Abstract
Background and purpose:
Research on new drugs with a natural source and low side effects is a priority in pharmacology studies. The present study was conducted to investigate the anti-inflammatory and anti-angiogenesis effects of bee pollen extract in the air pouch model of inflammation.
Experimental approach:
To achieve this goal, male rats were moderately anesthetized and then 20 and 10 mL of sterile air were subcutaneously injected into the intrascapular area of the back of the rat on first and third days, respectively. On day 6, inflammation was induced by intrapouch injection of carrageenan. Normal saline in the control group and bee pollen methanolic extract (50, 100, and 200 mg/pouch) were administered at day 6, simultaneously with carrageenan, and then for 2 consecutive days only normal saline and the extracts were injected. Following sacrificing the rats the pouch was opened and the exudate volume, leukocyte accumulation, granulation tissue weight, vascular endothelial growth factor (VEGF), interleukin 1beta, and tumor necrosis factor alpha (TNF-α) concentrations were determined 3 days after induction of inflammation. In order to investigate the angiogenesis, the granulation tissue was removed, homogenized in the Drabkin’s reagent, and then centrifuged. The supernatant was filtered and the hemoglobin concentration was determined using a spectrophotometer.
Results:
Bee pollen extract significantly decreased the exudate volume, leukocyte accumulation, granulation tissue weight, angiogenesis, VEGF, and TNF-α concentration.
Conclusion and implications:
The findings of the current study revealed that bee pollen methanolic extract has an anti-inflammatory and anti-angiogenesis effect, which could be attributed to the inhibition of VEGF and TNF-α production in the inflammatory exudates.
Keywords: Air pouch, Angiogenesis, Bee pollen, Inflammation, TNF-α, VEGF
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory and autoimmune disease affecting approximately 1% of the world’s population. The disease is characterized by chronic synovial inflammation and joint damage, which leads to pain, fatigue, and low quality of life (1). The use of corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs), which have been main intervention for RA treatment, are restricted due to their side effects. Today, attempts are being made to develop drugs with lower cost and minimal side effects but desirable and effective anti-inflammatory effects (2).
Apitherapy or treatment based on bee products has been used since ancient times to treat burns, ulcers, gastrointestinal disorders, and cancers in recent years (3,4). In addition to honey, bee products, bee pollen: royal jelly, propolis, and wax are not only edible but also have therapeutic activities (5).
Bee pollen, a mixture of pollen from plant flowers and bee salivary secretions, is consumed by humans as a food supplement (6).
Previous studies indicated that bee pollen contains proteins, carbohydrates, fatty acids, ascorbic acid, carotenoids, antioxidants such as phenolic acids, flavonoids, various types of vitamins such as B1, B2, E (5,6).
The present study was carried out to investigate the anti-inflammatory and anti-angiogenesis effects of bee pollen in the air pouch model of inflammation in rat. The disease is usually induced by subcutaneous injection of sterile air at the back of the rats. Following injection, a lining of primary macrophages and fibroblasts is formed in the pouch. The induced inflammation in this model is similar to joint inflammation in humans. This animal model of RA could be used to overcome the difficulties in sampling of the knee joint synovial environment. Injection of carrageenan into the pouch as a phlogistic agent causes inflammatory reaction characterized by accumulation of immune cells, increased exudates, and the production of inflammatory mediators such as prostaglandins, leukotrienes, and cytokines, as well as the formation of granulation tissue and angiogenesis (7). Progression of RA disease depends on the angiogenesis; therefore, angiogenesis inhibitors are considered as one of the therapeutic strategies for treatment of RA (8).
There are some evidences suggesting that angiogenesis and chronic inflammation are interdependent. Chronic inflammation is caused by the prolonged migration of inflammatory cells, and hypoxia occurring in the site of inflammation due to excessive cell proliferation and the production of inflammatory mediators. Then, hypoxia leads to an increased vascular endothelial growth factor (VEGF) expression, which is an important angiogenesis factor (9). The formation of new blood vessels results in high migration of inflammatory cells to the site of inflammation. Thus, in addition to the provision of food and oxygen needed for further proliferation of inflammatory cells, it seems that angiogenesis provides necessary conditions for the incidence of inflammation in the damaged area. Since tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) have a direct and indirect effect on RA and angiogenesis (1,10), the levels of VEGF, TNF-α, and IL-1 were also measured.
