Abstract
Neural activity within the ventromedial prefrontal cortex (vmPFC) is a critical determinant of stressor-induced anxiety. Pharmacological activation of the vmPFC during stress protects against stress-induced social anxiety suggesting that altering the excitatory/inhibitory (E/I) tone in the vmPFC may promote stress resilience. E/I balance is maintained, in part, by endogenous cannabinoid (eCB) signaling with the calcium dependent retrograde release of 2-arachidonoylglycerol (2-AG) suppressing presynaptic neurotransmitter release. We hypothesized that raising 2-AG levels, via inhibition of its degradation enzyme monoacylglycerol lipase (MAGL) with KML29, would shift vmPFC E/I balance and promote resilience. In acute slice experiments, bath application of KML29 (100nM) augmented evoked excitatory neurotransmission as evidenced by a left-shift in fEPSP I/O curve, and decreased sIPSC amplitude. In whole-cell recordings, KML29 increased resting membrane potential but reduced the after depolarization, bursting rate, membrane time constant and slow after hyperpolarization. Intra-vmPFC administration of KML29 (200ng/0.5μL/hemisphere) prior to inescapable stress (IS) exposure (25, 5s tail shocks) prevented stress induced anxiety as measured by juvenile social exploration 24h after stressor exposure. Conversely, systemic administration of KML29 (40mg/kg, i.p.) 2h before IS exacerbated stress induced anxiety. MAGL inhibition in the vmPFC may promote resilience by augmenting the output of neurons that project to brainstem and limbic structures that mediate stress responses.
Keywords: Monoacylglycerol, Stress, Endocannabinoids, Prefrontal cortex
1.1. Introduction
Exposure to stress is an important factor in the development of numerous psychiatric disorders, yet not all people who experience trauma develop psychopathology [1]. It is therefore critical to understand factors that predispose people towards resilience versus vulnerability to the negative impact of stressors. A wealth of data from a range of species implicates the ventromedial prefrontal cortex (vmPFC) in executive function, regulation of stress and emotion, and selecting appropriate behavioral strategies [2–4]. Traumatic stress disrupts vmPFC function leading to pathologies in these processes [5]. Under conditions that produce stress resistance, projection neurons from the vmPFC constrain the stress response and limit the development of anxiety by regulating the downstream activation of neural circuits in the brainstem, amygdala and hypothalamus which are involved in the generation of emotional changes from threat [6,7].
In rats, uncontrollable stress in the form of inescapable tail shocks results in failure to learn to escape in a shuttlebox, social anxiety, enhanced fear conditioning, and passive coping [8]. Prophylactic treatment with ketamine or behavioral control over the stressor blocks these negative sequelae of uncontrollable stress and both treatments increase activity within vmPFC neurons that project to the dorsal raphe nucleus [9]. vmPFC activation is both necessary and sufficient for the stress protective effects of these manipulations during uncontrollable stress [9,10]. Behavioral control over stress also increases the intrinsic excitability of putative prelimbic (PL) projection neurons [11] and leads to increased translation of proteins involved in synaptic plasticity [12]. Following social defeat stress, Syrian hamsters and mice both increase submissive and defensive behaviors, but dominant hamsters and mice housed in an enrichment environment or treated with prophylactic ketamine showed reduced behavioral consequences of social defeat, and increased neural activation in the PL and IL [13–15]. Lesioning of the IL blocked the reduction in behavioral consequences following social defeat conferred by environmental enrichment and pharmacological inactivation of the vmPFC blocked the social defeat buffering effects of dominance [13,14,16]. All of this evidence suggests that elevated vmPFC activity during or before stressor exposure favors resilience.
To identify novel strategies for prevention of stressor induced social anxiety, we investigated endocannabinoid (eCB) signaling in the vmPFC. eCBs may represent a mechanism underlying the previously observed increase in pyramidal neuron excitability [11] as eCBs are key regulators of cortical excitatory and inhibitory tone [17]. Specifically, the eCB 2-arachidonoylglycerol (2-AG), is released as a retrograde neurotransmitter when postsynaptic cells reach high intracellular Ca2+ concentrations (i.e. during bouts of high frequency firing). 2-AG inhibits presynaptic GABAergic interneurons by acting at the Gi-coupled CB1 receptor (CB1R) [17,18] resulting in a phenomenon of depolarization-induced suppression of inhibition [17]. CB1R mediated inhibition of GABAergic transmission and the subsequent increase in pyramidal neuron excitably can occur within the vmPFC [18], suggesting that raising 2-AG levels might be sufficient to increase the excitability of vmPFC neurons and produce resilience.
An ideal target for therapeutic intervention aimed at promoting stress resilience may be the enzymatic regulation of 2-AG. While deficits in 2-AG are anxiogenic, pharmacological elevation of 2-AG is anxiolytic [19,20]. Given that termination of 2-AG signaling is predominately achieved through 2-AG degradation via monoacylglycerol lipase (MAGL) [21], well characterized drugs that selectively inhibit MAGL, such as JZL184 and KML29, are effective tools for investigating the effects of increased 2-AG signaling [22,23]. Systemic inhibition of MAGL produces an anxiolytic-like phenotype in an elevated plus maze [24,25] and promotes resilience to foot shock [26]. Augmentation of eCB signaling, or direct activation of CB1Rs in the vmPFC promotes active coping during swim stress [27,28] and chronic stress [29], and promotes termination of the stress response [30]. As activation of CB1Rs in the vmPFC suppresses GABA release [30], these effects likely result from increased output from vmPFC projections. We used electrophysiology and behavioral pharmacology with vmPFC-specific and systemic administration of the MAGL inhibitor KML29 to test the hypotheses that raising 2-AG levels would reduce GABA transmission and shift vmPFC E/I balance, increase excitability of vmPFC pyramidal neurons, and promote resilience to uncontrollable stress.
2. Methods and Materials
2.1. Subjects
Male Fischer-344 rats weighing 225–250g upon arrival served as experimental subjects and male Fischer-344 rats aged approximately 21 days upon arrival served as conspecifics for social interaction were purchased from Envigo (Frederick, MD, USA). Rats were housed in pairs, maintained on a 12-h light/dark cycle (with lights on at 0700h) within the Boston College Animal Care Facility, and allowed 1 week to habituate to their home cages before the start of surgery, behavior or use for electrophysiology. All experimental protocols were reviewed and approved by the Boston College Institutional Animal Care and Use Committee (IACUC).
