Abstract
Background:
Limited evidence suggests that early cannabis use is associated with sleep problems. Research is needed to understand the developmental impact of early regular cannabis use on later adult sleep duration.
Methods:
In a sample of 1656 adult twins (56% female, Mean age = 25.79yrs), linear mixed effects models were used to analyze the influence of retrospectively assessed age of onset of regular cannabis use on adult sleep duration controlling for sex, depression, and current substance use. Twin analyses provided genetic and environmental variance estimates as well as insights into the association and potential casual relationships between these traits.
Results:
Earlier age of onset for regular cannabis use was significantly associated with shorter adult sleep duration on both weekdays (β = −0.13, 95% CI = [−0.23, −0.04]) and weekends (β = −0.18, 95% CI = [−0.27, −0.08]). Additive genetics significantly contributed to the onset of regular cannabis use (a2 = 76%, 95% CI = [68, 85]) and adult weekend sleep duration (a2 = 20%, 95% CI = [11, 32]). We found evidence of a significant genetic correlation (rA = −0.31, 95% CI = [−0.41, −0.15]) between these two traits and our best fitting model was consistent with early onset of regular cannabis use causing shorter adult weekend sleep duration (β = −0.11, 95% CI = [−0.18, −0.03]).
Conclusions:
Our results are consistent with the hypothesis that early onset of regular cannabis use may have a negative impact on adult sleep duration.
Keywords: Adolescence, Cannabis onset, Sleep duration, Twin models, Development
1. Introduction
Cannabis is one of the most widely used substances in the world with an estimated 147 million individuals using annually (World Health Organization (WHO), 2018). It is most frequently consumed by adolescents and young adults (Center for Behavioral Health Statistics and Quality (CBHSQ), 2017) with late adolescence being the highest point of risk for cannabis initiation (Chen et al., 2017). Early regular cannabis use has been linked to later life impairments such as psychological deficits (Ehrenreich et al., 1999; Fontes et al., 2011; Gruber et al., 2012), altered brain development (Jacobus and Tapert, 2014), psy-chopathology (Arseneault et al., 2002; Schubart et al., 2011), and substance use problems (Ellickson et al., 2004). To date, several studies focusing on cannabis initiation have found support for early cannabis use predicting later sleep deficits (Wong et al., 2009; Chheda et al., 2014) while two studies have failed to find similar effects (Pasch et al., 2012; Hasler et al., 2017), thus further research is needed to understand the developmental impact of early regular cannabis use on adult sleep.
Cannabis use amongst adults and adolescents has been linked to higher rates of insomnia (Roane and Taylor, 2008; Freeman et al., 2010; Alwan et al., 2011), sleep quality problems (Klonoff and Clark, 1976; Fakier and Wild, 2011), and shorter sleep duration times (Mednick et al., 2010; Glozier et al., 2010; Ebin et al., 2001; McKnight-Eily et al., 2011). Over a third of adults fail to get the recommended 7 h of sleep per night (Ford et al., 2015; Hirshkowitz et al., 2015) and shorter sleep duration is associated with deficits in psychological functioning (Kronholm et al., 2009; Banks and Dinges, 2007; Ferrie et al., 2011), work-related difficulties (Lian et al., 2015), and various maladaptive health outcomes (Steptoe et al., 2006; Gallicchio and Kalesan, 2009). While research demonstrates that baseline sleep problems are a significant predictor of later cannabis use (Weller and Halikas, 1982; Wong et al., 2004; Angarita et al., 2016) only a small selection of studies have found evidence for early cannabis use predicting later sleep factors (Wong et al., 2009; Pasch et al., 2012; Chheda et al., 2014). Given longitudinal associations in both directions, it is possible that sleep problems cause cannabis use, cannabis (or its consequences) cause sleep problems or a third underlying liability, like genetics, causes both and explains their correlation. Other factors known to influence sleep such as sex (Krishnan and Collop, 2006), depression (Tsuno et al., 2005), and substance use (Roehrs and Roth, 2001; McKnight-Eily et al., 2011; Boakye et al., 2018) are important to consider as well.
Twin studies estimate the role of genetic and environmental contributions to complex human traits. While no twin studies have been published focusing on the onset of regular cannabis use, moderate genetic contributions have been observed for cannabis initiation (a2 = 40–48%) (Verweij et al., 2010) and adult sleep duration (a2 = 29–44%) (Partinen et al., 1983; Genderson et al., 2013; Hublin et al., 2013; Watson et al., 2016). Environmental factors primarily contribute to the variance of early childhood sleep duration (c2 = 21% and e2 = 78) (Gregory et al., 2006), but as age increases unique environment largely contributes to the majority of the variance in adult sleep with a smaller contribution from additive genetics (e2 = 61–70% and a2 = 29–39%) (Genderson et al., 2013; Hublin et al., 2013; Watson et al., 2016). It is unknown whether sleep duration and cannabis share common genetic and/or environmental etiologies and twin modeling can be used to estimate the genetic/environmental overlap and liability between traits.
Using a young adult twin sample, we set out to investigate and dissect the relationship between the onset of regular cannabis use and adult sleep duration. We first tested if age of onset of regular cannabis use is associated with adult weekend and weekday sleep duration controlling for sex, depression, and current tobacco/alcohol/cannabis use. Then, we used classical univariate twin analyses to partition the variance into genetic and environmental components for adult sleep duration and onset of regular cannabis use separately. Next, we tested a series of nested bivariate twin models that decomposed the genetic and environmental overlap between sleep and cannabis. Finally, we used a causally informative extension of the twin design to test whether the relationship between onset of regular cannabis use and adult sleep duration is consistent with causality.
2. Methods
2.1. Participants and design
We used a sample of 1656 twins (403 monozygotic pairs and 425 dizygotic pairs) recruited from the Longitudinal Twin Sample (LTS) and Colorado Twin Study (CTS) (Rhea et al., 2006) as part of the third wave of the Center for the Genetics of Antisocial Drug Dependence [CADD; PI: Hewitt]. The sample was 56% female (n = 932) with an average age of 25.79 (SD = 1.99, range = 23–30).
2.2. Measures
2.2.1. Onset of regular Cannabis use
Data for onset of regular cannabis use (M = 17.58, SD = 3.09, Median = 17) was collected from a supplement derived from the Composite International Diagnostic Interview Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989; Cottler, 2000) and was assessed via a self-report question, “How old were you when you began using marijuana on a regular basis, that is at least once per month?” For our twin modeling analysis, onset of regular cannabis use was treated as an ordinal variable by coding twins who had never used cannabis regularly as 2 (n = 1142), twins who reported age of first regular use after age 17 as 1 (late onset) (n = 282), and if they reported age of first regular use at age 17 or earlier as 0 (early onset)1 (n = 232). This categorization was chosen based on the mean and median age of 17 and because it divided the distribution of age of onset into approximately equal groups of early and late onset. Binary measures of onset have been shown to bias genetic and environmental correlations in bivariate designs. Using a three-category ordinal variable that includes a third group who have not begun regular use and whose implied age of onset would therefore be the latest minimizes these biases and improves the performance of genetic and environmental modeling (Heath et al., 2002). Furthermore, using this ordinal measure, rather than a continuous measure for onset of regular cannabis use in our twin model, allowed us to boost our sample size by including this third group consisting of those who have not started regular cannabis use. We ran separate sets of regressions for onset of regular cannabis use coded as both an ordinal (reflecting our twin analyses) and continuous variable. Onset of regular cannabis use is quite distinct from onset of any use and occasional use across lifespan. In our sample of people who had never used cannabis regularly, by age 26 close to 85% had used cannabis less than 20 times in their life. This implies that those who have not initiated regular use have not engaged in a large amount of cannabis use across their lifespan and we would not expect this low-level usage to influence sleep.
2.2.2. Adult sleep duration
Adult sleep duration data was assessed using the Jessor Health Questionnaire (Jessor and Jessor, 1977), which asked, “How many hours of sleep you typically get on a weekend” and “How many hours of sleep you typically get on a weekday” with responses being “5 h or less”, “6 h”, “7 h”, “8 h”, “9 h”, “10 h”, or “11 h or more”. Sleep duration responses were treated as quasi-continuous variables. We reverse coded sleep duration for our regressions and bivariate twin analysis. Weekday and Weekend sleep duration were analyzed separately to account for possible differences as a result of work obligations or the flexibility of weekend schedules (Roepke and Duffy, 2010) as well as evidence that separate questions prompt more thoughtful and detailed responses (Lauderdale, 2014). However, we also conducted an additional analysis regarding the onset of regular cannabis use and an average of weekend and weekday sleep duration that is included in a supplement; the results mirror those for weekend sleep.
2.2.3. Covariates of sleep
Our phenotypic mixed effects regression models adjusted for covariates that have shown associations with sleep, including frequency of tobacco (Boakye et al., 2018), alcohol, (Roehrs and Roth, 2001) and cannabis use (included to separate the effects of onset of regular use from frequency of cannabis use) (Mednick et al., 2010; Glozier et al., 2010; Ebin et al., 2001; McKnight-Eily et al., 2011) which we defined as the number of days a substance was used in the past 6 months. We also controlled for sex (Krishnan and Collop, 2006) and depression (Tsuno et al., 2005) which was quantified via a composite score using responses from the Center for Epidemiological Studies-Depression (CES-D) scale (Radloff, 1977). We removed the sleep disturbance question from the CES-D composite score (range = 0–56) due to its redundancy with our outcome measure.
