Abstract
Opioids are widely prescribed for chronic pain, including neuropathic pain, despite growing evidence of long-term harm. Previous preclinical studies have documented exacerbation of nociceptive hypersensitivity, including that induced by peripheral nerve injury, by morphine. The present series of behavioral studies sought to replicate and extend our prior research, which demonstrated a multimonth exacerbation of nociceptive hypersensitivity by a 5-day course of morphine initiated 10 days after nerve injury. The current studies demonstrate that enduring exacerbation of nociceptive hypersensitivity is not restricted to morphine, but rather is also created by the clinically relevant opioids fentanyl and oxycodone when these are likewise administered for 5 days beginning 10 days after nerve injury. Furthermore, enduring exacerbation of nociceptive hypersensitivity is also observed when the same dosing regimen for either morphine, fentanyl, or oxycodone begins 1 month after nerve injury. Finally, a striking result from these studies is that no such exacerbation of nociceptive hypersensitivity occurs when either morphine, fentanyl, or oxycodone dosing begins at the time of nerve injury. These results extend our previous findings that morphine exacerbates nociceptive hypersensitivity to the clinically relevant opioids fentanyl and oxycodone when administered after the development of nociceptive hypersensitivity, while also providing possible clinically relevant insight into when these opioids can be safely administered and not exacerbate neuropathic pain.
Keywords: rats, oxycodone, fentanyl, morphine, neuropathic pain, chronic constriction injury, behavior, allodynia
1. Introduction
Approximately one-third of Americans suffer from chronic pain, and this proportion is increasing.41,43 Chronic pain, including neuropathic pain from peripheral nerve injury, is frequently treated with opioids.3,12,41 Despite widespread opioid prescriptions, there is no evidence of the efficacy of long-term opioid use for management of chronic pain.6 Furthermore, there is growing evidence of harm.2,23,46,50
In addition to clinical studies, preclinical studies demonstrate that opioids may cause long-term harm in models of chronic pain. We and others have shown that opioids such as morphine can paradoxically exacerbate nociceptive hypersensitivity for weeks to months after treatment ends in a range of experimental pain models. These models include inflammatory and postoperative pain,11,14,25,35,36,52 and peripheral and centrally induced neuropathic pain.10,18–20,24,25,36 We have also shown that exacerbation of allodynia by morphine is dependent on inflammatory signaling in the spinal cord because inhibition of microglia or proinflammatory cytokines during opioid administration can prevent exacerbation of such allodynia.10,18–20 These pronociceptive spinal inflammatory signaling pathways have a well-documented role in the development and maintenance of chronic pain.15,30,34,45
Notably, the pattern-recognition receptor toll-like receptor 4 (TLR4) orchestrates the exacerbation of allodynia by morphine. This conclusion is based on the finding that morphine non-stereoselectively activates TLR4 and induces proinflammatory cytokines.17,28,29,51 We showed that blockade of TLR4 during morphine treatment prevented exacerbation of nociceptive hypersensitivity without interfering with analgesia.10,19,20 Importantly, the deleterious effects of morphine seemed to be independent of actions on mu-opioid receptors20 because opioid-induced exacerbation of allodynia still occurs: (1) despite knockdown of spinal mu-opioid receptors, and (2) on administration of (+)-morphine, which activates TLR4 but not mu-opioid receptors because the latter are highly stereoselective for (−)-opioid isomers.8
The present series of behavioral studies focus on 3 interrelated objectives. The first objective arises from our prior demonstration that morphine enhanced allodynia in Sprague-Dawley rats after sciatic chronic constriction injury (CCI).20 However, this single 4–0 diameter chromic gut suture model was constrained by a floor effect for measuring allodynia using the von Frey test. To overcome this constraint, the first objective of this study was to develop a model of morphine enhancement of CCI allodynia with greater dynamic range. The second objective arises as our previous studies of opioid exacerbation of allodynia focused exclusively on administration of morphine. It is important to define whether exacerbation of allodynia is restricted to morphine. To address this, the second objective was to extend our initial studies20 so to now examine the effect of oxycodone and fentanyl, opioids that are widely used to treat chronic pain and that activate TLR4.27,29,51 The third objective also arises from our previous studies of morphine exacerbation of nerve injury–induced allodynia because the original design was a short course (5 days) of opioid beginning on robust expression of allodynia, that is, initiating dosing 10 days after CCI. Thus, this last objective explores the important issue of whether opioid-induced exacerbation of allodynia also occurs when dosing occurs at other times relative to nerve injury.
