ABSTRACT
Invasive Staphylococcus aureus infections are a leading cause of morbidity and mortality in both hospital and community settings, especially with the widespread emergence of virulent and multi-drug resistant methicillin-resistant S. aureus strains. There is an urgent and unmet clinical need for non-antibiotic immune-based approaches to treat these infections as the increasing antibiotic resistance is creating a serious threat to public health. However, all vaccination attempts aimed at preventing S. aureus invasive infections have failed in human trials, especially all vaccines aimed at generating high titers of opsonic antibodies against S. aureus surface antigens to facilitate antibody-mediated bacterial clearance. In this review, we summarize the data from humans regarding the immune responses that protect against invasive S. aureus infections as well as host genetic factors and bacterial evasion mechanisms, which are important to consider for the future development of effective and successful vaccines and immunotherapies against invasive S. aureus infections in humans. The evidence presented form the basis for a hypothesis that staphylococcal toxins (including superantigens and pore-forming toxins) are important virulence factors, and targeting the neutralization of these toxins are more likely to provide a therapeutic benefit in contrast to prior vaccine attempts to generate antibodies to facilitate opsonophagocytosis.
Keywords: Staphylococcus aureus, MRSA, vaccine, immunity, genetics, evasion
This review summarizes the data from humans regarding the immune responses that protect against invasive Staphylococcus aureus infections as well as host genetic factors and bacterial evasion mechanisms, which form the basis for a hypothesis that future vaccines and immune-based therapies that target the neutralization of staphylococcal toxins superantigens and pore-forming toxins are more likely to provide a therapeutic benefit.
INTRODUCTION
The mortality of Staphylococcus aureus invasive infections has fallen from ∼80% in the pre-antibiotic era (Smith and Vickers 1960) to 16%–30% over the past two decades (van Hal et al. 2012; Nambiar et al. 2018; Kourtis et al. 2019). Further reductions in mortality below 20% have remained elusive despite the introduction of new antibiotics to address antibiotic-resistant isolates, rapid diagnostic and susceptibility testing, widespread antibiotic stewardship programs and improvements in therapeutic supportive care (Holland, Arnold and Fowler 2014; Tong et al. 2015). While vaccine development has lowered the mortality of other bacterial infections, all vaccination attempts aimed at preventing S. aureus invasive infections have failed in human trials, especially all vaccines aimed at generating high titers of opsonic antibodies against S. aureus surface antigens to facilitate antibody-mediated bacterial clearance (Daum and Spellberg 2012; Fowler and Proctor 2014; Proctor 2015; Giersing et al. 2016; Missiakas and Schneewind 2016; Mohamed et al. 2017; Proctor 2019). A major impediment to the development of a successful vaccine against S. aureus is an incomplete understanding of protective immune mechanisms and biomarkers that clearly indicate durable and long-term protective immunity against S. aureus infections in humans. This impediment stems in part from relatively limited information about the specific immune responses in humans that protect against invasive S. aureus infections (Miller and Cho 2011; Fowler and Proctor 2014; Montgomery, David and Daum 2015; Proctor 2019).
The development of human vaccines against S. aureus infections has relied primarily on data from preclinical animal models. Unfortunately, animal models in general, and murine models in particular, have failed to translate into successful S. aureus vaccines in humans (Proctor 2012; Proctor 2012). For example, none of the 15 S. aureus antigenic targets identified to date from initial efficacy studies in murine models were ultimately shown to be effective vaccine targets in 12 human clinical trials (in both active and passive immunization approaches) (Fowler and Proctor 2014; Yeaman et al. 2014; Redi et al. 2018). This is likely in part due to the attenuated activity of many S. aureus superantigens (SAgs) and pore-forming toxins (PFTs) in murine and other animal models of infection (Bubeck Wardenburg et al. 2008; Diep et al. 2010; Loffler et al. 2010; Salgado-Pabon and Schlievert 2014). All of these trials have shared a common approach of inducing opsonophagocytosis of S. aureus by eliciting antibodies that bind to the bacterial surface and promote bacterial killing. Unfortunately, none of these opsonic antibody-based vaccine candidates were protective in clinical trials, and some were harmful when a S. aureus infection ultimately did occur (Fowler et al. 2013).
