Abstract
The risk of recurrence of venous thromboembolism (VTE) persists after interruption of the initial anticoagulation therapy. New evidence shows that direct oral anticoagulants are effective for extended treatment of VTE and may reduce the risk of all-cause mortality. The optimal duration of anticoagulation after VTE is, however, controversial and complicated by the need for individualised assessment and balance between thrombosis and bleeding risks. Three direct oral anticoagulants (rivaroxaban, apixaban and dabigatran) have been studied for extended treatment of VTE. Dabigatran was shown to be safer than vitamin K antagonists and similarly effective for the prevention of recurrent VTE. Dabigatran, apixaban and rivaroxaban resulted in significant decreases in the rate of recurrent symptomatic VTE when compared to placebo, without a statistically significant difference in the risk of major bleeding. The latest guidelines of the American College of Chest Physicians suggest the use of low-dose aspirin to prevent VTE recurrence in patients who want to stop anticoagulation. In the randomised, double-blind, phase 3 EINSTEIN CHOICE trial, once-daily rivaroxaban at doses of 20 mg or 10 mg and 100 mg of aspirin were compared in VTE patients for whom there was clinical equipoise for extended anticoagulation. Either a treatment dose (20 mg) or a prophylactic dose (10 mg) of rivaroxaban significantly reduced the risk of VTE recurrence without a significant increase in bleeding risk compared with aspirin. The EINSTEIN CHOICE trial included patients with provoked or unprovoked VTE. Patients with VTE provoked by minor persistent or minor transient risk factors enrolled in this trial had not-negligible VTE recurrence rates. These new findings on extended therapy suggest the possibility of anticoagulation regimens at intensities tailored to the patients’ risk profiles and VTE characteristics, with a shift of the risk-benefit balance in favour of extended treatment.
Keywords: venous thromboembolism, extended treatment, direct oral anticoagulants
INTRODUCTION
The optimal duration of anticoagulation therapy for venous thromboembolism (VTE) is controversial. After a first acute episode, VTE tends to recur. The likelihood of recurrence varies according to the characteristics of the disease as well as those of the patient1. After anticoagulant withdrawal, the estimated cumulative incidence of recurrence is 11.0% at 1 year, 19.6% at 3 years, 29.1% at 5 years and 39.9% at 10 years. The risk of recurrence is particularly elevated in patients with cancer-associated thrombosis and it is greater after an unprovoked VTE than after a VTE associated with removable risk factors2. The risk of recurrence in patients with VTE provoked by a non-surgical trigger is estimated to be 5% within 1 year and 15% within 5 years. Conversely, the risk of recurrence of VTE provoked by a major reversible risk factor, such as recent surgery, is very low, with estimated rates being 1% within 1 year and 3% within 5 years3.
The extension of anticoagulant treatment for a defined period of time over the first 3–6 months (e.g., up to 1 year) delays recurrences but does not reduce the risk of recurrence after suspension of the anticoagulant4,5. Therefore, treatment is recommended for at least 3 months in all patients after a first acute VTE. After this standard period, determining the optimal duration of anticoagulation treatment for secondary VTE prevention requires an individualised balance between the long-term risk of recurrence and bleeding to properly evaluate both the benefit and the risk of extended treatment3. An assessment of the patient’s preferences should also be pursued. A major challenge is to identify patients who would benefit most from extended treatment. In the decision-making process, it should be considered that the risk of recurrence is greatest in the first months after the withdrawal of anticoagulant therapy, with an incidence of approximately 5–7% per year in patients with a first unprovoked event6. However, the haemorrhagic risk remains unchanged over time7. Furthermore, the risk of death associated with recurrence of VTE (case-fatality rate: 5.1%) tends to decrease over time. Conversely, the mortality rate associated with haemorrhagic complications (9.1–11%) remains stable8,9. As a result, the optimal strategy for the prevention of VTE recurrence should also take into account the incidence of bleeding complications. However, individual haemorrhagic risk is difficult to predict10,11, and currently, the available bleeding risk scores have only a modest predictive value for patients with VTE12,13.
The 2016 American College of Chest Physicians (ACCP) guidelines14 recommend anticoagulation therapy for 3 months for provoked VTE and suggest extended treatment for patients with unprovoked VTE and a low or intermediate bleeding risk.