2. Materials and methods
2.1. Animals
Pathogen-free adult male Sprague-Dawley rats (Envigo, Indianapolis, IN), 10 weeks old on arrival, were used in all experiments. Rats were pair-housed in temperature-controlled (23 ± 3°C) and light-controlled (12-hour light–dark cycle; lights on at 07:00 hours) rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder. This study adhered to the ARRIVE guidelines.
2.2. Chronic constriction injury surgery
Neuropathic pain was induced by a modification of the CCI model.1 Briefly, surgery was performed under isoflurane anesthesia at the midthigh level of the left hind leg. The shaved skin was treated with Nolvasan, and the surgery was performed aseptically. The sciatic nerve was gently isolated, and a single sterile chromic gut suture of varying diameter (5–0 and 6–0 cuticular WebGut; Medtronic, Minneapolis, MN; 7–0 chromic gut; Ethicon, Somerville, NJ) was loosely tied around the nerve. Experiment 1 tested a single 5–0, 6–0, or 7–0 chromic gut suture, based on the observations that “dose”-dependent allodynia can be achieved by varying suture diameter37 or suture number.16 All following experiments used 1 × 6–0 sutures. Rats were monitored postoperatively until fully ambulatory before being returned to their home cage.
2.3. Drug administration
Morphine, fentanyl, and oxycodone were gifted from the National Institute on Drug Abuse Drug Supply Program Division of Therapeutics and Medical Consequences, Research Triangle Institute, NC. Morphine was administered subcutaneously (s.c.) at 5 mg/kg, twice daily for 5 days.20 Oxycodone was administered s.c. at 2 mg/kg, twice daily for 5 days.9,26 Fentanyl was continuously infused at 0.01 mg/kg/h using a s.c. osmotic minipump (Alzet, Cupertino, CA, model 2001) for 5 days.38 Osmotic minipumps were implanted subcutaneously under brief isoflurane anesthesia and became active at the time of implantation. Because the half-life of fentanyl is shorter than morphine or oxycodone, fentanyl was administered through continuous infusion to avoid increased injections or more frequent withdrawals. Pilot studies were conducted to optimize dosage of oxycodone and fentanyl to confirm analgesia without observed side effects or overdose (data not shown). Drugs were prepared and are reported as free base concentrations. Equivolume saline vehicle was used as the control. Drug administration began 1 hour, 10 days, or 4 weeks after CCI surgery.
2.4. Mechanical allodynia
Rats received at least three 60-minute habituations to the test environment before behavioral testing. The von Frey test5 was performed as previously described in detail4,40 within the sciatic innervation region of the hind paws. Assessments were made before CCI surgery (baseline), before opioid administration, at day 1 after opioid completion, and then at weekly intervals. Three separate experimenters, blinded to group assignments, performed von Frey testing: experimenter #1 performed testing for the data presented in Figures 1, 2, and 3; experimenter #2, the data in Figure 4, and experimenter #3, the data in Figures 5 and 6. A logarithmic series of 10 calibrated Semmes–Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to the left vs right hind paws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs ranged from manufacturer-designated 3.61 (0.40 g) to 5.18 (15.14 g) filaments. The behavioral responses were used to calculate the absolute threshold (the 50% probability of response) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method21,49 as described previously.39,40 This fitting method allowed parametric analyses that otherwise would not be statistically appropriate.21,49
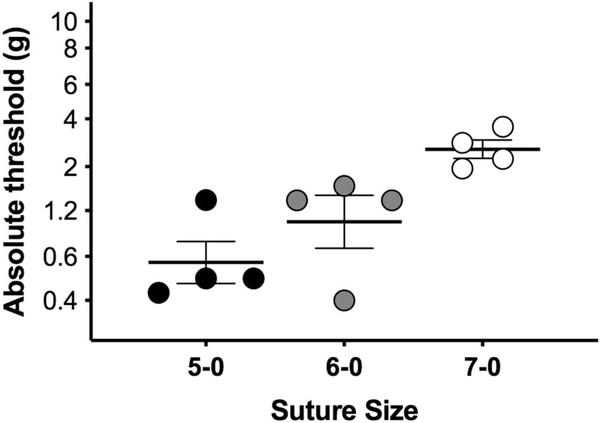
Figure 1.
