Compelling epidemiologic data strongly implicate obstructive sleep apnea (OSA) in the development of cardiovascular disease (CVD) independent of obesity, diabetes, hypertension, and other documented risk factors (1). On the basis of studies of animals exposed to chronic intermittent hypoxia (CIH) and humans with OSA, several potential mechanisms have been proposed, including oxidative stress, systemic inflammation, increased sympathetic nervous system activity, activation of the renin-angiotensin-aldosterone system (RAAS), peripheral chemoreceptor reflex neuroplasticity, dysfunction of nitric oxide (NO) regulation, increased expression of cell adhesion molecules, and dyslipidemia (2). Despite this substantial body of evidence, OSA is not incorporated into any contemporary cardiovascular risk algorithm (3). The consequence of the absence of this “risk equivalent” may be the underestimation, in patients with OSA but without comorbidities such as hypertension and diabetes, of future but modifiable risk. In addition, no randomized controlled trial to date has documented a significant reduction in cardiovascular events when OSA is treated by continuous positive airway pressure (CPAP). Therefore, the current clinical equipoise invites the hypothesis that the coadministration of pharmacologic adjunctive therapy could counter this increased cardiovascular risk by targeting adverse downstream physiologic and biochemical consequences of incompletely treated OSA.
The main utility of CPAP is in effectively relieving OSA-associated symptoms. However, in the general population and in clinic populations, many patients with OSA do not complain of sleepiness (4), and the overall usefulness of CPAP in these patients is unclear. In the SAVE (Sleep Apnea Cardiovascular Endpoints) study (5), patients with a history of coronary artery/cerebrovascular disease and moderate to severe OSA were randomized to CPAP or usual care alone. In the intention-to-treat analysis, CPAP prescription did not reduce the rate of the composite cardiovascular events compared with usual care alone, with a hazard ratio of 1.10 (95% confidence interval, 0.91–1.32; P = 0.34). Of note, the average adherence to CPAP was only 3.3 h/night, or less than half the time asleep, suggesting that significant periods of OSA remained untreated. Furthermore, a recent systematic review and meta-analysis (10 clinical trials; N = 7,266) concluded that the use of CPAP compared with no or sham treatment was not associated with reduced risk of cardiovascular outcomes or death in patients with OSA (6). These results create an opportunity for other approaches for stand-alone therapy (e.g., in patients who cannot adhere to CPAP) or as adjunct therapy to CPAP to reduce cardiovascular events in these patients.
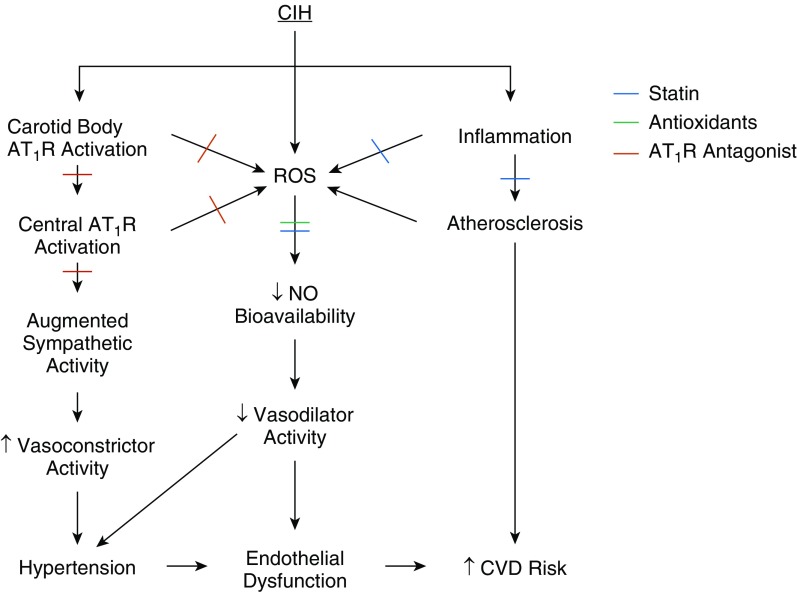
In this pulmonary perspective article, we describe a potentially innovative treatment approach with the goal of CVD reduction in OSA. Specifically, adjunctive pharmaceutical agents (alone or in combination) could target the downstream consequences of OSA. We focus on three drug classes (statins, antioxidants, and angiotensin receptor antagonists) that may be of particular relevance (Figure 1). We acknowledge that this is not an exhaustive list and that other drugs, such as β-blockers, cyclooxygenase inhibitors, vitamin D, angiotensin-converting enzyme inhibitors, imidazoline receptor antagonists, and mineralocorticoid receptor antagonists, may also be useful in this regard. For example, aspirin has both antiinflammatory and antithrombotic properties. Even though recent trials have suggested minimal effects in terms of primary CVD prevention with excessive bleeding risks (7), these patients were not screened for OSA, and aspirin might still be beneficial in OSA, particularly if given before bedtime (8, 9). Also, our discussion does not include drugs aimed at improving OSA by stimulating upper airway tone or improving breathing stability (10), nor does it include lifestyle interventions such as diet or exercise.
