Abstract
Background and purpose:
Because of the high prevalence, diabetes is considered a global health threat. Hence, the need for effective, cheap, and comfortable therapies are highly felt. In previous study, a novel oligosaccharide with strong anti-diabetic activity in the crude extract of Rosa canina fruits, from the rosacea family, was identified. The present study was designed to ensure its efficacy using in vivo and in vitro studies.
Experimental approach:
Crude extract and its purified oligosaccharide were prepared from corresponding herb. Adult male Wistar rats were randomly divided into four groups of 10 each, as follows: group 1, healthy control rats given only sterile normal saline; group 2, diabetic control rats received sterile normal saline; group 3, diabetic rats treated with crude extract of Rosa canina (40% w/v) by oral gavage for 8 weeks; group 4, diabetic rats treated with purified oligosaccharide of Rosa canina (2 mg/kg) by oral gavage for 8 weeks. After treatment, body weight, fasting blood glucose, serum insulin levels and islet beta-cell repair and proliferation were investigated. The possible cytoprotective action of oligosaccharide was evaluated in vitro. The effect of oligosaccharide on apoptosis and insulin secretion in cell culture media were examined. Real-time PCR was used to determine the expression level of some glucose metabolism-related regulator genes.
Findings / Results:
In the animal model of diabetes, the insulin levels were increased significantly due to the regeneration of beta-cells in the islands of langerhans by the purified oligosaccharide. In vitro cell apoptosis examination showed that high concentration of oligosaccharide increased cell death, while at low concentration protected cells from streptozotocin-induced apoptosis. Molecular study showed that the expression of Ins1 and Pdx1 insulin production genes were increased, leading to increased expression of insulin-dependent genes such as Gck and Ptp1b. On the other hand, the expression of the Slc2a2 gene, which is related to the glucose transporter 2, was significantly reduced due to insulin concentrations.
Conclusion and implications:
The purified oligosaccharide from Rosa canina was a reliable anti-diabetic agent, which acted by increasing insulin production in beta-cells of the islands of Langerhans.
Keywords: Apoptosis, Cell viability, Diabetes, Insulin, Rosa canina
INTRODUCTION
Diabetes mellitus is a metabolic diseases generally characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. This hyperglycemia eventually leads to damage of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels. It is caused by several pathogenic processes such as autoimmune destruction of the beta-cells of the pancreas and insulin resistance (1). Estimations showed that there were about 451 million people with diabetes worldwide in 2017 and approximately 5 million deaths attributable to it.
It is predicted that the incidence of diabetes will increase to 693 million by 2045 and half of them (49.7%) will be undiagnosed (2). Currently, the main treatment option for diabetes is the use of insulin and hypoglycemic drugs, but these compounds also have many adverse side effects (3). Despite recent advances in the field of anti-diabetic drugs, this disease is still associated with high mortality. Considering the global statistics of diabetes, it seems necessary to develop new strategies for its treatment.
Over the last decade, there has been an increasing interest in the use of herbal medicine for the management of diseases such as cancers and diabetes (4). It is reported that up to 72.8% of people with diabetes used herbal medicine (5). Ethnobotanical information indicates that more than 800 plants are used as traditional remedies for the treatment of diabetes (6).
Rosaceae family is a group of plants with the hypoglycemic effects (7). From this family Rosa canina has been suggested as a strong anti-diabetic natural remedy in traditional medicine. In addition, a number of studies reported the hypoglycemic and anti- diabetic activities of Rosa canina fruits extract (8,9,10,11,12). The results of a clinical trial about the impact of the administration of aqueous extract of Rosa canina fruits on patients with type 2 diabetes indicated the reduction of fasting blood glucose and serum total cholesterol/high density lipoprotein-chlosterol without any side effect in patients (8). Also, a significant hypoglycemic effect was observed in streptozotocin (STZ)-induced diabetic rats (9). In another study, the intraperitoneal administration of hydroethanolic extract of Rosa canina fruits to alloxan-induced diabetic rats, decreased serum levels of glucose, low density lipoprotein- chlosterol, triglyceride, total cholesterol, urea, uric acid, creatinine, and alkaline phosphatase, and at once increased serum high density lipoprotein-chlosterol levels (10). Study of the anti-diabetic and anti-hyperlipidemic effects of Rosa canina fruits extract in STZ-induced diabetic rats showed a significant reduction in both serum glucose and triglyceride levels.
Furthermore, there was remarkable improvement in islets necrosis in diabetic rats treated with Rosa canina fruits extract (11). An in vitro study proposed that the anti-diabetic effects of this plant might be related to the enhanced proliferation of pancreatic beta-cells (12).
In the present study, the mechanism of action of Rosa canina extract and its purified oligosaccharide in managing diabetes mellitus was evaluated.
MATERIAL AND METHODS
Plant collection and preparation of crude extract
The ripe fruits of Rosa canina were collected and the crude extract and its purified oligosaccharide were prepared according to the US Patent by Bahrami Gh, (9296831, March 29, 2016).
Animals and experimental protocol
Adult male wistar rats (initial weight: 320-350 g) were purchased from Pasteur Institute of Iran (Tehran, I.R. Iran) housed and maintained at a constant temperature of 24 ± 1 °C with a relative humidity of 55% and standard 12/12-h light/dark cycles. They had free access to standard food and tap water for a week before experiment.
