Abstract
Unsuppressed viral load (VL) in patients on antiretroviral therapy (ART) occurs when treatment fails to suppress a person’s VL and is associated with decreased survival and increased HIV transmission. The objective of this study was to evaluate factors associated with unsuppressed VL (VL > 400 copies/ml) in patients currently in care on first-line ART for 6 months attending South African public healthcare facilities. We analysed electronic medical records of ART patients with a VL result on record who started ART between January 2004 and April 2016 from 271 public health facilities. We present descriptive and multivariable logistic regression for unsuppressed VL at last visit using a priori variables. We included 244,370 patients (69% female) on first-line ART in April 2016 for ≥ 6 months. Median age at ART start was 33 years (7% were < 15 years old). Median duration on ART was 3.7 years. Adjusting for other variables, factors associated with having an unsuppressed VL at the most recent visit among patients in care included: (1) < 15 years old at ART start (adjusted odds ratio [aOR]=2.58; 95% CI = 2.37, 2.81) versus 15–49 years at ART start, (2) male gender (aOR = 1.29; 95% CI = 1.25, 1.35), (3) 6–12 months on ART versus longer (aOR = 1.34; 95% CI = 1.29, 1.40), (4) on tuberculosis (TB) treatment (aOR = 1.78; 95% CI = 1.48, 2.13), and (5) prior ART exposure versus none (aOR = 1.20; 95% CI = 1.08, 1.32). Approximately 85% of the ART cohort who were in care had achieved viral suppression, though men, youth/adolescents, patients with prior ART exposure, those with short duration of ART, and patients on TB treatment had increased odds of not achieving viral suppression. There is a need to develop and evaluate targeted interventions for ART patients in care who are at high risk of unsuppressed VL.
Keywords: HIV, AIDS, viral load, viral load suppression, South Africa, antiretroviral therapy, 90–90–90
Introduction
Globally, over 36.7 million people were living with HIV in 2015 (34.0–39.8 million), of which approximately 17 million people (46.3% of those infected with HIV) were on lifesaving antiretroviral therapy (ART).1 South Africa has the world’s largest HIV epidemic with approximately seven million people living with HIV in 2016. An estimated 19.2% of South Africans aged 15–49 years are HIV infected.2 South Africa launched its national HIV treatment programme in 2004. By 2016, over 3.4 million people, or 49% of those infected, were being treated with ART.1
HIV incidence has declined in South Africa from 2.2% among individuals aged 15–49 years in 2005 to 1.9% in 2012, and declined most among youth aged 15–24 from 2.8% in 2005 to 1.5% in 2012.3 Declining HIV incidence and an increased proportion of people on ART in South Africa have led to declines in HIV-related morbidity and mortality. When viral suppression is achieved and maintained, HIV transmission is substantially decreased, as is HIV-associated morbidity and mortality. However, virologic failure and acquired drug resistance may negatively affect these gains. ART failure is diagnosed clinically, immunologically, or virologically. Virologic failure occurs earliest, followed by immunological failure, then clinical failure. If virologic failure is not detected early it can lead to rapid health decline and death.4,5 The consequences of untimely detection of unsuppressed viral load (VL), virologic failure, and delay in switching to different ART drug regimens are increased accumulation of drug resistance mutations, limited subsequent treatment options, disease morbidity and mortality. Untimely detection of virologic failure also increases risk of HIV transmission to others.6
The World Health Organization (WHO) recommends VL testing as the preferred method for monitoring the clinical response of patients with HIV to ART. WHO recommends routine VL monitoring after six months on ART and then at least every 12 months to detect treatment failure earlier and more accurately.4 In patients with unsuppressed VL (e.g. >400 copies/ml), South African National Department of Health7 and WHO guidelines recommend adherence counselling and repeat testing.4 VL monitoring of patients on ART helps ensure early diagnosis and confirmation of ART failure and enables clinicians to take an appropriate course of action for patient management including transitioning those with drug resistance to expensive and complex second- and third-line ART regimens.