MATERIALS AND METHODS
Extraction using maceration method
Powdered bee pollen (350 g) was extracted with 2 L of methanol using maceration method. To prevent the chemical changes the extracted process was carried out in a dark place. The extraction was continued for 5 days at room temperature and then the resultant extract was filtered twice to obtain a clear liquid. The extract was concentrated using a rotary evaporator at 45 °C (11).
Determination of antioxidant activity
Free radical scavenging ability of the extracts was measured by a colorimetric assay, which is based on the reduction of 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) solution. A solution of DPPH (0.08 mg/mL) and the bee pollen extract (1 mg/ml) were prepared in methanol. Serial dilutions were made to obtain five different concentrations of 1.56 × 10-2, 3.13 × 10-2, 6.25 × 10-2, 12.50 ×1 0-2, and 25.00 × 10-2 mg/mL). Five mL of each diluted solutions were mixed with 5 mL DPPH and kept for 30 min at room temperature until completion of the chemical interactions. Then, the absorbance of the samples was determined using a Shimadzu UV/Visible spectrophotometer at 517 nm. The experiment was performed in triplicate and the percentage of reduction was plotted against the extract concentrations for calculation RC50 . The RC50 value expresses the extract concentration providing 50% loss of DPPH activity. Quercetin was used as a positive standard (12).
Determination of total phenolic content
Total phenolic content of bee pollen methanolic extract was measured using Folin- Ciocalteu test using gallic acid as a positive control. Samples containing higher amounts of phenolic compounds were reduced by Folin- Ciocalteu reagent, which produced a blue color. One mL of the extract (5 mg in acetone: water (60:40) v/v) was added to 0.2 Folin- Ciocalteu reagent (1:2 diluted with water) and 2% sodium carbonate mixture (1 mL), and after incubation of the samples at room temperature for 30 min, they were centrifuged (Eppendorf 5810R; Germany) at 10,000 rpm for 5 min. The supernatant absorbance was recorded at 750 nm. For the calibration curve, 10 mg of gallic acid was dissolved in 10 mL of acetone: water (60:40 v/v) as a stock solution. Different concentrations of gallic acid were prepared by serial dilution and determined by Folin- Ciocalteu method. Finally, a calibration curve was constructed and total phenolic values were expressed as mg of gallic acid equivalent to 100 g of powdered bee pollen. All tests were performed in triplicate (13).
Total flavonoid content measurement
Total flavonoid content of bee pollen methanolic extract was measured using a colorimetric assay (aluminum chloride assay) and rutin was used as a standard. The stock extract solution was prepared by dissolving 10 mg of bee pollen methanolic extract in 10 mL of methanol. Then, 0.5 mL of the stock solution was mixed with 2 mL of distilled water and 150 μL of sodium nitrate 5% into a 5-mL volumetric flask. After 6 min, 150 μL of aluminum chloride (10%) and 2 mL of sodium hydroxide (4%) were added to the mixture and the volume of samples brought to 5 mL using distilled water. After incubation of samples at room temperature for 15 min, the absorbance were determined at 510 nm. For calibration curve, different concentrations of rutin were prepared by serial dilution and measured by aluminum chloride assay. Finally, a calibration curve was sketched total flavonoid contents were expressed in 1 g of bee pollen (13).
Laboratory animal
Male Wistar rats (200-250 g, Pasture Institute, Tehran, I.R. Iran) were housed in standard polypropylene cages under a 12/12-h light/dark cycles with free access to food and water. The rats were kept at the animal house of the Faculty of Pharmacy affiliated with Tabriz University of Medical Sciences, Tabriz, Iran and acclimatized to the laboratory environment for at least 1 h before the experiments and used only once throughout the study. They were randomly assigned into four groups of 6 animals each as follows: a control group and 3 experimental groups receiving bee pollen methanolic extract (50, 100, and 200 mg/mL/pouch, respectively). The animal care and handling procedures were conducted in accordance with the Animal Ethics Guidelines at Tabriz University of Medical Sciences and Principles of laboratory animal care (NIH publication No. 86-23, revised 1985). Ethical approval was obtained from the Ethics Committee of the Tabriz University of Medical Sciences (Ethical No. 657552).
Air pouch animal model of inflamation
Rats were moderately anesthetized through inhalation of an anesthetic agent, and the injection site on the back of the animals were shaved and disinfected by ethanol 70%. Then, 20 mL of sterile air was injected subcutaneously into the back of the animals. Three days later, again 10 mL of the air was injected into the same region after anesthetizing the animals. On the day 6 following the first injection, 2 mL of 2% carrageenan suspension (Sigma; Germany), which was autoclaved at 121°C for 15 min to prevent the probable infection, was injected into the previously formed pouch (7).
