Abstract
Aim
This research protocol addresses the development of web-based modules for the ‘Creating Opportunities for Personal Empowerment: Symptom and Technology Management Resources’ intervention with caregivers of children who require medical technology. The commonly experienced symptoms of fever and increased respiratory symptoms (coughing, wheezing, increased secretions) and the care of technologies (tracheostomy tubes, respiratory equipment and feeding tubes) are addressed in this nurse-led and nurse-developed intervention.
Design
The purpose of this study was development of the web-based intervention modules and obtaining review by expert review and caregiver reviewers using a systematic, structured process and form.
Methods
The intervention includes evidenced-based, theory-based, modules that address the child’s most common emotions and behavioral responses with the management of their symptoms and technologies using a web-based format. To establish fidelity of the intervention, expert reviewers and caregiver reviewers (e.g., caregivers of children with multiple complex chronic illnesses and technologies) will review the modules that will then be refined prior to feasibility testing. Funding for the study began in July 2018.
Discussion
The intervention development led by nurses entails an evidence-based literature review, development of scripts with appropriate health literacy level and content by experts, photography and videography, production of video modules and creation of a website for modules.
Impact
This nursing intervention addresses the educational needs and skills considered essential and most applicable to caregivers of children who require medical technology to improve self-management of their child’s symptoms and technology in the home setting. The information obtained from this study will be valuable to nursing, other health care providers and health care systems in planning and implementing programs and services for these children and for nurse researchers designing intervention studies for children with multiple complex chronic illnesses.
Trial registration
This study is not designated as a clinical trial per NIH/NINR study and grant proposal guidelines.
Keywords: complex chronic illness, children, caregivers, medical technology, tracheostomy, feeding tube, technological dependence, intervention development, nursing
Introduction
Children who require medical technology have multiple complex chronic conditions and are part of the estimated 11 million children (15%) with special health care needs in the United States (U.S.; 2009–2010 National Survey of Children with Special Health Care Needs, Child and Adolescent Health Measurement Initiative, 2011). While exact estimates of the numbers of these children internationally are unknown, populations of children who require various medical technologies have been described in Ireland (Nicholl, Doyle, Moran, & Guilfoyle, 2013), Canada (Amin, Sayal, Syed, Chaves, Moraes, & MacLusky, 2014) and Brazil (Schweiger, Manica, Becker, Abreu, Manzini, Sekine, & Kuhl, 2017). This medical technology (e.g., tracheostomy and feeding tubes) enables children to live at home. They require intensive, specialized care from informal caregivers (e.g., parents, foster/adoptive parents, grandparents) who need to be acutely aware of changes in the child’s condition, including symptoms experienced by the child and maintain familiarity with the medical technology used by the child. Despite diligence in care by caregivers, these children have frequent emergency department (ED) visits and hospitalizations due to commonly experienced symptoms and commonly used technologies at home in the U.S. and other countries (Lindahl, & Lindblad, 2013; Musil, Zauszniewski, Burant, Toly, & Warner, 2015; Russell & Simon, 2014; Spratling, 2017). When presenting to the ED, children are often admitted to the hospital due to the complexity of their multiple chronic conditions and their need for medical technology at home. When stable and well, they require varying amounts and types of technological intervention and care at home throughout the day and night. Improving caregivers’ education and self-management of their child’s symptoms and medical technology at home may contribute to fewer disruptive, avoidable ED visits and hospitalizations.
To address the educational and skill needs identified for caregivers of children who require medical technology, a web-based intervention was developed by nurses to improve caregivers’ management of their child’s symptoms and medical technology needs at home. This intervention, entitled ‘Creating Opportunities for Personal Empowerment: Symptom and Technology Management Resources’ (COPE-STAR), focuses on commonly experienced symptoms and medical technology used at home by caregivers. COPE-STAR builds on a previously developed, reproducible intervention program initially designed to improve the care of hospitalized, critically ill children and their parents (Melnyk, 1994; Melnyk, Alpert-Gillis, Hensel, Cable-Billing, & Rubenstein, 1997). The web-based modules developed for the study are topics identified by caregivers in previous nursing studies examining electronic health records (EHR) and caregivers’ perspectives at home (Spratling, 2017; Spratling & Lee, 2019) and are essential and applicable to care for most children using medical technology. COPE-STAR was designed to improve caregivers’ self-management of their child’s symptoms and medical technology in the home setting. COPE-STAR includes the child’s most common behavioral responses and provides suggestions for caregivers on how they can best help their child with care at home using a web-based format that is compatible with computers, tablets and handheld devices or smart phones and with a targeted lower literacy level. COPE-STAR is evidence-based and developed by nurses using advice from content experts, a health literacy expert and caregivers, called caregiver reviewers, that evaluated the web-based intervention content prior to feasibility testing.
