the potential approaches for malignant glioblastoma therapies 92, 93. For instance, Yu et al. designed an organoplatium (II) metallacage (M) coordinated by photosensitizer 5, 10, 15, 20-tetra(4-pyridyl)porphyrin (TPP), therapeutic drug cis-(PEt3)2Pt(OTf)2 (cPt), and disodium terephthalate (DSTP) 94. The metallacage self-assembled with mPEG-b-PEBP and RGD-PEG-b-PEBP to form metallacage-loaded nanoparticles (MNPs) for long blood circulation and active targeting (Figure 6). The MNPs exhibited superior anticancer results against U87MG with no recurrence after single injection, which attributed to the synergistic photochemotherapy.
Figure 6.
Schematic diagrams of the MNPs served as a multifunctional theranostic platform. The structures of TPP, cPt, DSTP, M, mPEG-b-PEBP and RGD-PEG-b-PEBP were illustrated. Adapted with permission from 94. Copyright 2018 Springer Nature.
The inherent proteins were applied as drug carriers due to their superior biocompatibility. Liu's group utilized the human serum albumin (HSA) as the carrier to conjugate with the photosensitizer chlorin e6 (Ce6) and self-assemble with the anticancer drug paclitaxel (PTX) 95. An acyclic Arg-Gly-Asp (cRGDyK) peptide, targeting to ανβ3-integrin overexpressed on tumor angiogenic endothelia, was chosen as the active targeting (HSA-Ce6-PTX-RGD). The accumulation of the nanoparticles was about 2.4 times higher than the non-targeting one in vivo. The survival time after the combination therapy prolonged to 40 days without a single death, while the mice treated with control groups exhibited lifetimes about 15-30 days.
Gold nanoparticles were the attractive photothermal agents for cancer therapies due to their strong absorbance of NIR light and higher heat conversion efficiency 96-99. Yongwei Hao et al. developed a tumor-targeting hybrid nanosystem by modifying Au on the surface of docetaxel (DTX)-loaded poly(lactide-co-glycolide) (PLGA) nanoparticles (ANG/GS/PLGA/DTX NPs) 100. The U87MG cells treated with nanosystem showed a striking temperature increase for 12.3°C when exposed to 808 nm irradiation. And the tumor inhibition rate of the ANG/GS/PLGA/DTX NPs under irradiation was about 70%, which was the highest among all of the control groups for in vivo studies.
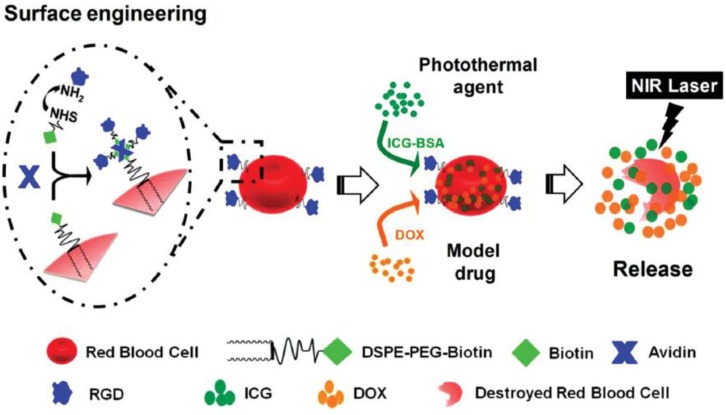
The biomimetic drug delivery systems coated with native cell membranes also attracted researchers' attentions to fight against cancers with phototherapy 101, 102. Liu's group exploited a RBC-based system (IB&D@RBC-RGD) with a tumor angiogenesis targeting (Figure 7) 103. Anticancer drug DOX and photothermal agent indocyanine green-bovine serum albumin nanocomplexes were co-loaded into the modified RBCs. With deep penetrability of near-infrared (NIR) light, the IB&D@RBC-RGD significantly released the cargos and enhanced cytotoxicity against U87MG and 4T1 cells.
Figure 7.
Schematic illustration for the preparation of the RBC-based system (IB&D@RBC-RGD) and the remotely controlled drug release under the NIR laser. Adapted with permission from 103. Copyright 2015 WILEY‐VCH Verlag GmbH & Co.
Phototherapy for GBM was restricted due to the limited tissue penetration and photosensitizers' delivery. Nanosystems were applied in photosensitizers' delivery for targeting transportation and reducing the non-specific accumulation in normal tissues. However, due to the highly invasive growth of GBM, there usually had tumor recurrence and metastasis after phototherapy. Combined therapies by nanotechnology maximized the anti-glioma efficacy, which were attributed to the significant increase of chemotherapeutic drugs and photosensitizers/photothermal agents in the tumor cells. And these were confirmed by a growing amount of studies. But the challenges for this combined-therapeutic strategy against GBM still remained, including the ratio of the therapeutic drugs and photosensitizers/photothermal agents, the drug-to-light interval and the synergistic interaction of the therapeutic methods. All of these should greatly affect the anti-glioma effects and need comprehensive studies in future.
Combined chemotherapy with immunotherapy
Immunotherapy had received tremendous attentions in the treatment of cancers in the decades 104. It could activate the body's own immune systems and induce the specific immune responses with tumor antigens to eliminate the tumor cells 105, 106. Most importantly, it had the potential for long-term reduction of cancer metastasis and recurrence 107. The immunotherapeutic strategies included the cancer vaccines, monoclonal antibodies, oncolytic virus, engineered T-cells and immunomodulation 11, 107, 108. However, the major challenges of immunotherapy for glioblastoma were the lack of specific tumor antigens, limited immunogenicity of the cancer cells and the immunosuppressive environment of the tumors 109-111. Intensive studies had been reported to promote the specific immune responses and exhibit effective immunotherapy for glioblastoma 112, 113.
The epidermal growth factor receptor variant III (EGFRvIII), an oncogenic variant of the EGFR, expressed in 20-30% of all glioblastoma 114. It was exploited to cancer vaccine (Rindopepimut) and showed an encouraging progression-free survival in clinical trials 115, 116. However, the combination of Rindopepimut and temozolomide in phase 3 trial did not show significant difference in overall survival for EGFRvIII-expressing glioblastoma 117. This might be due to inappropriate combination of the drugs, which counteracted the immunological and chemical effects. And the unspecific tumor antigens, which were not sufficient to cover the heterogeneous brain tumors, also influenced the therapeutic effects of GBM.
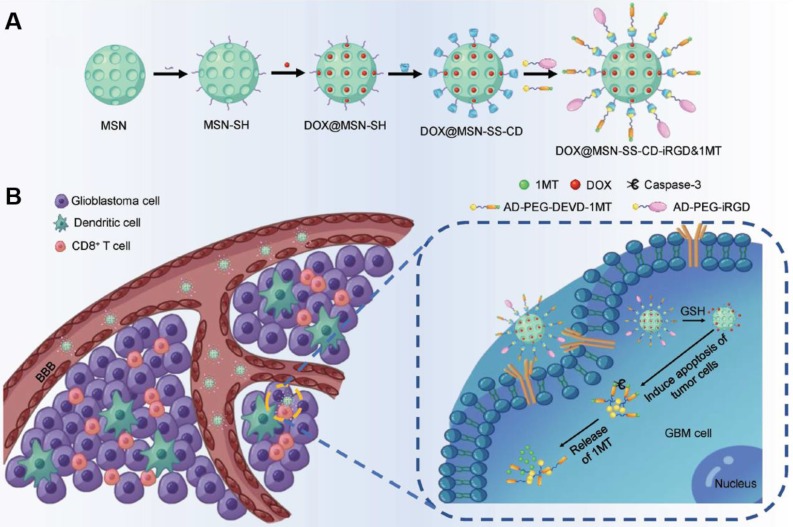
The immune checkpoint was a costimulatory factor for regulating the antigen recognition in the process of immune responses. The inhibition of the checkpoint could enhance the anticancer immune activation 118-120. Combination of immune checkpoint with chemotherapy could stimulate the tumor immunity and sensitize the tumor to chemical agents 121-123. For instance, Jing Kuang et al. designed an iRGD-modified silica nanoparticles (DOX@MSN-SS-iRGD&1MT) to simultaneously deliver the chemical agent doxorubicin (DOX) and the immune checkpoint inhibitor 1-methyltryptophan (1MT) for anti-glioma treatment (Figure 8) 119. The active targeting iRGD guided the nanoparticles to penetrate across BBB and improved drug accumulation in orthotopic brain tumors. The nanoparticles induced antitumor immune responses and regulated the immunosuppressive microenvironment. The chemo-immunotherapeutic therapy significantly extended the medium survival with a 50% durable cure rate.