The control group was treated with normal saline. One mL of bee pollen extract (50, 100, and 200 mg) was injected into the pouch in the experimental groups immediately before injection of carrageenan, 24 and 48 h later.
Investigation of inflammatory parameters
Carrageenan resulted in exudate accumulation, leukocyte infiltration, and granulation tissue formation by inducing inflammatory reaction inside the pouch. To measure the volume of inflammatory exudate, 3 days after induction of inflammation, the animals were killed using high doses of anesthetic agent and the pouch region was disinfected with ethanol 70%. Then, phosphate-buffered saline (PBS) was injected into the pouch cavity and the area was gently massaged for 30 sec. Finally, the pouch fluid was drained by a syringe and its volume was measured. The derived inflammatory exudate was diluted using PBS and the number of leukocytes was counted by a neobar lam (Boec, Germany) under a light microscope (Olympus, Japan). The tissue formed around the pouch was carefully separated, the outer skin removed, the pouch isolated from the surrounding tissue, and the isolated tissue was weighed out (7).
Measurement of VEGF, IL-Ιβ, and TNF-α in the inflammatory exudate
The drained exudate was transferred into test tubes and centrifuged at 1000 rpm and 4 °C for 10 min. The levels of VEGF, IL-1β, and TNF-α were determined with a special enzyme-linked immunosorbent assay (ELISA) kit (Eastbiopharm, China) using an ELISA plate reader in the supernatant-free inflammatory exudate cells (7).
Angiogenesis measurement
To evaluate the angiogenesis, the isolated granulation tissue was gently rinsed in PBS solution. It was then cleaned and dried with a filter paper and cut into small pieces. It was then homogenized in Drabkin’s solution (Zistchemist, Iran) by a homogenizer (Heidolph D-91126, Germany) at 15,000 rpm in an ice substrate (4 °C) for 4 min. Following this, the homogenate was centrifuged at 10,000 RCF and 4 °C for 30 min. Then, the supernatant was filtered using a 0.22-μm filter (Millipore, Germany), and hemoglobin concentration as an angiogenesis index was determined spectrophotometrically at 540 nm by a hemoglobin assay kit (ZiestChem Diagnostics, Iran). Finally, the amount of hemoglobin in the granulation tissue was expressed as mg hemoglobin/100 g of wet tissue (14).
Statistical analysis
The results were presented as mean ± SEM. Inter-group comparison was carried out using one-way ANOVA followed by LSD post hoc test using SPSS software Ver. 16. P-value < 0.05 was considered statistically significant.
RESULTS
Bee pollen extraction efficiency
A total of 248.9 g of methanolic extract was obtained from 350 g of bee pollen which corresponds to 71.1% production yield.
Amount of antioxidant in bee pollen extract
The results of the antioxidant test are expressed as RC50. RC50 is an extract concentration neutralizing 50% of the free radicals’ activity (DPPH). The RC50 value of the bee pollen methanolic extract was 0.064 ± 0.0028 mg/mL.
Total phenol content of bee pollen extract
Gallic acid solution was prepared in different concentrations and their absorbance was determined at 750 nm. Then, a calibration curve was constructed and its equation was determined. Total phenolic content was equivalent to 47.98 mg gallic acid/g of the extract, which corresponds to 4.80% of phenol relative to gallic acid.
Effect of bee pollen extract on inflammation
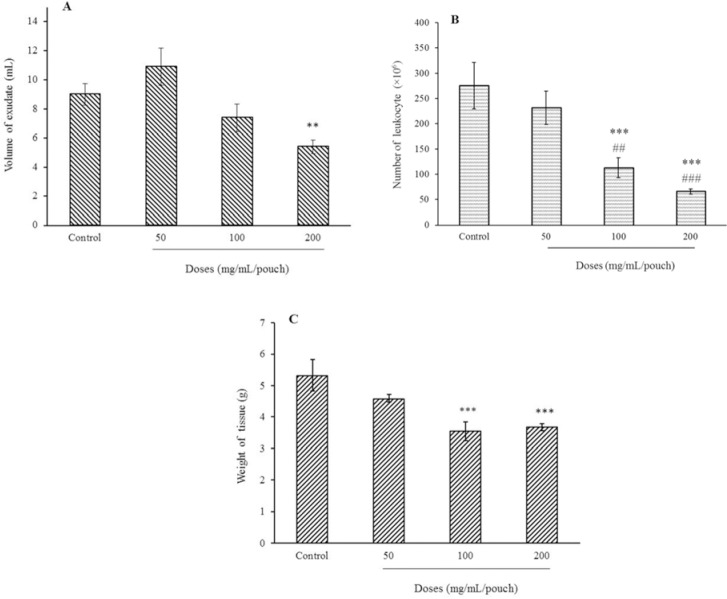
As shown in Fig. 1A, injection of 200 mg bee pollen extract into the pouch the exudate volume was reduced by 40% (P < 0.01) compared to control carrageenan group. However, no significant differences between the experimental groups treated with 50 and 100 mg of the extract and the control group was observed.