2.3. Analysis
2.3.1. Mixed effects models
We assessed descriptive statistics and mixed-effects regressions via R version 3.4.4 (Team, RC, 2018). Individual-level regressions were estimated via mixed effects models to account for the nested nature of twin data and standardized regression coefficients were estimated from the lme4 package of R studio (Bates et al., 2014).
2.3.2. Univariate twin models
Using biometrical twin models (Neale and Cardon, 2013) we evaluated the contributions from genetic and environmental factors to the onset of regular cannabis use and adult sleep duration. Traditional twin models assume that the variation in traits can be attributed to three latent factors: additive genetic effects (A), shared environmental (C) and nonshared or unique environmental factors (E). Monozygotic (MZ) twins are assumed to be correlated perfectly for A. Dizygotic (DZ) twins, on average, share half of their alleles identical by descent and are assumed to correlate 0.5 for A. By definition C is set to correlate perfectly for MZ and DZ pairs. Analyzing the variation and covariation between MZ and DZ pairs provides estimates of the amount that each latent variable contributes to a specific trait. Genetic influences would be inferred when MZ correlations are larger than DZ correlations. Structural equation modeling was conducted via Mplus version 8 (Muthén, 2007). We tested for differences across sex for all traits, testing mean differences for weekday and weekend sleep duration (quasi-continuous variables) and threshold differences for onset of regular cannabis use (an ordinal variable). We also tested whether the magnitudes of A, C, and E estimates were different across males and females (scalar sex differences). To determine the best fitting and most parsimonious univariate model we used χ2 difference tests to see whether individual parameter estimates (i.e., A, C or E) could be dropped without a significant decrement in model fit. Biometrical twin models utilized robust maximum likelihood and weighted least squares means and variances estimators to properly account for nonnormality (Asparouhov and Muthén, 2010) for sleep and cannabis models, respectively. We used scaled and approximated chi squared difference tests to test significance for continuous models and ordinal models, respectively. Chi squared difference tests for the E pathway cannot be identified in classic univariate twin designs for continuous models, thus p values cannot be estimated for significance.
2.3.3. Bivariate models
Cholesky decomposition models were used to decompose the environmental/genetic variance and covariance amongst onset of regular cannabis use and adult sleep duration in order to estimate unique and overlapping genetic/environmental contributions. Once the genetic overlap is estimated using the Cholesky Decomposition, a simple conversion of the Cholesky path coefficients can provide the proportion of genetic variation shared between two traits, i.e. the Genetic Correlation with confidence intervals derived from the Cholesky estimates. Similar to our univariate analysis, we tested for scalar sex differences and used χ2 difference tests to see whether individual parameter estimates (i.e., A, C or E) could be dropped without a significant decrement in model fit for bivariate models. Additionally, we tested a potential causal model (Heath et al., 1993; Verhulst and Estabrook, 2012; Minică et al., 2018; Torvik et al., 2019) in which the onset of regular cannabis use directly predicted adult sleep duration. Our data would be consistent with causality if we observed a non-significant reduction in model fit when comparing the causal model to the Cholesky decomposition model. This causal model comparison tested whether the A and E associations in a model can be accounted for by a directed phenotypic association between two traits. In order to verify that our causal model results were not due to a lack of power or other formality of data fitting, we compared models with opposite regression paths for both weekday and weekend sleep duration (adult sleep duration predicting onset of regular cannabis use) to our best fitting Cholesky models. Multivariate twin models controlled for fixed effect of sex but not for behavioral correlates.
All multivariate models were fit using weighted least squares means and variances estimators to account for nonnormality and we utilized χ2 difference tests to test significance for all multivariate trait models. The best fitting models were determined via χ2 difference tests to determine whether individual parameters could be dropped without a significant reduction in model fit. χ2 is sensitive to sample size, and to account for this we also used root-mean-square error of approximation (RMSEA) < 0.06 as an indicator of good fit (Hu and Bentler, 1998). We generated 95% confidence intervals with the CINTERVAL (bootstrap) command in Mplus.
3. Results
3.1. Individual-level analyses
Table 1 contains descriptive statistics of all study variables for the total sample (as well as for each separate sex). Table 2 shows the partial and full model regression outputs for onset of regular cannabis use (both as a continuous and ordinal variable). Controlling for depression, sex, and current cannabis/alcohol/tobacco use, age of onset of regular cannabis (as a continuous variable) was significantly associated with shorter adult sleep duration on both weekends (β = −0.18, 95% CI = [−0.27, −0.08], p < 0.001) and weekdays (β = −0.13, 95% CI = [−0.23, −0.04], p = 0.007). When onset of regular cannabis use was coded ordinally (reflecting our twin analyses) and controlling for depression, sex, and current alcohol/cannabis use, early onset of regular cannabis use was significantly associated with shorter adult sleep duration on weekends (β = −0.07, 95% CI = [−0.15, −0.01], p = 0.043) but not weekdays (β = −0.03, 95% CI = [−0.10, 0.04], p = 0.422), however this association for weekend sleep became non-significant when including tobacco in the full model (β = −0.03, 95% CI = [−0.11, 0.04], p = 0.351). Current frequency of tobacco use was significantly correlated with early onset of regular cannabis use (r = −0.38, 95% CI = [−0.42, −0.34], p < 0.001) and weekend sleep duration (r =−0.14, 95% CI = [−0.18, −0.09], p < 0.001). Table 3 displays the descriptive statistics of each cannabis onset group as well as the results of a one-way ANOVA group difference tests between the cannabis groups for all variables in the study. Fig. 1 plots the means and standard errors for both weekday and weekend sleep duration for each onset group. We found the largest difference in weekend sleep duration was between early onset and late onset of regular cannabis users. No-tably, there was very little difference in weekend sleep duration between late onset user and those who never initiated onset of regular cannabis use.
Table 1.
Descriptive Statistics for all Study Variables in the Full Sample.
| Sample Characteristics [M (SD)] | |||
|---|---|---|---|
| Full Sample N = 1656 |
Female N = 932 |
Male N = 724 |
|
| Age | 25.79 (1.99) | 25.77 (1.99) | 25.82 (2.01) |
| Age of Onset of Regular Cannabis Use | 17.58 (3.09) | 17.73 (3.27) | 17.47 (2.95) |
| Weekday Sleep in hours | 7.15 (1.05) | 7.21 (1.08) | 7.07 (1.02) |
| Weekend Sleep in hours | 7.81 (1.31) | 7.81 (1.35) | 7.82 (1.25) |
| Number of Days of Tobacco Use in the past 180 days | 37.37 (69.17) | 29.56 (63.29) | 47.43 (74.94) |
| Number of Days of Alcohol Use in the past 180 days | 28.35 (36.80) | 22.00 (30.49 | 36.53 (42.24) |
| Number of Days of Cannabis Use in the past 180 days | 13.86 (42.24) | 9.61 (36.18) | 19.32 (48.44) |
| Number of Depression symptoms (CES-D) | 29.33 (9.23) | 30.19 (9.63) | 28.21 (8.57) |
Table 2.
Partial and Full Model Regression Estimates Controlling for Covariates, for Onset of Regular Cannabis Use (Coded as both a Continuous and Ordinal variable) with Weekend/Weekday Sleep Duration.
| Onset of Regular Cannabis Use as a Continuous variable (Weekend Sleep Duration) | Estimate | 95 % Confidence Interval | p value | |
|---|---|---|---|---|
| Model 1 | Weekend Sleep | −0.19* | [−0.28, −0.09] | < 0.001 |
| Model 2 | Weekend Sleep, Sex | −0.18* | [−0.28, −0.09] | < 0.001 |
| Model 3 | Weekend Sleep, Sex, Cannabis | −0.19* | [−0.28, −0.09] | < 0.001 |
| Model 4 | Weekend Sleep, Sex, Cannabis, Alcohol | −0.19* | [−0.28, −0.09] | < 0.001 |
| Model 5 | Weekend Sleep, Sex, Cannabis, Alcohol, Depression | −0.19* | [−0.28, −0.09] | < 0.001 |
| Model 6 | Weekend Sleep, Sex, Cannabis, Alcohol, Depression, Tobacco | −0.18* | [−0.27, −0.08] | < 0.001 |
| Onset of Regular Cannabis Use as a Continuous variable (Weekday Sleep Duration) | Estimate | 95% Confidence interval | p value | |
| Model 1 | Weekday Sleep | −0.16* | [−0.25, −0.07] | < 0.001 |
| Model 2 | Weekday Sleep, Sex | −0.16* | [−0.25, −0.07] | < 0.001 |
| Model 3 | Weekday Sleep, Sex, Cannabis | −0.16* | [−0.25, −0.07] | < 0.001 |
| Model 4 | Weekday Sleep, Sex, Cannabis, Alcohol | −0.16* | [−0.25, −0.07] | < 0.001 |
| Model 5 | Weekday Sleep, Sex, Cannabis, Alcohol, Depression | −0.15* | [−0.24, −0.06] | 0.002 |
| Model 6 | Weekday Sleep, Sex, Cannabis, Alcohol, Depression, Tobacco | −0.13* | [−0.23, −0.04] | 0.007 |
| Onset of Regular Cannabis Use as an Ordinal variable (Weekend Sleep Duration) | Estimate | 95 % Confidence Interval | p value | |
| Model 1 | Weekend Sleep | −0.09* | [−0.15, −0.02] | 0.007 |
| Model 2 | Weekend Sleep, Sex | −0.09* | [−0.16, −0.03] | 0.005 |
| Model 3 | Weekend Sleep, Sex, Cannabis | −0.09* | [−0.16, −0.03] | 0.009 |
| Model 4 | Weekend Sleep, Sex, Cannabis, Alcohol | −0.09* | [−0.16, −0.02] | 0.012 |
| Model 5 | Weekend Sleep, Sex, Cannabis, Alcohol, Depression | −0.07* | [−0.15, −0.01] | 0.043 |
| Model 6 | Weekend Sleep, Sex, Cannabis, Alcohol, Depression, Tobacco | −0.03 | [−0.11, 0.04] | 0.351 |
| Onset of Regular Cannabis Use as an Ordinal variable (Weekday Sleep Duration) | Estimate | 95 % Confidence Interval | p value | |
| Model 1 | Weekday Sleep | −0.06 | [−0.12, 0.01] | 0.070 |
| Model 2 | Weekday Sleep, Sex | −0.05 | [−0.12, 0.02] | 0.149 |
| Model 3 | Weekday Sleep, Sex, Cannabis | −0.05 | [−0.12, 0.02] | 0.166 |
| Model 4 | Weekday Sleep, Sex, Cannabis, Alcohol | −0.05 | [−0.12, 0.01] | 0.134 |
| Model 5 | Weekday Sleep, Sex, Cannabis, Alcohol, Depression | −0.03 | [−0.10, 0.04] | 0.422 |
| Model 6 | Weekday Sleep, Sex, Cannabis, Alcohol, Depression, Tobacco | 0.01 | [−0.07, 0.08] | 0.863 |
Substance Use = Number of days used in the past 6 months.
