Abstract
Background
Caffeine has a variety of pharmacological effects; it is a weak bronchodilator and it also reduces respiratory muscle fatigue. It is chemically related to the drug theophylline which is used to treat asthma. It has been suggested that caffeine may reduce asthma symptoms and interest has been expressed in its potential role as an asthma treatment. A number of studies have explored the effects of caffeine in asthma; this is the first review to systematically examine and summarise the evidence.
Objectives
To assess the effects of caffeine on lung function and identify whether there is a need to control for caffeine consumption prior to either lung function or exhaled nitric oxide testing.
Search methods
We searched the Cochrane Airways Group trials register and the reference lists of articles (August 2011), an updated search in June 2011 yielded one potentially relevant article which has been added to 'studies awaiting classification'. We also contacted study authors.
Selection criteria
We included randomised trials (RCTs) of oral caffeine compared to placebo or coffee compared to decaffeinated coffee in adults with asthma.
Data collection and analysis
Two review authors independently carried out trial selection, quality assessment and data extraction.
Main results
We included seven trials involving a total of 75 people with mild to moderate asthma. The studies were all of cross‐over design.
Six trials involving 55 people showed that in comparison with placebo, caffeine, even at a 'low dose' (less than 5 mg/kg body weight), appears to improve lung function for up to two hours after consumption. Forced expiratory volume in one second (FEV1) showed a small improvement up to two hours after caffeine ingestion (standardised mean difference 0.72; 95% confidence interval 0.25 to 1.20), which translates into a 5% mean difference in FEV1. However in two studies the mean differences in FEV1 were 12% and 18% after caffeine. Mid‐expiratory flow rates also showed a small improvement with caffeine and this was sustained up to four hours.
One trial involving 20 people examined the effect of drinking coffee versus a decaffeinated variety on the exhaled nitric oxide levels in patients with asthma and concluded that there was no significant effect on this outcome.
Authors' conclusions
Caffeine appears to improve airways function modestly, for up to four hours, in people with asthma. People may need to avoid caffeine for at least four hours prior to lung function testing, as caffeine ingestion could cause misinterpretation of the results. Drinking caffeinated coffee before taking exhaled nitric oxide measurements does not appear to affect the results of the test, but more studies are needed to confirm this.
Plain language summary
The effect of caffeine in people with asthma
Caffeine is found in coffee, tea, cola drinks and cocoa. Caffeine is a drug that is very similar to theophylline. Theophylline is a bronchodilator drug that is taken to open up the airways in the lungs and therefore relieve the symptoms of asthma, such as wheezing, coughing and breathlessness. Scientists are interested in finding out whether caffeine has the same effect on the lungs as theophylline.
There are two major reasons why it is important to know if caffeine is a bronchodilator. The first is because it may be beneficial for asthmatics to take caffeine in order to relieve the symptoms of asthma. The second is because consuming caffeine may affect the results of important tests that determine how bad someone's asthma is.
If caffeine acts as a bronchodilator and widens the airways, then a patient who has consumed caffeine before taking the test would show a better result in a lung function test than they would have if they had not consumed any caffeine. The potential problem with this is that if the test results are better than expected doctors may prescribe a lower dose or a weaker drug than is really necessary, which can lead to problems with asthma management.
This review carefully examines all the available high‐quality clinical trials on caffeine in asthma. This review was conducted to discover if people should avoid consuming caffeine before taking lung function tests.
This review found that even small amounts of caffeine can improve lung function for up to four hours. Therefore caffeine can affect the result of a lung function test (e.g. spirometry) and so caffeine should be avoided before taking a lung function test if possible, and previous caffeine consumption should be recorded.
It is not known if taking caffeine leads to improvements in symptoms. It may be that in order to improve the symptoms of asthma, caffeine is needed in such large amounts that the drug's adverse effects would become a problem, so more research is needed.
Another clinical trial looked at the effect of caffeine on exhaled nitric oxide levels and found that there is no significant effect, so it appears unlikely that patients would need to avoid caffeine before taking this type of test. However, this is the result of just a single study so more research is needed to clarify this.
Background
Caffeine has been widely consumed throughout the world for centuries. It is used for both non‐medical and medical purposes. It is ubiquitous, being found in coffee, tea, cola‐flavoured soft drinks and compounds containing cocoa. Caffeine and its derivatives have therapeutic uses and are contained in medicines such as analgesics and cold remedies.
The general pharmacological effects of caffeine have been extensively investigated and are described in several reviews (e.g. Curatolo 1983; Stephenson 1977). Early studies reported that caffeine improved mental performance and increased motor activity (Cheney 1935) and this has been confirmed in other studies. Caffeine ingestion has also been shown to elevate oxygen consumption (Grollman 1930), increase respiratory rates in disease‐free patients (Robertson 1978) and increase ventilation in patients with coronary obstructive pulmonary disease (COPD) (Woodcock 1981).
Caffeine belongs to a group of chemicals called methylxanthines, along with the bronchodilator drug theophylline. As a class, these drugs have a history of use in respiratory disorders. The mechanism of action of the methylxanthines is uncertain, but is possibly due to their inhibition of the enzyme phosphodiesterase. Phosphodiesterase hydrolyses cyclic adenosine monophosphate (cAMP) which is a messenger within the cell that regulates many functions including the contraction and relaxation of smooth muscle. Methylxanthines are also competitive antagonists for adenosine receptors. One of the effects of adenosine, a chemical regulator, is that of bronchoconstriction. Methylxanthines are known to be weak bronchodilators and they also interact with respiratory muscles to reduce respiratory muscle fatigue. Some believe the latter to be more important than the former in the treatment of respiratory diseases.
Thus, interest has been expressed in the potential role of caffeine as a treatment in respiratory disease (Pagano 1988). As early as 1859, Salter (in Becker 1984) recommended coffee as one of the best remedies for asthma. A study of the general Italian population in 1983 by Pagano 1988 found an inverse relationship between the prevalence of bronchial asthma and the amount of coffee consumed. From this, the authors suggested that caffeine may reduce asthma symptoms.
Why it is important to do this review
Despite the amount of information regarding caffeine and its potential effectiveness in obstructive airways diseases, no reviews have been conducted that examine the evidence in a systematic fashion. The results from such a review could have important implications for research and clinical practice.
Objectives
To identify all published randomised controlled trials of caffeine in the management of asthma.
To assess the methodological quality of these randomised controlled trials.
To estimate the overall effect of caffeine upon lung function and exhaled nitric oxide (FeNO).
To test whether there is a need to control for caffeine consumption prior to lung function testing and testing exhaled nitric oxide (FeNO).
To examine the need for further research into the effects of caffeine in asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials (RCTs) only.
Types of participants
We included adults (older than 18 years) with previously documented asthma of any level of severity.
Types of interventions
We included the following comparisons:
oral caffeine versus placebo; and
coffee versus decaffeinated coffee.
Types of outcome measures
We did not use outcome measures to decide if a study was eligible for inclusion in the review.
We did not include challenge test data in this review.