2.2. Electrophysiology solutions and drugs
All chemicals were purchased from Fisher Scientific, Sigma-Aldrich or Tocris and standard artificial cerebrospinal fluid (aCSF) and recording solutions were used as previously [11,31]. aCSF recording composition was (in mM) NaCl 125, KCl 2.5, NaHCO3 25, NaH2PO4 1.25, MgCl2 1, CaCl2 2 and glucose 10; pH = 7.40; 310 mOsm; aCSF cutting solution was: sucrose 75, NaCl 87, KCl 2.5, NaHCO3 25, NaH2PO4 1.25, MgCl2 7, CaCl2 0.5, glucose 25 and kynurenic acid 1; pH = 7.40, 312 mOsm. The internal recording solution consisted of (in mM) potassium gluconate 115, KCl 20, HEPES 10, Mg-ATP 2, Na-GTP 0.3 and sodium phosphocreatine 10; pH = 7.30; 278mOsm with 0.1% biocytin. Kynurenic acid (1mM) and SR-95531 (2μM) were added to the recording aCSF to block synaptic transmission for intrinsic recordings. KML29 was purchased from Tocris, dissolved in DMSO, and stored in aliquots at −20°C. On the day of recordings, aliquots were thawed and diluted in warm aCSF to a working concentration of 100nM KML29.
2.3. vmPFC slices
Adult male rats were anesthetized with isoflurane, intracardially perfused with chilled (4 °C), oxygenated aCSF cutting solution and quickly decapitated. Coronal slices (300μm) containing the vmPFC were made on a vibratome (VT-1000S, Leica Microsystems, Nussloch, Germany). The slices were placed in oxygenated aCSF cutting solution (95% O2 and 5% CO2) at 37 °C for 30 min and then at room temperature (approximately 23°C) for a minimum of 30 min before slices were used for electrophysiological recordings.
2.4. Extracellular multiple electrode array recordings
Evoked field excitatory postsynaptic potentials (fEPSPs) were recorded on a 6 × 10 perforated multiple electrode array (model: MCSMEA-S4-GR, Multichannel Systems) with integrated acquisition hardware (model: MCSUSB60) and analyzed with MC_Rack software (Version 3.9). Slices were placed on the array and adhered by suction of the perfusion through the perforated substrate. Bath solutions were as above and perfused through the slice from above. A stimulating electrode was selected in the deep layers of PL, and fEPSPs were recorded after stimulation (0 to 5 V, biphasic 220 μs, 500-mV increments) before and during application of 100nM KML29 (more than double the IC50 for MAGL [22]). Each step in the I/O curve was repeated 3 times (20s interstimulus interval) and each family of steps was replicated 3 times in each phase of the experiment. fEPSPs from channels displaying clear synaptic responses (as in Fig. 1B) and in the vicinity of the stimulating electrode were normalized to the individual channel’s maximum response to 5V stimulation at baseline; channels from the same slice were averaged for group analysis. These experiments were replicated to test the dependence of KML29 effects on CB1Rs, by co-application of the CB1R antagonist/inverse agonist AM251 (2μM) and the dependence on GABAA receptors, by adding the GABAA receptor antagonist SR-95531 (20μM) in the bath solution. All dependent measures were normalized to pre-drug baselines for analysis. Analyses were performed using custom software written for Python and Igor Pro (Wavemetrics Inc., Lake Oswego, OR).
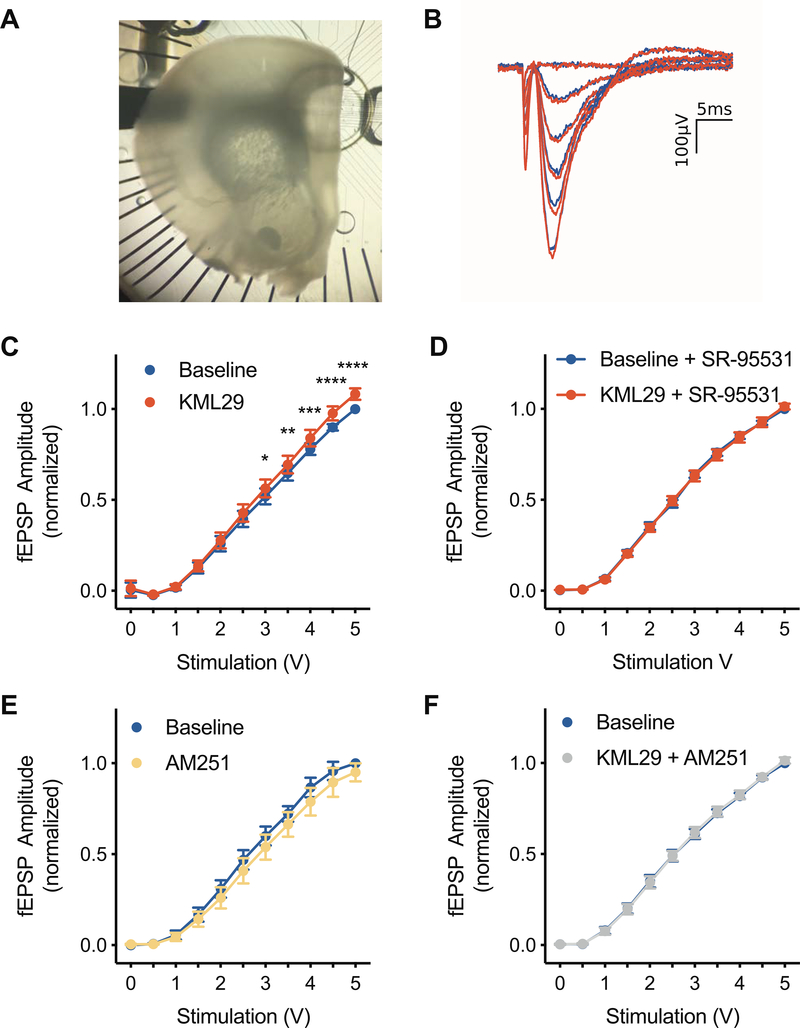
Figure 1.
Effects of KML29 on input output (I/O) curves of field potentials recorded from the PL. (A) Top view of acute extracellular recordings of the PL using a 60 channel microelectrode array. (B) Representative fESPS evoked by biphasic stimulation during baseline (blue) or during application of 100nM KML29. (C) Bath application of KML29 increased the amplitude of evoked field excitatory postsynaptic potentials (fEPSP) at stimulations intensities of 3V and above. (D) The effects of KML29 were also blocked in the presence of the GABAA receptor antagonist, SR-95531. (E) Bath application of CB1 receptor inverse agonist, AM251, did not alter I/O curves. (F) Co-application of KML29 and AM251 blocked the effects of KML29. Error bars represent SEM. *, **, ***, and **** denote p<0.05, p<0.01, p<0.001, and p<0.0001 respectively.
