Abstract
Background and purpose:
Doxorubicin (DOX) is an effective agent for the treatment of many neoplastic diseases. Cardiotoxicity is the major side effect of this drug and limits its use. Vanillic acid (VA) is a pharmaceutical compound from the phenolic acids family. The present study is an attempt to investigate the possible helpful effects of VA against DOX-induced cardiotoxicity in rats.
Experimental approach:
For induction of cardiotoxicity, male Wistar rats received total of six doses of DOX (2.5 mg/kg i.p.) three times per week from days 14 to 28. Treatment groups received daily oral doses of VA (10, 20, and 40 mg/kg) two weeks before DOX injection and then plus DOX for 2 weeks. At the end of experiment, systolic blood pressure (SBP) and heart rate (HR) were detected using tail-cuff method. Lactate dehydrogenase (LDH), creatine phosphokinase-MB (CK-MB), serum glutamic oxaloacetic transaminase (SGOT), malondialdehyde (MDA), and ferric reducing antioxidant power (FRAP) were measured in serum samples. Troponin-I and toll-like receptor 4 (TLR4) were measured in cardiac tissue. All the measurements processed spectrophotometrically using commercial ELISA kits. Cardiac tissue was also processed for histopathological examination.
Findings / Results:
Treatment with VA significantly increased SBP compared to the DOX group and restored HR near to the normal level. Administration of VA at all of doses, decreased serum levels of LDH, SGOT, CK-MB, MDA, cardiac troponin-I, cardiac TLR4 and increased FRAP value.
Conclusion and implications:
These results suggest that VA may exert cardioprotective effects against DOX-induced cardiotoxicity by decreasing oxidative stress and biomarkers of cardiotoxicity, suppression of TLR4 signaling and consequently inflammation pathway.
Keywords: Antioxidant, Cardiotoxicity, Doxorubicin, TLR4, Vanillic acid
INTRODUCTION
Doxorubicin (DOX) is a powerful, well-established, and highly efficacious drug in many kinds of cancers like solid tumors, leukemia, soft tissue sarcoma, breast cancer, small cell carcinoma of the lung, and esophageal carcinoma. However its clinical usefulness is still restricted due to the specific toxicities in cardiac tissues (1). Cardiomyopathy and heart failure are dose- dependent adverse effects of DOX administration (2). The possible mechanisms proposed for cardiotoxic effects of DOX include free radical-induced myocardial injury, lipid peroxidation (3), mitochondrial damage (4) decreased activity of Na+-K+ adenosine triphosphate (5), release of vasoactive amines (6), increased oxidative stress including release of free radicals, such as super oxide anions (7) and other reactive oxygen intermediates as well as endogenous antioxidant deficits (8,9).
Several Toll-like receptors (TLRs) are expressed in cardiomyocytes, including TLR2 and TLR4. These TLRs respond to endogenous and exogenous signals and lead to pathophysiological changes during cardiomyopathy (10). TLR4 signaling induces nuclear factor kappa B (NF-kβ) expression and activation which is contributed to the release of pro-inflammatory cytokines such as, interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-a (TNF-α) in cardiac tissue (11). The roles of TLR, in this process-in particular TLR4 as heart failure was completely unobserved in TLR4-knockout mice treated with DOX (12). Therefore the inhibition of TLR4/NF-kβ signaling has been suggested as one of the most important mechanisms against DOX-induced cardiotoxicity. Increased generation of reactive oxygen species (ROS) is recognized as the underlying cause for DOX-induced cardiotoxicity, prevention of the cardiotoxicity using a variety of natural or synthetic compounds have been investigated. Probucol and statins as lipid lowering drugs, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers have prevented DOX-mediated cardiotoxicity and heart failure without compromising the antitumor property of the drug. Several natural compounds such as vitamins, resveratrol, oleuoprein, polyphenols, and flavonoids have been investigated as adjunct therapies to prevent DOX-induced cardiotoxicity (13). Meta-analysis from seven randomized clinical trials suggested beneficial role of dexrazoxane (DEX) in reducing DOX- induced cardiotoxicity in breast cancer (14). The use of DEX is recommended as cardioprotective effect in advanced or metastatic breast cancer who has already received a minimum cumulative dose of 300 mg/m2 of DOX (15). DEX, a cyclic derivative of ethylenediaminetetraacetic acid, has the ability to chelate iron and hence reduces the formation of ROS. It is the only cardioprotective drug licensed in USA, Canada, and Europe for reduction of DOX-induced cardiotoxicity and clearly provides long-term cardioprotection without compromising anthracyclines antitumor efficacy (14,15). In recent years, there is a growing interest in the usage of natural antioxidants as a protective strategy against cardiovascular-related problems such as heart failure and drug-induced cardiotoxicity. Some studies have demonstrated the protective effects of antioxidants such as polyphenolic compounds in DOX-induced cardiotoxicity without reducing the efficacy of this antineoplastic agent (16).