“Dose”-dependent allodynia in response to sciatic CCI. Chronic constriction injury surgeries performed with one 5–0, 6–0, or 7–0 chromic gut sutures tied around the sciatic nerves of mail rats. Von Frey thresholds measured at day 14 after CCI size. n = 4/group. CCI, chronic constriction injury.
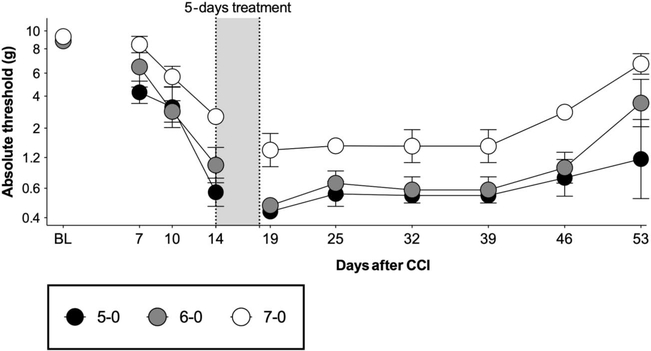
Figure 2.
Validation of the attenuated model of CCI for avoiding behavioral floor effects in the measurement of allodynia. Chronic constriction injury surgeries performed with one 5–0, 6–0, or 7–0 chromic gut suture. Morphine (5 mg/kg twice per day) was administered for 5 days, beginning at day 14 after surgery. Von Frey testing was conducted before and after CCI surgery, and after completion of opioid administration. BL: presurgery baseline. n = 4/group. CCI, chronic constriction injury.
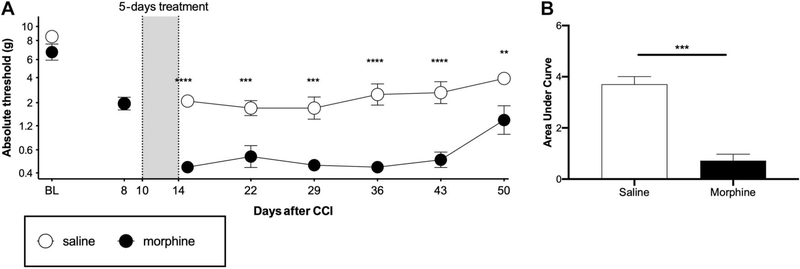
Figure 3.
Morphine exacerbates CCI allodynia when dosing begins 10 days after injury. Chronic constriction injury surgeries were performed with one 6–0 chromic gut suture. Morphine (5 mg/kg twice per day) or equivolume saline was administered for 5 days, beginning at day 10 after surgery. (A) Von Frey testing was conducted before and after CCI surgery, and after completion of opioid administration. (B) Area-under-the-curve analysis of von Frey threshold of all timepoints after morphine treatment. BL: baseline, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 6/group. CCI, chronic constriction injury.
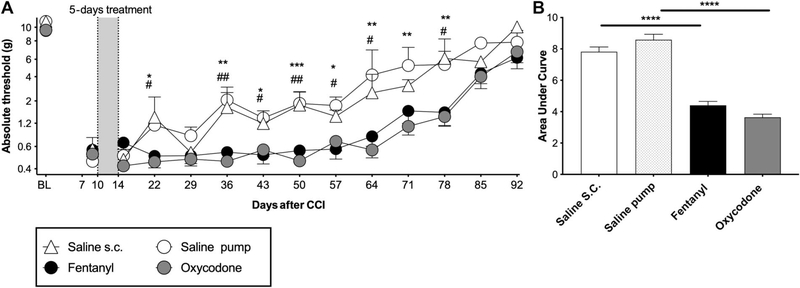
Figure 4.
Exacerbation of CCI allodynia by oxycodone and fentanyl when dosing begins 10 days after injury. Chronic constriction injury was performed with one 6–0 chromic gut suture. Fentanyl (0.01 mg/kg/h), oxycodone (2 mg/kg twice per day), or equivolume saline was administered for 5 days, beginning at day 10 after surgery. (A) Von Frey testing was conducted before and after CCI surgery, and after completion of opioid administration. (B) Area-under-curve analysis of von Frey threshold of all timepoints after opioid treatment. *Oxycodone vs all saline, #fentanyl vs all saline. BL, baseline, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 6/group. CCI, chronic constriction injury.
