Abstract
Background
More than two‐thirds of pregnant women experience low‐back pain and almost one‐fifth experience pelvic pain. The two conditions may occur separately or together (low‐back and pelvic pain) and typically increase with advancing pregnancy, interfering with work, daily activities and sleep.
Objectives
To update the evidence assessing the effects of any intervention used to prevent and treat low‐back pain, pelvic pain or both during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth (to 19 January 2015), and the Cochrane Back Review Groups' (to 19 January 2015) Trials Registers, identified relevant studies and reviews and checked their reference lists.
Selection criteria
Randomised controlled trials (RCTs) of any treatment, or combination of treatments, to prevent or reduce the incidence or severity of low‐back pain, pelvic pain or both, related functional disability, sick leave and adverse effects during pregnancy.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
We included 34 RCTs examining 5121 pregnant women, aged 16 to 45 years and, when reported, from 12 to 38 weeks’ gestation. Fifteen RCTs examined women with low‐back pain (participants = 1847); six examined pelvic pain (participants = 889); and 13 examined women with both low‐back and pelvic pain (participants = 2385). Two studies also investigated low‐back pain prevention and four, low‐back and pelvic pain prevention. Diagnoses ranged from self‐reported symptoms to clinicians’ interpretation of specific tests. All interventions were added to usual prenatal care and, unless noted, were compared with usual prenatal care. The quality of the evidence ranged from moderate to low, raising concerns about the confidence we could put in the estimates of effect.
For low‐back pain
Results from meta‐analyses provided low‐quality evidence (study design limitations, inconsistency) that any land‐based exercise significantly reduced pain (standardised mean difference (SMD) ‐0.64; 95% confidence interval (CI) ‐1.03 to ‐0.25; participants = 645; studies = seven) and functional disability (SMD ‐0.56; 95% CI ‐0.89 to ‐0.23; participants = 146; studies = two). Low‐quality evidence (study design limitations, imprecision) also suggested no significant differences in the number of women reporting low‐back pain between group exercise, added to information about managing pain, versus usual prenatal care (risk ratio (RR) 0.97; 95% CI 0.80 to 1.17; participants = 374; studies = two).
For pelvic pain
Results from a meta‐analysis provided low‐quality evidence (study design limitations, imprecision) of no significant difference in the number of women reporting pelvic pain between group exercise, added to information about managing pain, and usual prenatal care (RR 0.97; 95% CI 0.77 to 1.23; participants = 374; studies = two).
For low‐back and pelvic pain
Results from meta‐analyses provided moderate‐quality evidence (study design limitations) that: an eight‐ to 12‐week exercise program reduced the number of women who reported low‐back and pelvic pain (RR 0.66; 95% CI 0.45 to 0.97; participants = 1176; studies = four); land‐based exercise, in a variety of formats, significantly reduced low‐back and pelvic pain‐related sick leave (RR 0.76; 95% CI 0.62 to 0.94; participants = 1062; studies = two).
The results from a number of individual studies, incorporating various other interventions, could not be pooled due to clinical heterogeneity. There was moderate‐quality evidence (study design limitations or imprecision) from individual studies suggesting that osteomanipulative therapy significantly reduced low‐back pain and functional disability, and acupuncture or craniosacral therapy improved pelvic pain more than usual prenatal care. Evidence from individual studies was largely of low quality (study design limitations, imprecision), and suggested that pain and functional disability, but not sick leave, were significantly reduced following a multi‐modal intervention (manual therapy, exercise and education) for low‐back and pelvic pain.
When reported, adverse effects were minor and transient.
Authors' conclusions
There is low‐quality evidence that exercise (any exercise on land or in water), may reduce pregnancy‐related low‐back pain and moderate‐ to low‐quality evidence suggesting that any exercise improves functional disability and reduces sick leave more than usual prenatal care. Evidence from single studies suggests that acupuncture or craniosacral therapy improves pregnancy‐related pelvic pain, and osteomanipulative therapy or a multi‐modal intervention (manual therapy, exercise and education) may also be of benefit.
Clinical heterogeneity precluded pooling of results in many cases. Statistical heterogeneity was substantial in all but three meta‐analyses, which did not improve following sensitivity analyses. Publication bias and selective reporting cannot be ruled out.
Further evidence is very likely to have an important impact on our confidence in the estimates of effect and change the estimates. Studies would benefit from the introduction of an agreed classification system that can be used to categorise women according to their presenting symptoms, so that treatment can be tailored accordingly.
Plain language summary
Treatments for preventing and treating low‐back and pelvic pain during pregnancy
Review question
We looked for evidence about the effects of any treatment used to prevent or treat low‐back pain, pelvic pain or both during pregnancy. We also wanted to know whether treatments decreased disability or sick leave, and whether treatments caused any side effects for pregnant women.
Background
Pain in the lower‐back, pelvis, or both, is a common complaint during pregnancy and often gets worse as pregnancy progresses. This pain can disrupt daily activities, work and sleep for pregnant women. We wanted to find out whether any treatment, or combination of treatments, was better than usual prenatal care for pregnant women with these complaints.
Study characteristics
The evidence is current to 19 January 2015. We included 34 randomised studies in this updated review, with 5121 pregnant women, aged 16 to 45 years. Women were from 12 to 38 weeks’ pregnant. Studies looked at different treatments for pregnant women with low‐back pain, pelvic pain or both types of pain. All treatments were added to usual prenatal care, and were compared with usual prenatal care alone in 23 studies. Studies measured women's symptoms in different ways, ranging from self‐reported pain and sick leave to the results of specific tests.
Key results
Low‐back pain
When we combined the results from seven studies (645 women) that compared any land‐based exercise with usual prenatal care, exercise interventions (lasting from five to 20 weeks) improved women's levels of low‐back pain and disability.
Pelvic pain
There is less evidence available on treatments for pelvic pain. Two studies found that women who participated in group exercise and received information about managing their pain reported no difference in their pelvic pain than women who received usual prenatal care.
Low‐back and pelvic pain
The results of four studies combined (1176 women) showed that an eight‐ to 12‐week exercise program reduced the number of women who reported low‐back and pelvic pain. Land‐based exercise, in a variety of formats, also reduced low‐back and pelvic pain‐related sick leave in two studies (1062 women).
However, two other studies (374 women) found that group exercise plus information was no better at preventing either pelvic or low‐back pain than usual prenatal care.
There were a number of single studies that tested a variety of treatments. Findings suggested that craniosacral therapy, osteomanipulative therapy or a multi‐modal intervention (manual therapy, exercise and education) may be of benefit.
When reported, there were no lasting side effects in any of the studies.
Quality of the evidence and conclusions
There is low‐quality evidence suggesting that exercise improves pain and disability for women with low‐back pain, and moderate‐quality evidence that exercise results in less sick leave and fewer women reporting pain in those with both low‐back and pelvic pain together. The quality of evidence is due to problems with the design of studies, small numbers of women and varied results. As a result, we believe that future studies are very likely to change our conclusions. There is simply not enough good quality evidence to make confident decisions about treatments for these complaints.
Summary of findings
Summary of findings for the main comparison. Low‐back pain: any exercises + usual prenatal care versus usual prenatal care.
| Low‐back pain: any exercises + usual prenatal care versus usual prenatal care | ||||||
| Patient or population: pregnant women with back pain Intervention: low‐back pain: any exercises + usual prenatal care versus usual prenatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual prenatal care | Any exercises + usual prenatal care | |||||
| Pain intensity measured by a number of different measurements; lower score = better. Follow‐up was measured between 8 and 24 weeks after randomisation across studies. Treatments varied from 5 to 20 weeks in duration. | The mean pain intensity in the control groups was 16.14 | The mean pain intensity in the intervention groups was 0.64standard deviations lower
(1.03 to 0.25 lower) (SMD ‐0.64, 95% CI ‐1.03 to ‐0.25; participants = 645; studies = 7) |
SMD ‐0.64, 95% CI ‐1.03 to ‐0.25 |
645 (7 studies) | ⊕⊕⊝⊝ low1,2 | A standard deviation of 0.64 lower represents a moderate difference between groups, and may be clinically relevant. However, there was considerable clinical heterogeneity amongst the participants, interventions and outcome measures. |
| Disability measured by Roland Morris Disability Questionnaire and Oswestry Disability Index; lower score = better. Follow‐up was measured from 5 to 12 weeks after randomisation across studies. Treatments varied from 5 to 8 weeks in duration. | The mean disability in the control groups was 26.64 | The mean disability in the intervention groups was 0.56standard deviations lower (0.89 lower to 0.23 higher) | SMD ‐0.56; 95% CI ‐0.89 to ‐0.23 | 146 (2 studies) | ⊕⊕⊝⊝ low1,3 | A standard deviation of 0.56 lower represents a moderate difference between groups and may be clinically relevant. |
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Poor or no description of randomisation process, allocation concealment, or blinding of research personnel in most of the studies in the meta‐analyses. 2 One study reported results in the opposite direction. 3 Imprecision (< 400 participants).
4 The assumed risk was calculated by measuring the mean pain intensity and the mean disability for the control groups.
Summary of findings 2. Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care.
| Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care | ||||||
| Patient or population: pregnant women with, or at risk of developing, pelvic and back pain Intervention: pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk (moderate risk population)3 | Corresponding risk | |||||
| Usual prenatal care | Any exercises + usual prenatal care | |||||
| Number of women who reported pain on Visual Analogue Scale. Follow‐up was measured immediately after the intervention. Treatments ran from 8 to 12 weeks in duration. | Study population | RR 0.66 (0.45 to 0.97) | 1176 (4 studies) | ⊕⊕⊕⊝ moderate1 | mean reduction of 34% across studies | |
| 708 per 1000 | 467 per 1000 (318 to 686) | |||||
| Number of women who reported LBPP‐related sick leave. Follow‐up was measured immediately after the intervention, which ran for 12 weeks. | Study population | RR 0.76 (0.62 to 0.94) | 1062 (2 studies) |
⊕⊕⊕⊝ moderate2 | mean reduction of 24% across studies | |
| 288 per 1000 | 219 per 1000 (178 to 270) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was a mix of potential biases among the four studies: no allocation concealment (1); no blinding of research personnel (all); poor/no description of drop‐outs, co‐interventions and baseline inequality (mixed). 2 No blinding of research personnel; poor description of attrition; some differences in co‐interventions.
3 Moderate risk population chosen as the assumed risk because studies included pregnant women who did not have serious, systemic morbidities and entered at different points of their pregnancies, with varying levels of pain and disability.
Background
Description of the condition
Low‐back pain (LBP) and pelvic pain (PP) are common during pregnancy and tend to increase as pregnancy advances; in some cases, the pain radiates into the buttock, leg and foot. However, much still remains unclear about these very distinct but related conditions (Vermani 2010; Vleeming 2008). For many women, pain can become so severe that it interferes with ordinary daily activities, disturbs sleep and contributes to high levels of sick leave (Kalus 2007; Mogren 2006; Sinclair 2014; Skaggs 2007). Global prevalence is reported to range from 24% to 90%, in part, because there is currently no universally recognised classification system for the condition (Vermani 2010; Vleeming 2008). A prospective study of 325 pregnant women from the Middle East found that almost two‐thirds reported LBP, PP or both, during their current pregnancy (Mousavi 2007), with similar proportions reported by a sample of pregnant women (N = 599) in the United States (Skaggs 2007). Relapse rates are high in subsequent pregnancies (Mogren 2005; Skaggs 2007), and a postpartum prevalence of 24.7% (range 0.6% to 67%) (Wu 2004) underlines the importance of developing effective treatment programmes for this condition. Despite these figures, it is estimated that over 50% of women receive little or no intervention from healthcare providers (Greenwood 2001; Sinclair 2014; Skaggs 2007). These numbers suggest that more studies are needed to establish the underlying aetiology and pathogenesis of the conditions (Miquelutti 2013; Mørkved 2007). Current theories include: altered posture with the increased lumbar lordosis (exaggerated curvature of the lower spine) necessary to balance the increasing anterior weight of the womb, and inefficient neuromuscular control (Vleeming 2008). Several risk factors have also been identified, including increased weight during pregnancy, previous history of LBP and low job satisfaction (Albert 2006; Vleeming 2008).
Whilst LBP and PP may occur together during pregnancy, PP (posterior pain arising from the region of the sacroiliac joints, anterior pain from the pubic symphysis, or both) can often occur on its own, along with residual symptoms postpartum. A follow‐up to a cohort study of 870 pregnant women with PP found that 10% still experienced moderate or severe pain 18 months after delivery, and were seriously hindered in more than one activity (Rost 2006). Estimates of the prevalence of pregnancy‐related PP vary (depending on the type of study, diagnostic criteria and precision of identifying the pain), however, the best evidence suggests a point prevalence of 20% (Vleeming 2008). Van de Pol 2007 also reported that, whilst prognosis was generally good, those women reporting PP were less mobile than those reporting LBP only, and experienced more co‐morbidity and depressive symptoms; these findings are supported by a recent review (Vermani 2010). The need for a uniform terminology in order to promote research and management of these conditions is widely recognised. There are a number of tests validated for distinguishing LBP from PP; Vermani 2010 and Vleeming 2008 provide details of these tests.
Description of the intervention
European guidelines recommend that LBP (Airaksinen 2006) and PP (Vleeming 2008), are managed by providing adequate information and reassurance to patients that it is best to stay active, continue normal daily activities and work if possible, and by offering individualised exercises where appropriate. Similarly, prenatal practitioners in the United Kingdom and Nordic countries give women information about how to manage LBP, PP or both during their pregnancy and may refer them to physiotherapy for a more specific treatment programme. However, in the United States, women are taught that LBP is a normal part of pregnancy. Interventions that have been used to date to help manage the pain include exercises, frequent rest, hot and cold compresses, abdominal or pelvic support belts, massage, acupuncture, chiropractic, aromatherapy, relaxation, herbs, yoga, Reiki, paracetamol, and non‐steroidal anti‐inflammatory drugs (Sinclair 2014; Vermani 2010).
For this review, we conducted a broad search for studies that assessed the effects of any intervention that prevented or treated LBP, PP, or a combination of both for women at any stage of their pregnancy. We identified studies investigating the effects of: exercise (land‐ or water‐based), pelvic belts, osteopathic manipulative therapy (OMT), spinal manipulative therapy (SMT), neuro emotional technique (NET), craniosacral therapy (CST), transcutaneous electrical nerve stimulation (TENS), kinesiotaping (KT), yoga, acupuncture, acupuncture plus exercises, and a multi‐modal approach incorporating manual therapy, exercise and education.
How the intervention might work
Exercise (land‐ or water‐based)
Exercise therapy is a management strategy that is supervised or 'prescribed' and encompasses a group of interventions ranging from general physical fitness or aerobic exercise, to muscle strengthening, various types of flexibility/stretching or progressive muscle relaxation exercises. It is further defined as any program in which, during the therapy sessions, the participants were required to carry out repeated voluntary dynamic movements or static muscular contractions (in each case, either 'whole‐body' or 'region‐specific' (Cochrane Back Review Group). Regular exercise can have both physical and psychological benefits, depending on the content of each programme, and the individual’s adherence (ACSM 2006). The exercises recommended for pregnancy‐related LBP are similar to those used for non‐specific LBP, with minor modifications, and are thought to have a similar mechanism of action (Vermani 2010).
Yoga
Yoga is a form of complementary and alternative medicine that incorporates a fluid transition through a number of poses (Asanas), to promote improvements in joint range of motion, flexibility, muscular strength and resistance, balance, concentration and self‐confidence, and a series of breathing exercises (Pranayamas) that facilitate mental relaxation and introspection (Martins 2014).
Progressive muscle relaxation (PMR)
Progressive muscle relaxation (PMR) aims to relax muscles, reduce stress responses and decrease pain sensations. The technique involves deep breathing and progressive relaxation of major muscle groups, which promotes systematic relaxation (McGuigan 2007).
Manual therapy (SMT, OMT, CST, NET)
Spinal manipulative therapy (SMT) is defined as a high velocity thrust performed to a joint beyond its restricted range of movement. Spinal mobilisation involves low‐velocity, passive movements within or at the limit of joint range (Cochrane Back Review Group). Most studies do not make a clear distinction between these two, because in clinical practice, these two techniques are often part of a 'manual therapy package', which may also include soft tissue/myofascial release. Manual therapy is thought to influence the spinal ‘gating’ mechanism and the descending pain suppression system at spinal and supraspinal levels to decrease pain. In addition, it is thought to return a vertebra to its normal position or restore lost mobility (Maigne 2003).
Osteopathic manipulative therapy (OMT) is a hands‐on, whole body approach to diagnose, treat, and prevent illness or injury, during which the osteopathic physician moves muscles and joints using techniques including stretching, gentle pressure and resistance (American Osteopathic Association).
Craniosacral therapy (CST), like OMT, is a hands‐on, whole body approach using light touch to release tension build‐up in different areas of the body and mind. For LBP and PP, CST techniques can be used to release tension build‐up in the connecting fascia, ligaments and muscles of the low‐back and pelvis, promoting a feeling of relaxation and enhanced body‐awareness. Other experiences reported include: sense of ease; warmth and tingling sensations, feeling accepted, a sense of harmony and peace, a feeling of letting go, feeling balanced, and increased energy (Craniosacral Therapy UK).
Neuro emotional technique (NET) is a mind‐body technique that combines relaxed breathing and visualisation with manual adjustment of the spinal levels that innervate the organ thought to be disturbing the body's balance of yin and yang. The underlying theory is that pain is caused by neurological imbalances related to the physiology of unresolved stress, therefore, by finding and removing the imbalances, the symptoms can be resolved (Bablis 2008; Neuro Emotional Technique; Peterson 2012a).
Acupuncture, alone or with exercises
Acupuncture is needle puncture at classical meridian points, aimed at promoting the flow of ‘Qi’ or energy. The acupuncturist must avoid certain acupuncture points in pregnancy that supply the cervix and uterus (which have been used to induce labour), but the technique in general is considered to be safe (Moffatt 2013; Vermani 2010). Needles may be stimulated manually or electrically. Acupuncture is thought to stimulate the body’s own pain relieving opioid mechanisms (Lin 2008). Placebo or sham acupuncture is needling of traditionally unimportant sites, superficial insertion or non‐stimulation of the needles once placed. There is some evidence that sham acupuncture may produce similar results to real acupuncture, raising the possibility that the effect of acupuncture may be a result of the stimulation of pressure receptors, regardless of their location (Field 2008).
Multi‐modal approach, including manual therapy, exercise and education
A combination of aspects of manual therapy and exercise, along with education that includes information about correct posture, relaxation techniques (Vermani 2010), instructions to keep the knees together and bent when turning in bed, and to avoid activities such as jarring, bouncing and stretching joints to their extreme (Lile 2003; Mens 2009).
Pelvic belts
Pelvic belts are a form of lumbar or pelvic support that can help to: (1) correct deformity; (2) limit spinal motion; (3) stabilise the lumbar spine and/or pelvis; (4) reduce mechanical loading; and (5) provide miscellaneous effects such as massage, heat or placebo. They may be made of flexible or rigid materials (Cochrane Back Review Group). Mens 2006 suggests that pelvic belts may stimulate the action of the corset muscle around the tummy and stabilising muscles of the spine along with the pelvic floor muscles.
Kinesio tape
Kinesio tape is an elastic therapeutic tape used for treating sports injuries and a variety of other disorders. Kinesiotaping (KT) techniques were developed by a Chiropractor, Dr Kenso Kase, in the 1970s. It is claimed that KT supports injured muscles and joints and helps relieve pain by lifting the skin and allowing improved blood and lymph flow (Williams 2012).
Transcutaneous electrical nerve stimulation (TENS)
Transcutaneous electric nerve stimulation is a therapeutic non‐invasive modality, which is primarily used for pain relief. It consists of electrical stimulation of peripheral nerves via skin surface electrodes, typically placed over the painful area (Cochrane Back Review Group). A variety of TENS applications can be used depending on patient comfort and nerve accommodation, all with the aim of inhibiting pain transmission via the activation of the inhibitory interneurons in the substantia gelatinosa of the spinal cord dorsal horn, and/or via the body's descending pain suppression system.
Why it is important to do this review
The number of new studies investigating the effectiveness of interventions for preventing and treating LBP and PP during pregnancy is increasing rapidly. The most recent update of this review was published in 2013, therefore, in order to appropriately reflect the current evidence available and inform decisions about the care of pregnant women with these conditions, it was essential to update this review again.
Objectives
To update the evidence assessing the effects of any interventions used to prevent and treat LBP, PP and LBPP during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that evaluated any intervention for preventing or treating LBP, PP and LBPP during pregnancy. We excluded quasi‐randomised studies (those which use techniques for allocating participants to groups that may be prone to bias), and cross‐over designs, as they are not considered a valid study design for Pregnancy and Childbirth reviews, since women's physical characteristics change rapidly during pregnancy.
Types of participants
Studies that included pregnant women at any point in their pregnancy who were at risk of developing, or suffering from LBP, PP or the two in combination as reported symptomatically by the women or diagnosed by clinicians using specific tests.
Types of interventions
Studies that examined any intervention intended to reduce the incidence or severity of LBP, PP or both during pregnancy. We did not put parameters on the types of interventions. We grouped the studies to allow us to examine interventions that specifically addressed LBP, PP or the two in combination. Under each population, we grouped the interventions under exercise, yoga, manual therapy, acupuncture, multi‐modal approach, pelvic belts, Kinesio tape and transcutaneous electrical nerve stimulation (TENS), depending on the studies identified. Interventions were added to usual prenatal care and compared to usual prenatal care (in some studies referred to as 'no treatment'), or usual prenatal care plus another intervention.
Types of outcome measures
We included studies that started interventions and measured outcomes at least once post treatment during pregnancy. We excluded studies that diagnosed LBP, PP or LBPP, identified LBP, PP or LBPP as intermediate or proxy outcomes only, started interventions prior to pregnancy but measured the LBP, PP or LBPP during pregnancy, or started the study during pregnancy when their goal was to assess postpartum outcomes, and therefore the only measurements conducted during pregnancy were baseline values.
Primary outcomes
We included outcomes that were measured with validated measurement tools and included women’s own rating of usefulness of a treatment, reduction of symptoms, participation in usual daily activities and adverse effects (reported by women and assessors) measured at the end of treatment, during pregnancy. If outcomes were measured by both women and assessors, we only used data provided by the women.
Pain intensity (pain levels).
Back‐ or pelvic‐related functional disability/functional status (ability to perform daily activities).
Days off work/sick leave.
Adverse effects for women and infants; as defined by trialist.
Secondary outcomes
We did not identify or analyse secondary outcomes.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (19 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We also identified potential studies by searching the Trials Register of the Cochrane Back Review Group (CBRG) by contacting the Trials Search Co‐ordinator, most recently on 19 January 2015, and by following up on studies that were listed as 'ongoing' in prior literature searches. The CBRG Trials Register is populated by the results of monthly electronic database searches (MEDLINE, Embase, CINAHL, PsycInfo, Index to Chiropractic Literature), by handsearching selected journals and conference proceedings, by quarterly searches of CENTRAL and international healthcare guideline sources and by the results of specific searches and reference checks of included studies for individual reviews (Cochrane Back Review Group). Regular searches for ongoing studies of back and neck pain treatments are conducted in the U.S. National Institute of Health database of clinical trials, ClinicalTrials.gov, as well as via the World Health Organization’s International Clinical Trials Registry Platform Search Portal (ICTRP)
Searching other resources
We checked the reference lists of included studies and related systematic reviews (Airaksinen 2006; Anderson 2005; Ee 2008; Field 2008; Franke 2014; Richards 2012; Vermani 2010).
We did not apply any language or date restrictions.
Data collection and analysis
For this update and the previous version of this review (Pennick 2013), the following methods were used for assessing all reports that were identified as a result of all searches. For methods used in previous versions of the review, see Pennick 2007.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed, for inclusion, all the potential studies identified as a result of the search strategy. We obtained the full text of any articles identified that appeared to meet the inclusion criteria, or lacked sufficient information to make a decision. We made all final decisions for inclusion after reading the full‐text of the article. We resolved any disagreement through discussion or, if required, we consulted a third assessor.
Data extraction and management
We used the data abstraction form developed by the Cochrane Pregnancy and Childbirth Group to extract data. For eligible studies, both review authors independently extracted the data. Any discrepancies were resolved through discussion or, if required, we consulted a third assessor. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted the authors of the original reports to provide further details. For articles that were published in non‐English languages, we sought the assistance of individuals who were experienced in systematic reviews and fluent in the language of interest; where possible, we used Google Translate (Google Translate) to assist with the translations and then verified key results with our translators.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described, for each included study, the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias (e.g. study reports that participants were randomised but does not give details of the method used).
(2) Allocation concealment (checking for possible selection bias)
We described, for each included study, the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth, case record numbers);
unclear risk of bias (e.g. study does not report any concealment approach, or only states that a list or table was used).
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described, for each included study, the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and whether reasons were given, and the numbers included in the analysis at each stage (compared with the total randomised participants). We also noted if missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; greater than 20% drop‐out; ‘as treated’ analysis done with substantial departure from intervention received, compared to that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described, for each included study, how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described, for each included study, any important concerns we had about other possible sources of bias.
We assessed other bias as:
low, high or unclear risk of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, assuming there are adequate data, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using GRADE
We assessed the quality of the overall body of evidence for all primary outcomes for all comparisons, using the GRADE approach as outlined in the GRADE Handbook. We also included 'Summary of findings' tables for the following two comparisons for which we had sufficient data to conduct meta‐analyses.
Comparison 1: Low‐back pain: any exercises + usual prenatal care versus usual prenatal care ('Summary of findings' table 1)
Pain intensity measured after end of treatment by a number of different measurements (lower score = better)
Disability measured after end of treatment by Roland Morris Disability Questionnaire and Oswestry Disability index (lower score = better)
Comparison 7: Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care ('Summary of findings' table 2)
Number of women who reported pain on a Visual Analogue Scale (VAS)
Number of women who reported LBP/PP‐related sick leave
GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence is downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risks of bias: indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. See Appendix 1 for further explanation of the quality of the evidence.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (95% CI).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between studies. We used the standardised mean difference (SMD) for studies that measured the same outcome, but used different methods. We presented both summary results with 95% CIs. As in the 2013 update, we used Cohen's three levels to guide our classification of the estimates of effect as small (< 0.5), medium (0.5 to < 0.8) or large (≥ 0.8; Cohen 1988). To determine if an estimate of effect was clinically important, we were guided by the work conducted in LBP research; a 30% change in pain (VAS/NRS (numerical rating scale)) was considered clinically important (Ostelo 2008), and two to three points on the Roland Morris Disability Questionnaire (RMDQ), or 8% to 12% on the Oswestry Disability Index (ODI) (Bombardier 2001).
Unit of analysis issues
Cluster‐randomised trials
We did not include any cluster‐RCTs in this review.
If we identify cluster‐randomised trials in future updates, we will include them in the analyses along with individually‐randomised studies. We will adjust their sample sizes or standard errors using the methods described in the Handbook, Section 16.3.4 or 16.3.6 (Higgins 2011), using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the study (if possible), from a similar study or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised and individually‐randomised studies, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
Studies in Pregnancy and Childbirth may include outcomes for multiple pregnancies. If we identify studies with multiple pregnancies in future updates of this review, we will outline analytical methods as per the Pregnancy and Childbirth Group Methodological Guidelines and Handbook sections 9.3.7 and 16.3 (Higgins 2011).
If we identify studies with more than two treatment groups, we will divide the control group by the number of intervention groups if data from all groups are used in the same meta‐analysis, as per Handbook Section 16.4.7 (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included in analyses, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis (seeSensitivity analysis). For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each study was the number randomised minus any participants whose outcomes were known to be missing.
If data were unclear or missing, we contacted the authors of the included studies for further details.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics provided in RevMan. We regarded heterogeneity as substantial if an I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. We noted potential sources of differences between studies where analyses had high heterogeneity. In future updates, with sufficient data, we plan to explore heterogeneity by subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases, such as publication bias, using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). Had it been reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where studies were examining the same intervention, and the studies’ populations and methods were judged to be sufficiently similar, we would have used fixed‐effect meta‐analysis for combining data.