Figure 8.
A) The synthetic route of the iRGD-modified silica nanoparticles DOX@MSN-SS-iRGD&1MT. B) The illustration of DOX@MSN-SS-iRGD&1MT showed active targeting and drug release. Adapted with permission from 119. Copyright 2018 WILEY‐VCH Verlag GmbH & Co.
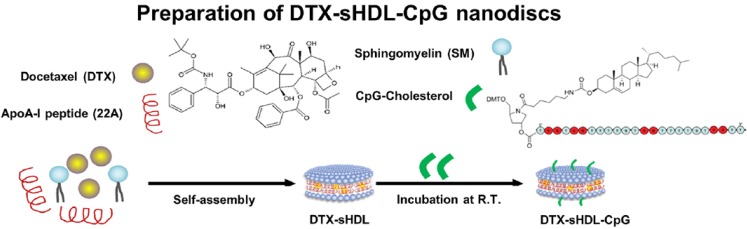
The immunomodulation of the microenvironment of the glioblastoma was another promising approach for tumor regression 124. For example, Padma Kadiyala et al. reported a nanodiscs (DTX-sHDL-CpG) based on high-density lipoprotein (HDL), the synthetic apolipoprotein-I (ApoA-1) 125, which encompassed the anticancer drug docetaxel (DTX) and the immune activator CpG (Figure 9). The nanosystem could elicited antitumor CD8+ T cell responses and developed the anti-GBM immunological memory. The DTX-sHDL-CpG showed moderate effect on tumor growth, and the combination of DTX-sHDL-CpG with radiation significantly increased the long-term survival in 80% of GBM-bearing mice.
Figure 9.
The formulation route of the nanodiscs DTX-sHDL-CpG. Adapted with permission from 125. Copyright 2019 American Chemical Society.
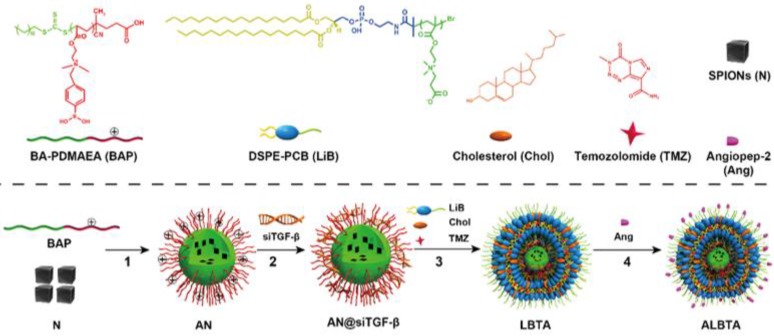
Current advances demonstrated that the chemotherapy with temozolomide (TMZ) could induce and aggravate the immunosuppressive tumor microenvironment 126, 127. Regulating the tumor microenvironment by immunotherapy enabled the GBM more sensitive to chemotherapy. Qiao et al. in our team exploited a dual targeting and ROS responsive nanosystem (ALBTA) for immunochemotherapy (Figure 10) 128. SiRNA against tumor growth factor β (siTGF-β) was employed to modify the immune microenvironment of glioblastoma and improve the efficacy of TMZ. To deliver these two drugs in a controlled manner, firstly, ROS-responsive poly[(2-acryloly)ethyl(p-boronic acid benzyl) diethylammonium bromide] (BAP) was chosen to assemble with siTGF-β and triggered to release in cytoplasm of tumor cells. Secondly, zwitterionic lipid distearoyl phosphoethanol-aminepolycarboxybetaine (DSPE-PCB) based envelope (ZLE) was selected to enhance the delivery of TMZ and BAP/siTGF-β (AN@siTGF-β) into cytoplasm. The core-shell structural nanoparticles (ALBTA) significantly improved the immunosuppressive microenvironment in vivo, and increased the medium survival time from 19 d (for untreated control group) to 36 d without obvious systemic toxicity in the intracranial glioblastoma mice.
Figure 10.
The chemical structural formula of the components and the preparation route of the nanosytem ALBTA. Adapted with permission from 128. Copyright 2018 WILEY‐VCH Verlag GmbH & Co.
Despite the impressive advances in GBM treatments, the efficacy of immunotherapy still needed further improvements for desirable overall survival. For example, the identification of the specific glioblastoma antigens was required to reinforce the specific immunity responses. Combination of immunotherapy with chemotherapy via nanotechnology obviously enhanced the sensitivity of the GBM cells to chemotherapeutic drugs, and regulated the microenvironment of tumors. However, the timing of the immunotherapy for combination 127, 129 and the delivery strategies had critical influences on the therapeutic effects, which needed more efforts to investigate.
Combined chemotherapy with magnetothermal therapy
Hyperthermia therapy had been developed as a safe and effective complementary therapy for cancer treatments 130. Several magnetic nanomaterials had been used in hyperthermia applications, including iron oxide nanoparticles, cobalt ferrite, iron platinum nanoparticles and so on 131-133. Iron oxide nanoparticles were usually applied as the thermo-agents in cancer treatments and entered into clinical trials 134-136. Since the most thermo-agents were the metal nanomaterials, they were preferable for phagocytose by macrophages rather than the glioblastoma cells. Moreover, the challenges for magnetothermal therapy were the focused heating on the tumor sites with optimum temperature, and not harmed the surrounding normal tissues 137, 138. Hence, nanotechnology was introduced to this therapy for active targeting and inducing more thermo-agents into diseased areas, which promoted the anti-tumor effects 139-141.
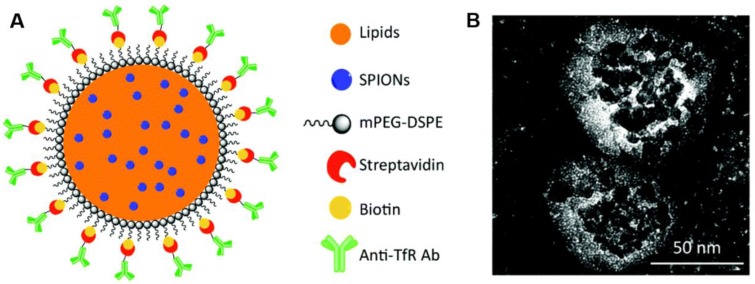
Plenty of evidences showed that the magnetothermal therapy against GBM was more effective by combining with chemotherapy 142. They could enhance the sensitivity of the tumor cells to therapeutic drugs. For example, Gianni Ciofani's group developed a lipid magnetic nanovectors (LMNVs) with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-conjugated poly(ethylene glycol) (DSPE-PEG) (Figure 11) 143, 144. It was functionalized with the antibody against the transferrin receptor (TfR) for dual targeting to endothelial cells of BBB and GBM cells. The superparamagnetic iron oxide nanoparticles (SPIONs) and temozolomide (TMZ) were co-loaded in the lipid nanovectors (LMNVs). The anti-TfR antibodies facilitated targeting to glioblastoma spheroids in multi-cellular in vitro models, with 40.5% vs. 8.1% for non-target nanovectors. The combined treatment with magnetothermal hyperthermia was able to efficiently disintegrate the GBM spheroids and induce significant tumor cell death. Under magnetic fields, the nanovector induced mild hyperthermia (43°C) and enhanced anticancer effect against U87 MG cells.