Figure 5.
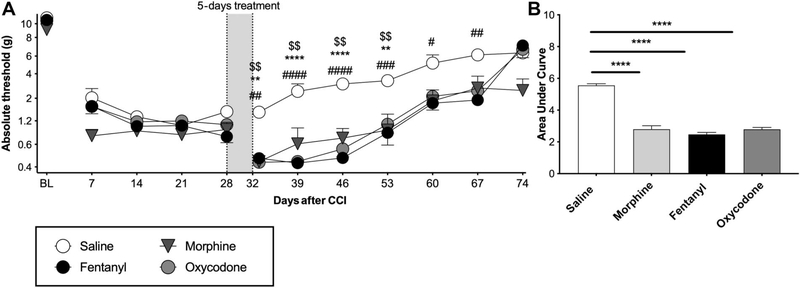
Exacerbation of CCI allodynia by morphine, oxycodone, or fentanyl when dosing begins 1 month after injury. Chronic constriction injury was performed with one chromic gut 6–0 suture. Morphine (5 mg/kg twice per day), fentanyl (0.01 mg/kg/h), oxycodone (2 mg/kg twice per day), or equivolume saline was administered for 5 days, beginning at day 28 after surgery. (A) Von Frey testing was conducted before and after CCI surgery, and after completion of opioid administration. (B) Area-under-curve analysis of von Frey threshold of all timepoints after opioid treatment. *Oxycodone vs all saline, #fentanyl vs all saline #, $morphine vs all saline, BL: baseline, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 6/group. CCI, chronic constriction injury.
Figure 6.
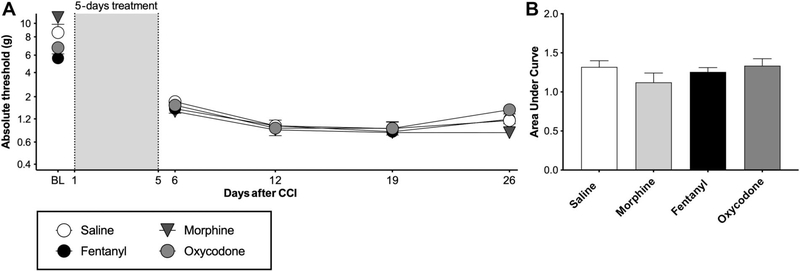
No exacerbation of CCI allodynia by morphine, oxycodone, or fentanyl when dosing begins 1 hour after injury. Chronic constriction injury was performed with one chromic gut 6–0 suture. Morphine (5 mg/kg twice per day), (fentanyl (0.01 mg/kg/h), oxycodone (2 mg/kg twice per day), or equivolume saline was administered for 5 days, beginning at one-hour after surgery. (A) Von Frey testing was conducted before and after CCI surgery, and after completion of opioid administration. (B) Area-under-curve analysis of von Frey threshold of all timepoints after opioid treatment. BL, baseline. n = 6/group. CCI, chronic constriction injury.
2.5. Statistics
Mechanical allodynia was analyzed as the interpolated 50% thresholds (absolute threshold). A Spearman correlation was used to determine the correlation between suture size and withdrawal thresholds, and a one-way analysis of variance (ANOVA) was used to determine differences between groups. One-way ANOVA was used to determine baseline differences between groups. One-way repeated-measures ANOVA was used to determine the effect of morphine on mechanical allodynia, compared with pretreatment thresholds, in suture dose–response tests. Differences in mechanical allodynia between treatment groups after opioid administration were determined using two-way repeated-measures ANOVA followed by Sidak post hoc test of opioids vs saline control. Unpaired t-tests were used to analyze area under curve for opioids vs saline control treatments.