Since there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between studies, we used random‐effects meta‐analysis to produce an overall summary when an average treatment effect across studies was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between studies. Where the average treatment effect was not clinically meaningful, we did not combine studies. When we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Although we identified heterogeneity in gestational age, duration and content of exercise regimens across studies, we did combine data with random‐effects meta‐analyses to determine the overall estimate of effect for any type of exercise intervention for women with LBP and those who had both LBP and PP. We were unable to investigate subgroup analyses due to insufficient data. For outcomes with high heterogeneity, we discussed possible sources of differences between studies in the text where results are reported.
Had we had sufficient data, we would have carried out the following subgroup analyses.
Gestational age by trimester per comparison and outcome
Different durations of interventions per comparison and outcome
Different content of interventions per comparison and outcome
Had we had sufficient data, we would have examined the following outcomes in subgroup analyses.
Pain intensity (pain levels)
Low‐back‐ or pelvic‐related functional disability/functional status (ability to perform daily activities)
Days off work/sick leave
We would further assess subgroup differences by interaction tests available within RevMan (RevMan 2014), reporting the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We conducted sensitivity analyses to explore the effect of study quality, assessed by concealment of allocation, high attrition rates, or both, by excluding studies with a high or unclear risk of bias from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
The review completed in 2002 (Young 2002) contained three studies: two examined interventions for women with LBP (Kihlstrand 1999; Thomas 1989), one examined an intervention for a mixed population with both LBP and PP (Wedenberg 2000). One study was excluded because it used a quasi‐randomised sequence generation.
The first update of the review (Pennick 2007) included eight studies (1305 women), described in nine publications. Seven were randomised controlled trials (RCTs), and the eighth used a cross‐over design. The literature search, updated to February 6, 2006 had identified 11 potentially relevant reports; five RCTs, described in six reports, were included: two studies examined women with LBP (Garshasbi 2005; Suputtitada 2002), one examined women with PP (Elden 2005), and two more examined a mixed population with LBP and PP (Kvorning 2004; Martins 2005); two studies were identified as ongoing, and three were excluded because they used a quasi‐randomised study design.
The 2013 update (Pennick 2013) included 26 studies, described in 30 reports. The literature search, updated to July 18, 2012, identified 47 potentially relevant reports from both the Cochrane Pregnancy and Childbirth and the Cochrane Back Review Groups' Trials Registers.
This 2015 update identified 19 potentially relevant reports from the Cochrane Pregnancy and Childbirth Review Group's Trials Register to 31 August 2014 and two reports that were identified through personal searching by the co‐author (VEP) to follow up on abstracts and reports of ongoing studies to 14 October 2014. One more report (Gundermann 2013) was identified by scanning the references in a recent systematic review (Franke 2014), identified by the Cochrane Back Review Group's Trials register to 19 January 2015 (Figure 1).
1.
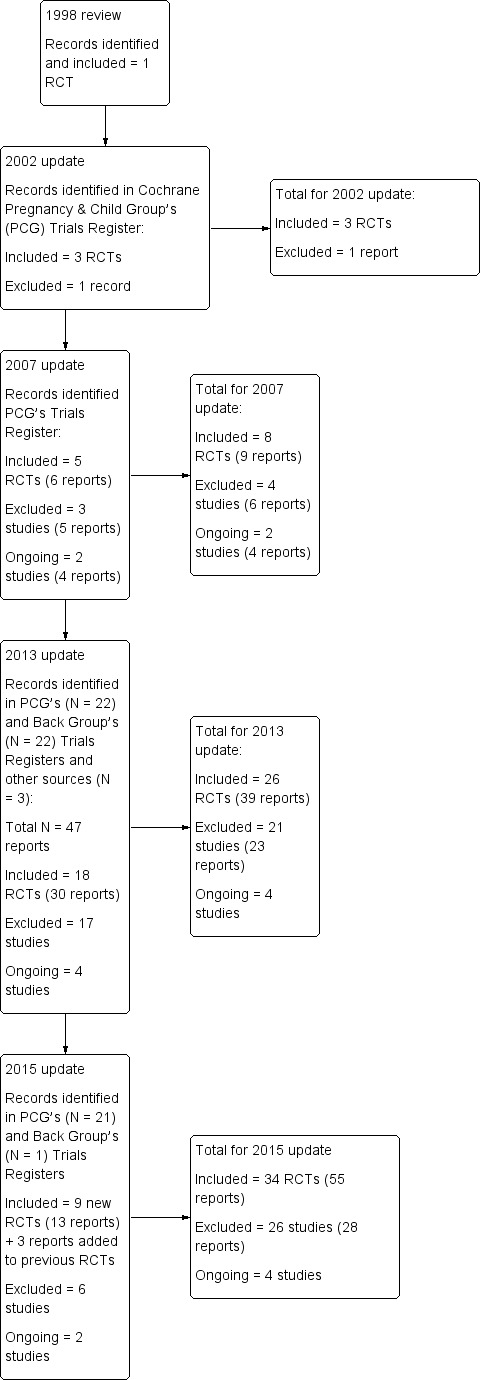
Study flow diagram.
Included studies
We included nine new RCTs in this update, reported in 13 publications: three were reported in multiple publications: (Kordi 2013; Martins 2014; Miquelutti 2013); six were published in single reports (Akmese 2014; Elden 2013; Gundermann 2013; Hensel 2014; Kaya 2013; Keskin 2012). Four reports were ongoing studies (Freeman 2013; Greene 2009; Moholdt 2011; Vas 2014).
This review now includes 34 RCTs examining 5121 pregnant women, aged between 16 to 45 years.
Population
Gestational age, when reported, ranged from 12 to 38 weeks. Fifteen RCTs examined women with low‐back pain (LBP) (N = 1847 randomised; 1687 analysed): Akmese 2014; Bandpei 2010; Garshasbi 2005; Gil 2011; Gundermann 2013; Hensel 2014; Kalus 2007; Kashanian 2009; Kaya 2013; Keskin 2012; Kihlstrand 1999; Licciardone 2010; Peterson 2012; Sedaghati 2007; Suputtitada 2002); six looked at pelvic pain (PP) (N = 889 randomised; 791 analysed; Depledge 2005; Elden 2005; Elden 2008; Elden 2013; Kordi 2013; Lund 2006); and 13 studies examined women with pelvic‐ and LBP (LBPP), reported separately or together (N = 2385 randomised; 2160 analysed; Eggen 2012; Ekdahl 2010; George 2013; Kluge 2011; Kvorning 2004; Martins 2005; Martins 2014; Miquelutti 2013; Mørkved 2007; Peters 2007; Stafne 2012; Wang 2009a; Wedenberg 2000).
Interventions
Seven studies investigated the effects of exercise on LBP, either on land (N = 713 randomised; 645 analysed; Bandpei 2010; Garshasbi 2005; Gil 2011; Kashanian 2009; Miquelutti 2013; Sedaghati 2007; Suputtitada 2002), or in water (N = 258 randomised; 241 analysed; Kihlstrand 1999). In each study, exercise was added to usual prenatal care and compared with prenatal care alone. Akmese 2014 (N = 73 randomised; 66 analysed) compared progressive muscle relaxation with music (PMR) added to usual prenatal care with prenatal care plus instructions to rest twice a day. Peterson 2012 (N = 57 randomised; 50 analysed) compared the effects of exercise, spinal manipulative therapy (SMT) and neuro emotional technique (NET), and Kalus 2007 (N = 115 randomised; 94 analysed) compared the effects of the BellyBra against those of Tubigrip. Kaya 2013 (N = 29 randomised and analysed) compared kinesiotaping (KT) with exercise, and Keskin 2012 (N = 88 randomised; 79 analysed) investigated the effects of transcutaneous electrical nerve stimulation (TENS) compared with acetaminophen, exercise or usual prenatal care. Three further studies (Gundermann 2013; Hensel 2014; Licciardone 2010, N = 587 randomised; 585 analysed) added osteopathic manipulative therapy (OMT) to usual prenatal care and compared it with sham ultrasound (sham US) added to usual prenatal care or usual prenatal care by itself.
Of the six studies investigating pregnant women with PP, Depledge 2005 (N = 90 randomised; 87 analysed) compared the effects of two types of pelvic support belt (rigid versus non‐rigid) added to exercise with exercise alone. Elden 2013 (N = 123 randomised and analysed) examined the effects of craniosacral therapy (CST) added to, and compared with, usual prenatal care. Elden 2008 and Lund 2006 (N = 185 randomised; 155 analysed) compared different acupuncture techniques, and Elden 2005 (N = 386 randomised; 330 analysed) added acupuncture or stabilising exercises to, and compared them with, usual prenatal care. Kordi 2013 (N = 105 randomised; 96 analysed) added a lumbo‐pelvic belt to information about managing PP, and compared this to exercise plus information, or information alone. Miquelutti 2013 compared a Birth Preparation Program (incorporating exercise and information) with usual prenatal care and included women with LBP and PP who were analysed separately. For women with PP, 29 were randomised; 45 analysed, i.e. more reported PP at the end of the study.
Women who had both LBP and PP, reported separately or together, were given a range of exercise interventions (Eggen 2012; Kluge 2011; Martins 2005; Mørkved 2007; Stafne 2012; N = 1532 randomised; 1390 analysed), or exercises as part of a yoga program (Martins 2014; N = 60 randomised; 45 analysed), OMT (Peters 2007; N = 60 randomised; 57 analysed), a multi‐modal intervention that included manual therapy, exercise and education (George 2013; N = 169 randomised and analysed); or acupuncture alone (Ekdahl 2010; Kvorning 2004; Wang 2009a; Wedenberg 2000; N = 359 randomised; 302 analysed).
The control groups used were generally described as usual prenatal care. The more recent acupuncture studies used sham acupuncture as a control (Elden 2008; Wang 2009a), tested the optimal time to start treatment with acupuncture (Ekdahl 2010), examined different depths of acupuncture stimulation (Lund 2006), or its relative effectiveness against physiotherapy (Wedenberg 2000). Relative effectiveness was examined between SMT and NET (Peterson 2012) and between two types of pelvic belts (Depledge 2005; Kalus 2007). Sham US was used as a control against OMT (Hensel 2014; Licciardone 2010), and exercise as a control against either KT (Kaya 2013) or a lumbo‐pelvic belt (Kordi 2013).
All studies looked at treatment; two studies also looked at interventions that may prevent LBP (Licciardone 2010; Sedaghati 2007) and four explored what may prevent LBPP (Eggen 2012; Miquelutti 2013; Mørkved 2007; Stafne 2012).
See tables of Characteristics of included studies; Characteristics of ongoing studies for further details.
Excluded studies
For this update, we excluded five studies after review of the full‐text: Hensel 2013; McCullough 2014; Moffatt 2014; Thomas 1989; Torstensson 2013. Thomas 1989 used a cross‐over design to investigate the effects of different pillows on LBP, and this study had been included in this review up to and including the 2013 update. There were always concerns about the appropriateness of including the study due to study design and methods of analyses. The review authors decided to exclude this study in the 2015 update because of these concerns.
See table of Characteristics of excluded studies for details about reasons for exclusion for this and previous updates.
Risk of bias in included studies
Overall, the risks of bias were high, raising concerns about the confidence we could put in the estimates of effect. See Figure 2 for a summary of these risks of bias in each study; see the 'Risk of bias' tables in the Characteristics of included studies for details.
2.
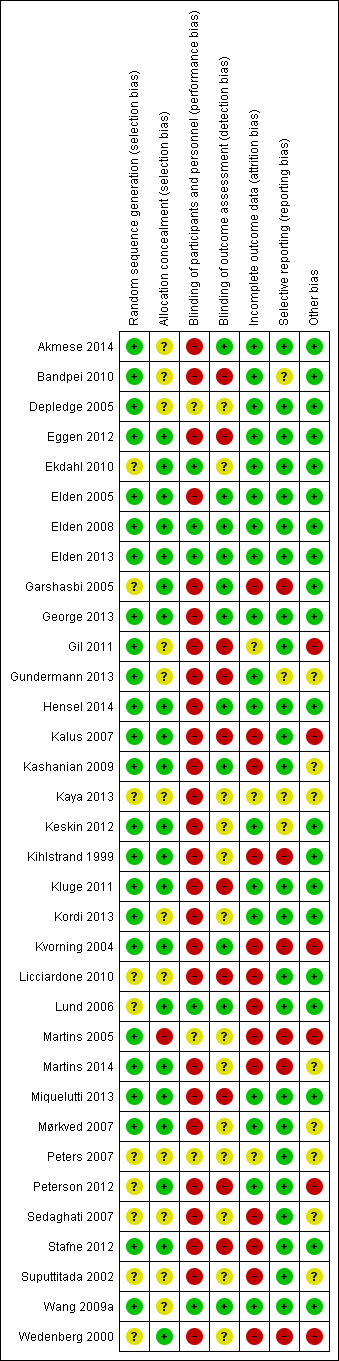
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 34 studies included in this update, 24 (71%) adequately reported on the method of randomisation employed, despite the fact that all were identified as 'randomised controlled trials'; 21 (62%) studies adequately reported on an appropriate method of allocation concealment. This represents an improvement when compared to the last update, in which only 13 out of 26 studies (50%) adequately reported on the method of randomisation, and 14 out of 26 studies (54%) on the method of allocation concealment.
Blinding
Blinding is very difficult to carry out in non‐pharmacological studies, especially when symptoms are the outcomes of interest; nonetheless, lack of blinding of research personnel and participants still has the potential to introduce bias. Five studies (15%) reported that the providers and the participants were blinded, while 11 (32%) reported that the outcome assessors were blinded. Of these, only two reduced the bias for blinding by asking all participants if they felt the treatment they had received was credible (Elden 2013; Wang 2009a).
Incomplete outcome data
Eighteen studies (53%) reported that the women were analysed in the groups to which they were randomised; most of the studies only analysed data from those who completed the study, although two (Licciardone 2010; Peterson 2012) imputed missing data in order to present a full data set. Attrition rates ranged from zero to 33% (Lund 2006). In seven of the study reports, it was difficult to determine the exact numbers randomised and withdrawn, reasons for the withdrawal and the group membership of those who withdrew. Seventeen (50%) of the more recent publications included a CONSORT flow chart that traced the enrolment, randomisation, follow‐up and analysis of participants (Schulz 2010).
Selective reporting
We did not specifically search for protocols to determine what outcomes had been planned, however five studies were identified from study registration databases during the literature search for the 2013 update (Eggen 2012; Elden 2008; Kalus 2007; Licciardone 2010; Wang 2009a); four studies were identified as ongoing for this update (Freeman 2013; Greene 2009; Moholdt 2011; Vas 2014), and two studies that were identified as 'ongoing' in the last update were included as completed studies for this update (Abolhasani 2010 (included study Kordi 2013); Hensel 2008 (included study Hensel 2014)). Only 19 studies (compared to 17 listed in the 2013 update) provided data on the outcomes they had identified in the description of the methods in either the publication or the study registration report, in a form that enabled us to include them in analyses; for one study, we calculated the end of treatment score and standard deviation using the RevMan calculator to enable us to include the data (Bandpei 2010).
Other potential sources of bias
Fourteen studies were either at high (N = 6) or unclear (N = 8) risk of bias for this section. Four of these were dissimilar at baseline in important prognostic characteristics (Gil 2011; Martins 2005; Peterson 2012; Wedenberg 2000), despite the fact that Gil 2011; Martins 2005 described adequate randomisation techniques; 13 reported very different co‐interventions between the groups, did not describe co‐interventions, reported co‐interventions that would make it difficult to determine the real effect of the intervention, or did not describe compliance (Gil 2011; Gundermann 2013; Kalus 2007; Kashanian 2009; Kaya 2013; Kvorning 2004; Martins 2005; Martins 2014; Mørkved 2007; Peters 2007; Sedaghati 2007; Suputtitada 2002; Wedenberg 2000). Three of the above studies were either abstracts or short communications, for which we were unable to obtain the full reports (Gundermann 2013; Kaya 2013; Peters 2007).
Effects of interventions
Low‐back pain (LBP)
Comparison 1. Low‐back pain: any exercise + usual prenatal care versus usual prenatal care
There was low‐quality evidence (study design limitations, inconsistent results) from seven studies (Bandpei 2010; Garshasbi 2005; Gil 2011; Kashanian 2009; Miquelutti 2013; Sedaghati 2007; Suputtitada 2002; N = 645 analysed) that any land‐based exercise, added to usual prenatal care, significantly reduced pain (standardised mean difference (SMD) ‐0.64, 95% confidence interval (CI) ‐1.03 to ‐0.25; participants = 645; studies = 7; I² = 81%; Analysis 1.1) more than usual prenatal care by itself. A SMD of 0.64 represents a moderate difference between groups. Heterogeneity was high for this analysis, and pain was measured between eight and 24 weeks after randomisation across the included studies. The gestation at which pain was measured may be one source of heterogeneity; elements of the exercise regimens may be another. When we conducted a sensitivity analysis, based on the 'Risk of bias' domain of allocation concealment, heterogeneity was not improved by excluding studies with unclear risk of bias (Tau² = 0.23; I² = 83%). In both Gil 2011 and Suputtitada 2002, it appeared that the standard deviation (SD) reported in the study report was actually a standard error (SE), however, after discussion with the statisticians, we used the published data in the meta‐analysis. We are concerned about the accuracy of reporting in these studies.
1.1. Analysis.
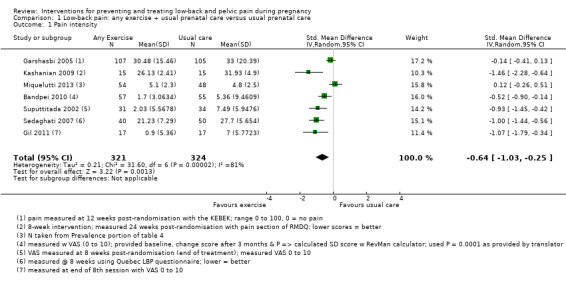
Comparison 1 Low‐back pain: any exercise + usual prenatal care versus usual prenatal care, Outcome 1 Pain intensity.
There was low‐quality evidence (study design limitations, imprecision) from two studies (Bandpei 2010; Gil 2011; N = 146 analysed) that land‐based exercise also reduced functional disability (SMD ‐0.56; 95% CI ‐0.89 to ‐0.23; I² = 0%; Analysis 1.2) more than usual prenatal care by itself (Table 1). Again, we recalculated the SD stated in Gil 2011 because it appeared to be a SE.
1.2. Analysis.
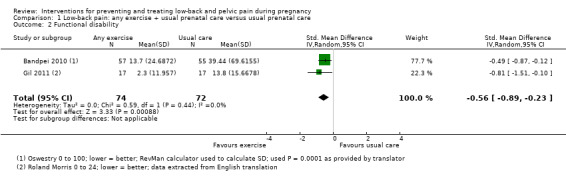
Comparison 1 Low‐back pain: any exercise + usual prenatal care versus usual prenatal care, Outcome 2 Functional disability.
All but one of the studies (Miquelutti 2013) reported effects in the same direction, so land‐based exercise seemed to reduce pain and functional disability, but there is considerable uncertainty about the size of the effect, due to the risks of bias in the included studies and concern about the accuracy of reporting in Bandpei 2010, Gil 2011 and Suputtitada 2002. See further details in the Discussion. None of the interventions, gestational ages or outcomes was sufficiently similar, nor were sufficient data provided to allow us to perform a meta‐analysis to determine the estimate of effect of any specific exercise for a specific group of pregnant women.
None of the studies included in this comparison reported the primary outcome of days off work/sick leave. Four studies (Garshasbi 2005; Gil 2011; Miquelutti 2013; Suputtitada 2002) reported that no adverse effects occurred as a result of the intervention.
Comparison 2. Low‐back pain: water gymnastics + usual prenatal care versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Kihlstrand 1999; N = 241 analysed) that water‐based exercise, added to usual prenatal care, reduced LBP‐related sick leave more than usual prenatal care by itself; women who exercised were 60% less likely to take sick leave due to their LBP at 32 weeks' gestation (risk ratio (RR) 0.40; 95% CI 0.17 to 0.92; Analysis 2.1).
2.1. Analysis.
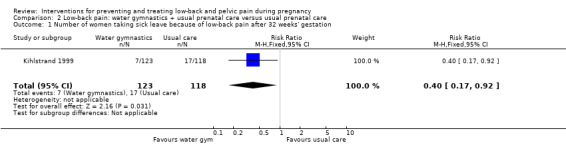
Comparison 2 Low‐back pain: water gymnastics + usual prenatal care versus usual prenatal care, Outcome 1 Number of women taking sick leave because of low‐back pain after 32 weeks' gestation.
This study reported the primary outcome of pain intensity on a graph of mean values and did not report on the other primary outcome of functional disability. No adverse effects occurred as a result of the intervention.
Comparison 3. Low‐back pain: usual prenatal care + support belts ‐ Bellybra versus Tubigrip
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Kalus 2007; N = 94 analysed) that there was no significant difference between the BellyBra's and the Tubigrip's ability to relieve pain (mean difference (MD) ‐0.20; 95% CI ‐1.19 to 0.79) or to decrease functional disability (activities of daily living) (MD ‐0.90; 95% CI ‐1.81 to 0.01; Analysis 3.1).
3.1. Analysis.
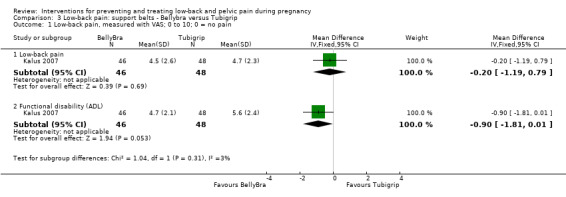
Comparison 3 Low‐back pain: support belts ‐ Bellybra versus Tubigrip, Outcome 1 Low‐back pain, measured with VAS; 0 to 10; 0 = no pain.
This study did not report the primary outcomes of days off work/sick leave, or adverse effects.
Comparison 4. Low‐back pain: group exercise + education + usual prenatal care versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from two studies (Eggen 2012; Miquelutti 2013; N = 374 analysed) that group exercise added to information about how to manage pregnancy‐related LBP was no better at preventing LBP than usual prenatal care alone (RR 0.97; 95% CI 0.80 to 1.17; Analysis 4.1). There were no adverse effects resulting from the interventions in either study.
4.1. Analysis.
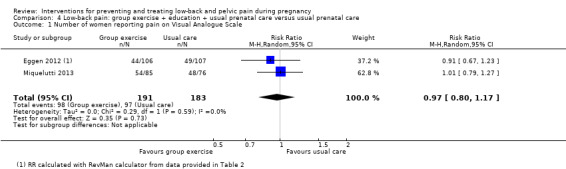
Comparison 4 Low‐back pain: group exercise + education + usual prenatal care versus usual prenatal care, Outcome 1 Number of women reporting pain on Visual Analogue Scale.
Eggen 2012 found no significant difference between groups for disability. Neither study reported the primary outcome of days off work/sick leave.
Additional results for low‐back pain
All results below were extracted directly from the papers as the presentation of results in each paper prevented pooling of data.
Progressive muscle relaxation (PMR) with music + usual prenatal care versus advice to rest + usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Akmese 2014; N = 66 analysed) that PMR, accompanied by music, significantly decreased pain more than lying down for the same amount of time each day. The study did not report the primary outcomes of functional disability, days off work/sick leave or adverse effects.
Manual therapy + usual prenatal care versus usual prenatal care
Four studies examined the effects of manual therapy, specifically: osteomanipulative therapy (OMT) (Gundermann 2013; Hensel 2014; Licciardone 2010), spinal manipulative therapy (SMT), or neuro emotional technique (NET) (Peterson 2012), which were compared with usual prenatal care alone or with another intervention.
There was moderate‐quality evidence (study design limitations) from one study (Hensel 2014; N = 400 analysed) that OMT added to usual prenatal care improved pain (effect size ‐7.11; 95% CI ‐10.30 to ‐3.93) and functional disability (effect size ‐2.25; 95% CI ‐3.18 to ‐1.32) significantly more than usual prenatal care alone, but not more than usual prenatal care plus placebo ultrasound (US). The paper did not specify the measure of treatment effect (i.e. MD or SMD), referring instead to 'effect size'. There were no adverse effects resulting from the interventions used in this study.
Low‐quality evidence (study design limitations, imprecision of effect estimates) from another study (Licciardone 2010; N = 144 analysed) also found no significant difference in pain relief (effect size 0.14; 95% CI ‐0.26 to 0.53) or functional disability (effect size 0.35; 95% CI ‐0.06 to 0.76) between usual prenatal care plus OMT and usual prenatal care plus placebo ultrasound. In further analysis of the same data, the authors concluded that OMT was more effective than usual prenatal care alone for preventing progressive functional disability as a result of LBP (RR 0.4; 95% CI 0.2 to 0.7). The paper did not specify the measure of treatment effect (i.e. MD or SMD), referring instead to 'effect size'. Adverse effects were reported as being similar across groups but no further detail was provided.
Low‐quality evidence (study design limitations, imprecision of effect estimates) from a third small study (Gundermann 2013; N = 41 analysed) concluded that OMT was more effective in reducing pain than usual prenatal care (between‐group difference of means 3.5; 95% CI 2.4 to 4.6). This study did not report on the other primary outcomes of functional disability, days off work/sick leave or adverse effects.
Low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Peterson 2012; N = 50 analysed) found that, while the majority of women in each of the groups (exercise, NET and SMT) improved in functional disability and pain, there was no statistically significant difference between the groups. Days off work/sick leave was not compared between groups before and after treatment, however the authors did state that they had measured sick leave due to pregnancy‐related LBP at the beginning of their study. Some post‐treatment soreness was reported in all groups but no adverse effects occurred as a result of the interventions.
Transcutaneous electrical nerve stimulation (TENS) + usual prenatal care versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Keskin 2012; N = 79 analysed) that TENS improved pain and functional disability significantly more than usual prenatal care. This study also included two additional active treatment groups (exercise; acetaminophen), which also resulted in significant pre‐ versus post‐treatment improvements in pain and functional disability when compared to usual prenatal care (pain/VAS P < 0.001; disability/RMDQ P < 0.001). There was no significant difference in these outcomes when acetaminophen was compared to exercise (pain/VAS P = 0.694; disability/RMDQ P = 0.506), despite the fact that baseline VAS scores (pain) were significantly higher in the TENS group (P = 0.004). There were no adverse effects resulting from TENS.
Keskin 2012 did not report on the primary outcome of days off work/sick leave.
Acetaminophen + usual prenatal care versus usual prenatal care
There was low‐quality evidence from one study (Keskin 2012) as described above, that acetaminophen improved pain and functional disability (measured on the Roland Morris Disability Questionnaire (RMDQ)) significantly more than usual prenatal care. There was no significant difference in these outcomes, also noted above, when acetaminophen was compared to exercise.
Kinesiotaping (KT) + usual prenatal care versus usual prenatal care + exercise + acetaminophen
One abstract (Kaya 2013; N = 29 analysed) compared KT with exercise plus acetaminophen and reported that pain and functional disability were significantly lower in both groups, however, these outcomes improved more in the group that received KT (P < 0.001); insufficient information was available in the abstract to draw any further conclusions. The abstract did not report on the primary outcome of days off work/sick leave. There were no adverse effects resulting from the KT intervention.
Adverse effects
When reported, there were no serious adverse effects noted for either the mother or the neonate in any of the studies (Eggen 2012; Gil 2011; Garshasbi 2005; Hensel 2014; Kaya 2013; Keskin 2012; Kihlstrand 1999; Licciardone 2010; Miquelutti 2013; Peterson 2012; Sedaghati 2007; Suputtitada 2002). Women who participated in water‐based exercise did not develop any more urinary tract or uterine infections than those who received usual prenatal care (Kihlstrand 1999). There were no data reported on the (primary) preventative aspects of any of these interventions, although there was a sense that they may have prevented further development of pain and disability, and therefore may have had some secondary preventative consequences.
Pelvic pain (PP)
Comparison 5. Pelvic pain: deep acupuncture + usual prenatal care versus superficial acupuncture + usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Lund 2006; N = 47 analysed) that there was no significant difference in evening pain between women who received deep acupuncture and those who received superficial acupuncture (RR 1.06; 95% CI 0.73 to 1.54; Analysis 5.1). Data were not provided for the primary outcomes of functional disability, days off work/sick leave or adverse effects.