Background
Children who require medical technology have multiple, complex chronic conditions and often are geographically distant from health care specialists that can best monitor and manage health problems (Spratling, 2017). The children’s health care entails many primary and specialty clinic visits, emergency department (ED) visits and hospitalizations for acute exacerbations. Our preliminary nursing research indicated that 37% of these ED visits and hospitalizations are preventable (Spratling). These ED visits and hospitalizations are costly and disruptive to the daily lives of the children and families (Cohen, Kuo, Agrawal, Berry, Bhagat, Simon, & Srivastava, 2011; Hudson, Mueller, Hester, Magwood, Newman, & Laken, 2014; Lindahl, & Lindblad, 2013; Russell & Simon, 2014). Other preliminary nursing research explored health care needs from caregivers’ perspectives who described symptoms and technology problems at home (Spratling & Lee, 2019). Caregivers of children who require medical technology reported that they have initial education about their child’s care prior to discharge; however, reinforcement and more skills are needed (Spratling & Lee). In addition, caregivers of children with multiple complex chronic conditions requiring medical technology experience anxiety and depressive symptoms and have doubts in their beliefs about managing their child’s care (Musil et al., 2015; Oswalt, Bonds McClain, & Melnyk, 2013; Toly & Musil, 2015; Toly, Musil, & Zauszniewski, 2014). Thus, it is important for nurses to enhance caregivers’ self-management for their children who require medical technology.
Theoretical framework
COPE-STAR is based on the Creating Opportunities for Personal Empowerment (COPE) intervention which is an existing theory-based, reproducible intervention program that uses a combination of Self-Regulation Theory (Johnson, 1999), control theory (Carver & Scheier, 1982) and the emotional contagion hypotheses (Jimerson, 1987). The COPE intervention was developed by a nurse and has been effective in nursing studies in increasing parental beliefs about their child’s care, decreasing anxiety and depression in caregivers and parents, improving parent interaction with their child and improving parent and caregiver support during illness (Lu et al., 2016; Melnyk, Amaya, Szalacha, Hoying, Taylor, & Bowersox, 2015; Militello, Melnyk, Hekler, Small, & Jacobson, 2016). The COPE intervention has previously been used in web-based format in other populations (Melnyk et al.; Militello et al.).
COPE focuses on caregivers using concrete, objective information about the care needed for the child. The intervention focuses on enhancing behaviors and thus reduces the discrepancy between caregiver expectations and actual caregiver role in caring for their child. Caregivers are better able to understand the experience of the child and manage future experiences with their child’s care. The COPE intervention includes education and skills about the child’s health problems and addresses the child’s most common emotions and behavioral responses with illness and hospitalization (Melnyk, 1994; Melnyk et al., 2015; Militello et al., 2016).
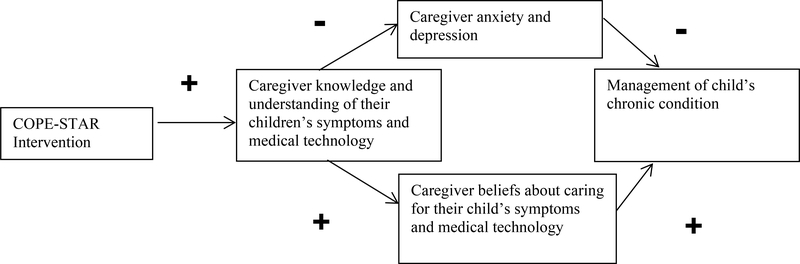
The COPE-STAR intervention extends the previous COPE intervention (Oswalt et al., 2013; Melnyk, 1994; Melnyk et al., Melnyk et al., 1997; Militello et al., 2016) by: 1) focusing the intervention for caregivers of children who require medical technology based on their needs for education; 2) incorporating the use of a web-based platform accessible regardless of geographic location; 3) providing caregivers with tailored education on how to manage common symptoms and medical technology problems for children who require medical technology; 4) incorporating content and education at the health literacy levels of caregivers; and 5) obtaining measures of caregiver management of chronic conditions and beliefs and reducing negative caregiver psychosocial outcomes of anxiety and depression (see Figure 1). This intervention includes the child’s most common emotions and behavioral responses with the management of their symptoms and technologies at home and provide suggestions for caregivers on how they can best help their child when the child experiences symptoms or problems with medical technology. The symptoms of fever and increased respiratory symptoms (coughing, wheezing, increased secretions) and the care of technologies (tracheostomy tubes, respiratory equipment and feeding tubes) are addressed in the intervention. These symptoms and technologies were the most commonly found concerns and described by the caregivers’ themselves in our previous work (Spratling, 2017; Spratling & Lee, 2019).