2.5. Whole Cell Recordings
Whole-cell current-clamp recordings were obtained at 30 ± 2 °C. Patch-clamp electrodes were pulled (P-1000, Sutter Instruments, CA) from 1.5mm outer diameter borosilicate glass (Sutter Instruments, CA) and filled with intracellular solution. Electrode resistance was 3–5MΩ in the bath, and recordings were only included if the series resistance remained less than 30MΩ with less than 10% change from baseline throughout the experiment. Slices were visualized using a 40× (0.75 NA) water-immersion objective under infrared differential interference contrast imaging on an upright microscope (AxioExaminer D1, Zeiss, Germany). All recordings were obtained with an Axon 700B amplifier and pClamp 10 (Molecular Devices), using appropriate bridge balance and electrode-capacitance compensation. After achieving a whole-cell configuration, baseline recordings were made in aCSF until 10 min of stable baseline were observed, at which point KLM29 (100nM) was added to the bath. Analyses were performed using custom software written for Igor Pro (Wavemetrics Inc., Lake Oswego, OR; code freely available by request).
As described previously [11,31], action potential properties were quantified by holding the neuron at −67mV while a single 2.5ms current pulse was injected to elicit an AP. Passive properties were measured by holding the membrane potential at −67mV and injecting 1s current pulses through the patch electrode in whole cell current-clamp configuration. The amplitudes of the current injections were between −300pA and +400pA in 50pA steps. All traces in which no APs were elicited were used; depolarizing traces where APs were elicited were used to generate input-output curves (total number of APs per second plotted against the current injected). Spontaneous excitatory postsynaptic currents (sEPSCs) were record in whole-cell voltage-clamp configuration with the same internal solution but adding 2uM SR95531 to the aCSF to block inhibitory synaptic currents; spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded using a 70mM K-Gluconate + 70mM KCl internal solution and held in voltage-clamp at −90mV and 5uM NBQX were added to the aCSF to block excitatory synaptic events. Cells were allowed to stabilize for 10min and recordings were made for 10min, 100nM KML29 was bath-applied for 10min before data was collected. sEPSCs and sIPSCs were analyzed with the mini analysis program (Synaptosoft). After recording, the slice was fixed in 4% paraformaldehyde and biocytin was visualized using the ABC method and NovaRed (Vector labs, Burlingame, CA). Only neurons with a pyramidal morphology and soma in deep layers of PL just dorsal to the IL/PL border were included for analysis.
2.6. Surgical implantation of microinjection cannula
Rats were anesthetized using isoflurane (3% in O2) and mounted in a stereotaxic apparatus. An incision was made in the center of the scalp to expose bregma and lambda. Bilateral stainless-steel guide cannula (22g, 1mm spacing; Plastics One, Roanoke, VA, USA) were implanted within the vmPFC (coordinates: 3.0mm anterior to bregma, 3.4mm ventral to bregma, ±0.5mm lateral to bregma) according to the atlas of Paxinos and Watson [32] to target the PL. Cannulas were fixed to the skull using stainless steel screws and acrylic cement. A stylet extending 1 mm ventral to the tip of the cannula was guided into each side and tightened to the top of the fixture to ensure patency. After surgery, each rat received 1 dose each of loxicom (1mg/kg), penicillin G procaine (15,000Units), and 5mL of lactated Ringers’ solution (Henry Schein, Albany, NY, USA) to aide in recovery. The next day, rats were administered a second dose of loxicom in accordance with the policy of the Boston College IACUC. All animals were allowed one week of post-operative recovery before the start of behavioral testing. During the recovery period, each rat was periodically handled and stylets checked to acclimate the animals to this type of contact, and also to confirm that cannulas remained clear. Microinjections were conducted as described in [42]. KML29 (200ng [33]) in 0.5μL of vehicle (0.05% DMSO in saline) or vehicle alone was administered 45 min before stress exposure. At the end of the experiment, rats were asphyxiated with CO2 and brains were removed and processed for cresyl violet verification of cannula placement. Cannula found to fall outside of the vmPFC were considered “misses”. Systemic injection of KML29 (4mg/Kg/mL, or 40mg/Kg/mL) were dissolved in a solution containing a ratio 1:1:18 of DMSO, Tween-80, Saline. The injections were given into the intraperitoneal cavity 2h before stress exposure. The dose of was chosen 40mg/Kg/mL because it significantly raises 2-AG in brain when 2h after administration [23]. A second low dose of 4mg/Kg/mL was chosen as a ten-fold decrease over the high dose.
2.7. Stress procedure
Rats were placed in wheel turn chambers (Model ENV-586B, Med Associates, St. Albans, VT), and rats’ tails were tapped to the tail restraint rods and affixed with copper electrodes. Twenty-five, 5s tail shocks (1mA) with a random ITI ranging from 30–90s and an average ITI of 60s (Precision Animal Shocker Model H13–15, Coulbourn Instruments). As it has been shown that animals that receive tail restraint without shocks appear behaviorally identical to rats left in the home cage [34], non-shocked home-cage control rats remained undisturbed in the colony. After stress treatment, rats were returned to their home cages.
2.8. Juvenile Social exploration
Social exploration was conducted at 0800h (~1 hours prior to stress) and 24 h after the stress procedure as previously described [35]. Briefly, test rats were placed into the test cage (45 cm length, 24.5cm width, 20cm height), and after 1h of acclimation, a 28±2day-old juvenile was introduced to the cage for 3min. An observer, blind to treatment, timed exploratory behaviors (sniffing, pinning, and allogrooming) initiated by the adult. Juveniles were used for multiple tests but were never used twice for the same adult rat. The testing order was counterbalanced for stress and drug treatments.
2.9. Data Analysis
Prior to analysis data were inspected for normality and homogeneity of variance and deemed suitable for analysis with parametric statistics. I/O Curves were analyzed via repeated measures ANOVA. Cumulative distributions of synaptic measures in whole cell recording experiments were compared by Kolmogorov-Smirnov tests. Following significant differences in K-S tests, means of 50 random events per cell were analyzed with dependent samples t-tests. Intrinsic measures in whole cell recording experiments were analyzed with one-tailed dependent samples t-tests. Social exploration scores were analyzed as seconds spent investigating and by percent of baseline social exploration, calculated for each rat as percentage of baseline social exploration the rats displayed during the second JSE test. Seconds of social exploration in the systemic administration experiment were analyzed with one-way ANOVA followed by Sidak’s post hoc test, and percent of baseline social exploration was analyzed via one-way ANOVA followed by Tukey’s HSD post hoc test. One sample t-tests were used to determine if percent of baseline social exploration was significantly different from 100 (i.e. no change in social exploration). In the intra-vmPFC administration experiment, seconds of social exploration were analyzed via three-way repeated measures ANOVA (stress-by-drug-by-day) followed by Sidak’s post hoc tests, and percent of baseline social exploration was analyzed via two-way repeated measures ANOVA (drug-by-day) followed by Tukeys’s post hoc tests. Animals in the miss condition were excluded from analysis in the ANOVA and were compared to animals in the stress condition with cannula in the vmPFC by students t-test. All analyses were made using GraphPad Prism 8.0 with experiment-wise error set to α = 0.05.