5.1. Analysis.
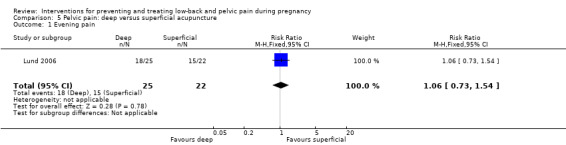
Comparison 5 Pelvic pain: deep versus superficial acupuncture, Outcome 1 Evening pain.
Comparison 6. Pelvic pain: usual prenatal care + group exercise + education versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from two studies (Eggen 2012; Miquelutti 2013; N = 374 analysed) that group exercise added to information about how to manage pregnancy‐related PP was no better at preventing pain than usual prenatal care alone (RR 0.97; 95% CI 0.77 to 1.23; Analysis 6.1).
6.1. Analysis.
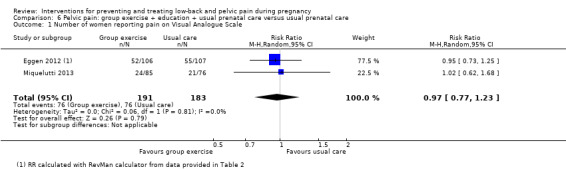
Comparison 6 Pelvic pain: group exercise + education + usual prenatal care versus usual prenatal care, Outcome 1 Number of women reporting pain on Visual Analogue Scale.
Neither study reported the primary outcome of days off work/sick leave. Eggen 2012 found no difference between groups for functional disability. No adverse effects occurred as a result of either intervention.
Additional results for pelvic pain
All results below were extracted directly from the papers as the presentation of results in each paper prevented pooling of data.
We were unable to pool the data from Elden 2005 and Elden 2008, since acupuncture was compared with different interventions in these two studies; data were extracted directly from the published reports.
Acupuncture + usual prenatal care versus sham acupuncture + usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Elden 2008; N = 108 analysed) that there was no significant difference in pain relief between acupuncture plus usual prenatal care and non‐penetrating sham acupuncture plus usual prenatal care (median evening pain on VAS = 36 and 41 respectively, P = 0.483); acupuncture plus usual prenatal care showed significant improvement in some daily functions, as measured using the Disability Rating Index (DRI), over non‐penetrating sham acupuncture plus usual prenatal care (median DRI 44 and 55 respectively, P = 0.001), which was further illustrated in the two groups by the number of women who worked regularly (28/57 (acupuncture) versus 16/57 (sham acupuncture), P = 0.041).
Acupuncture + usual prenatal care versus stabilising exercises + usual prenatal care or usual prenatal care alone
Elden 2005 (N = 330 analysed) examined the effects on PP of adding either acupuncture or stabilising exercises to usual prenatal care versus usual prenatal care alone. There was moderate‐quality evidence (imprecision of effect estimates) that, after one week of treatment, those who received usual prenatal care reported significantly more intense evening PP than those who had received either acupuncture (difference of medians: 27; 25th to 75th percentiles 13.3 to 29.5; P < 0.001) or stabilising exercises (difference of medians:13; 25th to 75th percentiles 2.7 to 17.5; P = 0.0245). Those who received acupuncture reported significantly less intense evening PP than those who received stabilising exercises (difference of medians: ‐14; 25th to 75th percentiles ‐18 to ‐3.3; P = 0.0130). Data were not provided for the primary outcomes of disability or days off work/sick leave.
Rigid pelvic belts + exercise + usual prenatal care versus non‐rigid pelvic belt + exercise + usual prenatal care versus exercise + usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Depledge 2005; N = 87 analysed) that there was a significant reduction of average pain in the group that received exercise alone (31.8% reduction in pain) or exercise plus a rigid belt (29% reduction), and no significant pain reduction in the group that had exercise plus a non‐rigid belt (13% reduction); there were no data provided that compared results between groups. There was no significant difference between the three groups in functional disability.
Low‐quality evidence (study design limitations, imprecision of effect estimates) from another study (Kordi 2013; N = 96 analysed) suggested that the use of a non‐rigid lumbo‐pelvic belt significantly reduced pain (P < 0.001) and functional disability (P = 0.008) more than exercise at the final six‐week follow‐up.
In both of these studies belts and exercise were added to information about how to manage pregnancy‐related PP; both resulted in better outcomes than information alone. Neither study measured the other primary outcomes of days off work/sick leave or adverse effects.
Craniosacral therapy (CST) + usual prenatal care versus usual prenatal care
There was moderate‐quality evidence (imprecision of effect estimates) from one study (Elden 2013; N = 123 analysed) that the addition of CST to usual prenatal care significantly improved morning PP (P = 0.017) and functional disability (P = 0.016) more than usual prenatal care alone. There were no significant differences between groups in evening pain or days off work/sick leave.
Prevention ‐ Birth Preparation Program versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Miquelutti 2013; N = 44 analysed (PP)) that a Birth Preparation Program (incorporating exercise and information about how to manage PP) was no more effective than usual prenatal care at decreasing pain (MD ‐0.38; 95% CI ‐2.09 to 1.33). Data were not provided for functional disability or days off work/sick leave.
Adverse effects
There were no lasting adverse effects noted; complaints of needle pain, slight bleeding, fainting, and sleepiness were noted for both acupuncture and sham acupuncture, and drowsiness, belt discomfort or a temporary increase in PP were noted for CST. Kordi 2013 reported one episode of vaginal bleeding and pre‐eclampsia, however these effects occurred in the information (control) and exercise groups respectively.
Mixed population with pelvic and low‐back pain (LBPP; reported separately or together)
Comparison 7. Pelvic + low‐back pain: any exercise + usual prenatal care versus usual prenatal care
There was moderate‐quality evidence (study design limitations) from four studies (N = 1176 analysed) that an eight‐ to 12‐week exercise program reduced the risk of women reporting LBPP by 44% (average RR 0.66; 95% CI 0.45 to 0.97; Tau² = 0.12; I² = 88%; Analysis 7.1; Table 2). Heterogeneity was high for this analysis. When we conducted a sensitivity analysis, based on the 'Risk of bias' domain of allocation concealment, heterogeneity was not appreciably improved by excluding studies at high risk of bias (Tau² = 0.07; I² = 83%). Two studies (Martins 2005; Martins 2014) measured pain between 12 and 32 weeks' gestation, while Mørkved 2007 and Stafne 2012 measured pain at 32 to 36 weeks and at 36 weeks. These differences in gestation, the elements of the exercise regimens, and the small sizes of the studies conducted by Martins et al (N = 69 and 45 analysed respectively), may account for the heterogeneity in this outcome.
7.1. Analysis.
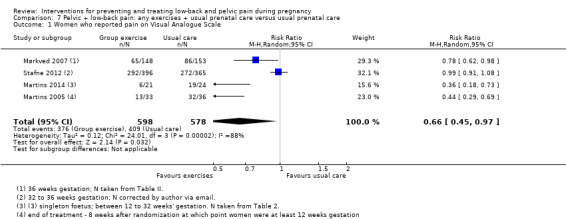
Comparison 7 Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care, Outcome 1 Women who reported pain on Visual Analogue Scale.
There was moderate‐quality evidence (study design limitations) from two studies (Mørkved 2007; Stafne 2012; N = 1062 analysed) that a 12‐week exercise program reduced the risk of women reporting LBPP‐related sick leave by 24% (RR 0.76; 95% CI 0.62 to 0.94; Tau² = 0.00; I² = 0%; Analysis 7.2), and improved functional disability (results could not be pooled). As with the LBP studies, there was insufficient clinical homogeneity amongst the studies investigating exercise interventions to be able to analyse or support a specific set of exercises for a specific group of pregnant women.
7.2. Analysis.
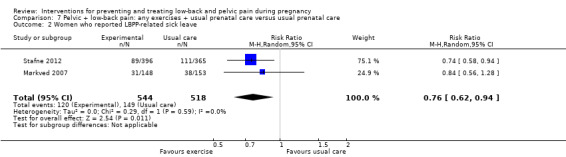
Comparison 7 Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care, Outcome 2 Women who reported LBPP‐related sick leave.
The studies included in this comparison did not all report the primary outcome of functional disability; the studies that did measure functional disability did not provide data that could be pooled.
Comparison 8. Pelvic + low‐back pain: acupuncture + usual prenatal care versus usual prenatal care
Four studies measured the effects of adding acupuncture to usual prenatal care. However, because of differences in interventions, comparisons, acupuncture techniques and outcome measures, we were unable to pool any of the results. Therefore, there is only low‐quality evidence (study design limitations, imprecision of effect estimates) for any of the outcomes, although each study reported positive results in favour of acupuncture for pain reduction and improved functional disability.
In one study (Kvorning 2004; N = 72 analysed), 60% of the women who completed their acupuncture treatment reported their pain intensity had decreased, compared to only 14% of the control group, who received usual prenatal care, suggesting a four‐fold benefit from acupuncture (RR 4.16; 95% CI 1.77 to 9.78; Analysis 8.1). The women who received usual prenatal care also used analgesics (5/35), TENS (6/35), physiotherapy (6/35) and a sacroiliac belt (15/35) to help them relieve the pain. Four out of the 37 women in the acupuncture group also used a sacroiliac (pelvic support) belt.
8.1. Analysis.
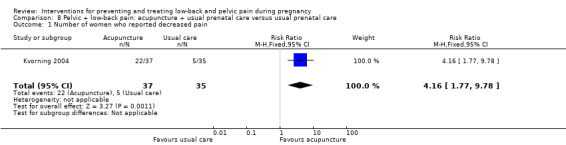
Comparison 8 Pelvic + low‐back pain: acupuncture + usual prenatal care versus usual prenatal care, Outcome 1 Number of women who reported decreased pain.
This study did not report the primary outcome of days off work/sick leave.
Comparison 9. Pelvic + low‐back pain: acupuncture + usual prenatal care versus individual physio + usual prenatal care
Women who received either acupuncture or physiotherapy (Wedenberg 2000; N = 46 analysed), all reported a reduction in evening pain intensity and functional disability after completing their program, with the acupuncture group reporting significantly less intense pain (P < 0.01) and lower functional disability scores than the physiotherapy group. Neither summary data nor analyses were provided for pain. Of note: none of the 30 participants were lost to follow‐up in the acupuncture group (two were not analysed because they received both treatments), while 12/30 were lost to follow‐up in the physiotherapy group; for those who completed the study, there was no significant difference between groups for satisfaction with treatment, with a RR of 1.24 (95% CI 0.96 to 1.60; Analysis 9.1).
9.1. Analysis.
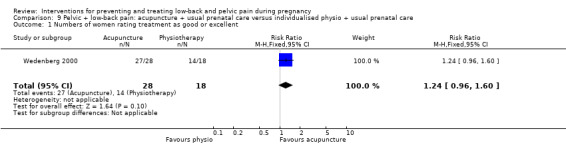
Comparison 9 Pelvic + low‐back pain: acupuncture + usual prenatal care versus individualised physio + usual prenatal care, Outcome 1 Numbers of women rating treatment as good or excellent.
This study did not report the primary outcome of days off work/sick leave.
Comparison 10. Pelvic + low‐back pain: multi‐modal intervention (MOM) versus standard obstetric care (STOB)
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (George 2013; N = 169 analysed) that women who received either a multi‐modal intervention that included manual therapy, exercise and education (MOM) or usual prenatal care reported significantly improved functional disability, but only those in the MOM group reported improved pain (MD ‐2.70; 95% CI ‐3.54 to ‐1.86; Analysis 10.1) and functional disability (MD ‐1.40; 95% CI ‐2.09 to ‐0.71; Analysis 10.2). There was no significant difference in days off work/sick leave between groups (MD 0.10; 95% CI ‐1.12 to 1.32; Analysis 10.3).
10.1. Analysis.
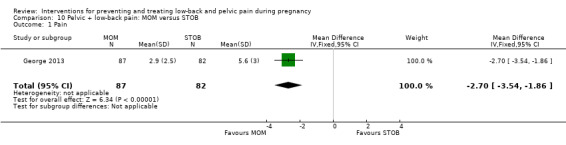
Comparison 10 Pelvic + low‐back pain: MOM versus STOB, Outcome 1 Pain.
10.2. Analysis.
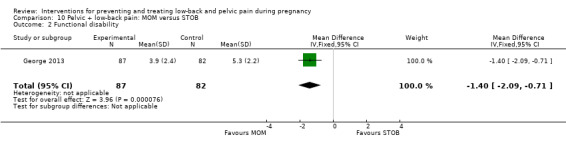
Comparison 10 Pelvic + low‐back pain: MOM versus STOB, Outcome 2 Functional disability.
10.3. Analysis.
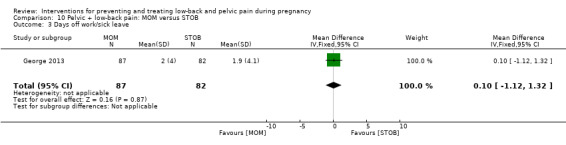
Comparison 10 Pelvic + low‐back pain: MOM versus STOB, Outcome 3 Days off work/sick leave.
Additional results for pelvic + low‐back pain
All results below have been extracted directly from the papers.
Usual prenatal care + acupuncture started at 20 weeks' gestation versus usual prenatal care + acupuncture started at 26 weeks' gestation
One small study (Ekdahl 2010; N = 32 analysed) examined the difference between acupuncture started at 20 weeks' and 26 weeks' gestation. They found that both regimens relieved pain, but significantly more in the group that started later. The later group also reported improvement in functional disability despite increased physical restrictions, but data were not provided for between‐group comparisons. This study also did not report the primary outcome of days off work/sick leave.
Usual prenatal care + auricular acupuncture versus usual prenatal care + sham auricular acupuncture versus usual prenatal care + waiting list
Another study (Wang 2009a; N = 152 analysed) compared the effects of auricular (ear) acupuncture, sham auricular acupuncture and a waiting list control. All women reported pain relief and improved functional disability, but those in the ear acupuncture group reported significantly more pain relief and functional improvement than those in either the sham ear acupuncture or control group; data were not provided for between‐group comparisons. Sixty‐eight per cent of those in the ear acupuncture group reported a clinically significant improvement in pain after two weeks of treatment (paper states 30% reduction is clinically significant), as compared to 32% in the sham ear acupuncture group (P = 0.02) and 18% in the control group (P < 0.001). These data were extracted directly from the paper. This study did not report on the primary outcome of days off work/sick leave.
Manual therapy (osteomanipulative therapy (OMT) + usual prenatal care versus usual prenatal care + waiting list
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Peters 2007; N = 57 analysed) that OMT significantly reduced pain (68% improvement versus 0%; P < 0.0005) and improved functional disability (28% improvement versus 20% deterioration) over those on a waiting list. This study did not report on the primary outcome of days off work/sick leave.
Prevention ‐ 12‐week exercise program + usual prenatal care versus usual prenatal care
There was low‐quality evidence (study design limitations, imprecision of effect estimates) from one study (Mørkved 2007; N = 301 analysed) that suggested a 12‐week exercise program prevented lumbo‐pelvic pain in one in 8.1 women treated (number needed to treat (NNT) analysis) over usual prenatal care. This study did not provide the 95% CI for number needed to benefit (NNTB) and did not report NNT or NNTB for the primary outcomes of functional disability or days off work/sick leave.
Adverse effects
Adverse effects were not reported in Gundermann 2013, Martins 2005 and Peters 2007. There were minor, transient adverse effects reported by those who received acupuncture (small subcutaneous haematomas/bruises at insertion site) in Wedenberg 2000 and Wang 2009a. Although the adverse effects reported by those women who received physiotherapy (preterm uterine contractions, pre‐eclampsia) were unlikely to have been caused by the physiotherapy, they withdrew from the study (Wedenberg 2000). Thirty‐eight per cent of the women who received acupuncture in Kvorning 2004 also reported some minor, transient adverse effects (local pain, heat or sweating, local haematoma, tiredness, nausea, weakness). There were no reported problems with any of the deliveries or neonates.
Discussion
Summary of main results
We included 34 randomised controlled trials (RCTs) examining 5121 pregnant women aged between 16 to 45 years in this updated review. Fifteen studies examined low‐back pain (LBP; N =1847 randomised; 1687 analysed); six looked at pelvic pain (PP; N = 889 randomised; 791 analysed); and 13 examined women with pelvic‐ and low‐back pain, reported either separately or together (LBPP; N = 2385 randomised; 2160 analysed). Overall, 91% of the women were included in the analyses; the number of women lost to follow‐up ranged from none reported to 33% (Lund 2006).
In summary, for LBP , results from meta‐analyses provided low‐quality evidence (study design limitations, inconsistent results), suggesting that land‐based exercise, in a variety of formats, significantly reduced pain and functional disability more than usual prenatal care. Low‐quality evidence (study design limitations, imprecision of effect estimates) also suggested there was no significant difference in the number of women reporting low‐back pain between group exercise, added to information about pain management, compared to usual prenatal care. Moderate‐quality evidence (study design limitations) from one large study showed that osteomanipulative therapy (OMT) added to usual prenatal care relieved pain and functional disability better than usual prenatal care alone, but the addition of OMT did not significantly improve outcomes any more than adding placebo ultrasound (US) to usual prenatal care.
The remaining evidence was of low‐quality (study design limitations, imprecision of effect estimates) and from single studies, suggesting that: water gymnastics significantly reduced sick leave resulting from LBP more than usual prenatal care; TENS significantly improved pain and functional disability more than either acetaminophen, exercise or usual prenatal care; a supervised progressive muscle relaxation programme with music was more effective at significantly reducing pain and functional disability than usual prenatal care plus instructions to rest for 20 minutes twice a day; there was no significant difference in pain relief or functional disability (activities of daily living) between women who wore two types of pelvic support belt or between women who received exercise, neuro emotional technique (NET) or spinal manipulative therapy (SMT). Low‐quality evidence (unclear risk of bias, indirectness) from one small study suggested that Kinesio tape (KT) might significantly provide more pain relief than exercise.
In summary, for PP , results from a meta‐analysis provided low‐quality evidence (study design limitations, imprecision of effect estimate) of no significant difference in the number of women reporting PP between group exercise, added to information about managing pain, compared to usual prenatal care.
Evidence from a single large study provided moderate‐quality evidence (imprecision of effect estimates) that craniosacral therapy (CST) significantly improved morning PP and functional disability, but not evening PP, more than usual prenatal care, and that acupuncture was better than stabilising exercises at reducing evening PP. The remaining evidence from single studies was of low quality (study design limitations, imprecision of effect estimates) and suggested that the addition of a rigid pelvic support belt to exercise plus information did not enhance the pain‐relieving effects of exercise plus information alone; a non‐rigid lumbo‐pelvic belt plus information significantly reduced pain and functional disability more than exercise plus information, for up to six weeks after treatment; a Birth Preparation Program (consisting of exercise plus information) was no better than usual prenatal care for preventing or decreasing PP; acupuncture was no better than sham acupuncture for improving PP or functional disability; and evening pain relief was the same following either deep or superficial acupuncture.
In summary, for LBPP , results from meta‐analyses provided moderate‐quality evidence (study design limitations) that an eight‐ to 12‐week exercise program significantly reduced the risk of women reporting LBPP, but group exercise added to information was no better than usual prenatal care at preventing either pelvic‐ or LBP. There was also moderate‐quality evidence (study design limitations) that land‐based exercise significantly reduced sick leave resulting from LBPP, and improved LBPP‐related functional disability.
The remaining evidence, from single studies, was of low‐quality (study design limitations, imprecision of effect estimates) and suggested that a multi‐modal intervention (manual therapy, exercise and education (MOM)) significantly improved pain and related functional disability, but not sick leave, more than usual prenatal care. Studies investigating acupuncture suggested it reduced pain better than usual prenatal care, when started at 26 weeks' rather than 20 weeks' gestation, and it significantly reduced pain and improved women's ability to carry out daily activities better than either physiotherapy, sham acupuncture or usual prenatal care. The results of a small study suggested that OMT significantly improved pain and related functional disability more than a waiting list control.
Prevention: six studies sought an effective intervention to prevent pelvic‐ or LBP (LBPP). There was low‐quality evidence (study design limitations, imprecision of effect estimates) of conflicting results on the effectiveness of exercise programmes for preventing LBP, PP or LBPP, related functional disability and days off work/sick leave; while OMT, provided during the third trimester of pregnancy, appeared to prevent 40 cases of LBP‐related functional disability for every 100 pregnant women receiving OMT.
Overall completeness and applicability of evidence
The studies included in this updated review were conducted in Iran, Brazil, USA, Sweden, Thailand, Turkey, Australia, New Zealand, Norway, South Africa and Germany, which would suggest that the women who participated broadly represented pregnant women in general. However, women entered the studies at various times in their pregnancies and were diagnosed with LBP, PP or both (reported separately or together) using a variety of methods ranging from self‐reported symptoms to the findings of specific clinical tests (depending on the study), making the internal validity and reliability of the classification of each condition questionable. Of note, there were no studies included from the UK. Based on the findings of a recent survey which revealed the high incidence of both conditions during pregnancy, along with the high levels of pain and distress women experience (Sinclair 2014), more efforts to explore ways of preventing or managing symptoms would be welcome in the UK health system, particularly as women with a history of LBP are more likely to develop pregnancy‐related LBP (Bishop 2014).
Inclusion criteria were quite different across studies; women were admitted at different points in their pregnancy, 'diagnoses' of LBP and PP ranged from self‐reported symptoms to clinical interpretation of the results of a variety of pain provocation tests, such as the posterior pelvic pain provocation test (Östgaard 1994), resulting in a heterogenous population. Primary outcomes of interest for this review were pain intensity, low‐back‐ or pelvic‐related functional disability, days off work/sick leave and adverse events. While pain was measured in all the studies, functional disability was not, nor was sick leave or adverse effects. Pain and related functional disability were measured in a variety of ways; pain was measured as intensity, prevalence, change in pain within groups, numbers or percentage who reported improvement; and functional disability was measured as back‐specific function, general function, disability, ability to perform activities of daily living, pain levels during activities of daily living, change in abilities within groups, numbers or percentage who reported improved functional status and sleep disturbance. Outcomes were measured daily, weekly, in the morning, in the evening, over the course of the pregnancy and during the postpartum period; the latter outcomes were outside the scope of this review. Days off work/sick leave was reported as number of days off work or whether (or not) the woman had taken sick leave. Only six studies (Elden 2008; Elden 2013; George 2013; Kihlstrand 1999; Mørkved 2007; Stafne 2012) reported on the impact of the interventions on the women's absenteeism from work due to their symptoms. Considering the number of women who now participate in the paid workforce, assessing absenteeism from work should be addressed in future studies. The other primary outcome of adverse effects (to mother, infant or both) was reported as; whether (or not) adverse effects occurred, were similar between groups, or specific details about adverse effects, the numbers of women involved and whether (or not) they subsequently withdrew from the study. Of the 24 studies that reported on this outcome, effects were minor and transient and, in the main, experienced by those who received acupuncture (small subcutaneous haematomas at the insertion site, tiredness, transient ear tenderness) in Kvorning 2004; Wedenberg 2000 and Wang 2009a. A systematic review on the safety profile of acupuncture for LBP concluded, from reports on over 100,000 patients from the US, UK, and Sweden, that reported incidents from acupuncture were, on the whole, minor and transient. They listed fainting (10 patients), unexpected exacerbation of symptoms (12 patients), pain at site of needle insertion (six patients), needle left in place (five patients), seizure after needle insertion (one patient with known epilepsy), slurred speech (one patient), pneumothorax (two patients), broken needle (two patients) and minor bleeding at the needle insertion site (15% of treatments) as the most notable problems (Cherkin 2003). More recently Moffatt 2013 has concluded that there is no firm evidence to suggest that acupuncture treatment can cause detrimental effects for the fetus or the maintenance of pregnancy. Therefore, provided that certain points that supply the cervix and uterus are avoided Vermani 2010, the current evidence would suggest that acupuncture is relatively safe for pregnant women with no other complications.
Women who participated in additional exercise programs (of variable content and duration), received acupuncture or OMT, generally expressed satisfaction with the interventions and felt they would consider them in subsequent pregnancies. In general, women in the studies who received more than usual prenatal care appeared to experience some pain relief, although the results varied. Four pooled estimates of effect were moderate in size (SMD 0.5 to < 0.8, Analysis 1.1; Analysis 1.2; RR 0.5 to 0.8, Analysis 7.1; Analysis 7.2; Cohen 1988) and may be considered clinically significant. In Kordi 2013, the pain relief experienced by the group receiving the lumbo‐pelvic belt was almost three times that of the exercise group (mean VAS = 11.0 versus 31.1), and more than four times that for the group receiving information alone (mean VAS = 11.0 versus 45.2). On the other hand, had the potential for risks of bias been lower, the estimates of effect may also have been lower, since it has been shown that studies with lower risks of bias tend to have lower effect sizes (Van Tulder 2009). This was borne out by the sensitivity analyses conducted for Analysis 1.1; Analysis 1.2. When data from studies that had an unclear or high risk of bias for allocation concealment were removed, the pooled estimate of effect for pain dropped to ‐0.36; 95% CI ‐0.98 to 0.25 (three studies, 344 analysed) and was no longer significant; the SMD for functional disability would have had no data since both studies in the original analysis would have been removed. For Analysis 7.1, the SMD rose to 0.77, 95% CI 0.54 to 1.09, but was no longer statistically significant; Analysis 7.2 remained the same.
Considering the quality of the evidence in this review, these results must be considered with caution and generalising the results to all pregnant women is likely premature.
Incorporating the evidence into clinical practice may be challenging since 'usual prenatal care' and 'standard physiotherapy' are not described in sufficient detail in the studies and are likely to vary across jurisdictions. Similarly, there were insufficient details provided about other interventions that would make it difficult to replicate in another clinical setting. Akmese 2014 is just one example of the variability typically observed in the content of studies investigating the effectiveness of exercise for the management of LBP; therefore, it would seem prudent to avoid the same pitfalls when conducting future studies on low‐back and pelvic pain during pregnancy.
Quality of the evidence
No outcomes were supported by high‐quality evidence and only six (largely from individual studies) by moderate‐quality evidence (exercise for LBPP and related sick leave (Table 2); OMT for LBP (Hensel 2014); acupuncture for PP (Elden 2005) and craniosacral therapy for PP and related functional disability (Elden 2013). Overall, there was low‐quality evidence for outcomes because of high risks of bias, imprecision of effect estimates and inconsistent results. Studies were generally small (range 29 to 855 women; with only four including more than 300 women, the GRADE rule of thumb for imprecision (GRADE Handbook). In all but the exercise interventions for LBP, LBPP and the prevention of PP, clinically heterogeneous populations, interventions, comparisons and outcome measures precluded pooling the results to arrive at overall estimates of effect.
Besides the paucity of usable data, the risks of bias contribute to the lack of confidence we have in the results. Overall, reports were poorly written and, in some cases it was difficult to follow the analyses, although the more recent studies have improved in their design and analyses. We only included RCTs in this review, but only 24 of the studies provided the methods of randomisation and only 21 outlined the methods of allocation concealment. On the other hand, we excluded eight studies because the techniques they described for randomisation were at high risk for bias, or allocation procedures were simply unclear. Current wisdom suggests that randomisation and concealment of allocation are key study characteristics that reduce the potential for bias. Blinding of personnel remains difficult in non‐pharmaceutical studies, a reality that increases the risk of bias, especially in self‐reported measures of symptoms. Some of the more recent studies did attempt to minimise bias by recruiting, for example, only participants who were naive to acupuncture (Elden 2005; Elden 2008), or by conducting credibility checks (Elden 2013; Wang 2009a) to determine the participants' expectations of the study interventions they were offered. In four studies (Ekdahl 2010; Gil 2011, Keskin 2012; Martins 2005), baseline pain was different between groups, and Martins 2014 did not provide any information about baseline similarities. Akmese 2014 provided low‐quality evidence (study design limitations, imprecision of effect estimates) suggesting that progressive muscle relaxation (PMR) reduced pain intensity more than lying down for the same time each day, however they also noted that the use of relaxing music during the PMR sessions may have contributed to their findings. This factor prevented us from being able to include this study in the meta‐analysis for any exercise for LBP (Analysis 1.1). In Wedenberg 2000, 12 of 30 women dropped out of the physiotherapy group, while none withdrew from the acupuncture group (although two were excluded from analysis due to receiving both treatments), leading to potential attrition bias. Based on baseline data, there were no obvious reasons for the difference in withdrawals between the two groups.