Depression = Summed scores from the CES-D (with the exclusion of a sleep question).
Table 3.
Descriptive Statistics for each Onset of Regular Cannabis Use Group as well as a Group Difference Test Between the Groups for each Study Variable.
| Sample Characteristics [M (SD)] | ||||
|---|---|---|---|---|
| Early Onset of Regular Cannabis Use (17 or earlier) N = 232 |
Late Onset of Regular Cannabis use (after 17) N = 282 |
Never used Cannabis regularly N = 1142 |
Group Difference F Ratio Test(p value) | |
| Weekday Sleep in Hours | 6.98 (1.15) | 7.28 (1.07) | 7.16 (1.02) | 3.66 (0.056) |
| Weekend Sleep in Hours | 7.55 (1.41) | 7.91 (1.40) | 7.86 (1.25) | 9.27 (0.002) |
| Number of Days of Tobacco Use in the Past 180 Days | 89.08 (84.12) | 57.05 (78.38) | 20.61 (54.16) | 284.90 (< 0.001) |
| Number of Days of Alcohol Use in the Past 180 Days | 38.73 (45.89) | 46.25 (47.82) | 22.16 (29.20) | 79.17 (< 0.001) |
| Number of Days of Cannabis Use in the Past 180 Days | 39.66 (65.33) | 49.82 (67.48) | 0.18 (0.78) | 367.00 (< 0.001) |
| Number of Depression Symptoms (CES-D) | 31.31 (10.38) | 31.26 (10.37) | 28.45 (8.54) | 29.54 (< 0.001) |
Fig. 1.
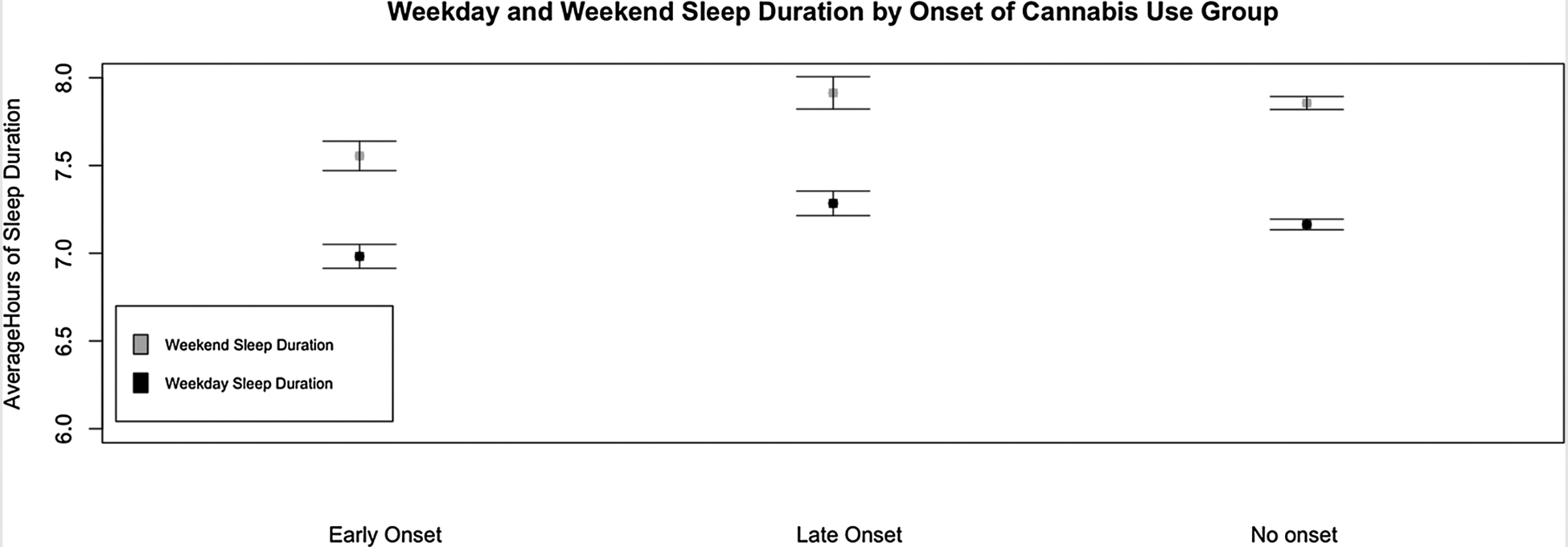
Plot of Means and Standard Errors of both Weekday and Weekend Sleep Duration for all Three Onset Groups.
3.2. Twin correlations
All three phenotypes demonstrated MZ correlations that were larger than DZ correlations, indicating the presence of genetic influences. Table 4 shows the cross-twin and cross-twin cross-trait correlations between onset of regular cannabis use and adult sleep duration.
Table 4.
Cross-Twin Correlations (95% CI) for Onset of Regular Cannabis Use with Both Adult Weekend and Weekday Sleep Duration. MZ = Monozygotic; DZ = Dizygotic.
| Onset of Regular Cannabis Use | Adult Weekend Sleep Duration | Adult Weekday Sleep Duration | |
|---|---|---|---|
| MZ | 0.74 [0.64, 0.82] | 0.21 [0.07, 0.32] | 0.28 [0.15, 0.39] |
| DZ | 0.49 [0.35, 0.60] | 0.09 [−0.02, 0.20] | 0.05 [−0.05, 0.16] |
| Cross-Twin Cross-Trait Correlations (95% CI) | ||
|---|---|---|
| Onset of Regular Cannabis Use and Adult Weekend Sleep Duration | Onset of Regular Cannabis Use and Adult Weekday Sleep Duration | |
| MZ | −0.12 [−0.21, −0.02] | −0.06 [−0.17, 0.05] |
| DZ | −0.06 [−0.16, 0.03] | 0.01 [−0.07, 0.09] |
3.3. Univariate twin models
To determine the genetic/environmental contributions to the variance of each individual trait we conducted univariate twin models. Table 5 includes model fit and comparison statistics for all univariate twin models. Sex differences were found when testing thresholds of univariate twin models for onset of regular cannabis use (χ2diff (2) = 42.05, p < 0.001) but not for adult weekend and weekday sleep duration (all χ2diff (1) < 0.16, p > 0.689). Therefore, thresholds were estimated separately by sex for onset of regular cannabis use, but the means were constrained to be the same for adult sleep duration. No significant sex differences were found in the degree to which genetic and environmental factors contributed to cannabis and sleep outcomes (scalar sex differences; all χ2diff (3) < 3.78, p > 0.286); thus, male and female estimates were constrained to be the same. Table 6 includes standardized estimates for all possible parameter combinations of the univariate models constrained across sex. C could be dropped in all univariate models (all p > 0.073) but not A or E parameters (all χ2diff (1) > 6.31, p < 0.012 and χ2diff (2) > 34.15, p < 0.001). Our best fitting univariate models consisted of only A and E parameters for onset of regular cannabis use (χ2 (23) = 16.58, p = 0.829, RMSEA = < 0.001), adult weekend sleep duration (χ2 (22) = 13.81, p = 0.908, RMSEA = < 0.001), and weekday sleep duration (although all univariate models for weekday sleep duration demonstrated poor overall fit; all χ2 > 40.69, p < 0.009, RMSEA > 0.072). Genetic and unique environmental factors significantly contributed to the variance for onset of regular cannabis use (a2 = 76%, 95% CI = [68, 85], χ2diff (1) = 411.580, p < 0.001, and e2 = 24%, 95% CI = [16, 32]), adult weekend sleep duration (a2 = 20%, 95% CI = [11, 32], χ2diff (1) = 20.08, p < 0.001, and e2 = 80%, 95% CI = [70, 90]), and adult weekday sleep duration (a2 = 20%, 95% CI = [11, 29], χ2diff (1) = 21.13, p < 0.001, and e2 = 80%, 95% CI = [71, 89]), with the caveat that for weekday sleep duration the AE model gave a poor fit as reported above. Overall, we found that additive genetics and nonshared environmental factors contribute to the etiology of the onset of regular cannabis use and adult sleep duration.