Figure 1.
COPE-STAR theoretical model
Intervention format
The intervention modules were developed on a web-based platform so that future modules can be easily added to COPE-STAR. The modules are in a web-based platform that can be accessed using a computer, tablet or handheld device, or smart phone. A secure, password accessible website is being used for the modules. The website exists as a framework for the modules and an initial platform where modules can be easily added to expand the intervention in the future. The principal investigator (PI) who is a nurse worked with graphic designers, videographers, film production experts and information technologists in the university to develop the video modules. The module design incorporated PowerPoint summary content and video demonstrations. Video demonstrations use simulation patients and live models acting as caregivers, not actual patients.
Module content and development
The COPE-STAR intervention consists of six web-based modules developed for the management of common symptoms (fever, increased respiratory symptoms, cough, wheeze, increased secretions), common technologies (tracheostomy tubes, respiratory equipment, feeding tubes) and a module for community resources. The focus and key points of the modules are summarized in Table 1. These videos also include the child’s most common emotions and behavioral responses with the management of their symptoms and technologies and suggestions for caregivers on how they can best help their child with management of care at home. For example, the tracheostomy tube care module includes information on what to look for in responses of the child as well as the steps of tracheostomy stoma care.
Table 1.
COPE-STAR module content.
| Module title | Module focus | Modules include key points using expert explainations and/or actual demonstrations, review of child’s most common emotions and behavioral responses, and suggestions for caregivers on how they can best help their child with care at home • Contacting emergency services for emergency and when to seek emergent care (*at the beginning of all modules) |
| Fever | Fever and associated symptoms | • Obtaining accurate temperature (oral, axillary, rectal, forehead, ear) • Fever interventions (fluids, rest, cool cloths/lukewarm bath) • Fever medications (general use) • Concerning symptoms associated with fever (lethargy, pallor, neck or abdominal pain) |
| Increased respiratory symptoms | Respiratory symptoms (coughing, wheezing, increased respiratory/tracheal secretions) | • Symptom reducing interventions • Respiratory medications (general use) • Concerning associated symptoms (blood in secretions, pallor/cyanosis) |
| Tracheostomy tubes | Tracheostomy tube care | • Tracheostomy tube basics ○ Stoma care ○ Suctioning • Tracheostomy tube change • Distracting child during tube change • Troubleshooting (increased respiratory secretions, tracheal plugging) |
| Respiratory equipment | Use of nebulizer, oxygen, humidifier, and pulse oximeter | • Respiratory medications (general use) • Delivering oxygen and humidification • Troubleshooting (alarms, settings, connections) |
| Feeding tubes | Feeding tube care | • Gastrostomy and jejunostomy, tube basics • Replacing tube as needed • Distracting child during tube replacement • Troubleshooting (clogged or dislodged tube) |
| Resources | Rehabilitation therapies and community resources | • Speech, occupational, and physical therapies overview • Common health care providers in specialty care (nurse practitioners, physician assistants, and physicians) |
Caregivers are directed to seek emergent care in the event of an emergency or life-threatening event at the beginning of all modules. The focus of these modules is routine care of common symptoms and medical technologies and reinforcement of education received by caregivers prior to hospital discharge. The use of the modules in an emergency is discouraged for the safety and well-being of the child. Each module is approximately five to seven minutes in time length. Each module has reflective questions for caregivers about module content to engage participants.
The Study
Aims
The initial study aim is the content development of the web-based COPE-STAR intervention modules at lower health literacy levels. The focus is obtaining fidelity and usability through expert and caregivers’ review using a systematic, structured process. The secondary aim in the future includes feasibility testing that will examine caregiver acceptance and caregiver outcomes of management of the child’s chronic condition, caregiver beliefs about caring for the child’s symptoms and medical technology, anxiety and depressive symptoms.