Potential biases in the review process
This review was updated using the updated Cochrane methodology for assessing risk of bias and quality of the evidence. The international literature was searched and studies in languages other than English were retrieved. However, while the English articles were independently identified and assessed by two review authors, the three non‐English reports were only reviewed by one person (Bandpei 2010; Gil 2011; Martins 2005); Gil 2011 was also translated using Google Translate (Google Translate). Three non‐English reports were also excluded by one person only (Chitryniewicz 2010; Momoi 1999; Zand 2011). Information from two German PhD theses was taken from the English abstracts only (Gundermann 2013; Peters 2007). This has the potential to lead to some errors, but considering the lack of overall data in the English reports, this is not likely to make a substantial change in the results or quality of the evidence.
Agreements and disagreements with other studies or reviews
Pennick 2007 included eight RCTs (1305 women with LBP, PP or both) and found that adding pregnancy‐specific exercises, physiotherapy, acupuncture and pillows to usual prenatal care reduced pain intensity, functional disability, and absenteeism. However, seven of the eight studies had moderate to high risks of bias, and readers were advised to view the results with caution.
A number of non‐Cochrane systematic reviews have been published since Pennick 2007. While they do have different foci and different search dates, Ee 2008; Franke 2014; Kanakaris 2011; Richards 2012; Vermani 2010 and this current review are essentially in agreement for the aspects of overlap. Field 2008 reviewed research on the effectiveness of complementary and alternative medicine for pregnancy and labour and concluded that the evidence suggests they are effective for reducing pregnancy‐related back and leg pain, amongst other symptoms and bio‐markers outside the scope of this review, but 'the research has several methodological limitations'. Anderson 2005 investigated the effectiveness of complementary and alternative medicine in obstetrics and included two of the studies included in this review that examined the effects of acupuncture on pregnancy‐related LBP (Kvorning 2004; Wedenberg 2000). They came to the same conclusions as we did. Franke 2014 reviewed the effects of OMT for LBP, which included a separate analysis for pregnant women with LBP. They included data from the same trials as we included in this review; the results of their meta‐analysis were strongly supportive of the positive effects of OMT on pain and disability.
Authors' conclusions
Implications for practice.
The quality of the evidence included in this review ranged from moderate to low, raising concerns about the confidence we have in the estimates of effect. Clinical heterogeneity precluded pooling of results in many cases and statistical heterogeneity was substantial in all but three meta‐analyses, which did not improve following sensitivity analyses. When compared to usual prenatal care, the variety of interventions reported largely improved pain, related functional disability or sick leave and with minor, transient adverse effects. However, publication bias and selective reporting cannot be ruled out.
Whilst exercise in a variety of formats appears to improve LBP and related functional disability, the quality of the evidence was low and, due to the high statistical and clinical heterogeneity of studies, we could not determine the estimate of effect of any specific exercise for a specific group of pregnant women.
There was less evidence available for PP. Of note, there was moderate‐quality evidence from single studies that acupuncture significantly improves evening PP better than stabilising exercises or usual prenatal care; and craniosacral therapy significantly improves morning PP and related functional disability more than usual prenatal care. Although the difference in treatment outcomes between exercise for LBP and PP cannot be directly compared it would appear that, in contrast to LBP, exercise does not appear to improve outcomes any better than usual prenatal care. The above observations would suggest that establishing the anatomical source of symptoms is paramount for tailoring treatment accordingly. Currently, this does not appear to be a part of routine clinical practice for these complaints (Sinclair 2014).
The issue of effectively diagnosing the source of symptoms is also relevant for those women with LBPP, however there was moderate‐quality evidence from meta‐analyses supporting the use of an exercise program, lasting eight to 12 weeks, for reducing the number of women reporting LBPP, and land‐based exercise, in a variety of formats, for reducing LBPP‐related sick leave. A multi‐modal intervention (manual therapy, exercise and education (MOM)) may also improve LBPP and related functional disability, however the evidence was of low‐quality and from only one study (George 2013).
The remaining evidence from individual studies, examining a number of other interventions, included moderate‐quality evidence (study design limitations or imprecision) that osteomanipulative therapy added to usual prenatal care relieves LBP and related functional disability better than usual prenatal care alone but not significantly better than the addition of placebo ultrasound to usual prenatal care (Hensel 2014).
Findings from smaller individual studies provided low‐quality evidence suggesting that; TENS or progressive muscle relaxation accompanied by music, may relieve pelvic‐ or low‐back pain more than usual prenatal care; acupuncture is better than physiotherapy at relieving evening LBPP and related functional disability, and improves pain, but not women's ability to carry out daily activities, when started at 26‐ rather than 20‐weeks' gestation. The addition of a lumbo‐pelvic belt to information significantly relieves PP more than exercise plus information or information alone, however no additional pain relief occurs with the use of a rigid pelvic support belt added to exercise plus information. There is no significant difference in LBP and related functional disability when using different support belts, between exercise, NET or SMT, or in evening PP between deep and superficial acupuncture.
Further research is likely to change our confidence in the estimates of effect of the above treatments.
Implications for research.
Given the high incidence of LBP and PP during pregnancy, and the distress this causes many women in late pregnancy, more research would be helpful to inform the advice given by prenatal practitioners. Future studies would benefit from an agreed classification system for categorising women according to their presenting symptoms. Possible foci of future research might include: developing and validating a classification system for pregnancy‐related LBP and PP, testing the efficacy and safety of analgesics in late pregnancy, and standardisation of outcome assessment. More and better designed studies that build on the current evidence, investigating the effects of physiotherapy, acupuncture and other conservative and complementary treatments already being used by pregnant women (Wang 2004) are also needed. Preventive studies beginning early in pregnancy would be welcome to see if any of these interventions will really prevent the development of either of these conditions, given the conflicting findings on prevention reported in this review. In order to establish the safety of interventions we recommend that future studies routinely measure the presence or absence of adverse effects. In addition, by incorporating validated outcome measures into study designs that include work‐related absence along with pain and low‐back‐ or pelvic‐related functional disability, future reviews may use meta‐analyses to help determine the most effective interventions.
Feedback
Herxheimer, September 1998
Summary
Characteristics of included studies: Thomas 1989 was a cross‐over study, was it reported as such? The outcome for the first cross‐over should be reported separately from the second cross‐over. Data for women who did not complete the second period could then be included for the first period. More information about when and for how long women used the pillows would be useful, and at what gestation. Information about how to get the OZZLO pillow should be presented, and whether it is a patented design. A drawing of the pillow would also be helpful.
Results: If the reviewers have contact with the trialists it would be useful to know whether they still use the OZZLO pillow, and if not why not.
Reply
These comments have now been incorporated into the updated review. It is not possible to provide a drawing of the OZZLO pillow within the Cochrane review but we have mentioned in the update that a drawing can be found in the original study, which is referenced.
[reply from Gavin Young, October 2001]
Contributors
Comments received from Andrew Herxheimer, September 1998.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2015 | New citation required but conclusions have not changed | Nine new randomised controlled trials (RCTs) added, but the conclusions remain largely the same. Heterogeneity in population, interventions, comparisons, outcomes and outcome reporting precluded further meta‐analyses over the 2013 update, despite the inclusion of additional exercise studies for low‐back pain or low‐back and pelvic pain. Evidence from single studies ranged from low to moderate quality. |
| 29 January 2015 | New search has been performed | Search updated and 22 reports identified. We included nine new RCTs in this update, reported in 13 publications: three were reported in multiple publications: (Kordi 2013; Martins 2014; Miquelutti 2013); six were published in single reports (Akmese 2014; Elden 2013; Gundermann 2013; Hensel 2014; Kaya 2013; Keskin 2012). Four reports were ongoing studies (Greene 2009; Freeman 2013; Moholdt 2011; Vas 2014). Five studies have been excluded (Hensel 2013; McCullough 2014; Moffatt 2014; Thomas 1989; Torstensson 2013.), one of these was previously included (Thomas 1989) in this review but, due to concerns with the use of a cross‐over design, we have excluded it in this update. This review now includes 34 RCTs. Sarah Liddle took over as Contact person for this 2015 update. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 14 December 2012 | New citation required but conclusions have not changed | With the addition of new trials, there is now more evidence for interventions aimed at preventing and treating low‐back pain, pelvic pain and a combination of both (lumbo‐pelvic pain). |
| 18 July 2012 | New search has been performed | Searches updated. Since the last update in 2007, 47 reports of potentially relevant studies have been identified and of these: 18 new trials included (Bandpei 2010; Depledge 2005; Eggen 2012; Ekdahl 2010; Elden 2008; Gil 2011; George 2013; Kalus 2007; Kashanian 2009; Kluge 2011; Licciardone 2010; Lund 2006; Mørkved 2007; Peters 2007; Peterson 2012; Sedaghati 2007; Stafne 2012; Wang 2009a); 17 studies excluded (Beyaz 2011; Chitryniewicz 2010; de Jonge‐Vors 2011; Field 1999a; Field 2012; Foxcroft 2011; Granath 2006; Haugland 2006; Kohama 2006; Ladefoged 2012; Mens 2012; Momoi 1999; Schoenfeld 2011; Singh 2008; Thorell 2012; Torstensson 2009; Zand 2011); and four trials identified as ongoing (Abolhasani 2010; Greene 2009; Hensel 2008; Moholdt 2011). |
| 9 June 2008 | Amended | Converted to new review format. |
| 15 April 2006 | New citation required but conclusions have not changed | A new author, Victoria Pennick, joined the review team and is now the guarantor of the review. |
| 8 February 2006 | New search has been performed | This updated review (February 2006) includes an updated search, which identified five new trials that met the inclusion criteria: two studies examined women with low‐back pain (Garshasbi 2005; Suputtitada 2002); one study examined women with pelvic pain (Elden 2005); and two studies examined a mixed population with pelvic and back pain (Kvorning 2004;Martins 2005). In total, we included nine reports (1305 participants), describing eight studies. One report was the abstract of one of the published articles and only gave preliminary results. Despite the addition of these studies, the conclusions remain essentially the same. The specially‐designed Ozzlo pillow was more effective than a regular one in relieving back pain, but is no longer commercially available. Pregnant‐specific exercise programs, physiotherapy and acupuncture added to usual prenatal care all appeared to reduce back or pelvic pain more than usual prenatal care. However, all but one study had moderate to high potential for bias, prohibiting full confidence in these results. The updated search also identified three new reports, which we excluded because they are quasi‐randomized controlled trials (Ciardi 2002C; da Silva 2004; Nilsson‐Wikmar 2005) and two ongoing trials (Quinlivan 2005a; Wang 2005a). |
| 31 October 2001 | New search has been performed | Search updated. Two new studies are included which assess the role of acupuncture versus physiotherapy, and water gymnastics versus no treatment. |
| 31 October 2001 | New citation required but conclusions have not changed | The background section has been enlarged, giving more information about prevalence and prognosis. A distinction is made between pain arising from the lumbo‐sacral region (low‐back pain) and pain in the region of the sacro‐iliac joints and pubic symphysis (pelvic pain). Two new studies are included which assess the role of acupuncture versus physiotherapy, and water gymnastics versus no treatment. |
| 1 October 2001 | Feedback has been incorporated | Authors replied to feedback. |
| 9 January 1998 | Feedback has been incorporated | Feedback received from Andrew Herzheimer. |
Acknowledgements
For this update, we would like to acknowledge the support from the World Health Organization, specifically Nancy Medley, for her assistance with the analyses, and preparation of the review. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
For the 2013 update, as part of the pre‐publication editorial process, the review was commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
For the 2007 update: Victoria Pennick (VEP) and Gavin Young (GY) selected and assessed the methodological quality of the articles and extracted and analysed the data. VEP wrote the first draft of the review; GY reviewed and offered his comments. Victoria Pennick would like to thank Andrea Furlan for her helpful comments and assistance with translation.
We were able to exclude studies and include data from non‐English studies due to the gracious assistance of Andrea Furlan, Ivan Steenstra, Jacob Etches, Ayako Kitta, Reza Yousefi‐Nooraie and Tomasz Kotwicki. Thanks also to Kelly An who helped with 'Risk of bias' assessment and data extraction for the 2007 update, Lynn Hampson, Rachel Couban and Shireen Harbin who helped identify and obtain copies of the studies.
And finally, we would like to acknowledge the contributions made by David Jewell to the first two versions of this review, Gavin Young for his contributions to the first three versions; the Scientific Foundation Board of the Royal College of General Practitioners for financial support of the 2002 update and Paul Shekelle of the Cochrane Back Review Group who helped with the 2002 analyses.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. GRADE criteria
As outlined in the GRADE Handbook
Limitations in study design or execution (risk of bias) ‐ overall, the studies that measured the outcome were assessed to have sufficiently high risk of bias that we did not have full confidence in the results.
Inconsistency of results ‐ studies that measured the outcome had widely differing estimates of treatment effect.
Indirectness of evidence ‐ studies did not measure the outcome directly.
Imprecision of effect estimates (imprecision) ‐ studies that measured the outcome included few patients (< 400) or few events (< 300) and/or wide confidence intervals (included both appreciable harm and appreciable benefit that was greater than 25%).
Publication bias ‐ overall, the studies that measured the outcome showed a systematic selective publication bias.
Data and analyses
Comparison 1. Low‐back pain: any exercise + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 7 | 645 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.03, ‐0.25] |
| 2 Functional disability | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.89, ‐0.23] |
Comparison 2. Low‐back pain: water gymnastics + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women taking sick leave because of low‐back pain after 32 weeks' gestation | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.17, 0.92] |
Comparison 3. Low‐back pain: support belts ‐ Bellybra versus Tubigrip.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low‐back pain, measured with VAS; 0 to 10; 0 = no pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐back pain | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.19, 0.79] |
| 1.2 Functional disability (ADL) | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.81, 0.01] |
Comparison 4. Low‐back pain: group exercise + education + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women reporting pain on Visual Analogue Scale | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
Comparison 5. Pelvic pain: deep versus superficial acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Evening pain | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.73, 1.54] |
Comparison 6. Pelvic pain: group exercise + education + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women reporting pain on Visual Analogue Scale | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
Comparison 7. Pelvic + low‐back pain: any exercises + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women who reported pain on Visual Analogue Scale | 4 | 1176 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.97] |
| 2 Women who reported LBPP‐related sick leave | 2 | 1062 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.62, 0.94] |
Comparison 8. Pelvic + low‐back pain: acupuncture + usual prenatal care versus usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women who reported decreased pain | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [1.77, 9.78] |
Comparison 9. Pelvic + low‐back pain: acupuncture + usual prenatal care versus individualised physio + usual prenatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Numbers of women rating treatment as good or excellent | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.96, 1.60] |
Comparison 10. Pelvic + low‐back pain: MOM versus STOB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐3.54, ‐1.86] |
| 2 Functional disability | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.09, ‐0.71] |
| 3 Days off work/sick leave | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.12, 1.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akmese 2014.
| Methods | 8‐week prospective RCT conducted in the Antenatal Care Unit of the Department of Obstetrics and Gynecology, Faculty of Medicine, Ege University, Turkey. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 37/33 analysed): PMR; 20‐minute session completed by each participant at home twice a day (morning and evening) for 8 weeks. At baseline, each participant attended a 2‐hour education session at the obstetrics department to check they understood and could complete the PMR exercises and breathing techniques. Each participant received handbook and a CD (prepared by Turkish Psychological Association). At 4‐weeks a follow‐up session took place at the obstetrics dept. to give feedback and check exercises. Control group (N = 36/33 analysed): participants instructed to lie down twice a day (morning and evening) for 20 minutes. At the end of the study participants received the same CD and handbook as the intervention group. Routine daily activity was not restricted for any group. Participants were asked to record their performance of the specified activities on a calendar in an attempt to encourage compliance. They were 'warned not to use any complementary treatment for LBP' during the study. |
|
| Outcomes |
Primary outcome: pain (VAS 0 to 10), completed at baseline, weeks 4 and 8. Secondary outcome: Generic Health Status (Short Form‐36), completed at baseline, weeks 4 and 8. Personal information form (PIF), completed at baseline: demographics, pregnancy and obstetric history. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Financial support was provided by Ege University Scientific Research Project (No. 2007/ASYO/004). Note: on page 2, higher score for SF‐36 was attributed to deterioration, while the reverse is true, which was borne out in the results section, where improvement is illustrated with a higher score. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random number table used to assign participants to control and experimental groups' but no further details on how the random number table was used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Experimental group were aware of the benefit of exercise for their condition. Providers of training for PMR could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No assistance was given by staff to any participants completing assessment forms/questionnaires. At the end of the study (week 8) the study co‐ordinator, who was blinded to group and timing of assessment, collected all assessment forms. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | There was a 8% drop‐out rate from each group that were not accounted for in the analysis, nor were reasons provided for drop‐out. |
| Selective reporting (reporting bias) | Low risk | Means and SD were provided for primary and secondary outcomes at each time point (weeks 4 and 8) for all participants completing the study, N = 33 in experimental and N = 33 in control group. |
| Other bias | Low risk | VAS scores were similar between groups at baseline, however SF‐36 scores were not similar at baseline. Sample size very small, however there were very specific inclusion criteria and the authors controlled for use of co‐interventions in both groups and participants reported similar adherence to program. |
Bandpei 2010.
| Methods | 'Following ethical approval and through a randomised controlled clinical trial, 120 pregnant women with LBP were recruited into experimental and control groups.' Conducted in Iran. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 60/57 analysed):
Control group (N = 60/55 analysed): Usual prenatal care. |
|
| Outcomes | Pain (VAS 0 to 10) and disability (ODI) were measured in both groups but, in the results, only the baseline and the difference from baseline was reported in each study group (with no report of SD). The comparison of all changes between 2 study groups were statistically significant with P < 0.0001. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Change score from immediately after treatment was subtracted from the baseline pain score for an 'immediately after treatment' VAS score; reported lost to follow‐up was assumed to have happened during treatment; RevMan calculator was used to calculate SD to allow results to be included in meta‐analysis for 'any exercise vs usual prenatal care', analysis 1.1. Translated from Arabic by single Iranian researcher. Funding = no information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with matching (stratification?) for age, gestational age, and BMI. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in paper. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were not blinded. Nothing mentioned about blinding of providers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nothing mentioned about blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | 120 patients were enrolled (60 in each group). 3 patients in intervention group, 5 in control group missed. In all cases the researchers lost track of the patients due to the change in living location. |
| Selective reporting (reporting bias) | Unclear risk | The outcomes were also measured at 6 months and 1 year after delivery. But only the results for immediately after treatment, and 3 months after delivery were reported. Pain (VAS) and disability (ODI) were measured in both groups. But in the results, only the baseline and the difference from baseline, with no report of SD, were reported in each study group. The comparison of all changes between 2 study groups were statistically significant with P < 0.0001. |
| Other bias | Low risk | Nothing noted in the paper. |
Depledge 2005.
| Methods | 'Prospective masked randomised experimental clinical trial', carried out at the National Women's Hospital, Physical Therapy outpatient department, Auckland, NZ. Those meeting inclusion criteria were assessed by 1 of 4 therapists (identically trained); 36 withdrew prior to randomisation because they did not meet the inclusion criteria. To see a small to medium effect size (0.35) on a modified RMDQ, with power set at 0.8 and alpha at 0.05, 30 women were needed per group. Number randomised = 90; number analysed = 87. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention groups: 1. Exercise plus non‐rigid support belt (N = 29 analysed): participants received same information and exercises as the control group, plus a non‐rigid neoprene support belt (Smiley Belt) and logbook for recording number of hours the belt was worn and number of times exercises done. 2. Exercise plus rigid support belt (N = 28 analysed): participants received same information and exercises as the control group, a rigid belt (Lifecare Pubic Belt) and a logbook for recording number of hours the belt was worn and number of times exercises done. Control group: Exercise only (N = 30 analysed): participants received an exercise booklet with 5 exercises aimed to increase the stability of the pelvic bones. A trained physical therapist demonstrated the exercises and checked that they were being performed correctly. Exercises needed to be completed 3 times daily for 1 week. Participants were given logbook to record the frequency they exercised. Participants also received verbal and written education about the anatomy and pathology of symphysis pubis dysfunction and self‐help management. See study’s Appendix 2 and 3 for specific exercises and self‐help management techniques). |
|
| Outcomes | Average and worst pain in last week ‐ NRS (0 to 100); modified RMDQ; Patient Specific Functional Scale; measured at baseline, after treatment. | |
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | There were no significant differences between the groups in adherence to their exercise program or belt wearing. The adherence rate was acceptable (average for all participants: Exercises = 16.5/21 times, Number of hours belt worn/week = 44.2). Funding = Maurice and Phyllis Paykel Trust for a Research Scholarship. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Randomization process involved the use of a table of 3 randomly permuted blocks'. |
| Allocation concealment (selection bias) | Unclear risk | Not specifically mentioned: patients assigned to groups by independent person (not connected to study) but unclear how this was actually done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients not blinded; therapists providing exercise therapy were unaware of the intervention groups to which participants were assigned. However, unclear as to who distributed the belts. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors did not specify who collected the outcomes (outcomes were self‐report measures). |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | No withdrawals in the control group. 1 woman in the non‐rigid support belt group delivered her baby before the post‐intervention assessment. 2 women in the rigid support belt group delivered their babies before their post‐intervention assessment. 1 woman refused to be in the study as she was 'not prepared to be in the exercise‐only group'. No exclusions mentioned. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes as indicated in methods. |
| Other bias | Low risk | Groups similar at baseline; adherence similar between groups; outcomes taken at same time for each group, co‐interventions likely to be similar. |
Eggen 2012.
| Methods | Observer‐blinded RCT. | |
| Participants | 257 women were randomised. Inclusion criteria: Healthy Norwegian speaking women between 18 to 40 years from 2 Maternity Care Units (within the Norwegian Public Health System). Exclusion criteria: 1. Pregnant women carrying twins. 2. Inflammatory rheumatic disorders. 3. Risk factors for miscarriage. |
|
| Interventions |
Intervention group (N = 129/106 analysed): Participants, referred to 1 of 2 specially trained physical therapists, received tailored supervised group exercise once a week, along with advice to do daily HEP. Specific attention to body awareness and ergonomic advice in real‐life situations; the main focus of the intervention being the specific training of the transversely oriented abdominal muscles with co‐activation of the lumbar multifidus at the lumbosacral region, and stretching the hip abductors. Intervention took place for a maximum of 16 weeks, between 20 to 36 weeks' gestation, with no follow‐up after 36 weeks' gestation. Control group (N = 128/107 analysed): Usual prenatal care. |
|
| Outcomes |
Primary outcome: The proportion of women experiencing pain in the lumbar spine/pelvic girdle. Secondary outcomes: 1. Functional disability measured with the modified RMDQ (0 to 24). 2. LBP and LBPP measured using the VAS scale (0 to 10). 3. Health‐related quality of life measured with the SF‐8 Health Survey. All outcomes measured at 24, 28, 32, and 36 weeks' gestation. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Funding/sponsor: Norwegian Fund for Postgraduate Training in Physiotherapy (Norway). Lead author contacted to clarify the number analysed in intervention group; she confirmed that it should be 106, not 103 as stated in the Figure and tables. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomisation procedure was computer generated by the statistician not involved in data collection.' |
| Allocation concealment (selection bias) | Low risk | 'group allocation was concealed in consecutively numbered, sealed, opaque envelopes.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were self‐reported, therefore not blinded; however the midwives who distributed the questionnaires to the women were not aware of their group allocation. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Treatment group lost 22/129 (17.8%) and the control group lost 21/128 (16.4%) by the end of follow‐up at 36 weeks' gestation. |
| Selective reporting (reporting bias) | Low risk | Results provided for all the outcomes outlined in the trial registration (ISRCTN95014448). |
| Other bias | Low risk | Groups were similar at baseline except that the training group had significantly higher BMI; almost twice as many women in the training group had experienced moderate to severe PGP in a previous pregnancy but this was adjusted for in the outcome analyses; adherence to exercises did not seem to vary between groups, nor did consultation with healthcare providers. |
Ekdahl 2010.
| Methods | 40 pregnant women 'from the same demographic area' in Sweden; N = 20 in each group (group 1 and 2). After diagnosis with pelvic and LBP, women were referred to the acupuncturist at the hospital 'where randomisation was carried out; women were phoned to give them the dates for their acupuncture; baseline data were collected when they came for treatment'. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Both groups received the same treatment; 8 acupuncture treatments over a 6‐week period (2 treatments per week in the first 2 weeks and once per week thereafter) with first treatment lasting 20 minutes and number of needles limited to 5, and remainder 30 minutes with maximum 10 needles. Intervention group 1 (N = 20/16 analysed): mean age 28.6 years, started treatment at 20 weeks' gestation and Intervention group 2 (N = 20/16 analysed): mean age 27.9 years, started treatment at 26 weeks' gestation. No control group: acupuncture intervention was started either at 20 (group 1) or 26 weeks' (group 2) gestation. |
|
| Outcomes | Short Form Health survey questionnaire (SF‐36), Short Form Magill Pain Questionnaire (MPQ), Pain‐o‐meter, fetal sound measured at baseline, at 4th and 8th treatment sessions, at same times for each group; qualitative data collected via telephone interviews 2 to 3 months after delivery. Both groups had similar experience of acupuncture (from qualitative interviews). Small number of study participants acknowledged by authors. Non compliance in both groups reported. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Funding = Council of Research and Development (FoU‐centrum), Landstinget Kronoberg, Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information given about sequence generation; in the discussion it states that 'the women were chosen randomly'. |
| Allocation concealment (selection bias) | Low risk | Telephone allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'both groups had similar experience with acupuncture when asked at end of treatment'; unclear if acupuncturists were informed of gestation, or if they were able to determine by observation, however, the difference was only 20 to 26 weeks, therefore likely not a big issue. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided on who collected the self‐report outcomes. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Both groups had 4 drop‐outs with reasons given ‐ did not appear to be related to intervention. |
| Selective reporting (reporting bias) | Low risk | Qualitative data supports quantitative data ‐ however the telephone interviews were completed by the study author. |
| Other bias | Low risk | Co‐interventions and adherence similar across groups, timing of outcome assessment same across groups, mean pain intensity was significantly lower in group 1 than group 2 at baseline. |
Elden 2005.
| Methods | Single‐blind RCT; N = 386 women consecutively selected by doctors and midwives and randomised to 3 groups; acupuncture, stabilising exercises and standard treatment.
Participants and caregiver not blinded; assessor blinded. ITT: those who finished the study were analysed in the group to which they had been assigned. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group 1: acupuncture (N = 125/110 analysed).