Table 5.
Model Fit and Comparison Statistics for all Univariate Models. Threshold Testing is at 1.
| Trait and model | χ2 | df | p | RMSEA |
|---|---|---|---|---|
| Onset of Regular Cannabis Use | ||||
| Full A,C,E Model With Scalar Sex Differences | 13.24 | 19 | 0.826 | < 0.001 |
| Sex Thresholds Differences | 42.05 | 2 | < 0.001 | |
| No Scalar Sex Differences | 0.61 | 3 | 0.894 | |
| AE | 3.22 | 1 | 0.073 | |
| CE | 12.51 | 1 | < 0.001 | |
| E | 414.80 | 2 | < 0.001 | |
| Adult Weekend Sleep Duration | ||||
| Full A,C,E Model With Scalar Sex Differences | 8.97 | 18 | 0.960 | < 0.001 |
| No Sex Threshold Differences | 0.16 | 1 | 0.689 | |
| No Scalar Sex Differences | 3.78 | 3 | 0.286 | |
| AE1,2 | 0.00 | 1 | 1 | |
| CE | 67.52 | 1 | < 0.001 | |
| E | 34.15 | 2 | < 0.001 | |
| Adult Weekday Sleep Duration | ||||
| Full A,C,E Model With Scalar Sex Differences | 33.20 | 18 | 0.016 | 0.071 |
| No Sex Threshold Differences | .032 | 1 | 0.857 | |
| No Scalar Sex Differences | 2.61 | 3 | 0.457 | |
| AE | 1.28 | 1 | 0.257 | |
| CE1 | 6.31 | 1 | 0.012 | |
| E | 42.3 | 2 | < 0.001 |
Due to the scaling factor MLR produced a negative chi squared difference. To test this parameter, we had to use maximum likelihood estimation which would have given us a conservative estimation of the chi squared difference, but the effect was so strong is remained significant.
We observed no difference in the chi squared values between the weekend sleep duration model with A, C, and E paths and the weekend sleep duration model with only A and E paths, thus 0 was estimated in a chi squared difference test.
Table 6.
Standardized Estimates (and 95% CIs) for all Univariate Raw Models Constrained Across Sex.
| Trait | Model | Genetic and Environmental Estimates | Model fit Information | |||||
|---|---|---|---|---|---|---|---|---|
| a2 (95% C.I) | c2 (95% C.I) | e2 (95% C.I) | χ2 | df | p | RMSEA | ||
| Onset of Regular Cannabis Use | ACE | 0.51[0.23, 0.90] | 0.23[0.02, 0.67] | 0.26[0.18, 0.35] | 13.79 | 22 | 0.9089 | < 0.001 |
| AE | 0.76[0.68, 0.85] | – | 0.24[0.16, 0.32] | 16.58 | 23 | 0.8295 | < 0.001 | |
| CE | – | 0.67[0.60, 0.74] | 0.33[0.27, 0.41] | 24.26 | 23 | 0.3894 | 0.018 | |
| Adult Weekday Sleep Duration | ACE | 0.20[0.11, 0.32] | 0[−0.01, 0.01] | 0.80[0.71, 0.89] | 40.69 | 21 | 0.0061 | 0.075 |
| AE | 0.20[0.11, 0.29] | – | 0.80[0.71, 0.89] | 40.69 | 22 | 0.0090 | 0.072 | |
| CE | – | 0.14[0.07, 0.23] | 0.86[0.79, 0.93] | 47.01 | 22 | 0.0015 | 0.083 | |
| Adult Weekend Sleep Duration | ACE | 0.20[0.05, 0.47] | 0[−0.05, 0.05] | 0.80[0.69, 0.90] | 13.81 | 21 | 0.8778 | < 0.001 |
| AE | 0.20[0.11, 0.32] | – | 0.80[0.70, 0.90] | 13.81 | 22 | 0.9082 | < 0.001 | |
| CE | – | 0.14[0.08, 0.24] | 0.86[0.78, 0.93] | 17.41 | 22 | 0.7402 | < 0.001 | |
A = additive genetic influences.
C = common environmental influences.
E = unique environmental influences.
3.4. Bivariate twin models
To disentangle overlapping and/or unique genetic/environmental contributions among the onset of regular cannabis use and adult sleep duration we fit bivariate Cholesky decomposition models. Table 7 includes model fit and comparison statistics for bivariate twin models. Similar to our univariate analyses, we found that bivariate models assuming no genetic and/or environmental sex differences did not yield a significant decrement in model fit (all χ2diff (9) < 7.76, p > 0.559). We then employed a model comparison approach to determine whether we could remove individual A, C and E parameters from multivariate models. C could be dropped without a significant deterioration of fit in our bivariate Cholesky decomposition models for weekend and weekday sleep (all χ2diff (3) > 3.16, p > 0.306), but not A or E parameters (all χ2diff (3) > 16.55, p < 0.001). Fig. 2 displays the best fitting A and E Cholesky model for weekend sleep (χ2 (63) = 39.44, p = 0.992, RMSEA = < 0.01), with Cholesky decomposition pathways suggesting a significant genetic correlation (A path = −0.14, 95% CI = [−0.24, −0.04], rA = −0.31, 95% CI = [−0.41, −0.15], χ2diff (1) = 9.59, p = 0.002) between onset of regular cannabis use and adult weekend sleep duration. Fig. 3 displays the best fitting A and E Cholesky model for weekday sleep (χ2 (63) = 70.36, p = 0.245, RMSEA = 0.027) but suggested no significant genetic and/or environmental correlations (all χ2diff (1) < 0.98, p > 0.321) between onset of regular cannabis use and weekday sleep duration, as would be expected given the non-significant phenotypic regression analysis. Table 8 displays the additive genetic pathways and significance tests needed from the standard bivariate Cholesky decompositions for calculating the genetic correlations.
Table 7.
Model Fit and Comparison Statistics for all Bivariate models. Threshold testing is at 1.
| Trait and model | χ2 | df | p | RMSEA |
|---|---|---|---|---|
| Onset of Regular Cannabis Use and Adult Weekend Sleep Duration | ||||
| Full Cholesky Model with Scalar Sex Differences | 27.55 | 51 | 0.997 | < 0.001 |
| No Scalar Sex Differences | 7.76 | 9 | 0.559 | |
| Dropping all A Paths | 16.55 | 3 | < 0.001 | |
| Dropping all C Paths | 3.16 | 3 | 0.368 | |
| Dropping all E Paths | 322.22 | 3 | < 0.001 | |
| Difference test of A and E Cholesky vs Casual (Going from Cannabis to Sleep) | 2.44 | 1 | 0.118 | |
| Difference test of A and E Cholesky vs Reverse Causal (Going from Sleep to Cannabis) | 21.12 | 1 | < 0.001 | |
| Trait and model | χ2 | df | p | RMSEA |
| Onset of Regular Cannabis Use and Adult Weekday Sleep Duration | ||||
| Full Cholesky Model with Scalar Sex Differences | 61.62 | 51 | 0.147 | 0.035 |
| No Scalar Sex Differences | 5.76 | 9 | 0.764 | |
| Dropping all A Paths | 21.77 | 3 | < 0.001 | |
| Dropping all C Paths | 3.62 | 3 | 0.306 | |
| Dropping all E Paths | 301.98 | 3 | < 0.001 | |
| Difference test of A and E Cholesky vs Casual (Going from Cannabis to Sleep) | 0.08 | 1 | 0.783 | |
| Difference test of A and E Cholesky vs Reverse Causal (Going from Sleep to Cannabis) | 6.211 | 1 | 0.013 |
Fig. 2.