Design
This nursing intervention study will include the development of the web-based COPE-STAR intervention modules. Subsequent feasibility testing will use a using a quasi-experimental one-group design with measurements at baseline and at 4 weeks post-intervention in a sample of caregivers of children ages 1–5 years who require medical technology.
Setting
Caregivers will be recruited from a clinic at a regional pediatric health care system in the Southeast that serves over 200 patients. All patients seen at this clinic require a tracheostomy and our preliminary work noted that 88% require a feeding tube as well (Spratling, 2017). Successful recruitment strategies used in prior studies that are being used in this study are: 1) recruitment cards mailed by the nurse PI and also provided to potential participants by health care providers and staff at clinic visits; 2) health care provider referrals to the PI where providers and staff will notify the PI of potential participants so that the PI or research assistants may contact and/or be present at clinic visit; and 3) PI availability at clinic to discuss study and prescreen potential participants (Spratling, 2013).
Sample participants
Caregivers will be the self-identified primary caregivers of an active clinic patient ages 1 to 5 years requiring both a tracheostomy and feeding tube. Inclusion criteria are caregivers: 1) who are either parents, foster or adoptive parents, grandparents, or any other non-compensated adult providing care to children ages 1–5 years who require both a tracheostomy and feeding tube in the home setting; 2) must be age 18 or older; 3) must be able to read, write, speak and understand English; and 4) must have access to a computer, tablet or handheld device, or smart phone. Exclusion criteria include those who are employed as paid caregivers (home nurses, home health aides) and those who have been providing care less than 1 year.
Sample size determination
We will recruit a convenience sample of 6 caregiver reviewers and later 30 caregiver participants, for a total of 36 caregivers. To clarify, caregiver reviewers are part of expert review for intervention fidelity in the initial aim. Caregiver participants are for feasibility testing in the secondary aim in the near future. We believe this sample size is adequate for assessing the feasibility of the COPE-STAR intervention based on prior recruitment by the PI and dialogue with co-investigators, including our biostatistician and consultants. Since this is a feasibility study, limited preliminary outcome data precluded sample size calculations using a power analysis (Kistin & Silverstein, 2015; Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006; Leon, Davis, & Kraemer, 2011).
Expert review and caregiver reviewers
To establish fidelity of COPE-STAR modules, the modules will be reviewed by content experts and caregivers of children with multiple complex chronic illnesses and technologies, called caregiver reviewers and refined prior to feasibility testing. Once the web-based modules are developed and reviewed by the nurse PI and co-investigators in nursing and health literacy, they will be reviewed by nurse consultants with expertise in the COPE intervention and children who require medical technology, nurse practitioners and physicians that are clinical experts, information technology specialists, caregiver reviewers and graduate research assistants who are also nurses (GRAs). Caregiver or parent reviewers have been used to assess content and usability in development of interventions for parents in skills training for chronic pain in youth (N = 6; Palermo, Law, Essner, Jessen-Fiddick, & Eccleston, 2014), interventions for adolescents with epilepsy (N = 9; Wagner, Smith, Ferguson, van Bakergem, & Hrisko, 2011) and development of instruments for interventions in caregivers of those with head and neck cancer (N = 13; Bond et al., 2016). Six caregiver reviewers will review modules in the study. They will be provided information about the study, provide written informed consent and complete demographic and health literacy measure (described later). In addition to completing the COPE-STAR expert review feedback form, they will also be asked to make notes of any words they do not know or understand. The expert reviewers and caregiver reviewers will systematically review the modules using the COPE-STAR expert review feedback form (described below). The feedback will be incorporated into the final modules that will be used for feasibility testing with caregiver participants in the secondary aim.
The COPE-STAR expert review feedback form was adapted from an existing investigator-developed rating form used in the web-based care partner focused intervention (CARE-CITE) and based on a form for the Telephone Assessment and Skill-Building Kit (TASK) intervention, both for caregivers (Bakas, Farran, Austin, Given, Johnson, & Williams, 2009; Blanton, Clark, & Dunbar, 2017; Lu et al., 2016). All reviewers, experts and caregivers, will use the form to assess the intervention content and the modules in areas of Usefulness, Ease of Use and Acceptability (Blanton et al., 2017). The form includes questions about time required to review and complete each module and Likert scales specific to questions on accuracy of specific module content, ease of use, helpfulness, accuracy and acceptability for caregivers of children who require medical technology. The form concludes with open-ended questions on helpfulness, areas of concern and recommendations for module improvement. The COPE-STAR expert review feedback form was reviewed by both authors of existing expert review instruments (Bakas et al.; Blanton et al.). The expert review feedback data will be summarized and analyzed for recommended changes to COPE-STAR intervention. Final revisions will be made to the COPE-STAR intervention based on expert review feedback prior to implementation of the feasibility study in the secondary aim.