3. Results
3.1. Behavioral characterization of morphine-potentiated allodynia in a mild CCI model
3.1.1. Experiment 1: comparison of chronic constriction injury allodynia induced by 3 suture sizes
The classic CCI model involves loose ligation of the sciatic nerve with four 4–0 chromic gut ligatures.1 The maximal allodynia produced by this model confounds assessment of possible amplified mechanical allodynia by morphine; the drug may decrease the absolute thresholds below the limit of detection by von Frey filaments. To overcome this issue, we altered the CCI model to induce submaximal allodynia and therefore increase the dynamic range of allodynia. Previous studies have shown that allodynia induced by CCI can be attenuated if the suture size of the 4 ligatures is reduced,37 or if the number of 4–0 chromic gut sutures is decreased.16 Here, we combined the 2 approaches by applying only single ligatures of decreasing diameter (5–0, 6–0, or 7–0) to the sciatic nerve (n = 4/group). When tested at 14 days after CCI surgery, withdrawal threshold significantly correlated with suture size, with 7–0 suture producing the mildest allodynia (Fig. 1, Spearman R = 0.791, P = 0.0022; main effect of suture size F (2.00, 9.00) = 8.04, P = 0.0099). Post hoc tests did not reveal significant differences between groups.
3.1.2. Experiment 2: comparison of chronic constriction injury + morphine allodynia induced by 3 suture sizes
To test whether expression of mechanical allodynia after morphine in the mild CCI model fell within the measurable range so to avoid floor effects, morphine (5 mg/kg, twice per day) was administered for 5 days, beginning 14 days after surgery (Fig. 2). There was a main effect of treatment for the 7–0 (F (2.03, 6.08) = 10.7, P = 0.0102) and 6–0 (F (2.05, 6.16) = 9.79, P = 0.012), but not for the 5–0 suture (F (1.20, 3.60) = 1.36, P = 0.33).
3.1.3. Experiment 3: comparison of allodynia induced in 6–0 suture chronic constriction injury rats administered a 5-day regimen of morphine vs saline
This study was conducted to assess whether morphine administered for 5 days beginning at day 10 after surgery could significantly increase magnitude and/or duration of allodynia, compared with saline in a mild CCI model. The 6–0 suture was chosen for the following experiments because it avoided behavioral floor effects before and after morphine treatment in the previous experiment (yet still had a suitable dynamic range for future assessment of reversal of allodynia, unlike 7–0 suture). Morphine significantly amplified mechanical allodynia in this model for at least 5 weeks after morphine completion (Figs. 3A and B; main effect of morphine F (1.00, 6.00) = 32.7, P = 0.0012, main effect of time F (5.00, 30.00) = 13.9, P < 0.0001, interaction F (5.00, 30.00) = 2.63, P = 0.0435). Thus, this optimized CCI procedure using 6–0 suture, which avoids behavioral floor effects, replicates and importantly improves detection sensitivity over our prior observation of morphine amplification of CCI allodynia in Sprague-Dawley rats.20 With this improved procedure, the remaining studies examine generalization across opioids and time.
3.2. Fentanyl and oxycodone amplify chronic constriction injury allodynia when administered for 5 days beginning 10 days after mild chronic constriction injury
To define whether commonly used opioids other than morphine also exacerbate nerve injury–induced allodynia, the effects of fentanyl and oxycodone were tested. These opioids were administered for 5 days beginning day 10 after injury in our mild model of CCI (a single 6–0 ligature). Due to the short half-life, fentanyl and the vehicle control were continuously infused through a subcutaneous osmotic minipump. Both opioids amplified mechanical allodynia for approximately 9 weeks after treatment concluded, compared with saline treatment (Figs. 4A and B; main effect of opioids F (2.00,19.00) = 13.8, P = 0.0002, main effect of time F (6.04,114.8) = 59.0, P < 0.0001, interaction F (22.00,209.0) = 2.16, P = 0.0028). Hence, amplification of allodynia by opioids is not restricted to morphine (20 and experiment 3 above), importantly demonstrating broad generality of this phenomenon to other clinically relevant opioid medications.