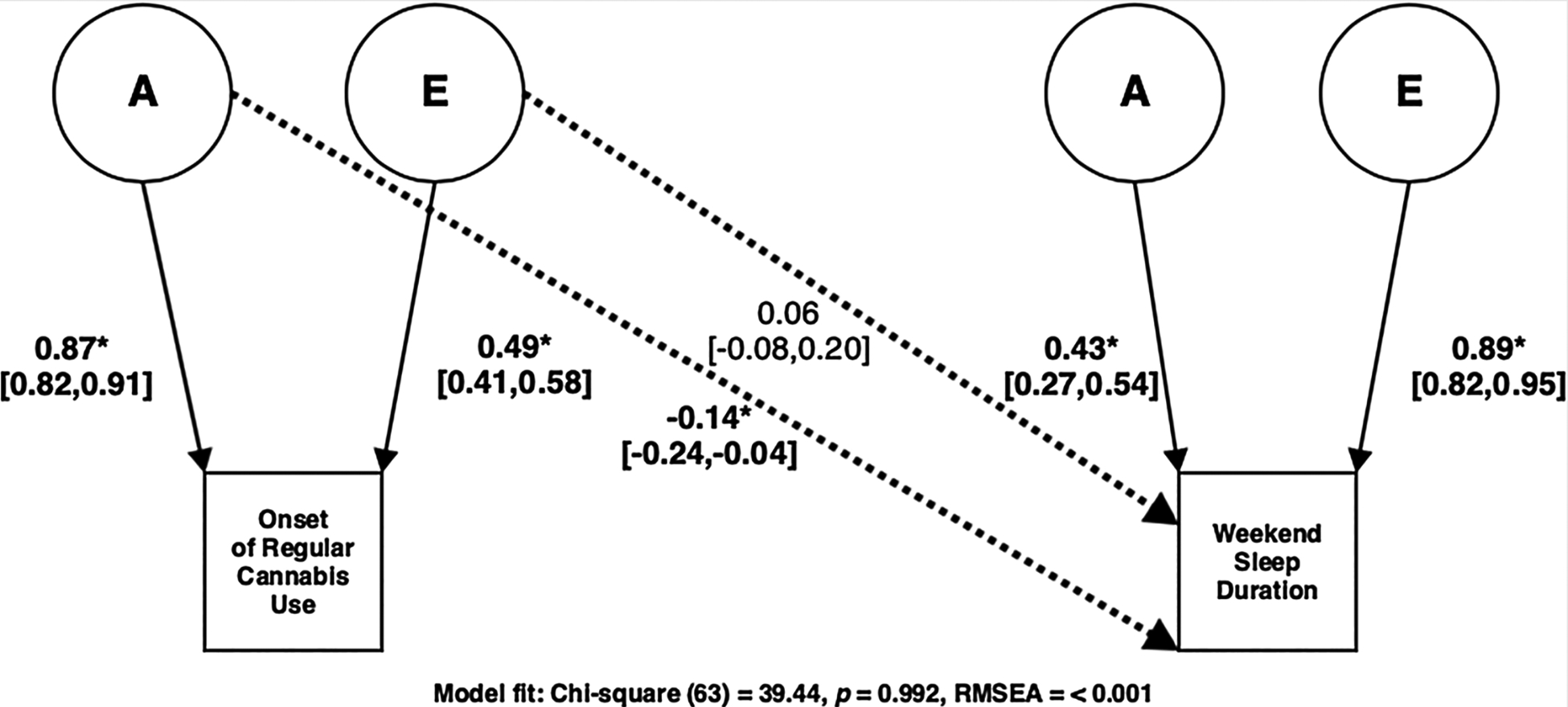
Best fitting A and E Cholesky model for Onset of Regular Cannabis Use and Weekend Sleep Duration.
Fig. 3.
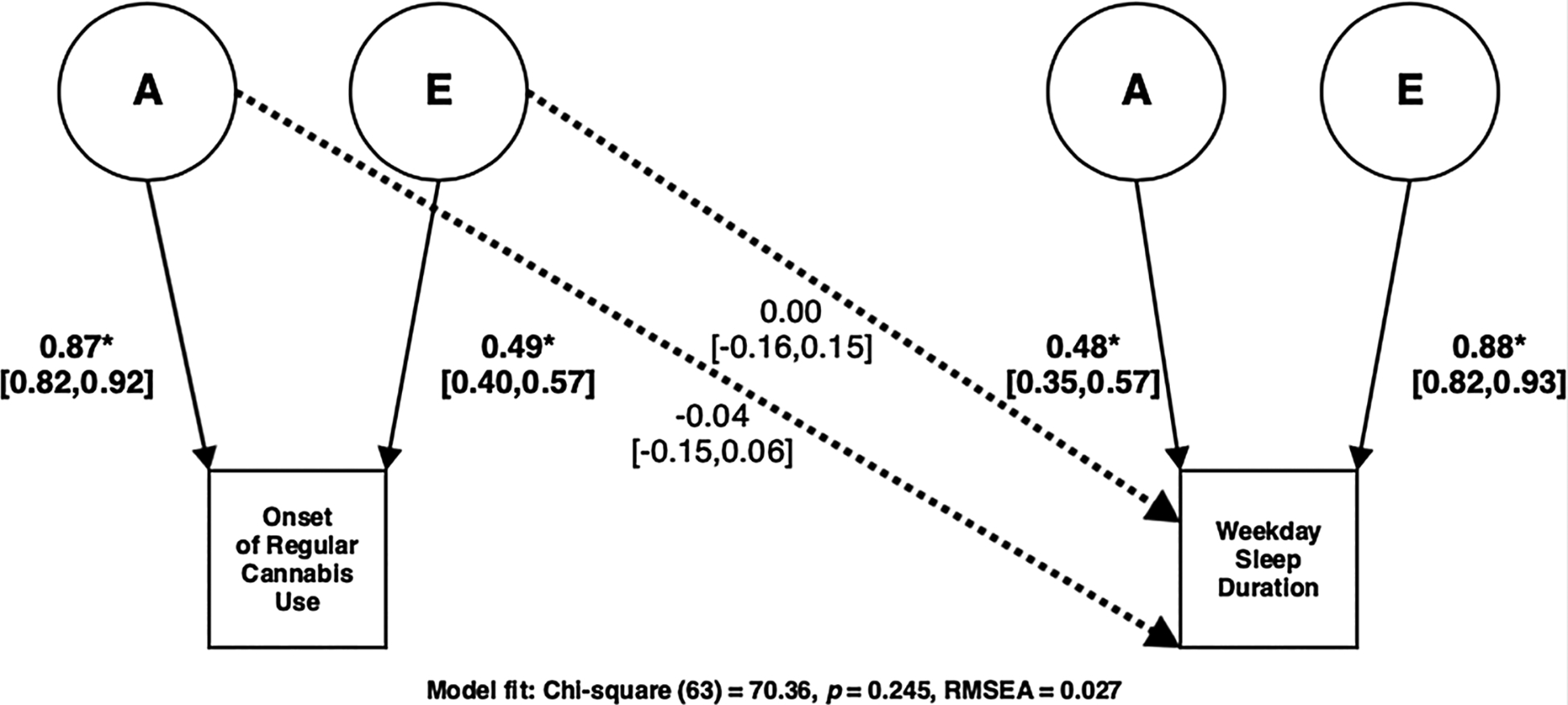
Best fitting A and E Cholesky model for Onset of Regular Cannabis Use and Weekday Sleep Duration.
Table 8.
Pathways and Significance tests for Genetic Association Between Onset of Regular Cannabis Use and Weekday/Weekend Sleep Duration.
| Variable | PathA21 | PathA22 | rA | P value |
|---|---|---|---|---|
| Weekend Sleep Duration | −0.14 [−0.24, −0.04] | 0.43 [0.27, 0.54] | −0.31 [−0.41, −0.15] | 0.002 |
| Weekday Sleep Duration | −0.04 [−0.15, 0.06] | 0.48 [0.35, 0.57] | −0.08 [−0.25, 0.17] | 0.321 |
rA represents the genetic correlation. All values were derived from the best fitting bivariate Cholesky decomposition. The A21 path signifies the A cross-path from the Cholesky model between cannabis and weekday/weekend sleep duration. The A22 path signifies the weekday/weekday sleep duration standardized A estimate. Squaring this value yields the heritability estimate. The p-value for the genetic associations was estimated via a 1-df chi-square difference test of the best fitting Cholesky cross path.
We then compared a potential causal model, with the onset of regular cannabis use predicting adult sleep duration, to our best fitting Cholesky decomposition model for both adult weekday and weekend sleep duration and there was no decrement in fit (all χ2diff (1) < 2.44, p > 0.118). The causal models demonstrated good fit (all χ2 (64) < 70.96, p > 0.257, RMSEA < 0.026) and are consistent with a causal relationship of onset of regular cannabis use predicting weekend sleep duration (β = −0.11, 95% CI = [−0.18, −0.03], χ2diff (1) = 11.78, p < 0.001) but the effect on weekday sleep duration was not significant (β = −0.04, 95% CI = [−0.11, 0.04], χ2diff (1) = 1.793, p = 0.181), which again was expected given the non-significant phenotypic association. Fig. 4 illustrates the best fitting causal model for onset of regular cannabis use and weekend sleep duration. We tested reverse causal models (sleep duration causing onset of cannabis use) against our best fitting Cholesky models and these comparisons did result in significant decrements in fit (all χ2diff (1) > 6.211, p < 0.013), suggesting that early monthly cannabis use predicts sleep duration and not the other way around. While these latter models are not plausible due to the time frames of the measures, they demonstrate that the study does have sufficient power to reject inappropriate causal models.
Fig. 4.