Measures
Caregiver and child characteristics and caregiver health literacy will be obtained from the caregiver reviewers.
Caregiver and child characteristics
Caregiver and child characteristics will be measured using self-report. Caregiver and child characteristics will be examined to describe the health care needs of the child who requires medical technology and the demographics of the caregiver. This questionnaire has been previously used by the PI in studies with caregivers of children who require medical technology, capturing essential data about the caregiver and the health care needs of the child. Caregiver characteristics include household income, number of persons living in the home, duration of caregiving and caregiver age, sex, education and race/ethnicity. Child characteristics include age, sex, race/ethnicity, medical diagnoses, medical technologies required, length of time the child has required medical technologies and other care (trained caregivers, home nursing care and rehabilitation therapies).
Caregiver health literacy
Caregiver health literacy will be measured with the Newest Vital Sign (NVS; Weiss et al., 2005). The NVS measures three different components of health literacy for adults – prose, numeracy and documents. A participant reads an ice cream label and answers six questions relating to the label. The NVS is normed for ages 18 and older. Construct validity has been established with existing health literacy measures and the internal consistency reliability is .76 (Rowlands, Khazaezadeh, Oteng-Ntim, Seed, Barr, & Weiss, 2013; Weiss et al., 2005).
Data analysis
Descriptive statistics will be used to summarize responses on the expert review feedback form, sample characteristics and the health literacy questionnaire (NVS). Data will be analyzed using SPSS Version 24 with guidance and support from a member of the research team who is a biostatistician.
Future module feasibility testing
The secondary aim which will be commencing in the near future focuses on the acceptance and overall effectiveness of COPE-STAR by caregivers. Intervention recruitment, retention and adherence rates will be measured, and caregiver satisfaction will be measured using an investigator-developed COPE-STAR satisfaction questionnaire and an investigator-developed exit interview. In addition, the measures of management of child’s chronic condition, caregiver beliefs about the child’s symptoms and medical technology, anxiety and depressive symptoms, health literacy and caregiver and child characteristics will be evaluated as process measures of the intervention.
Ethical considerations
Institutional Review Boards (IRB) approval have been obtained from the health care system and the university. All participants must meet study eligibility criteria and complete the written informed consent process. All data will be coded by participant study identification (ID) numbers. Only the PI will have access to each participant’s identity. ID numbers will be stored separately from consent forms. Research Electronic Data Capture (REDCap) will be used to capture and manage data (Harris, Taylor, Thielke, Payne, Gonzalez, & Conde, 2009).
Considering the study rationale, population, procedures and the risk, this study is considered minimal risk. Although likely that there will be no adverse events during the study period, we will monitor for adverse events and we have proposed a Data Safety and Monitoring Plan, which details the response to any adverse events. Because a measure of depressive symptoms will be obtained, referrals to health care providers will be made for any concerning results (i.e., score ≥ 16 on CES-D considered at risk for possible depression). There will be a statement in the consent form that indicates if the participant responses indicate that they may benefit from further evaluation for depression, they will be referred to their health care provider. Caregivers will also be directed to seek emergent care in the event of an emergency or life-threatening event at the beginning of all modules; their use in an emergency situation is discouraged for the safety and well-being of the child.
Rigour
Rigour in this study is evident in the development of a theory-based intervention for caregivers of children who require medical technology, implementation of the intervention and the experienced nursing research team. The COPE-STAR intervention is based on an existing theory-based COPE intervention that has been reproduced in a variety of settings and populations in nursing studies. In addition, the modules have been developed and stored with details of modules and intervention so that others may use in the future. We are conducting two home visits for data collection to reduce missing data. We are also calling participants weekly to remind them to review modules and answer questions. All data collected is checked by the PI after data collection is completed by the PI or trained research assistants. Any inconsistencies in the data collected are discussed by the research team and addressed accordingly.