3.3. Opioids still amplify allodynia when dosing begins 1 month after chronic constriction injury, but not when dosing begins at the time of surgery
3.3.1. Experiment 1: morphine, fentanyl, and oxycodone amplify allodynia when administered for 5 days beginning 28 days after mild chronic constriction injury
Our previous publications10,17–19 and the experiments above focused on assessing the impact of opioid administration at a time when allodynia is recently fully expressed (ie, day 10 after CCI). To test whether these opioids also amplify allodynia at a more extended timepoint, we administered a 5-day course of each opioid (same doses as above) beginning 28 days after mild CCI. Because no differences were found with saline vehicle delivered by injection vs osmotic minipump in the previous experiment, all saline vehicles were delivered by minipump in this experiment to control for the more invasive drug delivery method. Morphine, fentanyl, and oxycodone each amplified allodynia for approximately 5 weeks after treatment concluded (Figs. 5A and B; main effect of opioids F (3.00, 21.0) = 12.7, P < 0.0001, main effect of time F (6.00, 126.0) = 96.7, P < 0.0001, interaction F (18.0, 126.0) = 4.13, P < 0.0001).
3.3.2. Experiment 2: morphine, fentanyl, and oxycodone do not amplify allodynia when administration begins 1 hour after chronic constriction injury
To determine whether opioids would also amplify CCI allodynia when delivery begins before the onset of nerve injury-induced allodynia, morphine, oxycodone, or fentanyl was administered for 5 days beginning 1 hour after CCI surgery. Under these conditions, we did not observe an increase in allodynia after treatment conclusion, compared with saline treatment (Figs. 6A and B, main effect of opioids F (3.00, 26.0) = 0.431, P = 0.733, main effect of time F (3.00, 78.0) = 26.6, P < 0.0001, interaction F (9.00, 78.00) = 1.07, P = 0.395).
5. Discussion
We have previously shown that a short course of morphine, beginning 10 days after CCI, can exacerbate nociceptive hypersensitivity induced by nerve injury for weeks to months after treatment concludes.20 Here, we extend these observations to demonstrate that oxycodone and fentanyl—both widely prescribed opioids for pain treatment—induce similar effects in our rat model of neuropathic pain. We also demonstrate that exacerbation of allodynia occurs if morphine, oxycodone, or fentanyl is administered at an extended timepoint (ie, 28 days after CCI), in addition to early after allodynia develops (ie, 10 days after CCI). Importantly, none of these opioids exacerbated allodynia when administration began an hour after nerve injury.
Our working model for opioid exacerbation of nociceptive hypersensitivity is a “two-hit” hypothesis, that peripheral nerve injury (hit 1) confers a heightened neuroinflammatory response to subsequent opioids (hit 2).7,13,20 This is based on literature of glial priming, wherein a first glial activating event (hit 1) primes glia to overrespond to a subsequent challenge (hit 2).42 We argue that spinal TLR4 gates the immune signaling in our model because it is activated by damage-associated molecular patterns released by injured afferents as well as by opioids.15,17,19,34 The exacerbation of nociceptive hypersensitivity by oxycodone and fentanyl is consistent with this working model because both of these opioids also activate TLR4 as does morphine.27,29,51 Proinflammatory cytokines are produced as a consequence of TLR4 activation, which subsequently promote central sensitization through neuromodulation and dysfunctional synaptic plasticity. For example, tumor necrosis factor and interleukin-1β (IL-1β) enhance excitatory neurotransmission by mechanisms including neurotransmitter exocytosis and increased synaptic strength.31,33,44,53 Glutamate homeostasis is also impaired by these mediators, which induce downregulation and posttranslational modifications of glutamate transporters and glutamine synthetase.31,33,44,53 Our previous work indicates that these pronociceptive mechanisms are potentiated when opioids are administered after injury.10,14,19,20
The timing of opioid administration, relative to peripheral nerve injury, determines whether subsequent allodynia is exacerbated. Here, we found that 5 days of opioids only exacerbated allodynia when allodynia was already established at days 10 or 28 after CCI. We have previously found that a 5-day morphine treatment that ends 2 days before CCI surgery can also exaggerate ensuing allodynia.36 In this study, we unexpectedly found that when morphine, oxycodone, and fentanyl administration was initiated one hour after CCI, subsequent allodynia was not adversely affected. This observation also raises other intriguing questions to be addressed in future studies. For example, whether immediate exposure to opioids after injury protects against the deleterious effects on subsequent reexposure. Also, importantly, we note that this temporally dependent result with the CCI neuropathic pain model contrasts with that from a laparotomy postoperative pain model. In this model, when morphine was administered for 7 days beginning immediately after surgery, morphine more than doubled the duration of allodynia induced by laparotomy.14 It is not yet clear if our divergent results are due to the slightly longer course of morphine treatment in the laparotomy study, or if they are related to inherent differences between these models.