Patients with cancer are generally prescribed an extended anticoagulation treatment with low molecular weight heparin as long as they have active malignancy, irrespective of the bleeding risk. Similarly, patients suffering from a second VTE episode or those with severe thrombophilia (antiphospholipid syndrome or deficiency of protein C, protein S, or anti-thrombin) are usually candidates for extended treatment due to the higher risk of recurrence related to these conditions.
The aim of this narrative review is to discuss the latest evidence on extended treatment of VTE with a special focus on insights from the EINSTEIN CHOICE study15.
VITAMIN K ANTAGONISTS
The evidence and rationale for the long-term treatment of VTE come from previous experience with vitamin K antagonists (VKA). Indeed, trials comparing long-term with short-term treatment in patients with unprovoked VTE who completed at least 3 months of initial treatment have confirmed that extended anticoagulant therapy with VKA reduces the incidence of VTE recurrence by 90% compared to recurrence in control groups4,5,16,17. Unfortunately, extended therapy with VKA is associated with an increased risk (approximately 3% per year) of major bleeding14. Consequently, various therapeutic strategies have been studied to reduce haemorrhagic complications of extended treatment. However, warfarin at a reduced intensity (international normalised ratio target: 1.5–2.0 or 1.8–2.2) is less effective than warfarin at a conventional intensity (international normalised ratio target: 2.0–3.0) without reducing haemorrhagic complications18,19.
LOW-DOSE ASPIRIN
Antiplatelet treatment with aspirin may also play a role in the prevention of recurrence in patients with unprovoked VTE20–22. A meta-analysis of clinical trials of low-dose aspirin vs placebo in patients who completed standard anticoagulation treatment for a first unprovoked VTE demonstrated a 35% relative risk reduction in recurrent VTE among people taking aspirin23. Nevertheless, the thromboembolic risk reduction observed with aspirin is lower than that of anticoagulant therapy. Therefore, the most recent guidelines do not consider aspirin as a reasonable alternative to anticoagulation in patients who want extended therapy. However, for patients who decided to stop anticoagulants, they suggest considering the prevention of recurrent VTE as one of the benefits of aspirin that needs to be balanced against the risk of bleeding and the inconvenience caused by taking aspirin.
Furthermore, if aspirin has been temporarily stopped during anticoagulation, it should be reintroduced when patients stop anticoagulant therapy, as indicated in the guidelines14.
SULODEXIDE
Sulodexide is a natural glycosaminoglycan with antithrombotic and profibrinolytic activity. In the Survet trial, 615 patients with first-ever unprovoked VTE, who had completed 3 to 12 months of oral anticoagulant treatment with VKA, were randomly assigned to sulodexide 500 lipasemic units twice daily or placebo for 2 years, in addition to elastic stockings. VTE recurred in 15 of the 307 patients who received sulodexide and in 30 of the 308 patients who received placebo. No major bleeding episodes occurred; two patients in each treatment group had a clinically relevant bleeding episode. The hazard ratio (HR) for clinically relevant bleeding was 0.97 (95% confidence interval [95% CI]: 0.14–6.88; p=0.98). Treatment with oral sulodexide decreased the incidence of VTE recurrences (HR: 0.49; 95% CI: 0.27–0.92; p=0.02), with no apparent increase in bleeding.
While interesting, these results require confirmation in further studies. Indeed, the proportion of enrolled patients with pulmonary embolism as the index event was low (7.6%) in the Survet trial. Therefore, these findings could be considered poorly applicable to this specific subpopulation. Future investigations could examine whether specific subgroups are more or less likely to benefit from sulodexide or other treatments and whether a comparable effect can be obtained after treatment of the index event with direct oral anticoagulants (DOAC)24.