In this review, we propose a paradigm for S. aureus vaccine development based upon the latest available evidence in humans. This paradigm can be categorized into three main areas: (i) What can we learn about immunity to invasive S. aureus infections from humans with congenital or acquired immune defects that lead to an increased susceptibility to or reduced clearance of S. aureus infections? (ii) What can we learn from the human antibody, cytokine and immune cell profiles during invasive S. aureus infections to provide a greater understanding of protective versus deleterious immune responses in otherwise healthy humans? and (iii) Which specific human immune responses and human genetic makeups reduce the severity of invasive S. aureus infections?
While the reasons for the lack of progress in developing successful vaccines against S. aureus invasive infections are multifactorial, this review will include the most recent evolving evidence regarding human immunity against S. aureus and provide suggestions for how this information could help guide future vaccine development efforts. In addition, clinical data regarding the association of certain deleterious immune responses and poor clinical outcomes in patients with invasive S. aureus infections (especially S. aureus bacteremia [SAB]) will also be described. Finally, we will examine the role of anti-toxin antibodies in modulating the severity of S. aureus infections. Based upon these data, we propose a hypothesis that S. aureus vaccines aimed at neutralizing the activity of S. aureus toxins are more likely to provide a therapeutic benefit in humans than those targeting opsonophagocytosis.
IMMUNE CELLS, CYTOKINES AND SIGNALING PATHWAYS IMPLICATED IN PROTECTION AGAINST S. aureus INFECTIONS AND EVASION MECHANISMS THAT COUNTERACT THESE RESPONSES
In this section, the early innate immune mechanisms mediated by keratinocytes and mucosal epithelial cells as well as phagocytic cells (including neutrophils, monocytes/macrophages and dendritic cells) will be reviewed. This will also include a thorough analysis of adaptive immune responses, mediated primarily by B and T cells as well as immune responses mediated by unconventional T cells, including γδ T cells and mucosal-associated invariant T (MAIT) cells. For each of these cellular immune responses, the evasion mechanisms that S. aureus utilizes to counteract these host immune responses will be discussed. Importantly, the findings from humans with genetic mutations and polymorphisms in cytokines, receptors and signaling molecules that have shed light on the host responses implicated in mediating protective immunity against S. aureus infections will be described.
Keratinocytes in innate immunity against S. aureus
Staphylococcus aureus causes the vast majority of skin and soft tissue infections and consequently our first line of defense against S. aureus occurs at our skin and mucosal surfaces. Moreover, S. aureus nasal mucosal colonization is a known risk factor for the development of ensuing bacteremia (von Eiff et al. 2001; Marzec and Bessesen 2016). At these epithelial sites, keratinocytes and mucosal epithelial cells produce host defense peptides (HDPs) that provide innate antimicrobial activity (bacteriostatic and bactericidal) against S. aureus (Table 1) (Miller and Cho 2011; Liu, Mazhar and Miller 2018). Several HDPs have been shown to