The mechanisms underlying the temporal differences remain unclear: spinal TLR4 mRNA is upregulated within 4 hours of peripheral nerve and persists for more than 28 days.48 It is possible that other signaling components are not upregulated at this early timepoint after injury. For example, the purinergic receptor P2X7R is not upregulated in the lumbar spinal cord until 3 days after peripheral nerve injury.22,32 Importantly, we have shown that P2X7R is required together with TLR4 for opioid exacerbation of allodynia because such potentiation is dependent on inflammasomes.20 This could be one explanation for the lack of effect of opioid administration early after injury. Additional studies will be required to explore the important mechanistic differences underlying the lack of opioid effects when administered at the time of peripheral nerve injury vs after the expression of neuropathic pain.
We did note some between-experimenter variability in von Frey testing; the pretreatment thresholds in Figure 4 were slightly lower than those in other figures (~0.6 vs ~1.4 g). Such variability has been documented before.47 However, the lower thresholds were also accompanied by a longer recovery in this experiment (~10 vs ~5 weeks). These differences may be explained by the greater detection sensitivity of this experimenter.
Despite being classified as third-tier therapeutics for the treatment of peripheral neuropathic pain, opioids are among the most common treatments prescribed for such pain.3,12,41 The preclinical evidence presented here indicates that commonly used opioids may have adverse long-term consequences for the treatment of established neuropathic pain. By contrast, our results suggest that opioids may be appropriate to manage trauma pain acutely after peripheral nerve injury. The extent to which our findings extend from mechanical allodynia to other indices of neuropathic pain (eg, thermal hyperalgesia and ongoing pain) will be investigated in future studies. Overall, these studies provide a framework for a better understanding of the timing of administration and specific opioid medications that could lead to significantly different clinical treatment outcomes. These observations warrant future clinical testing in patients with chronic pain.
Acknowledgements
Morphine, fentanyl, and oxycodone were gifted from the National Institute on Drug Abuse Drug Supply Program (NDSP) Division of Therapeutics and Medical Consequences, Research Triangle Institute, NC. This work was supported by Department of Defense award number W81XWH-16–1-0161 (L.R.W.), NIH R01 DA044934 (L.R.W.), and University of Texas Rising STARs Award (P.M.G.).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- [1].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. PAIN 1988;33: 87–107. [DOI] [PubMed] [Google Scholar]
- [2].Bonnie RJ, Kesselheim AS, Clark DJ. Both urgency and balance needed in addressing opioid epidemic: a report from the national academies of sciences, engineering, and medicine. JAMA 2017;318:423–4. [DOI] [PubMed] [Google Scholar]
- [3].Callaghan BC, Reynolds E, Banerjee M, Kerber KA, Skolarus LE, Burke JF. Longitudinal pattern of pain medication utilization in peripheral neuropathy patients. PAIN 2019;160:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. PAIN 2001;94:231–44. [DOI] [PubMed] [Google Scholar]
- [5].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53:55–63. [DOI] [PubMed] [Google Scholar]
- [6].Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015;162: 276–86. [DOI] [PubMed] [Google Scholar]
- [7].Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience 2002;112:7–11. [DOI] [PubMed] [Google Scholar]
- [8].Cox BM. Recent developments in the study of opioid receptors. Mol Pharmacol 2013;83:723–8. [DOI] [PubMed] [Google Scholar]
- [9].Der-Avakian A, Bland ST, Rozeske RR, Tamblyn JP, Hutchinson MR, Watkins LR, Maier SF. The effects of a single exposure to uncontrollable stress on the subsequent conditioned place preference responses to oxycodone, cocaine, and ethanol in rats. Psychopharmacology (Berl) 2007;191:909–17. [DOI] [PubMed] [Google Scholar]
- [10].Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, Ayala M, Hutchinson MR, Falci S, Rice KC, Maier SF, Watkins LR. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav Immun 2016;58:348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feehan AK, Zadina JE. Morphine immunomodulation prolongs inflammatory and postoperative pain while the novel analgesic ZH853 accelerates recovery and protects against latent sensitization. J Neuroinflammation 2019;16:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice ASC, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun 2007;21: 47–59. [DOI] [PubMed] [Google Scholar]
- [14].Grace PM, Galer EL, Strand KA, Corrigan K, Berkelhammer D, Maier SF, Watkins LR. Repeated morphine prolongs postoperative pain in male rats. Anesth Analg 2019;128:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE. A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J Neurosci Methods 2010; 193:47–53. [DOI] [PubMed] [Google Scholar]