DIRECT ORAL ANTICOAGULANTS
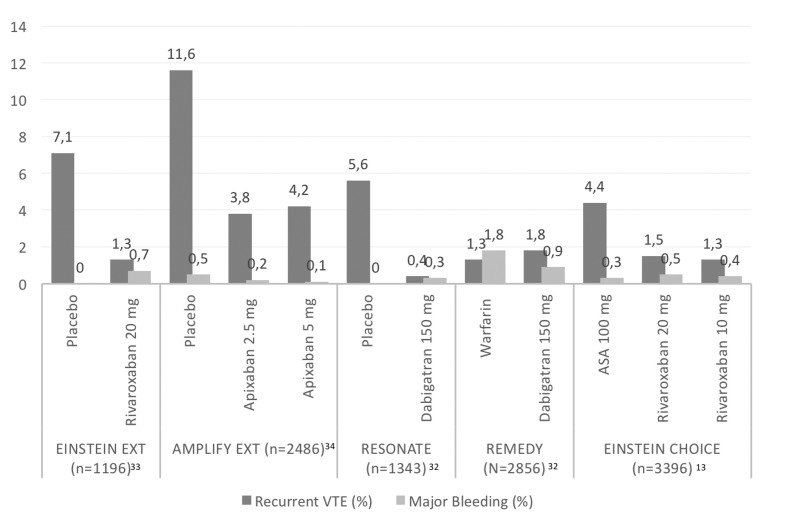
DOAC have altered the standard of care among patients with VTE25–29. According to the guidelines, for patients without cancer, DOAC might be considered as a first-line treatment14,30. Three DOAC (rivaroxaban, apixaban and dabigatran) have been studied for extended treatment of VTE31 (Figure 1). Dabigatran was shown to be safer than VKA and similarly effective for the prevention of recurrent VTE32. Dabigatran, apixaban and rivaroxaban resulted in 80–90% decreases in the rate of recurrent symptomatic VTE when compared to the rate in placebo-treated patients, with a similar risk of major bleeding33.
Figure 1.
Venous thromboembolism recurrence and major bleeding rates in extension studies with direct oral anticoagulants.
VTE: venous thromboembolism; ASA: aspirin.
Indeed, in the REMEDY trial, patients were randomised to receive either dabigatran at a dose of 150 mg twice daily or warfarin for 6–36 months, following at least 3–12 months of initial anticoagulation therapy. Recurrent or fatal VTE occurred in 1.8% and 1.3% of the patients on dabigatran and warfarin, respectively (HR: 1.44; 95% CI: 0.78–2.64; p=0.01 for non-inferiority). Major bleeding events did not differ significantly between the two groups. Conversely, major bleeding or clinically relevant non-major bleeding (CRNMB) was significantly lower in the dabigatran group than in the warfarin group (HR: 0.54; 95% CI: 0.41–0.71).
In the placebo-controlled RE-SONATE trial, recurrent VTE occurred in 0.4% and 5.6% of patients in the 150 mg twice-daily dabigatran group and in the placebo group, respectively (HR: 0.08; 95% CI: 0.02–0.25; p<0.001). Major bleeding occurred in two patients in the dabigatran group (0.3%) and no patients in the placebo group. Major bleeding or CRNMB occurred in 36 patients in the dabigatran group (5.3%) and 12 patients in the placebo group (1.8%) (HR: 2.92; 95% CI: 1.52–5.60). The REMEDY and RE-SONATE studies did not explicitly present the number of patients with unprovoked VTE. However, based on the patients’ characteristics presented, it should be assumed that the majority of patients had unprovoked VTE34.
The EINSTEIN EXTENSION study35 compared a once daily dose of 20 mg of rivaroxaban with placebo for an additional 6 or 12 months in 1,196 patients (mean age: 58.3±15.8 years) who had completed 6–12 months of treatment and for whom there was equipoise regarding the continuation or cessation of therapy. Overall, 881 (73.7%) patients had unprovoked VTE. The primary efficacy endpoint of recurrent VTE occurred in eight patients (1.3%) receiving rivaroxaban and in 42 patients (7.1%) receiving placebo (HR: 0.18; 95% CI: 0.09–0.39; p<0.001). The principal safety outcome of major bleeding occurred at a similar rate in both treatment groups (4/602 patients in the rivaroxaban group vs none of the 594 patients in the placebo group; p=0.11). The outcome of a net clinical benefit, defined as the composite of the primary efficacy outcome or major bleeding, occurred in 12 patients (2.0%) receiving rivaroxaban and in 42 patients (7.1%) receiving placebo (HR: 0.28; 95% CI: 0.15–0.53; p<0.001).