General information about PGP, the anatomy of the back and pelvis. Participants given advice about ADL, a pelvic belt and a HEP by a physiotherapist + acupuncture treatment given twice a week over 6 weeks using 10 local acupuncture points in sensitive spots + 7 extra‐segmental points. Needles inserted to evoke De qi, left in situ for 30 minutes and stimulated every 10 minutes. Acupuncture given by 2 experienced medical acupuncturists. Intervention group 2: stabilising exercises (N = 131/112 analysed). General information about PGP, the anatomy of the back and pelvis. Participants given advice about ADL, a pelvic belt and a HEP by a physiotherapist + individual stabilising exercises (modified for pregnancy) for a total of 6 hours over 6 weeks. Stabilising exercises provided by 2 experienced physiotherapists. Control group: standard treatment (N = 130/108 analysed). General information about PGP, the anatomy of the back and pelvis. Participants given advice about ADL, a pelvic belt and a HEP by a physiotherapist (provided by 3 experienced physiotherapists). |
|
| Outcomes | Pain intensity (VAS 0 to 100): self‐report each am and at 1 week post‐treatment: examiner assessment of recovery from symptoms ‐ positive pain drawing; examiner assessment of recovery from symptoms ‐ P4 test; examiner assessment of recovery from symptoms ‐ pain when turning in bed. Adverse events: none reported for any of the 3 groups. |
|
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | Women recruited from East Hospital, Sahlgrenska Academy and 27 maternity care centres in the hospital's reference area in Gothenburg, Sweden between 2000 and 2002. Funding = The Vardal Foundation, the Dagmar Foundation, the Trygg‐Hansa Insurance Company, the Sahlgrenska University Foundation. 14 March 2012 ‐ email and LinkedIn message sent to Dr Elden to clarify number of participants in Table 3; response received ‐ clarified that there were 130 in the standard group and 131 in the exercise group; other data are correct. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table to determine the allocation sequence before the study. |
| Allocation concealment (selection bias) | Low risk | Pre‐sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Results coded and entered by personnel from independent institution; Statistician blinded to group and treatment.' |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Standard treatment group; randomised = 130; analysed = 108 [83.0%] (lost to follow‐up: declined treatment N = 15, early delivery N = 3, declined visit N = 3, moved from area N = 1). Acupuncture group: randomised = 125; analysed = 110 [88%] (lost to follow‐up: declined treatment N = 10, declined visit N = 1, early delivery N = 4). Stabilising exercises: randomised = 131; analysed = 112 [85.5%] (lost to follow‐up: declined treatment N = 9, moved from area N = 1, early delivery N = 4, declined visit N = 5). ITT: analysed participants measured 1 week post‐treatment against those randomised. |
| Selective reporting (reporting bias) | Low risk | Data presented for a priori determined outcomes. |
| Other bias | Low risk | Table 3 seems to have the number of women reversed between 'Standard' and 'Exercise' groups. Author clarified this to be so. |
Elden 2008.
| Methods | 'Randomised double‐blind controlled trial.' N = 115 randomised; N = 58 to standard treatment plus acupuncture; N = 57 to standard treatment plus non‐penetrating acupuncture. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 58/56 analysed): standard treatment + penetrating acupuncture Standard treatment: general information about condition and anatomy of back and pelvis and a pelvic belt, gave advice and HEP designed to increase strength in the abdominal and gluteal muscles. Information was supplemented by a leaflet. Also instructed to avoid other treatments during intervention period. Penetrating acupuncture: see study methods for exact acupuncture points used. Sterilised disposable needles were used and inserted intramuscularly to depth of 15‐50 mm. Needles were left in situ for 30 minutes and manually stimulated every 10 minutes. Control group (N = 57/52 analysed): standard treatment + non‐penetrating acupuncture Standard treatment: identical to experimental group Non‐penetrating acupuncture: used a validated sham acupuncture device (which looks like real acupuncture needles but the tip of needle is blunted). The shaft of the sham needle did not penetrate the skin, it collapsed into the handle and creates an illusion of insertion. Needles were left in situ for 30 minutes and manually stimulated every 10 minutes. |
|
| Outcomes | EQ‐5d questionnaire and EQ‐5d VAS; pain (VAS 0 to 100) in the morning and evening; ODI (back specific function); frequency of sick leave; Disability Rating Index (DRI) measured at baseline, after treatment and 1‐week follow‐up. Adverse events: transient, tingling, needle pain, slight bleeding, fainting, sleepiness. |
|
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | Pain severity diagnosed with ASLR and P4 tests. N = 165 women assessed for eligibility (N = 50 did not meet inclusion criteria). All women acupuncture naive and singleton fetus. No serious adverse events reported. Same contact time, manual contact during search and stimulation of needles, interaction between patient and therapist in both groups. Drop‐outs reported with reasons. Funding = grants from the Foundation of the Health and Medical care committee of the Region of Västra Götaland (Sweden), grants from the Swedish Medical Research Council and Swedish government grants to researchers in the public health service. Women recruited between June 2006 and May 2007 from 25 units within the Västra Götaland region, Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Computer‐generated random table was used'. |
| Allocation concealment (selection bias) | Low risk | Statistician who was not involved in the study administered pre‐coded numbered identical opaque envelopes to assign participants to the intervention groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Participants: low risk as only LI4 (on hand) not blinded. 'Women were blinded to whether they were receiving sham or active treatment.'
Therapist remained neutral for both groups.
Women were treated in a prone position, i.e. unable to see the needles except ones on hand.
Sham needle collapses into a handle to create illusion of insertion.
Reported that most participants believed they received the penetrating acupuncture. Providers: high risk Not blinded. Same therapist administered sham and active treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Assessors: low risk Blinded to treatment allocation, doctors handling decisions about sick‐listing were also blinded. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk |
Drop‐outs: 2 in treatment and 5 in control dropped intervention because it 'violated protocol'. At follow‐up: 3 drop‐outs in treatment group due to early birth and declined visit, 2 in control group due to declined visit. Low drop‐out rate, and similar reasons between the groups. No exclusions mentioned. Numbers add up. For missing data and those who withdrew, ITT analysis applied to outcome data using last recorded data. Low risk: attrition and drop‐outs reported and reasons, numbers at each stage add up, ITT ‐ last value carried forward. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes it said it would report in methods. All outcome data are found in tables. |
| Other bias | Low risk | Randomisation procedure successful (however more in control group on sick leave?). |
Elden 2013.
| Methods | Multicentre single‐blind RCT. | |
| Participants |
Inclusion criteria:
Participants were diagnosed according to the European PGP Guidelines. Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 63 randomised/analysed): craniosacral therapy (CST) as adjunct to standard treatment.
Control group (N = 60 randomised/analysed):
All information provided as standard treatment was supplemented by a leaflet. |
|
| Outcomes |
Primary outcomes: pain intensity (VAS 0 to 100) in the morning and evening; sick leave. Secondary outcomes: function (modified ODI); Disability Rating Index; Quality of Life. (EuroQol‐5d); unpleasantness of pain (VAS); helpfulness of treatment. Physical tests used: Faber Test, 4P test, Modified Trendelenberg Test, Symphysis Pubis Pressure Test, ASLR. Women also completed a pain drawing denoting the location of their symptoms. |
|
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | Study supported by research grants from the Health and Medical Care Committee of the Regional Executive Board, Region Vastra Gotaland (Sweden), Grant No. [VGFOUREG‐155171]. Study conducted in Gothenberg, Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table used. Stratified balanced randomisation was used to guarantee balance between groups for frequency of sick leave. |
| Allocation concealment (selection bias) | Low risk | Research assessor not involved in the study administered pre‐coded, numbered identical opaque envelopes to assign participant to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding not possible for participants or providers, however the researchers did assess the credibility of treatment to reduce the effect of treatment preference for participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent observer measured and entered VAS without knowledge of group assignment; Statistican blinded to group allocation and treatments. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attrition and exclusions given with reasons, ITT analysis used (last value carried forward). 6 women withdrew from CST. |
| Selective reporting (reporting bias) | Low risk | Results presented as described in the methods. All outcome data are found in tables. |
| Other bias | Low risk | Women asked to conceal information about their treatment during assessment. Interventions carried out by 2 experienced craniosacral therapists who met to ensure consistent approach throughout study. No serious adverse events ‐ 5 minor adverse events reported including only partial pain relief (N = 1), belt discomfort (N = 1), drowsiness (N = 3). |
Garshasbi 2005.
| Methods | 266 randomised: those who could not exercise were excluded from the exercise group, but it is unclear why 54 people dropped out of exercise group and none dropped out of control group. Excluded before randomisation = 14 with UTI, threatened abortion, lack of time, leaving 266 to be randomised. Analysis of pain and flexibility measures were conducted on those who completed the intervention in the group to which they had been randomised. | |
| Participants | 280 women invited to participate from those who registered at the hospital (no details about how they were selected from the 2358 who had registered at the clinic during the study period). Inclusion criteria:
Exclusion criteria:
Baseline characteristics: 2 groups similar in age, weight, height, BMI. In exercise group 73 women (68%) had LBP during pregnancy. In control group 78 women (70.5%) had LBP during pregnancy. |
|
| Interventions |
Intervention group (N = 161/107 analysed): (54 who could not participate in exercises = 107).
Exercises recommended by Tarbiat Modares Faculty of Sport and tested for pregnant women by physiotherapists, to strengthen abdominal muscles, hamstring muscles and increase traction of iliopsoas and para vertebral muscles.
12‐week exercise program: 15 movements in 60 minutes: 5 minutes of slow walking, 5 minutes of extension movements, 10 minutes of general warming up, 15 minutes anaerobic exercise, 20 minutes of specific exercise, 5 minutes return to the 1st position ‐ offered to exercise 3 times a week ‐ supervised by midwife ‐ intensity of exercises controlled by maternal pulse rate ‐ stopped if > 140/minute. Control group (N = 105 randomised/analysed?): usual prenatal care. |
|
| Outcomes | No scales/units given for outcomes measured, but may be assumed they are reporting the group mean, measured on the KEBEK questionnaire (Iranian version of Quebec Questionnaire for assessing pain; range 0 to 100, higher = worse pain); change scores do not appear to be included; the degree of lordosis and degree of flexibility of the spine. Outcomes assessed at baseline and after 12 weeks for both groups. Adverse events: none reported. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Numbers do not add up; there are contradictions in text; we tried unsuccessfully to clarify data with lead author during the 2007 update. Funding: not stated. Study conducted at Hazrat Zaynab Hospital prenatal clinic in Tehran, Iran. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘prospective randomised study’ but method of randomisation not described. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Report states that the outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Difficult to assess since numbers do not add up; appears that 14 withdrew prior to randomisation; about 20% withdrew/dropped out after randomisation; it appears that 54 dropped out of the intervention group and none out of the control group. |
| Selective reporting (reporting bias) | High risk | Results are difficult to interpret and appear to be reversed. |
| Other bias | Low risk | Nothing more to add. |
George 2013.
| Methods | Prospective RCT including 169 pregnant women recruited from 3 obstetric centres. Women randomised into experimental (N = 87) and control (N = 82) groups. | |
| Participants |
Inclusion criteria:
Women were evaluated by their obstetric provider. Those with symptoms were screened by a dedicated study co‐ordinator. Women were not excluded if they had lower limb symptoms or radiculopathy. Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 87 randomised/analysed): multi‐modal musculoskeletal and obstetric management (MOM) ‐ standard obstetric care PLUS a chiropractic specialist provided manual therapy, stabilisation exercises and patient education based on the biopsychosocial model. Women attended weekly for MOM until 33 weeks' gestation, and were expected to complete home exercises twice a day. The aim was for women to receive 4 to 6 treatments each but number actually received was not recorded. NB: sacroiliac belts were reserved for women with severe hypermobility. Control group (N = 82 randomised/analysed): Standard Obstetric Care (STOB). Frequency of visits at the discretion of the obstetrics provider who also could recommend 1 or more of the following; rest, aerobic exercise, heat pad application (maximum 10 minutes), use of acetaminophen or narcotics if severe comfort not relieved with other methods, or onward referral. |
|
| Outcomes |
Primary outcomes: pain intensity (NRS 0 to 10), Disability (Quebec Disability Questionnaire ‐ QDQ). Secondary outcomes: personal pain history (PPH), SLR, P4 test, ASLR, long dorsal ligament test, sick leave, Patients' Global Impression of Change (patients' perception of clinical improvement), use of over‐the‐counter medications, trouble sleeping. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | This study is the full paper associated with Gross (2012) reported in the previous update of this review (presented at the 32nd Annual Meeting for the Society for Maternal‐Fetal Medicine, Dallas in February, 2012). The study was funded by the Health Resources and Services Administration, Grant number: R18HP07640. Study took place in St Louis, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation scheme used across the 3 recruitment sites using a computer‐generated list of randomised numbers. |
| Allocation concealment (selection bias) | Low risk | Online Web Data Entry System used to allocate women to experimental or control groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No report on blinding of providers or participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Chiropractic specialist performing baseline evaluation and follow‐up exams 'single masked'. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Data from all recruited participants were analysed, but there was 24% drop‐out from MOM group and only 13% drop‐out from control group; reasons for drop‐out not provided. |
| Selective reporting (reporting bias) | Low risk | NRS, QDQ, PPH, SLR (left leg only) and ASLR measured at baseline and 33 weeks. Means and SD were presented along with P values however, there were 10 more drop‐outs from the experimental group than the control group. Query re: number of individuals approached versus number randomised ‐ first author (JG) confirmed via email, 30 January 2015 (N = 2510 approached, N = 2341 excluded). |
| Other bias | Low risk | Groups were demographically similar and baseline evaluation showed no differences in pain, disability, physical assessments or other secondary outcomes between groups. |
Gil 2011.
| Methods | Potential women were identified through obstetric records and approached, in person or by phone, to determine if they met the inclusion criteria. 41 women were invited to attend; 4 declined, 3 did not attend the first follow‐up. 34 women randomised to either Global Postural Re‐education (GPR) treatment or usual prenatal care. |
|
| Participants | Women selected from those receiving prenatal care in 3 health centres and those who attended lectures in preparation for birth at a private hospital in Campinas, Brazil. Both groups of women were similar in most of the characteristics studied on admission to the study: in the GPR group 10 women came from a private hospital and 7 from a health (public) centres. In the control group there were 6 women from the private hospital and 11 from health (public) centres. Inclusion criteria:
Differentiation made between LBP and posterior PP at baseline physiotherapy assessment. |
|
| Interventions |
Intervention group = GPR (N = 17 analysed): Weekly 40‐minute sessions for 8 weeks. Stretching of the muscles of the posterior chain ‐ angle closure coxo‐femoral and abduction of the upper limbs and closing angle coxo‐femoral with adduction of the upper limbs. Control group (N = 17 analysed): usual prenatal care. |
|
| Outcomes |
GPR group: Intensity of LBP, using VAS (0 to 10) at baseline, before/after each treatment session. Back‐related functional disability, using the RMDQ at baseline, before/after each session. Control group: Intensity of LBP (0 to 10) and RMDQ measured at baseline, at 4 and 8 weeks. Use of pain medication collected for both study groups. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Used Google Translate (Google Translate) to translate from Portuguese; verified by single Portuguese researcher. Paper stated that there was no external funding. Data needed for the meta‐analyses appeared to be incorrectly reported in the paper and we re‐analysed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomisation was performed by using a list of random numbers generated by computer.' |
| Allocation concealment (selection bias) | Unclear risk | No mention in translated version of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'these professionals had lagged randomisation, so did not know to which group each woman was allocated' ... however, those who provided the exercise therapy and those who received it would have known to which group they were allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'at the end of the participation on each woman in the study, they conducted a professional full re‐evaluation of LBP' ... however, the women were the ones who reported their symptoms via the VAS and RMDQ. |
| Incomplete outcome data (attrition bias) effect of intervention | Unclear risk | 3 participants are reported as lost to follow‐up; there is no real clarification of their initial group ‐ it could be control group, but the 17 in each group do not seem to take any losses into consideration. |
| Selective reporting (reporting bias) | Low risk | Data provided for baseline and after intervention outcome measures for pain and disability. |
| Other bias | High risk | Not similar at baseline for education or age (intervention group was better educated and older), but similar in other prognostic factors; women in control group used more pain medication (87% versus 12% in intervention group); no information provided on compliance, co‐interventions or use of pain medication; the control group was only measured twice after baseline, the intervention group was measured 8 times, but all within the same time‐frame. |
Gundermann 2013.
| Methods | Randomised trial conducted in private practice in Jena, Germany. Group allocation was 'by external randomisation'. | |
| Participants |
Inclusion criteria:
|
|
| Interventions |
Intervention group = OMT (N = 21 randomised/analysed): usual prenatal care plus 4 custom tailored osteopathic treatments in 2‐week intervals based on osteopathic principles. Control group (N = 20 randomised/analysed): usual prenatal care; also received osteopathic treatment after an 8‐week untreated waiting period. |
|
| Outcomes |
Primary outcomes: pain intensity (VAS 0 to 10); pain frequency measured using a Likert scale; Secondary outcomes: functional disability (RMDQ), parturition characteristics and frequency of osteopathic dysfunctions. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Full thesis is in German and has not been requested as of Jan 2015; information and data extracted from the English abstract and the systematic review by Franke 2014. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomised via external randomisation.' |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment not noted in abstract, but Franke et al assessed as low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'One trained osteopath conducted the study in her private practice in Jena, Germany.' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'One trained osteopath conducted the study in her private practice in Jena, Germany.' |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | No attrition reported in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | Data only provided for pain intensity in the abstract; in Franke 2014, data for pain intensity and RMDQ provided in forest plots, Figures 6 and 7; Franke 2014 assessed as low risk. |
| Other bias | Unclear risk | It was difficult to note because of limited information in the abstract, but Franke 2014 assessed as low risk. |
Hensel 2014.
| Methods | A randomised, controlled, clinical study (PROMOTE). Clinical personnel referred interested women to the research co‐ordinators for screening. N = 400 randomised. | |
| Participants |
Inclusion criteria:
|
|
| Interventions |
All groups received usual prenatal care. Intervention group 1 (N = 136): Osteopathic Manipulative Therapy (OMT) to specific body regions x 7 treatments of approximately 20 minutes each at weeks 30, 32, 34, 36, 37, 38 and 39. Intervention group 2 (N = 131): placebo ultrasound (US) incorporating tactile and manual stimulation of the same body regions as OMT, using steady, circular movements with an US wand, and the usual auditory and visual cues of an US machine, but not emitting any US waves. Each treatment lasted approximately 20 minutes and was conducted at the same weeks' gestation as the OMT group. Control group (N = 133): Usual prenatal care only. This group did not spend any additional time with, or intervention from the treating physician. |
|
| Outcomes |
Primary outcomes: collected at baseline, and each visit.
Secondary outcomes: collected from participants' clinical notes after delivery.
Additional baseline data collected:
|
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Study conducted from 2007 to 2011 at 3 Obstetrics and Gynecology clinics in Texas, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation program used to allocate women, in blocks of 15, by clinical obstetric clinic. |
| Allocation concealment (selection bias) | Low risk | No specific information provided. However, the authors state that a 'randomisation envelope' was opened to reveal participant's group assignment, which would suggest appropriate concealment method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants in the OMT and placebo US groups received same duration of treatment, number of treatments and to same bodily regions, however it was not possible to blind to intervention. Providers could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After delivery, the research co‐ordinator collected data from each participant's medical record in paper format. Data were then transferred to an electronic data set using 'double data entry'. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attrition and exclusions reported, including number and reasons. An ITT analysis was completed initially (imply last measure carried forward), followed by a PPA as high numbers did not complete the protocol (all 7 visits) in each group. Each type of analysis gave similar results. |
| Selective reporting (reporting bias) | Low risk | All data reported as stated in methodology. |
| Other bias | Low risk | Outcomes assessed at same time points; co‐interventions controlled as women unable to continue in the study if they reported using any other body‐based therapies. Characteristics similar at baseline except BMI. At baseline, pain at best higher in OMT group versus usual prenatal care only, and 'pain now' higher in OMT versus placebo US. Results showed no difference in improvements in pain and function between OMT and placebo US groups. |
Kalus 2007.
| Methods | RCT conducted in a tertiary referral hospital in Australia (N = 115 women randomised). | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 55/46 analysed): BellyBra. A nylon/spandex undergarment worn like a vest, has a 1‐way stretch panel across the thoracolumbar back that is designed to provide support and assisted by the involvement of shoulder straps, to improve posture. A wide elastic band sits below the abdomen supporting the uterus and lifting weight off the pelvis. Worn for 3 weeks, did not specify how often to be worn. Control group (N = 60/48 analysed): Tubigrip. More generic form of support. Worn as a double layer and extends from the mid‐thoracic spine to the sacral spine and pelvis. Worn for 3 weeks, did not specify how often to be worn. |
|
| Outcomes | VAS (0 to 10 cm), physical activity including work, satisfaction with life survey (SWLS), use of analgesic medication, usefulness of garment at baseline, completion of 3‐week intervention, 'on a return visit to the antenatal clinic' ‐ ? timing. | |
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Although the primary aim was to assess the severity of LBP and posterior PP, the PP was primarily due to pain in the sacroiliac joint. Conducted in Australia. Funding = no funding or support was provided for any of the authors; Furtile Mind Pty Ltd (retailers for maternity and postpartum clothes, supplies) provided the BellyBras used in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Participants were randomised...by means of computer‐generated numbered, sealed, opaque envelopes'. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants, providers mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors mentioned. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | 9 participants (16%) in intervention group were lost at follow‐up (2 delivered within study period, 7 failed to attend appointment and could not be contacted). 12 participants in control group (20%) were lost at follow‐up (3 delivered within study period, 9 failed to attend their follow‐up appointment and could not be contacted). No exclusions mentioned; 14% were lost to follow‐up with no reason. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes it said it would report in methods. |
| Other bias | High risk | 11 women (23.9%) in intervention group and 23 women (47.9%) in control group reported the use of other treatments for their back pain during the study period, including the use of analgesic medication, physiotherapy, acupuncture, massage, etc. (co‐interventions make it difficult to attribute change to the intervention). Most noticeably, 3 in the intervention and 14 in the control group used analgesic medication during the study period. 44 (95.7%) women in intervention group stated that they wore the garment at least once a week compared with 33 (68.8%) in the control group. High risk: co‐interventions and compliance different. |
Kashanian 2009.
| Methods | 43 women 'randomly assigned' to study group; 30 women completed the study and were analysed. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 15 analysed): exercise. 1 hour introduction session with 7 exercises and relaxation movements taught. Each exercise session lasted 30 minutes x 3/week x 8 weeks. Exercise included warm up (4.5 minutes) walking, stretching (spine extensors, hamstrings, thigh adductors, lumbar paravertebral muscles), strengthening (thigh extensors and abdominal obliques) x 21 minutes, relaxation x 4.5 minutes. Control group (N = 15 analysed): routine prenatal care ‐ did not perform any of the study exercises. |
|
| Outcomes | Disability (RMDQ); lumbar lordosis using flexible ruler and formula measured at baseline, after 1 and 2 months. | |
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | This paper was part of the journal's 'brief communication' section only, so biases are difficult to assess; there was no reference to other publications on this study and none were identified. Assume the study was carried out in Iran, since all authors were affiliated with Iranian universities. 14 March 2012 ‐ email and Linked‐In message sent to lead author, requesting more information. 10 January 2014 ‐ author sent answer to questions on ROB and copy of paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned' ‐ details not provided in paper. From author: Randomization was performed using 4 parts, block sealed, sequentially distributed envelopes to which the letters A, B, C and D had been allocated: the letters A and C to the exercise program group and the letters B and D to the control group. |
| Allocation concealment (selection bias) | Low risk | Details not provided in paper. From author: The patients chose one of the envelopes which were opened by the investigator’s colleague and according to the letters, the groups of patients were determined. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Details not provided in paper. From author: therapists were blinded, they were different and knew the groups of women as group 1 and group 2; women were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Details not provided in paper. From author: assessors were blinded, they were different and knew the groups of women as group 1 and group 2. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Details not provided in paper, but number randomised are included in the results table. From author: 43 women were selected for the study and 30 women finished the study, and we analysed the 15 [in each group] who finished the study. High risk because > 20% drop‐out rate. |
| Selective reporting (reporting bias) | Low risk | Limited data provided; e.g. states that RMDQ was used, but no values given for this outcome. From author: RMDQ [for pain] and lordosis results provided in the paper forwarded by author. |
| Other bias | Unclear risk | This was part of the journal's 'brief communication' section only, so biases are difficult to assess; there was no reference to other publications on this study and none were identified during a Google search 13 March 2012. In January 2014, author responded to request for more information, which is inserted above. |
Kaya 2013.
| Methods | A prospective, randomised study to compare the efficiency of Kinesio Tape, compared to exercise and acetaminophen (Ex + A) for the treatment of pregnancy‐related LBP during the third trimester of pregnancy. | |
| Participants |
Inclusion criteria:
|
|
| Interventions |
Intervention group: Kinesio Tape (N = 15): no further detail provided. Control group: Ex + A (N = 14): no further detail provided. |
|
| Outcomes |
Pain: VAS (0 to 10). Functional disability: RMDQ. Outcomes completed before and 7 days after treatment. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Poster presentation to the 24th National Physical Medicine and Rehabilitation Congress. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available from abstract. Abstract only available. |
| Allocation concealment (selection bias) | Unclear risk | No information available from abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel to an exercise intervention, or Kinesio Tape. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available from abstract. |
| Incomplete outcome data (attrition bias) effect of intervention | Unclear risk | As above. |
| Selective reporting (reporting bias) | Unclear risk | Only P values provided. Abstract reports a significant decrease in VAS and RMDQ in both groups (P < 0.001) with a significantly greater degree of pain relief in the Kinesio Tape group (P < 0.001). |
| Other bias | Unclear risk | No adverse events of Kinesio Tape reported. No further detail provided about other possible sources of bias. |
Keskin 2012.
| Methods | Prospective randomised study. | |
| Participants | Women were identified via self‐report of LBP symptoms: this was followed up with physical tests of palpation, spinal range of motion and P4 test to rule out PP. N = 88 randomised to 1 of 3 groups. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
TENS (N = 22/20 analysed): dual channel portable TENS, using 4 x 5 cm2 electrodes placed over pain lumbar region. High frequency stimulation to achieve a tingling sensation 2 to 3 times above the sensory threshold. Each participant received 6 treatments (2 x/week for 3 weeks), no detail on duration of each treatment. Exercise (N = 22/19 analysed): HEP prescribed by a physiotherapist incorporating pelvic tilting, lower limb stretching, postural exercises, isometric abdominal contractions. Each exercise to be completed 10 times, twice a week for 3 weeks. Acetaminophen (N = 22/19 analysed): 1 500 mg paracetamol tablet 2 x/day for 3 weeks. Control group (N = 22/21 analysed): usual prenatal care |
|
| Outcomes | The following outcomes were collected at baseline and 3 weeks' after treatment. Pain: VAS (0 to 10); functional disability (RMDQ); palpation of symphysis pubis, sacroiliac joint and gluteal regions for tenderness/reproduction of symptoms. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Study conducted at Department of Obstetrics and Gynecology, Fatih University, Turkey. No funding information provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided randomly into 1 of 4 groups, by drawing a sealed opaque envelope with group names ‐ prepared by 1 author and opened by another. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, prepared by 1 of the authors of the study from a box containing group names, opened by another study author who was blinded to the contents of the envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded nor could providers due to nature of interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessment. An experienced physical medicine and rehabilitation specialist carried out the physical assessments, i.e. palpation, ROM, posterior pain provocation tests, however unclear whether this person also collected pain and disability assessments. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attrition and exclusions reported with number and reasons provided. Numbers included in the analysis at each stage add up. Authors did not specifically indicate how they dealt with missing data however, given that analyses were conducted on those participants completing the 3‐week study period only, it appears they completed a PPA. (Small numbers did not complete the 3 week study period from each group; N = 2 TENS; N = 3 acetaminophen; N = 3 exercise; N = 1 control). |
| Selective reporting (reporting bias) | Unclear risk | Median scores provided as along with P values for both pain (VAS) and disability (RMDQ). |
| Other bias | Low risk | 3 women reported adverse events. Groups similar at baseline for all variables with the exception of VAS, which was higher in the TENS and acetaminophen groups at baseline. Compliance reported as > 90% in all groups. No information about controlling for co‐interventions. |
Kihlstrand 1999.
| Methods | Preventive RCT. 329 women invited to participate, 258 were randomised 'using sealed envelopes'. Enrolment was done in segments of time, since only 60 women could participate in the pool program at the same time. | |
| Participants | Women registering at 1 of 6 maternity clinics (N = 967) run by Falun County Health Care Board in Sweden and had their ultrasound between gestational age 15 to 18 weeks.
From 329 women invited, 60 declined because they could not participate in water gymnastics.
258 randomised to 2 groups of 129 each. Inclusion criteria:
Exclusion criteria:
Drop‐outs due to inability to participate in water gymnastics, recurrent UTIs, shift work, baby‐sitting problems, miscarriage, intrauterine death, lack of time, invited to participate after date of closure. |
|
| Interventions |
Intervention group (N = 129/123 analysed):
20 1‐hour weekly water gymnastics classes involving exercise (tested for pregnant women) and relaxation in water (32 to 34 degrees), with music.