- [17].Grace PM, Maier SF, Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache 2015;55:475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grace PM, Strand KA, Galer EL, Maier SF, Watkins LR. MicroRNA-124 and microRNA-146a both attenuate persistent neuropathic pain induced by morphine in male rats. Brain Res 2018;1692:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grace PM, Strand KA, Galer EL, Rice KC, Maier SF, Watkins LR. Protraction of neuropathic pain by morphine is mediated by spinal damage associated molecular patterns (DAMPs) in male rats. Brain Behav Immun 2018;72:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 2016; 113:E3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput 1986;18:623–32. [Google Scholar]
- [22].He WJ, Cui J, Du L, Zhao YD, Burnstock G, Zhou H-D, Ruan H-Z. Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav Brain Res 2012;226:163–70. [DOI] [PubMed] [Google Scholar]
- [23].Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017;74:773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res 2007;179:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience 2010;169:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang L, Edwards SR, Smith MT. Comparison of the pharmacokinetics of oxycodone and noroxycodone in male dark agouti and Sprague—Dawley rats: influence of streptozotocin-induced diabetes. Pharm Res 2005;22:1489–98. [DOI] [PubMed] [Google Scholar]
- [27].Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 2012;32:11187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 2011;63:772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 2010;24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28:5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 2011;504:57–61. [DOI] [PubMed] [Google Scholar]
- [33].Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016;354:1144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther 2018;184:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li WW, Irvine KA, Sahbaie P, Guo TZ, Shi XY, Tawfik VL, Kingery WS, Clark JD. Morphine exacerbates postfracture nociceptive sensitization, functional impairment, and microglial activation in mice. Anesthesiology 2019;130:292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Loram LC, Grace PM, Strand KA, Taylor FR, Ellis A, Berkelhammer D, Bowlin M, Skarda B, Maier SF, Watkins LR. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain Behav Immun 2012;26:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. PAIN 1993;54:57–69. [DOI] [PubMed] [Google Scholar]
- [38].Megens AAHP, Artois K, Vermeire J, Meert T, Awouters FHL. Comparison of the analgesic and intestinal effects of fentanyl and morphine in rats. J Pain Symptom Manage 1998;15:253–7. [DOI] [PubMed] [Google Scholar]
- [39].Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res 2000;861:105–16. [DOI] [PubMed] [Google Scholar]
- [40].Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci 2001;21:2808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-year trends in the prevalence of, and health Care use for, noncancer pain in the United States: data from the medical expenditure panel survey. J Pain 2019;20: 796–809. [DOI] [PubMed] [Google Scholar]
- [42].Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014;10:217–24. [DOI] [PubMed] [Google Scholar]
- [43].Pizzo PA, Clark NM. Alleviating suffering 101—pain relief in the United States. N Engl J Med 2012;366:197–9. [DOI] [PubMed] [Google Scholar]
- [44].Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000;4:247–57. [DOI] [PubMed] [Google Scholar]
- [45].Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23:1018–27. [DOI] [PubMed] [Google Scholar]
- [46].Sommer C Peripheral neuropathies: long-term opioid therapy in neuropathy: benefit or harm? Nat Rev Neurol 2017;13:516–17. [DOI] [PubMed] [Google Scholar]
- [47].Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JCS, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014;11:629–32. [DOI] [PubMed] [Google Scholar]
- [48].Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int 2004;45:397–407. [DOI] [PubMed] [Google Scholar]
- [49].Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys 1999;61:87–106. [DOI] [PubMed] [Google Scholar]
- [50].Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med 2017;377:391–4. [DOI] [PubMed] [Google Scholar]
- [51].Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 2012;109:6325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wilson NM, Ripsch MS, White FA. Impact of opioid and nonopioid drugs on postsurgical pain management in the rat. Pain Res Treat 2016;2016: 8364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yan X, Weng HR. Endogenous interleukin-1β in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J Biol Chem 2013;288:30544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]