Primary outcomes
Lung function outcomes used were: forced expiratory volume in one second (FEV1), maximum mid‐expiratory flow (FEF25‐75) and specific airway conductance (Gaw/VL)
Exhaled nitric oxide concentration (FeNO)
Secondary outcomes
Forced vital capacity (FVC)
Maximal expiratory flow rates at 25% and 50% of vital capacity (Vmax50 and Vmax25 respectively)
Exercise‐induced bronchoconstriction
PC20
Carbachol challenge
Pulse
Blood pressure
Symptoms
Serum caffeine levels
Side effects and adverse effects.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1). We searched all records in the Specialised Register coded as 'asthma' using the following terms:
caffeine* or *caffeine or coffee or tea or chocolate or cola.
We did not exclude trials on the basis of language. We searched the CAGR up to August 2011.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references.
We contacted authors of identified trials and asked them to identify other published and unpublished studies.
Data collection and analysis
Selection of studies
Two of us (AB, EB) independently reviewed the title, abstract and key words of the references obtained from the literature search. For the 2009 and 2011 update this was done by EJW and CC. We excluded all studies that were not randomised trials or that clearly did not fit the inclusion criteria. Two of us reviewed the full text of the remaining articles. Complete agreement was achieved at all stages.
Data extraction and management
We contacted trial authors in an effort to obtain raw and missing data for the original review. Two of us (AB, EB) independently extracted means and standard deviations or standard errors. We converted standard errors to standard deviations. Two research staff from the Division of Physiological Medicine, St. George's Hospital Medical School (Sally Spencer and Catherine O'Leary) extracted data visually from graphs. There was little variation in the data extracted by the four review authors. We used the mean figures from the four independently extracted sets in this review.
Only data from cross‐over studies were available for inclusion in this review. Since caffeine is a short‐acting agent and most studies reported washout periods, 'carryover' and 'period' effects were not considered to affect the results in an important way. They were treated as parallel designs in the analyses for the original review, but for the 2009 update we have used paired t‐test results with Generic Inverse Variance pooling. In addition, since no results were reported from parallel‐group studies, there was no need to provide subgroup analyses on the basis of design.
We entered extracted data into the Cochrane Collaboration software program (RevMan 5.1).
In one paper (Bukowskyj 1987) the mean value provided in the table and that in the corresponding figure were inconsistent (value for % change FEF25‐75 at 0.5 hours); this was assumed to be a misprint and therefore omitted from the meta‐analysis.
Assessment of risk of bias in included studies
For this 2009 update, two of us (EW and CC) updated the risk of bias according to four domains:
allocation generation and concealment;
blinding;
handling of missing data; and
selective reporting bias.
For each domain we judged the risk of bias as being high, low or unclear risk of bias in line with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Measures of treatment effect
We reported individual and pooled statistics as odds ratios (OR) with 95% confidence intervals (95% CI). We used weighted mean difference (MD) when identical units of measurement were reported. To allow the combination of studies where different units (e.g. actual values, change scores, % predicted values) were reported for a particular pulmonary function test (e.g. FEV1 or FEF25‐75), we performed analyses using the standardised mean difference (SMD).
Unit of analysis issues
Outcomes were measured and reported at a variety of different time points and following different doses of caffeine. For the purposes of this review and meta‐analysis, we grouped data for each outcome according to time of measurement. We divided data into three time frames labelled as follows: 'short' (less than or equal to two hours); 'medium' (greater than two hours and less than or equal to four hours); and 'long' (greater than four hours). In the 2009 review a comparison of all doses at two hours was included; where no data at two hours were given we used the nearest data point and recorded the time in Table 2.
1. Characteristics of studies used in meta‐analysis.
| Study | Dose of caffeine | Formulation of dose |
Mean baseline % predicted FEV1 |
Time of reading used in meta‐analysis |
| Bukowskyj 1987 | 5 mg/kg | Aqueous solution | 48 | 2 h |
| Colacone 1990 | 5 mg/kg | Aqueous solution | 84 | 2h |
| Crivelli 1986 | 6 mg/kg | Aqueous solution | ‐‐ | 45 min |
| Duffy 1991 | 5 mg/kg ('low') 10 mg/kg ('high') |
Capsules | 92.9 | 90 min |
| Gong 1986 | 7.2 mg/kg | Decaffeinated coffee plus caffeine |
56 | 2 h |
| Kivity 1990 | 3.5 mg/kg ('low') 7 mg/kg ('high') |
Capsules | 78.8 | 2 h |
| Taylor 2004 | 15 g coffee | Coffee of decaffeinated coffee |
94 | ‐‐ |
Dealing with missing data
Since the trials were run over a few hours there were few dropouts. Out of a total of 75 patients only six dropped out.
Data synthesis
We analysed continuous data using the inverse‐variance fixed‐effect method in RevMan 5.1.
Subgroup analysis and investigation of heterogeneity
For each outcome in each time frame, we performed subgroup analyses to test for differences between 'high' and 'low' doses of caffeine. Using the median value to divide the data, we defined doses as: 'high' (greater than 5 mg/kg (mg per kg of body weight)); or 'low' (lower than 5 mg/kg).
Results
Description of studies
Results of the search
An all years literature search to 2011 returned 23 references. We discarded 13 on the basis of the title, abstract or title and abstract. We obtained full papers for the remaining 10 references. We identified 17 additional references by searching the bibliographies of the retrieved studies.
Seven trials fulfilled the inclusion criteria and were included in this review. Complete agreement was achieved between the review authors.
Included studies
Six studies were included in the original review and an update search conducted in August 2009 identified one additional study which met the inclusion criteria (Taylor 2004). All studies are outlined in the Characteristics of included studies table.
Six studies tested for the effects of caffeine on pulmonary function, although the main aim of these studies differed. Three studies of these six additionally tested the influence of caffeine on bronchial provocation challenge tests, one using histamine (Colacone 1990), one carbachol (Crivelli 1986) and one using eucapnic voluntary hyperventilation (EVH) (Duffy 1991). We did not include challenge test data in this review. One study also tested the effect of caffeine on exercise‐induced bronchoconstriction (Kivity 1990). One study also compared caffeine to aminophylline (Gong 1986), but these data were not analysed as it was considered to be beyond the scope of this review.
One study (Taylor 2004) assessed the effects of coffee on exhaled nitric oxide (FeNO).
Participants
There were 55 (39 male) adult participants in the six included studies testing for pulmonary function in the original review. These patients were all described as having stable, mild to moderate asthma. Further details of baseline lung function are found in Table 2.
There were 20 adult participants (gender not specified) in the study testing for exhaled nitric oxide included in the 2009 update. The severity of their asthma was not described, but there were 10 steroid‐naive and 10 steroid‐treated patients.
Interventions
Caffeine and matched placebos were administered orally (as a solution = three studies, capsule = two studies, decaffeinated coffee plus caffeine = one study, caffeine versus decaffeinated coffee = one study). Two studies contributed to the 'low' dose comparison: Bukowskyj 1987 (5 mg/kg) and Colacone 1990 (5 mg/kg). Four studies contributed to the 'high' dose comparison: Crivelli 1986 (6 mg/kg), Duffy 1991 (10 mg/kg), Gong 1986 (7.2 mg/kg) and Kivity 1990 (7 mg/kg).