Approximately 14% of patients on first-line ART experience viral failure, defined as the inability to achieve or maintain suppression of viral replication to an HIV RNA level <400 copies/ml.8,9 Previous studies in South Africa and other African countries have demonstrated that predictors of first-line ART failure include gender, age, clinic attendance, prior ART exposure, prior prevention of mother-to-child transmission (PMTCT) exposure (South African PMTCT guidelines provided women with single-dose nevirapine [NVP] from 2002 to 201310), ART adherence and general health (including baseline CD4 cell count).8,5–17 However, few studies have included patients on ART for >10 years in resource-limited settings. The aim of this study is to evaluate factors associated with unsuppressed VL at a recent clinical visit in a large cohort of patients followed from 2004 in South Arica who were on first-line ART in order to inform interventions to improve adherence, and address viral failure where present, in patients on ART.
Methods
Data source and study population
We conducted a cross-sectional analysis of an observational cohort using routinely collected HIV medical records of patients in care from the BroadReach clinical HIV cohort from 271 public health facilities in five districts in South Africa (Sedibeng in Gauteng Province, Ugu and King Cetshwayo Districts in KwaZulu Natal Province, Alfred Nzo in Eastern Cape Province and Gert Sibande in Mpumalanga Province). The electronic medical record system captures basic information on:
demographics (e.g. gender, date of birth),
clinical information (e.g. weight, height, pregnancy status, tuberculosis (TB) symptoms, TB treatment, CD4 cell count, and VL),
- prior ART exposure (e.g. post-exposure prophylaxis, PMTCT, PMTCT and prior ART, or prior ART for >30 days),
- For the prior ART exposure, a woman who initiated lifelong ART for PMTCT (option B+) would be counted as ‘PMTCT and prior ART’.If a woman initiated monotherapy before option B+, the prior ART exposure was classified as ‘PMTCT’.
date of clinical and laboratory visits,
other diagnoses (e.g. TB),
and type of HIV treatment.
The electronic medical records did not include the date of prior ART exposure (or PMTCT) nor any adherence measures such as pill counts or on time drug pickups.
We included patients who had initiated ART between January 2004 and April 2016 and were on ART for six or more months. We excluded patients who (1) did not have VL results available in the data-set, (2) were no longer in care (defined as not visiting the clinic within six months of last clinical visit), (3) had transferred to another health facility, or (4) died. The standard first-line regimen in South Africa changed over time but as of the date of analysis (April 2016) the first-line regimen included: two nucleoside reverse transcriptase inhibitors (zidovudine or tenofovir (TDF) plus lamivudine or emtricitabine [FTC]) and one non-nucleoside reverse transcriptase inhibitor (either efavirenz [EFV] or NVP). Our analysis does not include information on those who were excluded from our cohort of patients in care (e.g. those without a VL done, those lost to follow-up, those who died). The objective of our study was to compare patients currently in ART care (on first-line ART) who were not virally suppressed and compare them to other patients in care who were virally suppressed in order to inform interventions among patients in ART care.
Study variables
Factors associated with unsuppressed VL at last clinical visit
Variables came from clinic data at ART initiation and most recent clinic visit, including gender, age at ART start, CD4 cell count at ART start, where initiated ART (e.g. in facility or transferred in from another facility), current age, duration on ART, TB status, pregnancy status at ART start and prior ART exposure (including: >30 days prior ART exposure, ART during pregnancy or post-exposure prophylaxis).
Outcomes
Our primary outcome was an unsuppressed VL among patients currently in care on first-line ART for six or more months. Unsuppressed VL was defined using South African National Department of Health guidelines, as the most recent VL result of >400 copies/ml.7 We included time on ART in the multivariable regression models to control for time on ART. Therefore, a patient who previously had a VL >400 copies/ml who continued on first-line ART and was suppressed at the most recent visit would be reported as suppressed in this cross-sectional analysis.