The AMPLIFY-EXT study was the first randomized, double-blind study comparing either a treatment dose (5 mg of apixaban twice daily) or a thromboprophylactic dose (2.5 mg of apixaban twice daily) with placebo. Patients with symptomatic VTE who had completed 6–12 months of standard anticoagulation therapy were considered eligible for 12 months of extended therapy if there was equipoise about the continuation or cessation of anticoagulant therapy. Overall, 2,482 patients (mean age 56.7±15.3 years) were assigned, in a 1:1:1 ratio, to receive active treatment or placebo. Over 90% of patients had a history of unprovoked VTE. Compared with placebo, the prophylactic dose of apixaban was as effective as the treatment dose for the prevention of recurrence (5 mg: 4.2 vs 11.6%; relative risk [RR]: 0.36; 95% CI: 0.25–0.53; 2.5 mg: 3.8 vs 11.6%, RR: 0.33; 95% CI: 0.22–0.48; p<0.001 for both comparisons). Major bleeding did not differ significantly, occurring in 0.5% of patients in the placebo group, in 0.2% of patients in the 2.5 mg apixaban group, and in 0.1% of the 5 mg apixaban group. The composite of major bleeding CRNMB occurred in 22 patients (2.7%) receiving placebo, in 27 patients (3.2%) receiving 2.5 mg of apixaban (RR: 1.20; 95% CI: 0.69–2.10 vs placebo) and in 35 patients (4.3%) receiving 5 mg of apixaban (RR: 1.62; 95% CI: 0.96–2.73 vs placebo)36.
A meta-analysis37 performed to assess the clinical benefit of DOAC for the extended treatment of VTE which included 7,877 participants reported that DOAC significantly lowered the risk of recurrent VTE or VTE-related death compared to placebo/warfarin (odds ratio [OR]: 0.25; 95% CI: 0.07–0.86; number needed to treat = 30). However, these drugs caused a significantly higher rate of CRNMB or major bleeding compared to the placebo (OR: 2.69; 95% CI: 1.25–5.77; number needed to harm = 39).
Subsequently, the Hokusai-VTE trial38 was designed to compare edoxaban with active treatment using the minimum duration of 3 months in all patients and allowing a flexible duration thereafter for up to 12 months based on the physicians’ practice. Indeed, when the Hokusai-VTE study was planned, there was a body of consistent randomised, placebo-controlled data on both VKA6 and DOAC34–36 for extended therapy to prevent recurrent VTE.
Therefore, as explained by the authors, doing another placebo-controlled study seemed not only unnecessary, but potentially unethical. The duration of extended therapy in the placebo-controlled DOAC trials34–36 was 6–12 months.
In the Hokusai-VTE trial, the median duration of edoxaban treatment was 8.8 months, and 1,661 (46%) of 3,633 patients completed a 12-month course of therapy. In the on-treatment, post-hoc analysis of the study38, the cumulative incidence of recurrent VTE between 3 and 12 months was 0.3% in the edoxaban-treated group and 0.4% in the warfarin-treated group (HR: 0.78, 95% CI: 0.36–1.72). The cumulative incidence of CRNMB between 3 and 12 months was 3.9% in the edoxaban group and 4.1% in the warfarin-treated group (HR: 0.97, 95% CI: 0.77–1.22); the cumulative incidence of major bleeding was 0.3% in the edoxaban group and 0.7% in the warfarin-treated group (HR: 0.45; 95% CI: 0.22–0.92).