Fig. 1.
Effect of bee pollen methanolic extract on (A) exudate volume, (B) leukocyte accumulation, and (C) granulation tissue weight three days after injection of carrageenan in the inflammatory air pouch model in male rats (n = 6). **P < 0.01 and ***P < 0.001 indicate significant difference compared to the control group (normal saline + carrageenan); and ###P < 0.001 in comparison with extract at 50 mg/mL/pouch.
Number of leukocytes in exudate was reduced respectively by 63% and 74% when 100 and 200 mg of the extract per pouch was used. Moreover, a significant decrease in leukocyte count between groups treated with 100 and 200 mg of extract in experimental groups was observed (Fig. 1B). As shown in Fig. 1C granulation tissue weight was reduced by 34 and 30.2% following treatment with 100 and 200 mg of the pollen extract.
Effect of bee pollen extract on the angiogenesis and VEGF
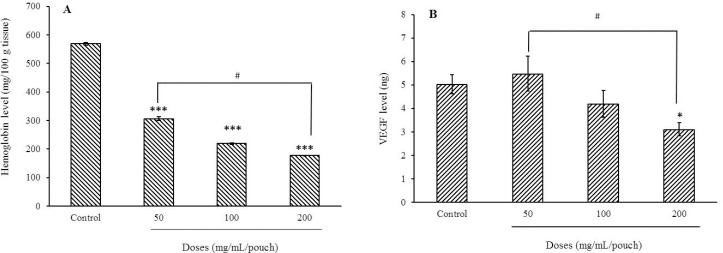
As illustrated in Fig. 2A, the tissue hemoglobin levels, as an angiogenesis index, were decreased to 46.2%, 61.5%, and 68.9% by bee pollen extracts at 50, 100, and 200 mg per pouch, respectively (compared to the control group). Comparison of hemoglobin values between 50 and 200 mg of the extract per pouch was also significant.
Fig. 2.
Effect of bee pollen methanolic extract on (A) hemoglobin level as angiogenesis marker and (B) VEGF level of inflammatory exudates 72 h following carrageenan injection. Values represent mean ± SEM. *P < 0.05 and ***P < 0.001 indicate significant difference compared to the control group (normal saline + carrageenan); and #P < 0.05 shows significant differences between indicated groups. VEGF, vascular endothelial growth factor.
A significant reduction in VEGF concentrations was achieved following administration of 200 mg of the pollen extract per pouch as compared with control and 50 mg pollen extract (Fig. 2B).
Effect of bee pollen extract on IL-1β and TNF-α
To investigate the modulatory effect of bee pollen extract on the overall effects of inflammatory cytokines, the production of IL- 1β and TNF-α was determined in the inflammatory exudate.
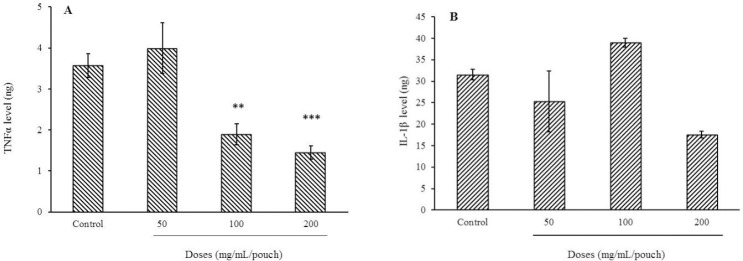
Intra-pouch injection of bee pollen extracts at 100 and 200 mg per pouch decreased TNF-α levels by 47.5 and 60%, respectively (Fig. 3). IL-1 is an inflammatory mediator playing an important role in maintaining the vascular permeability, angiogenesis, and proliferation of fibroblasts. IL-1 has two forms, IL-1α and IL-1β, the former being biologically active (15). The amount of IL-1β was 31.5 ± 1.2 ng in the control group, while injection of the bee pollen methanolic extract at 50 and 200 mg/pouch lowered its levels to 25.3 ± 7 and 17.6 ± 0.8 ng, respectively, though nonsignificant.