Statistical analysis
Data analysis was carried out using STATA/SE statistical software package version 14.0 (StataCorp., College Station, TX, USA). Because the data were not normally distributed, continuous variables were described as medians and inter-quartile ranges (IQRs). Binary variables were described using frequency and percentages. Logistic regression modelling was used to analyse the contribution of the following variables, selected a priori: gender, age at ART start, current age, CD4 cell count at ART start, exposure to prior ART, method into ART programme, duration on ART and current TB status (e.g. not on treatment, on treatment, symptomatic and not on treatment), to the development of a recent unsuppressed VL.
Results
Study sample
Characteristics of patients on first-line ART for six or more months are shown in Table 1. The study sample consisted of 244,370 patients. Almost three-quarters of patients had initiated ART between 2011 and 2016 (74%) and the remaining patients initiated ART from 2004 to 2011 (26%) (Figure 1). The median age at ART start was 33 years (IQR=27–42 years), while the median current age (at date of analysis, April 2016) was 38 years (IQR = 30–46). Median duration on ART was 3.7 years (IQR = 1.9–5.8). At ART start the median CD4 cell count was 197 cells/mm3 (IQR = 108–312) and the most recent CD4 cell count was 455 cells/mm3 (IQR = 300–637).
Table 1.
Characteristics of patients on first-line ART for six or more months (n = 244,370).
| Median (IQR) | |
|---|---|
| Age (years) | |
| At ART start | 33 (27–42) |
| Current | 38 (30–46) |
| CD4 cell count (cells/mm3) | |