Further, this study will take a rigorous approach to intervention development and has content experts, a health literacy expert and caregivers that will evaluate the web-based intervention content prior to feasibility testing. Existing online videos often are informal, and layperson developed, not evidence-based and without evidence of expert review. Some are only actual demonstrations of using technologies without addressing how to care for the child. COPE-STAR will also use a multi-media, web-based format that is compatible with mobile devices (smart phones and tablets).
Discussion
Thus far, the study has focused on intervention development and expert review, including recruitment and enrollment of six caregiver reviewers. The COPE-STAR intervention development began with a thorough review of the evidence-based literature, which was summarized and translated into scripts using caregiver language. The scripts were reviewed by a health literacy expert (co-investigator), nursing experts in interventions (co-investigator, consultants) and clinical experts, including nurses and nurse practitioners, in pediatric complex care and use of medical technology at home for content (co-investigator, consultants). The modules were developed by nurses based on the scripts using videography, photography, PowerPoint file and voice-over actors. The modules were produced and loaded onto a web-based platform. Then, experts in nursing interventions with children with chronic illness, including the creator of the COPE intervention, nursing care experts of children who require medical technology, clinical experts described above and clinic physicians and nurse practitioners and GRAs who are nurses and nursing student volunteers reviewed the modules and provided feedback. In addition, six caregiver reviewers were recruited and enrolled for initial feedback on the intervention as part of the expert review.
In preparation for feasibility testing, study accomplishments have included the development of a detailed procedures manual and intervention integrity format, establishment of a data management program in Research Electronic Data Capture (REDCap) and recruitment and enrollment of caregiver reviewers as participants.
The COPE-STAR intervention development entailed an evidence-based literature review, development of scripts that were reviewed for health literacy level and content by experts, photography and videography, production of modules and creation of a website for modules. The intervention required approximately nine months to develop; six months of time had been projected in the study proposal. At this time, intervention development and expert review has been completed and feasibility testing will be commencing soon.
Limitations
Developing the modules required substantial time. A detailed project-time line with benchmarks was set up at the beginning of the study. The PI has closely monitored the timeline and study activities to maintain necessary progress. Recruitment of these caregivers can be challenging as this is a small population; however, there is a network of clinics in the healthcare system that provides care to children with multiple chronic conditions and if needed, we will expand recruitment to other clinics. In addition, the PI has experience in recruitment and success with this population using a variety of recruitment strategies and has conducted studies with children and adolescents who require medical technology and their caregivers. The research team also has experience with recruitment and data collection with similar populations.
The sample of caregivers are from the same state, in a clinic with providers in the same health care system and insured by entities in the same state; thus, these caregivers may experience a similar trajectory of care, including training in the hospital, hospital length of stay and discharge based on hospital policies and insurance guidelines. These similarities may affect caregiver education at discharge and follow up and subsequently caregivers’ self-management of symptoms for their children who require medical technology at home. However, the focus of this study is to improve caregivers’ management of their child’s symptoms and medical technology at home common to all caregivers.
Conclusion
The COPE-STAR intervention addresses the caregivers of children who require medical technology to improve self-management of their child’s symptoms and technology in the home setting. This nursing intervention provides education to the caregivers who provide intensive, specialized care to their children. The initial intervention modules developed for the study are those educational needs and skills considered essential and most applicable to these children; however, the modules have been developed so that modules can be easily added for use with a wider caregiver audience in future nursing studies. For example, additional modules might address education prior to hospital discharge for caregivers of children who require additional medical technology such as infusion pumps for medication delivery. Other essential module topics may include related education, support, skill building and training following discharge from the hospital. Future goals include expanding the use of this nursing intervention to additional centers with populations of children with multiple, complex chronic conditions and special health care needs. In addition, the knowledge obtained from this study will be valuable to nursing, other health care providers and health care systems in planning and implementing programs and services for these children, specifically tailored interventions for caregivers of children who require medical technology. For nurse researchers, the current study may be a useful guide in design of nurse-led and nurse-developed intervention studies using web-based formats for a variety of populations of children with multiple complex chronic illnesses.
Acknowledgement of funding support
Funding was provided by the National Institute of Nursing Research, National Institutes of Health Grant Number 1R15NR018037-01.
Footnotes
Anonymised Conflict of Interest Statement
The authors declare no conflicts of interest.
Disclosures
The authors declare no conflicts of interest.
Contributor Information
Regena SPRATLING, School of Nursing, Byrdine F. Lewis College of Nursing and Health Professions, Georgia State University, 140 Decatur Street, Urban Life Building Room 950, Atlanta, GA 30303, USA.