Vanillic acid (VA) is a natural compound of phenolic acids family. It is a form of vanillin oxide and is produced when vanillin converted to ferulic acid. VA has several pharmacological effects including anti-metastatic (17), anti-melanogenesis (18), antioxidant, anti-angiogenesis (19), and anti-apoptotic effects (20). Recent study has shown the cardioprotective effect of VA in ischemia-reperfusion through decreasing oxidative stress and improving myocardial dysfunction (21). Other investigations revealed some phenolic acid such as ferulic acid suppressed TLR4-induced inflammatory responses against acetaminophen-induced liver injury (22). Therefore, the present attempt has been made to investigate the possible cardioprotective effects of VA against DOX-induced cardiotoxicity.
MATERIALS AND METHODS
Chemicals
DOX was purchased from Tehran Darou Pharmaceutical Co. (Tehran, I.R. Iran). VA and DEX were obtained from Merck Co. (Darmstadt, Germany). The standard kits for analyzing of lactate dehydrogenase (LDH), creatine phosphokinase-MB (CK-MB), serum glutamic oxaloacetic transaminase (SGOT) were obtained from Pars Azmun Co. (Tehran, I.R. Iran). The assay kits for evaluation of oxidative stress including malondialdehyde (MDA) and ferric reducing antioxidant power (FRAP) were purchased from Hakiman Shargh Research Co. (Isfahan, I.R. Iran). Rat TNNI3/cardiac troponin-I enzyme-linked immunosorbent assay (ELISA) kit was obtained from MyBioSource Inc. (San Diego, USA). TLR4 ELISA kit was obtained from AVIVA Systems Biology Co. (San Diego, USA).
Animals and treatment
Male Wistar albino rats weighing 200 ± 20 g were obtained from the animal house of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences, I.R. Iran. The animals had free access to water and standard rodent diet and were kept under standard laboratory condition including room temperature of 20-25 °C and a 12/12-h light/dark cycle. Rats were acclimatized for 1 week before the experiment. All animal experiments were approved by the Ethics Committee of Isfahan University of Medical Sciences (Ethical approval ID: IR.MUI.REC.1396.3.573) and performed in accordance with National Institute of Health Guide for the Care and Use of Laboratory Animals. In this study, thirty-six rats were randomly divided in to 6 groups. Group 1, animals received total of six doses of DOX (2.5 mg/kg i.p.) three times per week from days 14 to 28; groups 2 to 4 pretreated with daily doses of VA (10, 20, and 40 mg/kg/day, p.o.) for 14 days and then received DOX simultaneously for 2 weeks; group 5 (reference) received DEX at 50 mg/kg i.p., 30 min before DOX administration; and group 6 (normal control) received single oral daily doses of normal saline (NS) 1 mL/kg. The body weight was measured on alternative days. Twenty-four h after the last day of experiment, blood samples were taken from orbital sinus plexus under mild anesthesia and serum was collected for biochemical analysis. After scarification, the hearts were separated and weighted out. Small pieces of heart were kept in 10% neutral buffered formalin solution and were further processed for histopathological examinations and other pieces were first freezed in liquid nitrogen for 10 min and then kept in a deep freezer (-70 °C) until analyzed for troponin-I and TLR4.
Measurement of systolic blood pressure and heart rate
On the first and last days of experiment, systolic blood pressure (SBP) and heart rate (HR) was measured using noninvasive tail cuff method (AD Instrument Power Lab Data Acquisition System, Australia) in rats. Three days before the last treatment, the training of rats in different groups for SBP and HR measurements was started. This training consisted of the regular handling of the animals and getting used to the restraining cage with a cuff around the end of proximal of the tail. After placing of the cuff, a pulse transducer was used around the end of the tail. Then the tail cuff was inflated using the related button on the apparatus and data were performed by the computerized system power lab. The mean values of the six SBP and HR records were used for each animal.
Biochemical analysis
The serum levels of LDH, SGOT, and CK-MB as the sensitive but nonspecific biomarkers for detection of cardiotoxicity were spectrophotometrically measured using commercial ELISA kits. The biomarkers expressed as enzyme unit activity (U) per 1 mL of sample (23).
Measurement of cardiac troponin-I
Cardiac troponin-I levels were estimated by rat TNNI3/cardiac troponin-I ELISA kit. Anti-cTn-I/TNNI3 antibody was pre-coated onto 96-well plates. According to the manufacturer’s protocol, briefly, the standards, test samples, antibody, and the kit reagents were added to the wells. After adding stop solution the color changed. The density of final color product was proportional to the cTn-I/TNNI3 amounts of samples captured in the plate. Spectrophotometric absorbance was read at 450 nm and the concentrations of cTn-I/TNNI3 were calculated (24).
Measurement of cardiac TLR4
Cardiac TLR4 levels were measured by rat TLR4 ELISA kit. In the following kit manufacturer’s instructions, the specific antibody had been pre-coated onto a 96-well plate and the standards, test samples and the kit reagents were added to the wells. Finally after adding stop solution, the color density was calculated as the sum of absorbance at 450 nm, and it was quantitatively proportional to the TLR4 amounts of samples captured in the wells (25).