First 10 sessions with exercises suitable for early pregnancy; last 10 sessions with exercises suitable for later pregnancy.
Hour session divided into 30 minutes exercise + 30 minutes relaxation. Control group (N = 129/118 analysed): no water gymnastics. |
|
| Outcomes | LBP (VAS 0 to 10). (LBP was not measured until 1 week postpartum, which is outside the timelines of this review); number of days off work because of LBP in pregnancy. Adverse events: no excess risk for pregnancy associated with water gymnastics observed: no differences with gyn/UTI infections, maternal weight gain, gestational age at delivery, weight/height of neonate, delivery characteristics. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Funding: Dalarna Research Institute; Local Insurance Office. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Preventive randomised controlled trial' randomised 'using sealed envelopes' ‐ actual method of randomisation not described, but it was conducted 'by a mid‐wife when the women had their ultrasound.' |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregiver not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding unclear. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Participants who completed the study were analysed in the groups to which they were randomised; less than 5% reported as lost to follow‐up; numbers do not always add up ‐ query if N for outcomes are based on those who answered specific questions on follow‐up?. |
| Selective reporting (reporting bias) | High risk | Not enough data were given to allow use of the VAS; pain data provided in graphs from which one cannot extract exact values. Difficult to follow the path of recruitment, drop‐outs since numbers given in text do not add up. |
| Other bias | Low risk | Nothing noted. |
Kluge 2011.
| Methods | RCT (N = 50 women). | |
| Participants | South African women of 20 to 40 years between 16 and 24 weeks' gestation; LBP/PP (with or without radiation to the knee) that had started during current pregnancy (72% of sample had LBP). | |
| Interventions |
Intervention group (N = 26/24 analysed): exercise. 1 formal exercise class lasting 30 to 45 minutes with warm‐up and cool‐down periods incorporated. Handout illustrating and explaining the exercise program which consisted of postural, transversus abdominis and pelvic floor exercises to train correct isolation and isometric contraction. Exercises then individually progressed to increase level of difficulty and facilitate co‐contraction of transversus abdominis and PFM with gluteals, quadriceps and other muscle groups. Follow‐up class every second week for 10 weeks. Women also asked to complete a daily HEP and record their goals in their training diary. Verbal information on basic back care and posture during pregnancy and an information pamphlet. Control group (N = 24/22 analysed): verbal information on basic back care and posture during pregnancy and an information pamphlet as for exercise group but no specific instructions given to participants regarding whether to perform any exercise. |
|
| Outcomes | Pain intensity (NRS 0 to 10); functional ability (Likert modified RMDQ). | |
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Neurological exam was completed at assessment along with erector spinae palpation, sacroiliac palpation, P4 test and passive SLR however, apart from erector spinae palpation eliciting LBP symptoms, the positive yield of these tests for subtyping of symptoms was low. Conducted in South Africa. Funding not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in balanced blocks of 20. |
| Allocation concealment (selection bias) | Low risk | Sealed numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | ITT analysis completed; less than 10% of sample lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified. |
| Other bias | Low risk | Groups similar at baseline regarding most important prognostic indicators; outcomes assessed at same time for both groups; compliance reported in detail. |
Kordi 2013.
| Methods | RCT conducted at the obstetric clinic of the Imam Hospital Complex, Tehran. Women randomly allocated into 1 of 3 groups; Exercise plus information (Ex), Lumbo‐pelvic belt plus information (Belt), or Information only (Control). | |
| Participants |
Inclusion criteria:
Diagnosis of PGP based on self‐reported pain (using a pain diagram) in the lumbar region between the gluteal folds and the posterior iliac crest, and a positive result from 1 of the following physical tests:
Exclusion criteria:
|
|
| Interventions |
Intervention group 1 (Ex) (N = 35/31 analysed): in addition to information provided to control group, this group were given a HEP including exercises to strengthen the pelvic girdle muscles, such as back pressing, pelvic tilting, leg‐lifting each held for 3 to 10 seconds and completed twice a day, 3 days per week; to encourage aerobic activity of at least 64 to 76% of their maximum heart rate for 25 minutes/day x 3 per week, e.g. walking, and stretching exercises for the lower limbs and trunk each held for 10 to 20 seconds and completed twice a day, 3 to 5 x per week. Intervention group 2 (Belt) (N = 35/34 analysed): in addition to information provided to control group, this group received a non‐rigid lumbo‐pelvic belt and asked to use it throughout the course of the study with the exception, should they wish, to remove it for sleeping. Control group (N = 35/31 analysed): this group received general information about the anatomy, body posture and ergonomic advice about sitting, walking and lying. |
|
| Outcomes |
Primary outcomes: pain intensity (VAS 0 to 100); functional disability (Validated Persion version of ODI). Secondary outcomes: Quality of Life (World Health Organisation's Quality of Life Questionnaire) ‐ this covers physical and psychological health along with social and environmental conditions. All outcomes assessed at baseline, week 3 and week 6 of the study. |
|
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | Funding = Tehran University of Medical Sciences. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation sequence (block sizes 15 participants each). |
| Allocation concealment (selection bias) | Unclear risk | No information provided about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided about blinding; however participants and providers knew interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided about blinding. Not even clear who collected the outcomes. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attrition and exclusions were presented along with reasons, and numbers included in the analysis at each stage add up. The authors are not clear about how they dealt with missing values but appear to have used a per protocol analysis as those lost to follow‐up are excluded from the final analyses. Control and exercise had 11% drop‐out; belt group had only 3% drop‐out. |
| Selective reporting (reporting bias) | Low risk | Means and SDs for each group along with 95% CIs provided for the primary and secondary outcomes at all time points along with P values. Comparisons are also presented to identify where the significant differences occur, i.e. between which groups. |
| Other bias | Low risk | Use of pain provocation tests as well as self‐report to diagnose PP increases validity of diagnosis. No significant differences in any of the primary or secondary outcomes at baseline. Adverse events described (one participant each in control and exercise group). No information presented on participants' use of co‐interventions or researchers attempts to control for these. |
Kvorning 2004.
| Methods | 100 women, enrolled and randomised to 1 of 2 groups. The code for group allocation was obtained in advance by throwing dice in pairs of 10, and enclosed in advance in an envelope, marked with the order number of inclusion and opened consecutively by midwife on inclusion to the study.
Those who finished the study were analysed in the assigned groups. Duration of study or follow‐up not given. |
|
| Participants | Pregnant women presenting at the maternity ward centres in southern Sweden. Inclusion criteria:
Exclusion criteria:
Baseline: 2 groups did not differ significantly in age (30 ± 5.0 years); gestational week at first visit (30 ± 4.2 weeks); employed (75%); had acupuncture before (20%); negative attitude to acupuncture (20%). Pain in sacroiliac region or over symphysis with no motor or sensory disturbances: acupuncture = 78%; control = 80%. Duration of pain: acupuncture = 8.8 ± 5.6 weeks; control = 6.0 ± 3.8 weeks (P < 0.001). Duration of pain in past 24 hours: acupuncture = 9.8 ± 7.1 hours; control = 9.2 ± 7.4 hours. Number of participants on analgesics: acupuncture = 1; control = 0. |
|
| Interventions |
Intervention group (N = 50/37 analysed): Acupuncture given according to written instructions and periosteal stimulation. Started with LR3 and GV20 points + local tender points, added BL60, SI3 and 1 of lumbar and sacral bladder points (BL22 to 26) if needed; stimulated to De qi, needles left in place for increasing length of time. Time: patient received acupuncture twice a week during first 2 weeks; after this, they only received it once a week (note ‐ no total duration of treatment time given). Control group (N = 50/35 analysed): usual prenatal care. |
|
| Outcomes | Pain increased, pain unchanged, pain decreased, no pain during last 3 weeks of pregnancy, pain on activity decreased, Visits to maternity centres, number of participants who used analgesics, number of participants who used TENS, number of participants who used sacroiliac belt, number of participants who used physiotherapy, baby's birthweight, baby's Apgar at 1/5/10 minutes. Adverse events: reported by 38% of acupuncture group ‐ local pain (6); heat or sweating (5); local haematoma (2); tiredness (2); nausea (2); weakness (1). |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | No mention of funding. Study took place in Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘code for group obtained in advance by throwing dice in pairs of 10.’ |
| Allocation concealment (selection bias) | Low risk | 'Predetermined code enclosed in advance in envelop, marked with the order number of inclusion and opened consecutively by midwife on inclusion.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No explicit mention in the report, but it seems unlikely that either the women, midwives or acupuncturists were unaware of inclusion into the acupuncture or control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'two blinded investigators independently assessed the development of the patients' individual VAS scoring over time with a kappa coefficient of 0.68% (95% CI 0.54 to 0.83).' |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Over 20% lost to follow‐up in each group. 1 ward closed to recruitment after 12 months because women no longer wished to be included in the study => excluded 12 participants who had been enrolled by this clinic, leaving 44 in each group. Acupuncture group ‐ lost 3 because they delivered, 2 did not like acupuncture, 1 did not complete assessment correctly, 1 lost due to vacation of midwife (7) ‐ analysed 37/50. Control group ‐ lost 5 ‐ did not complete forms correctly, 3 insisted on acupuncture, 1 was admitted to hospital for pain management and rest (9) ‐ analysed 35/50. |
| Selective reporting (reporting bias) | High risk | Data provided on outcomes listed in methods section but at times they are difficult to follow and not presented in a fashion that allow analyses. |
| Other bias | High risk | Variety of other treatments used by the women to relieve symptoms (analgesics, TENS, pelvic belt, physio); length of study unclear. |
Licciardone 2010.
| Methods | RCT; N = 146 randomised (group 1: N = 49; group 2: N = 48; group 3 (controls): N = 49). Participants stratified by age and gravida. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group 1 (N = 49 randomised): usual obstetric care plus Osteomanipulative Therapy (OMT). Intervention group 2 (N = 48 randomised): usual obstetric care plus sham ultrasound (sham US). Control group (N = 49 randomised): usual obstetric care. Groups 1 and 2 received treatments each lasting 30 minutes at 30, 32, 34, 36, 37, 38, 39 weeks' gestation (in conjunction with usual obstetric care). OMT = included any of the following modalities: soft tissue, myofascial release, muscle energy, range of motion mobilisations used in a systematic manner by all providers*. No manipulations used as these pose a risk to mother and fetus. Sham US = using a non‐functional ultrasound (US) therapy unit that provided the usual visible and auditory cues provided by a normal therapeutic ultrasound unit. The US head was applied over clothing at body areas corresponding to the OMT protocol. Usual obstetric care = no study treatments provided but usual 7 visits in total, in accordance with usual obstetric care, at 30, 32, 34, 36, 37, 38, 39 weeks' gestation. |
|
| Outcomes | Average pain intensity: NRS (0 to 10). Back‐specific function (RMDQ) measured at baseline and after 7th (last) treatment session; at same times for each group. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | *Treatment providers met regularly to ensure consistency in duration, type, anatomic location and manner of OMT provided. OMT and Sham US provided by same physicians with same amount of attention given to both groups. 2 from each treatment group missed more than 50% of treatments. Compliance best in control group. Funding = grants from the Osteopathic Heritage Foundation and the National Center for Complementary and Alternative Medicine at the National Institutes of Health. The study took place in Texas, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients randomly assigned and stratified by age and gestation, but no other information given about the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind patients or care providers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measures were by self‐report, but high risk because patients not blinded. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | ITT analysis = 144 participants; last observation carried forward, attrition and exclusions reported but query the reliability of imputing over 1/2 of the data (actual data for 146 ‐ 83 = 63). (23 (16%) withdrew before visit 7; a further 60 (42%) withdrew due to delivery). Adherence reported as greater than 80% in both treatment groups for those who continued the intervention. |
| Selective reporting (reporting bias) | Low risk | Several approaches used to decrease risk of bias from last observation carried forward method. |
| Other bias | Low risk | Similar compliance in treatment groups, baseline measurements similar, co‐interventions controlled, outcomes taken at same time points. |
Lund 2006.
| Methods | Prospective single‐blind RCT. Women recruited from 2 different maternity healthcare departments and randomised to superficial or deep acupuncture. 106 women examined; 70 women randomised; 23 dropped out; analyses conducted on 47 women who completed the study. |
|
| Participants |
Inclusion criteria:
Physical examination confirming provoked PP: (i) In 1 of 3 tests: P4 test, standing on 1 leg, Patrick’s/Fabere test; (ii) In palpating tissue over: the sacroiliac joints, the symphysis pubis, or Gluteus maximus/medius muscles. Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 35/25 analysed): deep stimulation acupuncture. 10 acupuncture treatments of 30 minutes each, given twice weekly for 5 weeks by a registered physiotherapist. See study for exact location of acupuncture points used. Longer and thicker needles were inserted intramuscularly. Needles were stimulated 5 times during the treatment sessions by manually twirling the needles 180° back and forth until patient reported sensations ofDe qi. Control group (N = 35/22 analysed): superficial stimulation acupuncture. 10 acupuncture treatments of 30 minutes each, given twice weekly for 5 weeks by a registered physiotherapist. See study for exact location of acupuncture points used. Shorter and thinner needles were inserted subcutaneously and left in place until end of treatment. To mimic the procedure of deep stimulation, therapist sat down by patient 4 additional times during treatment without manipulating the needles. |
|
| Outcomes | VAS pain (at rest and during 3 daily activities); Nottingham Health Profile measured 5 days prior to and 5 days after treatment; at same time for both groups. |
|
| Condition (LBP, PP, LBPP) | PP. | |
| Notes | Funding = research grants from Praktikertjänst AB and the National Security in Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'women ... were randomised ...' but randomisation procedure not described. Unclear risk ‐ as above, randomisation procedure not explained. |
| Allocation concealment (selection bias) | Low risk | Quote: 'Sealed envelopes with labels for determination of treatment were used in randomisation provided by a statistician not involved in the study'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both groups given acupuncture so could not tell difference as patients were acupuncture naive; care providers knew whether they gave superficial or deep acupuncture but acted the same towards the patient regardless. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes collected from patients who were unaware of their treatment group. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk |
Drop‐out rate: 23 participants out of 70 (13 in Superficial group, 10 in Deep group). Reasons for drop‐outs listed; reasons similar for both groups. It does not seem that the grouping affected the drop‐out reasons, and although almost 1/3 dropped out from each group, the over‐riding reason was non‐compliance with completing pain diaries. No excluded data mentioned ‐ and it appears that analyses only done on complete data sets. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes it said it would report in methods. |
| Other bias | Low risk | No other bias. Women all acupuncture naive. Groups similar at baseline. |
Martins 2005.
| Methods | 'Randomised controlled study'. The physiotherapist conducting the research randomised the women into 2 groups by means of a 'raffle' or 'lottery'. Exercise group = 33; control group = 36. There appeared to be no drop‐outs, and although analysis is unclear, there appears to be no contamination of groups in analysis; outcomes for control group not reported. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 33 randomised/analysed): exercises in groups for 'global activity and stretching'. Control group (N = 36 randomised/analysed): routine medical recommendations. |
|
| Outcomes | Proportion of women with improvement in VAS categorised as VAS = 0; 1 to 3; 4 to 5; 6 to 8; 9 to 10 after 8 weeks. Adverse events: not reported. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Funding: not reported. Translated from Portugese by single Portuguese researcher. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a 'Raffle' or 'lottery'. |
| Allocation concealment (selection bias) | High risk | Physiotherapist who was doing the research allocated to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Description of blinding for participants, caregiver not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Description of blinding for assessors not provided. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Outcome table appears to indicate no drop‐outs; report appears to indicate that there is no contamination between groups, but none of this is clearly described. |
| Selective reporting (reporting bias) | High risk | Results are incomplete (only intervention group's improvement reported, no data for control group). |
| Other bias | High risk | Other treatments not described; baseline data were not comparable: Exercise group = 48% greater than 5 on VAS 0 to 10; Usual care group = 61% greater than 5 on VAS 0 to 10. |
Martins 2014.
| Methods | Randomised controlled clinical trial (N = 60 randomised). Study conducted in 'Basic Healthcare Units' in São Paulo, Brazil. | |
| Participants |
Inclusion criteria:
For women reporting LBP only, an increase in pain precipitated by bending forward, circling the trunk or on palpation of paraspinal muscles confirmed the diagnosis. The 4P test was used to assist diagnosis of PGP. Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 30/21 analysed): Hatha yoga. 10 sessions, once a week, each lasting 1 hour (up to 10 participants/group). Class consisted of a 10‐minute warm‐up to gain focus, 40 minutes of poses and breathing exercises focusing on stretching, strengthening, endurance, muscle resistance, self‐control, concentration and self‐confidence, and a 10‐minute relaxation at the end. Control group (N = 30/24 analysed): information pamphlet on postural orientation and advice on ADL's, sleeping positions sitting with adequate foot and lumbar support, |
|
| Outcomes | Pain intensity (VAS 0 to 10) with facial expressions positioned at 3 points on the scale corresponding to weak/median/severe pain. This was assessed at the beginning and end of each yoga session. 4P test, lumbar flexion test. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Study conducted in Brazil; part of Doctoral thesis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers for 60 participants using SAS software. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No detail provided on blinding. however it is not possible to blind a yoga intervention. there was also no mention of whether participants were yoga naive. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided on blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Attrition and exclusions were reported along with reasons, and the numbers included in the analysis add up. Lost to follow‐up: N = 9 in the yoga group, N = 6 in the control group, therefore risk is high (20%+). |
| Selective reporting (reporting bias) | High risk | The sample included N = 10 (17%) with LBP, N = 12 (20%) PGP, and N = 38 (63%) with LBPP however the authors did not present baseline data according to intervention groups and did not present any findings for those who had combined LBPP. |
| Other bias | Unclear risk | Adverse events were described and similar between groups. Interim data collection was not completed for control group, just the intervention group. |
Miquelutti 2013.
| Methods | Prospective RCT conducted at the Women's Integral Health Care Hospital, University of Campinas, Sao Paulo and 4 municipal primary healthcare centres in Sao Paulo, Brazil. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 103/97 analysed): Birth Preparation Program (BPP). HEP including pelvic floor muscle contractions, daily aerobic exercise encouraged, 2 stretches to decrease back pain, information about the physiology of labour and practice of non‐pharmacological techniques for pain control. Control group (N = 102/100 analysed): routine information about breastfeeding, signs and symptoms of labour and a visit to the delivery ward. Supervised practice of the HEP (experimental group) only occurred on the days of scheduled medical visits, which were monthly up until 30 weeks' gestation, fortnightly between 31 to 36 weeks' and weekly from 37 weeks onwards. |
|
| Outcomes |
Primary outcomes: average LBP/PP during preceding days (VAS 0 to 10) and marking of pain location on body chart; urinary incontinence; physical activity performed at home (PPAQ); anxiety (STAI). Secondary outcomes: perinatal variables. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Financial support was provided by the Foundation for the Support of Research of the State of São Paulo (Fundação de Amparo à Pesquisa do Estado de São Paulo–FAPESP) grant #08/10392‐5. The first author received partial financial support from the Coordination for the Improvement of Higher Education Personnel (CAPES). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence of numbers. Randomisation was 1:1. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque, consecutively numbered envelopes prepared by an individual not directly involved with the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors acknowledge in the discussion that lack of participant blinding may have led to women giving what they felt were favourable answers to the questions about urinary incontinence and physical activity completed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided on outcome assessor or analyst; participants provided self‐assessment data. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attirtion and exclusion reported with reasons and numbers included in the analysis add up. ITT analysis completed, with participants analysed according to group assignment but final analyses only included those who completed study. |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods with number (%) of participants, means (+/‐ SD) and 95% CIs for VAS (LBP/PP) at 3 time points, and RR (95% CI) for prevalence of LBP or PP. Only missing data for secondary outcomes. |
| Other bias | Low risk | Co‐interventions controlled between groups. Groups similar at baseline and weight gain during course of study similar between groups. Collection of outcomes at 3 time points (same for each group). |
Mørkved 2007.
| Methods | RCT conducted at Trondheim University Hospital and 3 outpatient physiotherapy clinics. 1533 pregnant women in and around Trondheim, Norway were invited to join => 301 were randomised. Primary outcome was prevention and treatment of urinary incontinence; secondary outcome was prevention and treatment of LBPP. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group ‐ exercise training (N = 148 randomised/analysed): Training with a physical therapist in groups of 10 to 15 women for 60 minutes once per week for 12 weeks, where training focused on PFM and other exercises. Women were encouraged to perform 8 to 12 intensive PFM contractions twice per day at home. Motivation was strongly emphasised. Each training sessions consisted of: 15 to 20 minutes aerobic activity, 30 to 35 minutes of exercises, 5 to 10 minutes of light stretching, body awareness, and breathing and relaxation exercises. Women were given general advice related to ergonomics and daily life activities in pregnancy. Control group ‐ usual prenatal care (N = 153 randomised/analysed): Women received customary information given by their midwife or general practitioner. They were not discouraged from exercising on their own. |
|
| Outcomes | Self‐reported pain in the low‐back area lasting for ≥ 1 week; pain drawing, off sick due to low‐back/PP (yes/no); Disability Rating index (DRI); pelvic floor muscle strength measured at baseline (20 weeks' gestation); 36 weeks' gestation, 3 months' postpartum. | |
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Adherence to training protocol was registered based on the women’s personal training diary (must do 2 sets of 8 to 12 contractions of PFM per day) and reports from the physical therapists that led the group training (participation in ?6 group training sessions).
120 of the 148 women (81%) in training group followed the training protocol. Funding = Norwegian Fund for Postgraduate Training in Physiotherapy and the Norwegian Women's Public Health Association. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Randomisation was done in blocks of 32 with the use of opaque sealed envelopes', did not specify method used to select the blocks, but likely OK, given the fact that they used other safeguards. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes used. Quote: 'A secretary with no other involvement in the study prepared the envelopes. Each woman opened 1 of the envelopes herself and was enrolled by the secretary in the secretary's office'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and care providers were aware of treatments (exercise vs usual care). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: 'The principal investigator was not involved in the training of the women and was blinded to group allocation while making the assessments and plotting the data'. However, the outcomes were self‐report and the women were not blinded to their treatment; unclear if those who received usual care were aware of other options. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | 7 participants in control and 5 in training group withdrew after the first assessment. Reasons for withdrawal were diseases connected to pregnancy (N = 6) or personal reasons (N = 6). It does not seem that the grouping affected the drop‐out reasons. No excluded data mentioned. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes it said it would report in methods. |
| Other bias | Unclear risk | Unclear risk: Influence of co‐interventions, adherence not reported in results. |
Peters 2007.
| Methods | 'Randomized controlled clinical trial based on the classical 'waiting list' design. Carried out by 2 osteopaths in their offices in Űberlingen and Műlheim, Germany. 60 pregnant women were recruited from 'a number of midwives and gynaecologists.' |
|
| Participants |
Inclusion criteria: Pregnant women with LBPP that had lasted at least a week and was at least VAS > 3. Average age 30 years, mean gestation 25 weeks. |
|
| Interventions |
Intervention group (N = 30 randomised/analysed): received 4 osteopathic treatments in weekly intervals. Waiting list (control) group (N = 30/27 analysed), after 5 weeks on the waiting list they received osteopathic treatment that was reported as 'having no relevance for the study'. |
|
| Outcomes | Pain, measured with VAS; interference with ADL, measured with Quebec Back Pain Disability Scale at baseline and end of first 5 weeks (end of treatment for intervention group). | |
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Information taken from an abstract of an unpublished thesis that is available in German, for a cost, from Akademie für Osteopathie (AFO), Deutschland (funds not available to obtain full manuscript). Funding not reported. Abstract initially translated from German by single German‐speaking researcher, then English abstract found on‐line. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized controlled clinical trial' ‐ methodology not reported in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Waiting list comparison group, after 5 weeks waiting list they get a treatment that is reported as having no relevance for the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding in abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding in abstract. |
| Incomplete outcome data (attrition bias) effect of intervention | Unclear risk | 3 patients in the control group dropped out; no information provided on exclusions or analyses. |
| Selective reporting (reporting bias) | Low risk | Data provided for pain and Quebec Back Pain Disability Scale. |
| Other bias | Unclear risk | Difficult to assess since we were unable to access the full thesis. |
Peterson 2012.
| Methods | Pilot RCT (57 participants randomised). All participants screened initially by phone and all treatments described prior to randomisation. No limit on what stage in pregnancy women could enter the study. Before randomisation all participants identified their treatment preference. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Exercise group (control; N = 22/16 analysed): exercise booklet provided with specific exercises and recommendations for postural and movement patterns to alleviate LBP, and advice on when to stop exercising. Individualised stretching and strengthening exercises were prescribed, demonstrated and practiced at each study visit. Exercisies took approximately 15 minutes to perform and participants were asked to exercise 5 x/week. Spinal manipulative therapy (N = 15 randomised/analysed): high velocity, low amplitude thrust applied to isolated joint to move it just past physiological end range in side‐lying position. Direction, velocity and amplitude determined by the clinician from palpation findings. Neuro emotional technique (NET; N = 20/19 analysed): chiropractic mind‐body technique using relaxed breathing and visualisation techniques with elements of traditional Chinese medicine (such as association of emotions with certain organs or meridians) and chiropractic medicine (adjustment of spinal levels innervating specific organs. The NET standard protocol was followed (Bablis 2008). Maximum number of treatments per participant = 8 with very few in any group reaching this amount. Co‐interventions controlled. |
|
| Outcomes |
Primary outcome: RMDQ (back‐specific function). Secondary outcome: Pain intensity (NRS 0 to 10); Sick leave due to pregnancy‐related LBP (assessed but not listed as 1 of the outcomes). |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | 138 participants screened; sick leave not listed in methods as one of the outcomes but reported in Table 2; higher drop‐out from exercise group however adherence to exercise did not affect outcomes. Funding provided by The One Foundation, the research division of the Neuro Emotional Technique; 'The One Foundation did not contribute to the study in any other way'. Conducted at Oregon Health & Science University, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used to generate the allocation sequence was not described ‐ 'before being randomised, participants identified their treatment preference ... she would open the consecutive envelope in her preference strata in the presence of the researcher ... women were randomly allocated into 1 of 3 treatment groups'. |
| Allocation concealment (selection bias) | Low risk | 'the randomisation schedule was completed prior to initiating the study and was concealed from all study staff by using consecutively numbered, sealed, opaque envelopes for each strata of preference group.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and practitioners were not blinded to treatment group after randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were outcome assessors. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | ITT analysis performed. Missing data ‐ last observation carried forward. Minor inaccuracies noted in number excluded prior to randomisation, and between text and Figure 1 in drop‐outs from exercise group (N = 1). |
| Selective reporting (reporting bias) | Low risk | Last observation carried forward may limit data but carried out to replicate methods used in an earlier study by Licciardone and colleagues (2010). Sensitivity analysis completed providing similar results to primary outcome analysis. |
| Other bias | High risk | Participants randomised according to their treatment preference, entered the study at different gestational points, groups were not similar at baseline for all prognostic factors, and were paid to participate (USD$20 per visit). |
Sedaghati 2007.
| Methods | 'Prospective randomised controlled study'. 100 women invited and divided into 2 groups; 10 withdrawn from exercise group prior to intervention => 90 analysed. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (Exercise program): N = 50/40 analysed. Program consisted of a 15‐minute warm‐up and cool‐down plus 30 minutes cycling in the range of 55% to 65% of the maximal heart rate with respect to the age. Exercises were prescribed by a physical training specialist. The exercise sessions were 3 times a week for 8 weeks. Control group: N = 50 randomised/analysed. The study did not specify what the control group was. |
|
| Outcomes | Pain, measured with Quebec questionnaire, measured at baseline and 8 weeks after start of program; demographic data collected at baseline; P value < 0.05 considered to be statistically significant. | |
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Email to the corresponding author for clarification failed to elicit a response. Funding = grant from Sports Medicine Research Center and Vice Chancellor for Research at Tehran University of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation were described except 'the total numbers of 100 invited were divided into two exercise and control groups'. 'Randomised' was only mentioned in the abstract. Unclear risk: randomisation procedure not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned, but assume not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Quote: 'every woman missing three session of exercise was excluded from the study' ‐ but unclear how many this affected. Drop‐outs/withdrawals from study not mentioned, however, 10 women who were randomised did not proceed to the intervention because they were unable to participate in the exercises. Did not specify how they dealt with the missing/excluded data. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes it said it would report in methods. |
| Other bias | Unclear risk | Unclear risk: compliance not reported, nor co‐interventions. |
Stafne 2012.
| Methods | 2‐armed, 2 centre RCT ‐ 855 women randomised. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention group (N = 429/396 analysed): exercise. 60‐minute exercise sessions 1 x/week for 12 weeks between 20 to 36 weeks' gestation led by a physiotherapist in groups of 8 to 15 participants. Each session consisted of moderate intensity (13‐14 on Borg scale) aerobic activity, strength training and balance exercises. 45‐minute home exercise session 2 x/week consisting of 30 minutes of aerobic activity and 15 minutes of strengthening and balance exercises. Adherence monitored throughout. Control group (N = 426/365 analysed): standard antenatal care; not discouraged from exercising. Both groups given written information on pelvic floor exercises, diet and pregnancy‐related LBPP. |
|
| Outcomes | LBPP: VAS (0‐100) ‐ morning and evening, sick leave due to lumbo‐PP, Disability Rating Index (DRI), Fear Avoidance Beliefs Questionnaire. | |
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Additional outcomes related to a related study: gestational diabetes, glucose metabolism. Approximately 60% of women who enrolled reported lumbo‐PP at time of inclusion. Funding sources: Norwegian University of Sciences and Technology, Norweigian Fund for Postgraduate Training in Physiotherapy, Liason Committee for Central Norway Regional Health Authority. 30 October 2012 ‐ email sent to lead author to clarify correct number analysed in the intervention group ‐ 396 or 397; author confirmed that there were 396 women in the intervention group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. |
| Allocation concealment (selection bias) | Low risk | 'concealed randomisation' by a web‐based computerised procedure; ... personnel had no influence over randomisation.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While personnel had no influence over the process of randomisation, the physiotherapists who delivered the programmes were aware of the end results ... i.e. they were providing the participants with the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were self‐reported symptoms, therefore the women were the outcome assessors and they knew whether they were receiving exercise therapy or not. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Exercise group = 8% drop‐out/loss to follow‐up; control group = 14% drop‐out/loss to follow‐up with a large proportion of these giving no reason and not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as specified in methods. |
| Other bias | Low risk | Groups similar at baseline even when not including those lost to follow‐up. Co‐interventions avoided or similar between groups and compliance with exercise assessed against specified level of 3 x/week. Timing of outcome assessment same for both groups. |
Suputtitada 2002.
| Methods | 74 women were allocated to experimental or control groups by using a 'random sampling technique' (no description). Exercise group: randomised = 37; analysed = 32 (76.2%). Control group: randomised = 37; analysed = 35 (83.3%). Lost to follow‐up: toxaemia (3), would not deliver at hospital (3), preterm labour due to oligohydramnios (1), group membership not noted, nor the reasons for the other losses. | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Women were similar at baseline for all factors except job activities: exercise group sat more often at work (N/S); control group stood more often at work and income: exercise group were in higher paid jobs than the control group (P = 0.008). |
|
| Interventions |
Intervention group (N = 37/32 analysed): exercise.