Fig. 3.
Effect of bee pollen methanolic extract on (A) TNF-α and (B) IL-1β, 72 h following carrageenan injection. Values are the mean ± SEM shown. **P < 0.01 and ***P < 0.001 indicate significant differences from control group (normal saline + carrageenan). TNF-α, tumor necrosis factor alpha; IL-1β, interleukin 1 beta.
DISCUSSION
Increasing awareness about the efficacy of herbal medicines on one hand, and the multiple side effects of synthetic drugs on the other hand, has led the researchers to pay more attention to new drugs of a natural origin with minor side effects (16). Bee pollen has antimicrobial, antifungal, and antioxidant properties, and has beneficial protective effects against some pathologic conditions such as atherosclerosis, respiratory, and prostate diseases. In addition, bee pollen seems to improve the function of the cardiovascular system, gastrointestinal tract, and the immune system (17).
The results of this study showed that bee pollen contains 71.1% of total methanolic extract indicating its proper cost-effectiveness. The extract had an antioxidant effect (0.064 mg/mL), which is a significant amount compared to quercetin (0.0039 mg/mL) (13). Moreover, our study revealed that bee pollen extract contained more phenolic compound and flavonoid exhibiting higher antioxidant effect.
The present study investigated the effect of bee pollen extract on the inflammation and angiogenesis in the air pouch model of inflammation, which is an acceptable animal model for studying joint inflammation. This model possess many advantages such as evaluation of the inflammatory cells responses to various substances causing inflammation at different time intervals, the study of both acute and chronic inflammations, ease of exudates and granulation sampling, measurement of inflammatory mediators and angiogenesis (7).
Carrageenan, as an inflammatory agent, increases leukocyte accumulation and inflammatory exudate volume in the pouch by enhancing the vascular permeability and stimulating neutrophil migration (18).
The presence of flavonoids in the bee pollen methanolic extract reduces nitric oxide production and inhibits cyclooxygenases activity (6). In the present study, bee pollen extract reduced the volume of inflammatory exudates and inhibited leukocyte accumulation more likely through inhibition of nitric oxide production due to the presence of high amounts of flavonoids. Phenolic compounds present in many medicinal plants seem to play an important role in the treatment of disorders related to oxidative stress and inflammation. Therefore, antioxidant activity is likely one of the possible mechanisms of anti-inflammatory effect of bee pollen (17).
During inflammation, regulation of cyclooxygenases 2 and nitric oxide levels is controlled by NF-κB proinflammatory transcription factor (19). As described earlier, oxidative stress affects the onset and persistence of inflammatory conditions and stimulates NF-κB inflammatory pathways, which play a fundamental role in regulation of gene expression involved in the inflammatory response, especially the expression of monocyte chemoattractant protein-1. Since bee pollen methanolic extract decreased the inhibition of leukocyte migration, the reduced expression of the monocyte chemoattractant protein-1 is probably one of the involved mechanisms as confirmed in a study by Ahmed et al. (20).
Formation of granulation tissue is one of the characteristics of chronic inflammation. Accumulation of fibroblasts, macrophages, neutrophils, monocytes, and eosinophils, collagen synthesis, and angiogenesis are among important features of granulation tissue (21).
Synovial tissue is formed in response to stretch and disorder in the connective tissue due to the accumulation of macrophages, fibroblasts, and blood vessels, and may be found in any anatomical region exposed to an appropriate mechanical stimulus. In the air pouch model of inflammation, frequent subcutaneous injection of air into the back of animal leads to the mechanical disorder in the connective tissue and formation of a cavity with a cellular structure similar to that of the synovial tissue (22). In this study, bee pollen extracts significantly decreased granulation tissue weight compared to the control group. Some studies have stated that macrophage- derived cytokines and monokines consisting of IL-1 and TNF-α are vitally important in the formation of granulation tissue under in vitro conditions such that these cytokines are called granulomatogenic cytokines (21).