Figure 11.
A) The illustration of the lipid magnetic nanovectors (LMNVs) loaded with SPIONs and functionalized with an anti-transferrin receptor antibody (anti-TfR Ab). B) The high magnification transmission electron microscopy (TEM) images of functionalized nanovectors (AbLMNVs). Adopted with permission from 143. Copyright 2019 The Royal Society of Chemistry.
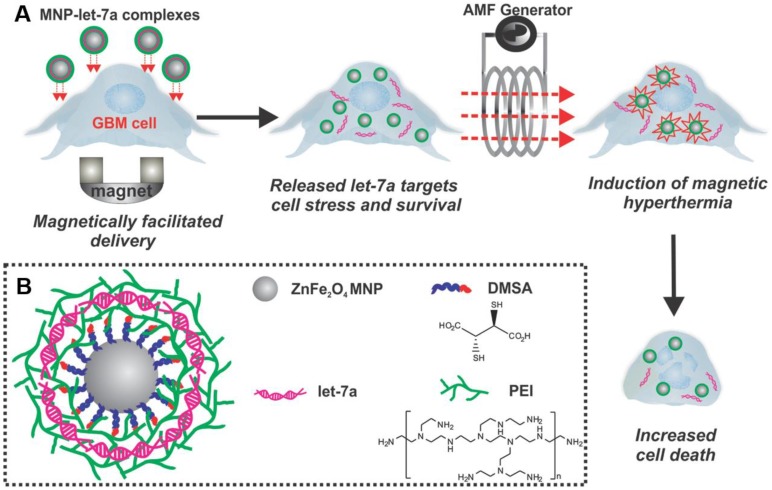
Besides the lipid nanovectors, many synthetic polymeric nanosystems were also exploited as delivery vectors for combined treatments. Yin et al. exploited a highly magnetic zinc-doped iron oxide nanoparticle (ZnFe2O4) decorated with branched polyethyleneimine (PEI) to deliver lethal-7a miRNA (let-7a) (MNP-PEI/miRNA/PEI complexes), which was a tumor suppressor that inhibited malignant growth by targeting the downstream effectors of heat shock proteins (HSPs) (Figure 12) 145. The nanosystem MNP-PEI/miRNA/PEI exhibited obvious apoptosis of brain cancer cells and led to caspase-3 mediated apoptosis by combining with magnetic hyperthermia.
Figure 12.
The MNP-PEI/miRNA/PEI complexes enhanced the treatment against brain cancer. A) The MNP complexes were first delivered to GBM cells, which was enhanced by magnetofection. Once inside the cells, let-7a miRNA was released for targeting downstream effectors of HSPs. This sensitized the cancer cells to subsequent magnetic hyperthermia for enhanced apoptosis. B) MNPs were complexed with let-7a miRNA branched PEI via a layer-by-layer approach. Adapted with permission from 145. Copyright 2014 WILEY‐VCH Verlag GmbH & Co.
Wen-Chia Huang et al. reported a tumortropic adipose-derived stem cells (ADSCs) encapsulating the nanotherapeutics (SPNPs) for chemo-thermotherapy 146. The nanotherapeutics were co-assembled with poly(γ-glutamic acid-co-distearyl γ-glutamate), poly(lactic-co-glycolic acid), the anticancer drug paclitaxel (PTX) and oleic acid-coated superparamagnetic iron oxide NPs. With the guide of ADSCs to tumor sites, hyperthermia was activated by the high frequency magnetic field (HFMF) and triggered drug release in tumor cells. The data in vivo demonstrated that the combined therapy extended the lifespan of the brain tumor-bearing mice almost 3 folds, from 11 to 31 days, in comparison with the PBS control group. Whereas, the ADSCs without HFMF activation was only 15 days.
Although the combination of magnetothermal therapy with chemotherapy showed better inhibition against the GBM than single therapy, the combined strategy had not reached the required threshold for efficient clinical applications in GBM treatments. The reasons for this were as follows. Firstly, the magnetothermal efficiency of the magnetic systems was insufficient for clinical applications. The amount and characteristics of magnetic materials and their intratumoral distribution were the key factors for therapeutic effects. Secondly, the timing of the magnetothermal therapy and chemotherapy should greatly influence the overall results of treatments, which needed further investigations. Finally, the design of the delivery systems for the combination was very important, since these would directly affect the anti-tumor effects. Albeit these challenges, such combinations offered multimodal therapeutic strategies in a single treatment. The thermo-agents in the magnetothermal therapy, like the iron oxide nanoparticles, could also exhibit magnetic resonance imaging for tracing the combined nanosystems and monitoring the drug accumulation in in vivo distribution.
Combined phototherapy with immunotherapy
Near-infrared photoimmunotherapy (NIR-PIT) was a recently emerging therapy for cancers 147. It employed a conjugate, which consisted of a near-infrared photosensitizer and a target-specific antibody. They could selectively kill the cancer cells and activate the body's antitumor immune responses. And few of the photo-immunoconjugates displayed toxicity until the activation by the light with specific wavelength. The NIR-PIT was applied for clinical trials against inoperable head and neck cancers by using cetuximab-IR700 (RM1929), and they exhibited promising results 148, 149.
Utilizing the unique character of NIR-PIT, Jing et al. chose the AC133 monoclonal antibody to conjugate with the photosensitizer IR700 (AC133-IR700) for glioblastoma treatments 150. The AC133 could recognize the CD133 on human cancer stem cells, and enhance the active targeting to orthotopic AC133+ brain tumor. The treated group extended the median survival time to 33.5 days in comparison with non-irradiated control group (with 15 days of survival time). The epidermal growth factor receptor (EGFR) was the general genetic mutation in GBM. Hence, an EGFR-specific antibody (ZEGFR-03115) was conjugated to the photosensitizer IR700DX for glioblastoma treatments 151. The tumor distribution of the ZEGFR-03115-IR700DX was 6-fold higher than that of the control group ZTaq-IR700DX. And they exhibited a significant inhibition of tumor growth in U87-MGvIII bearing mice over a period of 18 days.
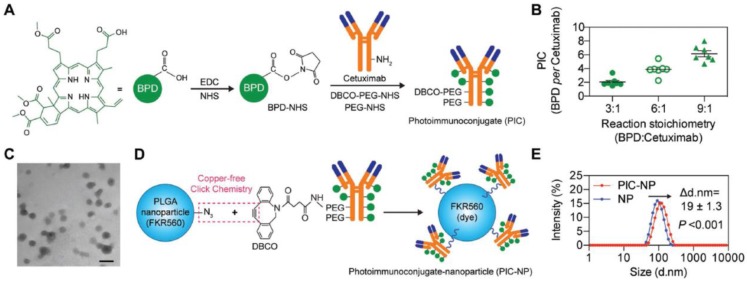
However, due to the limited ratio of the photosensitizer-to-antibody, the amount of photo-immunoconjugates delivered to tumor cells was not sufficient. Hence, Huang et al. adopted poly(lactic-co-glycolic acid) (PLGA) nanoparticles to overcome the obstacles 152. The photo-immunoconjugates benzoporphyrin derivative (BPD)-cetuximab was bound to PLGA nanoparticles by click coupling (Figure 13). The photo-immunoconjugate nanoparticle (PIC-NP) could significantly enhance the photosensitizers to cancer cells, and increase light-activated cytotoxicity in EGFR-overexpressing U87 cells. The nanotechnology improved the overall treatment outcomes comparing with the photo-immunoconjugates.