Measurement of oxidative stress markers
The level of lipid peroxidation was indicated by the content of MDA. Serum MDA content was determined using the thiobarbituric acid reactive substance assay that is reacted with MDA. According to the kit manufacturer’s instructions, briefly, serum and kit reagents were added and the absorbance of the samples was read at 532 nm by a spectrophotometer (26). Total antioxidant capacity of serum samples indicated by FRAP value and it was measured based on the reduction of ferric- tripyridyltriazine complex to ferrous form using a standard assay kit. Briefly, the FRAP reagent was added to plasma samples and incubated for 40 min in 40 °C. The absorbance of colored samples was read at 570 nm using a spectrophotometer/microplate reader. The values were calculated against the standard curve of FeSO4.7H2O concentrations and expressed as micromole of ferrous ion equivalents per liter (27).
Histopathological examination
The fixed heart tissue specimens in 10% neutral buffer formalin solution were additionally processed for histopathologic assessment. Then, five μm-thick sections were prepared from tissue paraffin block and stained with hematoxylin and eosin (H&E) for pathological evaluation under light microscopic examinations.
Statistical analysis
Data are presented as mean ± standard error of the means (SEM). One-way analysis of variance (ANOVA) followed by post hoc Tukey test using the Statistical Package for the Social Sciences (SPSS software V. 20.0) were performed for statistical analysis. P values < 0.05 were considered as the significant level.
RESULTS
Effect of vanillic acid on systolic blood pressure and heart rate
Significant decrease in SBP and HR was observed in DOX group compared to the NS control group on day 28 (P < 0.001). As shown in Table 1, all doses of VA significantly increased the SBP. It also reversed the HR near to normal value at the doses of 20 and 40 mg/kg. However DEX had no valuable effect on SBP and HR.
Table 1.
Effect of VA and DEX on SBP and HR in DOX-induced cardiotoxicity at day 1 and 28. Values are mean ± SEM, n = 6.
| Groups (mg/kg) | SBP 1 (mmHg) | SBP 28 (mmHg) | P value | HR 1 (bpm) | HR 28 (bpm) | P value |
|---|---|---|---|---|---|---|
| Normal saline | 121.9 ± 2.3 | 122.5 ± 4.5 | 0.54 | 342 ± 14.3 | 339 ± 16.1 | 0.16 |
| DOX | 121.3 ± 2.7 | 88 ± 4.1### | 0.000 | 360 ± 20.1 | 276 ± 15.5### | 0.000 |
| DEX + DOX | 118.9 ± 4.5 | 87± 2.3 | 1.2 | 365 ± 17.2 | 280 ± 14.8 | 2.2 |
| VA 10 + DOX | 124 ± 3.2 | 99 ± 3.7* | 0.03 | 350 ± 16 | 278 ±17.3 | 1.2 |
| VA 20 + DOX | 117.5 ± 3.7 | 109 ± 4.9*** | 0.000 | 355 ± 20.1 | 319.4 ± 17*** | 0.000 |
| VA 40 + DOX | 122.3 ± 4.1 | 114 ± 3.1*** | 0.000 | 350 ±15.3 | 334 ±13.3*** | 0.000 |
VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; SBP, systolic blood pressure, HR, heart rate; ###P < 0.001 indicates significant differences versus normal saline control; and *P < 0.05 and ***P < 0.001 versus DOX group.
Effect of vanillic acid on serum levels of cardiac enzyme biomarkers
The activities of LDH, SGOT, and CK-MB were significantly increased in DOX group (P < 0.001). Treatment with DEX (50 mg/kg) and VA at all doses significantly decreased the serum levels of these non-specific cardiotoxicity biomarkers (Table 2).
Table 2.
Effect of VA and DEX on serum level of cardiac enzyme biomarkers. Values are mean ± SEM, n = 6.
| Groups (mg/kg) | LDH activity (IU/L) | SGOT activity (IU/L) | CK-MB activity(IU/L) |
|---|---|---|---|
| Normal saline | 114 ± 15.9 | 67 ± 4.8 | 191 ± 25.2 |
| DOX | 378 ± 32.56### | 93 ± 7.8### | 448 ± 33.1### |
| DEX + DOX | 200 ± 30.1*** | 65 ± 10*** | 240 ±16.2*** |
| VA10 + DOX | 277 ± 22*** | 87 ± 5.6** | 275 ± 28* |
| VA20 + DOX | 221 ± 18*** | 76 ±10.3*** | 204 ± 30*** |
| VA40 + DOX | 211 ± 25*** | 65 ± 5.8*** | 202 ± 48*** |
VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; LDH, lactate dehydrogenase; SGOT, serum glutamic oxaloacetic transaminase; CK-MB, creatine phosphokinase-MB; ###P < 0.001 indicates significant differences in comparison with normal saline control, *P < 0.05, **P < 0.01, and ***P < 0.001 versus DOX group
Effect of vanillic acid on cardiac troponin-I
Cardiac troponin-I levels were measured in cardiac samples as a specific marker of cardiac injury by ELISA method. DOX administration significantly increased cardiac troponin-I levels compared to the NS control group (P < 0.001). Treatment with VA at all doses significantly decreased cardiac troponin-I levels. DEX also brought back this indicator to near normal levels (P < 0.001, Fig. 1).