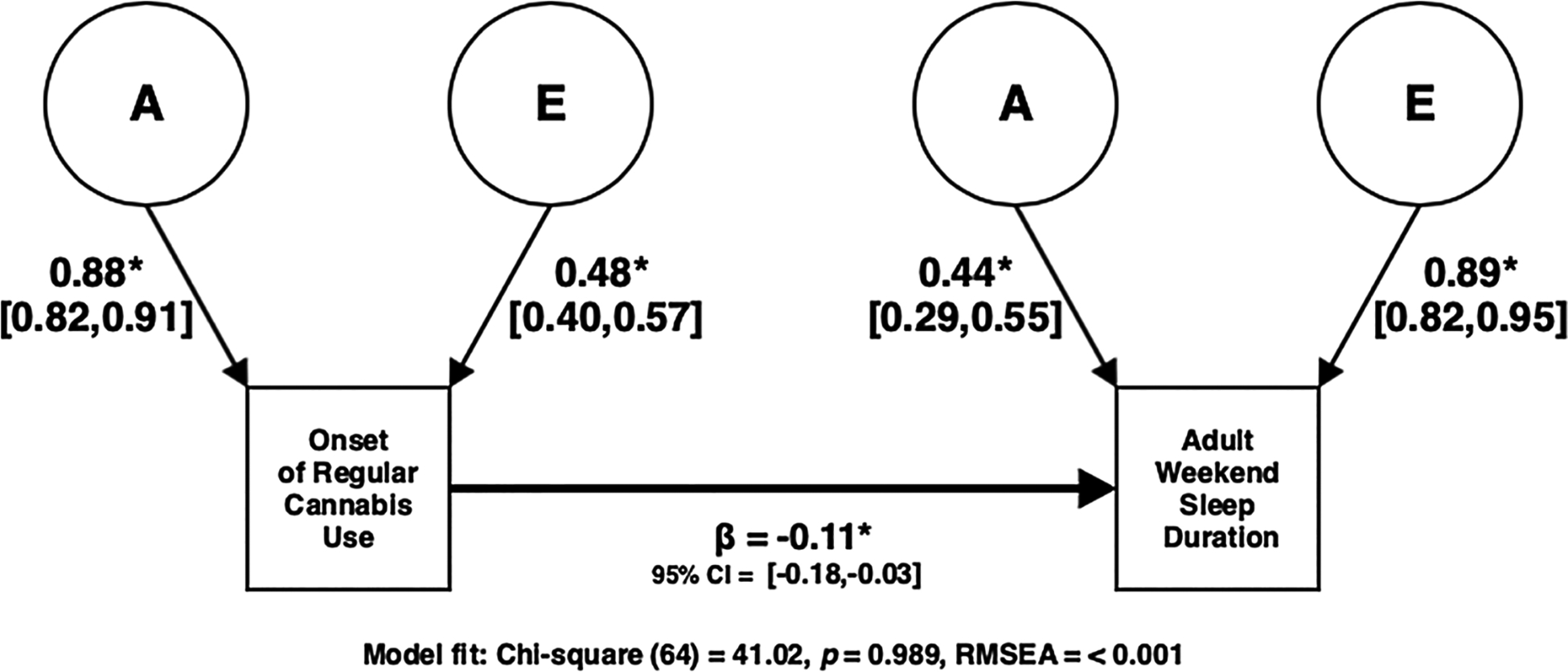
Best Fitting Causal Model (Collapsing Across Genetic and Environmental Influences) in which Onset of Regular Cannabis Use Predicts Adult Weekend Sleep Duration.
4. Discussion
We set out to uncover the etiological relationship between the onset of regular cannabis use and adult sleep duration. Earlier onset of regular cannabis use (continuous) was associated with shorter weekday and weekend sleep duration when controlling for sex, depression, and current alcohol/cannabis/tobacco use. Early onset of regular cannabis use (coded ordinally) was associated with shorter adult sleep duration on the weekends when controlling for sex, depression, and current alcohol/cannabis use. We found significant genetic and unique environmental contributions to the onset of regular cannabis use and adult weekend sleep duration independently. We discovered initial evidence of a significant overlapping additive genetic influence between onset of regular cannabis use and weekend sleep implying a common genetic liability, but ultimately a causal twin model comparison determined that onset of regular cannabis use predicting weekend sleep had the best fit and suggested that these results are consistent with causality.
Our variance contributions estimated for weekend sleep duration are consistent with previous adult twin studies (Genderson et al., 2013; Hublin et al., 2013; Watson et al., 2016) which have found that (unique) environment explains a much larger portion of the variance than additive genetics and that shared environment does not make a significant contribution. We are not aware of any twin studies specifically focusing on the onset of regular cannabis use and our findings of significant genetic contribution are consistent with studies of cannabis initiation (Verweij et al., 2010). Our univariate analysis is novel in that we assess the age at when individuals began using cannabis on a regular (at least monthly) basis and separate regular users from experimental or one-time users. Binary measures of initiation that do not utilize age are often listed as a limitation and problem to address in twin studies (Miles et al., 2001; Agrawal et al., 2004; Lessem et al., 2006; Fowler et al;, 2007). To our knowledge, this is the first twin study to specifically investigate the age of onset of regular cannabis use and find support of significant genetic influence.
Our findings contribute to the small collection of studies investigating the effects of early cannabis use on later sleep and are consistent with some (Chheda et al., 2014; Wong et al., 2009) but not all studies (Pasch et al., 2012; Hasler et al., 2017) suggesting that early cannabis use predicts later sleep deficits in adolescence and later adulthood. We extend these findings by providing evidence of a potentially causal relationship between the onset of regular cannabis use and shorter weekend sleep duration in adulthood. The temporal associations between these variables demonstrate possible long-lasting effects of early regular cannabis use on later sleep. Additionally, the study shows that early (regular) cannabis use is significantly associated with later sleep beyond current frequency of cannabis use and other known factors that are highly associated with sleep duration. Lastly, this is the first study to find evidence of a potential genetic correlation and common genetic liability between onset of regular cannabis use and weekend sleep duration, indicating either a pleiotropic influence on both traits or a causal influence of genetically influenced early regular cannabis use on adult sleep duration.
There are several possible explanations for this relationship. There could be developmental consequences of early cannabis use, such that early substance users might develop maladaptive behaviors or long-lasting sleep problems that continue into adulthood effecting sleep (Pasch et al., 2012). Another possibility is that early cannabis use could result in altered brain development that effects later sleep. Imaging studies have found both functional and structural alterations in the prefrontal cortex (PFC) for early cannabis users (Jacobus and Tapert, 2014) and there is evidence that sleep deprivation (Chee, 2004; Drummond et al., 2005; Yoo et al., 2007; Chee and Chuah, 2009; Simon et al., 2015), altered sleep patterns (Hasler et al., 2012a), and poor sleep quality (Tashjian et al., 2018) are associated with alterations in functional PFC connectivity with other parts of the brain and in networks involving the PFC. Additionally, there could be potential developmental disruptions in circadian rhythm as a result of early cannabis use, as there is evidence of circadian pathway disturbances resulting from substance use (Shibley et al., 2008; Hasler et al., 2012b, 2015).
Lastly, while our best fitting model was a casual model, there are limitations to how we interpret this result (Coventry and Keller, 2005) and we cannot reject the possibility of a common genetic liability between these traits, i.e. pleiotropic influence of genes on both traits that explains this relationship. Consistent with this common genetic liability theory, the endocannabinoid system is believed to be involved in the circadian sleep–wake cycle (Vaughn et al., 2010; Murillo-Rodriguez et al., 2011) and several large genome-wide association studies of lifetime cannabis use (Stringer et al., 2016; Gage et al., 2017; Pasman et al., 2018) have found significant genes associated that are believed to be involved circadian rhythm and sleep behaviors (Sato et al., 2004; Yan et al., 2008; Stadler et al., 2010; Tabuchi et al., 2013; Liu et al., 2014; Jiang et al., 2015; Young et al., 2016; Uetani et al., 2017; Yang et al., 2018). Additionally, a recent study found that several clock gene polymorphisms were significant risk factors for cannabis addiction (Saffroy et al., 2019). However, consistent with a potential causal relationship, there is evidence of chemicals in cannabis that change stability in circadian rhythm (Perron et al., 2001) and alter the expression of clock genes on a molecular level (Lafaye et al., 2018). Thus to explain the relationship between cannabis use and sleep, there is evidence for both common genetics and potential causal paths, and these mechanisms need not be mutually exclusive.
There are several limitations of this study. First, there are issues with the time specific validity of the cannabis and sleep measures that could have led to recall bias or error. This measurement error could be responsible for the poor fit of the univariate weekday sleep duration models. Second, a limitation of causal twin model analyses is that results can be misleading if the measures have large amounts of error, especially if one of the two measures has a considerably larger amount than the other. In such cases, the measure with more error is an unlikely candidate for a causal variable. While our best fitting model for adult weekend sleep duration had a high contribution of E (80%) (which could be seen as a concern for unreliability), prior twin studies on adult sleep duration have estimated similar patterns in variance contributions along with no contribution from the shared environment (Genderson et al., 2013; Hublin et al., 2013; Watson et al., 2016). Thus, based on the available literature this is not likely a major concern for error or unreliability in our study. Third, is the lack of a significant environmental correlation in our bivariate twin model for onset of regular cannabis use and weekend sleep duration. In the case of a causal effect, we would expect the bivariate twin model to show both genetic and environmental correlations. Even though the directional causal model was our best fitting and most parsimonious model by means of a χ2 difference test, the absence of a significant environmental correlation is a noteworthy limitation, possibly reflecting the power limitations of this study despite the substantial sample size. While these causal model comparisons provide additional and novel information, we cannot make conclusive or definitive statements regarding causality. We are confident in the results of our genetic association, but further research is needed to solidify the inference of a causal relationship for these traits. Fourth, is the possible role of tobacco in this relationship. The influence of the onset of regular cannabis use (as an ordinal variable) on weekend sleep remained significant when controlling for sex, current cannabis/alcohol use, and depression but not when tobacco use was included in the model. Frequency of tobacco use was correlated with onset of regular cannabis use and weekend sleep, and it is entirely plausible that tobacco could play a role in this relationship via possible moderation or mediation. Further research is needed to understand its influence on this association. Fifth, all of our measures of sleep, depression, and substance use were self-report measures and could be prone to report bias or memory discrepancies. Lastly, while our continuous mixed effects regression analysis controlled for several known sleep correlates, we did not control for other factors that may influence sleep such as pre-existing sleep conditions, additional forms of substance use, or other potential factors nor did we control for these covariates in our twin models. Forthcoming studies should consider appropriate controls in their analysis as well as specifications of measures to address the mentioned concerns.
