Abstract
Objectives:
To report the respiratory function of school aged children with infantile Pompe disease (IPD) who started enzyme replacement therapy (ERT) in infancy and early childhood.
Study design:
This is a retrospective chart review of pulmonary function tests (PFT) of: 1) IPD patients 5 to 18 years of age 2) who were not ventilator dependent, and 3) were able to perform upright and supine spirometry. Subjects were divided into a younger (5-9 years) and older cohort (10-18 years) for the analysis. Upright and supine forced vital capacity (FVC), maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were analyzed.
Results:
Fourteen patients, all cross-reactive immunologic material (CRIM)-positive, met the inclusion criteria and were included in this study. Mean upright and supine FVC were 70.3% and 64.9% predicted, respectively, in the 5-9 years old cohort; and 61.5% and 52.5% predicted, respectively, in the 10-18 years old group. Individual patient trends showed stability in FVC over time in six of the 14 patients. MIPs and MEPs were consistent with inspiratory and expiratory muscle weakness in the younger and older age group but did not decline with age.
Conclusion:
Data from this cohort of CRIM-positive IPD patients showed that ERT is able to maintain respiratory function in a subgroup of patients whereas others had a steady decline. There was a statistically significant decline in FVC from the upright to supine position in both the younger and older age groups of CRIM-positive ERT treated patients. Prior to ERT, patients with IPD were unable to maintain independent ventilation beyond the first few years of life.
Keywords: Pulmonary Function testing, Infantile Pompe Disease, Enzyme Replacement Therapy
Introduction:
Pompe disease is an autosomal recessive lysosomal storage disease caused by a deficiency of acid alpha-glucosidase (GAA) – an enzyme that hydrolyzes lysosomal glycogen. Deficiency of GAA results in lysosomal glycogen accumulation in many organ systems including the heart, skeletal muscle, smooth muscle, and central nervous system (1, 2). In the respiratory system, lysosomal glycogen accumulation occurs in the muscles of respiration as well as the motor neurons innervating these muscles and patients eventually develop respiratory failure (3). Pompe disease can be broadly classified into infantile-onset and late-onset forms. The Infantile-onset form is further sub-divided into classic and non-classic forms (2). Classic infantile-onset Pompe disease (IPD) occurs in infants with little to no GAA activity and results in severe cardiomyopathy and weakness within the first few days to weeks of life (2, 4). Non-classic IPD patients have less severe cardiac involvement in the first year of life and have a longer survival (2, 5). In contrast, patients with late-onset Pompe disease (LOPD) have a more variable presentation, absence of cardiomyopathy in first year of life and can present anywhere from the first year to sixth decade of life (6-8).
Respiratory insufficiency occurs in both IPD and LOPD patients as a result of progressive diaphragm and accessory muscle weakness (3, 9-11). As a result of this muscle weakness, hypoventilation initially occurs at night and then progresses to daytime hypoventilation. In addition, restrictive lung disease occurs as evidenced by a reduced total lung capacity secondary to the inability of the respiratory muscles to generate adequate lung volumes (9, 10). The progressive muscle weakness also manifests as a decreased forced vital capacity (FVC), a drop in FVC from the upright to supine position and also a decreased maximal inspiratory and expiratory pressures (MIP and MEP). A drop in supine FVC is often one of the initial indications of diaphragmatic weakness(10). Lingual weakness is also noted in patients with Pompe disease(12, 13). Furthermore, with progressive weakness of the tongue and respiratory muscles, there is an inability to protect the upper airway and coughing is impaired. This combination of factors can result in aspiration pneumonia and inadequate airway clearance.
Enzyme replacement therapy (ERT) with recombinant human acid alfa-glucosidase was granted FDA approval for patients with Pompe disease in 2006 and has significantly improved prognosis in both IPD and LOPD patients (14-17). Prior to ERT, patients with IPD became ventilator dependent or died within their first 2 years of life (6, 18, 19). ERT has significantly changed the course of the disease in IPD with children surviving ventilator-free well beyond infancy (14-16). However, as these children are living longer, new long-term medical issues are unmasked (20). One of these issues is respiratory muscle strength weakenss and respiratory insufficiency. ERT slows the decline of pulmonary function in LOPD patients who are not ventilator dependent (21). However, response to treatment is variable in IPD patients and there are several factors that can impact the response to ERT including age at ERT start, stage of the disease, and cross-reactive immunologic material (CRIM) status (18, 22, 23). ERT is less effective in patients that are CRIM-negative, due to the antibody response against the ERT (18, 19, 24). Finally, ERT cannot cross the blood-brain barrier to treat respiratory motor neuron pathology (25, 26). Although there have been reports of long-term survivors of IPD patients on ERT, the pulmonary status of those patients who are ventilator-free into childhood is unclear. To date, no study has examined the impact of ERT on respiratory function in long-term surviving IPD patients. The purpose of this study is to report the respiratory function of school-aged IPD children 5 years of age and older who were diagnosed and initiated on ERT in infancy and early childhood.