3. Results
3.1. KML29 leads to GABAa receptor- and CB1 receptor-dependent increase in PL excitability
Given the known role of eCBs in the regulation of E/I balance, we first examined the effects of MAGL inhibition in the PL. The relationships between fEPSP amplitude and stimulation strength (I/O curves) was significantly altered by bath application 100nM KML29 (F(1,8)=6.601, p<0.05, Figure 1C), but not in the presence of the GABAA receptor antagonist SR-95531 (F(1,8)=0.0117, p=0.917, Figure 1D). I/O Curves were not altered by application of the CB1R inverse agonist AM251 (F(1,6)=3.323, p=0.118, Figure 1E), nor by co-application of 100 nM KML29 with 2μM AM251 (F(1,7)=0.0468, p=0.835, Figure 1F).
3.2. KML29 modulates spontaneous excitatory and inhibitory synaptic currents
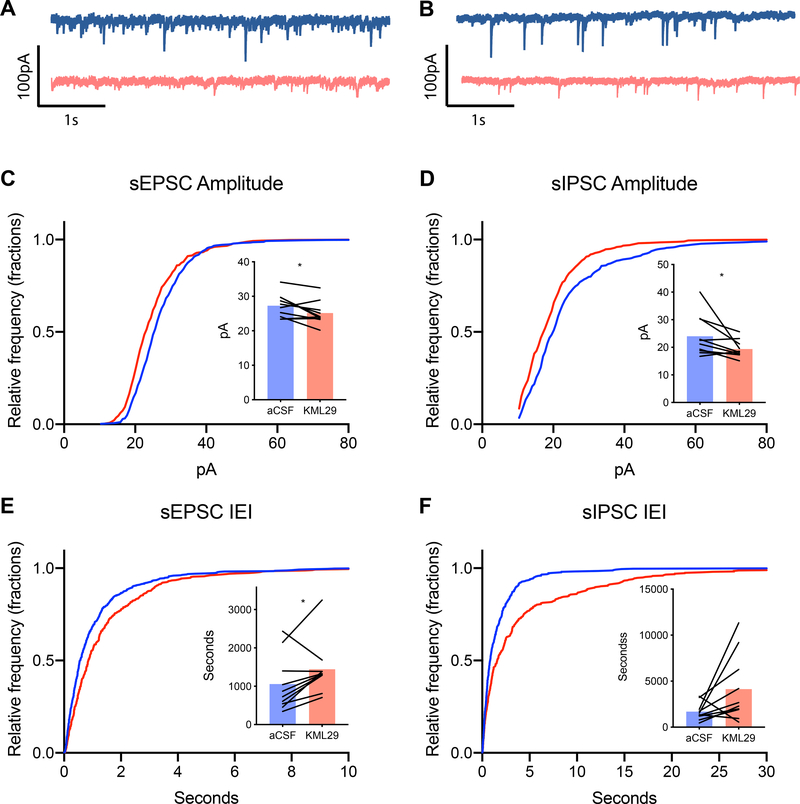
Because KML29 increased fEPSP amplitude, we hypothesized that KML29 would alter E/I balance through decreased inhibitory tone on vmPFC neurons. Therefore, we investigated the effects of KML29 on spontaneous excitatory and inhibitory currents in deep layer PL neurons. KML29 resulted in a leftward shift in the cumulative distribution of sIPSC amplitude (Kolmogorov-Smirnov (K-S) test, p<0.001, Figure 2B) and rightward shift in sIPSC interevent interval (K-S test, p<0.001, Figure 2D). KML29 also reduced the mean sIPSC amplitude (t(9)=2.284, p<0.05), and showed reduced mean sIPSC interevent interval (t(9)=2.147, p<0.05). KML29 resulted in a leftward shift in the cumulative distribution of sEPSC (K-S test, p<0.001, Figure 2A) amplitude and a rightward shift in sEPSC interevent interval (K-S test, p<0.0001). Mean sEPSC amplitude (t(9)=2.517, p<0.05) was reduced while mean interevent interval (t(9)=2.367, p<0.05) was increase by KML29. Thus, KLM29 reduced the frequency and size of both inhibitory and excitatory inputs onto PL pyramidal neurons.
Figure 2.
Effect KML29 on spontaneous synaptic events. (A) Representative spontaneous excitatory post synaptic currents (sEPSC) traces and (B) spontaneous inhibitory post synaptic currents (sIPSC) during baseline (blue) and during application of 100nM KML29 (red). (C) Bath application of KML29 resulted in leftward shift in the cumulative distribution of sEPSC amplitude corresponding with reduced mean sEPSC amplitude (p<0.05) and (E) a rightward shift in sEPCS amplitude corresponding with an increased mean interevent interval of sEPSCs (p<0.05) compared to baseline. (D) The cumulative distribution of sIPSC amplitude was shifted leftward following application of KML29, which corresponding with decreased decreased mean sIPSC amplitude (p<0.05). (F) KML29 application right-shifted the cumulative distribution of sIPSC interevent interval, and increased mean sIPSC interevent interval (p>0.05). * denotes p<0.05.
3.3. KML29 alters PL intrinsic excitability
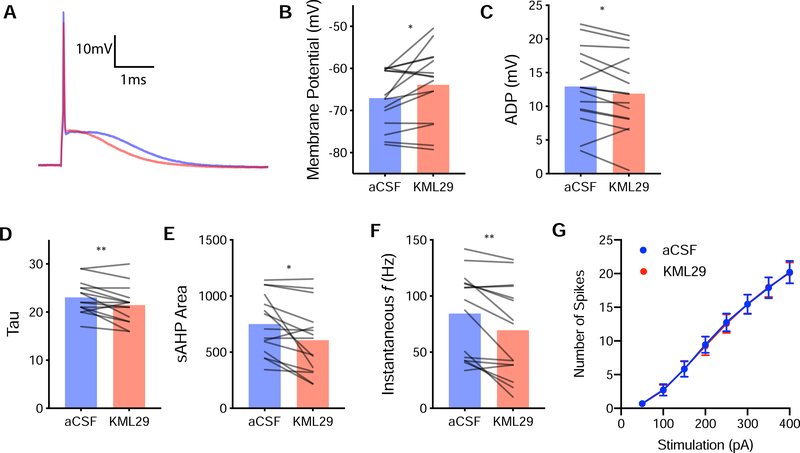
An increase in fEPSP amplitude could also be attributed to plasticity of vmPFC intrinsic excitability. Active and passive intrinsic properties were characterized after bath application of KML29 (100nM) in whole-cell pyramidal neuron recordings (Figure 3 and Table 1). KML29 caused depolarization of the resting membrane potential (t(14)=2.525, p<0.05, Figure 3B), decrease in membrane time constant (t(14)=3.055, p<0.01, Figure 3D), decrease in instantaneous frequency (t(14)=3.590, p<0.01, Figure 3F), decreased afterdepolarization (ADP) (t(14)=2.250, p<0.04, Figure 3C), and decrease slow after hyperpolarization (sAHP) (t(14)=2.908, p<0.05, Figure 3E).