Fig. 1.
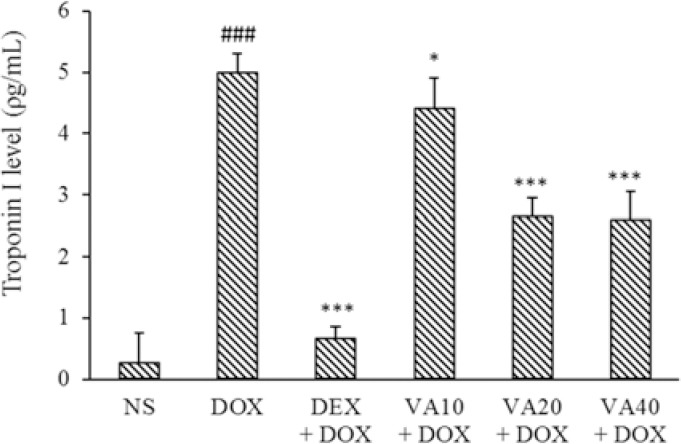
Effects of VA (10, 20, and 40 mg/kg) and DEX (50mg/kg) on cardiac levels of troponin-I in DOX-induced cardiotoxicity in rat. Values are mean ± SEM; n = 6. ###P < 0.001 Indicates significant differences compared to NS as a control group; and *P < 0.05 and ***P < 0.001 versus DOX group. VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; NS, normal saline.
Effect of vanillic acid on TLR4
TLR4 is expressed in cardiomyocytes and leads to the pathophysiological changes during cardiomyopathy. There was significant elevation of cardiac TLR4 levels in DOX-treated group compared with normal control rats (P < 0.001). Administration of VA at all doses significantly decreased the cardiac TLR4 levels compared to DOX group. DEX also significantly reduced this indicator (P < 0.001, Fig. 2).
Fig. 2.
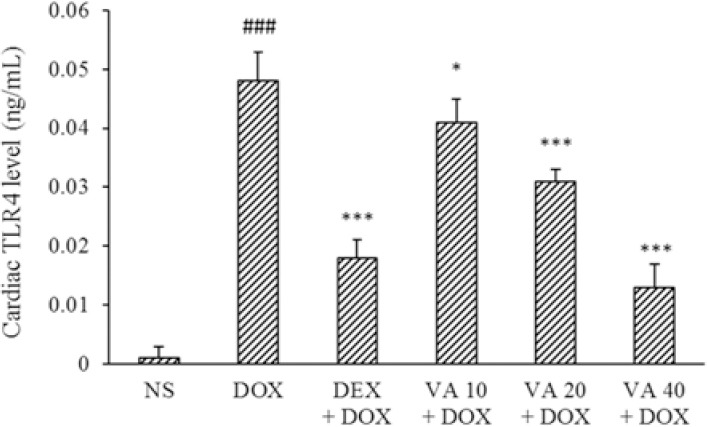
Effects of VA (10, 20, and 40 mg/kg) and DEX on cardiac TLR4 levels in DOX-induced cardiotoxicity. Values are mean ± SEM; n = 6. ###P < 0.001 Indicates significant differences compared with NS as control group; and *P < 0.05 and ***P < 0.001 versus DOX group. VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; NS, normal saline; TLR4, toll-like receptor4.
Effect of vanillic acid on lipid peroxidation
MDA concentrations were measured in serum samples as an indicator of lipid peroxidation. The serum levels of MDA were significantly increased in DOX-induced cardiotoxicity compared to the normal control group (P < 0.001). Treatment with VA at 20 and 40 mg/kg significantly decreased serum levels of MDA compared to the DOX group. DEX also brought back this indicator to near normal levels (P < 0.001, Fig. 3).
Fig. 3.
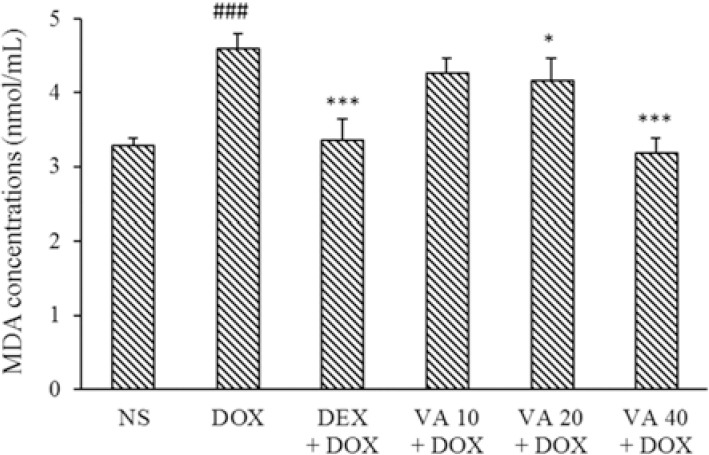
Effect of VA (10, 20, and 40 mg/kg) on serum MDA concentrations in DOX-induced cardiotoxicity in rats. Values are mean ± SEM; n = 6. ###P < 0.001 Shows significant differences in comparison with NS control; and *P < 0.05 and ***P < 0.001 versus DOX group. VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; NS, normal saline; MDA; serum malondialdehyde.