Methods:
Subjects:
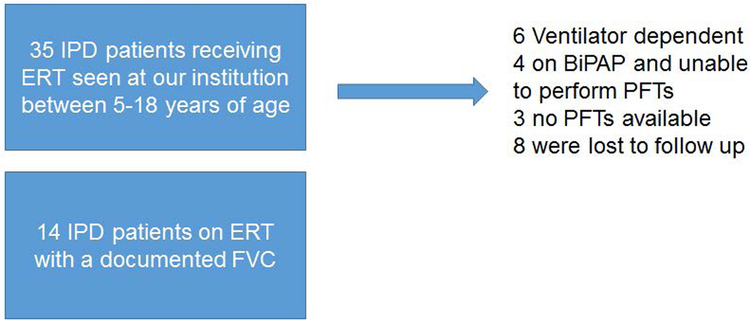
This study was approved by the Duke Institutional Review Board (Protocol #: Pro00010830). Patients were included based on the following criteria; 1) a confirmed diagnosis of IPD as previously described (27), 2) five to 18 years of age, who were diagnosed in the first year of life with cardiomyopathy, and 3) able to perform spirometry as well as maximal inspiratory pressure and maximal expiratory pressure (Figure 1). Patients were excluded if they had no pulmonary function testing (PFT) available on file, were unable to perform PFT and if they were ventilator dependent. Five years of age was chosen as the lower age cut off because with appropriate coaching, at this age they are often able to perform acceptable spirometry (28, 29). PFT results were interpreted by a pediatric pulmonologist (RMK) at the time of the visit, and confirmed by a pediatric pulmonologist (MKE) during the time of the retrospective chart review. Study relevant clinical data including demographics, GAA variants, CRIM status, ACE genotype, ERT dosing, and motor status were extracted from patient’s health records.
Figure 1:
Eligible patients for analysis
Pulmonary Function Tests:
FVC, MIPs, and MEPs were reviewed for each patient per year. If a patient performed spirometry more than once in a given year, the best spirometry values were used and spirometry was not included if the patient had an illness. In addition, PFTs that were performed during an illness were not used for this analysis. Data was then analyzed in groups and were combined into a younger age group – 5-9 year old where the last upright and supine PFT in that age group was used. Another group from 10-18 years old was also analyzed and the latest upright and supine PFTs recorded were used for each patient in this age group. The last available data point for any of the evaluated patients was at 17 years of age so the data is presented as “10-17 years”. Six patients had PFTs that spanned the two age groups and were included in both the 5-9 year age group and the 10-17 year age group. In order to take a closer look at individual patient trends and to eliminate the bias of group data, we also graphed the best FVC per year in each patient.
Statistics:
Normality testing was performed using the D’Agostino & Pearson normality test. Since a subset of FVC data did not pass the normality test, we used non-parametric testing to ensure uniform statistical analysis was used on all the data. All data were analyzed using Graph Pad Prism version 7.04. The upright and supine FVC were analyzed using the Wilcoxon test. MIPs for the older age group passed the normality test (p=0.16), but the MIPs did not (p=0.007). MEPs in the older age group did not pass normality testing (p=0.03) whereas MEPs in the younger age group passed the normality test (p=0.96)therefore MIPs and MEPs were also analyzed using a non-parametric test – the a Mann Whitney U test. Differences were considered statistically significant if p<0.05.
Results:
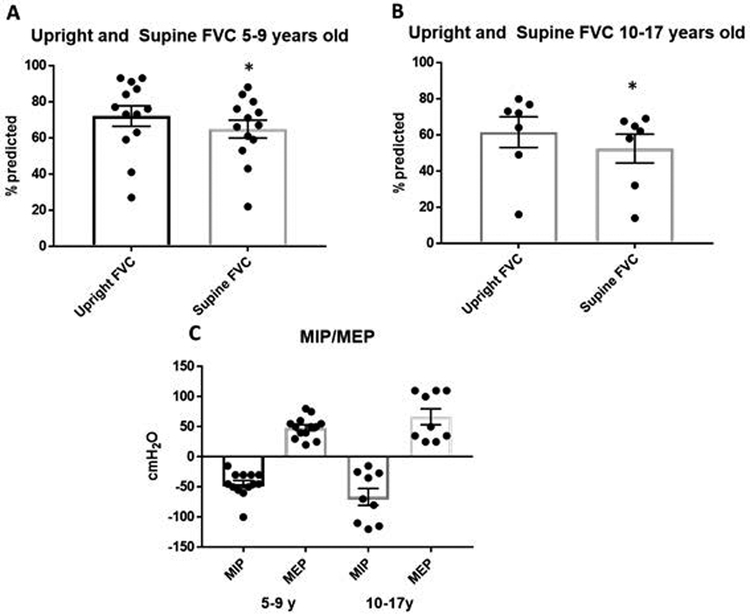
A retrospective chart review of 35 IPD patients who were seen at Duke Pompe disease clinic between 1999 and 2018 was conducted. Out of these 35 IPD patients, 14 patients, all CRIM-positive, met the inclusion criteria (Table 1). The median age of diagnosis was 2 months (range: prenatal to 18 months). The patient diagnosed at 18 months of age had non-classic IPD. The median age of ERT initiation was 3 months (range: 18 hours of life to 42 months). All patients, were on 40 mg/kg/week of ERT at the time of their latest PFT (Table 1). The changes in ERT doses can be seen in the individual patient plots (Figure 3). During their initial PFT, patients were on varying doses of ERT (Table 1). Forced vital capacity (FVC) was decreased in all patients in both the upright and supine position in both age groups. First, the group data was examined by looking at the latest recorded upright and supine FVC for each patient in the 5-9 year and the 10-18 year age group. The mean FVC in the 5-9 year old age group (n=13) was 70.3% predicted and 64.9% predicted in the upright and supine positions, respectively (Figure 2A, p=0.005). In the 10-17 year age group (n=7), the mean FVC was 61.5% predicted upright and 52.5% predicted in the supine position (Figure 2B, p=0.016). The decline in FVC between upright and supine spirometry was statistically significant in both age groups as can be expected in a child with myopathy or neuromuscular involvement (5-9: p=0.005 and 10-17:p=0.016, respectively).