Figure 1.
Proposed mechanisms whereby statins, antioxidants, and angiotensin II type I receptor (AT1R) antagonists might protect against the effects of chronic intermittent hypoxia (CIH). Cross-marks denote where each drug class may offer benefit. Blue = statin; green = antioxidant; red = angiotensin II type I receptor antagonist. CVD = cardiovascular disease; ROS = reactive oxygen species.
The Case for Statins
In addition to their powerful lipid-lowering effects, statins (inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase) have pleiotropic properties, including reducing oxidative stress, atherosclerotic plaque inflammation, vascular smooth muscle proliferation, and platelet aggregation. In addition, they improve endothelial function (11) and reduce blood pressure (12) and central sympathetic outflow (13). Totoson and colleagues (14) studied the impact of CIH for 14 and 28 days on vascular injury in rats and determined whether statin administration prevented vascular injury in the setting of CIH. They found that systolic arterial pressure was significantly higher in rats exposed to 14 days of CIH; however, simultaneous atorvastatin treatment eliminated the CIH-induced increase. Atorvastatin prevented adverse changes in carotid artery compliance and endothelial function after 28 days of CIH. Moreover, the increases in carotid intima–media thickness in rats exposed to CIH were annulled by atorvastatin.
Emin and colleagues (15) studied venous endothelial cells from 76 untreated patients with OSA and 52 without OSA. Patients with OSA had increased internalization of the antiinflammatory cell surface protein CD59 compared with patients without OSA. This decrease in the cell surface concentrations of CD59 leaves endothelial cells in patients with OSA more prone to inflammatory damage. However, patients with OSA treated with statins had preserved concentrations of surface CD59 and were similar to those of the control patients without OSA, suggesting that concomitant statin therapy in OSA was beneficial.
To date, only one multicenter randomized controlled trial of statin therapy has examined the impact of short-term statin use in untreated patients with OSA (16). In this study, 51 patients with severe OSA (defined as an apnea–hypopnea index ≥30/h) were randomized to atorvastatin (40 mg/d) or placebo for 12 weeks. In the intention-to-treat analysis, atorvastatin did not significantly improve the primary endpoint (reactive hyperemia index using peripheral arterial tonometry, a surrogate of endothelial function). However, systolic blood pressure and cholesterol levels improved significantly with atorvastatin. Whether the combination of CPAP and statin therapy would have beneficial effects on CVD in OSA is unknown and requires further investigation (17). Furthermore, initiating statin therapy earlier may help mitigate the proatherosclerotic influence of OSA, thereby minimizing the vascular remodeling induced by CIH.
The Case for Antioxidants
Reactive oxygen species (ROS) are highly reactive molecules characterized by an unpaired electron in their outer atomic shell (18). Under conditions of oxidative stress, concentrations of ROS can increase dramatically and can overwhelm the antioxidant capacity for detoxification (19). ROS can damage a variety of cellular molecules, including proteins, DNA, RNA, and lipids, which in turn can cause membrane damage, cell death, apoptosis, and activation of inflammation (20). Oxidative stress is a contributor to CVD, and ROS play a role in mediating the adverse effects of many cardiovascular risk factors, including diabetes, obesity, smoking, and air pollution (21).
Owing to ischemia and subsequent reperfusion, patients with OSA have increased degrees of oxidative stress in response to CIH (22). Oxidative stress in OSA correlates with markers of CVD, including reduced flow-mediated dilation, arterial thickening, and hypertension (23). Furthermore, rodents exposed to CIH consistently show increased oxidative stress (24), in addition to systemic inflammation, vascular endothelial dysfunction, and atherosclerosis (25, 26).
