2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/ computed tomography (FDG PET/CT) is routinely used in clinical oncology for the diagnosis of many different types of cancers, taking advantage of the high glucose avidity of neoplastic cells 1. It is indeed notable that, cancer cells display an “addiction” to “aerobic” glycolysis, the so-called “Warburg effect”. However, plasticity in the metabolic reprogramming of cancer cells is far more complex, as genetic and phenotypic changes lead to metabolic heterogeneity of tumor cell populations. Factors in the microenvironment, such as the level of nutrients, oxygen, pH, and the presence of host stromal cells may influence cancer cell metabolism. As a consequence, a low FDG uptake by cancer cells may be related to a low glycolytic rate or to the use of alternative substrates. However, in certain tumors, the diagnostic accuracy of FDG-PET may be affected either by the size of lesion (if below the spatial resolution of PET/CT), or by the physiological urinary excretion rate of the tracer, or by the relatively high metabolic activity of surrounding tissue. Indeed, in the case of bladder, prostate, kidney, breast and endocrine gland cancers, FDG/PET analysis is not recommended for diagnosis 2.
In order to improve the accuracy of FDG PET/CT imaging analysis, it is essential to seek further clarification in particular about the way in which cancer cells adapt to different metabolic profiles. In recent years, many authors have examined cancer metabolism and its reprogramming when cells are exposed to the influence of critical features of the tumor microenvironment, such as hypoxia and acidosis. Proliferating cancer cells use Warburg's aerobic glycolysis to move to “anaerobic” glycolysis when challenged by hypoxia. Both of these metabolic conditions lead to glucose exhaustion and low extracellular pH (pHe), due to lactic acid secretion associated with a reduction in the draining activity of the lymphatic system. Acidosis in the tumor microenvironment, in turn, reprograms cancer cell metabolism towards a more oxidative phenotype. Thus, the microenvironment may lead to different FDG avidity of cancer cells.
It has been reported quite recently that lactic acidosis inhibits breast cancer cell FDG uptake (e.g. in the human MDA-MB-231 triple-negative breast cancer cell line) 3. Single cell suspensions were obtained from core and peripheral regions of subcutaneous tumors removed from mice. Next, FDG uptake was determined in cells of the two regions by radioluminescence (RLM) imaging. FDG uptake was significantly higher in cells isolated from the periphery of the tumors than in those from the core. Accordingly, there was evidence of mitochondrial redox differences between the core and the periphery of tumors in human breast cancer mouse xenografts 4. Therefore, the microenvironment conditions which are considered capable of driving a change of FDG uptake are proliferation, hypoxia and acidosis. To corroborate RLM results further, the authors used gamma radiation counting, observing that the reduction of FDG uptake by cells from the core was promoted by either extracellular acidosis (both lactic and hydrochloric acidosis) or by sodium lactate, (although only at a high dosage). Several in vitro reports have shown that a transient (24 hour) 5 or a chronic (8-10 week) 6 exposure to an acidic medium (pH 6.7-6.8) causes a shift of cancer cell metabolism from glycolytic to respiratory. Accordingly, this shift is associated with a decrease of glucose transporters, thus accounting for the reduction of FDG uptake.
A further question is the case of oncogene-driven tumors. The constitutive activation of oncogene-dependent pathways might reinforce glycolytic metabolism and downregulate respiratory metabolism in cancer cells, via the reduction of both pyruvate kinase M2 (PKM2)-dependent conversion of phosphoenolpyruvate to pyruvate, and pyruvate flux to mitochondria 7. This might explain the reverse association, observed in vivo, between FDG uptake and extracellular pH (pHe) in human epidermal growth factor receptor-2-positive (HER2+) breast cancer grafts. In this case, HER2 regulates key effectors of cellular metabolism such as the serine/threonine kinase Akt, increasing glucose metabolism and explaining the acidic pHe. As a consequence, only the inhibition of oncogene expression could lead to a significant shift from glycolysis to oxidative phosphorylation.