Sitting pelvic tilt exercise: week 1 = do 4 cycles (hold position for 5 seconds then relax for 5 seconds) of exercises each morning and evening; increase by 2 cycles/session in weeks 2 to 4, until you are doing 10 cycles/session, then continue at this level for the next 4 weeks.
Exercises should be done twice a day, 5 days/week (twice under supervision of exercise instructor at the hospital; 3 times unsupervised at home) for a total of 8 weeks.
Record kept of exercises done; instructor checked agility and overall fitness when at clinic. Control group (N = 37/35 analysed): no treatment (nothing noted in article). |
|
| Outcomes | Pain improved, pain worsened, pain measured with VAS, gestational age at birth, baby's Apgar score at 1 minute, baby's Apgar score at 5 minutes. Adverse events: 'no negative effects on mother or fetus; no preterm labour; no premature rupture of membranes'. |
|
| Condition (LBP, PP, LBPP) | LBP. | |
| Notes | Numbers are not consistently reported throughout the article; total number of participants seems to range from 73 to 84, with most mention of 74 randomised, which is the number we used. Data needed for the meta‐analyses appeared to be incorrectly reported in the paper and were re‐analysed. Funding: not mentioned. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘random sampling technique’ but not described; in discussion section, the authors state 'the inclusion and exclusion criteria were used to match these two groups as closely as possible and scrutinize the variables that may contribute to the impact of physical conditioning or pregnancy outcomes'... which doesn't sound like 'randomisation'... |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ 'allocated to experimental or control groups'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear about outcome assessors. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | 17% loss of participants from control group; 24% loss of participants from intervention group; details for withdrawals not clearly described. Analysis on 67 completers only. |
| Selective reporting (reporting bias) | Low risk | Reported on LBP for mothers, 1‐minute and 5‐minute Apgar scores and birthweight of babies. |
| Other bias | Unclear risk | Exercise diary kept and checked by exercise instructor; co‐interventions not described. |
Wang 2009a.
| Methods | RCT conducted at Yale‐New Haven Hospital, USA. Women recruited by prenatal healthcare providers in the area; women called the hotline and spoke with the research assistant. N = 159 women randomised/152 analysed. |
|
| Participants |
Inclusion criteria: Pregnant women; between 25 to 38 weeks' gestation; LBP and/or posterior PP. Exclusion criteria:
All patients acupuncture naive. Drop‐outs and exclusions reported with reasons. |
|
| Interventions |
Intervention group 1 (N = 58 randomised): auricular (ear) acupuncture x 7 days plus self‐care (AA). Used specific acupuncture points (kidney, analgesia, shenmen). Intervention group 2 (N = 54 randomised): sham auricular (ear) acupuncture x 7 days plus self care (Sham AA). Used non‐specific points (shoulder, wrist, extra auricular point). Control group (N = 47 randomised): self care only waiting list control (WL). Self care only. No acupuncture treatment received. Women just given advice. NB: all women given advice to rest if desired, take 650 mg acetaminophen every 6 hours if needed, use hot/cold compress as desired. |
|
| Outcomes | Pain: VAS (0 to 100 mm); Disability Rating Index (DRI) ‐ functional status; STAI, measured at baseline, after 7 days of continuous AA or Sham AA and at 1 week post treatment (for both groups). Days off work not included in outcomes. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | Funding = national Center for the Complementary and Alternative Medicine Grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned to one of the three treatment groups based on a computer generated randomisation sheet.' |
| Allocation concealment (selection bias) | Unclear risk | No information provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women had no previous experience with acupuncture and were asked to complete a credibility questionnaire after the removal of the needles. While not blinded, acupuncturist was skilled and trained and followed a strict script during treatment to avoid any nuances being picked up by the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors and statisticians were blinded; women who gave self‐reports were also blinded. |
| Incomplete outcome data (attrition bias) effect of intervention | Low risk | Attrition and exclusions reported, numbers add up in the analysis, authors indicate how they managed missing values in their analysis. |
| Selective reporting (reporting bias) | Low risk | Study reported all outcomes as indicated in the methods section. |
| Other bias | Low risk | Similar co‐interventions; groups similar at baseline, timing of outcome assessment same across groups and compliance acceptable across groups. |
Wedenberg 2000.
| Methods | RCT; conducted in the Eastern part of Östergötland, Sweden. N = 60 randomised/46 analysed. |
|
| Participants | Pregnant women with LBP or PP arising before 32 weeks' gestation. | |
| Interventions |
Acupuncture group (N = 28):
3 times/week for 2 weeks, then 2 times/week for 2 weeks = total 10; each session = 30 minutes.
2 to 10 needles used, started with fossa triangularis points in ear adding body points, local points as needed; needles were gently tapped or rotated 15 minutes after insertion until De qi reached. Physiotherapy group (N = 18): 1 to 2 times/week within 6 to 8 weeks = total 10 physiotherapy group sessions; 50 minutes each. Individualised treatment based on assessment + trochanter‐belt for pelvic support, warmth, massage, soft‐tissue mobilisation if needed. All were offered water gymnastics according to a defined program. |
|
| Outcomes | Pain (VAS 0 to 10), functional status (Disability Rating Index), and rating of overall percentage helpfulness of treatment ‐ assessed by the women in the study. Adverse events: no serious adverse effects reported, but 2 women reported small subcutaneous haematomas in the ear from acupuncture. |
|
| Condition (LBP, PP, LBPP) | LBPP. | |
| Notes | There was no comparison with no treatment. The pain and disability scales were not used in this review because of insufficient data. Study funded by the Council of Research and Development of Vrinnevi Hospital, Norrkoping, Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 60 women who accepted invitation to join study 'drew a closed envelope from a box to randomise to either the acupuncture or physiotherapy group', but method of randomisation not described. |
| Allocation concealment (selection bias) | Low risk | 'drew a closed envelope from a box.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregiver not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding unclear. |
| Incomplete outcome data (attrition bias) effect of intervention | High risk | Analysed those who completed the intervention in the group to which they had been randomised. 2 of 30 women were not analysed in the acupuncture group since they had both inadvertently received both acupuncture and physiotherapy. 12 of 30 women in the physiotherapy group dropped out: preterm contractions (3), delivered during study (1), pre‐eclampsia (1), no pain‐diary notes (1), failed to attend (3), inconvenient treatment hours (3). |
| Selective reporting (reporting bias) | High risk | Data for pain and disability outcomes not provided with sufficient detail to include in analyses. |
| Other bias | High risk | Statistically significant difference in the distribution of type of pain at baseline, women pursued different co‐treatments to relieve symptoms. |
AA: auricular acupuncture ACOG: American College of Obstetricians and Gynecologists ASLR: active straight leg raise ADL: activities of daily living BMI: body mass index CI: confidence interval gyn: gynaecological HEP: home exercise programme ITT: intention‐to‐treat kg/m2: kilogram/meters squared LBP: low‐back pain LBPP: low‐back and pelvic pain NET: neuro emotional technique NRS: numerical rating scale N/S: not significant NSAID: non‐steroidal anti‐inflammatory drug ODI: Oswestry Disability Index OMT: Osteopathic Manipulative Therapy P4: Posterior Pelvic Pain Provocation PGP: pelvic girdle pain PMR: progressive muscle relaxation PP: pelvic pain PPA: per‐protocol analysis PFM: pelvic floor muscles PPAQ: Pregnancy Physical Activity Questionnaire RMDQ: Roland Morris Disability Questionnaire PVD: peripheral vascular disease RCT: randomised controlled trial ROB: risk of bias ROM: range of motion SD: standard deviation SLR: straight leg raise test STAI: State‐Trait Anxiety Inventory TENS: transcutaneous electrical nerve stimulation UTI: urinary tract infection VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beyaz 2011 | CCT ‐ participants not randomised. |
| Chitryniewicz 2010 | QRCT ‐ participants not randomised. Translated from Polish by 1 Polish‐speaking researcher. |
| Ciardi 2002 | QRCT ‐ pilot study of 8 women assigned to groups based on ability to attend classes. |
| da Silva 2004 | QRCT ‐ women assigned to groups based on the day they attended the prenatal clinic ‐ Tuesday and Thursday were assigned to study group; Monday and Wednesday were assigned to control group. |
| de Jonge‐Vors 2011 | Not a clinical trial; publication reports on an audit/evaluation of a Midwifery Acupuncture Service. |
| Field 1999a | Trial studied the effect of massage on stress reduction in pregnancy; back pain was measured, but only as a stressor that was managed with massage, not as an outcome of real interest. Attempts to contact 1st author for clarification were unsuccessful. |
| Field 2012 | Intervention designed to study the effect of yoga or massage compared to standard prenatal care on depressed pregnant women; back and leg pain was measured but not an outcome of real interest and not listed as one of the outcomes in methods section. |
| Foxcroft 2011 | Participants not randomised. Secondary analysis of intervention to prevent gestational diabetes. |
| Granath 2006 | QRCT ‐ randomisation was by date of birth. |
| Haugland 2006 | Intervention was started during pregnancy, but goal and outcomes measured 6 and 12 weeks postpartum. |
| Hensel 2013 | Outcomes were blood pressure and heart rate, not LBP or PP. |
| Kohama 2006 | CCT ‐ sequence generation not described '...140 women were included in the study...80 patients were enrolled into the treatment group ... Pregnant women with the same pregnancy‐related pains were observed without Pycnogenol® treatment as a control group'. |
| Ladefoged 2012 | QRCT ‐ described as a 'Prospective controlled trial', with no details given for allocation. Report of conference proceedings, but unable to locate trial register (http://apps.who.int/trialsearch/Default.aspx; accessed 15 August 2012). |
| McCullough 2014 | Poster presentation of pilot study to assess the feasibility of using reflexology to manage LBPP in pregnant women in the third trimester; saliva, blood pressure, heart rate and pain reported as outcomes of interest, but no data provided; outcomes reported were feasibility of recruitment, intervention and outcome measures. |
| Mens 2012 | Cross‐sectional study to determine the sensitivity and specificity of specific tests for LBP/PP. No intervention involved. |
| Moffatt 2014 | Single intervention only; pilot study to determine the feasibility of undertaking a larger/longer trial to examine the effects of exercise and advice‐based physiotherapy on the prevention of pregnancy‐related LBP; no outcome analysis completed. |
| Momoi 1999 | CCT ‐ sequence generation not described ‐ attempts to contact the author for clarification unsuccessful. Translated from Japanese by 1 Japanese‐speaking researcher and a native Japanese non‐researcher. |
| Nilsson‐Wikmar 2005 | QRCT ‐ women stratified by previous pregnancies, then assigned to 1 of 3 treatment groups in sequence (1st primigravida to group 1, 2nd primigravida to group 2, 3rd primigravida to group 3, etc). |
| Ostgaard 1994 | QRCT ‐ 3 groups divided by whether date of birth was 1st to 10th day in the month, 11th to 20th or 21st to 31st. |
| Schoenfeld 2011 | Not a trial but an overview of the benefits of exercise in pregnancy. |
| Singh 2008 | Article is described as a 'Single center, prospective, randomised ,experimental study', but there are no details of allocation, the control group(s), or comparison of outcomes between groups. Results are provided for 15 participants who appear to be the only ones entered into the study. |
| Thomas 1989 | This cross‐over study was included in reviews up to and including the 2013 update. There were always concerns about the appropriateness of including this study due to study design and methods of analysis. The review authors decided to exclude this study in the 2015 update because of these concerns. |
| Thorell 2012 | Longitudinal cohort study that assessed peak oxygen uptake and incidence of back pain during and after pregnancy. |
| Torstensson 2009 | Women were not pregnant at the time of intervention, just at the inception of the LBP. |
| Torstensson 2013 | As for Torstensson 2009 ‐ women were not pregnant at the time of intervention, just at the inception of the LBP. |
| Zand 2011 | Not a RCT ‐ recruited pregnant women were allocated into study groups using block technique (AABB); acronym (AABB) implies that the block allocation was not probably random. Translated from Farsi by 1 Farsi‐speaking researcher. |
CCT: controlled clinical trial LBP: low‐back pain PP: pelvic pain RCT: randomised controlled trial QRCT: quasi‐randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Freeman 2013.
| Trial name or title | Management of Antenatal Pelvic Girdle Pain Study (MAPS): a single centre blinded randomised trial evaluating the effectiveness of two pelvic support garments. |
| Methods | Randomised double‐blind parallel assignment intervention study. |
| Participants | 72 pregnant women between 20 to 36 weeks' gestation who: report intermittent PGP or symphysis pubis dysfunction (commenced or aggravated during pregnancy), which causes walking and/or stair climbing to be bothersome and are positive on at least 3 out of 7 pain provocation tests. |
| Interventions | Eligible participants will be randomised to receive either a Customised Dynamic Elastomeric Fabric Orthoses (customised orthosis ‐ intervention group) or a Serola Sacroiliac Belt (rigid 'off the shelf' pelvic support belt ‐ control group) to wear for potentially 20 weeks during pregnancy. All participants will also be issued with standardised advice on PGP management via an information leaflet from the Association of Chartered Physiotherapists in Women's health website. |
| Outcomes |
Primary outcome: change in pain intensity a 2‐weekly intervals via numerical rating scale. Secondary outcomes: change in physical activity levels at 2‐weekly intervals via self‐report questionnaire, and change in quality of life at 2‐weekly intervals via Short Form 36 and the EuroQol‐5D. |
| Starting date | Start date = November 2012. Estimated end date = November 2013 (final data collection date for primary outcome measure). |
| Contact information | Jenny Freeman, University of Plymouth, UK. Tel: 01752 588835; Email: jenny.freeman@plymouth.ac.uk. |
| Notes | Trial registration: ClinicalTrials.gov NCT01820013. Study sponsored by the University of Plymouth, UK. |
Greene 2009.
| Trial name or title | Randomised controlled trial for the treatment of pelvic girdle pain in pregnancy. |
| Methods | Open‐label randomised controlled single‐centre trial. |
| Participants | 226 pregnant women (primigravida and multigravida; no age limits) from 20 to 35 weeks of gestation attending Cork University Maternity Hospital (CUMH) low‐risk antenatal clinics who are referred to the physiotherapy department by their healthcare provider or following self‐referral with back pain or pelvic pain will be assessed for inclusion in the study. Women referred to the physiotherapy department with symptoms of PGP will be assessed on presentation by a 1 of 6 departmental physiotherapists specialising in women's health. |
| Interventions | Following initial assessment participants will be randomly allocated to 1 of 2 treatment groups (randomisation ratio 1:1). Patients will be asked to keep a pain score diary where they will record their pain score using a VAS scoring system. Patients will be asked to record a score every morning and every evening during the treatment course. The first treatment in both treatment arms will be 1 week following initial assessment.
Individual care group: 3 sessions/week, approximately 45 minutes/session. Group care group: weekly group exercise classes for 4 weeks (1 hour/class), focusing on core stability and strengthening exercises. In both treatment groups pain scores will be followed up for 1 week post last treatment. |
| Outcomes | Primary outcome: a reduction in the current intensity of PGP related to motion on a 100‐point VAS in the morning and in the evening recorded in the patient's diaries (0 represented no pain and 100 represented worst conceivable pain). |
| Starting date | 01/04/2009 ‐ estimated end = 31/03/2010 ‐ trial completed. |
| Contact information | Prof Richard A Greene, Cork University Maternity Hospital (R.Greene@ucc.ie). |
| Notes | Sponsor: Cork University Maternity Hospital (Ireland); March 8, 2012 ‐ recruiting not yet started. |
Moholdt 2011.
| Trial name or title | Exercise training in pregnancy for obese women (ETIP). |
| Methods | Protocol for a randomised controlled trial. |
| Participants | 150 previously sedentary, pregnant women with a pre‐pregnancy BMI at or above 30 kg/m2; randomised to intervention and control groups. |
| Interventions |
Intervention group: organised exercise training 3 x/week starting in gestation week 14 (range 12 to 16 weeks' gestation). Control group: standard prenatal care. |
| Outcomes |
Primary outcome: weight gain from baseline to delivery. Secondary outcomes: changes in exercise capacity, physical activity level, endothelial function, body composition, incontinence, lumbo‐pelvic pain and cardiac function from baseline to gestation week 37 (range 36 to 38). Offspring outcome measures include anthropometric variables at birth, Apgar score. |
| Starting date | September 2010. |
| Contact information | Principal Investigator: Trine T Moholdt, PhD. |
| Notes | Trial registration: ClinicalTrials.gov: NCT01243554. Sponsor: Norwegian University of Science and Technology. |
Vas 2014.
| Trial name or title | Auricular acupuncture for primary care treatment of low‐back pain and posterior pelvic pain (LBPP) in pregnancy. |
| Methods | Protocol for a 4‐parallel arm, multicentre, randomised placebo‐controlled trial. |
| Participants | 212 pregnant women (24 to 36 weeks’ gestation), aged at least 17 years, with low back and/or PGP will be randomised into 1 of 3 intervention groups including standard antenatal care or standard antenatal care alone. |
| Interventions |
Intervention 1: verum auricular acupuncture (3 LBPP specific acupuncture points) x once/week x 2 weeks. Intervention 2: non‐specific auricular acupuncture (3 non‐specific acupuncture points) over the same intervention period. Intervention 3: placebo auricular acupuncture (3 non‐specific acupuncture points using the same auricular acupuncture devices but with no needle) over the same intervention period. Control group: standard antenatal care over the same period as intervention groups. |
| Outcomes |
Primary outcome: reduction in pain intensity at 2 weeks using the VAS. Secondary outcomes: LBPP‐related functional disability (RMDQ), Health‐related Quality of Life (Short‐Form 12) temporary occupational incapacity and reduction in analgesic use at 2 weeks. Change in pain intensity will also be recorded at 12 and 48 weeks (outside scope of this review). |
| Starting date | February 2014. |
| Contact information | Jorge Vas, Pain Treatment Unit. Doña Mercedes Primary Health Care Centre, Segovia s/n, Dos Hermanas 41701, Spain (jorgef.vas.sspa@juntadeandalucia.es) |
| Notes |
Trial registration: Current Controlled Trials ISRCTN41033073. Sponsor: Spanish Ministry of Health and Consumer Affairs. |
BMI: body mass index LBPP: low‐back and pelvic pain PGP: pelvic girdle pain VAS: visual analogue scale
Differences between protocol and review
The protocol was originally written in the 1990s. The methodology for conducting Cochrane reviews has changed substantially since then; this update reflects those changes. The current methods are based on the standard template of the Pregnancy and Childbirth Group.
In this update (2015) the outcome, "activities of daily living" has been re‐defined as, "back‐ or pelvic‐related functional disability/functional status (ability to perform daily activities)".
Contributions of authors
For the 2015 and 2013 updates, Victoria Pennick (VEP) and Dianne Liddle (SDL) selected and assessed the risks of bias of the articles and extracted and analysed the data. They both contributed to the writing of the review.
Sources of support
Internal sources
Institute for Work and Health, Canada.
External sources
Royal College of General Practitioners, UK.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akmese 2014 {published data only}
- Akmese ZB, Oran NT. Effects of progressive muscle relaxation exercises accompanied by music on low back pain and quality of life during pregnancy. Journal of Midwifery & Women's Health 2014;59(5):503‐9. [DOI] [PubMed] [Google Scholar]
Bandpei 2010 {published data only}
- Bandpei MAM, Ahmadshirvani M, Fakhri M, Rahmani N. The effect of an exercise program and ergonomic advices on treatment of pregnancy‐related low back pain: a randomized controlled clinical trial. Journal of Mazandaran University of Medical Sciences 2010;20(77):10‐9. [Google Scholar]
Depledge 2005 {published data only}
- Depledge J, McNair PJ, Keal‐Smith C, Williams M. Management of symphysis pubis dysfunction during pregnancy using exercise and pelvic support belts. Physical Therapy 2005;85(12):1290‐300. [PubMed] [Google Scholar]
Eggen 2012 {published data only}
- Eggen MH, Stuge B, Mowinckel P, Jensen KS, Hagen KB. Can supervised group exercises including ergonomic advice reduce the prevalence and severity of low back pain and pelvic girdle pain in pregnancy? A randomized controlled trial. Physical Therapy 2012;92(6):781‐90. [DOI] [PubMed] [Google Scholar]
- Hagen KB. Can tailored exercises in pregnancy prevent low back and pelvic girdle pain? A randomized controlled trial. http://www.controlled‐trials.com/isrctn/pf/95014448 (accessed 15 August 2012) 2010.
Ekdahl 2010 {published data only}
- Ekdahl L, Petersson K. Acupuncture treatment of pregnant women with low back and pelvic pain‐an intervention study. Scandinavian Journal of Caring Sciences 2010;24(1):175‐82. [DOI] [PubMed] [Google Scholar]
Elden 2005 {published data only}
- Elden H, Hagberg H, Olsen MF, Ladfors L, Ostgaard HC. Regression of pelvic girdle pain after delivery: follow‐up of a randomised single blind controlled trial with different treatment modalities. Acta Obstetricia et Gynecologica Scandinavica 2008;87(2):201‐8. [DOI] [PubMed] [Google Scholar]
- Elden H, Ladfors L, Olsen MF, Ostgaard HC, Hagberg H. Effects of acupuncture and stabilising exercises as adjunct to standard treatment in pregnant women with pelvic girdle pain: randomised single blind controlled trial. BMJ 2005;330:761‐5. [DOI: 10.1136/bmj.38397.507014.EO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden H, Ostgaard HC, Fagevik‐Olsen M, Ladfors L, Hagberg H. Treatments of pelvic girdle pain in pregnant women: adverse effects of standard treatment, acupuncture and stabilising exercises on the pregnancy, mother, delivery and the fetus/neonate. BMC Complementary and Alternative Medicine 2008;8:34‐46. [DOI: 10.1186/1472-6882-8-34] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladfors L, Elden H, Olsen MF, Ostgaard HC. Effects of acupuncture and specific stabilizing exercises among women with pregnancy‐related pelvic pain: a randomised single blind controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S77. [Google Scholar]
Elden 2008 {published data only}
- Elden H, Fagevik‐Olsen M, Ostgaard HC, Stener‐Victorin E, Hagberg H. Acupuncture as an adjunct to standard treatment for pelvic girdle pain in pregnant women: randomised double‐blinded controlled trial comparing acupuncture with non‐penetrating sham acupuncture. BJOG: an international journal of obstetrics and gynaecology 2008;115(13):1655‐68. [DOI] [PubMed] [Google Scholar]
- Hagberg H. Acupuncture as a complement to standard treatment for the treatment of well‐defined pelvic girdle pain in pregnant women. http://www.controlled‐trials.com/ISRCTN11374571 (accessed 15 February 2007).
Elden 2013 {published data only}
- Elden H, Ostgaard HC, Glantz A, Marciniak P, Linner AC, Olsen MF. Effects of craniosacral therapy as adjunct to standard treatment for pelvic girdle pain in pregnant women: a multicenter, single blind, randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2013;92(7):775‐82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Garshasbi 2005 {published data only}
- Garshasbi A, Faghih Zadeh S. The effect of exercise on the intensity of low back pain in pregnant women. International Journal of Gynecology & Obstetrics 2005;88:271‐5. [DOI: 10.1016/j.ijog.2004.12.001] [DOI] [PubMed] [Google Scholar]
George 2013 {published data only}
- George JW, Skaggs CD, Thompson PA, Nelson DM, Gavard JA, Gross GA. A randomized controlled trial comparing a multimodal intervention and standard obstetrics care for low back and pelvic pain in pregnancy. American Journal of Obstetrics and Gynecology 2013;208(4):295.e1‐7. [DOI: 10.1016/j.ajog.2012.10.869] [DOI] [PubMed] [Google Scholar]
- Gross G, George JW, Thompson PA, Nelson DM, Skaggs C. A randomized controlled trial comparing a multi‐modal intervention and standard obstetrical care for low back and pelvic pain in pregnancy. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S360. [DOI] [PubMed] [Google Scholar]
Gil 2011 {published data only}
- Gil VFB, Osis MJD, Faúndes A. Lumbar pain during pregnancy: efficacy of Global Postural Reeducation (GPR) treatment [Portuguese] [Lombalgia durante a gestação: eficácia do tratamento com Reeducação Postural Global (RPG)]. Fisioterapia e Pesquisa 2011;18(2):164‐70. [Google Scholar]
Gundermann 2013 {published data only}
- Gundermann S. Effectiveness of osteopathic treatment in pregnant women suffering from low back pain. A randomized controlled trial [Osteopathische Behandlung von Schwangeren mit Rückenschmerzen. Randomisierte kontrollierte Studie.[German]]. Unpublished D.O. Thesis.Akademie für Osteopathie; http://www.osteopathic‐research.com/index.php?option=com_jresearch&view=publication&task=show&id=15363&lang=en. (accessed 19 January 2015) 2013.
Hensel 2014 {published data only}
- Hensel K. Osteopathic manipulative medicine in pregnancy: physiologic and clinical effects. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 8 March 2012) 2008.