Oxidative stress could be an important pathogenic pathway for the development of premature CVD in patients with OSA. Targeting oxidative stress may be useful; however, few studies have addressed this issue (27). In rodent CIH studies, treatment with antioxidants (pitavastatin, allopurinol, vitamin C/E, and tempol) improves markers of oxidative stress (28). In a study of 20 patients with OSA who were randomized to the antioxidant N-acetylcysteine or control for 1 month, treatment reduced lipid peroxidation, a marker of oxidative stress (29). In another study, a single dose of intravenous vitamin C improved endothelial function as measured by brachial artery flow-mediated dilation in 10 patients with OSA (30).
Given the evidence described above, antioxidants may be beneficial in reducing oxidative stress and improving endothelial function in patients with OSA. Ideally, one consideration would be using an antioxidant with antiinflammatory properties that is able to access sites of ROS production. The antioxidant α-lipoic acid has advantages that may be useful in patients with OSA: It has little toxicity; is able to scavenge mitochondrial ROS; is able to upregulate the transcription of cellular antioxidant enzymes; can lead to regeneration of endogenous vitamin C; and, importantly, has potent antiinflammatory effects (31). Importantly, human trials of dietary antioxidants such as vitamins C and E have shown little improvement in cardiovascular health and may potentially be harmful (32). However, the prevalence of OSA in such trials is unknown, and the possibility that the benefits of antioxidants are specific to such individuals remains untested. A current concept is that antioxidant therapy is best targeted at patients who manifest increased oxidative stress; ROS are essential for cellular function, and thus antioxidant use in patients who are not in a state of oxidative stress could theoretically be harmful. Studying these types of agents in the context of OSA may thus be useful.
The Case for Angiotensin Receptor Blockers
An important driver of increased cardiovascular risk in OSA includes the activation of the sympathetic nervous system by the carotid chemoreceptor reflex through mechanisms dependent on activation of AT1R (Ang-II [angiotensin II] type I receptor). In OSA, higher sympathetic nerve activity is observed during the day, and it can be reduced by hyperoxia, suggesting that tonic carotid chemoreflex activation is an important upstream mediator (33). In animals and humans, CIH exposure leads to an increase in carotid chemoreflex activity that contributes to hypertension, oxidative stress, and endothelial dysfunction. CIH leads to greater activation of the renal (34) as well as local RAAS systems found within the carotid body and central cardiovascular centers (35–38). Circulating Ang-II activates the AT1R on the carotid body and centrally at the subfornical organ to further augment sympathetic nerve activity (37). Interestingly, CIH also increases AT1R expression on the endothelium and, when activated by Ang-II, leads to greater oxidative stress, reduced NO bioavailability, and endothelial dysfunction (39–41). Historical studies demonstrated that rodents exposed to CIH developed hypertension through a mechanism dependent on activation of the AT1R and the carotid chemoreflex (42). In this section, we review evidence for cardiovascular protection by AT1R blockade based on studies conducted in animals and humans exposed to CIH and in patients with OSA. Angiotensin-converting enzyme inhibitors may also be useful in this context, but they have not been as well studied as AT1R receptor blockers with respect to OSA.