Additional in vivo studies, dealing with the FGD uptake and pHe of cancer, have been carried out with conflicting results. In one study, the pHe of tumors, determined by chemical exchange saturation transfer (MRI-CEST) images, was found to be acidic (pH 6.7), and then FDG uptake was estimated by PET analysis. In order to validate CEST-pH mapping of tumors, animals were forced to drink bicarbonate-rich water to raise the pHe of tumors to almost neutral value (pH 7.0-7.1), demonstrating a significant increase of positive pixels in tumors from sodium bicarbonate-treated animals when compared to those from untreated animals. Upon this validation of the combination of PET/MRI-CEST images, the authors realized that tumors with higher FDG uptake were associated with a lower pHe, while tumors with lower FDG uptake displayed a less acidic microenvironment 8.
Although we could not exclude the possibility that some metabolic differences were related to the different tumors analyzed, it is very difficult to infer a clear conclusion from these data. After FGD phosphorylation, brought about by hexokinase or glucokinase, FDG-6P is not available for metabolic activity. This “metabolic trap” is fundamental for FDG/PET analysis. However, a low amount of intracellular FDG might be removed slowly from the cell by the action of 6-phosphatase on FDG-6P. An additional element of complexity is the possible decrease or absence of glucose-6-phosphatase (G6Pase) activity in tumor cells, which might play a role in the modulation of glucose fermentation, e.g. some tumor regions of a renal cancer with reduced G6Pase exhibit a high FDG uptake 9. The complex heterogeneity of cancer makes it difficult to predict a defined metabolic scenario.
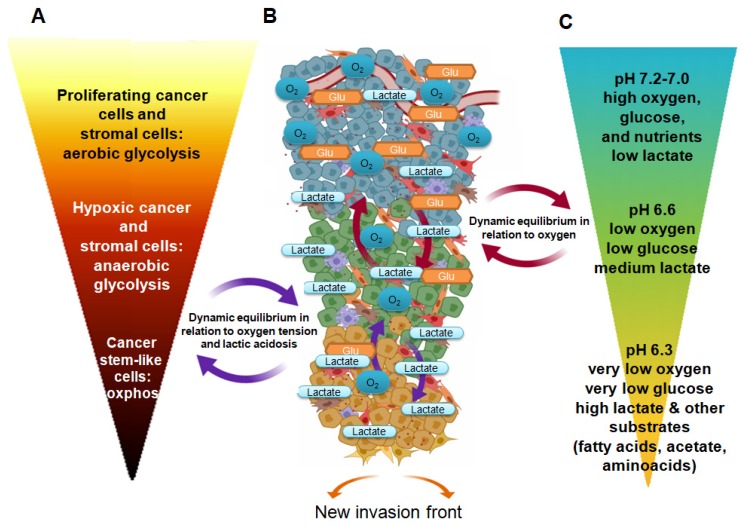
Figure 1 describes the active pathways of various cancer cell subpopulations, considering changes of major nutrients and the distance from blood supply, which are the prominent drivers of metabolic adaptation. Because of this, cancer cells located in proximity of a blood vessel are usually proliferative, when both PI3K/Akt signaling and c-Myc promote glucose uptake and glycolysis, and the already mentioned “Warburg effect” is present. This metabolic profile produces enough ATP at a fast rate but also generates important intermediates necessary for cell division. Further, glycolysis yields pyruvate, which, together with glutamine, sustains the TCA cycle for additional lipid and amino acid biosynthesis. However, most of pyruvate (80%) is converted to lactate and then excreted by specific transporters; this is necessary to maintain glycolytic flux. Although vasculature might remove the majority of protons and/or lactate, limiting the pH reduction, the extracellular medium becomes acidic.
Figure 1.
From proliferation to quiescence and aggressiveness: The figure describes the scenario of FDG uptake by the diverse metabolic cancer cell subpopulations inside a proliferative tumor. Changes in pHe and lactate due to local modification (e.g. high interstitial pressure, local ischemia) may drive a continuous metabolic reprogramming limiting FDG uptake. A, FDG uptake/ metabolism; B tumor microenvironment; C microenvironmental condition and nutrients.