| At ART start | 197 (108–312) |
| Most recent | 455 (300–637) |
| Viral load (copies/ml) | |
| On first-line treatment | 124 (124–125) |
| Duration on ART (years) | |
| On first-line treatment | 3.6 (1.9–5.8) |
ART: antiretroviral therapy; IQR: inter-quartile range.
Figure 1.
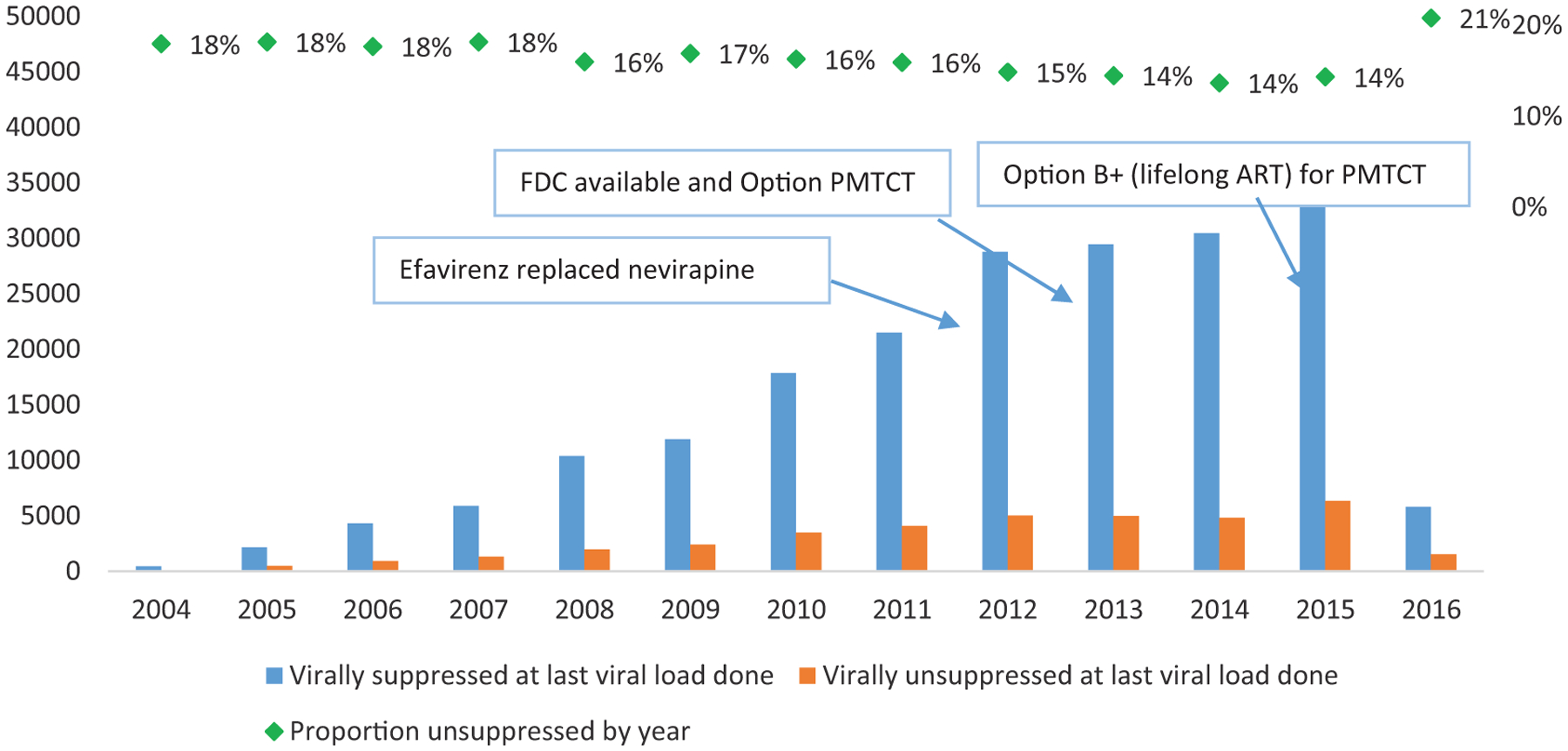
Number and proportion of patients on ART for >6 months who were virally unsuppressed (by year of ART initiation). ART: antiretroviral therapy; FDC: fixed dose combination; PMTCT: prevention of mother-to-child transmission.
After six or more months on ART, 15.3% (n = 37,487) of patients had a VL >400 copies/ml (e.g. unsuppressed VL) at the most recent visit (Table 2). Unsuppressed VL was more common in men than women (18.3% versus 14.0%), and in patients who started ART before 15 years old (31.8% versus 14.3% in patients who initiated between 15 and 49 years and 12.9% in patients who initiated ART when ≥50 years old). Approximately 21% of patients with a CD4 cell count of ≤100 cells/mm3 at ART start had a recent unsuppressed VL at last VL done, compared with 14.9% of those with a CD4 cell count of >100 cells/mm3. Almost one-third (32%) of patients on TB treatment had an unsuppressed VL compared to 15.4% of patients not on TB treatment. Over 17% of patients with 30 or more days of prior ART exposure and 20% with post-exposure prophylaxis had a recent unsuppressed VL. This was higher than women who had started ART during pregnancy (12.8% of women with prior PMTCT exposure had unsuppressed VLs).
Table 2.
Characteristics of virally suppressed (VL ≤ 400) and virally unsuppressed patients on ART for ≥6 months (n = 244,370).