CIH-induced increases in chemoreflex activity may contribute to sympathetic activation and hypertension in animals and humans. Repetitive stimulation of AT1R in the carotid body results in long-lasting afferent activity in the carotid body, called “sensory long-term facilitation” (43). This activity likely contributes to the long-lasting sympathetic outflow and the development of hypertension in patients with sleep-disordered breathing. When humans are exposed acutely to intermittent hypoxia (IH), sympathetic nerve activity is increased and remains elevated even after its removal, and this effect can be abolished by a single dose of losartan (100 mg) to block the AT1R (44). Likewise, humans exposed to IH for as short as 6 hours to as long as 28 days develop lasting elevations in blood pressure, similar to the hypertension induced by OSA (45–48). When the AT1R is blocked with losartan, the IH-induced augmentation in blood pressure is abolished (49). Similar improvements in blood pressure are observed in patients with OSA. In an 8-week randomized controlled crossover trial in 23 hypertensive patients with OSA (apnea–hypopnea index, 29 ± 18/h), valsartan, another AT1R blocker, was compared with CPAP with respect to blood pressure reduction (50). The reduction in mean arterial pressure with CPAP was similar to that in other trials (−2.1 ± 4.9 mm Hg; P < 0.01); however, the improvement in mean arterial pressure with valsartan was much greater (−9.1 ± 7.2 mm Hg), with a difference between treatments of −7.0 mm Hg (95% confidence interval, −10.9 to −3.1 mm Hg; P < 0.001). A more recent trial comparing the effectiveness of losartan in reducing blood pressure in patients with hypertension with (n = 55) and without OSA (n = 36) reported that losartan was less effective in reducing blood pressure in patients with OSA (51). However, supplemental CPAP treatment had no additional benefit in 24-hour mean arterial pressure (except in highly compliant patients), suggesting that losartan was as effective as CPAP in treating hypertension in OSA. Finally, losartan was tested in a 6-week randomized trial in 86 patients with hypertension who had moderate to severe OSA (52). AT1R blockade further reduced blood pressure in patients adequately treated with CPAP. Interestingly, chemoreflex sensitivity and sympathetic activity were not affected by AT1R blockade, but this may have been because patients had already been treated effectively with CPAP for more than 3 months before study enrollment.
Impaired endothelial function and vascular reactivity in patients with OSA may contribute to their heightened risk for CVD, and this too is modifiable by blockade of the AT1R. Activation of the AT1R leads to ROS generation, and therefore its blockade improves endothelial function (22). Endothelial dysfunction (including decreased bioavailability of NO) and inflammation can occur as a result of increased production of ROS after CIH. Indeed, animals and humans exposed to IH have increased markers of oxidative stress, nitrosative stress, and reduced NO bioavailability. Blockade of AT1Rs with losartan reduces ROS production, normalizes NO bioavailability, and restores endothelial function (39–41). Interestingly, IH exposure in humans augments the number of plasma exosomes released from the endothelium (53). When these exosomes are applied to IH-naive human endothelial cells in culture, they directly impair NO synthase expression. This effect is absent when IH-naive human endothelial cells are pretreated with losartan, suggesting a protective influence on the endothelium (54). In a small group of patients with OSA (n = 11) with low CVD risk, endothelial function (as measured by flow-mediated dilation) was reduced and AT1R expression on the vascular endothelium was upregulated compared with control patients without OSA (n = 10) (41). Both measurements were normalized by CPAP therapy. When ex vivo tissue samples were studied for superoxide production, application of losartan was beneficial, suggesting that oxidative stress in the vasculature can be improved by treatment with losartan.
A significant number of patients with OSA are already being preferentially prescribed AT1R blockers for the treatment of hypertension. By reducing sympathetic activation, decreasing oxidative stress, improving endothelial function, and preventing the hypertensive effects of IH, AT1R blockade may also benefit patients with OSA with normal blood pressure. It is unclear whether such benefits pertain to all AT1R antagonists as a class or are particular to specific blockers because such autonomic and vascular effects may relate to the magnitude of central nervous system penetration and pleiotropic properties, including ligand activity of the peroxisome proliferator-activated receptor-γ.
Conclusions
OSA exerts multiple adverse physiologic effects that could initiate or accelerate the progression of CVD. On the basis of animal studies, the CIH consequent to OSA probably has the greatest impact (55), but arousal and hypercapnia may also contribute to this increased risk (56, 57). Nevertheless, further preclinical and human work is required to assess through what mechanisms hypercapnia, arousal, and IH interact to predispose patients to future CVD. Because the onset of OSA usually precedes diagnosis by many years, the impact of chronicity of CIH and the temporal window during which cardiovascular risk may be reversed with pharmaceuticals or other interventions also need further study.
Contemporary guidelines advocating treatment of dyslipidemia, hypertension, and other disorders emphasize the estimate of a patient’s long-term cardiovascular risk rather than absolute quantitative thresholds (3). If OSA is given weight as an independent CVD risk factor, then its detection in a patient who would traditionally be considered low or borderline risk could trigger consideration of medication for primary prevention, in addition to CPAP treatment. The evidence reviewed supports the hypothesis that statins, antioxidants, and angiotensin receptor antagonists, alone or in combination, could benefit patients with OSA lacking contemporary clinical indications for their specific prescription.