Melissa Spezia FAULKNER, School of Nursing, Byrdine F. Lewis College of Nursing and Health Professions, Georgia State University.
Iris FEINBERG, Adult Literacy Research Center, College of Education and Human Development, Georgia State University.
Matthew J. HAYAT, Department of Population Health Sciences, School of Public Health, Byrdine F. Lewis College of Nursing & Health Professions (Joint), Georgia State University.
References
- Amin R, Sayal P, Syed F, Chaves A, Moraes TJ, & MacLusky I (2014). Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatric Pulmonology, 49(8), 816–824. 10.1002/ppul.22868 [DOI] [PubMed] [Google Scholar]
- Bakas T, Farran C, Austin J, Given B, Johnson E, & Williams L (2009). Content validity and satisfaction with a stroke caregiver intervention program. Journal of Nursing Scholarship, 41(4), 368–375. doi: 10.1111/j.1547-5069.2009.01282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton S, Clark P, & Dunbar S (2017). Feasibility of a carepartner integrated telehealth rehabilitation program for persons with stroke: A case series. American Physical Therapy Association Combined Sections Meeting; San Antonio, Texas. [Google Scholar]
- Bond SM, Schumacher K, Sherrod A, Dietrich MS, Wells N, Lindau RH, & Murphy BA (2016). Development of the Head and Neck Cancer Caregiving Task Inventory. European Journal of Oncology Nursing, 24, 29–38. doi: 10.1016/j.ejon.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Carver CS, & Scheier MF (1982). Control theory: A useful conceptual framework for personality-social, clinical and health psychology. Psychological Bulletin, 92(1), 111–135. [PubMed] [Google Scholar]
- Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SM, Simon TD, & Srivastava R (2011). Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics, 127(3), 529–538. doi: 10.1542/peds.2010-0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SM, Mueller M, Hester WH, Magwood GS, Newman SD, & Laken MA (2014). At-risk characteristics for hospital admissions and ED visits. Journal for Specialists in Pediatric Nursing, 19, 183–193. 10.1111/jspn.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM SPSS Inc. (2016). SPSS Base 24.0 for Windows User’s Guide. IBM SPSS Inc., Chicago IL. [Google Scholar]
- Jimerson SS (1987). Patterns of anxiety In Haber J, Hoskins PP, Leach AM, & Sideleau BF (Eds.), Comprehensive Psychiatric Nursing (pp. 545–563). New York: McGraw-Hill. [Google Scholar]
- Johnson JE (1999). Self-regulation theory and coping with physical illness. Research in Nursing & Health, 22(6), 435–448. [DOI] [PubMed] [Google Scholar]
- Leon AC, Davis LL, & Kraemer HC (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629. doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl B, & Lindblad B (2013). Being the parent of a ventilator-assisted child: Perceptions of the family–health care provider relationship when care is offered in the family home. Journal of Family Nursing, 19(4), 489–508. doi: 10.1177/1074840713506786 [DOI] [PubMed] [Google Scholar]
- Lu YY, Ellis J, Yang Z, Weaver MT, Bakas T, Austrom MG, & Haase JE (2016). Satisfaction with a Family-Focused Intervention for mild cognitive impairment dyads. Journal of Nursing Scholarship, 48(4), 334–344. doi: 10.1111/jnu.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, & Yesavage JA (2006). Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry, 63(5), 484–489. doi: 10.1001/archpsyc.63.5.484 [DOI] [PubMed] [Google Scholar]
- Kistin C, & Silverstein M (2015). Pilot studies: A critical but potentially misused component of interventional research. JAMA: Journal of the American Medical Association, 314(15), 1561–1562. doi: 10.1001/jama.2015.10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk BM (1994). Coping with unplanned childhood hospitalization: Effects of informational interventions on mothers and children. Nursing Research, 43(1), 50–55. [PubMed] [Google Scholar]
- Melnyk BM, Alpert-Gillis LJ, Hensel PB, Cable-Billing RC, & Rubenstein J (1997). Helping mothers cope with a critically ill child: A pilot test of the COPE intervention. Research in Nursing and Health, 20, 3–14. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Amaya M, Szalacha LA, Hoying J, Taylor T, & Bowersox K (2015). Feasibility, acceptability and preliminary effects of the COPE Online Cognitive-Behavioral Skill-Building Program on mental health outcomes and academic performance in Freshmen College Students: A Randomized Controlled Pilot Study. Journal of Child & Adolescent Psychiatric Nursing, 28(3), 147–154. doi: 10.1111/jcap.12119 [DOI] [PubMed] [Google Scholar]
- Militello LK, Melnyk BM, Hekler E, Small L, & Jacobson D (2016). Correlates of healthy lifestyle beliefs and behaviors in parents of overweight or obese Preschool children before and after a Cognitive Behavioral Therapy Intervention with text messaging. Journal of Pediatric Healthcare, 30(3), 252–260. doi: 10.1016/j.pedhc.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Musil CM, Zauszniewski JA, Burant CJ, Toly VB, & Warner CB (2015). Evaluating an online Resourcefulness Training Intervention pilot test using six critical parameters. International Journal of Aging & Human Development, 82(1), 117–135. doi: 10.1177/0091415015623552 [DOI] [PubMed] [Google Scholar]
- National Survey of Children with Special Health Care Needs. NS-CSHCN 2009/10. Data query from the Child and Adolescent Health Measurement Initiative, Data Resource Center for Child and Adolescent Health website. Retrieved September 1, 2019 from www.childhealthdata.org.