Figure 13.
The synthesis route of photo-immunoconjugate nanoparticles (PIC-NPs). A) The illustration of PIC synthesis. Benzoporphyrin derivative (BPD) photosensitizers were conjugated to PEGylated cetuximab via carbodiimide crosslinker chemistry. B) The varied stoichiometry of BPD reacted with cetuximab. C) Transmission electron microscopy (TEM) image of poly(ethylene glycol)-poly(lactic-co-glycolic acid) (PEG-PLGA) polymeric nanoparticles prepared via nanoprecipitation method. Scale bar 100 nm. D) Schematic depiction of PIC-NP synthesis via copper-free click chemistry. Azide-containing FKR560 dye-loaded PLGA nanoparticles were reacted with the dibenzocyclooctyne (DBCO)-containing PICs to form PIC-NPs. E) Covalent conjugation of PICs onto 80 nm PEG-PLGA NPs to form monodispersed PIC-NPs around 100 nm in diameter. Adapted with permission from 152. Copyright 2018 WILEY‐VCH Verlag GmbH & Co.
Compared to classic photodynamic therapy, the photoimmunotherapy utilized the specific antibodies that could facilitate targeting the tumor cells without damaging the normal cells. It also enhanced photosensitizer delivery into tumors and increased the light-activated cytotoxicity. Despite these promising advances, the limited ratio of photosensitizer-to-antibody restricted the amount of photosensitizers delivered by photo-immunoconjugates. Hence, the nanodelivery systems provided the larger loading capacity and increased accumulation in the tumors. They efficiently maximized the photo-toxicity to the targeted tissues. However, the photoimmunotherapy for glioblastoma was still in its infancy. The immune activation was not studied well for these anti-glioma treatments.
Combined gene therapy with immunotherapy
Gene therapy had emerged as a novel treatment for various human diseases including cancers. It could specifically regulate the oncogenes in the anti-tumor treatments 153, 154. The adenovirus-mediated gene therapy with sitimagene ceradenovec and ganciclovir after resection increased survival time of patients with newly diagnosed glioblastoma multiforme 155. Hence, the gene therapy was usually proposed as a useful adjuvant for the current glioblastoma treatments 156. The combination of gene therapy with immunotherapy should increase the whole outcomes of glioblastoma treatments 157, 158. For example, Maria-Carmela Speranza et al. utilized the nonreplicating adenovirus containing the HSV TK gene (AdV-tk) to potentiate the anti-PD-1 efficacy in syngeneic glioblastoma mouse models. The AdV-tk could upregulate the IFN signaling and increase the PD-L1 levels. And cytotoxic CD8+ T cells were induced to accumulate in tumors. The combination treatment significantly increased the percentage of long-term survival (LTS≥100 days) animals from 30-50% (single agents) to 88% 159.
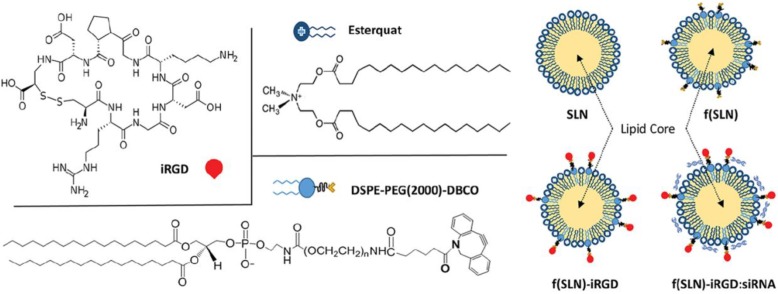
Despite the potentials of gene therapy, the intrinsic characteristics of the gene limited the efficient delivery to tumor sites and hindered their progress to the clinical applications 26, 160, 161. Especially the crossing through the BBB exhibited an extra challenge for anti-glioma treatments. Nanoparticles could compensate the shortages of the gene and safely deliver gene and immunotherapeutic agents to brain tumors 162. For instance, Gulsah Erel-Akbaba et al. developed a cyclic peptide iRGD (CCRGDKGPDC)-decorated solid lipid nanoparticle (SLN) to deliver siRNA against both the epidermal growth factor receptor (EGFR) and programmed cell death ligand-1 (PD-L1) for combined therapy (Figure 14) 163. The targeting nanoparticles f(SLN)-iRGD:siRNA led to 54.7% and 58.6% decrease for EGFR and PD-L1, respectively. Moreover, the median survival of the mice treated with f(SLN)-iRGD:siRNA with radiation increased to 38 days. These three combinations largely improved the survival of the GL261-bearing mice.
Figure 14.
The chemical structures of the DSPE-PEG(2000)-DBCO, Esterquat and iRGD peptide, and the construction of the nanoparticles SLN, f(SLN), f(SLN)-iRGD and f(SLN)-iRGD:siRNA. Adapted with permission from 163. Copyright 2019 American Chemical Society.
Evidences exhibited that the gene therapy was usually applied to regulate the immunosuppressive signals and enhance the systemic therapy in glioma treatments 164. These could synergistically augment the immune responses with immunotherapy. But the reported studies on this strategy were less. The gene therapy and immunotherapy usually served as adjuvant treatments in existing anti-glioma therapies. Moreover, the lack of specific genes and antigens was still the critical barriers for satisfied treatments.
Conclusion and Perspective
Glioblastoma is a complex and deadly disease in central nervous system. The high infiltration, heterogeneity, rapid growth rate and regeneration ability of the GBM stem cells result in poor prognosis and high recurrence. Furthermore, the existence of the blood-brain barrier (BBB) greatly hinders adequate accumulation of the therapeutic drugs in tumor sites, which also induces drug-resistance to therapy. With the deep understanding of glioblastoma, improved therapeutic strategies are exploited to enhance the efficacy against glioblastoma. They mainly include chemotherapy, gene therapy, radiotherapy, immunotherapy, phototherapy and magnetothermal therapy. Considering the intricate characteristics of GBM, combined-therapeutic strategies are developed for synergistic anti-glioma effects and reduced drug-resistance 165. And the pros and cons of the combined therapies are summarized and illustrated in Table 1.
Table 1.