TABLE 1:
Dose of enzyme replacement therapy in classic IPD patients during the time that pulmonary function testing was performed.
| ID/Gender | Complementary DNA change | ACE Genotype |
Age at diagnosis (months) |
Age at ERT initiation (months) |
Current age (years)a |
Cough assist device or NIPPV |
Mobility | |
|---|---|---|---|---|---|---|---|---|
| GAA Variant 1 | GAA Variant 2 | |||||||
| 1/M | c.1438-1G>T | c.1655T>C | I/I | Prenatal | 2 | 19 | NONE | Ambulatory with difficulties. Uses scooter |
| 2/M | c.1933G>A | c.1933G>A | N/A | 3 | 3 | 17 | NONE | Bilateral AFOs |
| 3/M | c.1327-2A>G | c.1327-2A>G | N/A | Prenatal | 0.03 | 11 | NONE | Bilateral AFOs |
| 4/F | c.1802C>T, c.1726G>A, c.2065G>A |
c.1099T>C | I/I | 6 | 6 | 15 | Cough assist device | Wheelchair |
| 5/M | c.525delT | c.1642G>T, c.1880C>T |
I/D | Prenatal | 0.5 | 12 | NONE | Bilateral AFOs |
| 6/M | c.1933G>A | c.1933G>A | I/D | 2 | 2 | 14 | Cough assist device | abnormal gait and uses wheelchair |
| 7/F | c.1293_1312del20 | c.1716C>G | N/A | 1.2 | 2.1 | 9 | NONE | Ambulates independently with AFOs |
| 8/M | c.307C>T | c.2219_2220delTG | I/D | 1 | 2 | 10 | NONE | Ambulate independently with frequent falls |
| 9/M | c.2297A>C | c.2297A>C | N/A | 6 | 6 | 15 | NONE | Wheelchair |
| 10/F | c.655G>A | c.655G>A | N/A | 7 | 7 | 13 | Cough assist device | Wheelchair |
| 11/M | c.2481+102_2646+331del | c.947A>G | N/A | 0.4 | 0.4 | 7 | Cough assist device | Uses Chipmunk shoe inserts wheelchair for long distance |
| 12/F | c.1447G>A | c.2560C>T | D/D | 13 | 13 | 6 | Cough assist device | Uses bilateral hinged AFO’s |
| 13/M | c.1408_1410delAAC | c.925G>A | D/D | 7 | 8 | 6 | Cough assist device | Unable to bear weight on legs. |
| 14/F | c.3G>A | c.923A>C | N/A | 18 | 42 | 19 | Cough assist device; BiPAP | Motorized wheelchair |
Abbreviations: ACE, angiotensin-converting enzyme; AFO, ankle-foot orthoses; BiPAP, bilevel positive airway pressure; ERT, enzyme replacement therapy; GAA, acid alpha-glucosidase; F, female; IPD, infantile Pompe disease; M, male; mo, month; N/A, not available; NIPPV, nasal intermittent positive pressure ventilation; wk, week; y, year.
All patients are currently receiving ERT at a dose of 40 mg/kg/wk.