| Virally suppressed | Virally unsuppressed | |
|---|---|---|
| n = 206,883 (84.7%) | n = 37,487 (15.3%) | |
| n (%) | n (%) | |
| Gender | ||
| Men | 61,793 (81.7) | 13,815 (18.3) |
| Women | 145,090 (86.0) | 23,672 (14.0) |
| Age at ART start | ||
| < 15 years | 11,173 (68.2) | 5221 (31.8) |
| 15–49 years | 179,861 (85.7) | 29,017 (14.3) |
| > 50 years | 21,849 (87.1) | 3249 (12.9) |
| CD4 cell count at ART start | ||
| ≤ 100 cells/mm3 | 10,164 (79.4) | 7832 (20.6) |
| > 100 cells/mm3 | 108,009 (85.1) | 18,930 (14.9) |
| Exposure to prior ART | ||
| No prior exposure | 133,624 (84.2) | 25,037 (15.8) |
| Post-exposure prophylaxis (PEP) | 285 (80.5) | 69 (19.5) |
| Prevention of mother-to child-transmission (PMTCT) | 10,896 (87.2) | 1596 (12.8) |
| Prior ART exposure >30 days | 4392 (82.4) | 939 (17.6) |
| Method into ART programme | ||
| Initiated at facility | 98,511 (83.7) | 19,191 (16.3) |
| Transferred from another facility | 50,935 (85.7) | 8498 (14.3) |
| Duration on ART | ||
| 6–12 months | 17,509 (81.7) | 3914 (18.3) |
| 1–5 years | 124,689 (85.8) | 20,577 (14.2) |
| 5–10 years | 59,513 (83.3) | 11,894 (16.7) |
| > 10 years | 5172 (82.4) | 1102 (17.6) |
| Current TB status | ||
| No TB symptoms | 181,194 (84.6) | 32,983 (15.4) |
| With TB symptoms | 944 (82.1) | 206 (17.9) |
| On TB treatment | 730 (68.1) | 342 (31.9) |
ART: antiretroviral therapy; VL: viral load.
The odds of an unsuppressed VL in patients in care and on first-line treatment for six or more months are shown in Table 3. In the full multivariate model, the odds of having a recent unsuppressed VL test were greater in men compared to women (adjusted odds ratio [aOR] = 1.29, 95% CI = 1.25, 1.35), younger than 15 years old at ART being initiation (OR = 2.58, 95% CI = 2.37, 2.81) compared to initiating ART at 15–49 years, being on ART for <1 year (aOR = 1.34, 95% CI = 1.29, 1.40) or more than ten years (aOR = 1.62, 95% CI = 1.46, 1.80) compared to an ART duration of 1–5 years, having a CD4 cell count of ≤100 (aOR = 1.46, 95% CI = 1.41, 1.52), prior ART exposure (>30 days prior exposure: aOR = 1.20, 95% CI = 1.08, 1.32), and being on TB treatment (aOR = 1.78, 95% CI = 1.48, 2.13) compared to not being on TB treatment. Protective factors against having a recent unsuppressed VL were older age (≥50 years = 0.90, 95% CI = 0.87, 0.95) and prior PMTCT exposure (0.90, 95% CI = 0.84, 0.97). Viral suppression by year of ART enrolment is in Figure 1, where 14% of patients who had initiated ART between 2013 and 2015 were not virally suppressed compared to 21% of patients who initiated in 2016 who were not virally suppressed (1527 of 5809 patients).
Table 3.
Odds ratios for virologic failure in patients on first-line treatment for six or more months using logistic regression modelling.
| Parameter | Univariate model | Full multivariate model | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Male gender (ref. women) | 1.36 (1.33–1.40) | <0.001 | 1.29 (1.25–1.35) | <0.001 |
| Age at ART start (Ref. 15–49 years): < 15 years | 2.44 (2.35–2.54) | <0.001 | 2.58 (2.37–2.81) | <0.001 |
| Age at ART start (ref. 15–49 years): 50 + years | 0.77 (0.75–0.80) | <0.001 | 0.90 (0.87–0.95) | <0.001 |
| Duration on ART (ref. 1–5 years): 6–12 months | 1.25 (1.21–1.31) | <0.001 | 1.34 (1.29–1.40) | <0.001 |
| Duration on ART (1–5 years): 5–10 years | 1.15 (1.13–1.18) | <0.001 | 1.28 (1.23–1.34) | <0.001 |
| Duration on ART (1–5 years): >10 years | 1.19 (1.11–1.27) | 0.004 | 1.62 (1.46–1.80) | <0.001 |
| CD4 cell count at ART start (ref. > 100) | 1.48 (1.44–1.53) | <0.001 | 1.46 (1.41–1.52) | <0.001 |
| Initiated at facility (ref. transferred from another facility) | 1.17 (1.14–1.20) | <0.001 | 1.39 (1.33–1.45) | <0.001 |
| TB status: TB symptoms (ref. no symptoms) | 1.18 (1.02–1.38) | 0.031 | 1.03 (0.84–1.27) | 0.767 |
| TB status: On TB treatment (ref. no symptoms) | 2.56 (2.25–2.92) | <0.001 | 1.78 (1.48–2.13) | <0.001 |
| Prior ART: PEP (ref. no prior exposure to ART) | 1.31 (1.01–1.71) | 0.041 | 0.99 (0.64–1.54) | 0.961 |
| Prior ART: PMTCT (ref. no prior PMTCT) | 0.78 (0.74–0.82) | <0.001 | 0.90 (0.84–0.97) | 0.003 |
| Prior ART: Prior ART >30 days (ref. no prior exposure to ART) | 1.15 (1.07–1.23) | 0.001 | 1.20 (1.08–1.32) | 0.001 |
ART: antiretroviral therapy; CI: confidence interval; OR: odds ratio; PEP: post-exposure prophylaxis; PMTCT: prevention of mother-to-child transmission.