A single intraperitoneal injection (45 mg/kg) of STZ (Sigma Ltd., USA), dissolved in cold normal saline, was used for induction of diabetes mellitus in the rats. Sterile normal saline was injected to nondiabetic rats. Diabetes was confirmed by measuring blood glucose levels 48 h after injection. Blood glucose was measured by glucose oxidase method using a glucometer (Gluco Dr, South Korea) and rats with a blood glucose level above 250 mg/dL were considered diabetic. Then, the rats were randomly divided into four groups of 10 each, as follows: group 1, healthy control rats given only sterile normal saline; group 2, diabetic control rats received sterile normal saline; group 3, diabetic rats treated with crude extract of Rosa canina (40% w/v) by oral gavage for 8 weeks; group 4, diabetic rats treated with purified oligosaccharide of Rosa canina (2 mg/kg) by oral gavage for 8 weeks (13).
Measurement of insulin and fasting blood glucose
After 8 weeks, the fasting blood glucose was measured using a glucometer (Gluco Dr, South Korea) and blood insulin was measured by a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (insulin ELISA kit, Ab100578, Abcam, Cambridge, UK) according to their manufacturer’s protocol. Blood samples were collected from the rat’s tail vein.
Immunohistochemical technique and hematoxylin-eosin staining
At the end of experiments, the rats were anesthetized with ether and sacrificed. Tissue samples were taken from the pancreas and then fixed by 10% buffered formalin. Alcohol dehydration process was performed automatically in tissue processing machine. Subsequently samples were embedded in paraffin using routine procedures. Paraffin embedded tissues were cut with microtome at a thickness of 3 μm and then deparaffinized in xylene and rehydrated through graded ethanol. They were stained with insulin antibody according to Dako (Denmark) immunohistochemical kit protocol and quantitative evaluation was performed to detect the repair and increase islet beta-cells. Also, they were stained with hematoxylin and eosin and examined for islet beta cell repair and proliferation.
Cell culture
The RIN5-F cells, prepared from the Cell Bank of the Iranian Biological Resources Center (Tehran, I. R.Iran) were transferred to flasks containing Roswell Park Memorial Institute (RPMI) and 15% fatal bovine serum (FBS), incubated at 37 °C with 5% CO2 and 95% humidity. After 24 h, the morphology of the cells was checked by light microscopy and the new medium was added to the cells. After this, the culture medium was changed every 24 h.
Dose optimization with viability assay
The cells were cultured in 96-well plates (15 × 103 cells/well) and then, incubated with various concentrations of oligosaccharide (1, 5, 10, 15, 20, 30, 40, 60, 80, 100, 200, 400, and 800 μg/mL) for 24 h. Also, the cells were treated with STZ at 10-80 mM, which was dissolved in cell culture medium, and incubated 24 h. After that, the viability of the cells was evaluated using MTT assay (11). The IC50 value of STZ was determined by nonlinear regression using GraphPad Prism 5 (GraphPad Software Inc, San Diego, USA).
Protective effect of oligosaccharide on STZ-induced cytotoxicity
The cells were cultured in 96-well plates and treated with optimal concentrations (1, 5, 15, 30, and 60 μg/mL) of oligosaccharide and IC50 value of STZ at different periods of incubation. In the pre-treatment study, cells were first incubated with different concentrations of oligosaccharide for 5 h. Then, medium was removed and replaced with fresh medium containing STZ and incubated for 24 h. In simultaneous treatment study, the cells were incubated with medium for 5 h, and then oligosaccharide and STZ were added simultaneously and incubated for 24 h. In post- treatment the cells were treated with STZ for 24 h. Then, medium was removed and replaced with fresh medium containing oligosaccharide and incubated for an additional 5 h. At last for all treatments, MTT assay was performed.
Apoptosis induction effect of oligosaccharide
The cells were plated in a 24-well plate (7 × 105 cells/well). After attachment, cells were treated with oligosaccharide (80, 100, 200, 400, and 800 μg/mL) as described above. For observation of the intact, apoptotic, and necrotic cells under the fluorescent microscope, acridine orange/ethidium bromide (AO/EB) double staining (AO/EB dye containing 100 μg/mL of AO and 100 μg/mL of EB in phosphate buffered saline) was performed. The cells were observed under a fluorescent microscope (14).
Insulin secretion assay
RIN-5F cells were cultured in 24-well plates. After attachment, the medium was removed and replaced with fresh medium with and without glucose (20 mM) supplemented with purified oligosaccharide (30 μg/mL) and glibenclamide (100 μg/mL). After 6 and 24 h, the medium of all wells were collected to determine the concentration of insulin with the ELISA kit according to the manufacturer’s instructions. The insulin secretion levels in treated cells were compared to the normal cells (15).
Real-time polymerase chain reaction
The effects of purified oligosaccharide (30 μg/mL) on the expression level of some genes involving in production, secretion, and regulation of insulin were analyzed by real-time polymerase chain reaction (RT-PCR). After 6 and 24 h treatment total RNA from cells was extracted by total RNA isolation kit (DENAzist, Tehran, I.R. Iran) and complementary DNA (cDNA) synthesis was carried out using cDNA synthesis kit (Vivantis Technologies, Selangor DE. Malaysia). RT-PCR was performed using SYBR Premix Ex Taq technology (TaKaRa Bio Inc., Otsu, Shiga, Japan) on the Applied Biosystems StepOne RT-PCR system. Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was served as an internal control and the fold change in relative expression of each target mRNA was calculated based on comparative Ct (2-ΔΔct) method (15). The primers sequences, purchased from Qiagen, are provided in Table 1.