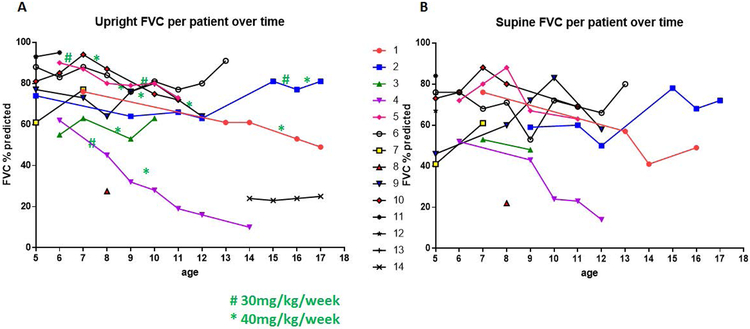
Figure 3:
Panels A & B: Upright and Supine Forced Vital Capity per patient over time shows the variable progression of IPD pulmonary function over time and the response to different doses of ERT. Of note, patients did not always undergo supine spirometry when they performed upright spirometry. For example, patient 14 did not have any supine spirometry measurements and patient 4 did not have any supine spirometry measures beyond 12 years of age. Green # and * depict the change in ERT dose to 30mg/kg/week and 40mg/kg/week, respectively.
Figure 2:
Forced Vital Capacity (FVC), Maximal Inspiratory Pressure (MIP) and Maximal Expiratory Pressure (MEP) in classic IPD patients on Enzyme Replacement therapy. Panels A and B shows the latest upright and supine FVC data point in the 5-9 year old and 10-17 year old patients. Panel C shows MIPs and MEPs at 5-9 years and again at 10-17 years. Data represented as mean ± SEM; paired t-test analysis; *p<0.05. Table 2 shows the age and the ERT dose for these patients during the time that the upright and supine FVC graphed in Figure 1 were measured. Patient 14 did not have a supine PFT so was not included in this table.
Next, we examined the individual upright and supine FVC for each of the patients in the study (Figure 3) and correlated this with the dose of ERT. Nine of the fourteen patients had spirometry performed over several years. Of these nine patients, three (patients 2, 3, and 6) had improving upright FVC over time and this was enhanced by increasing the ERT dose to 40 mg/kg/week. Patient 2 had an improvement of 74% at 5 years of age to 91 % at 17 years of age. Patient 3 had an improvement of FVC from 55% at 6 years of age to 63% at 10 years of age. Patient 6 had an improvement of FVC from 88% at 5 years of age to 91 % at 13 years of age. However, there was a decline in upright FVC for patient 1, patient 4, and patient 5. All the patients with an improvement in their FVC had ERT commenced in the first 3 months of life. Patient 14 was diagnosed at a later age and started ERT much later than the rest of the IPD patients described here which may explain why the FVC of this patient is low. However, her FVC remained stable without further deterioration.
We also analyzed maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) (30, 31). MIPs and MEPs are less reliable because they depend on a patient’s motivation and are subject to more variability (32-34). MIPs and MEPs in the younger age group were consistent with significant inspiratory and expiratory strength weakness. These values did not decline with age, but instead, they seemed to improve slightly (mean MIP improved from −45mmHg to −66mmHg; mean MEP improved from 48mmHg to 67mmHg). However, a Mann-Whitney U test shows that MIPs did not significantly decrease with age (U=46.5, p=0.44); similarly MEPs did not significantly deteriorate with age (U=29.5; p = 0.57).
Discussion
Historically, patients with IPD were either deceased or ventilator dependent within the first two years of life (18, 19). The advent of ERT has resulted in ventilator-free survival in these CRIM-positive Pompe disease patients. Despite the significant improvement in ventilator-free survival in CRIM positive patients with Pompe disease, there continues to be evidence of respiratory insufficiency. The respiratory system is affected in Pompe disease with glycogen accumulation occurring in the diaphragm (3), the tongue (35), the tracheal smooth muscle (36), and in the respiratory motor neurons (35, 37). In this current case series, there is a variable course with some patients showing an annual decline in FVC and others showing an improvement. It is unclear whether the improvement is secondary to improved diaphragmatic strength but nonetheless it shows that there is stability of lung function in these patients who historically would have been ventilator dependent if they were not on ERT.
A few of our IPD patients continued to have a decline in FVC despite increasing the dose of ERT to 40 mg/kg/week. This deterioration occurred in some patients despite an early diagnosis and early ERT initiation (17). For example, patient 8 was diagnosed at 1 month of life and started ERT at 2 months of life but his FVC was significantly lower than the other patients who started ERT later. Patient 4 had a significant decline in FVC over time despite increasing ERT dose and she is the only patient in this study who is also BiPAP dependent. She was diagnosed at 6 months of age and so had a significant delay in ERT initiation. In addition, patients 1 and 4 had a decline in FVC despite the fact that patient 1 was diagnosed in utero and started ERT at 2 months. In addition, both patients 1 and 4 have an I/I ACE genotype and were the only patients in this group with I/I ACE genotype. The I/I ACE genotype has previously been correlated with a delay in muscle weakness and disease onset but in these two patients it does not appear to be protective and their FVC continued to decline (22, 38, 39). Thus, in this case series, age at ERT initiation and ACE genotyping have only a slight correlation with the rate of FVC decline. When all the data were grouped and averaged into younger and older spirometry groups, there was a significant drop in supine FVC at both time points. This is expected with the neuromuscular phenotype of these patients. However, when averaged upright spirometry in the younger age groups was compared to that of the older age group FVC did not significantly decline. This can possibly be explained by the optimization of ERT dosing with 40 mg/kg/week effectively which blunted further respiratory decline (14, 40, 41).