One study, Taylor 2004, assessed drinking a cup of coffee (intervention group) versus decaffeinated coffee (placebo group) prepared using a standard quantity (15 g) of either caffeine‐containing coffee or decaffeinated coffee.
Outcomes
Pulmonary function tests were the only outcomes suitable for entry into the meta‐analysis.
See Characteristics of included studies for details of secondary outcomes of trials.
Excluded studies
From examination of the full papers of the of the potentially eligible references, we excluded two studies (Becker 1984; Henderson 1993). One potentially eligible reference was returned from the bibliographic search and this was excluded on retrieval of the full paper (Simmons 1983). See Characteristics of excluded studies.
Risk of bias in included studies
Complete agreement was reached by the review authors for both assessments. See Characteristics of included studies for 'Risk of bias' tables for individual studies and Figure 1 for an overview.
1.
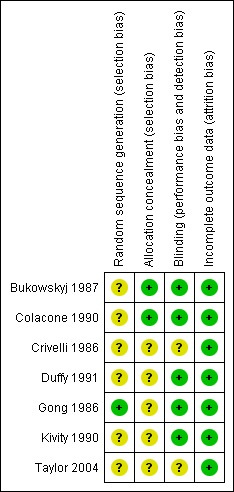
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All the papers stated that the trials were randomised. One trial reported computerised sequence generation which was judged to have a low risk of bias (Gong 1986), while the remaining six trials were unclear. Two trials reported adequate allocation concealment (Bukowskyj 1987; Colacone 1990) while the remaining five were unclear.
Blinding
All the studies were described as double‐blind. Blinding of the patient is important so that they put the same effort into lung function testing regardless of intervention, however they would presumably be able to detect if they had ingested any caffeine due to side effects. Five studies were judged to have a low risk of bias with respect to blinding (Bukowskyj 1987; Colacone 1990; Duffy 1991; Gong 1986; Kivity 1990). None of the papers described blinding of the investigator administering the caffeine or placebo to the patient or the investigator taking the outcome readings.
Incomplete outcome data
Since the trials took place over relatively short time frames there were few dropouts and only one missing data point throughout all the studies. All trials were judged to be of low risk of bias with respect to dealing with incomplete data.
Other potential sources of bias
All the included studies used a cross‐over design. In all cases the cross‐over rule was time. The time period between study days was not always stated but, where details were provided, ranged from consecutive days to within two weeks. To control for the effects of circadian rhythms, tests took place at the same time on each day in all studies. Few studies comment on the existence or effect of outlying values.
Effects of interventions
Outcomes relating to lung function
The description of the analysis will follow the list of comparisons used in this Cochrane Review and will concentrate on caffeine versus placebo results. Subgroup analyses will highlight the 'low' dose versus 'high' dose and time of final assessment comparisons.
Results:
Forced expiratory volume in one second (FEV1): 'short' (standardised mean difference (SMD) 0.72; 95% confidence interval (CI) 0.25 to 1.20; six studies on 78 participants), 'medium' (mean difference (MD) 12.66; 95% CI ‐0.34 to 25.67; two studies on 34 participants), 'long' (MD 11.00; 95% CI ‐6.49 to 28.49; one study on 16 participants).
Maximum mid‐expiratory flow (FEF25‐75): 'short' (MD 25.14; 95% CI 11.92 to 38.37; two studies on 34 participants), 'medium' (MD 32.72; 95% CI 16.26 to 49.17; two studies on 34 participants), 'long' (MD 26.00; 95% CI 5.02 to 46.98; one study on 16 participants).
Specific airway conductance (Gaw/VL): 'short' (MD 30.30; 95% CI 1.08 to 59.52; one study on 18 participants).
An improvement was seen for all outcomes after ingesting caffeine compared to placebo at all recorded time frames. This effect was statistically significant in all cases except for FEV1 at the 'medium' and 'long' time frames where the confidence intervals crossed the line of no effect.
Subgroup analysis: 'low' dose
Two studies on 36 participants reported FEV1 outcomes at 'low' dose. Data for the three time frame comparisons came from the following number of studies: 'short' = two studies, 'medium' = one study, 'long' = one study. For FEF25‐75 only one study on 16 participants contributed data at all time frames. There were no data for Gaw/VL at this dose.
All lung function parameters tended to improve post caffeine ingestion compared to placebo. For FEV1, this effect was clear only at the 'short' time frame. For FEF25‐75, the difference was clear at all times.
Subgroup analysis: 'high' dose
Four studies on 42 participants reported FEV1 outcomes at 'high' dose. Data were available for the meta‐analysis from two studies at the 'short' and one study at the 'medium' time frame. No FEV1 data were reported at the 'long' time frame. For FEF25‐75, one study on 16 participants only contributed data at the 'short' and 'medium' time frames. One study provided data for Gaw/VL at the 'short' time frame only.
Lung function was found to improve following a 'high' dose of caffeine compared to placebo for all measured outcomes. This effect was clear at the 'short' time frame only for FEV1 and FEF25‐75. A clear improvement in Gaw/VL was also seen at the 'short' time frame.
Two other studies (Crivelli 1986; Duffy 1991) tested the effect of 'high' dose caffeine at the 'short' time frame on FEV1, but no data were extracted for inclusion into the meta‐analysis in the original review. However, in correspondence, both authors reported no significant difference in bronchodilation between caffeine and placebo ingestion. For the 2009 update, data were extracted from Crivelli 1986 from the original patient data provided into FEV1 outcomes at two hours.
Subgroup analysis: FEV1 outcomes at two hours
In order to draw an overall conclusion we felt that it would be helpful to have a comparison with as many studies side by side as possible (2009 update). Peak FEV1 readings were recorded at around two hours, so this was chosen as the best time point (see Table 2). Crivelli 1986 reported a reading of FEV1 at 45 minutes rather than two hours and these data were included in the meta‐analysis. Data were extracted from the patient data provided in Crivelli 1986. Five studies on 88 participants gave FEV1 readings at 'high' doses and one study on 20 participants gave an additional reading at 'low' dose (Analysis 1.8). The forest plot shows improved FEV1 when patients had consumed caffeine at high dose prior to testing (SMD 0.76; 95% CI 0.32 to 1.20). There was no heterogeneity in the result (I2 = 0) indicating a good agreement in the outcome data between studies.
1.8. Analysis.
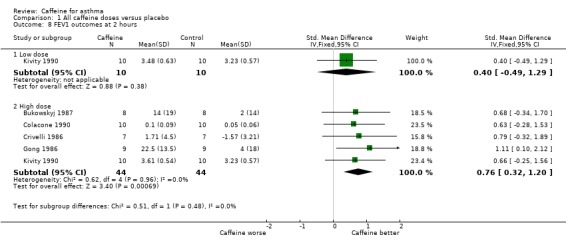
Comparison 1 All caffeine doses versus placebo, Outcome 8 FEV1 outcomes at 2 hours.
For this update we entered the paired t‐test results into the review using Generic Inverse Variance pooling for the two‐hour FEV1 outcome. The confidence intervals for each trial were fairly similar to those found previously, when the results had not been analysed with paired t‐tests. This provides reassurance that the previous conclusions of the review are valid.