More precise phenotyping of patients with OSA to identify molecular or physiologic biomarkers of heightened risk (e.g., hypoxic burden or inflammatory markers) (58) could help enrich future trials and may offer the potential for individualized precision therapy. Although OSA can cause inflammation, sympathetic activation, RAAS activation, and oxidative stress, there is likely substantial interindividual variability with respect to how OSA impacts a particular patient. Specifically, the impact on these markers could vary according to OSA severity, obesity, genetic (59), or other factors. Though speculative, a high degree of oxidative stress may identify a patient who may be more likely to respond to an antioxidant and a patient with greater concentrations of inflammatory markers who may be more likely to respond to a statin.
There will be many challenges and considerations in the design of future trials. One issue may be selection of patients because many patients with OSA likely have clinical indications for statins or other medications. The rigid exclusion of patients may be problematic for trial design. For example, given prior studies that have shown a beneficial impact of statin therapy in patients with elevated CRP (C-reactive protein) in large randomized controlled trials (60), it may be difficult to justify enrolling patients with elevated CRP in a trial of statin therapy. However, CRP may be the marker of the patients with OSA most likely to respond to a statin, and exclusion of these patients may result in a negative trial result. Other pertinent issues for trial design include whether a single agent or combination of agents should be tested; whether patients intolerant of CPAP should be specifically studied; and whether, in nonsleepy patients, the allocation of CPAP also should be randomized (i.e., in a two-by-two factorial test of CPAP and drugs, alone and in combination). Closer collaborations with the cardiology community will be important to further advance this field.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201811-2097PP on March 15, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122:1741–1764. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 2.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–1021. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Roure N, Gómez S, Mediano O, Duran J, Peña M de L, Capote F, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9:727–731. doi: 10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM. Should aspirin be used for primary prevention in the post-statin era? N Engl J Med. 2018;379:1572–1574. doi: 10.1056/NEJMe1812000. [DOI] [PubMed] [Google Scholar]
- 8.Bonten TN, Snoep JD, Assendelft WJJ, Zwaginga JJ, Eikenboom J, Huisman MV, et al. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension. 2015;65:743–750. doi: 10.1161/HYPERTENSIONAHA.114.04980. [DOI] [PubMed] [Google Scholar]
- 9.Snoep JD, Hovens MMC, Pasha SM, Frölich M, Pijl H, Tamsma JT, et al. Time-dependent effects of low-dose aspirin on plasma renin activity, aldosterone, cortisol, and catecholamines. Hypertension. 2009;54:1136–1142. doi: 10.1161/HYPERTENSIONAHA.109.134825. [DOI] [PubMed] [Google Scholar]
- 10.Jordan AS, O’Donoghue FJ, Cori JM, Trinder J. Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. Am J Respir Crit Care Med. 2017;196:814–821. doi: 10.1164/rccm.201612-2511PP. [DOI] [PubMed] [Google Scholar]
- 11.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(Suppl 1):III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 12.Briasoulis A, Agarwal V, Valachis A, Messerli FH. Antihypertensive effects of statins: a meta-analysis of prospective controlled studies. J Clin Hypertens (Greenwich) 2013;15:310–320. doi: 10.1111/jch.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavalipati N, Shah J, Ramakrishan A, Vasnawala H. Pleiotropic effects of statins. Indian J Endocrinol Metab. 2015;19:554–562. doi: 10.4103/2230-8210.163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Totoson P, Fhayli W, Faury G, Korichneva I, Cachot S, Baldazza M, et al. Atorvastatin protects against deleterious cardiovascular consequences induced by chronic intermittent hypoxia. Exp Biol Med (Maywood) 2013;238:223–232. doi: 10.1177/1535370212473696. [DOI] [PubMed] [Google Scholar]