However, ERT did not prevent a decline in respiratory function in all of our patients. This persistent decline may be due to inefficient targeting and uptake of ERT into the respiratory muscles such as the diaphragm and intercostal muscles (42). In addition, these patients most likely also had persistent respiratory neuropathology (25, 26, 43) since ERT does not cross the blood brain barrier.
Several preclinical studies from Pompe disease animal models (including the Gaa−/− mice and the acid-maltase deficient Japanese quail) reveal substantial glycogen accumulation in the motor neurons (44-46), and specifically the respiratory motor neurons (25, 35, 37, 47). In fact, when muscle was rescued in a muscle-specific GAA mice that only had CNS pathology, measurements of respiratory function revealed significantly blunted breathing at baseline and during a challenge with hypercapnia compared to wild type (25) This neurological pathology is also noted in patients with Pompe disease. Clinically, autopsy findings of patients with Pompe disease confirm anterior horn motor neurons and brain stem motor neuron glycogen storage(48-50). In addition, autopsy reports of Pompe patients that were on ERT also show persistent glycogen accumulation in the motor neurons of the cervical spinal cord where the phrenic motor neurons are located (25, 26, 51). These motor neuron findings are reported in both IPD (25, 51) and LOPD patients(26) and highlight the importance of treating the respiratory motor neurons in order to correct the respiratory pathology.
Nonetheless, although ERT does not cross the blood-brain barrier, its impact on the respiratory muscles appears to preserve some respiratory function in CRIM-positive patients. Our findings are consistent with those findings in LOPD patients which demonstrate a variable evolution of pulmonary function tests and respiratory strength (10, 52). Pellegrini et al reported that LOPD patients have a gradual decline in respiratory function over time with a weak correlation between respiratory and locomotor function (52). A recent study from the Pompe registry also reported stability in FVC over five years in 379 LOPD patients (53).
The limitations of this case series include a small sample size. In addition, since this study was mostly retrospective in nature, aspects of the data were missing for the older children in the study. As stated above, we also only evaluated patients that were able to perform pulmonary function testing which makes the study biased towards those patients with better respiratory function at 5 years of age. One point that needs to be made is we only looked at the patients who are currently alive and others may have died of respiratory failure or other causes. Furthermore, there were no CRIM-negative patients at the time of this study that had evaluable PFTs and therefore were not included in this study. However, recent advances in immune modulation with ERT have enhanced survival in CRIM-negative patients and these patients have a new and emerging phenotype but data on pulmonary function tests has yet to be collected (54). We will need to evaluate more of the IPD patients over time to assess the impact of genotype, ACE genotype, CRIM status, and dose escalation on the rate of decline of FVC (19, 22, 41). Finally, because these children had variable efforts with their maximal inspiratory and expiratory strengths measurements, these data were difficult to interpret. The higher MIPs and MEPs at the older age groups are most likely a reflection of improved effort rather than improved muscle strengths. A decrease in FVC from the upright to the supine position is a more sensitive indicator of diaphragm weakness than MIPs and MEPs (30, 31). MIPs and MEPs are difficult to perform reliably because they depend on effort and may be altered if there is any bulbar weakness (30).
In conclusion, this study is the first to report the respiratory function of CRIM-positive IPD patients who commenced ERT in the first year of life. In preparation for upcoming clinical trials involving novel ERTs and gene therapy (55, 56), this respiratory function information provides a baseline so we can better evaluate the impact of future therapies.
Table 2.
Age and ERT Dose for patients included in Figure 2.
| Patient | Age at PFT 1 (years) |
ERT dose at PFT 1 | Age at PFT 2 |
ERT dose PFT 2 |
|---|---|---|---|---|
| 1 | 7.1 | 20 mg/kg qoweek | 17.4 | 40 mg/kg/week |
| 2 | 9.0 | 20 mg/kg/qoweek | 16.3 | 30 mg/kg/week |
| 3 | 7.8 | 30 mg/kg/qoweek | 10.1 | 40 mg/kg/week |
| 4 | 9.0 | 30 mg/kg/week | 12.8 | 40 mg/kg/week |
| 5 | 8.1 | 30 mg/kg/qoweek | 11.5 | 40 mg/kg/week |
| 6 | 8.2 | 40 mg/kg/qoweek | 12.9 | 40 mg/kg/week |
| 7 | 7.8 | 40 mg/kg/week | ||
| 8 | 8.7 | 40 mg/kg/week | ||
| 9 | 7.8 | 30 mg/kg/qoweek | 12.3 | 40 mg/kg/week |
| 10 | 8.9 | 40 mg/kg/week | 11.2 | 40 mg/kg/week |
| 11 | 6 | 40 mg/kg/week | ||
| 12 | 5.9 | 40 mg/kg/week | ||
| 13 | 5.9 | 40 mg/kg/week |
Acknowledgements;
We would like to thank Drs. Kanecia Zimmerman and Karan Kumar for their statistical advice.