When analysed as % change in FEV1 the pooled result of three trials on 26 participants showed a significant benefit with caffeine at higher dose (MD 5.47%; 95% CI 1.43 to 9.52, Analysis 1.9) (see Figure 2). There was significant heterogeneity in this result (I2 = 61%), but this appears to come from Crivelli 1986; the participants in this trial were asymptomatic and not on treatment for asthma, so would have little potential to increase their FEV1 following caffeine. If the results of the two other trials (Bukowskyj 1987; Gong 1986) are combined this gives a larger mean difference with caffeine of around 15% difference in FEV1 (MD 14.54%; 95% CI 5.35 to 23.72).
1.9. Analysis.
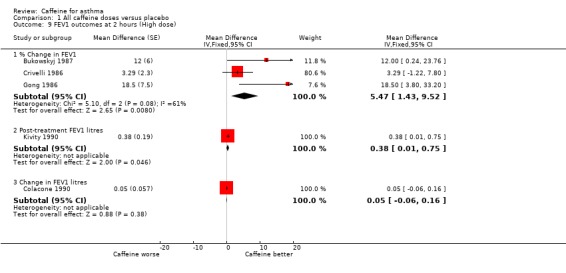
Comparison 1 All caffeine doses versus placebo, Outcome 9 FEV1 outcomes at 2 hours (High dose).
2.
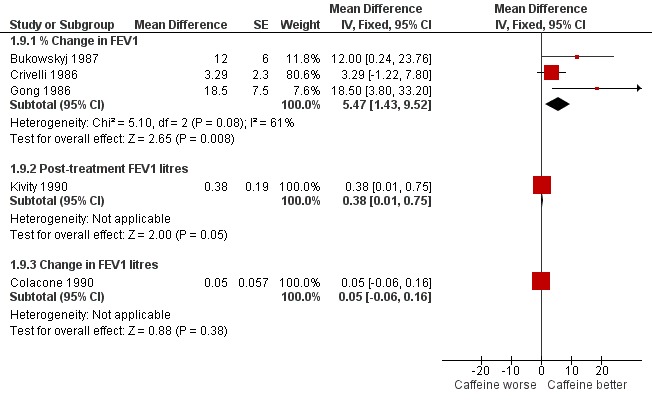
Forest plot of comparison: 1 All caffeine doses (highest dose from each study) versus placebo, outcome: 1.10 FEV1 outcomes at 2 hours (High dose).
Serum caffeine levels
The papers differed in their reporting of serum caffeine levels. After a dose of caffeine (5 mg/kg), Bukowskyj 1987 reported a peak serum level of 8.7 (SD = 1.7) μg/mL one hour after ingestion. Colacone 1990 used the same dose and reported a mean (but not peak) level at 1 hour 45 minutes of 5.4 (SD = 1.23) μg/mL. Duffy 1991 reported that peak serum levels of caffeine (mean 18.8; 95% CI 12.4 to 25.2 mg/L at 45 minutes) were observed 45 to 60 minutes after ingestion of caffeine (10 mg/kg).
Taylor 2004 reported that at 60 minutes, serum caffeine levels were higher after ingesting regular caffeine‐containing coffee than after decaffeinated coffee at 60 minutes (3.9 versus 0.4 mg/mL respectively). Statistical tests of significance were only reported for within‐group differences.
Side effects and adverse effects
Five of the studies commented on side effects, including heart rate and blood pressure changes although none contributed data that could be entered in a meta‐analysis. No side effects were reported after 'low' doses of caffeine. After ingestion of a 'high' dose of caffeine two patients reported mild tremor (Kivity 1990), three patients reported nervousness and gastrointestinal upset (Gong 1986), and one patient withdrew from the study because of nervousness and agitation (Duffy 1991), which was presumed to be due to the caffeine. Only one study (Gong 1986) reported significant changes in heart rate (a decrease up to 9%) and blood pressure (an increase up to 12%).
Outcomes relating to exhaled nitric oxide
The impact of caffeine on FeNO was assessed in one study on 20 participants (Taylor 2004). This small study reported no significant difference in exhaled nitric oxide (data reported in the text as non‐significant (P = 0.38) and presented graphically). Findings were not significantly different in subgroups for those treated with inhaled steroids and those not treated with steroids.
Discussion
The available evidence of the effect of caffeine compared to placebo on lung function and exhaled nitric oxide from randomised controlled trials is summarised in this systematic review.
Summary of main results
For all dose strengths, caffeine compared to placebo was found to significantly improve lung function measured in terms of FEV1, FEF25‐75 and Gaw/VL for up to two hours post ingestion. This effect was sustained for FEF25‐75 for over four hours. Improvement was also seen in FEV1 up to this time, however this effect did not reach statistical significance. No data were available for Gaw/VL after two hours. Bronchodilation was also seen after ingesting caffeine even following a 'low' dose (5 mg/kg). For FEV1, the difference between the caffeine and placebo groups was not significant after two hours. In contrast, for FEF25‐75 the effect was clear at all times, even over four hours. A clear increase following a 'high' dose of caffeine (> 5 mg/kg) compared to placebo was seen in FEV1, FEF25‐75 and Gaw/VL at up to two hours only. Gaw/VL outcomes were not recorded beyond two hours. At two hours, at all doses, there was an improvement in lung function after ingesting caffeine, and when reported as % increase in FEV1 this showed an average difference of 5% change (MD 5.47%; 95% CI 1.43 to 9.52). However when the study on patients who were asymptomatic and on no treatment (Crivelli 1986) was excluded, the change in FEV1 was higher after caffeine (MD 14.54%; 95% CI 5.35 to 23.72).
The size of improvements in lung function were small and at the margins of what would normally be considered clinical significance (FEV1: maximum 13.7% change from baseline (Gong 1986) or maximum 330 mL absolute change for caffeine versus placebo (Kivity 1990)). Only one study (Gong 1986) explicitly recorded the patients' perception: four of the nine participants reported improved breathing following ingestion of a 'high' dose of caffeine; the same information was not provided for the placebo group.
Concerns have been raised as to whether caffeine interferes with the measurement of exhaled nitric oxide levels, based on a randomised study in non‐asthmatic healthy volunteers (Bruce 2002). The only randomised study to date in a small sample of people with mild asthma did not identify a significant difference between a cup of coffee made with 15 g of grounds and a similar cup made with decaffeinated coffee (Taylor 2004). Additional studies would help to determine whether this finding is valid.
Overall completeness and applicability of evidence
Comparison of the findings across studies was complicated by the use of five different doses of caffeine, different outcome measures recorded at different times, and different methods of reporting outcomes. To permit the aggregation of data we grouped the trials according to dose and time. Similarly, we made analyses using the SMD where the combined trials used different units of measurement for the same variable. Despite this, few data were available to be combined in the meta‐analysis.
The dose of caffeine tested varied greatly between studies, from 5 to 10 mg/kg. In an effort to make this more meaningful in dietary terms, most authors related doses to cups of coffee. However, the average amount of caffeine per cup is quoted as between 30 to 150 mg, although 150 mg was most commonly stated. Consequently, the number of cups of coffee required to produce bronchodilation is reported from one to five cups.