THE EINSTEIN CHOICE STUDY
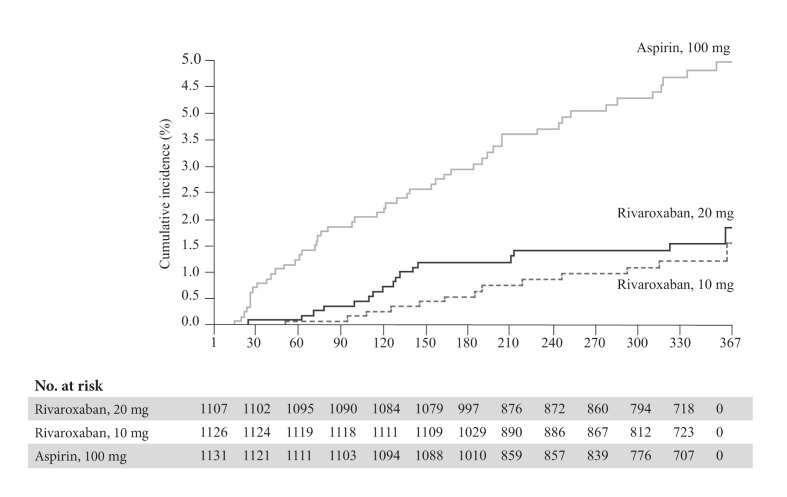
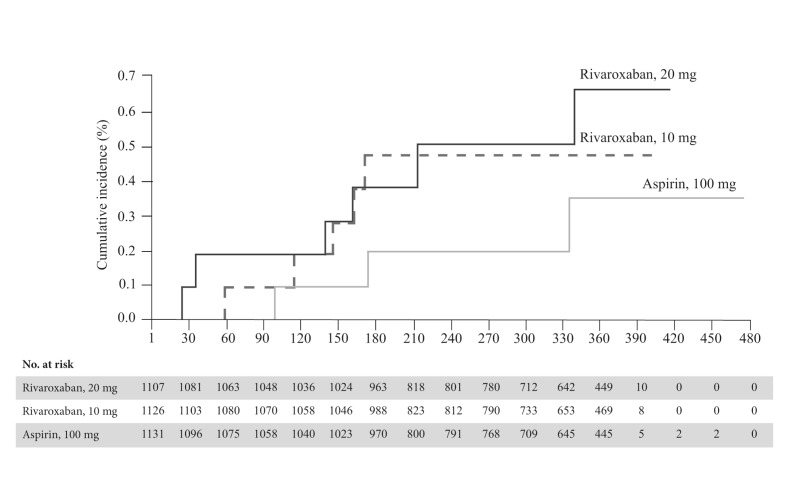
In the EINSTEIN CHOICE trial15, 3,396 patients (mean age 58.4±14.7 years), who had already completed 6–12 months of VTE treatment and were in equipoise regarding the need for continued anticoagulation treatment, were randomised into three treatment groups: 10 mg of rivaroxaban daily, 20 mg of rivaroxaban daily and 100 mg of aspirin daily for 12 months of therapy. The baseline characteristics of patients enrolled in the EINSTEIN CHOICE trial are reported in Table I. A composite of symptomatic, recurrent fatal or nonfatal VTE and unexplained death was chosen as the primary efficacy endpoint and major bleeding as the primary safety outcome. The rate of the composite efficacy endpoint was significantly lower with the 10 mg rivaroxaban dose (1.2%; HR: 0.26; 95% CI: 0.14–0.47) and the 20 mg dose (1.5%; HR: 0.34; 95% CI: 0.20–0.59) than with aspirin (4.4%; p<0.001 for both comparisons) (Figure 2). There was no significant difference between the two studied doses of rivaroxaban (HR: 1.34; 95% CI: 0.65–2.75; p=0.42). Major bleeding occurred in six patients (0.5%) in the 20 mg group and in five patients (0.4%) in the 10 mg rivaroxaban group compared with three patients (0.3%) in the aspirin group (Figure 3). CRNMB occurred in 30 patients (2.7%) in the 20 mg group and in 22 patients (2.0%) in the 10 mg rivaroxaban group compared with 20 patients (1.8%) in the aspirin group. Myocardial infarction, stroke, or systemic embolism occurred in three patients (0.3%) in the 20 mg group, in five patients (0.4%) in the 10 mg rivaroxaban group, and in seven patients (0.6%) in the aspirin group. The rates of death from any cause were 0.7% and 0.2% in the 20 mg and 10 mg rivaroxaban groups, respectively, compared with 0.6% in the aspirin group. The rates of adverse events were similar in the three study groups.
Table I.
| Characteristics | Rivaroxaban 20 mg (n=1,107) | Rivaroxaban 10 mg (n=1,127) | Aspirin 100 mg (n=1,131) | |
|---|---|---|---|---|
| Index event (%) | Isolated deep vein thrombosis | 51.0 | 50.1 | 51.0 |
| Isolated pulmonary embolism | 34.4 | 33.8 | 32.4 | |
| Both | 14.0 | 15.9 | 16.0 | |
| Asymptomatic or unconfirmed | 0.5 | 0.2 | 0,6 | |
| Classification of index VTE (%) | Unprovoked | 39.8 | 42.6 | 41.4 |
| Provoked | 60.2 | 57.4 | 58.6 | |
| History of previous VTE (%) | 17.9 | 17.5 | 17.2 | |
| Known thrombophilia (%) | 7.1 | 6.6 | 6.2 | |
| Active cancer (%) | 2.3 | 2.4 | 3.3 | |
| Duration of study drug administration (median days) | 348 | 353 | 350 | |
There were no significant differences in baseline characteristics among the groups.
Adapted with permission from Weitz JI et al.15
VTE: venous thromboembolism.
Figure 2.
Primary efficacy endpoint (recurrent venous thromboembolism) in the EINSTEIN CHOICE trial. Kaplan-Meier curves.