Figure 3.
Effects of KML29 on deep layer PL pyramidal neurons. (A) Representative action potentials during baseline (blue) or during application of 100nM KML29 (red) (B) Compared to baseline, KML29 increased resting membrane potential (p<0.05), (C) decreased after-deploraization (ADP, p<0.05), (D) decreased membrane time constant (Tau, p,0.01), (E) slow after-hyperpolarization (sAHP, p<0.05), (F) increases instantaneous frequency (p<0.01). (G) KML29 did not alter the relationship between the number of spikes and the stimulation strength. * and ** denotes p<0.05 and p<0.01 respectively.
Table 1:
Electrophysiological properties of PL neurons
| Control | KML29 | T-test | |
|---|---|---|---|
| Passive Properties | |||
| Tau | 23.07±0.87 | 21.47±1.00 | t(14)=3.055, p<0.05 |
| IR | 147.33±10.89 | 155.67±14.44 | t(14)=1.341, p=0.201 |
| Rect | 0.72±0.02 | 0.75±0.03 | t(14)= 1.405, p=0.182 |
| Sag | 0.92±0.16 | 0.83±0.12 | t(14)=0.788, p=0.444 |
| sAHP | −750.33±68.86 | −606.67±78.58 | t(14)=2.908, p<0.05 |
| Action Potential | |||
| Vm | −67.05±1.74 | −63.89±2.26 | t(14)=2.525, p < 0.05 |
| ADP | 12.95±1.47 | 11.89±1.45 | t(14)=2.250, p<0.05 |
| Rheo | 1487.33±109.60 | 1434.00±113.57 | t(14)= 1.134, p=0.276 |
| Thresh | −36.69±2.25 | −37.57±2.03 | t(14)=0.702, p=0.495 |
| Spike amplitude | 65.95±4.15 | 65.24±3.96 | t(14)=0.390, p=0.702 |
| Spike width | 1.64±0.10 | 1.70±0.11 | t(14)=0.158, p=0.158 |
| Max rise rate | 143.93±14.78 | 138.13±14.92 | t(14)=0.901, p=0.383 |
| Min decay rate | −70.20±4.36 | −70.33±5.66 | t(14)=0.056, p=0.957 |
3.4. KML29 administered to the vmPFC mitigates stress induced anxiety
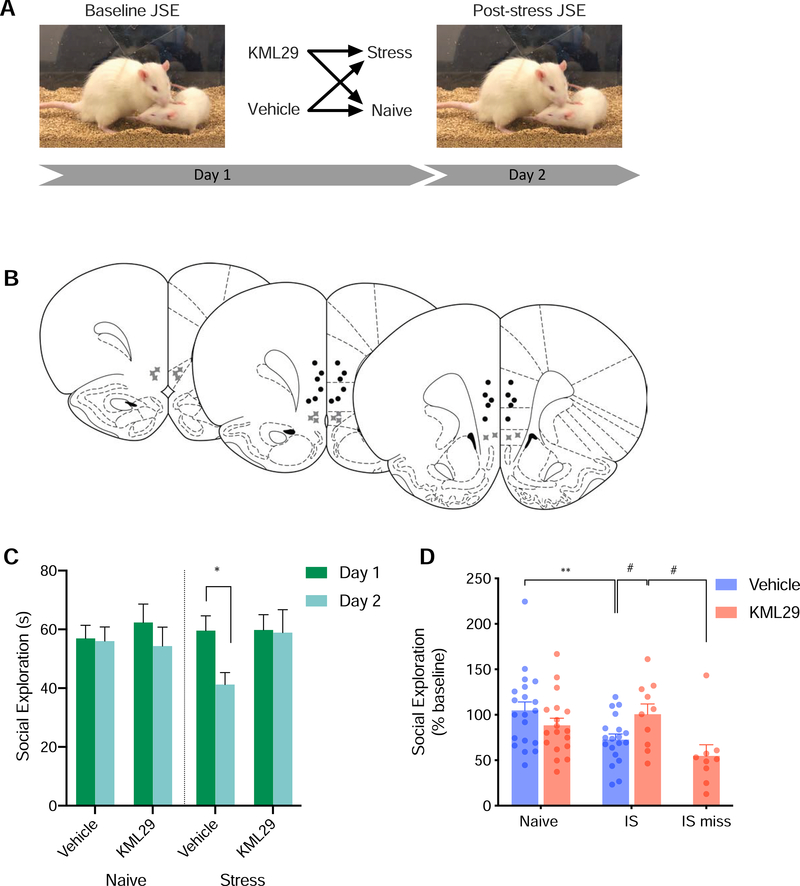
To investigate whether KML29 might confer stress resilience we made intra-vmPFC injections prior to uncontrollable stress. Intra-vmPFC administration of KML29 prior to stress resulted in a significant stress-by-drug-by-day (F(1,63)=4.977, p<0.05, Figure 4C). Rats that received vehicle injections prior to stress showed reduced social exploration post stress compared with pre-stress (p<0.05). However, post stress measures of social exploration did not differ from pre-stress measures in rats that received KML29 (p=0.999). When considering percent of baseline social exploration, intra-vmPFC administration of KML29 prior to stress resulted in a significant stress-by-treatment interaction (F(1,47)=6.883, p<0.05, Figure 4D). Rats that received saline injections prior to stress showed reduced social exploration compared to saline injected, stress-naive rats (p<0.05). When correcting for multiple comparisons, rats that received KML29 prior to stress showed a trend toward higher percent of baseline social exploration compared with saline injected stressed rats (p=0.0641). As this comparison was the impetus for the this experiment, we also report that this comparison is significant when compared using unprotected one-tailed t-test (t(22)=2.706, p<0.01). Rats that received KML29 prior to stress did not differ when compared with saline injected (p=0.8251) or KML29 injected (p=0.2605) stress-naive rats. KML29 administration without stress did not alter social exploration compared vehicle treated rats (p=0.288). Rats that received KML29 prior to stress also showed higher percent of baseline social exploration compared with rats in the miss condition, those that received stress and administration of KML29 through cannula that fell outside the vmPFC (t(17)=2.734, p<0.05). No differences were found between groups or days in the number of bouts (data not shown) in which rats engaged in social exploration.
Figure 4.
(A) Diagram of behavioral procedure. (B) Schematic of cannula placements. Circles denote placements within the vmPFC while “X” symbols denote missed placements. (C) Mean (+SEM) social exploration. KML29 administered into the vmPFC during stress alters the behavioral consequences of the stressor. Stress decreased seconds engaged in social exploration in vehicle treated rats (p<0.01), but not KML29 treated rats (p=0.999). (D) Mean (+SEM with individual replicates) social exploration expressed as percent of baseline. Among rats that received vehicle injections, stress exposure (25 inescapable tail shocks) decreased social exploration compared with stress naïve rats (p<0.05). Rats in the miss condition received KML29 outside the vmPFC and showed less social exploration than rats that received KML29 in the vmPFC. * denotes p<0.05 with Sidak’s post hoc (C) Tukey’s post hoc (D). # denotes p<0.05 unprotected students t-test (D).