Ferric reducing antioxidant power assay
FRAP method was used for evaluation of total antioxidant capacity of VA extract. There was significant reduction in the FRAP value in DOX-induced cardiotoxicity in rats compared to the NS control group (P < 0.001). Administration of VA at all doses and DEX (50 mg/kg) significantly increased FRAP value compared to the DOX group (P < 0.001, Fig. 4).
Fig. 4.
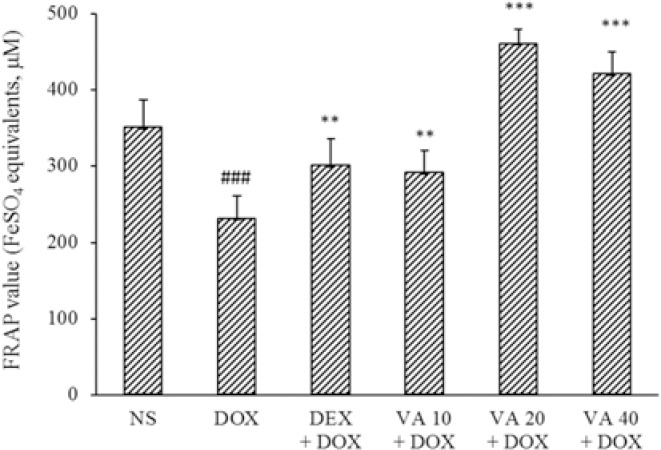
Effects of VA (10, 20, and 40 mg/kg) and DEX (50 mg/kg) on serum FRAP value in DOX-induced cardiotoxicity. Values are mean ± SEM; n = 6. ###P < 0.001 indicates significant differences in comparison with NS control, and **P < 0.01 and ***P < 0.001 versus DOX group. VA, Vanillic acid; DEX, dexrazoxane; DOX, doxorubicin; NS, normal saline; FRAP, ferric reducing antioxidant power.
Effect of vanilic acid on heart histopathology
Histopathological examination of cardiac sections of DOX-treated animals (Fig. 5) showed (A1) rupture of cardiac muscle fibers, (A2) hemorrhage, (A3) myocyte wavy degeneration, (A4) necrosis and inter-fibrillar congestion; (B) DEX group showed mild hemorrhage; (C) treatment with VA at dose of 10 mg/kg showed mild inter-fibrillar congestion and mild wavy degeneration. However treatment with (D and E) VA at 20 and 40 mg/kg as (F) NS control rats group showed normal architecture of the myocardium.
Fig. 5.
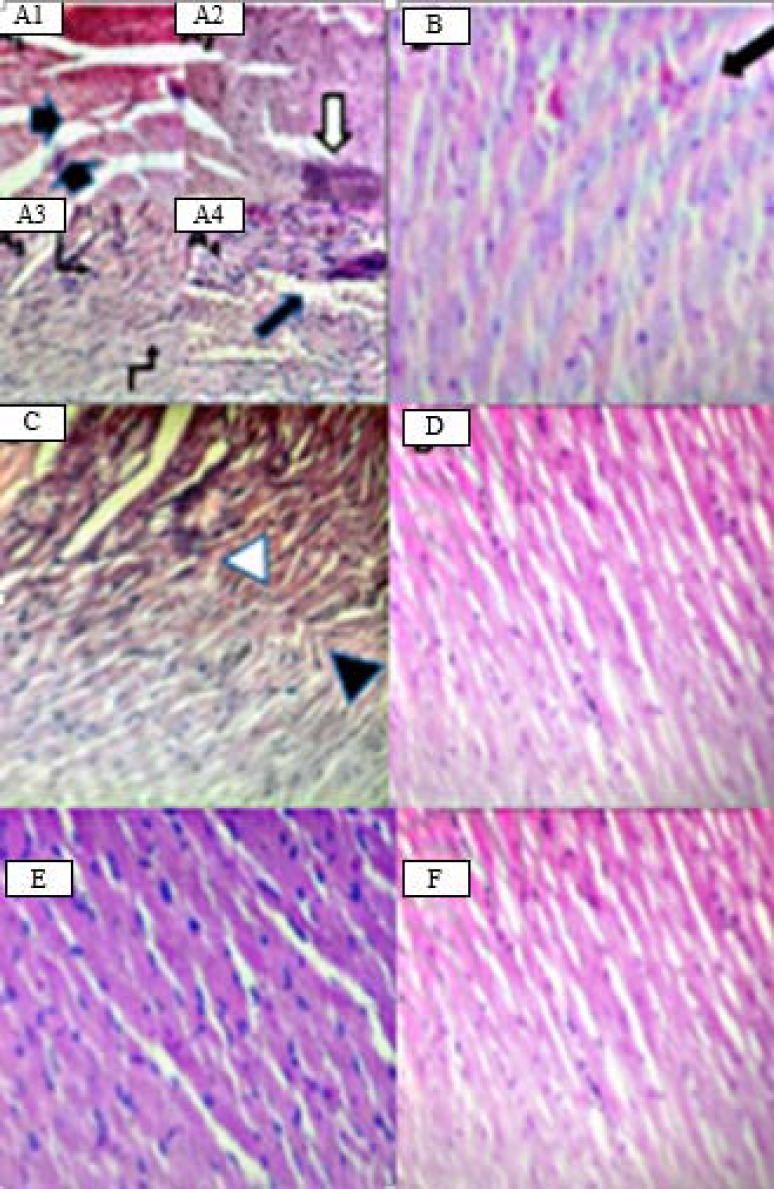
Representative hematoxylin and eosin (H&E) histological sections of the cardiac tissue of (A) DOX showing rupture of cardiac muscle fibers (black arrows in A1), hemorrhage (white arrow in A2) myocyte wavy degeneration (angled arrows in A3), necrosis and inter-fibrillar congestion (black arrow in A4). (B) Dexrazoxane (50 mg/kg) showing mild hemorrhage (black arrow). (C) Vanillic acid at dose of 10 mg/kg showing mild inter-fibrillar congestion (white arrowhead) and mild wavy degeneration (black arrowhead). (D and E) Vanillic acid at doses of 20 and 40 mg/kg and (F) normal saline control group, showing normal cardiac tissue architecture; ×40 magnification.