- Hensel KL, Buchanan S, Brown SK, Rodriguez M, Cruser DA. Pregnancy research on osteopathic manipulation optimizing treatment effects: the PROMOTE study. American Journal of Obstetrics and Gynecology 2014 July 25 [Epub ahead of print];212(1):108.e1‐108.e9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalus 2007 {published data only}
- Kalus SM, Kornman L, Quinlivan JA. Managing back pain in pregnancy using a support garment: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2007;115:68‐75. [DOI: 10.1111/j.1471-0528.2007.01538.x] [DOI] [PubMed] [Google Scholar]
- Kalus SM, Kornman LH, Quinlivan JA. Evaluating the impact of the belly bra on back pain in pregnancy. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:219.
- Quinlivan J, Kornman L. Evaluating the impact of a belly bra on back pain in pregnancy. Australian Clinical Trials Register. http://www.actr.org.au (accessed 6 December 2005).
Kashanian 2009 {published data only}
- Kashanian M, Akbari Z, Alizadeh MH. The effect of exercise on back pain and lordosis in pregnant women. International Journal of Gynecology & Obstetrics 2009;107(2):160‐1. [DOI: 10.1016/j.ijgo.2009.06.018] [DOI] [PubMed] [Google Scholar]
Kaya 2013 {published data only}
- Kaya E, Yosunkaya N. The effect of Kinesiotaping on low back pain during pregnancy. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2013;59(Suppl 1):248. [Google Scholar]
Keskin 2012 {published data only}
- Keskin EA, Onur O, Keskin HL, Gumus II, Kafali H, Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecologic and Obstetric Investigation 2012;74(1):76‐83. [DOI: 10.1159/000337720] [DOI] [PubMed] [Google Scholar]
Kihlstrand 1999 {published data only}
- Kihlstrand M, Stenman B, Nilsson S, Axelsson O. Water‐gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstetricia et Gynecologica Scandinavica 1999;78(3):180‐5. [PubMed] [Google Scholar]
Kluge 2011 {published data only}
- Kluge J, Hall D, Louw Q, Theron G, Grové D. Specific exercises to treat pregnancy‐related low back pain in a South African population. International Journal of Gynecology & Obstetrics 2011;113(3):187‐91. [DOI: 10.1016/j.ijgo.2010.10.030] [DOI] [PubMed] [Google Scholar]
Kordi 2013 {published data only}
- Abolhasani M. Comparison between two types of treatment in pelvic girdle pain in pregnancy: abdomino lumbo‐pelvic belt and exercise therapy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
- Kordi R, Abolhasani M, Rostami M, Hantoushzadeh S, Mansournia MA, Vasheghani‐Farahani F. Comparison between the effect of lumbo pelvic belt and home based pelvic stabilizing exercise on pregnant women with pelvic girdle pain; A randomized controlled trial. Journal of Back and Musculoskeletal Rehabilitation 2013;26(2):133‐9. [DOI: 10.3233/BMR-2012-00357] [DOI] [PubMed] [Google Scholar]
- Shafiee M, Rostami M. Comparison between the effect of lumbo pelvic belt and home based pelvic stabilizing exercise on pregnant women with pelvic girdle pain; A randomized controlled trial. European Journal of Medical Research 2011;16(Suppl 1):35. [Google Scholar]
Kvorning 2004 {published data only}
- Kvorning N, Holmberg C, Grennert L, Aberg A, Akeson J. Acupuncture relieves pelvic and low‐back pain in late pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2004;83:246‐50. [DOI] [PubMed] [Google Scholar]
Licciardone 2010 {published data only}
- Licciardone JC. Study of the effectiveness of osteopathic manipulative treatment in pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 June 2007).
- Licciardone JC, Aryal S. Prevention of progressive back‐specific dysfunction during pregnancy: an assessment of Osteopathic manual treatment based on Cochrane Back Review Group criteria. JAOA: Journal of the American Osteopathic Association 2013;113(10):728‐36. [DOI: 10.7556/jaoa.2013.043] [DOI] [PubMed] [Google Scholar]
- Licciardone JC, Buchanan S, Hensel KL, King HH, Fulda KG, Stoll ST. Osteopathic manipulative treatment of back pain and related symptoms during pregnancy: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(1):43.e1‐8. [DOI: 10.1016/j.ajog.2009.07.057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lund 2006 {published data only}
- Lund I, Lundeberg T, Lonnberg L, Svensson E. Decrease of pregnant women's pelvic pain after acupuncture: a randomized controlled single‐blind study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(1):12‐9. [DOI: 10.1080/00016340500317153] [DOI] [PubMed] [Google Scholar]
Martins 2005 {published data only}
- Martins R, Pinto e Silva JL. An exercise method for the treatment of lumbar and posterior pelvic pain in pregnancy [Tratamento da lombalgia 3 dor pelvica posterior na gestacao por um metodo de exercisios]. Revista Brasileira de Ginecologia y Obstetricia 2005;27(5):275‐82. [Google Scholar]
Martins 2014 {published data only}
- Martins RF. Hatha yoga exercises in pelvic and lumbar back pain in pregnant women. ClinicalTrials.gov [accessed 21 May 2013] 2012. [NCT01576978]
- Martins RF, Pinto E Silva JL. Treatment of pregnancy‐related lumbar and pelvic girdle pain by the yoga method: a randomized controlled study. Journal of Alternative & Complementary Medicine 2014;20(1):24‐31. [DOI: 10.1089/acm.2012.0715] [DOI] [PubMed] [Google Scholar]
Miquelutti 2013 {published data only}
- Miquelutti MA, Cecatti JG, Makuch MY. Evaluation of a birth preparation program on lumbo pelvic pain, urinary incontinence, anxiety and exercise: a randomized controlled trial. BMC Pregnancy and Childbirth 2013;13:154‐61. [NCT01155804; http://www.biomedcentral.com/1471‐2393/13/154] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquelutti MA, Cecatti JG, Makuch MY. Evaluation of the efficacy of an antenatal birth preparation program. International Journal of Gynecology and Obstetrics. 2012; Vol. 119, issue Suppl 3:S414.
Mørkved 2007 {published data only}
- Mørkved S, Salvesen KA, Schei B, Lydersen S, Bø K. Does group training during pregnancy prevent lumbo pelvic pain? A randomized clinical trial. Acta Obstetricia et Gynecologica Scandinavica 2007;86(3):276‐82. [DOI: 10.1080/00016340601089651] [DOI] [PubMed] [Google Scholar]
Peters 2007 {published data only}
- Peters R, Linde M. Osteopathic treatment of women with low back pain during pregnancy. A randomized controlled trial. Osteopathy Today, 9th International Congress of the German Osteopathic Association (VOD); 2006 October 5‐8; Schlangenbad, Wiesbaden, Germany. Wiesbaden: German Academy of Osteopathy, 2006:7.
- Peters R, Linde M. Osteopathic treatment of women with back pain during pregnancy. A randomised controlled trial [Osteopathische Behandlung von Frauen mit Rückenschmerzen während der Schwangerschaft. Eine randomisierte kontrollierte Studie]. Osteopathische Medizin 2007;8(1):26. [Google Scholar]
Peterson 2012 {published data only}
- Peterson CD, Haas M, Gregory T. A pilot randomized controlled trial comparing the efficacy of exercise, spinal manipulation, and Neuro Emotional Technique for the treatment of pregnancy related low back pain. Chiropractic & Manual Therapies 2012;20(1):18‐30. [DOI: 10.1186/2045-709X-20-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CD, Haas M, Gregory WT. A pilot randomized controlled trial evaluating three treatments for pregnancy‐related low back pain: exercise, spinal manipulation, and neuro emotional technique. Journal of Midwifery & Women's Health 2012;57(5):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sedaghati 2007 {published data only}
- Sedaghati P, Ziaee V, Ardjmand A. The effect of an ergometric training program on pregnants weight gain and low back pain. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2007;166(6):209‐13. [Google Scholar]
Stafne 2012 {published data only}
- Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Mørkved S. Does regular exercise during pregnancy influence lumbo pelvic pain? A randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012 May;91(5):552‐9. [DOI: 10.1111/j.1600-0412.2012.01382.x] [DOI] [PubMed] [Google Scholar]
Suputtitada 2002 {published data only}
- Suputtitada A, Wacharapreechanont T, Chaisayan P. Effect of the "sitting pelvic tilt exercise" during the third trimester in primigravidas on back pain. Journal of the Medical Association of Thailand 2002;85 Suppl 1:S170‐S179. [PubMed] [Google Scholar]
Wang 2009a {published data only}
- Wang SM. Acupuncture and low back pain during pregnancy. National Centre for Complementary & Alternative Medicine. http://nccam.nih.gov/research/extramural/awards/2005/ (accessed 31 March 2006).
- Wang SM. Use ear acupuncture as treatment for low back pain during pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
- Wang SM, Caldwell‐Andrews AA, Fermo L, Sevarino F, Kain ZN. Auricular acupuncture as a treatment for gestational low back pain: preliminary findings [abstract]. Anesthesiology 2002;96 Suppl:A1025. [Google Scholar]
- Wang SM, Dezinno P, Lin EC, Lin H, Yue JJ, Berman MR, et al. Auricular acupuncture as a treatment for pregnant women who have low back and posterior pelvic pain: a pilot study. American Journal of Obstetrics and Gynecology 2009;201(3):271.e1‐9. [DOI: 10.1016/j.ajog.2009.04.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SM, Lin E, Braveman F, Kain Z. Auricular acupuncture as a treatment for posterior pelvic pain during pregnancy: a RCT. Anesthesiology 2007;107:Abstract no: A277. [Google Scholar]
Wedenberg 2000 {published data only}
- Wedenberg K, Moen B, Norling A. A prospective randomized study comparing acupuncture with physiotherapy for low‐back and pelvic pain in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2000;79:331‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Beyaz 2011 {published data only}
- Beyaz EA, Ozcan E, Ketenci A, Beyaz MM. The effectiveness of pregnancy rehabilitation: Effects on low back pain and calf cramps during pregnancy and pregnancy outcome. Nobel Medicus 2011;7(2):67‐74. [Google Scholar]
Chitryniewicz 2010 {published data only}
- Chitryniewicz J, Kulis A. The influence of physical activity and massage on low back pain in pregnant women [Wplyw aktywnosci ruchowej i zabiegow masazu na dolegliwosci bolowe kregoslupa ledz wiowego u kobiet w ciazy]. Ginekologia Praktyczna 2010;18(2):17‐22. [Google Scholar]
Ciardi 2002 {published data only}
- Ciardi S, Gozzo V, Wilmarth MA. Pregnant women's response to a prenatal body mechanics and exercise program for the prevention of low back pain: report on a pilot study. Journal of the Section on Women's Health 2002;26(4):17‐22. [Google Scholar]
da Silva 2004 {published data only}
- Silva JBG, Nakamura MU, Cordeiro JA, Kulay L Jr. Acupuncture for low back pain in pregnancy ‐ a prospective, quasi‐randomised, controlled study. Acupuncture in Medicine 2004;22(2):60‐7. [DOI] [PubMed] [Google Scholar]
de Jonge‐Vors 2011 {published data only}
- Jonge‐Vors C. Reducing the pain: midwifery acupuncture service audit in Birmingham. Practising Midwife 2011 Nov;14(10):22‐6. [Google Scholar]
Field 1999a {published data only}
- Field T, Hernandez‐Reif M, Hart S, Theakston H, Schanberg S, Kuhn C. Pregnant women benefit from massage therapy. Journal of Psychosomatic Obstetrics and Gynaecology 1999;20:31‐8. [DOI] [PubMed] [Google Scholar]
Field 2012 {published data only}
- Field T, Diego M, Hernandez‐Reif M, Medina L, Delgado J, Hernandez A. Yoga and massage therapy reduce prenatal depression and prematurity. Journal of Bodywork and Movement Therapies 2012;16(2):204‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foxcroft 2011 {published data only}
- Foxcroft KF, Rowlands IJ, Byrne NM, McIntyre HD, Callaway LK. Exercise in obese pregnant women: the role of social factors, lifestyle and pregnancy symptoms. BMC Pregnancy & Childbirth 2011;11(4):2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Granath 2006 {published data only}
- Granath AB, Hellgren MS, Gunnarsson RK. Water aerobics reduces sick leave due to low back pain during pregnancy. JOGNN: Journal of Obstetric, Gynecologic, & Neonatal Nursing 2006;35(4):465‐71. [DOI] [PubMed] [Google Scholar]
Haugland 2006 {published data only}
- Haugland KS, Rasmussen S, Daltveit AK. Group intervention for women with pelvic girdle pain in pregnancy. A randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2006;85(11):1320‐6. [DOI] [PubMed] [Google Scholar]
Hensel 2013 {published data only}
- Hensel L, Pacchia F, Smith L. Acute improvement in hemodynamic control after osteopathic manipulative treatment in the third trimester of pregnancy. Complementary Therapies in Medicine 2013;21(6):618‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohama 2006 {published data only}
- Kohama T, Inoue M. Pycnogenol alleviates pain associated with pregnancy. Phytotherapy Research 2006;20(3):232‐4. [DOI] [PubMed] [Google Scholar]
Ladefoged 2012 {published data only}
- Ladefoged ML, Bor P, Svendlund R, Sondergaard N, Hojgaard A. The effect of introducing regular exercise during pregnancy and postpartum in physically inactive women. Acta Obstetricia et Gynecologica Scandinavica 2012;39(Suppl 159):102‐3. [Google Scholar]
McCullough 2014 {published data only}
- McCullough J, Hughes C, Sinclair M. Complementary and alternative medicine (CAM) in the management of low back and/or pelvic pain in pregnancy. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:C148.
Mens 2012 {published data only}
- Mens JMA, Huis V, Pool‐Goudzwaard A. The Active Straight Leg Raise test in lumbo pelvic pain during pregnancy. Manual Therapy 2012;17(4):364‐8. [DOI] [PubMed] [Google Scholar]
Moffatt 2014 {published data only}
- Moffatt M, Flynn M. Prevention of pregnancy‐related lumbo pelvic pain, using a single exercise and advice‐based physiotherapy intervention in early pregnancy: A pilot study. Physiotherapy Practice & Research 2014;35(1):41‐8. [Google Scholar]
Momoi 1999 {published data only}
- Momoi M. Evaluation research on the use of the foot bath for pregnant women experiencing low back pain. Nihon Kangoka Gakkaishi (Journal of Japan Academy of Nursing Science) 1999;19(1):31‐41. [Google Scholar]
Nilsson‐Wikmar 2005 {published data only}
- Nilsson‐Wikmar L, Holm K, Oijerstedt R, Harms‐Ringdahl K. Effect of three different physical therapy treatments on pain and activity in pregnant women with pelvic girdle pain: a randomized clinical trial with 3, 6, and 12 months follow‐up postpartum. Spine 2005;30(8):850‐6. [DOI] [PubMed] [Google Scholar]
- Nilsson‐Wikmar L, Holm K, Oijerstedy R, Harms‐Ringdahl K. Effects of different treatments on pain and on functional activities in pregnant women with pelvic pain [abstract]. Third Interdisciplinary World Congress On Low Back & Pelvic Pain; 1998 Nov 19‐21; Vienna, Austria. 1998:330‐1.
Ostgaard 1994 {published data only}
- Ostgaard HC, Zetherstrom G, Roos Hansson E. Back pain in relation to pregnancy: a 6‐year follow‐up. Spine 1997;22(24):2945‐50. [DOI] [PubMed] [Google Scholar]
- Ostgaard HC, Zetherstrom G, Roos‐Hansson E, Svanberg B. Reduction of back and posterior pelvic pain in pregnancy. Spine 1994;19:894‐900. [DOI] [PubMed] [Google Scholar]
Schoenfeld 2011 {published data only}
- Schoenfeld B. Resistance training during pregnancy: safe and effective program design. Strength & Conditioning Journal (Allen Press) 2011;33(5):67‐75. [Google Scholar]
Singh 2008 {published data only}
- Singh N, Desai OP. Prevention and management of low backache in pregnant women through the use of exercise program and education booklet. Indian Journal of Occupational Therapy 2008;39(3):65‐72. [Google Scholar]
Thomas 1989 {published data only}
- Thomas IL, Nicklin J, Pollock H, Faulkner K. Evaluation of a maternity cushion (Ozzlo pillow) for backache and insomnia in late pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1989;29:133‐8. [DOI] [PubMed] [Google Scholar]
Thorell 2012 {published data only}
- Thorell E, Kristiansson P. Pregnancy related back pain, is it related to aerobic fitness? A longitudinal cohort study. BMC Pregnancy and Childbirth 2012;12 Article 30:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torstensson 2009 {published data only}
- Torstensson T, Lindgren A, Kristiansson P. Corticosteroid injection treatment to the ischiadic spine reduced pain in women with long‐lasting sacral low back pain with onset during pregnancy: a randomized, double blind, controlled trial. Spine 2009;34(21):2254‐8. [DOI] [PubMed] [Google Scholar]
Torstensson 2013 {published data only}
- Torstensson T, Lindgren A, Kristiansson P. Improved function in women with persistent pregnancy‐related pelvic pain after a single corticosteroid injection to the ischiadic spine: a randomized double‐blind controlled trial. Physiotherapy Theory & Practice 2013;29(5):371‐8. [DOI] [PubMed] [Google Scholar]
Zand 2011 {published data only}
- Zand S, Rafiei M, Zamani A. The effect of simple exercises and correct daily activities on backache and dependent variables during pregnancy period. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;14(2):48‐53. [Google Scholar]
References to ongoing studies
Freeman 2013 {published data only}
- Freeman J. Single centre blinded randomized controlled trial evaluating the effectiveness of two pelvic support garments (MAPS). ClinicalTrials.gov [accessed 21 May 2013] 2013. [NCT01820013]
Greene 2009 {published data only}
- Greene RA. Randomised controlled trial for the treatment of pelvic girdle pain in pregnancy. www.controlled‐trials.com/ (accessed 8 March 2012).
Moholdt 2011 {published data only}
- Moholdt TT, Salvesen K, Ingul CB, Vik T, Oken E, Morkved S. Exercise training in pregnancy for obese women (ETIP): Study protocol for a randomised controlled trial. Trials 2011;12:Article Number: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vas 2014 {published data only}
- Vas J, Aranda‐Regules JM, Modesto M, Aguilar I, Baron‐Crespo M, Ramos‐Monserrat M, et al. Auricular acupuncture for primary care treatment of low back pain and posterior pelvic pain in pregnancy: study protocol for a multi centre randomised placebo‐controlled trial. Trials 2014;15(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abolhasani 2010
- Abolhasani M. Comparison between two types of treatment in pelvic girdle pain in pregnancy: abdomino lumbo‐pelvic belt and exercise therapy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010. [IRCT138812113470N1]
ACSM 2006
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 7th Edition. Philadelphia, PA: Lippincott, Williams & Wilkins, 2006. [Google Scholar]
Airaksinen 2006
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber‐Moffett J, Kovacs F, et al. Chapter 4: European guidelines for the management of chronic non‐specific low back pain. European Spine Journal 2006;15(Suppl 2):S192‐S300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 2006
- Albert HB, Godskesen M, Korsholm L, Westergaard JG. Risk factors in pregnancy‐related pelvic joint pain. Acta Obstetricia et Gynecologica Scandinavica 2006;85(5):539‐44. [DOI] [PubMed] [Google Scholar]
American Osteopathic Association
- American Osteopathic Association. Osteopathic Medicine and your health. AOA Website; http://www.osteopathic.org/osteopathic‐health/treatment/Pages/default.aspx (Accessed May 22, 2013).
Anderson 2005
- Anderson FWJ, Johnson CT. Complementary and alternative medicine in obstetrics. International Journal of Gynaecology and Obstetrics 2005;91:116‐24. [DOI] [PubMed] [Google Scholar]
Bablis 2008
- Bablis P, Pollard H, Bonello R. Neuro Emotional Technique for the treatment of trigger point sensitivity in chronic neck pain sufferers: a controlled clinical trial. Chiropractic & Osteopathy 2008;16:4‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bishop 2014
- Bishop A, Holden M, Ogollah R, Foster NE. UK‐based physiotherapists' current management of pregnancy‐related back pain: a national survey. Rheumatology Journal 2014;53(1):123‐4. [DOI] [PubMed] [Google Scholar]
Bombardier 2001
- Bombardier C, Hayden J, Beaton DE. Minimal clinically important difference. Low‐back pain: outcome measures. Journal of Rheumatology 2001;28:431‐8. [PubMed] [Google Scholar]
Cherkin 2003
- Cherkin DC, Sherman KJ, Deyo RA, Shekelle PG. A review of the evidence for the effectiveness, safety and costs of acupuncture, massage therapy and spinal manipulation for back pain. Annals of Internal Medicine 2003;138:898‐906. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 1st Edition. New York, SanFrancisco, London: Academic press, 1988. [Google Scholar]
Craniosacral Therapy UK
- Craniosacral Therapy Association, UK. www.craniosacral.co.uk.
Ee 2008
- Ee CC, Manheimer E, Pirotta MV, White AR. Acupuncture for pelvic and back pain in pregnancy. American Journal of Obstetrics and Gynecology 2008;198(3):254‐9. [DOI] [PubMed] [Google Scholar]
Field 2008
- Field T. Pregnancy and labor alternative therapy research. Alternative Therapies in Health and Medicine 2008;14(5):28‐34. [PubMed] [Google Scholar]
Franke 2014
- Franke H, Franke J‐D, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta‐analysis. BMC Musculoskeletal Disorders 2014;15:286‐303. [DOI: 10.1186/1471-2474-15-286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Google Translate
- Google Translate. https://translate.google.ca/ (Accessed August 8, 2012).
Greenwood 2001
- Greenwood CJ, Stainton MC. Back pain / discomfort in pregnancy: invisible and forgotten. Journal of Perinatal Education 2001;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hensel 2008
- Hensel K. Osteopathic manipulative medicine in pregnancy: physiologic and clinical effects. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 8 March 2012) 2008. [NCT00426244]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kanakaris 2011
- Kanakaris NK, Roberts CS, Giannoudis PV. Pregnancy‐related pelvic girdle pain: an update. BMC Medicine 2011;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lile 2003
- Lile J, Perkins J, Hammer RL, Loubert PV. Diagnostic and management strategies for pregnant women with back pain. Journal of the American Academy of Physician Assistants 2003;16:31‐4. [PubMed] [Google Scholar]
Lin 2008
- Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms and actions. American Journal of Chinese Medicine 2008;36(4):635‐45. [DOI] [PubMed] [Google Scholar]
Maigne 2003
- Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine 2003;70(5):336‐41. [DOI] [PubMed] [Google Scholar]
McGuigan 2007
- McGuigan FJ, Lehrer PM. Progressive relaxation: origins, principles and clinical application. In: Lehrer PM, Woolfolk RL, Sime WE editor(s). Principles and Practice of Stress Management. 3rd Edition. NY: The Guilford Press, 2007:57‐87. [Google Scholar]
Mens 2006
- Mens JMA, Damen L, Snijders CJ, Stam HJ. The mechanical effect of a pelvic belt in patients with pregnancy‐related pelvic pain. Clinical Biomechanics 2006;21(2):122‐7. [DOI] [PubMed] [Google Scholar]
Mens 2009
- Mens JM, Pool‐Goudzwaard A, Stam HJ. Mobility of the pelvic joints in pregnancy‐related lumbo pelvic pain: a systematic review. Obstetrical & Gynecological Survey 2009;64:200‐8. [DOI] [PubMed] [Google Scholar]
Moffatt 2013
- Moffatt M, Flynn M. Safety of acupuncture in pregnancy. Journal of the Acupuncture Association of Chartered Physiotherapists 2013;Spring:41‐50. [Google Scholar]
Mogren 2005
- Mogren IM, Pohjanen A. Low back pain and pelvic pain during pregnancy. Spine 2005;30(8):983‐91. [DOI] [PubMed] [Google Scholar]
Mogren 2006
- Mogren IM. Perceived health, sick leave, psychosocial situation, and sexual life in women with low‐back and pelvic pain during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:647‐56. [DOI] [PubMed] [Google Scholar]
Mousavi 2007
- Mousavi SJ, Parnianpour M, Vleeming A. Pregnancy related pelvic girdle pain and low back pain in an Iranian population. Spine 2007;32(3):E100‐E104. [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33:90‐4. [DOI] [PubMed] [Google Scholar]
Peterson 2012a
- Peterson CD, Haas M, Gregory WT. A pilot randomized controlled trial evaluating three treatments for pregnancy‐related low back pain: exercise, spinal manipulation, and neuro emotional technique. Journal of Midwifery & Women's Health 2012;57(5):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2012
- Richards E, Kessel G, Virgara R, Harris P. Does antenatal physical therapy for pregnant women with low back pain or pelvic pain improve functional outcomes? A systematic review. Acta Obstetrica et Gynecologica Scandinavica 2012;91:1038‐45. [DOI: 10.1111/j.1600-0412.2012.01462.x] [DOI] [PubMed] [Google Scholar]
Rost 2006
- Rost CC, Jacqueline J, Kaiser A, Verhagen AP, Koes BW. Prognosis of women with pelvic pain during pregnancy: a long‐term follow‐up study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(7):771‐7. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Medicine 2010;7(3):e1000251. [DOI: 10.1371/journal.pmed.1000251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinclair 2014
- Sinclair M, Close C, McCullough JEM, Hughes C, Liddle SD. How do women manage pregnancy‐related low back and/or pelvic pain? Descriptive findings from an online survey. Evidence Based Midwifery 2014;12(3):76‐82. [Google Scholar]
Skaggs 2007
- Skaggs CD, Prather H, Gross G, George JW, Thompson PA, Nelson DM. Back and pelvic pain in an underserved United States pregnant population. Journal of Manipulative and Physiological Therapeutics 2007;30(2):130‐4. [DOI] [PubMed] [Google Scholar]
Van de Pol 2007
- Pol G, Brummen HJ, Bruinse HW, Heintz APM, Vaart H. Pregnancy‐related pelvic girdle pain in the Netherlands. Acta Obstetricia et Gynecologica Scandinavica 2007;86(4):416‐22. [DOI] [PubMed] [Google Scholar]
Van Tulder 2009
- Tulder MW, Suttorp M, Morton S, Bouter LM, Shekelle P. Empirical evidence of an association between internal validity and effect size in randomized controlled trials of low back pain. Spine 2009;34(16):1685–92. [DOI] [PubMed] [Google Scholar]
Vermani 2010
- Vermani E, Mittal R, Weeks A. Pelvic girdle pain and low‐back pain in pregnancy: a review. Pain Practice 2010;10(1):60‐71. [DOI] [PubMed] [Google Scholar]
Vleeming 2008
- Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. European Spine Journal 2008;17(6):794‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004
- Wang SM, Dezinno P, Maranets I, Berman MR, Caldwell‐Andrews AA, Kain ZN. Low‐back pain during pregnancy: prevalence, risk factors, and outcomes. Obstetrics & Gynecology 2004;104:65‐70. [DOI] [PubMed] [Google Scholar]
Wu 2004
- Wu WH, Meijer OG, Uegali K, Mens JMA, Dieen JH, Wuisman PI, et al. Pregnancy‐related pelvic girdle pain (PPP) i: terminology, clinical presentation, and prevalence. European Spine Journal 2004;13:575‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Östgaard 1994
- Östgaard HC, Zetherström G, Roos‐Hansson E. The posterior pelvic pain provocation test in pregnant women. European Spine Journal 1994;3(5):258‐60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pennick 2007
- Pennick V, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001139.pub2] [DOI] [PubMed] [Google Scholar]
Pennick 2013
- Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001139.pub3] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams S, Whatman C, Hume PA, Sheerin K. Kinesio taping in treatment and prevention of sports injuries: a meta‐analysis of the evidence for its effectiveness. Sports Medicine 1 February 2012;42(2):153‐64. [DOI: 10.2165/11594960-000000000-0000] [DOI] [PubMed] [Google Scholar]
Young 1995
- Young GL. Special vs standard pillow for backache in late pregnancy [revised 13 May 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Young 1998
- Young G, Jewell D. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001139] [DOI] [PubMed] [Google Scholar]
Young 2002
- Young G, Jewell D. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001139] [DOI] [PubMed] [Google Scholar]