Adapted with permission from Weitz JI et al.15
VTE: venous thromboembolism.
Figure 3.
Primary safety endpoint (major bleeding) in the EINSTEIN CHOICE trial. Kaplan-Meier curves.
Adapted with permission from Weitz JI et al.15
Of note, this study was designed to test the hypothesis that each dose of rivaroxaban would be superior to aspirin with respect to the primary efficacy outcome. Therefore, it was not powered to compare the two doses or to show the non-inferiority of the 10 mg dose compared to the established treatment regimen of 20 mg. Since both doses of rivaroxaban were more effective than aspirin, with low rates of major bleeding and CRNMB, similar to those occurring with aspirin, both the Food and Drug Administration and the European Medicines Agency approved the update of the label, including 10 mg rivaroxaban for extended prevention of recurrent VTE following 6 months of standard anticoagulation treatment39,40.
Interestingly, unlike other trials, in the EINSTEIN CHOICE study, approximately 58% of the included population had a history of provoked VTE. Consequently, data from the EINSTEIN EXTENSION and the EINSTEIN CHOICE trials were used to estimate the risk of recurrence according to the baseline risk factor profiles in a pre-specified analysis. Index VTE events were centrally classified as unprovoked or provoked by major transient or persistent or minor transient or persistent risk factors (Table II), and rates of recurrence at 1 year were calculated. Overall, 2,832 patients received rivaroxaban, 1,131 received aspirin and 590 received placebo. With unprovoked VTE, the rates of recurrence in the 1,173 patients given rivaroxaban, the 468 given aspirin, and the 243 given placebo were 2.0%, 5.9%, and 10.0%, respectively. There were no recurrences in patients with VTE provoked by major transient risk factors. With VTE provoked by minor persistent risk factors, recurrence rates in the 1,184 patients given rivaroxaban, the 466 given aspirin, and the 248 given placebo were 2.4%, 4.5%, and 10.7%, respectively. For patients with minor transient risk factors, recurrence rates were 0.4% in the 268 patients given rivaroxaban, 4.2% in the 121 given aspirin, and 7.1% in the 56 given placebo. Remarkably, recurrence rates in patients with VTE provoked by minor persistent or minor transient risk factors were not significantly lower than in those with unprovoked VTE (HR: 0.81, 95% CI: 0.56–1.16; and HR: 0.68, 95% CI: 0.32–1.30, respectively).
Table II.
Criteria for classification as unprovoked or provoked venous thromboembolism used in the EINSTEIN CHOICE and EINSTEIN EXTENSION studies41
| VTE provoked by a persisting major risk factor | Active cancer (diagnosis or treatment <6 months or recurrent or metastatic cancer), excluding basal cell or squamous cell carcinoma |
| VTE provoked by a major transient risk factor if within 3 months | Major surgery or major trauma, excluding leg injuries, including Caesarean section |
| VTE in association with a minor persisting risk factor |
|
| VTE provoked by a minor transient risk factor if within 3 months |
|
| Unprovoked VTE | All other patients |
VTE: venous thromboembolism; BMI: body mass index; CrCl: creatinine clearance.
Moreover, patients at the highest risk for recurrence may have been underrepresented in the EINSTEIN EXTENSION and the EINSTEIN CHOICE trials since both only enrolled patients with equipoise regarding the need for extended anticoagulant treatment. Therefore, as suggested by the authors, the rates of recurrence in such patients may be higher than those reported in the analysis41.
DISCUSSION
The management of patients with VTE is challenging, especially with regard to the assessments concerning the extension of treatment beyond the first 3 months. Guidelines suggest considering extended anticoagulation when the risk of recurrent VTE off treatment exceeds the risk of bleeding on treatment. Patients’ preference should also be considered14. Nevertheless, as recently pointed out by Palareti et al., the bleeding score suggested by the ACCP has insufficient predictive value for bleeding and can hardly be used to guide decision on extended treatment. Therefore, improving the long-term prognosis of patients with acute VTE remains a difficult task and new instruments for predicting bleeding risk during anticoagulation, including treatment with DOAC, may be advisable13.