Funding: NIH NICHD K08HD077040-01A1 (MKE); 1R01HD099486-01 (MKE and PSK); R21NS098131-01 (MKE); LDN grant (PSK)
Footnotes
Conflicts of interest: P.S.K. has received grant support from Sanofi Genzyme, Valerion Therapeutics, Shire Pharmaceuticals, and Amicus Therapeutics. P.S.K. has received consulting fees and honoraria from Sanofi Genzyme, Shire Pharmaceuticals, Amicus Therapeutics, Vertex Pharmaceuticals, and Asklepios BioPharmaceutical, Inc. (AskBio). P.S.K. is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme. P.S.K. has equity in Actus Therapeutics, which is developing gene therapy for Pompe disease.
REFERENCES
- 1.Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency In: Valle D, Scriver CR, editors. Scriver’s OMMBID the online metabolic & molecular bases of inherited disease. New York: McGraw-Hill; 2009. [Google Scholar]
- 2.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D, Infantile-Onset Pompe Disease Natural History Study G. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. The Journal of pediatrics 2006; 148: 671–676. [DOI] [PubMed] [Google Scholar]
- 3.Fuller DD, ElMallah MK, Smith BK, Corti M, Lawson LA, Falk DJ, Byrne BJ. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 2013; 189: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, Bakker HD, Loonen MC, de Klerk JB, Reuser AJ, van der Ploeg AT. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003; 112: 332–340. [DOI] [PubMed] [Google Scholar]
- 5.Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F. Identification of two subtypes of infantile acid maltase deficiency. J Pediatr 2000; 137: 283–285. [DOI] [PubMed] [Google Scholar]
- 6.Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case LE, Crowley JF, Downs S, Howell RR, Kravitz RM, Mackey J, Marsden D, Martins AM, Millington DS, Nicolino M, O’Grady G, Patterson MC, Rapoport DM, Slonim A, Spencer CT, Tifft CJ, Watson MS. Pompe disease diagnosis and management guideline. Genet Med 2006; 8: 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laforet P, Laloui K, Granger B, Hamroun D, Taouagh N, Hogrel JY, Orlikowski D, Bouhour F, Lacour A, Salort-Campana E, Penisson-Besnier I, Sacconi S, Zagnoli F, Chapon F, Eymard B, Desnuelle C, Pouget J, French Pompe Registry Study G. The French Pompe registry. Baseline characteristics of a cohort of 126 patients with adult Pompe disease. Rev Neurol (Paris) 2013; 169: 595–602. [DOI] [PubMed] [Google Scholar]
- 8.de Vries JM, van der Beek NA, Hop WC, Karstens FP, Wokke JH, de Visser M, van Engelen BG, Kuks JB, van der Kooi AJ, Notermans NC, Faber CG, Verschuuren JJ, Kruijshaar ME, Reuser AJ, van Doorn PA, van der Ploeg AT. Effect of enzyme therapy and prognostic factors in 69 adults with Pompe disease: an open-label single-center study. Orphanet journal of rare diseases 2012; 7: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson EM, Roberts M, Mozaffar T, Young P, Quartel A, Berger KI. Pulmonary function tests (maximum inspiratory pressure, maximum expiratory pressure, vital capacity, forced vital capacity) predict ventilator use in late-onset Pompe disease. Neuromuscul Disord 2016; 26: 136–145. [DOI] [PubMed] [Google Scholar]
- 10.Berger KI, Chan Y, Rom WN, Oppenheimer BW, Goldring RM. Progression from respiratory dysfunction to failure in late-onset Pompe disease. Neuromuscul Disord 2016; 26: 481–489. [DOI] [PubMed] [Google Scholar]
- 11.Alonso-Perez J, Segovia S, Dominguez-Gonzalez C, Olive M, Mendoza Grimon MD, Fernandez-Torron R, Lopez de Munain A, Munoz-Blanco JL, Ramos-Fransi A, Almendrote M, Illa I, Diaz-Manera J. Spanish Pompe registry: Baseline characteristics of first 49 patients with adult onset of Pompe disease. Medicina clinica 2019. [DOI] [PubMed] [Google Scholar]
- 12.Hobson-Webb LD, Jones HN, Kishnani PS. Oropharyngeal dysphagia may occur in late-onset Pompe disease, implicating bulbar muscle involvement. Neuromuscul Disord 2013; 23: 319–323. [DOI] [PubMed] [Google Scholar]
- 13.Dubrovsky A, Corderi J, Lin M, Kishnani PS, Jones HN. Expanding the phenotype of late-onset pompe disease: Tongue weakness: A new clinical observation. Muscle Nerve 2011. [DOI] [PubMed] [Google Scholar]
- 14.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, De la Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 2007; 68: 99–109. [DOI] [PubMed] [Google Scholar]
- 15.Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, Herman GE, Amalfitano A, Thurberg BL, Richards S, Davison M, Corzo D, Chen YT. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. The Journal of pediatrics 2006; 149: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolino M, Byrne B, Wraith JE, Leslie N, Mandel H, Freyer DR, Arnold GL, Pivnick EK, Ottinger CJ, Robinson PH, Loo JC, Smitka M, Jardine P, Tato L, Chabrol B, McCandless S, Kimura S, Mehta L, Bali D, Skrinar A, Morgan C, Rangachari L, Corzo D, Kishnani PS. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009; 11: 210–219. [DOI] [PubMed] [Google Scholar]