Table 1.
The primers characteristics of genes of interest.
| Genes | Cat numbers | Lot numbers |
|---|---|---|
| Ins1 | QT00373303 | 217332422 |
| Pdx1 | QT00405328 | 217332421 |
| Gck | QT00182966 | 217332423 |
| Ptp1b | QT00179193 | 217332220 |
| Slc2a2 | QT00192822 | 217332419 |
| Gapdh | QT00199633 | 217332300 |
Statistical analyses
All of the experiments were repeated at least three times independently. The data are presented as mean ± standard deviation (SD). Statistical evaluation was performed using one way analysis of variance (ANOVA) with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) software, and differences were considered not significant when P > 0.05.
RESULTS
Effect of oligosaccharide on weight, blood glucose, and insulin levels
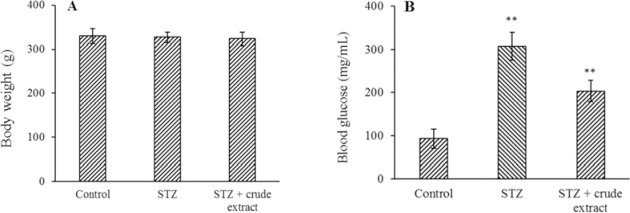
The weight changes of rats were subtle (Fig. 1A, P < 0.01). Fasting blood glucose showed a significant decrease in crude extract of Rosa canina and purified oligosaccharide groups (P < 0.01) (Figs. 1B and 2A).
Fig. 1.
The effect of treatment with crude extract (40% w/v) of Rosa Canina on (A) weight and (B) fasting blood glucose. The results represent the mean ± SD, n = 3. **P < 0.01 Indicates significant differences compared with control group. STZ, Streptozotocin.
Fig. 2.
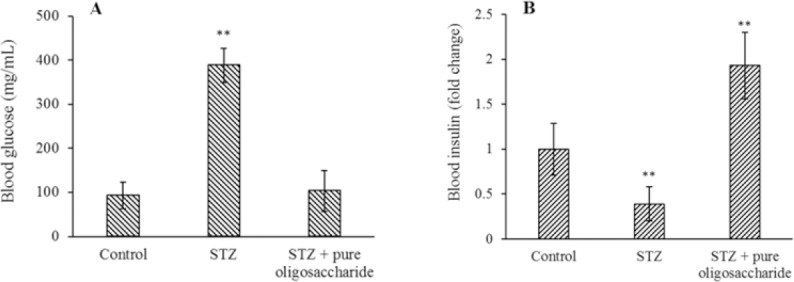
The effect of purified oligosaccharide (2 mg/kg) of Rosa Canina on (A) fasting blood glucose and (B) blood insulin levels in diabetic rats. The results show the mean ± SD, n = 3. **P < 0.01 Indicates significant differences compared with control group. STZ, Streptozotocin.
Figure 2B showed the changes in blood insulin levels (usually a function of the amount of production and secretion from the pancreas) in healthy, diabetic, and treated rats. The mean of blood insulin level of rats with STZ injection was lower than normal rats (P < 0.01). Decreased insulin levels in STZ-induced diabetic rats were probably due to injury of beta-cells and reduction of secreted insulin by these cells. On the other hand, symptoms of diabetes mellitus in rats were disappeared completely after 8 weeks of purified oligosaccharide reception. The results showed that the blood insulin levels were higher in rats treated with oligosaccharide relative to the STZ-induced diabetic group. Significant (P < 0.01) increase in blood insulin in the oligosaccharide recipient diabetic group compared to diabetic group indicated the ability of oligosaccharide to elevate blood insulin levels.
Effect of oligosaccharide on beta cell in the islands of Langerhans
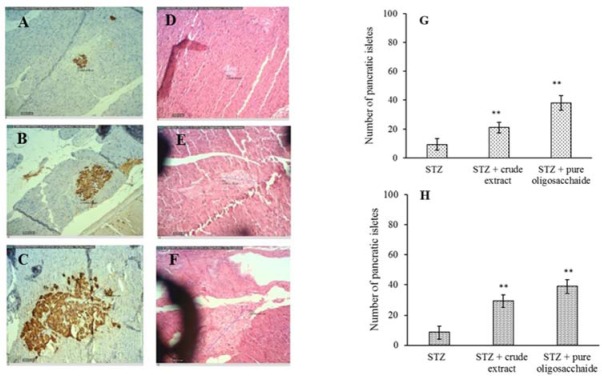
Pancreatic tissues of rats were isolated at the end of 8 weeks, stained with two methods of hematotoxin and eosin and insulin specific antibody (Fig. 3) and examined by light microscopy at magnification of 10 and 40. Islet counts with both staining methods had the same results and showed a significant increase in the mean number of islands by both treatments (P < 0.01). As can be seen in Fig. 3 the islets of Langerhans were approximately 2.5-fold larger in rats receiving both STZ and crude extract than rats receiving STZ alone. A 4-fold increase in the volume of islands was observed when rats treated with oligosaccharide compared to crude extract.