All DOAC studied for the secondary prevention of recurrent VTE resulted effective in the extended treatment setting; however, these drugs caused a significantly higher rate of CRNMB or major bleeding compared to placebo when used at full dose37. In the AMPLIFY-EXT trial, for the first time a lower dose of apixaban was evaluated for this indication. Both the treatment dose (5 mg) and the thromboprophylactic dose (2.5 mg) of apixaban reduced the risk of recurrent VTE and/or VTE-related death without increasing the rate of major bleeding36 as compared to that in placebo-treated patients.
According to the latest guidelines, aspirin may play a role over no treatment, mainly for patients who refuse extended anticoagulation, since it reduced the risk of recurrent VTE by 32% without significantly increasing the risk of major bleeding when compared with placebo22. However, in patients with equipoise about continuing or not continuing anticoagulation therapy, more recently the EINSTEIN CHOICE study showed that both doses of rivaroxaban (20 mg and 10 mg daily) reduce recurrent VTE with a favourable benefit-risk profiles compared to aspirin42. With the availability of the two doses of rivaroxaban, the intensity of extended anticoagulation therapy can be individualised based on the relative risks of recurrent VTE and bleeding. Specifically, patients with an ongoing indication for anticoagulation or those at high risk for recurrence (e.g., life-threatening VTE event, severe thrombophilia, recurrence during low-dose therapy) can benefit from rivaroxaban at a dose of 20 mg43. On the other hand, the low dose (10 mg once daily) should offer safety advantages over the usual intensity therapy for extended VTE treatment in patients at high risk of bleeding44.
In addition, the inclusion of patients with VTE provoked by minor persistent or transient risk factors in the EINSTEIN CHOICE trial and the high recurrence rates observed in this category of patients for the first time suggest reconsidering the optimal duration of treatment for this subgroup usually considered at “intermediate risk” of recurrence45.
According to this evidence, clinicians can be confident about anticoagulation with DOAC regimens tailored to both patients and VTE characteristics. Nevertheless, further studies are needed to assess the benefit of these strategies beyond one year of therapy as well as in subgroups of patients not included or under-represented in these trials.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
DI has received consultancy fees from Aspen, Bayer, Sanofi, BMS Pfizer, Daiichi-Sankyo, Boehringer Ingelheim and Werfen. The other Authors declare no conflicts of interest. Financial support for editorial services was provided by Bayer.
REFERENCES
- 1.Kyrle PA, Kammer M, Eischer L, et al. The long-term recurrence risk of patients with unprovoked venous thromboembolism: an observational cohort study. J Thromb Haemost. 2016;14:2402–9. doi: 10.1111/jth.13524. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123:1794–801. doi: 10.1182/blood-2013-12-512681. [DOI] [PubMed] [Google Scholar]
- 4.Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165–9. doi: 10.1056/NEJM200107193450302. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25. doi: 10.7326/0003-4819-139-1-200307010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ. 2011;342:d3036. doi: 10.1136/bmj.d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linkins L-A, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 8.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–89. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 9.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 10.Scherz N, Méan M, Limacher A, et al. Prospective, multicenter validation of prediction scores for major bleeding in elderly patients with venous thromboembolism. J Thromb Haemost. 2013;11:435–43. doi: 10.1111/jth.12111. [DOI] [PubMed] [Google Scholar]
- 11.Poli D, Antonucci E, Testa S, et al. The predictive ability of bleeding risk stratification models in very old patients on vitamin K antagonist treatment for venous thromboembolism: results of the prospective collaborative EPICA study. J Thromb Haemost. 2013;11:1053–8. doi: 10.1111/jth.12239. [DOI] [PubMed] [Google Scholar]
- 12.Riva N, Bellesini M, Di Minno MND, et al. Poor predictive value of contemporary bleeding risk scores during long-term treatment of venous thromboembolism. A multicentre retrospective cohort study. Thromb Haemost. 2014;112:511–21. doi: 10.1160/TH14-01-0081. [DOI] [PubMed] [Google Scholar]
- 13.Palareti G, Antonucci E, Mastroiacovo D, et al. The American College of Chest Physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism. J Thromb Haemost. 2018;16:1994–2002. doi: 10.1111/jth.14253. [DOI] [PubMed] [Google Scholar]
- 14.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. Chest. 2016;149:315–52. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–22. doi: 10.1056/NEJMoa1700518. [DOI] [PubMed] [Google Scholar]