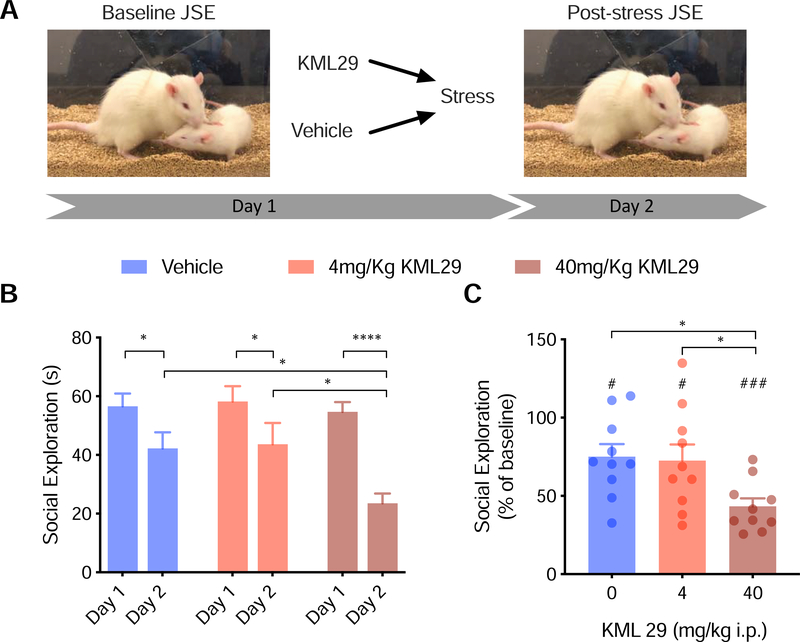
3.5. KML29 administered systemically exacerbates stress induced anxiety
To investigate if systemic administration of KML29 would confer resilience, we made i.p. injections of KML29 prior to uncontrollable stress. We found a main effect of day (F(1,27)=56.00, p<0.0001) and a significant drug-by-day interaction (F(2,27)=4.335, p<0.05, Figure 5B). On day 2, following stress, social exploration was reduced in rats treated with 0mg/Kg (p<0.05), 4mg/Kg KML29 (p<0.05), and 40mg/Kg (p<0.0001). In contrast to region specific manipulation, systemic administration of KML29 exacerbated the stress effect on social interaction such that rats that received the 40mg/Kg dose had significantly lower social exploration than rats that received 0mg/Kg (p<0.05) and 4mg/Kg (p<0.05) doses. Social exploration was not different between rats that received 0mg/Kg and rats that received 4mg/Kg (p=0.9957). Expressed as percent of baseline, drug treatment also altered the response to stress (F(2,27)=4.740, p<0.05, Figure 5C). Rats that received the 40mg/Kg dose had lower social exploration than rats that received 0mg/Kg (p<0.05) and 4mg/Kg (p<0.05) doses). Social exploration was not different between rats that received 0mg/Kg and rats that received 4mg/Kg (p<0.9742). As a percentage, social exploration was reduced from baseline in 0mg/Kg (t(9)=3.087, p<0.05), 4mg/Kg KML29 (t(9)=2.66, p<0.05), and 40mg/Kg KML29 (t(9)=11.16, p<0.0001) treated rats.
Figure 5.
(A) Diagram of behavioral procedure. (B) Mean (+SEM) Social exploration the day after stress was reduced compared to baseline in rats injected i.p. with either vehicle or KML29 prior to stress. (C) Mean (+SEM with individual replicates) social exploration expressed as percent of baseline. KML at 40mg/Kg exacerbated the stress induced deficit in social behavior compared to animals that received vehicle prior to stress (p<0.05). *,**, and *** denotes p<0.05, p<0.01, and p<0.001 respectively with Sidak’s post hoc (B) or Tukey’s post hoc (C).
4. Discussion
We investigated whether inhibition of MAGL would raise prefrontal excitability and promote resilience to stress. The MAGL inhibitor KML29 increased fEPSP amplitude in acute brain slices. Both CB1R antagonist/inverse agonist and GABAA receptor antagonist prevented this effect. KML29 decreased sIPSC amplitude and frequency. KML29 also decreased sEPSC amplitude and increased sEPSC inter-event interval. KML29 altered a number of pyramidal cell intrinsic properties including depolarization of resting membrane potential, reduced membrane time constant and instantaneous frequency while increasing sAHP. Finally, administration of KML29 to the vmPFC prior to stress prevented the stress-induced decrease in social exploration that typically follows stress, but somewhat paradoxically, systemic administration exacerbated the effect of stress on later social exploration. These results suggest that MAGL inhibition can augment evoked synaptic transmission through a combination of intrinsic plasticity and reduction in presynaptic inhibitory tone and that these changes may translate to a stress-protective effect when applied in vivo. However, caution is warranted with regard to the application of these findings to clinical treatments as systemic MAGL had an undesirable consequence of increasing stressor induced anxiety.
MAGL inhibition caused an increase in fEPSP amplitude in deep layers of the PL which we hypothesize to be a gain of function that could rectify the hypofrontality observed during uncontrollable stress. While, to our knowledge, it has not been directly tested, bath application of KLM29 likely leads to a steady rise in 2-AG in acute brain slices, including our PL slices. This is supported by the effect of KML29 on fEPSPs being blocked by co-application of AM251, which suggests a CB1R-dependent mechanism. In the vmPFC, the large majority of CB1Rs are localized to GABAergic terminals, where activation of CB1Rs results in both a transient suppression of GABA release and long term depression of inhibitory transmission [18,30,36]. Importantly, KML29 had no effect when administered with the GABAA receptor antagonist SR-95531. Next, in whole cell recordings, we quantified the effect of KML29 on sIPSCs and sEPSCs. Interestingly KML29 reduced both the frequency and amplitude of both forms of synaptic input. This effect is consistent with the location of CB1Rs which are expressed on GABAergic and glutamatergic presynaptic terminals, and with findings that eCBs regulate both DSI [37] and DSE [38] within the rodent cortex. That KML29 administration resulted in a net elevation of fEPSP amplitude, suggests that the global effect of CB1R activation in the vmPFC is to increase excitability, which is consistent with the multi-fold higher expression of CB1Rs on GABAergic versus glutamatergic terminals in the vmPFC.