5. Conclusion
Our data were consistent with the interpretation that there is a predictive and possibly causal relationship between early onset of regular cannabis use and sleep problems in adulthood, implying that shorter adult sleep duration is one of the growing developmental consequences for early cannabis use. Other implications include the possibility of overlapping genetics that drive this association and the need to explore genetic influences in this relationship more in depth. Education and prevention efforts should incorporate sleep loss as one of the possible side effects of early cannabis use.
Role of funding source
Supported by grants P60 DA011015, R01 DA042755, and T32 DA017637.
Footnotes
Ethical approval
Documented informed consent was obtained from all study participants and all procedures of this study followed the ethical standards of the University of Colorado Institutional Review Board.
Declaration of Competing Interest
No conflict Declared.
One twin pair was considered an outlier and was removed due to giving an implausible response on the age of first regular cannabis question. Removal from the data did not materially change any tests of significance, estimates, or conclusions.
References
- Agrawal A, Neale MC, Prescott CA, Kendler KS, 2004. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol. Med 34 (7), 1227–1237. 10.1017/S0033291704002545. [DOI] [PubMed] [Google Scholar]
- Alwan H, Viswanathan B, Rousson V, Paccaud F, Bovet P, 2011. Association between substance use and psychosocial characteristics among adolescents of the Seychelles. BMC Pediatr. 11 (1), 85 10.1186/1471-2431-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angarita GA, Emadi N, Hodges S, Morgan PT, 2016. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict. Sci. Clin. Pract 11 (1), 9 10.1186/s13722-016-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE, 2002. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 325 (7374), 1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, Muthén B, 2010. Weighted least squares estimation with missing data. Tech. Rep 1–10. [Google Scholar]
- Banks S, Dinges DF, 2007. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med 3 (05), 519–528. [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2014. Fitting Linear Mixed-effects Models Using lme4. arXiv preprint arXiv.1406.5823.. [Google Scholar]
- Boakye D, Wyse CA, Morales-Celis CA, Biello SM, Bailey MES, Dare S, Ward J, Gill JMR, Pell JP, Mackay DF, 2018. Tobacco exposure and sleep disturbance in 498 208 UK Biobank participants. J. Public Heal 40 (3), 517–526. 10.1093/pubmed/fdx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (CBHSQ), 2017. 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Chee MWL, 2004. Functional imaging of working memory after 24 hr of total sleep deprivation. J. Neurosci 24 (19), 4560–4567. 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Chuah LY, 2009. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr. Opin. Neurol 21 (4), 417–423. 10.1097/wco.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- Chen X, Yu B, Lasopa SO, Cottler LB, 2017. Current patterns of marijuana use initiation by age among US adolescents and emerging adults: implications for intervention. Am. J. Drug Alcohol Abuse 43 (3), 261–270. 10.3109/00952990.2016.1165239. [DOI] [PubMed] [Google Scholar]
- Chheda J, Chakravorty S, Grandner MA, 2014. Patterns of marijuana (cannabis) use and sleep symptoms in American adults. Sleep. 37, A286. [Google Scholar]
- Cottler LB, 2000. Composite International Diagnostic Interview—substance Abuse Module (SAM) Department of Psychiatry, Washington University School of Medicine, St. Louis, MO. [Google Scholar]
- Cottler LB, Robins LN, Helzer JE, 1989. The Reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br. J. Addict 84 (7), 801–814. 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Coventry WL, Keller MC, 2005. Estimating the extent of parameter bias in the classical twin design: a comparison of parameter estimates from extended twin-family and classical twin designs. Twin Res. Hum. Genet 8 (3), 214–223. 10.1375/1832427054253121. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Meloy MJ, Yanagi MA, Orff HJ, Brown GG, 2005. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. - Neuroimaging 140 (3), 211–223. 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ebin VJ, Sneed CD, Morisky DE, Rotheram-Borus MJ, Magnusson AM, Malotte CK, 2001. Acculturation and interrelationships between problem and health-pro-moting behaviors among Latino adolescents. J. Adolesc. Heal 28 (1), 62–72. 10.1016/S1054-139X(00)00162-2. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR, 1999. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl.) 142 (3), 295–301. 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Martino SC, Collins RL, 2004. Marijuana use from adolescence to young adulthood: multiple developmental trajectories and their associated outcomes. Heal. Psychol 23 (3), 299 10.1037/0278-6133.23.3.299. [DOI] [PubMed] [Google Scholar]
- Fakier N, Wild LG, 2011. Associations among sleep problems, learning difficulties and substance use in adolescence. J. Adolesc 34 (4), 717–726. 10.1016/j.adolescence.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A, 2011. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 34 (5), 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Bressan RA, Lacerda ALT, 2011. Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry 198 (6), 442–447. 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Ford ES, Cunningham TJ, Croft JB, 2015. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 38 (5), 829–832. 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, McBride A, Van Den Bree MBM, 2007. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 102 (3), 413–422. 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Brugha T, Meltzer H, Jenkins R, Stahl D, Bebbington P, 2010. Persecutory ideation and insomnia: findings from the second british national survey of psychiatric morbidity. J. Psychiatr. Res 44 (15), 1021–1026. 10.1016/j.jpsychires.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Jones HJ, Burgess S, Bowden J, Davey Smith G, Zammit S, Munafò MR, 2017. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol. Med. (Paris) 47 (5), 971–980. 10.1017/S0033291716003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B, 2009. Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res 18 (2), 148–158. 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Genderson MR, Rana BK, Panizzon MS, Grant MD, Toomey R, Jacobson KC, Xian H, Cronin-Golomb A, Franz CE, Kremen WS, Lyons MJ, 2013. Genetic and environmental influences on sleep quality in middle-aged men: a twin study. J. Sleep Res 22 (5), 519–526. 10.1111/jsr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozier N, Martiniuk A, Patton G, Ivers R, Li Q, Hickie I, Senserrick T, Woodward M, Norton R, Stevenson M, 2010. Short sleep duration in prevalent and persistent psychological distress in young adults: the DRIVE study. Sleep. 33 (9), 1139–1145. 10.1093/sleep/33.9.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Rijsdijk FV, Eley TC, 2006. A twin-study of sleep difficulties in school-aged children. Child Dev. 77 (6), 1668–1679. 10.1111/j.1467-8624.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE, 2012. Age of onset of marijuana use and executive function. Psychol. Addict. Behav 26 (3), 496 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR, 2012bb. Circadian rhythms, sleep, and substance abuse. Sleep Med. Rev 16 (1), 67–81. 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Phillips ML, Forbes EE, 2012aa. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol. Psychol 91 (3), 334–341. 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Franzen PL, de Zambotti M, Prouty D, Brown SA, Tapert SF, Pfefferbaum A, Pohl KM, Sullivan EV, De Bellis MD, Nagel BJ, Baker FC, Colrain IM, Clark DB, 2017. Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the national consortium on alcohol and neurodevelopment in adolescence study. Alcohol. Clin. Exp. Res 41 (6), 1154–1165. 10.1111/acer.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, Clark DB, 2015. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 49 (4), 377–387. 10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS, 1993. Testing hypotheses about direction of causation using cross-sectional family data. Behav. Genet 23 (1), 29–50. 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF, 2002. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 5 (2), 113–124. 10.1375/twin.5.2.113. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ, 2015. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Heal. 1 (1), 40–43. 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, 1998. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol. Methods 3 (4), 424 10.1037/1082-989X.3.4.424. [DOI] [Google Scholar]
- Hublin C, Partinen M, Koskenvuo M, Kaprio J, 2013. Genetic factors in evolution of sleep length - a longitudinal twin study in Finnish adults. J. Sleep Res 22 (5), 513–518. 10.1111/jsr.12051. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF, 2014. Effects of cannabis on the adolescent brain. Curr. Pharm. Des 20 (13), 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R, Jessor SL, 1977. Problem Behavior and Psychosocial Development: a Longitudinal Study of Youth. Acad. Press. [Google Scholar]
- Jiang P, Scarpa JR, Fitzpatrick K, Losic B, Gao VD, Hao K, Summa KC, Yang HS, Zhang B, Allada R, Vitaterna MH, Turek FW, Kasarskis A, 2015. A Systems Approach Identifies Networks and Genes Linking Sleep and Stress: Implications for Neuropsychiatric Disorders. Cell Rep. 11 (5), 835–848. 10.1016/j.celrep.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonoff H, Clark C, 1976. Drug patterns in the chronic marijuana user. Subst. Use Misuse 11 (1), 71–80. 10.3109/10826087109045531. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Collop NA, 2006. Gender differences in sleep disorders. Curr. Opin. Pulm. Med 12 (6), 383–389. 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T, 2009. Self-reported sleep duration and cognitive functioning in the general population. J. Sleep Res 18 (4), 436–446. 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Lafaye G, Desterke C, Marulaz L, Benyamina A, 2018. Cannabidiol affects circadian clock core complex and its regulation in microglia cells. Addict. Biol 24 (5), 921–934. 10.1111/adb.12660. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, 2014. Survey questions about sleep duration: does asking separately about weekdays and weekends matter? Behav. Sleep Med 12 (2), 158–168. 10.1080/15402002.2013.778201. [DOI] [PubMed] [Google Scholar]
- Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, Hewitt JK, 2006. Relationship between adolescent marijuana use and young adult illicit drug use. Behav. Genet 36 (4), 498–506. 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- Lian Y, Xiao J, Liu Y, Ning L, Guan S, Ge H, Li F, Liu J, 2015. Associations between insomnia, sleep duration and poor work ability. J. Psychosom. Res 78 (1), 45–51. 10.1016/j.jpsychores.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Liu S, Lamaze A, Liu Q, Tabuchi M, Yang Y, Fowler M, Bharadwaj R, Zhang J, Bedont J, Blackshaw S, Lloyd TE, Montell C, Sehgal A, Koh K, Wu MN, 2014. WIDE AWAKE mediates the circadian timing of sleep onset. Neuron. 82 (1), 151–166. 10.1016/j.neuron.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS, 2011. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev. Med. (Baltim). 53 (4–5), 271–273. 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Christakis NA, Fowler JH, 2010. The spread of sleep loss influences drug use in adolescent social networks. PLoS One 5 (3), e9775 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DR, Van den Bree MBM, Gupman AE, Newlin DB, Glantz MD, Pickens RW, 2001. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug Alcohol Depend. 62 (1), 57–68. 10.1016/S0376-8716(00)00165-4. [DOI] [PubMed] [Google Scholar]
- Minică CC, Dolan CV, Boomsma DI, de Geus E, Neale MC, 2018. Extending causality tests with genetic instruments: an integration of mendelian randomization with the classical twin design. Behav. Genet 48 (4), 337–349. 10.1007/s10519-018-9904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, 2007. Mplus: Statistical Analysis With Latent Variables (Version 7.31).