Moreover, in some tumor regions, despite the reduction in oxygen use, cancer cells move to HIF-1α-dependent anaerobic glycolysis when oxygen diffusion is limited and its percentage becomes less than 2%. In addition to this, the anaemia of cancer-bearing patients, occurring either as a paraneoplastic syndrome or as a common side effect of chemotherapy, contributes to the development of tissue hypoxia and to the activation of HIF-1α-dependent transcription factors. Major consequences of the switch to anaerobic glycolysis are a high and exclusive glucose uptake and the production of lactic acid, due to the HIF-1α promoting effect on glucose transporters and lactate dehydrogenase-A activity. The reduction of the proliferation level favors the resistance to apoptotic signals. Thus, both cancer cell sub-populations may acquire FDG but the anaerobic cells more so than the aerobic glycolytic cells. It should be noted that the tumor microenvironment contains host stromal cells, the so-called cancer-associated fibroblasts (CAF) which were observed to perform aerobic glycolysis (the so-called “reverse Warburg effect”), acquiring glucose and releasing lactate in normoxia 10. Therefore, hypoxic CAF, carrying out an anaerobic glycolysis, concur to consume glucose and discard lactate in the extracellular environment. Interestingly, CAF contribute to extend the heterogeneity of cancer cells promoting the FDG uptake in tumor cells, as observed by micro-PET analysis 11. Moreover, CAF influence FDG uptake in a model of non-small cell lung cancer. When the tumor-bearing animals were fed, FDG accumulated in noncancerous stroma of the tumors, whereas, when mice were made to fast, the predominant FDG uptake was ascribed to hypoxic cancer cells 12.
Furthermore, it has been demonstrated that the acidic microenvironment affects HIF-1α, which is the key glycolytic driver of hypoxic cancer cells. Indeed, when cancer cells are exposed, under hypoxic conditions (1% oxygen), to a slightly basic microenvironment (7.7 pHe), they express a very high level of HIF-1α as expected. The level of HIF-1α expression is not reduced when cells are exposed to 7.3 pHe, whereas it is severely reduced when cells are exposed to 6.8 pHe. HIF-1α completely disappears in cancer cells exposed to 6.3 pHe, under hypoxic conditions 13. In addition, during the early phase of tumor growth, when oxygen tension is still high enough (approx. 2.5% oxygen), pHe is already 6.6. When tumors are larger than 500 mm3, oxygen level is around 1% and the pHe is approximately 6.4, suggesting that low pHe not only influences hypoxic cancer cells but also the “Warburg-addicted” cancer cells of oxygenated regions 14. These findings indicate that acidosis, more than hypoxia, may represent the crucial factor in regulating cell metabolism, acting like a control trigger capable of switching cancer cell metabolism from glucose to other substrates.
Thus, a different degree of lactate accumulation may adjust the level of glucose uptake, as: (i) the low amount of lactate generated by low-glycolytic tumor cells does not modify the low level of FDG uptake; (ii) a medium amount of lactate, released by intermediate-glycolytic tumor cells, partially affects FDG uptake; (iii) a high amount of lactate, produced by high-glycolytic tumor/stromal cells, finally leads to a reduced glycolysis in adjacent tumor cell subpopulations, determining a significant reduction of FDG uptake.
The low pHe removes the inhibitory activity of the HIF-1α-dependent pyruvate dehydrogenase kinase-1 on pyruvate dehydrogenase, allowing the restoration of an electron flux through the oxidative phosphorylation. Thus, acidic cells, in a 0.5% (at least) oxygen microenvironment, may become oxidative using alternative substrates, whose lactate acquires a notable significance 15, 16.
In addition, acidic cancer cells reprogram their bioenergetic preferences toward glutamine and fatty acids, extending the range of their possible alternative sources 17. It is possible that chronically-addicted acidic cancer cells of a tumor mass, despite their reprogramming towards a more oxidative phosphorylation, maintain, in addition to the consumption of other substrates, some residual glucose uptake to be used for anaplerosis, which is critical for cell proliferation. Thus, chronic acidic cells might turn out to be weakly FDG positive.