Fig. 3.
The effect of crude extract and purified oligosaccharide on the islets of Langerhans. (A-C) immunohistochemical and (D-F) hematoxylin and eosin staining of the islets of Langerhans treatment by streptozotocin, crude extract, and purified oligosaccharide, respectively, and (G and H) columns of the number of the pancratic islets of immunohistochemical and hematoxylin and eosin staining. **P < 0.01 Indicates significant differences compared with STZ group.
Effect of oligosaccharide on cell viability
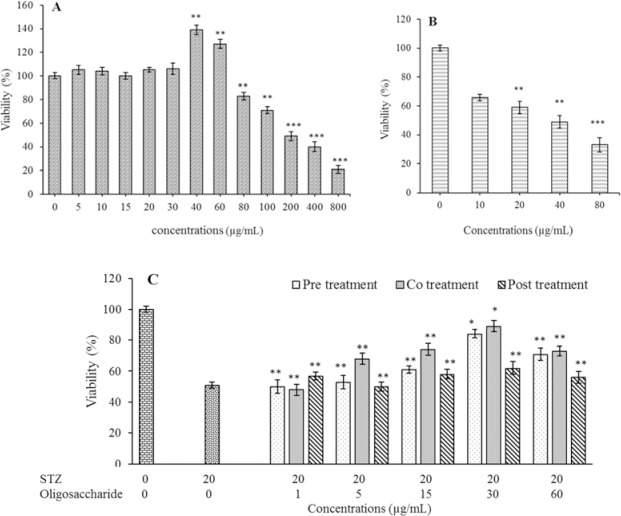
STZ significantly reduced cell viability in a dose-dependent manner (P < 0.01) and caused at least 50% cell death at the concentration of 20 μg/mL used in the experiments (P < 0.01). The treatment of the RIN5-F cell line with different concentrations of the oligosaccharide showed that up to 40 μg /mL no significant effect on cell death was observed (P > 0.05) and at concentrations of 40 and 60 μg/mL the number of living cells was significantly increased (P < 0.05). However, in concentrations higher than 80 μg/mL, a significant decrease in the number of living cells was seen (P < 0.01, Fig. 4).
Fig. 4.
The effect of (A) purified oligosaccharide, (B) STZ, and (C) co-treatment of oligosaccharide with STZ on viability of rat pancreatic β cells (RIN-5F). The viability was measured by MTT assay. Control wells were treated with equivalent amount of medium alone. The results show the mean ± SD, n = 3. *P < 0.05 and **P < 0.01 indicat significant differences compared with control group.
RIN5-F cells were exposed to higher concentrations of the oligosaccharide, because in order to introduce a therapeutic agent, its toxic effects should be tested. The concentrations higher than 100 μg/mL exerted a dose-dependent cytotoxicity on the cells, and the concentration of 800 μg/mL caused 75% cell death. The IC50 value of oligosaccharide was 376 μg/mL.
In co-treatment test, the simultaneous presence of oligosaccharide with STZ had a significantly better effect on cell viability than the other two conditions (P < 0.05). If oligosaccharide was added after STZ, it would have the least protective effect on the cells compared to the other two groups. When oligosaccharide was added to the culture medium before STZ intermediate cell viability was observed. Therefore, it seems that the simultaneous presence of both oligosaccharide and STZ had the most, and the addition of oligosaccharide after STZ had the least protective effects on the cells (Fig. 4).
Effect of oligosaccharide on apoptosis
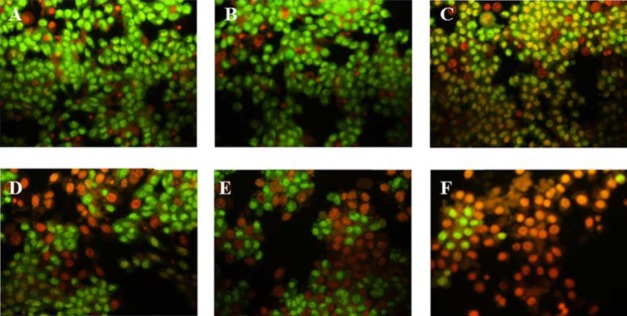
As indicated by the color of the cells (Fig. 5), in treated groups some cells were in the pre-apoptotic stage and some were in the apoptotic stage and at 800 μg/mL, most of the cells were apoptotic and dead. Therefore, apoptotic cell death was increased in a dose dependent manner.
Fig. 5.
The assessment of apoptotic effect of oligosaccharide at different concentrations (A, 0, control; B, 80; C, 100; D, 200; E, 400; and F, 800 μg/mL) on in rat pancreatic β cells (RIN-5F) using fluorescent microscope.
Effect of oligosaccharide on insulin secretion
Under normal glucose conditions, same as treatment with oligosaccharide and glibenclamide, insulin levels in the medium were significantly increased (P < 0.01). However, the presence of too much glucose in the medium the amount of insulin secreted was increased. However, the addition of oligosaccharide and glibenclamide did not significantly affect insulin levels (P < 0.01, Fig. 6).
Fig. 6.