- 15.Emin M, Wang G, Castagna F, Rodriguez-Lopez J, Wahab R, Wang J, et al. Increased internalization of complement inhibitor CD59 may contribute to endothelial inflammation in obstructive sleep apnea. Sci Transl Med. 2016;8:320ra1. doi: 10.1126/scitranslmed.aad0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyeux-Faure M, Tamisier R, Baguet JP, Dias-Domingos S, Perrig S, Leftheriotis G, et al. Response to statin therapy in obstructive sleep apnea syndrome: a multicenter randomized controlled trial. Mediators Inflamm. 2014;2014:423120. doi: 10.1155/2014/423120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toraldo DM, Benedetto M, Conte L, De Nuccio F. Statins may prevent atherosclerotic disease in OSA patients without co-morbidities? Curr Vasc Pharmacol. 2017;15:5–9. doi: 10.2174/1570161114666161007164112. [DOI] [PubMed] [Google Scholar]
- 18.Kanaan GN, Harper ME. Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta Gen Subj. 2017;1861:2822–2829. doi: 10.1016/j.bbagen.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Sack MN, Fyhrquist FY, Saijonmaa OJ, Fuster V, Kovacic JC. Basic biology of oxidative stress and the cardiovascular system: part 1 of a 3-part series. J Am Coll Cardiol. 2017;70:196–211. doi: 10.1016/j.jacc.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol. 2017;70:212–229. doi: 10.1016/j.jacc.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution. Part 3 of a 3-part series. J Am Coll Cardiol. 2017;70:230–251. doi: 10.1016/j.jacc.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavie L. Oxidative stress: a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Badran M, Ayas N, Laher I. Cardiovascular complications of sleep apnea: role of oxidative stress. Oxid Med Cell Longev. 2014;2014:985258. doi: 10.1155/2014/985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badran M, Abuyassin B, Golbidi S, Ayas N, Laher I. Uncoupling of vascular nitric oxide synthase caused by intermittent hypoxia. Oxid Med Cell Longev. 2016;2016:2354870. doi: 10.1155/2016/2354870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golbidi S, Badran M, Ayas N, Laher I. Cardiovascular consequences of sleep apnea. Lung. 2012;190:113–132. doi: 10.1007/s00408-011-9340-1. [DOI] [PubMed] [Google Scholar]
- 26.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lira AB, de Sousa Rodrigues CF. Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: a review article. Sleep Breath. 2016;20:1155–1160. doi: 10.1007/s11325-016-1367-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Zhao Y, Li X, Xu L, Jiang F, Hou W, et al. Effects of antioxidant tempol on systematic inflammation and endothelial apoptosis in emphysematous rats exposed to intermittent hypoxia. Yonsei Med J. 2018;59:1079–1087. doi: 10.3349/ymj.2018.59.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadasivam K, Patial K, Vijayan VK, Ravi K. Anti-oxidant treatment in obstructive sleep apnoea syndrome. Indian J Chest Dis Allied Sci. 2011;53:153–162. [PubMed] [Google Scholar]
- 30.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 31.Tibullo D, Li Volti G, Giallongo C, Grasso S, Tomassoni D, Anfuso CD, et al. Biochemical and clinical relevance of alpha lipoic acid: antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm Res. 2017;66:947–959. doi: 10.1007/s00011-017-1079-6. [DOI] [PubMed] [Google Scholar]
- 32.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JMO, et al. HOPE and HOPE-TOO Trial Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 33.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 34.Zalucky AA, Nicholl DDM, Hanly PJ, Poulin MJ, Turin TC, Walji S, et al. Nocturnal hypoxemia severity and renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med. 2015;192:873–880. doi: 10.1164/rccm.201502-0383OC. [DOI] [PubMed] [Google Scholar]
- 35.Allen AM. Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity. J Physiol. 1998;510:773–781. doi: 10.1111/j.1469-7793.1998.773bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung ML, Lam SY, Chen Y, Dong X, Leung PS. Functional expression of angiotensin II receptors in type-I cells of the rat carotid body. Pflugers Arch. 2001;441:474–480. doi: 10.1007/s004240000445. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Fong AY, Pilowsky PM, Abbott SBG. Sympathoexcitation following intermittent hypoxia in rat is mediated by circulating angiotensin II acting at the carotid body and subfornical organ. J Physiol. 2018;596:3217–3232. doi: 10.1113/JP275804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxena A, Little JT, Nedungadi TP, Cunningham JT. Angiotensin II type 1a receptors in subfornical organ contribute towards chronic intermittent hypoxia-associated sustained increase in mean arterial pressure. Am J Physiol Heart Circ Physiol. 2015;308:H435–H446. doi: 10.1152/ajpheart.00747.