The pros and cons of the combined treatments against glioblastoma.
| Combined treatments | Advantages | Disadvantages |
|---|---|---|
| Chemotherapies | Reduced drug resistance; Multiple mechanisms |
Non-specificity to tumor cells |
| Chemotherapy and radiotherapy | Increased tumor sensitivity to chemotherapeutic drugs | Hard to control radiation margin; Timing of radiotherapy |
| Chemotherapy and phototherapy | Multiple mechanisms | Limited tissue penetration of light; Drug to light interval |
| Chemotherapy and immunotherapy | Multiple mechanisms; Reduced metastasis and recurrence | Lack of specific antigens; Timing of immunotherapy |
| Chemotherapy and magnetothermal therapy | Multimodality; Theranostics | Thermal efficiency; Hard to focus heating on tumor sites |
| Phototherapy and immunotherapy | Enhanced targeting; Reduced metastasis and recurrence | Restricted ratio of photosensitizer to antibody; |
| Gene therapy and immunotherapy | Indirect anti-tumor effect; Reduced drug resistance |
Lack of specific genes and antigens |
In review of the intensive studies for GBM, some problems should be settled for optimizing GBM treatment. Firstly, the choices of the therapeutic agents in combined strategies are very important for overall treatment outcomes. The therapeutic mechanisms of the agents should not counteract the pharmaceutical effects for each other. Some of the combinations in clinical trials do not obtain the satisfied results compared with monotherapies 40, 166. It has been reported that the combination of erlotinib and sorafenib in phase II study exhibits significant pharmacokinetic interactions, which may display negative impacts on the therapeutic efficacy 167. Moreover, the recent therapeutic agents selected for therapy can not cover the critical points in the progression of GBM. Therefore, multi-modal combined strategies should be tesed in anti-glioma treatments. Secondly, the timing of the therapeutic strategies in combination needs more investigations. For instance, the administration timing of chemotherapy in combinations obviously affects the efficacy of immunotherapy 127. Hence, alternative therapeutic strategies are required to attempt for maximizing the therapeutic effects. Finally, the suitable delivery systems are demanded for effective drug delivery. Increasing studies demonstrate that nanosystems perform preferable therapeutic results in comparison with free therapeutic agents. The delivery platforms possess active brain targeting and diverse stimuli-responses 168, 169. Therefore, these strategies significantly promote the loading agents to cross BBB and accumulate in GBM cells. And they extend the overall survival time of GBM in animal experimental studies. However, glioblastoma remains a complicated cancer. The current reported targets are not specific for the endothelial cells of BBB and tumor cells. The further and better understanding of the intricate tumor microenvironment is still required. Hence, the specific targets and the suitable stimuli-responses of the GBM are required to exploit, which will facilitate the diverse agents to release in the right places and ensure sufficient therapeutic agents in GBM cells. Moreover, most of the biomaterials in studies have not been approved by FDA, which aggravate the difficulties for clinical translation.
Although the current combined-therapeutic strategies have enhanced the efficacy of GBM treatments, great efforts are still required on the understanding the complicated mechanisms of the growth, progression and invasion process of GBM. Multidisciplinary knowledge of GBM will guide the development of optimal therapeutic strategies and rational nanosystems for satisfied outcomes, and direct the investigation for other malignant diseases in central nerves system.
Acknowledgments
This work was financially supported by the Beijing Natural Science Foundation (L172046), the National Natural Science Foundation of China (31771095, 51573188 and 21875254), the National High Technology Research and Development Program (2016YFA0200303) and the Beijing Natural Science Foundation (2192057).
References
- 1.Reifenberger G, Wirsching H-G, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol. 2017;14:434–52. doi: 10.1038/nrclinonc.2016.204. [DOI] [PubMed] [Google Scholar]
- 2.Shergalis A, Armand Bankhead I, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70:412–45. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glas M, Happold C, Rieger J, Wiewrodt D, Bähr O, Wick JPS. et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27:1257–61. doi: 10.1200/JCO.2008.19.2195. [DOI] [PubMed] [Google Scholar]
- 4.Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko S, Eljamel MS. Fluorescence image-guided neurosurgery. Future Oncol. 2017;13:2341–8. doi: 10.2217/fon-2017-0194. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Rey-Dios R, Roberts DW, Valdés PA, Cohen-Gadol AA. Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg. 2014;82:175–85. doi: 10.1016/j.wneu.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-A review of the cutting edge. World Neurosurg. 2017;103:538–49. doi: 10.1016/j.wneu.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman FS, Wang M, Robins HI, Tsien CI, Walter J. Curran J, Werner-Wasik M, et al. Phase 2 study of radiation therapy plus low-dose temozolomide followed by temozolomide and irinotecan for glioblastoma: NRG oncology RTOG trial 0420. Int J Radiat Oncol Biol Phys. 2019;103:878–86. doi: 10.1016/j.ijrobp.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruhn H, Strandéus M, Milos P, Hallbeck M, Vrethem M, Lind J. Improved survival of Swedish glioblastoma patients treated according to Stupp. Acta Neurol Scand. 2018;138:332–7. doi: 10.1111/ane.12966. [DOI] [PubMed] [Google Scholar]
- 10.Warren KT, Liu L, Liu Y, Milano MT, Walter KA. The impact of timing of concurrent chemoradiation in patients with high-grade glioma in the era of the Stupp protocol. Front Oncol. 2019;9:186. doi: 10.3389/fonc.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15:422–42. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 12.Barani IJ, Larson DA. Radiation therapy of glioblastoma. USA: Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 13.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279–83. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127–36. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 15.Tsai Y-C, Vijayaraghavan P, Chiang W-H, Chen H-H, Liu T-I, Shen M-Y. et al. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics. 2018;8:1435–48. doi: 10.7150/thno.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Kim LJY, Wu Q, Wallace LC, Prager BC, Sanvoranart T. et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat Med. 2017;23:1352–61. doi: 10.1038/nm.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osuka S, Meir EGV. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127:415–56. doi: 10.1172/JCI89587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Wang G, Zhang H, Hu S, Liu X, Tang J. et al. The blood clearance kinetics and pathway of polymeric micelles in cancer drug delivery. ACS Nano. 2018;12:6179–92. doi: 10.1021/acsnano.8b02830. [DOI] [PubMed] [Google Scholar]
- 19.Parrish KE, Cen L, Murray J, Calligaris D, Kizilbash S, Mittapalli RK. et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol Cancer Ther. 2015;14:2735–43. doi: 10.1158/1535-7163.MCT-15-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Yan X, Jacobson O, Sun W, Wang Z, Tong X. et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 2017;7:319–28. doi: 10.7150/thno.18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 22.Kingwell K. Drug delivery: new targets for drug delivery across the BBB. Nat Rev Drug Discov. 2016;15:84–5. doi: 10.1038/nrd.2016.14. [DOI] [PubMed] [Google Scholar]
- 23.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–92. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 24.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 25.Zuchero YJY, Chen X, Bien-Ly N, Bumbaca D, K.Tong R, Gao X. et al. Discovery of novel blood-brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron. 2016;89:70–82. doi: 10.1016/j.neuron.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Y, Morshed R, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48:2967–3014. doi: 10.1039/c8cs00805a. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Wang C, Fitch S, Yang F. Targeting tumor hypoxia using nanoparticle-engineered CXCR4-overexpressing adipose-derived stem cells. Theranostics. 2018;8:1350–60. doi: 10.7150/thno.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao H, Zhang S, Cao S, Yang Z, Pang Z, Jiang X. Angiopep-2 and activatable cell-penetrating peptide dual-functionalized nanoparticles for systemic glioma-targeting delivery. Mol Pharm. 2014;11:2755–63. doi: 10.1021/mp500113p. [DOI] [PubMed] [Google Scholar]