Discussion
This study evaluated variables associated with an unsuppressed VL in a large cohort of South African patients currently in care and on first-line ART. We found that 85% of patients in care on ART for six or more months with a VL on record were virally suppressed (VL <400 copies/ml). Our analysis demonstrated that factors associated with unsuppressed VL were male gender, initiating ART before 15 years of age, being on ART for less than one year, or more than ten years, having a CD4 cell count of less than 100 at ART start and being on TB treatment.
Of particular concern is the finding that children and adolescents who start ART have increased odds of not achieving viral suppression. This could be due to behavioural factors, like not taking medication daily and as prescribed, or structural issues, like not being able to attend the health facility during the weekday because of school.18–22 Other behavioural and structural factors could include alcohol use or exposure to violence. For example, a recent study among Malawian youth found almost half of all adolescents living with HIV reported non-adherence to ART in the past month, and risk factors for poor adherence included violence in the home, recent alcohol use, and low treatment self-efficacy.22 A recent systematic review and meta-analysis in South Africa found that only 13% of HIV-infected youth (15–24 years old) were on ART and approximately 81% of youth on ART 15–24 were virally suppressed.21 Another key reason for poor adherence in children or failure to achieve viral suppression in children is the lack of child-friendly paediatric formulations.19,20 There is a need for further research to develop better paediatric formulations with improved palatability and randomised trials to test the newer drugs in adolescent populations.20 Programmes tailored to address barriers to accessing and staying adherent on ART among adolescents living with HIV may help improve adherence to ART, numbers of virally suppressed adolescents with a resulting reduction in transmission.
Our study demonstrated that prior exposure to ART during pregnancy was associated with decreased odds of unsuppressed VL in patients who returned to care after a prior break in care. There were significant changes in the roll-out of HIV prophylaxis and treatment in pregnant women from 2002 to 2016 highlighted below:
- In 2002, South Africa rolled out a national PMTCT programme.
- NVP was used as the first-line ART as per WHO guidelines.10
- The threshold for treatment was CD4 cell count of 350 cells/mm3 or lower.
In 2012, EFV was recommended over NVP for everyone, including pregnant women.
In 2013, South Africa changed the guidelines to option B in which all pregnant women could initiate ART regardless of CD4 cell count using a fixed dose combination (FDC) of TDF, FTC, and EFV.10
In January 2015, South Africa rolled out option B+ in which all pregnant and breastfeeding women were eligible to initiate lifelong ART.10
Our sample included women who had initiated ART from 2004 to 2011, when NVP was prescribed, and 2012–2016, when FDCs were given during pregnancy or for life after January 2015 (Option B+). Unfortunately, date of prior PMTCT exposure was not included in the electronic medical records so we do not know when prior ART exposure happened nor what the prior ART regimen was. Research has demonstrated that NVP drug resistance mutations are frequently detected after single-dose NVP, which remain detectable for several months and may affect future treatment of NVP-exposed women and children, especially in the case of recent exposure (6–12 months).23,24 The increased odds of achieving viral suppression among women in our study with prior ART exposure may be due to better treatment adherence or frequency of VL monitoring in pregnant and post-partum women.25 Further, our study excluded patients who were no longer in care, had died, or were on second- or third-line ART. Another explanation is that those patients who were excluded may have had increased drug resistance and viral failure, compared to those included in our sample.