Although the acidic cancer cells subpopulations express a more resistant and aggressive phenotype, often associated with an epithelial-to-mesenchymal transition, they are only weakly proliferative 18. Thus, we have suggested that an acidic microenvironment can constitute a perfect niche for dormant tumor cells endowed with all the characteristics useful for resistance to programmed cell death. Moreover, the niche of acidic cancer cells may support the re-expansion of cancer cells in the neighboring host tissues, which may be in search of new area of growth 19. It is possible that the new wave of tumor growth may start from the acidic/invasive front of the tumor, recapitulating a novel cell heterogeneity, indicating that an acidic microenvironment might be also a niche for the survival and renewal of cancer stem cells 20.
Beyond the points we have suggested here, there is still much to learn concerning tumor microenvironment. The rapid reprogramming of the metabolic profile of cancer cells is also involved in the emergence of a cancer stem cell-like phenotype 19, 20 and/or the acquisition of drug resistance 21, 22. A critical non-invasive evaluation of the different aspects of tumor microenvironment might predict cancer aggressiveness and patient prognosis 23, 24. Thus, in order to enhance the reliability of the crucial diagnostic tool represented by the use of FDG in medical imaging, we are convinced that the development of novel alternative tracers capable of estimating additional parameters such as intratumoral acidity should be pursued 25.
Biographies
 Dr Silvia Peppicelli obtained her Ph. D. degree in Biomedical Sciences, Experimental and Clinical Oncology, from the University of Florence, Italy. She spent most of her research efforts studying mechanisms of cancer progression focusing on the role of extracellular acidosis of tumor microenvironment. She is currently investigating the mechanisms underlying the sex disparity observed in melanoma progression considering the cell response to tumor microenvironment (hypoxia and acidosis) under the supervision of Prof. Lido Calorini.
Dr Silvia Peppicelli obtained her Ph. D. degree in Biomedical Sciences, Experimental and Clinical Oncology, from the University of Florence, Italy. She spent most of her research efforts studying mechanisms of cancer progression focusing on the role of extracellular acidosis of tumor microenvironment. She is currently investigating the mechanisms underlying the sex disparity observed in melanoma progression considering the cell response to tumor microenvironment (hypoxia and acidosis) under the supervision of Prof. Lido Calorini.
 Dr Elena Andreucci earned her Ph. D. degree in Biochemistry and Molecular Biology in 2015 at the University of Siena, Italy. During her Ph. D. she joined the Prof. Isacke CM's lab as visiting student at the Institute of Cancer Research in London, UK. Currently she is working on the role of tumor microenvironment on organotropism of melanoma metastasis in the lab of Prof. Lido Calorini. She is supported by an AIRC research fellowship. In 2019 she has been awarded by AMMI (Italian Physicians Association) for her research in the field of Gender Medicine and Pharmacology.
Dr Elena Andreucci earned her Ph. D. degree in Biochemistry and Molecular Biology in 2015 at the University of Siena, Italy. During her Ph. D. she joined the Prof. Isacke CM's lab as visiting student at the Institute of Cancer Research in London, UK. Currently she is working on the role of tumor microenvironment on organotropism of melanoma metastasis in the lab of Prof. Lido Calorini. She is supported by an AIRC research fellowship. In 2019 she has been awarded by AMMI (Italian Physicians Association) for her research in the field of Gender Medicine and Pharmacology.
 Dr Jessica Ruzzolini obtained her bachelor's degree from the School of Medical and Pharmaceutical Biotechnology, University of Florence in 2015 in Prof. Calorini's lab. She is currently a Ph. D. student under the supervision of Prof. Chiara Nediani and Prof. Calorini at the Department of Clinical and Experimental Biomedical Science at the University of Florence, Italy. Her research is centered on the role of Nutraceuticals in cancer chemosensitization.