be produced by human keratinocytes and other cells in the skin and promote bacteriostatic and bactericidal activity against S. aureus at the epithelial interface, including human β-defensins (HBDs) 1–4, cathelicidin (LL-37) and RNase 7, dermcidin, REG3A and resistin-like molecule α (RELMα) (Braff et al. 2005; Rieg et al. 2005; Minegishi et al. 2009; Gallo and Hooper 2012; Lai et al. 2012; Ommori et al. 2013; Harris et al. 2019). In particular, HBD3 has strong in vitro bactericidal activity against S. aureus (Harder et al. 2001) and human cathelicidin induced by vitamin D also has been shown to have potent antimicrobial activity against S. aureus (Braff et al. 2005; Schauber et al. 2007). Increased HBD3 expression in human skin and nasal mucosa, which can be induced by the T cell cytokines IFNγ as well as IL-17A, is associated with decreased nasal and skin S. aureus colonization (Nurjadi et al. 2016). Interestingly, if S. aureus invades into the subcutis, adipocytes can produce cathelicidin to help control the infection and prevent invasive spread (Zhang et al. 2015). HBD2 and cathelicidin also promote proinflammatory immune responses via their chemotactic activity for other immune cells by triggering CCR6 (expressed on T cells) and formyl peptide receptor-like 1 (FPRL1) (expressed on neutrophils, monocytes and T cells), respectively (Yang et al. 1999; De et al. 2000). Most recently, vitamin A was shown to increase keratinocyte expression of RELMα, which had antimicrobial activity against S. aureus (Harris et al. 2019). Perhaps the best evidence for a role of HDPs in immunity against S. aureus at the skin interface is that the affected skin of patients with atopic dermatitis, which is associated with high S. aureus skin colonization and skin superinfection by S. aureus (Kong et al. 2012; Byrd et al. 2017), has substantially reduced levels of HDPs (especially HBD2, HBD3, and LL-37) (Ong et al. 2002; Minegishi et al. 2009; Rangel and Paller 2018; Kim et al. 2019). Further evidence of the role of HDPs in immune protection against S. aureus is suggested by the presence of mechanisms that S. aureus utilizes to evade HDPs. For example, S. aureus-derived products of the dltABCD operon fosters D-alanylation of wall teichoic acid (WTA) resulting in a more positively charged cell wall and bacterial surface (Peschel et al. 1999) and Multiple peptide resistance Factor (MprF) is responsible for lysinylating phosphatidylglycerol and flipping it to the outer membrane to produce a relatively more positively charged cell membrane (Peschel et al. 2001), which inhibits the cationic-mediated activities of HDPs. Consequently, a mutant S. aureus strain deficient in D-alanylated teichoic acids (dltA mutant) was more susceptible to the antimicrobial activity of HBD2, HBD3, cathelicidin, RNase 7 and dermcidin (Simanski et al. 2013). S. aureus also produces iron surface determinant A (IsdA) that enhances its cellular hydrophobicity, which renders the S. aureus bacteria resistant to HBD2 and cathelicidin (Clarke et al. 2007). In addition, S. aureus produces aureolysin that inhibits cathelicidin antimicrobial activity (Sieprawska-Lupa et al. 2004). Finally, S. aureus secretes extracellular proteases that degrade and neutralize the activity of dermcidin (Lai et al. 2007).
Table 1.
Host Defense Peptides (HDPs) in human skin with activity against S. aureus.