One method of standardising the dose ingested between studies would be to examine the peak serum levels. The reported peak serum levels vary greatly: from 5.4 mg/L (Colacone 1990) to 18.8 mg/L (Duffy 1991). Interestingly, the study reporting serum caffeine level of 18.8 mg/L after a dose of caffeine (10 mg/kg) found no increase in FEV1, although a later protective effect against bronchoconstriction post broncho provocation challenge test was found (Duffy 1991). These data were not presented in a way that would enable extraction for entry into the meta‐analysis. Peak serum levels of caffeine tended to occur at 45 or 60 minutes post caffeine intake. However, a clear difference between caffeine and placebo was seen even four hours post ingestion (Bukowskyj 1987). Few studies reported measurements beyond four hours post dosing. However, serum caffeine was detected in some patients at baseline. It is not known whether this would affect the potential for bronchodilation, especially where lung function outcomes were reported in terms of change from baseline. Even consideration of the peak serum levels would not fully compensate for this.
The findings of any review are only applicable to the characteristics of the participants taking part in the included studies. Although a standardised definition was not used across the studies, participants were described as having mild or moderate asthma, therefore results may not be generalisable to those with more severe asthma. Most subjects were described as having stable or asymptomatic asthma, but how this was assessed was not described. Similarly, patients' treatment regimens varied greatly across the trials and the effect of this was not studied.
It could be argued that insufficient data were presented on side effects resulting from caffeine, however the studies were designed not to asses the long‐term effects of caffeine, but instead to assess the short‐term impact of ingesting caffeine on lung function and exhaled nitric oxide tests. The side effects of caffeine are similar to those of theophylline (tachycardia, palpitation, nausea and other gastrointestinal disturbances, headache, central nervous system stimulation, insomnia).
The issue arising from this review is not whether caffeine should or should not be used as a bronchodilator in preference for theophylline or whether the side effects are sufficient to override this benefit. Instead the issue is whether or not the amount of caffeine ingested in a cup of coffee (or two) is sufficient to alter the result of a lung function test. This may be important if coffee drinkers show better results in lung function tests than they would achieve if they did not drink coffee. The evidence presented here shows that taking a 'normal' amount of caffeine, equivalent to one to five cups of coffee, is enough to alter the results of a lung function test. Therefore patients should be advised not to drink coffee for four hours before taking a lung function test. Drinking coffee before taking a FeNO test does not affect the results according to the single‐study data presented here, although further data are necessary to clarify. The long‐term impact of drinking coffee was not studied in these trials or reviewed here.
Quality of the evidence
Interpretation of the results of this review must include consideration of methodological limitations. All of the studies employed a cross‐over design. Although the method of allocation was in all cases reported to be randomised, none of the authors assessed order effects, and only two authors explicitly stated that the patients could not discern which treatment they had received (Bukowskyj 1987; Gong 1986). Sample sizes were small and the existence or effect of outlying values were rarely discussed. Outlying values are important in small studies using cross‐over designs, as each subject provides a large proportion of the data.
This review was restricted to an analysis of clinically relevant data in terms of a patient response and excluded the scientific issue of whether caffeine affects airway response to bronchoconstricting agents.
Potential biases in the review process
The possibility of publication bias (non‐publication of negative studies) should be considered given that only small differences in bronchodilator effect were found between caffeine and placebo. However, we used a comprehensive search strategy and searched for unpublished trials in an attempt to minimise this bias.
Agreements and disagreements with other studies or reviews
There are no other published reviews.
Authors' conclusions
Implications for practice.
Caffeine, even at 'low' doses, has been found to improve lung function for at least four hours after ingestion. One trial using the most sensitive outcome measurements (FEF25‐75) showed that effects are sustained for over four hours post ingestion. It is therefore recommended that patients be advised to withhold caffeine for at least four hours prior to lung function testing. Alternatively lung function tests results should be considered in light of caffeine ingested within four hours of the start of the test, as coffee drinkers may present with a better lung function test result than if they had not consumed so much caffeine. Caffeine does not appear to have a significant effect on exhaled nitric oxide levels.
With regard to advice to patients, caffeine ingestion may improve lung function in the short term. However, these trial data do not indicate whether the effect reaches a threshold for clinical significance in terms of an improvement of symptoms or quality of life. This was not the purpose of trials examined in this review. It is not known if tolerance to the bronchodilatory effects of caffeine develops in habitual consumers, which is a concern given that tolerance has been found in studies of sleep and renal function (Curatolo 1983). The amount of dietary caffeine required and the true benefit of dietary caffeine intake would be difficult to calculate due to the varying levels of caffeine within different foods and beverages. It appears that a substantial intake of caffeinated products would be needed to achieve a beneficial bronchodilatory effect and that possible undesirable side effects may outweigh the benefits.
Implications for research.
That caffeine has a bronchodilatory effect in asthma is clear from existing research. Future studies could address the following.
Patients' perception of the effect of caffeine on their asthma and quality of life, as this has not been systematically studied.
The maximum length of time at which bronchodilation is sustained, as this cannot be determined from existing trials.
Effects on patients with different levels of asthma severity, since existing trials have only studied people with mild to moderate asthma.
The response to caffeine of people with well‐controlled asthmatics on anti‐inflammatory agents. Asthmatics using inhaled steroids may be less responsive and this needs further evaluation.
Differences in the bronchodilator effects of caffeine between habitual consumers and non‐consumers.
Whether caffeine ingestion alters management decisions in asthma (based on lung function measurements).
Feedback
Historical use of caffeine
Summary
Reading the "Background" in the Abstract, it seems that the interest by caffeine as a bronchodilator is new. As a matter of fact, caffeine is used for asthma since, at least, the last decades of 1800. We can find reference to this drug in Marcel Proust's "A l'Ombre de Jeunes Filles en Fleur". This author, an asthmatic, refers that when he was very young (he was born in 1871) he used caffeine "that was prescribed for help me breathing".
Reply
Thank you for this comment.