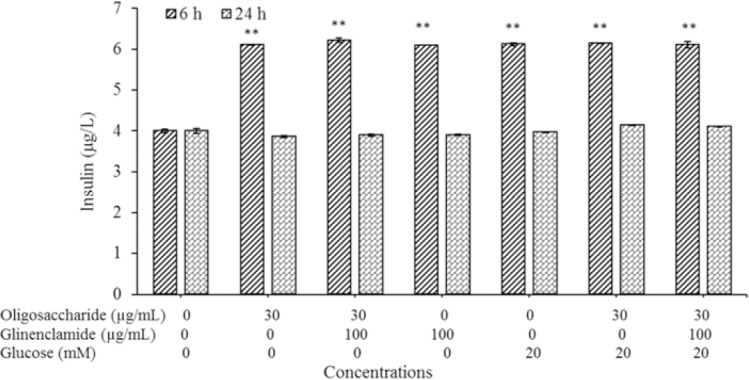
The effect of purified oligosaccharide on glucose stimulated insulin release (after 6 and 24 h) in rat pancreatic β cells (RIN-5F). The results represent the mean ± SD, n = 3. **P < 0.01 Indicates significance differences compared with control group.
Effect of oligosaccharide on gene expression:
In the present study, expression of Ins1, Pdx1, Ptp1b, Gck, and Slc2a2 genes in oligosaccharide-treated cells was also evaluated. These genes play important roles in insulin synthesis, the control of glucose metabolism and its transfer into the cell. Expression of Pdx1, Ptp1b, and Gck were increased after 6 and 24 h treatment with oligosaccharide compared to their corresponding control, whereas Slc2a2 gene expression was significantly decreased by about half compared to control group (P < 0.05) (Fig. 7). Ins1 gene expression was significantly increased in the first 6 h but in the next 24 h was significantly decreased (P < 0.05) (Fig. 7), which is consistent with the results of insulin measurements.
Fig. 7.
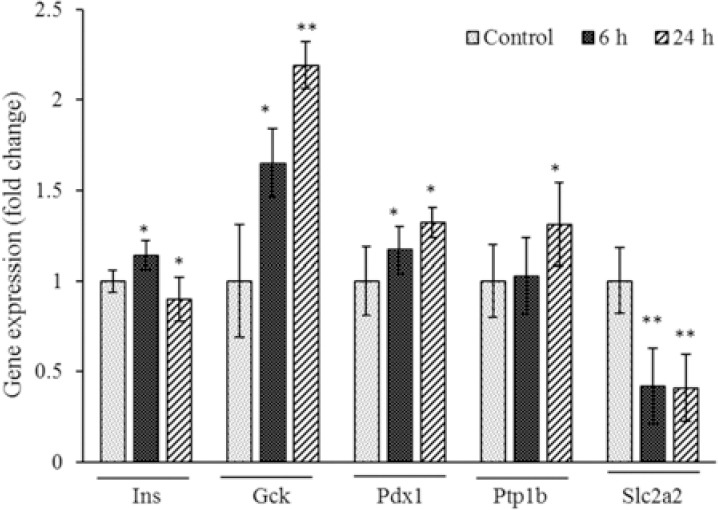
Expression level of Ins, GCK, Pdx1, Slc2a2, and Ptp1b genes in RIN5F 231 cells after 6 and 24 h treatment with oligosaccharide (30 μM) was evaluated by RT-PCR. *P < 0.05 and **P < 0.01 compared with the corresponding control group.
DISCUSSION
The findings of this study showed that oligosaccharide isolated from Rosa canina fruits had an anti-diabetic effect. Numerous studies have shown that plants derived polysaccharides, flavonoids, glycoproteins and polypeptides, steroids, alkaloids, and pectin can have hypoglycemic and hypolipidemic properties and are effective in treating diabetes (16,17,18,19,20). Most studies have used STZ injection to induce diabetes. It enters beta-cells by binding to glucose transporter 2 (GLUT-2) and destroys these cells (21,22). The effect of STZ is determined by changes in blood glucose levels in animals. A marked increase in blood glucose confirms the induction of diabetes and the effect of STZ.
The crude extract was gavaged to STZ-diabetic rats. Measurements of weight and blood glucose before and at the end of the treatment showed that there was no significant changes in body weight after crude extract administration, whereas the glucose levels of the treated groups with the crude extract of the fruits were reduced compared to control. Rosa canina are readily available and abundant; it can reduce the cost of producing oligosaccharide-based anti-diabetic drugs.
Since we intended to investigate the action mechanism of the pure active ingredient, the oligosaccharide was isolated using the method already described by Bahrami et al. (13). The use of pure entity allows for accurate and reliable assessment of any observed biological activities. Gavage of the oligosaccharide had similar but stronger effects than crude extract. Thus, without altering the body weight of the rats, it decreased blood glucose in the diabetic rats. This indicated that purified oligosaccharide is likely responsible for the anti-diabetic properties of the extract by lowering blood glucose. Decreased blood sugar of rats treated with oligosaccharide may be due to reduced sugar uptake, glucose biosynthesis, release of stored glucose, or increased glucose metabolism, storage of glucose in body organs such as liver and muscle, and the production or secretion of hormones such as insulin. Since insulin is the most powerful regulator of glucose metabolism, its levels were measured in rats. Insulin levels in normal rats (without oligosaccharide or STZ), after STZ injection and after oligosaccharide with STZ injection showed that STZ significantly reduced blood insulin levels compared to the control group. However, long-term use of the oligosaccharide significantly increased blood insulin levels compared to the STZ recipient group.