| Host Defense Peptide | Cellular expression in skin | Mechanisms of activity | S. aureus immune evasion mechanisms |
|---|---|---|---|
| HBD2 | Keratinocytes, monocytes/macrophages and DCs | Antimicrobial activity, chemotaxis of T cells and DCs | dltABCD operon, MprF, |
| HBD3 | Keratinocytes | Antimicrobial activity, chemotaxis of T cells and DCs | dltABCD operon |
| Cathelicidin (LL-37) | Keratinocytes, monocytes/macrophages, neutrophils, adipocytes | Antimicrobial activity, chemotaxis of neutrophils, monocytes and T cells | dltABCD operon, MprF, IsdA and aureolysin |
| Dermcidin | Eccrine sweat glands | Antimicrobial activity | dltABCD operon, extracellular proteases |
| RNase 7 | Keratinocytes | Antimicrobial activity | dltABCD operon |
| RELMα | Keratinocytes | Antimicrobial activity | staphyloxanthin |
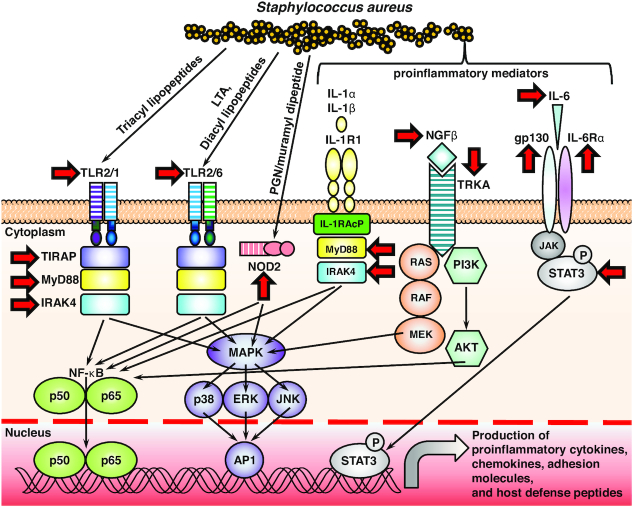
Human keratinocytes also express the pattern recognition receptor (PRR) Toll-like receptor 2 (TLR2), which heterodimerizes with TLR1 or TLR6 in host cell membranes, to recognize triacyl or diacyl lipopeptides, respectively (Fig. 1). TLR2 on keratinocytes can be activated by S. aureus lipopeptides and lipoteichoic acid (LTA) (which is diacylated), and this can result in increased production of proinflammatory cytokines such as IL-1β, IL-8 and TNF as well as HDPs (Mempel et al. 2003; Menzies and Kenoyer 2006). In addition to TLR2, nucleotide-binding oligomerization domain 2 (NOD2) is found in the cytosol of keratinocytes (and other cell types) where it can detect muramyl dipeptide, a breakdown product of peptidoglycan (PGN) from S. aureus (and other bacteria). Activation of NOD2 likely occurs when S. aureus muramyl dipeptide enters the cytoplasm of keratinocytes and results in activation of signaling pathways that promote production of proinflammatory cytokines, including IL-1β, TNF, IL-6 and IL-17C to promote host defense against S. aureus skin infections and prevent invasive spread of the bacteria (Muller-Anstett et al. 2010; Roth et al. 2014). Evidence for a potential role of both TLR2 and NOD2 in host defense against S. aureus infections has been further suggested by the identification of loss-of-function polymorphisms in TLR2 and NOD2 in patients with atopic dermatitis who have increased S. aureus skin colonization and impetiginization (Kabesch et al. 2003; Ahmad-Nejad et al. 2004; Potaczek et al. 2011). In addition, as an immune evasion mechanism, S. aureus produces SAg-like protein 3 (SSL3) and staphylococcal Toll/Interleukin-1 receptor (TIR) domain protein (TirS), which both interfere with the ability of TLR2 on keratinocytes to recognize a S. aureus infection and initiate innate immune mechanisms (Bardoel et al. 2012; Askarian et al. 2014).
Figure 1.
Host cell signaling pathways implicated in immunity against S. aureus infections. Toll-like receptor 2 (TLR2) (which heterodimerizes with TLR1 or TLR6 and the TLR2/6 heterodimer is activated by Staphylococcus aureus lipopeptides and LTA [lipoteichoic acid]) and interleukin–1 receptor 1 (IL–1R1) (which is activated by IL–1α and IL–1β) both signal through MyD88 (myeloid differentiation primary response protein 88) and IRAK4 (IL–1R–associated kinase 4) to trigger activation of NF–κB (nuclear factor-κB) and MAPK (mitogen-activated protein kinase) (including p38, ERK [extracellular signal–regulated kinase] and JNK [JUN N-terminal kinase]) signaling. An additional signaling adapter protein, TIRAP (Toll/interleukin-1 receptor [TIR] domain- containing adapter protein), is required for TLR2 signaling, and the IL-1 receptor accessory protein (IL-1RAcP), is required for IL-1R signaling. S. aureus also induces production of NGFβ (nerve growth factor β) that binds to its receptor TRKA (tyrosine kinase receptor A) to promote RAS/RAF/MEK and PI3K (phosphatidylinositol 3-kinase)/AKT (protein kinase B) signaling. Finally, IL-6, which binds to its receptor comprised of gp130 and the IL-6Rα activates JAK (Janus kinase) and STAT3 (signal transducer and activator of transcription 3) signaling. Each of these signaling pathways leads to transcription and translation of proinflammatory cytokines, chemokines, adhesion molecules and host defense peptides against S. aureus infections. Red arrows: The specific inflammatory mediators and signaling molecules in which loss-of-function mutations have been identified in humans that result in an increased susceptibility to S. aureus infections.