DISCUSSION
Some mechanisms have been proposed for DOX-induced cardiotoxicity and inflammation including increased levels of free oxygen radicals, alteration of myocardial cell membrane permeability due to lipid peroxidation, mitochondrial oxidative phosphorylation, and changes in electrolyte contents (28). In this study, administration of DOX increased serum levels of LDH, SGOT, CK-MB, cardiac troponin-I, and TLR4 as biomarkers of cardiotoxicity. DOX-induced cardiotoxicity was also confirmed biochemically by a significant elevation in oxidative stress and reduction in total antioxidant capacity (29). Lipid peroxidation is an important pathogenic event in cardiomyopathy and the accumulated lipid peroxides reflect the various stages of the disease and its complications (30). MDA assay provides information about free radicals activities in disease states (31). Total antioxidant capacity of serum samples is indicated by the content of FRAP as another biomarker of oxidative stress in various stages of cardiotoxicity (32). The histopathology of cardiac tissue in DOX-induced cardiotoxicity is associated with necrosis, rupture of cardiac muscle fibers, hemorrhagic myocyte wavy degeneration, and inter-fibrillar congestion of the heart. TLR4 which expressed in cardiomyocytes responds to endogenous or exogenous signals which may influence the pathophysiological alternations in cardiomyopathy (10). Previous investigations showed that DOX caused chronic and much more destructive inflammation thorough activation of TNF-a signaling pathway, generation of massive ROS and persistent expression of multiple pro-inflammatory cytokines in cardiac tissue which associated with cardiomyopathy (33). Elevated ROS levels could significantly increase the level of TLRs, specially TLR4 and subsequently TLR4/NF-κB signaling leading to elevated levels of pro-inflammatory cytokines such IL-1β, IL-6, and TNF-α (34). A recent study revealed that upregulation of TLR4 in cardiac inflammation participated in the pathogenesis and progression of heart failure which was completely unobserved in TLR4-knockout mice treated with DOX (12).
VA is a natural substance from phenolic acid family used in food, drug, beverages, cosmetics, and also is an established and potent antioxidant (35). The results of study of Radmanesh et al. revealed that VA scavenged superoxide radicals and reduced myocardial damage caused by free radicals in cardiac tissue due to ischemia-reperfusion event (36). Another investigation showed that antioxidant and anti-inflammatory effects of VA exhibited chemopreventive effect in experimentally induced carcinogenesis in rats (37). There is evidence suggesting that vanillin which VA is an oxidized form of that, shows anti-neuroinflammatory effects through suppression of ROS, inflammatory markers, and NF-κB signaling in lipopolysaccharide- stimulated microglia (38). Several studies have demonstrated that ferulic acid from phenolic acid family that VA derived from that, exerts cardioprotective effects in ischemia- reperfusion induced by anthracyclines via TLR4/NF-κB suppression (39). Another study demonstrated chemoprotective effect of caffeic acid from phenolic acid family, through inhibition of TLR4 and subsequently reduction of pro-inflammatory cytokines levels in hepatotoxicity (40). In our study, administration of VA increased SBP and HR near to normal levels. All doses of VA restored various altered biochemical parameters including serum LDH, SGOT, CK-MB, cardiac troponin-I, and TLR4 after 28 days treatment and also caused significant histopathological improvement in the cardiac tissue. VA at all doses reduced enormous amount of TLR4 and showed antioxidant effects based on reduction of MDA level and increased FRAP level. To our knowledge, this is the first report on the helpful effects of VA via inhibition of TLR4. The suppression of TLR4/NF-κB signaling and the subsequent reduction of pro-inflammatory cytokines and oxidative stress might be the key mechanisms of cardioprotective effects of VA in DOX-induced cardiotoxicity. It is important to note that the current findings do not represent detailed understanding of all the mechanisms of suppression of TLR4 activation by VA and further researches are required to elucidate the detailed signaling mechanisms for example, effect of TLR4/NF-κB signaling on apoptosis. Also, all the exact cardioprotective mechanisms of VA are needed to be investigated in future to prove usefulness of drug in reduction of DOX-induced cardiotoxicity in breast cancer patients.