Since this oligosaccharide has a significant effect on animal blood insulin levels, its effect on pancreatic islets was investigated. The number of these islands increased significantly after treatment with oligosaccharide. Thus, the increase in blood insulin levels appears to be due to an increase in the number of islets of the pancreatic and its beta-cells. On the other hand, a qualitative examination of the size of the islands under the microscope also confirmed this conclusion. An increase of 2 to 4 folds in the size of the islets indicates an increase in the number of beta-cells. Other study have shown that berberin had a protective effect on diabetes through increased insulin secretion, beta-cell regeneration, increased anti-oxidant enzyme activity, and decreased protective effect of lipid peroxidation (23). Ethanolic extract of Salvia hypoleuca had significant hypoglycemic effect in diabetic rats. Histological studies on the pancreas of diabetic rats treated with this extract also showed pancreatic beta-cell regeneration. These cases provide promising results to restore beta-cell function (24).
Data of in vitro tests showed that oligosaccharide up to 20 μg/mL did not affect cell viability, and at 30 μg/mL significantly increased cell viability and enhanced cell proliferation. Cell proliferation was increased up to 60 μg/mL and the concentration more than that showed a toxic effect and induced cell death.
The oligosaccharide increased insulin secretion from cells similar to glibenclamide. Oligosaccharide concentrations above 60 μg/mL caused cytotoxic effect, leading to induction of apoptosis in a dose-dependent manner possibly due to increased insulin production/secretion levels upon cellular consumption. In addition, glucose at 20 mg/dL caused insulin production/secretion similar to that produced at 30 μg/mL of oligosaccharide. This amount of glucose or oligosaccharide caused an increase in production/secretion of insulin, which may lead to a programmed cell death (apoptosis). In fact, although optimal concentration of insulin induces apoptosis by the Pdx1 gene and ERK and PI3K proteins, its high concentration induces apoptosis by an unknown mechanism, which is also dependent on glucose concentration (25). Simultaneous administration of oligosaccharide with STZ had the most protective effect, indicating that STZ-induced cell death was inhibited in the presence of oligosaccharide. Since STZ exerts its effect through nuclear factor kappa B (NFkB) activation (26), it is likely that oligosaccharide inhibited the STZ effect through a multifactorial process. This may include enhancing the transcription of genes related to glucose metabolism or inhibiting NFkB-dependent processes by increasing insulin production since insulin is a potent inhibitor of NFkB through the Raf-1 pathway (26).
On the other hand, because the activation of NFkB and the pathways leading to apoptosis by STZ are irreversible, addition of oligosaccharide had a minimal effect on cell viability after STZ treatment. Also, the addition of oligosaccharide prior to STZ is probably less effective than simultaneous administration due to the destruction of the oligosaccharide or its metabolism. Therefore, it seems that the best time to consume oligosaccharide is in the early stages of diabetes. In a previous study the effect of Rosa canina purified oligosaccharide on diabetes related gene expression of male rats was studied. It was observed that the expression of Pdx1, Ngn3, Nkx6.1, and insulin genes were significantly increased in the treated samples compared with the diabetic samples. However, in beta-cells, this increase in insulin expression was significant. On the other hand, the pancreas of animals has been studied pathologically to investigate the effect on beta-cell regeneration. Pathological data showed the improvement of this tissue after treatment. Pdx1 is required for human pancreatic development. Pdx1 -expressing cells, as pancreatic precursor cells, can produce all types of pancreatic cells, endocrine, extracellular, and ductal cells (27).
Pdx1 is essential for the expression of insulin, somatostatin, peptide amyloid, glucokinase, and GLUT-2 in adult. The Pdx1 transcription factor regulates the expression of these genes by binding to the promoter region of the insulin-glucokinase and glucocortin-2 genes (28). It has been shown that depletion of insulin and GLUT-2 mRNA due to cell aging is reversed by treatment with glucagon-like peptide-1 (29). Data showed that the expression of Ins1 and Pdx1 were markedly increased, suggesting that the the effect of oligosaccharide is possibly mediated by increased insulin production. On the other hand, Gck gene expression was also increased, indicating an increase in intracellular glucose concentration, which occurs naturally because of increased insulin levels due to the SREBP1c protein (30).
The effects of various medicinal plants and type 2 diabetes, as well as the cytotoxic effects of various compounds such as STZ, alloxan have been studied using RIN5-F cells. In addition to reducing the number of betacells, these cells show impaired function and lowered insulin production in diabetes (31,32,33). Therefore, by evaluating the expression of genes involved in the function of active beta-cells or important markers of active beta-cells, a proper assessment of the status of beta-cells in terms of their activity or functionality can be obtained. In this study, we investigated the effect of oligosaccharide on mRNA levels of insulin (Ins1), glucokinase (Gck), and GluT2 (Slc2as), which are active beta-cell markers. The increased expression of the mentioned genes indicated that the plant oligosaccharide increased the number of functional and activated cells.