Neutrophils and monocytes/macrophages in innate immunity against S. aureus
The important role of phagocytic cells such as neutrophils (polymorphonuclear leukocytes [PMNs]) and monocytes/macrophages in providing host defense against S. aureus infections is demonstrated in patients with congenital defects in neutrophil number or function, who are highly susceptible to skin, soft tissue and invasive S. aureus infections. Neutrophils and monocytes/macrophages are recruited from the bloodstream where they provide the initial host defense response against S. aureus by forming an abscess to surround and wall-off the infection to prevent invasive spread (Kobayashi, Malachowa and DeLeo 2015). Specific patients with identified congenital genetic mutations that result in defective phagocytosis, rendering these patients highly susceptible to S. aureus infections, including severe congenital neutropenia, patients with defective reactive oxygen species-mediated killing (e.g. chronic granulomatous disease, myeloperoxidase deficiency and glucose-6-phosphate dehydrogenase [G6PD] deficiency), patients with defective neutrophil chemotaxis from the bloodstream to the site of infection (e.g. leukocyte adhesion deficiencies, Wiskott-Aldrich syndrome and RAC2 deficiency), neutrophil granule disorders (e.g.neutrophil-specific granule deficiency and Chediak-Higashi syndrome) (Lakshman and Finn 2001; Andrews and Sullivan 2003; Bouma et al. 2010; Miller and Cho 2011) (Table 2). Moreover, humans with acquired defects in neutrophil number or function are also highly susceptible to invasive S. aureus infections, such as chemotherapy-induced neutropenia or patients with renal failure or diabetes that have multiple impairments in neutrophil function (Gonzalez-Barca et al. 2001; Chonchol 2006; Smit et al. 2016). Importantly, S. aureus possesses many different virulence factors against neutrophil-mediated killing. For example, S. aureus produces staphylokinase that binds to neutrophil α-defensins to inhibit and evade their antimicrobial activity (Jin et al. 2004). S. aureus also produces a number of factors such as extracellular fibrinogen-binding protein (Efb), extracellular complement-binding (Ecb) and complement 4 binding protein (C4BP), which all inhibit C3b-mediated opsonization and ensuing complement-mediated phagocytosis (Hair et al. 2012; Kuipers et al. 2016; Amdahl et al. 2017). Staphylokinase also inhibits C3b and IgG opsonization of S. aureus and subsequent phagocytosis by converting plasminogen into plasmin on the bacterial surface (Rooijakkers et al. 2005). Finally, S. aureus produces superoxide dismutase enzymes and the golden carotenoid pigment, staphyloxanthin, as potent antioxidants that inhibit reactive oxygen species (ROS) mediated neutrophil killing (Karavolos et al. 2003; Liu et al. 2005; Liu et al. 2008).
Table 2.