- Nicholl H, Doyle C, Moran S, & Guilfoyle M (2013). Identifying the types of technology that are used by children with intellectual disabilities and associated complex needs living at home in Ireland. British Journal of Learning Disabilities, 41(3), 229–236. 10.1111/bld.12045 [DOI] [Google Scholar]
- Oswalt KL, Bonds McClain D, & Melnyk B (2013). Reducing anxiety among children born preterm and their young mothers: The American Journal of Maternal Child Nursing, 38(3), 144–149. doi: 10.1097/NMC.0b013e318286140c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Law EF, Essner B, Jessen-Fiddick T, & Eccleston C (2014). Adaptation of Problem-Solving Skills Training (PSST) for parent caregivers of youth with chronic pain. Clinical Practice in Pediatric Psychology, 2(3), 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands G, Khazaezadeh N, Oteng-Ntim E, Seed P, Barr S, & Weiss BD (2013). Development and validation of a measure of health literacy in the UK: the newest vital sign. BMC Public Health, 13(116), (7 February 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CJ, & Simon TD (2014). Care of children with medical complexity in the hospital setting. Pediatric Annals, 43(7), e157–62. doi: 10.3928/00904481-20140619-09 [DOI] [PubMed] [Google Scholar]
- Schweiger C, Manica D, Becker CF, Abreu LSP, Manzini M, Sekine L, & Kuhl G (2017). Tracheostomy in children: a ten-year experience from a tertiary center in southern Brazil. Brazilian Journal of Otorhinolaryngology, 83(6), 627–632. 10.1016/j.bjorl.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratling R (2013). Recruitment of medically fragile children and adolescents: Lessons learned from qualitative research. Journal of Pediatric Health Care, 27(1), 62–65. doi.org/ 10.1016/j.pedhc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Spratling R (2017). Understanding the health care utilization of children who require medical technology: A descriptive study of children who require tracheostomies. Applied Nursing Research, 34, 62–65. 10.1016/j.apnr.2017.02.017 [DOI] [PubMed] [Google Scholar]
- Spratling R & Lee J (2019). Caregivers Experiences in Symptom Management for Their Children Who Require Medical Technology at Home. Journal for Specialists in Pediatric Nursing, manuscript accepted for publication. [DOI] [PubMed] [Google Scholar]
- Toly VB, & Musil CM (2015). Factors related to depressive symptoms in mothers of technology-dependent children. Issues in Mental Health Nursing, 36(7), 518–527. doi: 10.3109/01612840.2015.1009662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toly VB, Musil CM, & Zauszniewski JA (2014). Resourcefulness training intervention: A promising approach to improve mental health of mothers with technology-dependent children. Applied Nursing Research, 27, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JL, Smith G, Ferguson P, van Bakergem K, & Hrisko S (2011). Feasibility of a pediatric cognitive-behavioral self-management intervention: Coping openly and personally with epilepsy (COPE). Seizure, 20(6), 462–467. doi: 10.1016/j.seizure.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Weiss B, Mays M, Martz W, Castro K, DeWalt D, Mockbee J & Hale F (2005). Quick assessment of literacy in primary care: The newest vital sign. Annals of Family Medicine 3(6), 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]