- 16.Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–7. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 17.Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780–9. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–34. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 19.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–9. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 20.Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–67. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 21.Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–87. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 22.Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism. Circulation. 2014;130:1062–71. doi: 10.1161/CIRCULATIONAHA.114.008828. [DOI] [PubMed] [Google Scholar]
- 23.Castellucci LA, Cameron C, Le Gal G, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ. 2013;347:f5133. doi: 10.1136/bmj.f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreozzi GM, Bignamini AA, Davì G, et al. Sulodexide for the prevention of recurrent venous thromboembolism: the Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis (SURVET) study: a Multicenter, randomized, double-blind, placebo-controlled trial. Circulation. 2015;132:1891–7. doi: 10.1161/CIRCULATIONAHA.115.016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–75. doi: 10.1182/blood-2014-04-571232. [DOI] [PubMed] [Google Scholar]
- 26.Franchini M, Velati C. The use of novel oral anticoagulants: the debate continues! Blood Transfus. 2015;13:170–1. doi: 10.2450/2015.0059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prandoni P. The treatment of venous thromboembolism with novel oral anticoagulants: warnings and limitations. Blood Transfus. 2015;13:178–80. doi: 10.2450/2015.0002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riva N, Ageno W. Which patients with venous thromboembolism should receive non-vitamin K antagonist oral anticoagulants? The majority. Blood Transfus. 2015;13:181–3. doi: 10.2450/2015.0057-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripodi A, Ageno W, Ciaccio M, et al. Position paper on laboratory testing for patients on direct oral anticoagulants. A consensus document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus. 2018;16:462–470. doi: 10.2450/2017.0124-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 31.Franchini M, Liumbruno GM, Bonfanti C, Lippi G. The evolution of anticoagulant therapy. Blood Transfus. 2016;14:175–84. doi: 10.2450/2015.0096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imberti D, Pomero F, Benedetti R, Fenoglio L. Safety and efficacy of direct oral anticoagulants for extended treatment of venous thromboembolism. Intern Emerg Med. 2016;11:895–900. doi: 10.1007/s11739-016-1521-8. [DOI] [PubMed] [Google Scholar]
- 33.Sindet-Pedersen C, Pallisgaard JL, Olesen JB, et al. Safety and efficacy of direct oral anticoagulants compared to warfarin for extended treatment of venous thromboembolism -a systematic review and meta-analysis. Thromb Res. 2015;136:732–8. doi: 10.1016/j.thromres.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 35.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 36.Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 37.Sardar P, Chatterjee S, Mukherjee D. Efficacy and safety of new oral anticoagulants for extended treatment of venous thromboembolism: systematic review and meta-analyses of randomized controlled trials. Drugs. 2013;73:1171–82. doi: 10.1007/s40265-013-0082-7. [DOI] [PubMed] [Google Scholar]
- 38.Raskob G, Ageno W, Cohen AT, et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. 2016;3:e228–36. doi: 10.1016/S2352-3026(16)00023-5. [DOI] [PubMed] [Google Scholar]
- 39.Janssen Pharmaceuticals, Inc. FDA Approves new 10 mg dosing for XARELTO® (rivaroxaban) to reduce the continued risk of venous thromboembolism (VTE) [Accessed on 01/06/2018]. Available at: https://www.jnj.com/media-center/press-releases/fda-approves-new-10-mgdosing-for-xarelto-rivaroxaban-to-reduce-the-continued-risk-ofvenous-thromboembolism-vte.
- 40.Europeran Medicine Agency. Xarelto: EPAR - Summary for the public. [Accessed on 01/06/2018]. Availabe at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_the_public/human/000944/WC500057109.pdf.
- 41.Prins MH, Lensing AWA, Prandoni P, et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv. 2018;2:788–96. doi: 10.1182/bloodadvances.2018017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prandoni P, Lensing AWA, Prins MH, et al. Benefits and risks of extended treatment of venous thromboembolism with rivaroxaban or with aspirin. Thromb Res. 2018;168:121–9. doi: 10.1016/j.thromres.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Khorana A, Weitz J. Treatment challenges in venous thromboembolism: an appraisal of rivaroxaban studies. Thromb Haemost. 2018;118:S23–33. doi: 10.1160/TH17-09-0681. [DOI] [PubMed] [Google Scholar]
- 44.Vasanthamohan L, Boonyawat K, Chai-Adisaksopha C, Crowther M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2018;16:1288–95. doi: 10.1111/jth.14156. [DOI] [PubMed] [Google Scholar]
- 45.Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710–6. doi: 10.1001/archinternmed.2010.367. [DOI] [PubMed] [Google Scholar]