A shift in PL evoked fEPSPs could also be a consequence of 2-AG modulation of the intrinsic membrane properties of excitatory neurons. However, effects of eCBs on intrinsic properties are not well understood in the PL, or other brain areas. We quantified active and passive membrane characteristics in whole cell recordings of PL deep layer pyramidal neurons. Here, MAGL inhibition led to a number of interesting changes in PL properties. A depolarization of resting membrane potential, a decrease in the slow after hyperpolarization after a spike train, and a reduction in membrane time constant (Tau) all would suggest that KML29 would cause PL neurons might be more likely to fire action potentials in vivo. In contrast, KML29 reduced the after depolarization, which would reduce the likelihood of burst firing, and decreased the firing rate (instantaneous frequency). The observed changes in sAHP, membrane potential, and membrane time constant may reflect decreased M-current. Cannabinoids decrease persistent M-current in pyramidal neurons through a CB1R dependent mechanism [39] which would raise the intrinsic excitability of vmPFC neurons [40,41]. When looking at neuronal I/O curves, the KML29 had no effect, suggesting that the mix of depolarizing and hyperpolarizing effects of MAGL inhibition may only be apparent when looking at population or circuit level. However, a more powerful approach would have been a direct test of E/I balance, as has been used to demonstrate stress induced shifts in excitability in the prefrontal cortex [42]. Overall, the investigation of MAGL inhibition on PL intrinsic and synaptic physiology suggests a shift toward circuit excitation that is mediated by CB1R inhibition of presynaptic interneurons.
PL neuronal activity during stress is a correlate of stress resilience and pharmacological augmentation of the PL is stress protective [43]. Given the excitatory effect of KML29 on the PL in acute slices we administered KML29 to the vmPFC of rats prior to inescapable shock stress. IS typically leads to a reduction in social exploration, an index of anxiety-like behavior [44]. Here, IS reduced social exploration of a juvenile in rats that received vehicle injections but did not alter social exploration in rats that received KML29 in the vmPFC. Importantly, rats with cannulas found outside of the vmPFC (PL/IL regions) did not appear to be protected from the IS effect, indicating the anatomical specificity of this intervention. This result is consistent with prophylactic effects of intra-vmPFC ketamine [9,45], picrotoxin [46,47], and optogenetic stimulation [48], which all lessen the impact of IS on later anxiety like behaviors.
Administration of KML29 systemically prior to IS did not affect social behavior at a low dose (4mg/Kg), but had a stress-enhancing effect at a high dose (40mg/Kg). These results suggest that while elevation of 2-AG in areas such as the vmPFC may block negative consequences of stress, elevated 2-AG in other structures, may in fact enhance the anxiogenic effect. In support, systemic 2-AG augmentation enhances conditioned fear [49], impairs fear extinction [50] and stress induced release of 2-AG in the basolateral amygdala promotes anxiety [51]. Several brain regions known to be involved in the modulation of stress contain CB1 receptors including the BLA [52], DRN [53,54], and locus coeruleus [55]. Thus, the effects observed after systemic injection may result from elevated 2-AG within one or more of these regions. Interestingly, our result contrasts with recent work in mice in which systemic administration the MAGL inhibitor JZL184 led to stress resilience [26,56]. Dose, species specific roles of 2-AG, intrinsic differences in the MAGL inhibition or off-target effects of these drugs might explain the different behavioral results. Nonetheless, the available evidence suggests caution is needed when developing MAGL inhibitors for clinical use in stress related psychiatric illness.
The neural circuitry of resilience to uncontrollable stress is well elaborated. Behavioral or pharmacological treatments that augment PL activity lead to selective activation of deep layer pyramidal neurons that project to the dorsal raphe and inhibit 5-HT neuronal activity during stress [7,43]. These effects appear to be long lasting and, in some cases, sex specific [57]. We believe MAGL inhibition in the PL during uncontrollable stress in male rats activates this same descending pathway to promote resilience. However, future experiments will be required to demonstrate a causal link between physiological effects and stress protection. A limitation of our current study is that eCB augmentation prior to stress was only performed in male rats. Behavioral control over stress does not confer resilience in female rats and does not engage the same deep-layer outputs to the dorsal raphe [57]. Thus, it is possible that augmentation of eCB signaling would produce different effects in females, which represents an important avenue for future research.
Regarding eCBs and acute uncontrollable stressors, stress results in a loss of AEA tone [28] which, via release of tonic GABAergic inhibition, is thought to cause a reduction in PFC output [58]. On the other hand, exposure to stress has also been shown to increase 2-AG release within the vmPFC through a glucocorticoid-dependent mechanism, which acts to disinhibit pyramidal neurons in the PL and promote termination of the hypothalamic-pituitary-adrenocortical stress response [30]. Raising 2-AG levels by MAGL inhibition may counteract the net inhibitory effect of inescapable stress, and compensate for the loss of AEA, and thus enhance PL activity and bias the PL to control the activity of stress responsive circuits in the brainstem and limbic system. Consistent with these findings, activation of CB1R in the vmPFC enhances behavioral coping in swim stress models [27,28] and fosters active coping responses during chronic stress [29]. Chronic unpredictable stress and repeated restraint stress also alter 2-AG and AEA tone within the vmPFC [59]. However, to our knowledge, no one has investigated prophylactic treatment with MAGL inhibition in a repeated stress context, thus it remains an open area for future research. In sum, our data provide new routes for investigation into how to refine and augment eCB signaling within the vmPFC as a candidate pharmacotherapy that promotes resilience and stress coping.