- 31.Wanjale MV, Kumar GSV. Peptides as a therapeutic avenue for nanocarrier-aided targeting of glioma. Expert Opin Drug Deliv. 2017;14:811–24. doi: 10.1080/17425247.2017.1242574. [DOI] [PubMed] [Google Scholar]
- 32.Huang R, Harmsen S, Samii JM, Karabeber H, Pitter KL, Holland EC. et al. High precision imaging of microscopic spread of glioblastoma with a targeted ultrasensitive SERRS molecular imaging probe. Theranostics. 2016;6:1075–84. doi: 10.7150/thno.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64. doi: 10.1016/j.jconrel.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Yang W, Zhang J, Meng F, Zhong Z. Protein toxin chaperoned by LRP-1-targeted virus-mimicking vesicles induces high-efficiency glioblastoma therapy in vivo. Adv Mater. 2018;30:1800316. doi: 10.1002/adma.201800316. [DOI] [PubMed] [Google Scholar]
- 35.Sita TL, Kouri FM, Hurley LA, Merkel TJ, Chalastanis A, May JL. et al. Dual bioluminescence and near-infrared fluorescence monitoring to evaluate spherical nucleic acid nanoconjugate activity in vivo. Proc Natl Acad Sci USA. 2017;114:4129–34. doi: 10.1073/pnas.1702736114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangraviti A, Tzeng SY, Kozielski KL, Wang Y, Jin Y, Gullotti D. et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano. 2015;9:1236–49. doi: 10.1021/nn504905q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taal W, Oosterkamp HM, Walenkamp AME, Dubbink HJ, Beerepoot LV, Hanse MCJ. et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 38.Lun X, ConnorWells J, Grinshtein N, King JC, Hao X, Dang N-H. et al. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin Cancer Res. 2016;22:3860–75. doi: 10.1158/1078-0432.CCR-15-1798. [DOI] [PubMed] [Google Scholar]
- 39.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I. et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954–63. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 40.Galanis E, Anderson SK, Lafky JM, Uhm JH, Giannini C, Kumar SK. et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res. 2013;19:4816–23. doi: 10.1158/1078-0432.CCR-13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EQ, Reardon DA, Schiff D, Drappatz J, Muzikansky A, Grimm SA. et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17:862–7. doi: 10.1093/neuonc/nou350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verreault M, Wehbe M, Strutt D, Masin D, Anantha M, Walker D. et al. Determination of an optimal dosing schedule for combining Irinophore C™ and temozolomide in an orthotopic model of glioblastoma. J Control Release. 2015;220:348–57. doi: 10.1016/j.jconrel.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 43.Lundy DJ, Lee K-J, Peng I-C, Hsu C-H, Lin J-H, Chen K-H. et al. Inducing a transient increase in blood-brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme. ACS Nano. 2019;13:97–113. doi: 10.1021/acsnano.8b03785. [DOI] [PubMed] [Google Scholar]
- 44.Guo L, Fan L, Pang Z, Ren J, Ren Y, Li J. et al. TRAIL and doxorubicin combination enhances anti-glioblastoma effect based on passive tumor targeting of liposomes. J Control Release. 2011;154:93–102. doi: 10.1016/j.jconrel.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Sharpe MA, Marcano DC, Berlin JM, Widmayer MA, Baskin DS, Tour JM. Antibody-targeted nanovectors for the treatment of brain cancers. ACS Nano. 2012;6:3114–20. doi: 10.1021/nn2048679. [DOI] [PubMed] [Google Scholar]
- 46.Lam FC, Morton SW, Wyckoff J, Han T-LV, Hwang MK, Maffa A. et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9:1991. doi: 10.1038/s41467-018-04315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Y, Zhang J, Meng F, Zhong Z. Apolipoprotein E peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano. 2018;12:11070–9. doi: 10.1021/acsnano.8b05265. [DOI] [PubMed] [Google Scholar]
- 48.Locatelli E, Naddaka M, Uboldi C, Loudos G, Fragogeorgi E, Molinari V. et al. Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine. 2014;9:839–49. doi: 10.2217/nnm.14.1. [DOI] [PubMed] [Google Scholar]
- 49.Johannessen T-CA, Bjerkvig R, Tysnes BB. DNA repair and cancer stem-like cells-potential partners in glioma drug resistance? Cancer Treat Rev. 2008;34:558–67. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 50.Batista LFZ, Roos WP, Christmann M, Menck CFM, Kaina B. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res. 2007;67:11886–95. doi: 10.1158/0008-5472.CAN-07-2964. [DOI] [PubMed] [Google Scholar]
- 51.Ma J, Murphy M, O'Dwyer PJ, Berman E, Reed K, Gallo JM. Biochemical changes associated with a multidrug-resistant phenotype of a human glioma cell line with temozolomide-acquired resistance. Biochem Pharmacol. 2002;63:1219–28. doi: 10.1016/s0006-2952(02)00876-6. [DOI] [PubMed] [Google Scholar]
- 52.Murphy SF, Varghese RT, Lamouille S, Guo S, Pridham KJ, Kanabur P. et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;76:139–49. doi: 10.1158/0008-5472.CAN-15-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adair JE, Johnston SK, Mrugala MM, Beard BC, Guyman LA, Baldock AL. et al. Gene therapy enhances chemotherapy tolerance and efficacy in glioblastoma patients. J Clin Invest. 2014;124:4082–92. doi: 10.1172/JCI76739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma - look to the future. Nat Clin Pract Oncol. 2008;5:476–86. doi: 10.1038/ncponc1155. [DOI] [PubMed] [Google Scholar]
- 55.Kim S-S, Rait A, Kim E, Pirollo KF, Nishida M, Farkas N. et al. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano. 2014;8:5494–514. doi: 10.1021/nn5014484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, Hu X, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat Commun. 2019;10:1704. doi: 10.1038/s41467-019-09566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 58.Pan Y-J, Chen Y-Y, Wang D-R, Wei C, Guo J, Lu D-R. et al. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials. 2012;33:6570–9. doi: 10.1016/j.biomaterials.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13:204–12. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Guo Y, Huang R, Li J, Huang S, Kuang Y. et al. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials. 2012;33:4907–16. doi: 10.1016/j.biomaterials.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34:9142–8. doi: 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Zheng M, Liu Y, Wang Y, Zhang D, Zou Y, Ruan W, ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv Mater. 2019; 1903. 277. [DOI] [PubMed] [Google Scholar]
- 63.Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng M-Q, Duong N. et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci USA. 2013;110:11751–6. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esposito CL, Nuzzo S, Kumar SA, Rienzo A, Lawrence CL, Pallini R. et al. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J Control Release. 2016;238:43–57. doi: 10.1016/j.jconrel.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG. et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Bertoni H, Kozielski KL, Rui Y, Lal B, Vaughan H, Wilson DR. et al. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18:4086–94. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Z, Li Y, Shi Y, Li Y, Xiao Z, Zhang X. Traceable nanoparticles with spatiotemporally controlled release ability for synergistic glioblastoma multiforme treatment. Adv Funct Mater. 2017;27:1703967. [Google Scholar]
- 68.Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30:1706759. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–50. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pang H-H, Chen P-Y, Wei K-C, Huang C-W, Shiue Y-L, Huang C-Y. et al. Convection-enhanced delivery of a virus-like nanotherapeutic agent with dual-modal imaging for besiegement and eradication of brain tumors. Theranostics. 2019;9:1752–63. doi: 10.7150/thno.30977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alessandri G, Coccè V, Pastorino F, Paroni R, Cas MD, Restelli F. et al. Microfragmented human fat tissue is a natural scaffold for drug delivery: potential application in cancer chemotherapy. J Control Release. 2019;302:2–18. doi: 10.1016/j.jconrel.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X. et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci. 2019;6:1801899. doi: 10.1002/advs.201801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 74.Zou Y, Liu Y, Yang Z, Zhang D, Lu Y, Zheng M. et al. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv Mater. 2018;30:1803717. doi: 10.1002/adma.201803717. [DOI] [PubMed] [Google Scholar]
- 75.Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, L.West B. et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amsbaugh MJ, Yusuf MB, Gaskins J, Burton EC, Y.Woo S. Patterns of care and predictors of adjuvant therapies in elderly patients with glioblastoma: an analysis of the national cancer data base. Cancer. 2017;123:3277–84. doi: 10.1002/cncr.30730. [DOI] [PubMed] [Google Scholar]