Congenital and acquired diseases with impaired neutrophil number or function that are characterized by increased susceptibility to S. aureus infections.
| Neutrophil immune defect | Diseases |
|---|---|
| Neutropenia | Severe congenital neutropenia and acquired neutropenia in chemotherapy patients |
| Impaired reactive oxygen species (oxidative burst) | Chronic granulomatous disease (mutations in NADPH oxidase), myeloperoxidase (MPO) deficiency and glucose-6-phosphage dehydrogenase (G6PD) deficiency |
| Impaired neutrophil chemotaxis and recruitment to the site of infection | Leukocyte adhesion deficiencies I, II and III, Wiskott-Aldrich syndrome, RAC2 deficiency, MyD88-deficiency, IRAK4-deficiency and TIRAP-deficiency |
| Defective neutrophil granules | Neutrophil-specific granule deficiency and Chediak-Higashi Syndrome |
| Multiple defects in neutrophil function | Type I or II diabetes mellitus, renal failure patients on hemodialysis and cystic fibrosis patients |
There are many additional mechanisms that neutrophils and monocytes/macrophages utilize to provide antimicrobial activity in the innate immunes response against S. aureus, including recognition of S. aureus by various different PRRs, including TLR2 and NOD2 (similar to keratinocytes, as mentioned above) (Fig. 1). S. aureus also activates the inflammasome (which has been shown to be in part mediated by S. aureus-derived ATP, α-toxin, β-hemolysin, γ-hemolysin and PVL) that results in proteolytic processing and cellular release of IL-1β, which activates the IL-1R to induce production of proinflammatory and antimicrobial immune responses against S. aureus (Mariathasan et al. 2006; Franchi et al. 2007; Miller et al. 2007; Craven et al. 2009; Munoz-Planillo et al. 2009; Holzinger et al. 2012). The importance of TLRs and IL-1Rs in host defense against S. aureus is further supported by the identification of individuals with genetic defects in TLR/IL-1R signaling molecules that increase the susceptibility to S. aureus skin, mucosal as well as invasive infections (Table 2, Fig. 1). TLRs and IL-1R family members signal through MyD88 and IRAK4 signaling molecules to subsequently activate many downstream innate immune signaling pathways, including NF-kB and mitogen-activated protein kinases (MAPKs) to promote production of HDPs, cytokines, chemokines and other proinflammatory mediators (Casanova, Abel and Quintana-Murci 2011). In humans, pediatric patients with loss-of-function mutations in MyD88 or IRAK4 are highly predisposed to pyogenic (pus-forming bacterial infections), especially Streptococcus pneumoniae lung and systemic infections, S. aureus skin and mucosal infections and P. aeruginosa infections (von Bernuth et al. 2008; Picard et al. 2010; von Bernuth et al. 2012). Although these patients have defective TLR and IL-1R family signaling in many cell types, these patients have markedly impaired neutrophil migration to the site of infection and defective neutrophil phagocytosis (Bouma et al. 2009). As mentioned above, TLR2 is particularly important in the recognition of S. aureus lipopeptides and LTA (Casanova, Abel and Quintana-Murci 2011). However, TLR2 requires an additional adapter molecule TIRAP (also known as Mal = MyD88-adaptor-like) to initiate MyD88/IRAK4-signaling. Patients with loss-of-function mutations in TIRAP are highly predisposed to S. aureus infections (Israel et al. 2017). Interestingly, although about half of the pediatric patients with MyD88 or IRAK4 succumb to severe Streptococcus pneumoniae infections, those who survive into adulthood lose their susceptibility to pyogenic infections (Picard et al. 2010; Picard et al. 2011). The precise explanation for this clinical observation is unclear but can be attributed to compensatory immune responses that develop in these patients, including the markedly high titers of anti-S. aureus LTA antibodies in humans with loss-of-function mutations in TIRAP (that enhance macrophage function) (Israel et al. 2017) and the markedly expanded circulating Vδ2+ γδ T cells (the produce IFNγ and TNF to promote neutrophil recruitment) in humans with loss-of-function mutations in IRAK4 (Dillen et al. 2018).