CONCLUSION
In conclusion, the findings of the present study showed that VA offers cardioprotection by decreasing biomarkers of cardiotoxicity and oxidative stress, suppression of TLR4 signaling and consequently inflammation pathway. Further investigations are still needed to elucidate the detailed mechanisms of cardioprotective effect of VA and defining its clinical usefulness in the management of cardiotoxicity of DOX in human.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this study.
AUTHOR’S CONTERIBUTION
B. Baniahmad performed the measurements, processed the experimental data and statistical analysis, prepared the manuscript, and designed the tables and figures with support from L. Safaeian as corresponding author. L. Safaeian designed the main conceptual idea of study, developed the theory during clinical and experimental study, provided the intellectual input, designed and approved the protocol to be followed in the study. She was also responsible for manuscript editing, whole correspondence during the manuscript, submission, and handling the revisions. G. Vaseghi and M. Rabbani reviewed the manuscript and were in charge of overall direction and planning. B. Mohammadi as pathologist supported the laboratory experimental design of this study.
ACKNOWLEDGMENTS
The content of this paper is extracted from a PhD thesis submitted by Bahar Baniahmad which is financially (Grant No. 396573) supported by Vice Chancellor of Research, Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Zhou S, Palmeira CM, Wallace KB. Doxorubicin- induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121(3):151–157. doi: 10.1016/s0378-4274(01)00329-0. [DOI] [PubMed] [Google Scholar]
- 2.Gianny L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracy cline cardiotoxicity from bench to beside. J Clin Oncol. 2008;26(22):3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, et al. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res. 2007;38(4):380–385. doi: 10.1016/j.arcmed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev. 2018;2018:1–15. doi: 10.1155/2018/7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 6.Chung WB, Youn HJ. Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Korean J Intern Med. 2016;31(4):625–633. doi: 10.3904/kjim.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wold LE, Aberle NS, Ren J. Doxorubicin induced cardiomyocyte dysfunction via a p38 MAP kinase-dependent oxidative stress mechanism. Cancer Detect Prev. 2005;29(3):294–299. doi: 10.1016/j.cdp.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Kim SJ, Kim BJ, Rah SY, Chung SM, Im MJ, et al. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med. 2006;38(5):535–545. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed HH, Mannaa F, Elmegeed GA, Doss SH. Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorg Med Chem. 2005;13(5):1847–1857. doi: 10.1016/j.bmc.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Zhang X, Bao H, Mi S, Cai W, Yan H, et al. Toll-like receptor (TLR) 2 and TLR4 differentially regulate doxorubicin-induced cardiomyopathy in mice. PLoS One. 2012;7(7):1–10. doi: 10.1371/journal.pone.0040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nili-Ahmadabadi A, Ali-Heidar F, Ranjbar A, Mousavi L, Ahmadimoghaddam D, Larki-Harchegani A, et al. Protective effect of amlodipine on diazinon-induced changes on oxidative/antioxidant balance in rat hippocampus. Res Pharm Sci. 2018;13(4):368–379. doi: 10.4103/1735-5362.235164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riad A, Bien S, Gratz M, Escher F, Westermann D, Heimesaat MM, et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail. 2008;10(3):233–243. doi: 10.1016/j.ejheart.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Wang H, Xiang D, Guo W. Pharmaceutical measures to prevent doxorubicin-induced cardiotoxicity. Mini-Rev Med Chem. 2017;17(1):44–50. doi: 10.2174/1389557516666160621083659. [DOI] [PubMed] [Google Scholar]
- 14.Langer S W. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag Res. 2014;6:357–363. doi: 10.2147/CMAR.S47238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marty M, Espié M, Llombart A, Monnier A, Rapoport BL, Stahalova V, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17(4):614–622. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- 16.Psotova J, Chlopcikova S, Miketova P, Hrbac J, Simanek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes Part III Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytother Res. 2004;18(7):516–521. doi: 10.1002/ptr.1462. [DOI] [PubMed] [Google Scholar]