- 77.Perry JR, Laperriere N, O'Callaghan CJ, Brandes AA, Menten J, Phillips C. et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–37. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- 78.Stupp R, Hegi ME, Mason WP, Bent MJvd, Taphoorn MJB, Janzer RC. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 79.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 80.Shi J, Fan D, Dong C, Liu H, Jia B, Zhao H. et al. Anti-tumor effect of integrin targeted 177Lu-3PRGD2 and combined therapy with endostar. Theranostics. 2014;4:256–66. doi: 10.7150/thno.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamborini M, Locatelli E, Rasile M, Monaco I, Rodighiero S, Corradini I. et al. A combined approach employing chlorotoxin-nanovectors and low dose radiation to reach infiltrating tumor niches in glioblastoma. ACS Nano. 2016;10:2509–20. doi: 10.1021/acsnano.5b07375. [DOI] [PubMed] [Google Scholar]
- 82.Hottinger AF, Aissa AB, Espeli V, Squiban D, Dunkel N, Vargas MI. et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br J Cancer. 2014;110:2655–61. doi: 10.1038/bjc.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–37. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO. et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–81. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benachour H, Aymeric Sève3, Bastogne T, Frochot C, Vanderesse R, Jasniewski J. et al. Multifunctional peptide-conjugated hybrid silica nanoparticles for photodynamic therapy and MRI. Theranostics. 2012;2:889–904. doi: 10.7150/thno.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghosh S, Carter KA, Lovell JF. Liposomal formulations of photosensitizers. Biomaterials. 2019;218:119341. doi: 10.1016/j.biomaterials.2019.119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bechet D, Couleaud P, Frochot C, Viriot M-L, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–21. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Huang X, Zhang W, Guan G, Song G, Zou R, Hu J. Design and functionalization of the NIR-responsive photothermal semiconductor nanomaterials for cancer theranostics. Acc Chem Res. 2017;50:2529–38. doi: 10.1021/acs.accounts.7b00294. [DOI] [PubMed] [Google Scholar]
- 89.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 90.Luo D, Carter KA, Miranda D, Lovell JF. Chemophototherapy: an emerging treatment option for solid tumors. Adv Sci. 2016;4:1600106. doi: 10.1002/advs.201600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zou L, Wang H, He B, Zeng L, Tan T, Cao H. et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics. 2016;6:762–72. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Wang T, Liu H, Ren F, Qiu W, Sun Q. et al. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2-xSe nanoparticles. Nanoscale. 2019;11:7600–8. doi: 10.1039/c9nr01789e. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Li L, Guo Q, Wang L, Liu D, Wei Z. et al. Novel Cs-based upconversion nanoparticles as dual-modal CT and UCL imaging agents for chemo-photothermal synergistic therapy. Theranostics. 2016;6:1491–505. doi: 10.7150/thno.15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu G, Yu S, Saha ML, Zhou J, Cook TR, Yung BC. et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat Commun. 2018;9:4335. doi: 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Q, Wang X, Wang C, Feng L, Li Y, Liu Z. Drug-induced self-assembly of modified albumins as nanotheranostics for tumor-targeted combination therapy. ACS Nano. 2015;9:5223–33. doi: 10.1021/acsnano.5b00640. [DOI] [PubMed] [Google Scholar]
- 96.Deng H, Zhong Y, Du M, Liu Q, Fan Z, Dai F. et al. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-ray computed tomography imaging. Theranostics. 2014;4:904–18. doi: 10.7150/thno.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng H, Dai F, Ma G, Zhang X. Theranostic gold nanomicelles made from biocompatible comb-like polymers for thermochemotherapy and multifunctional imaging with rapid clearance. Adv Mater. 2015;27:3645–53. doi: 10.1002/adma.201501420. [DOI] [PubMed] [Google Scholar]
- 98.Song J, Yang X, Jacobson O, Lin L, Huang P, Niu G. et al. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9:9199–209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quintanilla M, García I, Lázaro Id, García-Alvarez R, Henriksen-Lacey M, Vranic S. et al. Thermal monitoring during photothermia: hybrid probes for simultaneous plasmonic heating and near-infrared optical nanothermometry. Theranostics. 2019;9:7298–312. doi: 10.7150/thno.38091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hao Y, Zhang B, Zheng C, Ji R, Ren X, Guo F. et al. The tumor-targeting core-shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J Control Release. 2015;220:545–55. doi: 10.1016/j.jconrel.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 101.Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P. et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–57. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 102.Lai J, Deng G, Sun Z, Peng X, Li J, Gong P. et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing Blood-Brain Barrier. Biomaterials. 2019;211:48–56. doi: 10.1016/j.biomaterials.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 103.Sun X, Wang C, Gao M, Hu A, Liu Z. Remotely controlled red blood cell carriers for cancer targeting and near-infrared light-triggered drug release in combined photothermal-chemotherapy. Adv Funct Mater. 2015;25:2386–94. [Google Scholar]
- 104.Wang C, Ye Y, Hu Q, Bellotti A, Gu Z. Tailoring biomaterials for cancer immunotherapy: emerging trends and future outlook. Adv Mater. 2017;29:1606036. doi: 10.1002/adma.201606036. [DOI] [PubMed] [Google Scholar]
- 105.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol. 2013;10:14–26. doi: 10.1038/nrclinonc.2012.204. [DOI] [PubMed] [Google Scholar]
- 107.Paggio JCD. Immunotherapy: cancer immunotherapy and the value of cure. Nat Rev Clin Oncol. 2018;15:268–70. doi: 10.1038/nrclinonc.2018.27. [DOI] [PubMed] [Google Scholar]
- 108.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boussiotis VA, Charest A. Immunotherapies for malignant glioma. Oncogene. 2018;37:1121–41. doi: 10.1038/s41388-017-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100–9. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 111.Oh T, Sayegh ET, Fakurnejad S, Oyon D, Lamano JB, DiDomenico JD. et al. Vaccine therapies in malignant glioma. Curr Neurol Neurosci Rep. 2015;15:508. doi: 10.1007/s11910-014-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen Q, Yang J, Liu R, Liu L, Zhang J, Shen S. et al. Hybrid 'clusterbombs' as multifunctional nanoplatforms potentiate brain tumor immunotherapy. Mater Horiz. 2019;6:810–6. [Google Scholar]
- 113.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–29. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gan HK, Burgess AW, Clayton AHA, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–30. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 115.Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD. et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17:854–61. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elsamadicy AA, Chongsathidkiet P, Desai R, Woroniecka K, Farber SH, Fecci PE. et al. Prospect of rindopepimut in the treatment of glioblastoma. Expert Opin Biol Ther. 2017;17:507–13. doi: 10.1080/14712598.2017.1299705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H. et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–85. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 118.Galstyan A, Markman JL, Shatalova ES, Chiechi A, Korman AJ, Patil R. et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10:3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuang J, Song W, Yin J, Zeng X, Han S, Zhao Y-P. et al. iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma. Adv Funct Mater. 2018;28:1800025. [Google Scholar]
- 120.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–14. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beug ST, Beauregard CE, Healy C, Sanda T, St-Jean M, Chabot J. et al. Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma. Nat Commun. 2017;8:14278. doi: 10.1038/ncomms14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao P, Wang Y, Kang X, Wu A, Yin W, Tang Y. et al. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage mediated immunotherapy. Chem Sci. 2018;9:2674–89. doi: 10.1039/c7sc04853j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li T-F, Li K, Wang C, Liu X, Wen Y, Xu Y-H. et al. Harnessing the cross-talk between tumor cells and tumor-associated macrophages with a nano-drug for modulation of glioblastoma immune microenvironment. J Control Release. 2017;268:128–46. doi: 10.1016/j.jconrel.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 125.Kadiyala P, Li D, Nuñez FM, Altshuler D, Doherty R, Kuai R. et al. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano. 2019;13:1365–84. doi: 10.1021/acsnano.8b06842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Candolfi M, Yagiz K, Wibowo M, Ahlzadeh GE, Puntel M, Ghiasi H. et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20:1555–65. doi: 10.1158/1078-0432.CCR-13-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mathios D, Kim JE, Mangraviti A, Phallen J, Park C-K, Jackson CM. et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180. doi: 10.1126/scitranslmed.aag2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y. et al. Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv Mater. 2018;30:1705054. doi: 10.1002/adma.201705054. [DOI] [PubMed] [Google Scholar]