Further support for the important role of TLR2 in host defense mechanisms of neutrophils and monocytes/macrophages against S. aureus is that the S. aureus-derived factors SSL3 and TirS interfere with TLR2 function to prevent the recognition and activation of neutrophils and monocytes/macrophages (similar to keratinocytes, above) during S. aureus infections (Bardoel et al. 2012; Askarian et al. 2014). Staphylococcus aureus also secretes cytolytic PFTs that damage the membranes of host cells, especially neutrophils and monocytes/macrophages, as an immune evasion mechanism to counter the activity of these phagocytic cells (Aman and Adhikari 2014; Spaan, van Strijp and Torres 2017). There are two main families of S. aureus PFTs: (1) single-component α-toxin (also called α-hemolysin or Hla) (Berube and Bubeck Wardenburg 2013) and (2) bicomponent leukotoxins, including Panton-Valentine Leukocidin (PVL), LukED, HlgAB and HlgCB (that comprise γ-hemolysin) and the more distantly related LukAB (also called LukGH) (Aman and Adhikari 2014; Seilie and Bubeck Wardenburg 2017; Spaan, van Strijp and Torres 2017). α-toxin is produced by nearly all S. aureus strains. Secreted as a monomer, it oligomerizes on the host cell surface upon interaction with its receptor the metalloproteinase ADAM10, resulting in pore formation (Inoshima et al. 2011). α-toxin promotes inflammatory responses and has cytolytic responses against a wide range of immune cells, such as monocytes/macrophages, T and B cells (Nygaard et al. 2012) and nonimmune cells such as epithelial and endothelial cells (Powers et al. 2012; Hermann et al. 2015). Also, α-toxin affects platelet activation and induces neutrophil inflammatory pathways to result in severe sepsis (Powers et al. 2015). Platelets possess diverse innate immune functions, so this further contributes to immune dysfunction (Deppermann and Kubes 2018). In addition, bicomponent leukocidins consist of two subunits: the receptor binding ‘S’ subunit and the oligomerization subunit ‘F’ (Aman and Adhikari 2014; Spaan, van Strijp and Torres 2017). For all these toxins (except for LukAB), the subunits are produced and released as monomers. The S subunit first binds to its cellular receptor and subsequently the F subunit binds to S and initiates octamerization and host membrane pore formation (Aman and Adhikari 2014; Spaan, van Strijp and Torres 2017). LukAB is produced as a dimer that upon binding to its receptor octamerizes to form a functional pore (Dumont et al. 2011). The toxins facilitate lysis of host cells, especially neutrophils and monocytes/macrophages, by interacting and binding to specific receptor targets that are present on the host cells, many of which have been recently discovered. PVL and HlgCB utilize C5aR1 and C5aR2 (Spaan et al. 2013a), LukED uses CCR5, CXCR1, and CXCR2 (Alonzo et al. 2013; Reyes-Robles et al. 2013), HlgAB and LukED share CXCR1 and CXCR2 as receptors but can also utilize CCR2 (Spaan et al. 2014), whereas LukAB binds to CD11b (DuMont et al. 2013). All bicomponent leukocidin lyse neutrophils and monocytes/macrophages and the specificity of LukED for CCR5 allows this toxin to also have cytolytic activity against dendritic cells (DCs), T cells, and NK cells (Spaan, van Strijp and Torres 2017). Finally, S. aureus possesses phenol soluble modulins (PSMs), including four PSMα peptides (PSMα1-PSM α4), PSMβ1, PSMβ2, and PSMδ (δ-toxin), which have the ability to lyse human erythrocytes and leukocytes, including neutrophils and monocytes/macrophages (Peschel and Otto 2013). Several different S. aureus PSMs at high concentrations have been shown to be recognized by human formyl peptide receptor 2 (FPR2) and this interaction inhibits neutrophil recruitment as a possible evasion mechanism (Kretschmer et al. 2010).