- 17.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, et al. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25(1):57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Chou TH, Ding HY, Hung WJ, Liang CH. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and VA from Origanum vulgare. Exp Dermatol. 2010;19(8):742–750. doi: 10.1111/j.1600-0625.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 19.Lirdprapamongkol K, Kramb JP, Suthiphongchai T, Surarit R, Srisomsap C, Dannhardt G, et al. Vanillic acid suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J Agric Food Chem. 2009;57(8):3055–3063. doi: 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- 20.Amin FU, Shah SA, Kim MO. Vanillic acid attenuates Aβ 1-42-induced oxidative stress and cognitive impairment in mice. Sci Rep. 2017;18(1):1–15. doi: 10.1038/srep40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dianat M, Hamzavi GR, Badavi M, Samarbaf-zadeh A. Effect of vanillic acid on ischemia-reperfusion of isolated rat heart: Hemodynamic parameters and infarct size assays. Indian J Exp Biol. 2015;53(1):641–646. [PubMed] [Google Scholar]
- 22.Krishnan DN, Prasanna N, Sabina EP, Rasool M. Hepatoprotective and antioxidant potential of ferulic acid against acetaminophen-induced liver damage in mice. Comp Clin Pathol. 2012;22(6):1177–1181. [Google Scholar]
- 23.Arunkumar P, Raju B, Vasantharaja R, Vijayaraghavan S, Preetham Kumar B, Jeganathan K, et al. Near infra-red laser mediated photothermal and antitumor efficacy of doxorubicin conjugated gold nanorods with reduced cardiotoxicity in swiss albino mice. Nanomed-Nanotechnol. 2015;11(6):1435–1444. doi: 10.1016/j.nano.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Adamcova M, Popelova-Lencova O, Jirkovsky E, Simko F, Gersl V, Sterba M. Cardiac troponins- translational biomarkers in cardiology: Theory and practice of cardiac troponin high-sensitivity assays. Biofactors. 2016;42(2):133–148. doi: 10.1002/biof.1261. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, et al. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF- KB pathway in H9c2 cells. Cell Physiol Biochem. 2016;40(6):1578–1590. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- 26.Taghiabadi E, Karimi G, Imenshahidi M, Sankian M, Mosafa F. The protective effect of silymarin on oxidative stress induced by acrolein in heart of mice. Res Pharm Sci. 2012;7(5):180. [Google Scholar]
- 27.Yarmohmmadi F, Rahimi N, Faghir-Ghanesefat H, Javadian N, Abdollahi A, Pasalar P, et al. Protective effects of agmatine on doxorubicin-induced chronic cardiotoxicity in rat. Eur J Pharmacol. 2017;796(1):39–44. doi: 10.1016/j.ejphar.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 30.Umlauf J, Horky M. Molecular biology of doxorubicin-induced cardiomyopathy. Exp Clin Cardiol. 2002;7(1):35–39. [PMC free article] [PubMed] [Google Scholar]
- 31.Erboga M, Donmez YB, Sener U, Erboga ZF, Aktas C, Kanter M. Effect of Urtica Dioica against doxorubicin-induced cardiotoxicity in rats through suppression of histological damage, oxidative stress and lipid peroxidation. Eur J Intern Med. 2016;13(2):139–144. [Google Scholar]
- 32.Chularojmontri L, Wattanapitayakul SK, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Srichairat Cardioprotective effects of Phyllanthus urinaria L on doxorubicin-induced cardiotoxicity. Biol Pharm Bull. 2005;28(7):1165–1171. doi: 10.1248/bpb.28.1165. [DOI] [PubMed] [Google Scholar]
- 33.Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13(7):1033–1049. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Pang XL, Shang WJ, Xie HC, Wang JX, Feng GW. Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-KB pathway by binding to CRY1. Mol Med. 2018;24(1):49–62. doi: 10.1186/s10020-018-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, et al. Natural antioxidants from residual sources. Food Chem. 2001;72(2):145–171. [Google Scholar]
- 36.Radmanesh E, Dianat M, Badavi M, Goudarzi G, Mard SA. The cardioprotective effect of vanillic acid on hemodynamic parameters, malondialdehyde, and infarct size in ischemia-reperfusion isolated rat heart exposed to PM10. Iran J Basic Med Sci. 2017;20(7):760–768. doi: 10.22038/IJBMS.2017.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince PS, Dhanasekar K, Rajakumar S. Preventive effects of vanillic acid on lipids, bax, bcl-2 and myocardial infarct size on isoproterenol-induced myocardial infarcted rats: a biochemical and in vitro study. Cardiovasc Toxicol. 2011;11(1):58–66. doi: 10.1007/s12012-010-9098-3. [DOI] [PubMed] [Google Scholar]
- 38.Kim ME, Na JY, Park YD, Lee JS. Anti-neuroinflammatory effects of vanillin through the regulation of inflammatory factors and NF-KB signaling in LPS-stimulated microglia. Appl Biochem Biotechnol. 2019;187(3):884–893. doi: 10.1007/s12010-018-2857-5. [DOI] [PubMed] [Google Scholar]
- 39.Aswar U, Mahajan U, Kandhare A, Aswar M. Ferulic acid ameliorates doxorubicin-induced cardiac toxicity in rats. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(1):659–668. doi: 10.1007/s00210-019-01623-4. [DOI] [PubMed] [Google Scholar]
- 40.Fadillioglu E, Oztas E, Erdogan H, Yagmurca M, Sogut S, Ucar M, et al. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 2004;24(1):47–52. doi: 10.1002/jat.945. [DOI] [PubMed] [Google Scholar]