Dr Jessica Ruzzolini obtained her bachelor's degree from the School of Medical and Pharmaceutical Biotechnology, University of Florence in 2015 in Prof. Calorini's lab. She is currently a Ph. D. student under the supervision of Prof. Chiara Nediani and Prof. Calorini at the Department of Clinical and Experimental Biomedical Science at the University of Florence, Italy. Her research is centered on the role of Nutraceuticals in cancer chemosensitization.
 Dr. Francesca Bianchini earned her Ph. D. degree in Experimental Pathology from University of Florence where she worked on the role of tumor microenvironment in cancer progression with Prof. Lido Calorini. She is a tenure track researcher at the Department of Clinical and Experimental Biomedical Science at the University of Florence, Italy, and member of the Interdepartmental Center for Molecular Preclinical Imaging Experimentation CISPIM, Italy. Currently she is exploring the opportunity to use integrin antagonist as molecular target for drug delivery in different diseases.
Dr. Francesca Bianchini earned her Ph. D. degree in Experimental Pathology from University of Florence where she worked on the role of tumor microenvironment in cancer progression with Prof. Lido Calorini. She is a tenure track researcher at the Department of Clinical and Experimental Biomedical Science at the University of Florence, Italy, and member of the Interdepartmental Center for Molecular Preclinical Imaging Experimentation CISPIM, Italy. Currently she is exploring the opportunity to use integrin antagonist as molecular target for drug delivery in different diseases.
 Prof. Lido Calorini is a professor of General Pathology at the University of Florence School of Medicine. He has co-authored over 80 publications on the role of host-tumor interaction in cancer progression. The current research interests in Professor Calorini's group include: (1) identification of the role of tumor microenvironment factors (pH, pO2, Glucose and Glutamine) in the progression to a metastatic disease; (2) uPAR expression during epithelial-to-mesenchymal transition and drug resistance; (3) development and biological characterization of integrin antagonist as a new imaging tracers; (4) biological characterization of Carbonic Anydrase IX inhibitors and complementary therapy; (5) nanomedicine and personalized cell-therapy.
Prof. Lido Calorini is a professor of General Pathology at the University of Florence School of Medicine. He has co-authored over 80 publications on the role of host-tumor interaction in cancer progression. The current research interests in Professor Calorini's group include: (1) identification of the role of tumor microenvironment factors (pH, pO2, Glucose and Glutamine) in the progression to a metastatic disease; (2) uPAR expression during epithelial-to-mesenchymal transition and drug resistance; (3) development and biological characterization of integrin antagonist as a new imaging tracers; (4) biological characterization of Carbonic Anydrase IX inhibitors and complementary therapy; (5) nanomedicine and personalized cell-therapy.
References
- 1.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K Eschner W. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–48. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Türkcan S, Kiru L, Naczynski DJ, Sasportas LS, Pratx G. Lactic acid accumulation in the tumor microenvironment suppresses 18F-FDG uptake. Cancer Res. 2019;79:410–9. doi: 10.1158/0008-5472.CAN-17-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HN, Nioka S, Glickson JD, Chance B, Li L. Quantitative mitochondrial redox imaging of breast cancer metastatic potential. J Biomed Opt. 2010;15:036010. doi: 10.1117/1.3431714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppicelli S, Toti A, Giannoni E, Bianchini F, Margheri F, Del Rosso M. et al. Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation. Cell Cycle. 2016;15:1908–18. doi: 10.1080/15384101.2016.1191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbet C, Draoui N, Polet F, Pinto A, Drozak X, Riant O. et al. The SIRT1/HIF2α axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 2014;74:5507–19. doi: 10.1158/0008-5472.CAN-14-0705. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP. et al. Reversal of Warburg Effect and Reactivation of Oxidative Phosphorylation by Differential Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:5110–20. doi: 10.1158/1078-0432.CCR-15-0375. [DOI] [PubMed] [Google Scholar]