Previous studies have shown that nitric oxide plays an important role in regulating the thickness of granulation tissue and the function of fibroblasts and inhibition of its synthesis may cause a decrease in production of collagen by fibroblasts. Studies have also identified the role of VEGF as the main regulator of granulation tissue formation, and stated that its production in granulation tissue is dependent on the active presence of nitric oxide synthase and nitric oxide production (23). On the other hand, TNF-α has been reported to play a role in nitric oxide production (24). The present study showed the inhibitory effects of bee pollen extract on the weight of granulation tissue, VEGF and TNF-α levels. This effect may be related to the simultaneous reduction of VEGF and TNF-α concentration.
Angiogenesis is involved in various pathological and physiological conditions such as tumor growth, metastasis, RA, organ development, wound healing, and reproduction. The imbalances between the angiogenesis inducing and inhibiting factors lead to the occurrence of certain diseases (25). VEGF acts as a mitogen for endothelial cells, which is derived from vessels, and increases the angiogenesis. Furthermore, VEGF has also been reported to increase the vascular permeability (26). Our study revealed that bee pollen extract could reduce the angiogenesis in all three doses used, while VEGF concentration was reduced only when 200 mg was used. The occurrence of a complete mismatch between reduction of angiogenesis and inhibition of VEGF at different extract doses is most likely attributed to other factors interfering with the angiogenesis pathway besides VEGF. Consistent with the results of present work, some previous in vitro studies have also reported that bee pollen reduced VEGF-induced angiogenesis (27). Recent studies have declared that VEGF is not only an angiogenic factor but also a proinflammatory mediator in the inflammation process. Croll et al., stated that VEGF increases the leukocyte migration by increasing ICAM-1 (28). Therefore, reduction of VEGF in the inflammatory fluid by bee pollen extract is involved in its anti-inflammatory activity.
Numerous preclinical and clinical studies have confirmed the vital role of TNF-α in the pathophysiology of RA, where anti-TNF-α drugs such as etanercept are used in controlling multiple inflammatory diseases including RA (1). On the other hand, TNF-α as a potent inflammatory mediator plays an important role in regulating the angiogenesis process (29). The results of the current study showed that administration of bee pollen extract decreased TNF-α levels in the exudate, which is consistent with the results of some previous studies (30).
The results of our previous study revealed that raw honey decreased the concentration of VEGF protein and prostaglandins E2, and consequently reduced angiogenesis in the air pouch model of inflammation (31). Kaczmarek et al., also showed that the prostaglandins E2 strongly induced VEGF secretion and VEGF mRNA expression (32). Another study indicated that, bee pollen is a potent cyclooxygenase enzyme inhibitor, which in turn, can inhibit the production of prostaglandins, especially prostaglandins E2, thereby inhibiting the expression of VEGF mRNA (6).
Reactive oxygen species is another angiogenesis-stimulating factor, which is produced by active macrophages and neutrophils. These oxidative agents enhance angiogenesis by stimulating VEGF production (33). Our study showed that bee pollen inhibits the inflammation and angiogenesis by preventing the leukocyte accumulation, probably through reduction of oxidative stress (34). Lee also indicated the antioxidant properties of pollen obtained from Darae and acorn flowers (30).
Despite the benefits of traditional medicine such as easy accessibility, local cultural beliefs, and increased demand for natural products, the safe use of these drugs must be confirmed by rigorous scientific research from the moral point of view. In the case of bee pollen, due to its nutritional value, good availability, low price, and absence of serious side effects, it seems that the usage of bee pollen is superior to the synthetic drugs such as corticosteroids and NSAIDs. Therefore, bee pollen can be used as an anti-inflammatory and anti-angiogenesis agent. However, it is difficult to draw a general conclusion concerning its potential therapeutic application without a detailed chemical analysis and clinical studies. Other animal models of inflammation and further detailed studies are recommended to identify the mechanism(s) of bee pollen in reducing the inflammation and angiogenesis.
CONCLUSION
The findings of the present study revealed that bee pollen methanolic extract exhibited good anti-inflammatory and anti-angiogenesis effects in the air pouch model of inflammation in the male rats. The observed effects are probably related to an inhibitory effect on the VEGF and TNF-α concentration by the extract. High quantity of flavonoids and phenolic compounds present in bee pollen are effective in reduction of the inflammatory parameters such as leukocyte accumulation, granulation tissue weight, inflammatory exudates, and angiogenesis. Our results may suggest that in addition to the nutritional considerations, pharmacological effects of bee pollen could have promising benefits in the treatment of many immune-related diseases, such as RA and cancer.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this study.