- Murillo-Rodriguez E, Poot-Ake A, Arias-Carrion O, Pacheco-Pantoja E, Fuente-Ortegon Ade L., Arankowsky-Sandoval G, 2011. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent. Nerv. Syst. Agents Med. Chem 11 (3), 189–196. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR, 2013. Methodology for Genetic Studies of Twins and Families. Methodology for Genetic Studies of Twins and Families. 10.1007/978-94-015-8018-2. [DOI] [Google Scholar]
- Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H, 1983. Genetic and environmental determination of human sleep. Sleep. 6 (3), 179–185. 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA, 2012. Longitudinal Bi-directional relationships between sleep and youth substance use. J. Youth Adolesc 41 (9), 1184–1196. 10.1007/s10964-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, Abdellaoui A, Nivard MG, Baselmans BML, Ong JS, Ip HF, van der Zee MD, Bartels M, Day FR, Fontanillas P, Elson SL, de Wit H, Davis LK, MacKillop J, Derringer JL, Branje SJT, Hartman CA, Heath AC, van Lier PAC, Madden PAF, et al. , 2018. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat. Neurosci 21 (9), 1161 10.1038/s41593-018-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron RR, Tyson RL, Sutherland GR, 2001. Δ9-Tetrahydrocannabinol increases brain temperature and inverts circadian rhythms. Neuroreport. 12 (17), 3791–3794. 10.1097/00001756-200112040-00038. [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas 19, 340–356. 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP, 2006. Colorado twin registry. Twin Res. Hum. Genet 9 (6), 941–949. 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- Roane BM, Taylor DJ, 2008. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 31 (10), 1351–1356. [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T, 2001. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med. Rev 5 (4), 287–297. 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Roepke SE, Duffy JF, 2010. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat. Sci. Sleep 2, 213 10.2147/NSS.S12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffroy R, Lafaye G, Desterke C, Ortiz-Tudela E, Amirouche A, Innominato P, Innominato P, Benyamina A, Lemoine A, 2019. Several clock genes polymorphisms are meaningful risk factors in the development and severity of cannabis addiction. Chronobiol. Int 36 (1), 122–134. 10.1080/07420528.2018.1523797. [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, Fitzgerald GA, Kay SA, Hogenesch JB, 2004. A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron. 43 (4), 527–537. 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Schubart CD, Van Gastel WA, Breetvelt EJ, Beetz SL, Ophoff RA, Sommer IEC, Kahn RS, Boks MPM, 2011. Cannabis use at a young age is associated with psychotic experiences. Psychol. Med. (Paris) 41 (6), 1301–1310. 10.1017/S003329171000187X. [DOI] [PubMed] [Google Scholar]
- Shibley HL, Malcolm RJ, Veatch LM, 2008. Adolescents with insomnia and substance abuse: consequences and comorbidities. J. Psychiatr. Pract 14 (3), 146–153. 10.1097/01.pra.0000320113.30811.46. [DOI] [PubMed] [Google Scholar]
- Simon EB, Oren N, Sharon H, Kirschner A, Goldway N, Okon-Singer H, Tauman R, Deweese MM, Keil A, Hendler T, 2015. Losing neutrality: the neural basis of impaired emotional control without sleep. J. Neurosci 35 (38), 13194–13205. 10.1523/jneurosci.1314-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler F, Schmutz I, Schwaller B, Albrecht U, 2010. Lack of calbindin-D28K alters response of the murine circadian clock to light. Chronobiol. Int 27 (1), 68–82. 10.3109/07420521003648554. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Peacey V, Wardle J, 2006. Sleep duration and health in young adults. Arch. Intern. Med 166 (16), 1689–1692. 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- Stringer S, Minică CC, Verweij KJH, Mbarek H, Bernard M, Derringer J, van Eijk KR, Isen JD, Loukola A, Maciejewski DF, Mihailov E, van der Most PJ, Sánchez-Mora C, Roos L, Sherva R, Walters R, Ware JJ, Abdellaoui A, Bigdeli TB, Branje SJT, Brown SA, Bruinenberg M, Casas M, Esko T, Garcia-Martinez I, et al. , 2016. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis consortium. Transl. Psychiatry 6 (3), e769 10.1038/tp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi S, Tsunematsu T, Kilduff TS, Sugio S, Xu M, Tanaka KF, Takahashi S, Tominaga M, Yamanaka A, 2013. Influence of inhibitory serotonergic inputs to Orexin/Hypocretin neurons on the diurnal rhythm of sleep and wakefulness. Sleep. 36 (9), 1391–1404. 10.5665/sleep.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian SM, Goldenberg D, Monti MM, Galván A, 2018. Sleep quality and adolescent default mode network connectivity. Soc. Cogn. Affect. Neurosci 13 (3), 290–299. 10.1093/scan/nsy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, RC, 2018. R: a Language and Environment for Statistical Computing.
- Torvik FA, Rosenström TH, Gustavson K, Ystrom E, Kendler KS, Bramness JG, Czajkowski N, Reichborn-Kjennerud T, 2019. Explaining the association between anxiety disorders and alcohol use disorder: a twin study. Depress. Anxiety 36 (6), 522–532. 10.1002/da.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K, 2005. Sleep and depression. J. Clin. Psychiatry 66 (10), 1254–1269. 10.4088/JCP.v66n1008. [DOI] [PubMed] [Google Scholar]
- Uetani N, Hardy S, Gravel S-P, Kiessling S, Pietrobon A, Wong NN, Chénard V, Cermakian N, St-Pierre J, Tremblay ML, 2017. PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms. JCI Insight. 2 (13), e91722 10.1172/jci.insight.91722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn LK, Denning G, Stuhr KL, De Wit H, Hill MN, Hillard CJ, 2010. Endocannabinoid signalling: has it got rhythm? Br. J. Pharmacol 160 (3), 530–543. 10.1111/j.1476-5381.2010.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Estabrook R, 2012. Using genetic information to test causal relationships in cross-sectional data. J. Theor 24 (3), 328–344. 10.1177/0951629812439348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM, 2010. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 105 (3), 417–430. 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Horn E, Duncan GE, Buchwald D, Vitiello MV, Turkheimer E, 2016. Sleep duration and area-level deprivation in twins. Sleep. 39 (1), 67–77. 10.5665/sleep.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RA, Halikas JA, 1982. Change in effects from marijuana: a five- to six-year follow-up. J. Clin. Psychiatry 43 (9), 362–365. [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA, 2004. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol. Clin. Exp. Res 28 (4), 578–587. 10.1097/01.ALC.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Zucker RA, 2009. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 10 (7), 787–796. 10.1016/j.sleep.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2018. Management of Substance Abuse Cannabis. [internet] Accessed on September 30, 2018 Available from:. WHO, Geneva: http://www.who.int/substance_abuse/facts/cannabis/en/. [Google Scholar]
- Yan J, Wang H, Liu Y, Shao C, 2008. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput. Biol 4 (10), e1000193 10.1371/journal.pcbi.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Baek JH, Cho Y, Cho EY, Choi Y, Kim Y, Park T, Hong KS, 2018. Effects of genetic variants of ST8SIA2 and NCAM1 genes on seasonal mood changes and circadian preference in the general population. Chronobiol. Int 35 (3), 405–415. 10.1080/07420528.2017.1410827. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP, 2007. The human emotional brain without sleep - a prefrontal amygdala disconnect. Curr. Biol 17 (20), R877–R878. 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Young AI, Wauthier F, Donnelly P, 2016. Multiple novel gene-by-environment interactions modify the effect of FTO variants on body mass index. Nat. Commun 7, 12724 10.1038/ncomms12724. [DOI] [PMC free article] [PubMed] [Google Scholar]