- 17.Kishnani PS, Corzo D, Leslie ND, Gruskin D, Van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, Bauer MS, Jokic M, Tsai CE, Tsai BW, Morgan C, O’Meara T, Richards S, Tsao EC, Mandel H. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatric research 2009; 66: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, Chen YT, Rosenberg AS, Kishnani PS. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genetics in medicine : official journal of the American College of Medical Genetics 2011; 13: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010; 99: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prater SN, Banugaria SG, DeArmey SM, Botha EG, Stege EM, Case LE, Jones HN, Phornphutkul C, Wang RY, Young SP, Kishnani PS. The emerging phenotype of long-term survivors with infantile Pompe disease. Genetics in medicine : official journal of the American College of Medical Genetics 2012; 14: 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regnery C, Kornblum C, Hanisch F, Vielhaber S, Strigl-Pill N, Grunert B, Muller-Felber W, Glocker FX, Spranger M, Deschauer M, Mengel E, Schoser B. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J Inherit Metab Dis 2012; 35: 837–845. [DOI] [PubMed] [Google Scholar]
- 22.De Filippi P, Saeidi K, Ravaglia S, Dardis A, Angelini C, Mongini T, Morandi L, Moggio M, Di Muzio A, Filosto M, Bembi B, Giannini F, Marrosu G, Rigoldi M, Tonin P, Servidei S, Siciliano G, Carlucci A, Scotti C, Comelli M, Toscano A, Danesino C. Genotype-phenotype correlation in Pompe disease, a step forward. Orphanet journal of rare diseases 2014; 9: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanovitch TL, Banugaria SG, Proia AD, Kishnani PS. Clinical and histologic ocular findings in pompe disease. J Pediatr Ophthalmol Strabismus 2010; 47: 34–40. [DOI] [PubMed] [Google Scholar]
- 24.Berrier KL, Kazi ZB, Prater SN, Bali DS, Goldstein J, Stefanescu MC, Rehder CW, Botha EG, Ellaway C, Bhattacharya K, Tylki-Szymanska A, Karabul N, Rosenberg AS, Kishnani PS. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med 2015; 17: 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH Jr., Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A 2009; 106: 9419–9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne BJ, Fuller DD, Smith BK, Clement N, Coleman K, Cleaver B, Vaught L, Falk DJ, McCall A, Corti M. Pompe disease gene therapy: neural manifestations require consideration of CNS directed therapy. Annals of translational medicine 2019; 7: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006; 148: 671–676. [DOI] [PubMed] [Google Scholar]
- 28.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, Ambrosius WT, Tepper RS. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med 2001; 163: 619–623. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 30.Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest 2002; 121: 436–442. [DOI] [PubMed] [Google Scholar]
- 31.Baydur A Respiratory Muscle Strength: A Reliable Index for Predicting Survival in Amyotrophic Lateral Sclerosis? Am J Respir Crit Care Med 2017; 195: 12–13. [DOI] [PubMed] [Google Scholar]
- 32.Aldrich TK, Spiro P. Maximal inspiratory pressure: does reproducibility indicate full effort? Thorax 1995; 50: 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hull J, Aniapravan R, Chan E, Chatwin M, Forton J, Gallagher J, Gibson N, Gordon J, Hughes I, McCulloch R, Russell RR, Simonds A. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax 2012; 67 Suppl 1: i1–40. [DOI] [PubMed] [Google Scholar]
- 34.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 35.Elmallah MK, Falk DJ, Nayak S, Federico RA, Sandhu MS, Poirier A, Byrne BJ, Fuller DD. Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice. Mol Ther 2014; 22: 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keeler AM, Liu D, Zieger M, Xiong L, Salemi J, Bellve K, Byrne BJ, Fuller DD, ZhuGe R, ElMallah MK. Airway smooth muscle dysfunction in Pompe (Gaa(−/−) ) mice. Am J Physiol Lung Cell Mol Physiol 2017; 312: L873–L881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, Greer JJ, Fuller DD. Ampakines Stimulate Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease. Am J Respir Cell Mol Biol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuperus E, van der Meijden JC, In ‘t Groen SLM, Kroos MA, Hoogeveen-Westerveld M, Rizopoulos D, Martinez MYN, Kruijshaar ME, van Doorn PA, van der Beek N, van der Ploeg AT, Pijnappel W. The ACE I/D polymorphism does not explain heterogeneity of natural course and response to enzyme replacement therapy in Pompe disease. PLoS One 2018; 13: e0208854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishnani PS, Beckemeyer AA, Mendelsohn NJ. The new era of Pompe disease: advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management. American journal of medical genetics Part C, Seminars in medical genetics 2012; 160C: 1–7. [DOI] [PubMed] [Google Scholar]