Previous studies have found that high rates of viral suppression can be achieved in routine care in South Africa, indicating the effectiveness of recommended ART regimens.26 Our study confirmed what other studies in South Africa have found, including that factors associated with unsuppressed VL include gender, age, and baseline CD4 cell count.8,11,12 Our study also identified that being on TB treatment at time of analysis and VL done and duration on ART (e.g. shorter [≤12 months] and longer [>5 years] duration) were risk factors for unsuppressed VL. The HIV Prevention Trials Network 052 trial demonstrated that 93% of participants on ART achieved viral suppression by 12 months and that the annual incidence of viral failure was 3.6%. In our study, patients who had been on ART for one year or longer (e.g. initiated ART from 2012 to 2015) had lower proportions of patients not virally suppressed (14%) compared to patients who initiated ART in 2016 and had their six-month VL test (21% not virally suppressed). Viral suppression was associated with similar factors found in our study including younger age and lower CD4 cell count at ART initiation. Their study also found that lower education level and lack of suppression by three months were also associated with longer time to viral suppression in early ART.27
Risk of HIV transmission to sex partners and infants increases with increasing VL values6 as does risk of mortality. There is a need to develop and evaluate specific interventions for patients at high risk of unsuppressed VL including adapted adherence counselling and targeted resistance testing to ensure that patients with acquired drug resistance are on the appropriate ART regimen.
The results of our study can help in identifying risk groups for virologic failure among patients on first-line ART and catalyse the development of improved interventions to those groups. Further, identifying patients at risk of virologic failure allows for targeted adherence interventions and counselling to avoid treatment failure and the need for second- or third-line therapy and more powerful and expensive drugs.
The findings in this study are subject to limitations. First, we only included patients who had a VL on file, and patients currently in care, which may underestimate the true proportion of patients on ART with an unsuppressed VL. Second, our analysis is cross-sectional which does not enable us to evaluate time-varying covariates or confounders which may affect viral suppression over time. Third, the medical records did not include any adherence measures such as pill counts or on-time drug pickups so we cannot evaluate the impact of those subjective measures of adherence on VL suppression.
Conclusion
In our cohort of patients on ART for six or more months, almost 85% had achieved viral suppression, which is in line with South Africa’s 90–90–90 targets. Our analysis identified at-risk groups who did not achieve viral suppression within six months or longer on ART, including children and adolescents, men, TB patients and those recently initiated on ART (less than one year), or on ART for ten or more years. Failure to achieve viral suppression in those high-risk patients must be addressed. Interventions such as targeted adherence counselling, differentiated care models (including community-based ART delivery), and targeted resistance testing are required to prevent virologic failure among these patients.
Acknowledgements
We would like to acknowledge our donor, U.S. Agency for International Development (USAID) (Cooperative Agreement No. AID-674-A-12–00016), and all of the patients, nurses, doctors, data capturers, and managers who support and implement the patient care and data management in the districts we support.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication of this manuscript was made possible by the support of the American People through the U.S. Agency for International Development (USAID) under the Cooperative Agreement No. AID-674-A-12–00016.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The contents of this article are the responsibility of BroadReach and do not necessarily reflect the views of USAID or the United States Government.
References
- 1.UNAIDS. AIDS by the Numbers, http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf (2016, accessed 10 January 2017).
- 2.Statistics South Africa. Statistical release: mid-year population estimate 2015, https://www.statssa.gov.za/publications/P0302/P03022015.pdf (2015, accessed 10 January 2017).