AUTHORS’ CONTRIBUTION
T. Eteraf-Oskouei proposed of the study, performed its design, statistical analysis, manuscript preparation and coordination. A. Shafiee-Khamneh carried out all the experiments. F. Heshmati-Afshar and A. Delazar performed the pharmacognosy studies.
ACKNOWLEDGEMENTS
The content of this paper was extracted from the Pharm. D thesis which was financially supported by the Faculty of Pharmacy, Tabriz University of Medical Sciences through the Grant No. 116. The authors would like to appreciate Drug Applied Research Center, Tabriz University of Medical Sciences, for their technical supports.
REFERENCES
- 1.Mattei RA, Dalmarco EM, Fröde TS. Etanercept administration prevents the inflammatory response induced by carrageenan in the murine air pouch model. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(12):1247–57. doi: 10.1007/s00210-015-1162-x. [DOI] [PubMed] [Google Scholar]
- 2.Negm AA, Furst DE. Nonsteroidal Anti-Inflammatory Drugs, Disease-Modifying Antirheumatic Drugs, Nonopioid Analgesics, & Drugs Used in Gout. In: Katzung BG, editor. Basic & Clinical Pharmacology. 14th ed. New York: McGraw-Hill Education; 2018. p. 643. [Google Scholar]
- 3.Yıldız O, Can Z, Saral Ö, Yuluğ E, Öztürk F, Aliyazıcıoğlu R, et al. Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/461478. 2013:461478,1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larki A, Hemmati AA, Arzi A, Borujerdnia MG, Esmaeilzadeh S, Zad Karami MR. Regulatory effect of caffeic acid phenethyl ester on type I collagen and interferon-gamma in bleomycin-induced pulmonary fibrosis in rat. Res Pharm Sci. 2013;8(4):243–252. [PMC free article] [PubMed] [Google Scholar]
- 5.Feás X, Vázquez-Tato MP, Estevinho L, Seijas JA, Iglesias A. Organic bee pollen: botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules. 2012;17(7):8359–8377. doi: 10.3390/molecules17078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama H, Sakamoto T, Araki Y, Hara H. Anti- inflammatory effect of bee pollen ethanol extract from Cistus sp of Spanish on carrageenan-induced rat hind paw edema. BMC Complement Altern Med. 2010;10(1):30–40. doi: 10.1186/1472-6882-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duarte DB, Vasko MR, Fehrenbacher JC. Models of inflammation: carrageenan air pouch. Curr Protoc Pharmacol. 2016;5:1–12. doi: 10.1002/0471141755.ph0506s72. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald IJ, Liu S, Su C, Wang Y, Tsai C, Tang C. Implications of angiogenesis involvement in arthritis. Int J Mol Sci. 2018;19(7):1–18. doi: 10.3390/ijms19072012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dana SHj, L Rafiee, N Antiangiogenic and antiproliferative effects of black pomegranate peel extract on melanoma cell line. Res Pharm Sci. 2015;10(2):117–124. [PMC free article] [PubMed] [Google Scholar]
- 10.Ashraf S, Mapp PI, Walsh DA. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 2010;62(7):1890–1898. doi: 10.1002/art.27462. [DOI] [PubMed] [Google Scholar]
- 11.Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:26–45. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delazar A, Shoeb M, Kumarasamy Y, Byres M, Nahar L, Modarresi M, et al. Two bioactive ferulic acid derivatives from Eremostachys glabra. DARU J Pharm Sci. 2004;12(2):49–53. [Google Scholar]
- 13.Afshar FH, Delazar A, Nazemiyeh H, Esnaashari S, Moghadam SB. CoComparison of the total phenol, flavonoid contents and antioxidant activity of methanolic extracts of Artemisia spicigera and A splendens growing in Iran. Pharm Sci. 2012;18(3):165–170. [Google Scholar]
- 14.Eteraf-Oskouei T, Akbarzadeh-Atashkhosrow A, Maghsudi M, Najafi M. Effects of salbutamol on the inflammatory parameters and angiogenesis in the rat air pouch model of inflammation. Res Pharm Sci. 2017;12(5):364–372. doi: 10.4103/1735-5362.213981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas A, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 8th ed. Philadelphia: Elsevier; 2014. pp. 82–85. [Google Scholar]