Contributors
Roni Marques, chest physician.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2012 | Review declared as stable | This review is no longer being updated. The review remains of historic interest. Because caffeine has a similar structure to other bronchodilators, trials have been conducted to examine the effects on asthma monitoring. However, caffeine is not a recognised treatment for asthma. |
| 15 June 2012 | New search has been performed | A literature search was conducted and one potentially eligible study was added to 'studies awaiting classification' but has not been fully incorporated in to the review. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 11 August 2011 | New search has been performed | New literature search run. No new eligible studies identified. Minor copy edits made. |
| 29 September 2009 | New citation required but conclusions have not changed | New authorship of the review. |
| 27 August 2009 | New search has been performed | New search conducted, added new included study (Taylor 2004), amendments made to Plain Language Summary, reformatted Results and Discussion and added 'Risk of bias' and 'Summary of findings' table. Conclusions unchanged. |
| 21 July 2008 | Amended | Converted to new review format. |
| 7 January 2005 | New search has been performed | New studies found and included or excluded: 8 January 2005 |
| 8 January 2003 | New search has been performed | New studies sought but none found: 8 January 2003 |
| 18 June 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Steve Milan and Jane Dennis for all their help, Brian Rowe and Paul Jones for their editorial input, and Sally Spencer and Catherine O'Leary for their assistance with the data extraction. We would also like to thank all the authors who responded to our enquiries: Dr E. Simons, Dr M. Bukowskyj, Dr A. Colacone, Dr S. Kivity, Dr H. Gong and Dr F. O'Connell.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (The Cochrane Library) | Quarterly |
| PSYCINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. All caffeine doses versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 outcomes at 'short' time frame | 6 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.25, 1.20] |
| 1.1 Low dose | 2 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.02, 1.38] |
| 1.2 High dose | 4 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.75 [0.08, 1.41] |
| 2 FEV1 outcomes at 'medium' time frame | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 12.66 [‐0.34, 25.67] |
| 2.1 Low dose | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.5 [‐7.44, 30.44] |
| 2.2 High dose | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 13.7 [‐4.18, 31.58] |
| 3 FEV1 outcomes at 'long' time frame | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐6.49, 28.49] |
| 3.1 Low dose | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐6.49, 28.49] |
| 3.2 High dose | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FEF 25‐75 outcomes at 'short' time frame | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 25.14 [11.92, 38.37] |
| 4.1 Low dose | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 23.33 [6.18, 40.48] |
| 4.2 High dose | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 27.8 [7.03, 48.57] |
| 5 FEF 25‐75 outcomes at 'medium' time frame | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 32.72 [16.26, 49.17] |
| 5.1 Low dose | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 35.5 [15.85, 55.15] |
| 5.2 High dose | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 26.20 [‐3.89, 56.29] |
| 6 FEF 25‐75 outcomes at 'long' time frame | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 26.0 [5.02, 46.98] |
| 6.1 Low dose | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 26.0 [5.02, 46.98] |
| 6.2 High dose | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Gaw/VL outcomes at 'short' time frame | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 30.30 [1.08, 59.52] |
| 7.1 Low dose | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 High dose | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 30.30 [1.08, 59.52] |
| 8 FEV1 outcomes at 2 hours | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Low dose | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.49, 1.29] |
| 8.2 High dose | 5 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.32, 1.20] |
| 9 FEV1 outcomes at 2 hours (High dose) | 5 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9.1 % Change in FEV1 | 3 | Mean Difference (Fixed, 95% CI) | 5.47 [1.43, 9.52] | |
| 9.2 Post‐treatment FEV1 litres | 1 | Mean Difference (Fixed, 95% CI) | 0.38 [0.01, 0.75] | |
| 9.3 Change in FEV1 litres | 1 | Mean Difference (Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
1.1. Analysis.
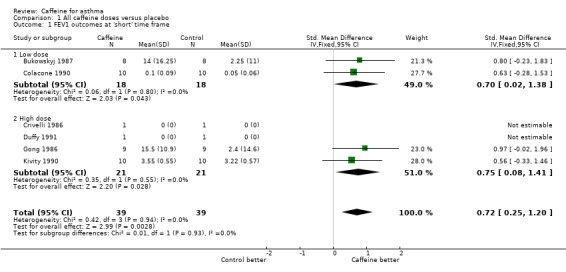
Comparison 1 All caffeine doses versus placebo, Outcome 1 FEV1 outcomes at 'short' time frame.
1.2. Analysis.
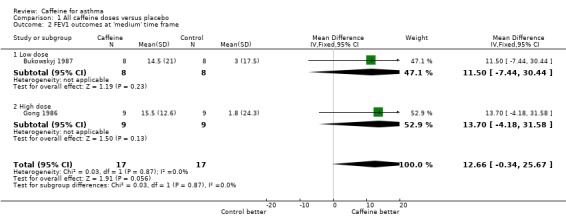
Comparison 1 All caffeine doses versus placebo, Outcome 2 FEV1 outcomes at 'medium' time frame.
1.3. Analysis.
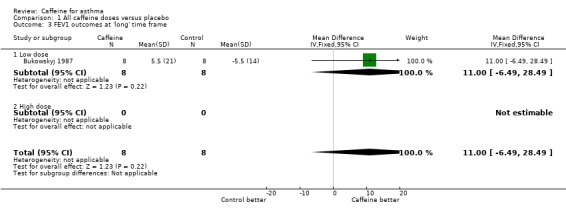
Comparison 1 All caffeine doses versus placebo, Outcome 3 FEV1 outcomes at 'long' time frame.
1.4. Analysis.
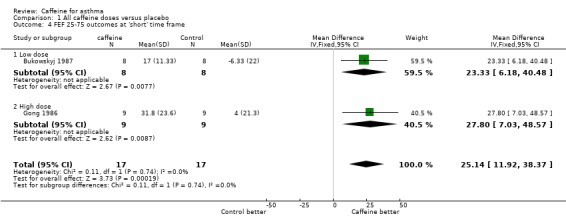
Comparison 1 All caffeine doses versus placebo, Outcome 4 FEF 25‐75 outcomes at 'short' time frame.
1.5. Analysis.
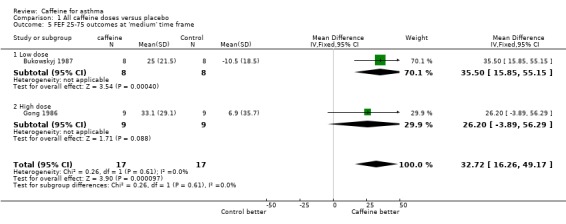
Comparison 1 All caffeine doses versus placebo, Outcome 5 FEF 25‐75 outcomes at 'medium' time frame.
1.6. Analysis.
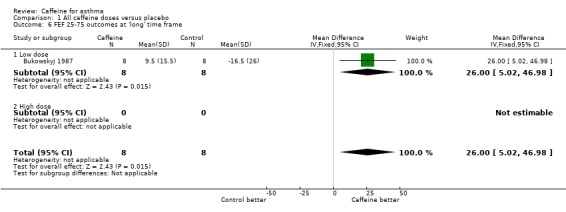
Comparison 1 All caffeine doses versus placebo, Outcome 6 FEF 25‐75 outcomes at 'long' time frame.
1.7. Analysis.
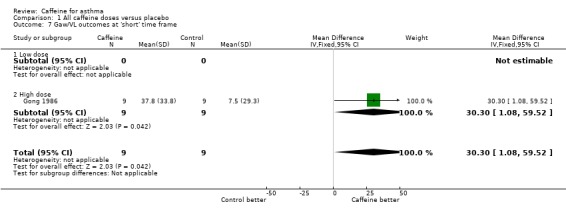
Comparison 1 All caffeine doses versus placebo, Outcome 7 Gaw/VL outcomes at 'short' time frame.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bukowskyj 1987.