Ptplb was the other gene examined in this study. Its expression was increased by oligosaccharide. Previous research has shown that transforming beta-cells into ductal epithelial cells occurs when cellular signaling pathways such as Akt become activated (34). Since there is an inverse association between Akt activity and Ptp1b gene expression, increased expression of this gene may reflect the conversion of non-beta cells into betacells. On the other hand, increased expression of Ptp1b gene is a concentration-dependent negative regulator of insulin (35). Increased expression of the insulin gene increases its intracellular concentration due to the restriction of its transport and outward secretion; it also increases Ptp1b expression with negative regulation. Slc2a2 gene expression, which is related to the GLUT-2, was also significantly reduced. This transporter is the major glucose transporter into the liver, and unlike GLUT-4, it is not insulin dependent. Therefore, because of the negative regulation of GLUT-2 gene expression by insulin, GLUT-2 gene expression decreases by increasing insulin gene expression and its intracellular high concentration (36). In addition, with the increase in insulin levels, the GLUT-4 transporter further mediates glucose transport.
CONCLUSION
We attempted to investigate the possible mechanism of a purified oligosaccharide on the cellular and molecular dimensions. It significantly reduced blood sugar levels in STZ-diabetic rats. Blood insulin measurement showed that blood insulin level was significantly increased in treated rats compared with control due to pancreatic islet beta-cell regeneration. A similar effect was observed on the insulin in culture media of RIN5-F cells after treatment with purified oligosaccharide. Evaluation of cell apoptosis showed that high concentrations of oligosaccharide might induce apoptosis due to overproduction of insulin. Molecular analysis revealed that oligosaccharide increased the expression of Ins1 and Pdx1 insulin- producing genes, whi ch led to an increase in the expression of insulin-dependent genes such as Gck and Ptp1b. On the other hand, the expression of the Slc2a2 gene, which is related to the GLUT-2 transporter, was significantly reduced by increased insulin concentrations. Overall, it seems that the oligosaccharide could be an acceptable anti-diabetic agent, which acts by enhancing insulin production in the pancreatic islet cells in diabetic models.
CONFLICT OF INTEREST STATMENT
The authors declare no conflict of interest for this study.
AUTHOR’S CONTRIBUTIONS
Gh. Bahrami designed the experiments and supervised the research. S. Sh. Miraghaee, R. Hatami, and S. Sajadimajd analysed data, and co-authored the manuscript. B. Mohammadi, M.T. Bahrami, and Gh. Taheripak performed all of the experiments and wrote the manuscript. S. Sajadimajd and A. Babaei edited the manuscript and performed gene expression analysis of this work. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was financially supported by Vice-Chancellor for Research of Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran under the Grant No. 94195.
REFERENCES
- 1.Davidson MB. Correction to the 2010 report on the diagnosis and classification of diabetes. Diabetes Care. 2010;33(4):e57. doi: 10.2337/dc09-2368. [DOI] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Das M, Sarma BP, Khan AK, Mosihuzzaman M, Nahar N, Ali L, Bhoumik A, Rokeya B. The antidiabetic and antilipidemic activity of aqueous extract of Urtica dioica L on type2 diabetic model rats. J Biosci. 2009;17:1–6. [Google Scholar]
- 4.Khazaei M, Pazhouhi M, Khazaei S. Evaluation of hydro-alcoholic extract of Trifolium Pratens L for its anti-cancer potential on U87MG cell line. Cell J. 2018;20(3):412–421. doi: 10.22074/cellj.2018.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Wallis M, Tiralongo E. Use of complementary and alternative medicine among people living with diabetes: literature review. J Adv Nurs. 2007;58(4):307–319. doi: 10.1111/j.1365-2648.2007.04291.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahangarpour A, Heidari H, Salehizade Junghani M, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin- induced type II diabetic male mice. Res Pharm Sci. 2017;12(5):416–424. doi: 10.4103/1735-5362.213987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pate DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabaghian HF, Abdollahifard M, Khalighi Sigarudi F, Taghavi Shirazi M, Shojaee A, Sabet Z, et al. Effects of Rosa canina L fruit on glycemia and lipid profile in type 2 diabetic patients: a randomized, double-blind, placebo-controlled clinical trial. J Med Plant. 2015;14(55):95–104. [Google Scholar]
- 9.Orhan N, Aslan M, Hosbas S, Deliorman OD. Antidiabetic effect and antioxidant potential of Rosa canina fruits. Pharmacogn Mag. 2009;5(20):309–316. [Google Scholar]
- 10.Ilchizadeh K, Eidi M, Ghahramani R, Sasaninejad Z, Ahmarinezhad Z. Antidiabetic effect of Rosa Canina L fruit in alloxan induced diabetic male rats. Qom Univ Med Sci J. 2015;9(5):23–34. [Google Scholar]
- 11.Taghizadeh M, Rashidi AA, Taherian AA, Vakili Z, Sajad Sajadian M, Ghardashi M. Antidiabetic and antihyperlipidemic effects of ethanol extract of Rosa canina L Fruit on diabetic rats: An experimental study with histopathological evaluations. J Evid Based Complementary Altern Med. 2016;21(4):NP25–NP30. doi: 10.1177/2156587215612626. [DOI] [PubMed] [Google Scholar]