- 8.Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M. et al. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res. 2016;76:6463–6470. doi: 10.1158/0008-5472.CAN-16-0825. [DOI] [PubMed] [Google Scholar]
- 9.Paul R, Johansson R, Kellokumpu-Lehtinen PL, Söderström KO, Kangas L. Tumor localization with 18F-2-fluoro-2-deoxy-D-glucose: comparative autoradiography, glucose 6-phosphatase histochemistry, and histology of renally implanted sarcoma of the rat. Res Exp Med (Berl) 1985;85:87–94. doi: 10.1007/BF01854893. [DOI] [PubMed] [Google Scholar]
- 10.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 11.Shangguan C, Gan G, Zhang J, Wu J, Miao Y, Zhang M. et al. Cancer-associated fibroblasts enhance tumor (18)F-FDG uptake and contribute to the intratumor heterogeneity of PET-CT. Theranostics. 2018;8:1376–88. doi: 10.7150/thno.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, Li J, Wang X, Ma Y, Yin X, Wang F. et al. The reverse Warburg effect and 18F-FDG uptake in non-small cell lung cancer A549 in mice: a pilot study. J Nucl Med. 2015;56:607–12. doi: 10.2967/jnumed.114.148254. [DOI] [PubMed] [Google Scholar]
- 13.Selfridge AC, Cavadas MA, Scholz CC, Campbell EL, Welch LC, Lecuona E. et al. Hypercapnia Suppresses the HIF-dependent Adaptive Response to Hypoxia. J Biol Chem. 2016;291:11800–8. doi: 10.1074/jbc.M116.713941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9(6):1957–71. [PubMed] [Google Scholar]
- 15.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG. et al. Lactate Metabolism in Human Lung Tumors. Cell. 2017;171:358–71. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W. et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–18. doi: 10.1038/nature24057. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, Feron O. Acidosis Drives the Reprogramming of Fatty Acid Metabolism in Cancer Cells through Changes in Mitochondrial and Histone Acetylation. Cell Metab. 2016;24:311–23. doi: 10.1016/j.cmet.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a "reappreciated" trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014;33:823–32. doi: 10.1007/s10555-014-9506-4. [DOI] [PubMed] [Google Scholar]
- 19.Peppicelli S, Andreucci E, Ruzzolini J, Laurenzana A, Margheri F, Fibbi G. et al. The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci. 2017;74:2761–71. doi: 10.1007/s00018-017-2496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rovida E, Peppicelli S, Bono S, Bianchini F, Tusa I, Cheloni G. et al. The metabolically-modulated stem cell niche: a dynamic scenario regulating cancer cell phenotype and resistance to therapy. Cell Cycle. 2014;13:3169–75. doi: 10.4161/15384101.2014.964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzzolini J, Peppicelli S, Andreucci E, Bianchini F, Margheri F, Laurenzana A. et al. Everolimus selectively targets vemurafenib resistant BRAF(V600E) melanoma cells adapted to low pH. Cancer Lett. 2017;408:43–54. doi: 10.1016/j.canlet.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Andreucci E, Peppicelli S, Carta F, Brisotto G, Biscontin E, Ruzzolini J. et al. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med (Berl) 2017;95:1341–53. doi: 10.1007/s00109-017-1590-9. [DOI] [PubMed] [Google Scholar]
- 23.Anemone A, Consolino L, Arena F, Capozza M, Longo DL. et al. Imaging tumor acidosis: a survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev. 2019;38:25–49. doi: 10.1007/s10555-019-09782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang CW, Hsieh WC, Hsu ST, Lin YW, Chung YH, Chang WC. et al. The Use of PET Imaging for Prognostic Integrin α (2)β(1) Phenotyping to Detect Non-Small Cell Lung Cancer and Monitor Drug Resistance Responses. Theranostics. 2017;7:4013–4028. doi: 10.7150/thno.19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovhannisyan N, Fillesoye F, Guillouet S, Ibazizene M, Toutain J, Gourand F. et al. [(18)F] Fludarabine-PET as a promising tool for differentiating CNS lymphoma and glioblastoma: Comparative analysis with [(18)F]FDG in human xenograft models. Theranostics. 2018;8:4563–73. doi: 10.7150/thno.26754. [DOI] [PMC free article] [PubMed] [Google Scholar]