| Methods | Randomised controlled trial Cross‐over design ‐ double‐blinded, rule = time (1 week) |
|
| Participants | Ten patients admitted, 8 (4 male) completed the study Mean age 64.8 (SD = 8) years Severity of asthma: Inclusion criteria: reversible obstructive airway disease, clinically stable, FEV1 < 75% predicted value Exclusion criteria: congestive heart failure, hepatic disease, ingestion of cimetidine, ingestion of oral contraceptives Prescribed medication: 7 patients took oral theophylline and salbutamol; 3 patients took inhaled beclomethasone dipropionate; 1 patient took inhaled beclomethasone dipropionate and prednisolone Control measure: refrained from caffeine, other methylxanthine‐containing substances and orally‐administered beta‐agonists for 12 h prior to and throughout 8 h study. Refrained from inhaling beta‐agonists for 6 h prior to and throughout the 8 h study period. |
|
| Interventions | 5 mg/kg caffeine versus placebo as a solution in a juice drink | |
| Outcomes | % change FEV1, % change FVC, % change FEF25‐75, % change Vmax50, % change Vmax25, FEV1 % predicted, pulse, blood pressure | |
| Notes | Administration of corticosteroids was continued unchanged in those patients receiving long‐term therapy with these drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Allocated by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blinded" Quote: "The medication code was known only to the hospital pharmacists." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The two patients who withdrew did so because they found the repeated spirometric tests unacceptably tiring. There was one patient who completed the study except for the last four h of the placebo day when she developed dyspnoea." No data used from these patients |
Colacone 1990.
| Methods | Randomised controlled trial Cross‐over design, rule = time (variable, within 2 weeks) |
|
| Participants | Ten adults (7 male) completed the study Mean age 46 (SD = 17) years Asthma severity: mild. Nine patients had previously documented increased airways reactivity and one had seasonal asthma Symptom‐free Prescribed medication: 7 patients took inhaled beta‐agonist, 5 patients took theophylline, 4 patients took inhaled corticosteroid and 2 required no medication Control measures: no caffeine 48 h before study. Fasted for 8 h. Withheld antiasthmatic medications according to standard guidelines for histamine broncho provocation testing. Beta‐agonists and anticholinergic drugs withheld for 8 to 12 h and theophylline for 12 h before testing. Slow‐release theophylline and antihistamines withheld 48 h before testing. Steroids continued as normal. |
|
| Interventions | 5 mg/kg caffeine versus placebo in a juice drink solution indistinguishable in taste and smell Histamine broncho provocation challenge | |
| Outcomes | Change in FEV1, PC20 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Low risk | Allocated by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Caffeine and corresponding placebo were prepared in solution and coded by the hospital pharmacy." Quote: "Both solutions were indistinguishable by taste, colour and smell." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Crivelli 1986.
| Methods | Randomised controlled trial Cross‐over design rule = time (2 consecutive days) |
|
| Participants | Seven adults (6 male) completed the study Age range 27 to 40 years Asthma severity: asymptomatic Included: patients with documented asthmatic airway obstruction Excluded: pregnant women asthmatic patients with concomitant liver and/or cardiovascular diseases, and patients treated with drugs affecting the hepatic microsomal enzyme system (barbiturates, phenytoin, rifampicin etc.) Prescribed medication: no bronchodilators, sodium cromoglycate or steroids for at least 2 weeks before the investigation Control measures: withheld all caffeine and methyl xanthine‐containing foods and beverages at least 12 h prior to the experiment |
|
| Interventions | 6 mg/kg caffeine versus placebo. Orange juice drink containing caffeine or a placebo drink containing solvent, i.e. saline. Carbachol challenge |
|
| Outcomes | FEV1, SGaw | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation unknown |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" but caffeine or saline given in orange juice so may not have tasted the same |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Duffy 1991.
| Methods | Randomised controlled trial Cross‐over design ‐ double‐blinded, rule = time (not stated) |
|
| Participants | 12 adults (11 male) admitted, 11 males completed the study Age range 18 to 42 years Inclusion criteria: FVC and FEV1 > 80% predicted and at least 10% fall in FEV1 in response to EVH (eucapnic voluntary hyperventilation) broncho provocation. Non‐smokers. Exclusion criteria: upper respiratory tract infection or influenza vaccination within 6 weeks before testing, an episode of asthma requiring hospitalisation or steroids within the previous 6 weeks before testing, pregnancy, or other cardiovascular disease apart from asthma Prescribed medication: no daily asthma medication Control measures: refrain from caffeine and methylxanthine‐containing substances for 12 hours, and food and cigarettes for 4 hours before testing |
|
| Interventions | 5 mg/kg, 10 mg/kg, placebo EVH broncho provocation | |
| Outcomes | FEV1, FVC, % dFEV (the percentage fall in FEV1, after EVH) | |
| Notes | Quote: 8 of 11 subjects had detectable caffeine levels on the day placebo was given, despite explicit instructions for avoidance of xanthine‐containing products | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "on three separate test days, each individual received, in random order, either placebo, 5 mg/kg caffeine or 10 mg/kg caffeine." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "After baseline pulmonary function tests, caffeine was given in a randomised, crossover, double blind fashion. Gelatin capsules were administered which contained either 0 mg, 5 mg/kg or 10 mg/kg caffeine." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout = 1 (female), due to side effects of nervousness and agitation presumably from the caffeine |
Gong 1986.
| Methods | Randomised controlled trial. Method of allocation computer program Cross‐over design ‐ double‐blinded, rule = time (at least 3 days) |
|
| Participants | Nine (4 male) Mean age = 35 (SD = 17) years Inclusion criteria: stable asthma (ATS criteria), 12 years of mild to moderately severe asthma, allergic in nature for 7 subjects, no other clinically evident disorders including hepatic disease or hypertension Exclusion criteria: no subject was receiving immunotherapy Prescribed medication: 7 = theophylline, 8 = sympathomimetic agents, 3 = inhaled corticosteroids, 1 = oral corticosteroids, 1 = cromolyn sodium Control measures: fasted for 4 hours prior to study and withheld the following prior to each day of study: theophylline compounds (48 h); adrenergic agents, oral (12 h) and inhaled (8 h); corticosteroids, oral (24 h) and inhaled (12 h); cromolyn sodium (24 h); antihistamines (48 h); caffeine‐containing beverages and medications (12 h) |
|
| Interventions | Decaffeinated coffee (containing ˜13 mg caffeine) plus a capsule containing aminophylline (200 mg)
Decaffeinated coffee (containing ˜13 mg caffeine) plus a placebo (lactose) capsule Decaffeinated coffee with additional 150 mg caffeine plus a placebo (lactose) capsule Decaffeinated coffee with additional 300 mg caffeine plus a placebo (lactose) capsule Decaffeinated coffee with additional 450 mg caffeine plus a placebo (lactose) capsule |
|
| Outcomes | % change FEV1, FVC, FEF25‐75, Gaw/VL (results given only for 7.2 mg/kg caffeine versus placebo + 200 mg aminophylline). Sampling of venous blood, whole body plethysmography, spirometry, respiratory rate, heart rate, sitting blood pressure, and symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer program |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although they went to lengths to blind the patients, there was no indication of method for blinding investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Kivity 1990.