- 12.Fattahi A, Niyazi F, Shahbazi B, Farzaei MH, Bahrami G. Antidiabetic mechanisms of Rosa canina fruits: an in vitro evaluation. J Evid Based Complementary Altern Med. 2017;22(1):127–133. doi: 10.1177/2156587216655263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami Gh. Herbal extract composition for the treatment of diabetes and a method of extracting the same 2016. Google Patents No US20140256673A1 [Google Scholar]
- 14.Khazaei M, Pazhouhi M. Temozolomide-mediated apoptotic death is improved by thymoquinone in U87MG cell line. Cancer Invest. 2017;35(4):225–236. doi: 10.1080/07357907.2017.1289383. [DOI] [PubMed] [Google Scholar]
- 15.Khazaei M, Pazhouhi M. Protective effect of hydroalcoholic extracts of Trifolium pratense L on pancreatic β cell line (RIN-5F) against cytotoxicty of streptozotocin. Res Pharm Sci. 2018;13(4):324–331. doi: 10.4103/1735-5362.235159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazhouhi M, Sariri R, Khazaei MR, Moradi MT, Khazaei M. Synergistic effect of temozolomide and thymoquinone on human glioblastoma multiforme cell line (U87MG) J Can Res Ther. 2018;14:1023–1028. doi: 10.4103/0973-1482.187241. [DOI] [PubMed] [Google Scholar]
- 17.De Paula AC, Sousa RV, Figueiredo-Ribeiro RC, Buckeridge MS. Hypoglycemic activity of polysaccharide fractions containing beta-glucans from extracts of Rhynchelytrum repens (Willd) CE Hubb, Poaceae. Braz J Med Biol Res. 2005;38(6):885–893. doi: 10.1590/s0100-879x2005000600010. [DOI] [PubMed] [Google Scholar]
- 18.Shukla A, Bigoniya P, Srivastava B. Hypoglycemic activity of Lepidium sativum Linn seed total alkaloid on alloxan induced diabetic rats. Res J Med Plant. 2012;6:587–596. [Google Scholar]
- 19.Manikandan R, Vijaya Anand A, Sampathkumar P, Manoharan N. Protective effect of Psidium guajava leaf ethanolic extract against streptozotocin-induced diabetes and lipidosis in rats. Indian J Anim Res. 2018;52:1190–1194. [Google Scholar]
- 20.Bhatia A, Mishra T. Hypoglycemic activity of Ziziphus mauritiana aqueous ethanol seed extract in alloxan-induced diabetic mice. Pharm Biol. 2010;48:604–610. doi: 10.3109/13880200903218935. [DOI] [PubMed] [Google Scholar]
- 21.Müller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- 22.Sun N, Yang G, Zhao H, Savelkoul HF, An L. Multidose streptozotocin induction of diabetes in BALB/c mice induces a dominant oxidative macrophage and a conversion of TH1 to TH2 phenotypes during disease progression. Mediators Inflamm. 2005;2005(4):202–209. doi: 10.1155/MI.2005.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, et al. Protective effect of berberine on beta-cells in streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606(1-3):262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 24.Javdan N, Estakhr J. Neuropharmacological and antidiarrhoeal activity of ethanolic extract of Salvia hypoleuca in rat. Pharmacologyonline. 2011;2:905–910. [Google Scholar]
- 25.Numata T, Araya J, Fujii S, Hara H, Takasaka N, Kojima J, et al. Insulin-dependent phosphatidylinositol 3-kinase/Akt and ERK signaling pathways inhibit TLR3-mediated human bronchial epithelial cell apoptosis. J Immunol. 2011;187(1):510–519. doi: 10.4049/jimmunol.1004218. [DOI] [PubMed] [Google Scholar]
- 26.Garg A, Aggarwal BB. Nuclear transcription factor- kappaB as a target for cancer drug development. Leukemia. 2002;16(6):1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 27.Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia. 2005;48(11):2296–2304. doi: 10.1007/s00125-005-1935-8. [DOI] [PubMed] [Google Scholar]
- 28.Cerf ME. Transcription factors regulating β-cell function. Eur J Endocrinol. 2006;155(5):671–679. doi: 10.1530/eje.1.02277. [DOI] [PubMed] [Google Scholar]
- 29.Aziz MT, El-Asmar MF, Rezq AM, Wassef MA, Fouad H, Roshdy NK, et al. Effects of a novel curcumin derivative on insulin synthesis and secretion in streptozotocin-treated rat pancreatic islets in vitro. Chin Med. 2014;9(1):3–14. doi: 10.1186/1749-8546-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansmannel F, Mordier S, Iynedjian PB. Insulin induction of glucokinase and fatty acid synthase in hepatocytes: analysis of the roles of sterol- regulatory-element-binding protein-1c and liver X receptor. Biochem J. 2006;399(2):275–283. doi: 10.1042/BJ20060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibowitz G, Oprescu AI, Uçkaya G, Gross DJ, Cerasi E, Kaiser N. Insulin does not mediate glucose stimulation of proinsulin biosynthesis. Diabetes. 2003;52(4):998–1003. doi: 10.2337/diabetes.52.4.998. [DOI] [PubMed] [Google Scholar]
- 32.Evans-Molina C, Garmey JC, Ketchum R, Brayman KL, Deng S, Mirmira RG. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes. 2007;56(3):827–35. doi: 10.2337/db06-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of β cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3(11):758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 34.Elghazi L, Weiss AJ, Barker DJ, Callaghan J, Staloch L, Sandgren EP, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology. 2009;136(3):1091–1103. doi: 10.1053/j.gastro.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol. 2005;25(2):819–829. doi: 10.1128/MCB.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta-cells: implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104(4):648–653. doi: 10.1016/j.ymgme.2011.08.026. [DOI] [PubMed] [Google Scholar]