- 16.Eteraf-Oskouei T, Allahyari S, Akbarzadeh- Atashkhosrow A, Delazar A, Pashaii M, Gan SH, et al. Methanolic extract of Ficus carica Linn leaves exerts antiangiogenesis effects based on the rat air pouch model of inflammation. Evid Based Complement Alternat Med. 2015;2015:1–9. doi: 10.1155/2015/760405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascoal A, Rodrigues S, Teixeira A, Feás X, Estevinho LM. Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem Toxicol. 2014;63:233–239. doi: 10.1016/j.fct.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Delves PJ, Martin SJ, Burton DR, Roitt IM. Roitt’s Essential Immunology. 1st ed. London: John Wiley & Sons; 2017. pp. 11–29. [Google Scholar]
- 19.Owczarek K, Lewandowska U. The impact of dietary polyphenols on COX-2 expression in colorectal cancer. Nutr Cancer. 2017;69(8):1105–1118. doi: 10.1080/01635581.2017.1367940. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed MA, ELosaily GM. Role of oxytocin in deceleration of early atherosclerotic inflammatory processes in adult male rats. Int J Clin Exp Med. 2011;4(3):169–178. [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Komatsu N, Higashi N, Imai Y, Irimura T. Granulation tissue formation by nonspecific inflammatory agent occurs independently of macrophage galactose-type C-type lectin-1. Clin Immunol. 2005;115(1):47–50. doi: 10.1016/j.clim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Colville-Nash P, Lawrence T. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol Biol. 2003;225:181–189. doi: 10.1385/1-59259-374-7:181. [DOI] [PubMed] [Google Scholar]
- 23.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50(1):49–59. [PubMed] [Google Scholar]
- 24.Muniz-Junqueira MI, de Paula-Coelho VN. Meglumine antimonate directly increases phagocytosis, superoxide anion and TNF-alpha production, but only via TNF-alpha it indirectly increases nitric oxide production by phagocytes of healthy individuals, in vitro. Int Immunopharmacol. 2008;8(12):1633–1638. doi: 10.1016/j.intimp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Balogh E, Biniecka M, Fearon U, Veale DJ, Szekanecz Z. Angiogenesis in inflammatory arthritis. Isr Med Assoc J. 2019;5(21):345–352. [PubMed] [Google Scholar]
- 26.Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, Houdijk AP, et al. A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis. 2017;20(4):557–565. doi: 10.1007/s10456-017-9567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izuta H, Shimazawa M, Tsuruma K, Araki Y, Mishima S, Hara H. Bee products prevent VEGF- induced angiogenesis in human umbilical vein endothelial cells. BMC Complement Altern Med. 2009;9:45–54. doi: 10.1186/1472-6882-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croll SD, Ransohoff RM, Cai N, Zhang Q, Martin FJ, Wei T, et al. VEGF-mediated inflammation precedes angiogenesis in adult brain. Exp Neurol. 2004;187(2):388–402. doi: 10.1016/j.expneurol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim TK, Park CS, Na HJ, Lee K, Yoon A, Chung J, et al. Ig-like domain 6 of VCAM-1 is a potential therapeutic target in TNFalpha-induced angiogenesis. Exp Mol Med. 2017;49(2):1–11. doi: 10.1038/emm.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH. Intracellular antioxidant activity and inhibition of bee pollens on the production of inflammatory mediators (P06-081-19) Curr Devel Nut. 2019;3(Suppl 1):296. [Google Scholar]
- 31.Eteraf-Oskouei T, Najafi M, Gharehbagheri A. Natural honey: a new and potent anti-angiogenic agent in the air-pouch model of inflammation. Drug Res (Stuttg) 2014;64(10):530–536. doi: 10.1055/s-0033-1363229. [DOI] [PubMed] [Google Scholar]
- 32.Kaczmarek MM, Blitek A, Kaminska K, Bodek G, Zygmunt M, Schams D, et al. Assessment of VEGF- receptor system expression in the porcine endometrial stromal cells in response to insulin-like growth factor-I, relaxin, oxytocin and prostaglandin E 2. Mol Cell Endocrinol. 2008;291(1-2):33–41. doi: 10.1016/j.mce.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Chakrabarti S, Rizvi M, Morin K, Garg R, Freedman JE. The role of CD40L and VEGF in the modulation of angiogenesis and inflammation. Vascular pharmacology. 2010;53(3-4):130–137. doi: 10.1016/j.vph.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Bridi R, Atala E, Pizarro PN, Montenegro G. Honeybee pollen load: phenolic composition and antimicrobial activity and antioxidant capacity. J Nat Prod. 2019;82(3):559–565. doi: 10.1021/acs.jnatprod.8b00945. [DOI] [PubMed] [Google Scholar]