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pialoux V, Foster GE, Ahmed SB, Beaudin AE, Hanly PJ, Poulin MJ. Losartan abolishes oxidative stress induced by intermittent hypoxia in humans. J Physiol. 2011;589:5529–5537. doi: 10.1113/jphysiol.2011.218156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, Morgan BJ. Effect of AT1 receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir Physiol Neurobiol. 2012;183:67–74. doi: 10.1016/j.resp.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khayat RN, Varadharaj S, Porter K, Sow A, Jarjoura D, Gavrilin MA, et al. Angiotensin receptor expression and vascular endothelial dysfunction in obstructive sleep apnea. Am J Hypertens. 2018;31:355–361. doi: 10.1093/ajh/hpx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher EC. Invited review: physiological consequences of intermittent hypoxia. Systemic blood pressure. J Appl Physiol (1985) 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 43.Roy A, Farnham MMJ, Derakhshan F, Pilowsky PM, Wilson RJA. Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naive carotid bodies. J Physiol. 2018;596:3149–3169. doi: 10.1113/JP275001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jouett NP, Moralez G, Raven PB, Smith ML. Losartan reduces the immediate and sustained increases in muscle sympathetic nerve activity after hyperacute intermittent hypoxia. J Appl Physiol (1985) 2017;122:884–892. doi: 10.1152/japplphysiol.00683.2016. [DOI] [PubMed] [Google Scholar]
- 45.Foster GE, Brugniaux JV, Pialoux V, Duggan CTC, Hanly PJ, Ahmed SB, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. 2009;587:3287–3299. doi: 10.1113/jphysiol.2009.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamisier R, Pépin JL, Rémy J, Baguet JP, Taylor JA, Weiss JW, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 47.Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299:H925–H931. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tremblay JC, Boulet LM, Tymko MM, Foster GE. Intermittent hypoxia and arterial blood pressure control in humans: role of the peripheral vasculature and carotid baroreflex. Am J Physiol Heart Circ Physiol. 2016;311:H699–H706. doi: 10.1152/ajpheart.00388.2016. [DOI] [PubMed] [Google Scholar]
- 49.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56:369–377. doi: 10.1161/HYPERTENSIONAHA.110.152108. [DOI] [PubMed] [Google Scholar]
- 50.Pépin JL, Tamisier R, Barone-Rochette G, Launois SH, Lévy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182:954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 51.Thunström E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193:310–320. doi: 10.1164/rccm.201505-0998OC. [DOI] [PubMed] [Google Scholar]
- 52.Morgan BJ, Teodorescu M, Pegelow DF, Jackson ER, Schneider DL, Plante DT, et al. Effects of losartan and allopurinol on cardiorespiratory regulation in obstructive sleep apnoea. Exp Physiol. 2018;103:941–955. doi: 10.1113/EP087006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalyfa A, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Andrade J, et al. Effect on intermittent hypoxia on plasma exosomal micro RNA signature and endothelial function in healthy adults. Sleep. 2016;39:2077–2090. doi: 10.5665/sleep.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khalyfa A, Youssefnia N, Foster GE, Beaudin AE, Qiao Z, Pialoux V, et al. Plasma exosomes and improvements in endothelial function by angiotensin 2 type 1 receptor or cyclooxygenase 2 blockade following intermittent hypoxia. Front Neurol. 2017;8:709. doi: 10.3389/fneur.2017.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jouett NP, Watenpaugh DE, Dunlap ME, Smith ML. Interactive effects of hypoxia, hypercapnia and lung volume on sympathetic nerve activity in humans. Exp Physiol. 2015;100:1018–1029. doi: 10.1113/EP085092. [DOI] [PubMed] [Google Scholar]
- 57.Taylor KS, Murai H, Millar PJ, Haruki N, Kimmerly DS, Morris BL, et al. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension. 2016;68:1467–1474. doi: 10.1161/HYPERTENSIONAHA.116.08212. [DOI] [PubMed] [Google Scholar]
- 58.Linz D, Baumert M, Catcheside P, Floras J, Sanders P, Lévy P, et al. Assessment and interpretation of sleep disordered breathing severity in cardiology: clinical implications and perspectives. Int J Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 59.Sandford AJ, Ha A, Ngan DA, Akhabir L, Saferali A, Fox N, et al. Adhesion molecule gene variants and plasma protein levels in patients with suspected obstructive sleep apnea. PLoS One. 2019;14:e0210732. doi: 10.1371/journal.pone.0210732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.