Augmenting endocannabinoid signaling with KML29 increases prefrontal excitability
KML29 alters intrinsic properties of prefrontal pyramidal neurons
Monoacylglycerol lipase inhibition in the prefrontal cortex promotes stress resilience
Systemic augmentation of 2-arachydonyl glycerol exacerbates the impact of stress
Acknowledgements
Funding for this work was provided by the NIH Grant MH110907 and the Gianinno Family to JPC, and the Canadian Institutes of Health Research to MNH. The authors have no competing financial or other conflicts of interest to declare. Nancy McGilloway and Todd Gaines oversaw animal husbandry in the Boston College Animal Care Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. [DOI] [PubMed] [Google Scholar]
- 2.Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14:922–932. [DOI] [PubMed] [Google Scholar]
- 3.Uylings HBM, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. [DOI] [PubMed] [Google Scholar]
- 4.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrewes DG, Jenkins LM. The Role of the Amygdala and the Ventromedial Prefrontal Cortex in Emotional Regulation: Implications for Post-traumatic Stress Disorder. Neuropsychol Rev. 2019. 2019. 10.1007/s11065-019-09398-4. [DOI] [PubMed] [Google Scholar]
- 7.Christianson JP, Greenwood BN. Stress-protective neural circuits: Not all roads lead through the prefrontal cortex. Stress. 2014;17:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. [DOI] [PubMed] [Google Scholar]
- 9.Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, et al. Previous Ketamine Produces an Enduring Blockade of Neurochemical and Behavioral Effects of Uncontrollable Stress. J Neurosci. 2016;36:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amat J, Baratta M V., Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. [DOI] [PubMed] [Google Scholar]
- 11.Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over Stress, But Not Stress Per Se Increases Prefrontal Cortical Pyramidal Neuron Excitability. J Neurosci. 2012;32:12848–12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, et al. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci. 2014;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulka BN, Koul-Tiwari R, Grizzell JA, Harvey ML, Datta S, Cooper MA. Dominance relationships in Syrian hamsters modulate neuroendocrine and behavioral responses to social stress. Stress. 2018;21:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann ML, Herkenham M. Environmental Enrichment Confers Stress Resiliency to Social Defeat through an Infralimbic Cortex-Dependent Neuroanatomical Pathway. J Neurosci. 2011;31:6159–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry. 2016;79:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison KE, Bader LR, McLaughlin CN, Cooper MA. Defeat-induced activation of the ventral medial prefrontal cortex is necessary for resistance to conditioned defeat. Behav Brain Res. 2013;243:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freund TF, Katona I, Piomelli D. Role of Endogenous Cannabinoids in Synaptic Signaling. Physiol Rev. 2003;83:1017–1066. [DOI] [PubMed] [Google Scholar]
- 18.Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;5:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guggenhuber S, Romo-Parra H, Bindila L, Leschik J, Lomazzo E, Remmers F, et al. Impaired 2-AG signaling in hippocampal glutamatergic neurons: Aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int J Neuropsychopharmacol. 2016;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shonesy BC, Bluett RJ, Ramikie TS, Báldi R, Hermanson DJ, Kingsley PJ, et al. Genetic Disruption of 2-Arachidonoylglycerol Synthesis Reveals a Key Role for Endocannabinoid Signaling in Anxiety Modulation. Cell Rep. 2014;9:1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn K, McKinney MK, Cravatt BF. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem Rev. 2008;108:1687–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglyceral lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2010;16:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JW, Niphakis MJ, Lum KM, Cognetta AB, Wang C, Matthews ML, et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busquets-Garcia A, Puighermanal E, Pastor A, De La Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. [DOI] [PubMed] [Google Scholar]
- 25.Sciolino NR, Zhou W, Hohmann AG. Inhibitor of the 2-Arachidonoylglycerol Hydrolyzing Enzyme Monoacylglycerol Lipase, Produces Anxiolytic Effects Under Conditions of High Environmental Aversiveness. Pharmacol Res. 2011;64:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedse G, Bluett RJ, Patrick TA, Romness NK, Gaulden AD, Kingsley PJ, et al. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl Psychiatry. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids Elicit Antidepressant-Like Behavior and Activate Serotonergic Neurons through the Medial Prefrontal Cortex. J Neurosci. 2007;27:11700–11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin RJ, Hill MN, Bambico FR, Stuhr KL, Gobbi G, Hillard CJ, et al. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol. 2012;22:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin RJ, Hill MN, Dang SS, Wainwright SR, Galea LAM, Hillard CJ, et al. Upregulation of CB1 receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav Brain Res. 2013;237:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill MN, McLaughlin RJ, Pan B, Fitzgerakd ML, Roberts CJ, Lee TTY, et al. Recruitment of prefrontal corical endocannabinoid signaling by glucocorticoids contributes to termination fo the stress response. J Neurosci. 2011;31:10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, Morgan MTM, Ritchey M, et al. Insular cortex mediates apprach and avoidance responses to social affective stimuli. vol. 21 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxnios G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. New York: Elsevier; 1998. [Google Scholar]
- 33.Qi M, Morena M, Vecchiarelli HA, Hill MN, Schriemer DC. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun Mass Spectrom. 2015;29:1889–1897. [DOI] [PubMed] [Google Scholar]
- 34.Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, et al. The Sensory Insular Cortex Mediates the Stress-Buffering Effects of Safety Signals But Not Behavioral Control. J Neurosci. 2008;28:13703–13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong P V., Paul ED, et al. 5- Hydroxytryptamine 2C Receptors in the Basolateral Amygdala Are Involved in the Expression of Anxiety After Uncontrollable Traumatic Stress. Biol Psychiatry. 2010;67:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiritoshi T, Sun H, Ren W, Stauffer SR, Lindsley CW, Conn PJ, et al. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology. 2013;66:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortin DA, Trettel J, Levine ES, Dale A, Trettel J, Levine ES. Brief Trains of Action Potentials Enhance Pyramidal Neuron Excitability Via Endocannabinoid-Mediated Suppression of Inhibition. J Neurophysiol. 2004:2105–2112. [DOI] [PubMed] [Google Scholar]
- 38.Fortin DA, Levine ES. Differential Effects of Endocannabinoids on Glutamatergic and GABAergic Inputs to Layer 5 Pyramidal Neurons. 2007. 2007. 10.1093/cercor/bhj133. [DOI] [PubMed]
- 39.Schweitzer P Cannabinoids Decrease the K + M-Current in Hippocampal CA1 Neurons. J Neurosci. 2018;20:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santini E, Porter JT. M-Type Potassium Channels Modulate the Intrinsic Excitability of Infralimbic Neurons and Regulate Fear Expression and Extinction. J Neurosci. 2010;30:12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng H, Bian XL, Ma FC, Wang KW. Pharmacological modulation of the voltage-gated neuronal Kv7/KCNQ/M-channel alters the intrinsic excitability and synaptic responses of pyramidal neurons in rat prefrontal cortex slices. Acta Pharmacol Sin. 2017;38:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwa LS, Pina MM, Pati D, Calloway R, Kash TL. Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacology. 2019:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolzani SD, Baratta MV., Moss JM, Leslie NL, Tilden SG, Sørensen AT, et al. Inhibition of a Descending Prefrontal Circuit Prevents Ketamine-Induced Stress Resilience in Females. Eneuro. 2018;5:ENEURO.0025–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baratta MV, Pomrenze MB, Nakamura S, Dolzani SD, Cooper DC. Control over stress accelerates extinction of drug seeking via prefrontal cortical activation. Neurobiol Stress. 2015;2:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llorente-Berzal A, Terzian ALB, Di Marzo V, Micale V, Viveros MP, Wotjak CT. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl). 2015;232:2811–2825. [DOI] [PubMed] [Google Scholar]
- 50.Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, Patel S. 2-Arachidonoylglycerol Signaling Impairs Short-Term Fear Extinction. Transl Psychiatry. 2016;6:e749–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, et al. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J Neurosci. 2016;36:8461–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geddes SD, Assadzada S, Lemelin D, Sokolovski A, Bergeron R, Haj-Dahmane S, et al. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal raphe nucleus by cannabinoids. Proc Natl Acad Sci. 2016;113:5429–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moldrich G, Wenger T. Localization of the CB1cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. [DOI] [PubMed] [Google Scholar]
- 55.Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: Relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bluett RJ, Báldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, et al. Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur J Neurosci. 2018;47:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. [DOI] [PubMed] [Google Scholar]
- 59.Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]