- 3.Shisana O, Rehle T, Simbayi LC, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSR Press, 2014. [Google Scholar]
- 4.World Health Organization; Consolidated ARV guidelines, 2013 Chapter 7.3: monitoring response to ART and the diagnosis of treatment failure, http://www.who.int/hiv/pub/guidelines/arv2013/art/artmonitoring/en/index3.html (2013, accessed 13 January 2017). [Google Scholar]
- 5.Barth RE, Tempelman HA, Moraba R, et al. Long-term outcome of an HIV-treatment programme in rural Africa: viral suppression despite early mortality. AIDS Res Treat 2011; 2011: 434375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 7.South African National Department of Health. National consolidated guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults, www.sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (2015, accessed 6 November 2017).
- 8.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010; 24: 563–572. [DOI] [PubMed] [Google Scholar]
- 9.Ribaudo H, Lennox J, Currier J, et al. Virologic failure endpoint definition in clinical trials: is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL? An analysis of ACTG studies 16th conference on retro-viruses and opportunistic infections, Montreal, Canada, 8–11 February 2009. [Google Scholar]
- 10.Burton R, Giddy J and Stinson K. Prevention of mother-to-child transmission in South Africa: an ever-changing landscape. Obstet Med 2015; 8: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox MP, Van Cutsem G, Giddy J, et al. IeDEA-SA collaboration. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012; 60: 42837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datay MI, Boulle A, Mant D, et al. Associations with virologic treatment failure in adults on antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010; 54: 48995. [DOI] [PubMed] [Google Scholar]
- 13.Greig JE, Du Cros PA, Mills C, et al. Predictors of raised viral load during antiretroviral therapy in patients with and without prior antiretroviral use: a cross-sectional study. PLoS One 2013; 8: e71407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastard M, Pinoges L, Balkan S, et al. Timeliness of clinic attendance is a good predictor of virological response and resistance to antiretroviral drugs in HIV-infected patients. PLoS One 2012; 7: e49091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One 2011; 6: e17518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan AT, Maskew M, Sanne I, et al. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc 2010; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldous AL and Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin Hiv AIDS 2009; 4: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahourou DL, Gautier-Lafaye C, Teasdale CA, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: challenges, youth-friendly models, and outcomes. J Int AIDS Soc 2017; 20: 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark D, Armstrong A, Andrade C, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc 2017; 20: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beghin JC, Yombi JC, Ruelle J, et al. Moving forward with treatment options for HIV-infected children. Expert Opin Pharmacother. Epub ahead of print 18 September 2017. DOI: 10.1080/14656566.2017.1377181. [DOI] [PubMed] [Google Scholar]
- 21.Zanoni BC, Archary M, Buchan S, et al. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the cresting wave. BMJ Glob Health 2016; 1: e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MH, Mazenga AC, Yu X, et al. High self-reported non-adherence to antiretroviral therapy amongst adolescents living with HIV in Malawi: barriers and associated factors. J Int AIDS Soc 2017; 20: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flys TS, Donnell D, Mwatha A, et al. Persistence of K103N-containing HIV-1 variants after single-dose nevirapine for prevention of HIV-1 mother-to-child transmission. J Infect Dis 2007; 195: 711. [DOI] [PubMed] [Google Scholar]
- 24.Stringer JSA, McConnell MS, Kiarie J, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med 2010; 7: e1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntington S, Thorne C, Anderson J, et al. UK collaborative HIV cohort (UK CHIC) Study. National Study of HIV in Pregnancy and Childhood (NSHPC). Response to antiretroviral therapy (ART): comparing women with previous use of zidovudine monotherapy (ZDVm) in pregnancy with ART naïve women. BMC Infect Dis 2014; 14: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecher S, Williams J, Fonjungo PN, et al. Progress with scale-up of HIV viral load monitoring – seven Sub-Saharan African Countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1332–1335. [DOI] [PubMed] [Google Scholar]
- 27.Eshleman SH, Wilson EA, Zhang XC, et al. Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clin Trials 2017; 18: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]