- 40.Yanovitch TL, Casey R, Banugaria SG, Kishnani PS. Improvement of bilateral ptosis on higher dose enzyme replacement therapy in Pompe disease. J Neuroophthalmol 2010; 30: 165–166. [DOI] [PubMed] [Google Scholar]
- 41.van Gelder CM, Poelman E, Plug I, Hoogeveen-Westerveld M, van der Beek NA, Reuser AJ, van der Ploeg AT. Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe disease: an open-label single-center study. Journal of inherited metabolic disease 2016; 39: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shea L, Raben N. Autophagy in skeletal muscle: implications for Pompe disease. Int J Clin Pharmacol Ther 2009; 47 Suppl 1: S42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuller DD, ElMallah MK, Smith BK, Corti M, Falk DJ, Byrne BJ. Pompe Disease and the Respiratory System Respiratory Physiology and Neurobiology 2013; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidman RL, Taksir T, Fidler J, Zhao M, Dodge JC, Passini MA, Raben N, Thurberg BL, Cheng SH, Shihabuddin LS. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J Neuropathol Exp Neurol 2008; 67: 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim JA, Yi H, Gao F, Raben N, Kishnani PS, Sun B. Intravenous Injection of an AAV-PHP.B Vector Encoding Human Acid alpha-Glucosidase Rescues Both Muscle and CNS Defects in Murine Pompe Disease. Mol Ther Methods Clin Dev 2019; 12: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui T, Kuroda S, Mizutani M, Kiuchi Y, Suzuki K, Ono T. Generalized glycogen storage disease in Japanese quail (Coturnix coturnix japonica). Vet Pathol 1983; 20: 312–321. [DOI] [PubMed] [Google Scholar]
- 47.Qiu K, Falk DJ, Reier PJ, Byrne BJ, Fuller DD. Spinal delivery of AAV vector restores enzyme activity and increases ventilation in Pompe mice. Mol Ther 2012; 20: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hogan GR, Gutmann L, Schmidt R, Gilbert E. Pompe’s disease. Neurology 1969; 19: 894–900. [DOI] [PubMed] [Google Scholar]
- 49.Teng YT, Su WJ, Hou JW, Huang SF. Infantile-onset glycogen storage disease type II (Pompe disease): report of a case with genetic diagnosis and pathological findings. Chang Gung Med J 2004; 27: 379–384. [PubMed] [Google Scholar]
- 50.Mancall EL, Aponte GE, Berry RG. Pompe’s Disease (Diffuse Glycogenosis) with Neuronal Storage. J Neuropathol Exp Neurol 1965; 24: 85–96. [DOI] [PubMed] [Google Scholar]
- 51.Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, Kishnani PS, O’Callaghan M. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Laboratory investigation; a journal of technical methods and pathology 2006; 86: 1208–1220. [DOI] [PubMed] [Google Scholar]
- 52.Pellegrini N, Laforet P, Orlikowski D, Pellegrini M, Caillaud C, Eymard B, Raphael JC, Lofaso F. Respiratory insufficiency and limb muscle weakness in adults with Pompe’s disease. Eur Respir J 2005; 26: 1024–1031. [DOI] [PubMed] [Google Scholar]
- 53.Stockton David W. MB, Byrne Barry, Llerena Juan Jr., Kishnani Priya, Roberts Mark, van der Ploeg Ans, Araujo Roberto, Maruti Sonia S., Thibault Nathan, Berger Kenneth I.. NYImpact of Time from Diagnosis to Treatment on Lung Function among Patients with Late-Onset Pompe Disease: Data from the Pompe Registry. LDN; 2019. [Google Scholar]
- 54.Kazi ZB, Desai AK, Berrier KL, Troxler RB, Wang RY, Abdul-Rahman OA, Tanpaiboon P, Mendelsohn NJ, Herskovitz E, Kronn D, Inbar-Feigenberg M, Ward-Melver C, Polan M, Gupta P, Rosenberg AS, Kishnani PS. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI insight 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keeler AM, Zieger M, Todeasa S, McCall A, Gifford J, Bircsak S, Choudhury SR, Byrne BJ, Sena-Esteves M, ElMallah MK. Systemic delivery of AAVB1-GAA clears glycogen and prolongs survival in a mouse model of Pompe disease. Hum Gene Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han SO, Ronzitti G, Arnson B, Leborgne C, Li S, Mingozzi F, Koeberl D. Low-Dose Liver-Targeted Gene Therapy for Pompe Disease Enhances Therapeutic Efficacy of ERT via Immune Tolerance Induction. Mol Ther Methods Clin Dev 2017; 4: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]