- 129.Fadul CE, Fisher JL, Gui J, Hampton TH, Côté AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang D, Lim M, Goos JACM, Qiao R, Ng YY, Mansfeld FM. et al. Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. 2018;9:831. doi: 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2008;24:467–74. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 132.Beik J, Abed Z, Ghoreishi FS, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A. et al. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced application. J Control Release. 2016;235:205–21. doi: 10.1016/j.jconrel.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 133.Fèvre RL, Durand-Dubief M, Chebbi I, Mandawala C, Lagroix F, Valet J-P. et al. Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics. 2017;7:4618–31. doi: 10.7150/thno.18927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5:173–86. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19:157–68. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Landeghem FKHv, Maier-Hauff K, Jordan A, Hoffmann K-T, Gneveckow U, Scholz R. et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–7. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 138.Kumar CSSR, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alphand E, Idbaih A, Adam C, Delattre J-Y, Schmitt C, Guyot F. et al. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials. 2017;141:210–22. doi: 10.1016/j.biomaterials.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 140.Chen L, Wu Y, Wu H, Li J, Xie J, Zang F. et al. Magnetic targeting combined with active targeting of dual-ligand iron oxide nanoprobes to promote the penetration depth in tumors for effective magnetic resonance imaging and hyperthermia. Acta Biomater. 2019;96:491–504. doi: 10.1016/j.actbio.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 141.Vverma J, Lal S, Noorden CJV. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863–77. doi: 10.2147/IJN.S57501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu YJ, Chuang EY, Cheng YH. et al. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem Eng J. 2019;373:720–33. [Google Scholar]
- 143.Marino A, Camponovo A, Degl'Innocenti A, Bartolucci M, Tapeinos C, Martinelli C. et al. Multifunctional temozolomide-loaded lipid superparamagnetic nanovectors: dual targeting and disintegration of glioblastoma spheroids by synergic chemotherapy and hyperthermia treatment. Nanoscale. 2019;11:21227–48. doi: 10.1039/c9nr07976a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tapeinos C, Marino A, Battaglini M, Migliorin S, Brescia R, Scarpellini A. et al. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale. 2019;11:72–88. doi: 10.1039/c8nr05520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin PT, Shah BP, Lee K-B. Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small. 2014;10:4106–12. doi: 10.1002/smll.201400963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang W-C, Lu I-L, Chiang W-H, Lin Y-W, Tsai Y-C, Chen H-H. et al. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J Control Release. 2017;254:119–30. doi: 10.1016/j.jconrel.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 147.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–91. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ogata F, Nagaya T, Nakamura Y, Sato K, Okuyama S, Maruoka Y. et al. Near-infrared photoimmunotherapy: a comparison of light dosing schedules. Oncotarget. 2017;8:35069–75. doi: 10.18632/oncotarget.17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagaya T, Okuyama S, Ogata F, Maruoka Y, Knapp DW, Karagiannis SN. et al. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody. Oncotarget. 2018;9:19026–38. doi: 10.18632/oncotarget.24876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jing H, Weidensteiner C, Reichardt W, Gaedicke S, Zhu X, Grosu A-L. et al. Imaging and selective elimination of glioblastoma stem cells with theranostic near-infrared-labeled CD133-specific antibodies. Theranostics. 2016;6:862–74. doi: 10.7150/thno.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Burley TA, Mączyńska J, Shah A, Szopa W, Harrington KJ, Boult JKR. et al. Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment. Int J Cancer. 2018;142:2363–74. doi: 10.1002/ijc.31246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang H-C, Pigula M, Fang Y, Hasan T. Immobilization of photo-immunoconjugates on nanoparticles leads to enhanced light-activated biological effects. Small. 2018;14:1800236. doi: 10.1002/smll.201800236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chou S-T, Patil R, Galstyana A, Gangalum PR, Cavenee WK, Furnari FB. et al. Simultaneous blockade of interacting CK2 and EGFR pathways by tumor-targeting nanobioconjugates increases therapeutic efficacy against glioblastoma multiforme. J Control Release. 2016;244:14–23. doi: 10.1016/j.jconrel.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kozielski KL, Ruiz-Valls A, Tzeng SY, Guerrero-Cázares H, Rui Y, Li Y. et al. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials. 2019;209:79–87. doi: 10.1016/j.biomaterials.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Westphal M, Ylä-Herttuala S, Martin J, Warnke P, Menei P, Eckland D. et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–33. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 156.Lozada-Delgado EL, Grafals-Ruiz N, Vivas-Mejía PE. RNA Interference for Glioblastoma therapy: Innovation ladder from the bench to clinical trials. Life Sci. 2017;188:26–36. doi: 10.1016/j.lfs.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Candolfi M, Kroeger KM, Muhammad AKMG, Yagiz K, Farrokhi C, Pechnick RN. et al. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr Gene Ther. 2009;9:409–21. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.S-S Harford JB, Moghe M Slaughter T, Doherty C Chang EH. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood-brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int J Cancer. 2019;145:2535–46. doi: 10.1002/ijc.32531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Speranza M-C, Passaro C, Ricklefs F, Kasai K, Klein SR, Nakashima H. et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20:225–35. doi: 10.1093/neuonc/nox139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhaskaran V, Nowicki MO, Idriss M, Jimenez MA, Lugli G, Hayes JL. et al. The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma. Nat Commun. 2019;10:442. doi: 10.1038/s41467-019-08390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.A. Hajj K, A. Whitehead K. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2:17056. [Google Scholar]
- 162.Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21–7. doi: 10.1016/j.jneuroim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 163.Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ. et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano. 2019;13:4028–40. doi: 10.1021/acsnano.8b08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samson A, Scott KJ, Taggart D, West EJ, Wilson E, Nuovo GJ. et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci Transl Med. 2018;10:aam7577. doi: 10.1126/scitranslmed.aam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu C, Xia Y. Biomimetics: reconstitution of low-density lipoprotein for targeted drug delivery and related theranostic applications. Chem Soc Rev. 2017;46:7668–82. doi: 10.1039/c7cs00492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Field KM, Simes J, Nowak AK, Cher L, Wheeler H, Hovey EJ. et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17:1504–13. doi: 10.1093/neuonc/nov104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peereboom DM, Ahluwalia MS, Ye X, Supko JG, Hilderbrand SL, Phuphanich S. et al. NABTT 0502: a phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013;15:490–6. doi: 10.1093/neuonc/nos322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gan HK, Bent Mvd, Lassman AB, Reardon DA, Scott AM. Antibody-drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat Rev Clin Oncol. 2017;14:695–707. doi: 10.1038/nrclinonc.2017.95. [DOI] [PubMed] [Google Scholar]
- 169.Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28:1457–72. doi: 10.1093/annonc/mdx106. [DOI] [PMC free article] [PubMed] [Google Scholar]