| Methods | Randomised controlled trial Cross‐over design ‐ double‐blinded, rule = time (within 2 weeks) |
|
| Participants | 13 admitted, 10 adults (7 male) completed the study Mean age = 19.5 (SD = 1.1) years Inclusion criteria: documented reversible obstructive airway disease with a 20% improvement in either FVC or FEV1 after bronchodilator therapy, exercise‐induced drop in FEV1 of ≥ 15% from baseline, all patients clinically stable Exclusion criteria: any other chronic illness or receiving medication other than for bronchial asthma Prescribed medication: 2 = slow release theophylline, 10 = inhaled salbutamol. None of the patients were receiving corticosteroids, cromolyn sodium or ketotifen. |
|
| Interventions | Opaque placebo capsule or opaque 3.5 mg/kg caffeine capsule or opaque 7 mg/kg caffeine capsule taken with 100 mL water. Exercise‐induced bronchoconstriction | |
| Outcomes | FEV1, pulse, blood pressure | |
| Notes | Patients refrained from caffeine for 12 h, from theophylline containing drugs for 48 h, and from inhaled beta‐agonists 8 h prior to the study. The patient did not have caffeinated drinks 24 h prior to the study day (conflicting information in paper). The patients did not eat during the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Caffeine and placebo were given through an opaque capsule together with 100 mL of water." Stated double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "three patients who withdrew from the study could not comply with multiple visits to the clinic". No data used from these patients. |
Taylor 2004.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants | 20 adults (gender not specified) completed the study Mean age: 37 (range 16 to 73) years Inclusion criteria: regular coffee drinkers; FeNO > 10 PPB. 10 steroid‐naive and 10 treated with ICS (mean dose 980 μg/d) Exclusion criteria: oral prednisone, oral theophylline or inhaled long‐acting beta‐agonist for 1 month prior to study Control measures: caffeine withheld for 24 h. Inhaled bronchodilators withheld 6 h. |
|
| Interventions | Intervention: 15 g caffeine‐containing coffee (Illy Espresso Caffe Macinato) prepared in an espresso coffee maker as a 200 mL cup of coffee Placebo: 15 g decaffeinated coffee (Illy Espresso Decaffeinated Macinato) prepared in an espresso coffee maker as a 200 mL cup of coffee |
|
| Outcomes | FeNO | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Stated randomised, no information given on method used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated double‐blind, but patient blinding depends on regular and decaffeinated coffee being indistinguishable by taste. No mention of researcher blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
ATS: American Thoracic Society EVH: eucapnic voluntary hyperventilation FEF25‐75: maximum mid‐expiratory flow FEV1: forced expiratory volume in one second FVC: forced vital capacity h: hour ICS: inhaled corticosteroids PPB: parts per billion SD: standard deviationVmax25/Vmax50: maximal expiratory flow rates at 25% and 50% of vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker 1984 | Participants are children Interventions are caffeine versus theophylline |
| Henderson 1993 | Histamine broncho provocation challenge (FEV1 measured after challenge) |
| Simmons 1983 | Not a randomised controlled trial |
Differences between protocol and review
The 2009 update included regular caffeine‐containing coffee versus decaffeinated coffee as a comparison type. The 2009 review also compared lung function at all doses at two hours.
Serum caffeine levels was included as an outcome.
Contributions of authors
Anna Bara and Elizabeth Barley extracted the data, did the meta‐analyses and drafted the original review.
Emma Welsh updated the review, reformatted and redrafted it, added a new included study (Taylor 2004) and added 'Risk of bias' tables. Chris Cates extracted data for the 'Risk of bias' table, carried out the Generic Inverse Variance analyses and edited the review update.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bukowskyj 1987 {published data only}
- Bukowskyj M, Nakatsu K. The bronchodilator effect of caffeine in adult asthmatics. American Review of Respiratory Disease 1987;135(1):173‐5. [DOI] [PubMed] [Google Scholar]
Colacone 1990 {published data only}
- Colacone A, Bertolo L, Wolkove N, Cohen C, Kreisman H. Effect of caffeine on histamine bronchoprovocation in asthma. Thorax 1990;45(8):630‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crivelli 1986 {published data only}
- Crivelli M, Wahllander A, Jost G, Preisig R, Bachofen H. Effect of dietary caffeine on airway reactivity in asthma. Respiration 1986;50(4):258‐64. [DOI] [PubMed] [Google Scholar]
Duffy 1991 {published data only}
- Duffy P, Phillips YY. Caffeine consumption decreases the response to bronchoprovocation challenge with dry gas hyperventilation. Chest 1991;99(6):1374‐7. [DOI] [PubMed] [Google Scholar]
Gong 1986 {published data only}
- Gong H Jr, Simmons MS, Tashkin DP, Hui KK, Lee EY. Bronchodilator effects of caffeine in coffee. A dose‐response study of asthmatic subjects. Chest 1986;89(3):335‐42. [DOI] [PubMed] [Google Scholar]
Kivity 1990 {published data only}
- Kivity S, Aharon YB, Man A, Topilsky M. The effect of caffeine on exercise‐induced bronchoconstriction. Chest 1990;97(5):1083‐5. [DOI] [PubMed] [Google Scholar]
Taylor 2004 {published data only}
- Taylor E, Smith AD, Herbison GP, Cowan JO, Taylor DR. Effect of caffeine on exhaled nitric oxide measurements in asthma [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P1165. [Google Scholar]
- Taylor ES, Smith AD, Cowan JO, Herbison GP, Taylor DR. Effect of caffeine ingestion on exhaled nitric oxide measurements in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2004;169(9):1019‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Becker 1984 {published data only}
- Becker AB, Simons KJ, Gillespie CA, Simons FE. The bronchodilator effects and pharmacokinetics of caffeine in asthma. New England Journal of Medicine 1984;310(12):743‐6. [DOI] [PubMed] [Google Scholar]
Henderson 1993 {published data only}
- Henderson JC, O'Connell F, Fuller RW. Decrease of histamine induced bronchoconstriction by caffeine in mild asthma. Thorax 1993;48(8):824‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 1983 {published data only}
- Simmons M, Gong H, Tashkin DP, Hui K, Lee E. Bronchodilator effects of coffee in asthmatics. Chest 1983;84:332. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Yurach 2011 {published data only}
- Yurach MT, Davis BE, Cockcroft DW. The effect of caffeinated coffee on airway response to methacholine and exhaled nitric oxide. Respiratory Medicine 2011;105:1606‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Bruce 2002
- Bruce C, Yates DH, Thomas PS. Caffeine decreases exhaled nitric oxide. Thorax 2002;57:361‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheney 1935
- Cheney RH. Ventricular response in caffeine‐nicotine antagonism. Journal of Pharmacology and Experimental Therapeutics 1935;54(1):42‐52. [Google Scholar]
Curatolo 1983
- Curatolo PW, Robertson D. The health consequences of caffeine. Annals of Internal Medicine 1983;98(1):641‐53. [DOI] [PubMed] [Google Scholar]
Grollman 1930
- Grollman A. The action of alcohol, caffeine, and tobacco, on the cardiac output (and its related functions) of normal man. Journal of Pharmacology and Experimental Therapeutics 1930;39(3):313‐27. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Pagano 1988
- Pagano R, Negri E, Decarli A, Vecchia C. Coffee drinking and prevalence of bronchial asthma. Chest 1988;94(2):386‐9. [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Robertson 1978
- Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, et al. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. New England Journal of Medicine 1978;298:181‐6. [DOI] [PubMed] [Google Scholar]
Stephenson 1977
- Stephenson PE. Physiologic and psychotropic effects of caffeine on man. Journal of the American Dietetic Association 1977;71:240‐7. [PubMed] [Google Scholar]
Woodcock 1981
- Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. New England Journal of Medicine 1981;305:1611‐6. [DOI] [PubMed] [Google Scholar]