Soon after the neutrophilic response, monocytes/macrophages and DCs are recruited to the site of infection to contribute to the early innate immune response against S. aureus. Monocytes/macrophages, like neutrophils, are phagocytic cells that engulf S. aureus bacteria and mediate bacterial killing (Spaan et al. 2013b; Foster et al. 2014) (Fig. 1). Neutrophil- and monocyte/macrophage-mediated phagocytosis of S. aureus is facilitated by the expression of Fc and complement receptors on their cell membranes, which recognize S. aureus bacteria opsonized with immunoglobulin (e.g. IgG) and complement component C3b, respectively (Spaan et al. 2013b; Foster et al. 2014). The important role of phagocytosis in host defense against S. aureus is supported by the numerous evasion mechanisms that S. aureus possesses to evade this critical host defense response (Foster et al. 2014). Specifically, S. aureus expresses protein A (SpA) and Sbi (second immunoglobulin-binding protein) that bind immunoglobulins (especially IgG) in the incorrect orientation so they can no longer be detected by Fc receptors on neutrophils and monocytes/macrophages (Atkins et al. 2008). Sbi also binds to and blocks the activity of the complement factor C3, as another evasion mechanism against C3b-mediated phagocytosis (Burman et al. 2008). In addition, S. aureus produces fibrinogen binding proteins and clumping factor A (ClfA), which bind fibrinogen and impair neutrophil and monocyte/macrophage phagocytosis (Palmqvist et al. 2004; Higgins et al. 2006).
An additional a role of neutrophils and monocytes/macrophages in innate immunity against S. aureus was identified in a study that uncovered nerve growth factor β (NGFβ) as a key mediator of host defense (Hepburn et al. 2014) (Fig. 1). S. aureus PGN, protein A, α-toxin and PSMs lead to production and release of NGFβ that binds to its receptor TRKA to mediate autocrine activity on macrophages and paracrine activity on neutrophils, which subsequently promoted enhanced phagocytosis, reactive oxygen species-dependent killing, increased proinflammatory cytokine production, and calcium-dependent neutrophil recruitment (Hepburn et al. 2014). Indeed, humans with loss-of-function mutations in the genes encoding NGFβ or TRKA are highly susceptible to recurrent and severe S. aureus infections of skin, teeth, joints and bone (Hepburn et al. 2014).
Dendritic cells (DCs) in innate immunity against S. aureus
DCs primarily function as professional antigen presenting cells (APCs) in which MHC molecules on the DCs present antigens to TCRs on T cells in adaptive immunity. For example, antigen delivery to DCs shapes human CD4+ and CD8+ T cell memory responses against S. aureus (Uebele et al. 2017). DCs can also directly mediate host defense against different bacterial insults to the skin, including S. aureus (Janela et al. 2019). In particular, conventional DC1 cells in the dermis of mouse and human skin in response to various different bacterial insults produced vascular endothelial growth factor-α (VEGF-α), which was critical for mediating neutrophil recruitment and host defense (Janela et al. 2019). Thus, cDC1s in the skin and potentially at other epithelial sites are essential regulators of neutrophil recruitment in the innate immune response to S. aureus (and other bacteria), providing evidence of a role for DCs beyond classical antigen presentation.
B cells in adaptive immunity against S. aureus
The adaptive immune response to S. aureus is mediated by B and T cells. The B-cell mediated immune response to S. aureus involves the production of specific antibodies against components of S. aureus, including differences in antibody titers in superficial versus deep-seated skin infections (Kumar et al. 2005, Holtfreter, Kolata and Broker 2010). The entire S. aureus antibody proteome includes antibodies against SAgs, PFTs, capsular polysaccharides, LTA and PGN, among many other antigens (Holtfreter, Kolata and Broker 2010). Studies using various animal models of S. aureus infection have suggested that antibodies against specific S. aureus components can provide varying degrees of immune protection (Spellberg and Daum 2012; Fowler and Proctor 2014). As mentioned above, these antibodies play an important role in opsonizing S. aureus and facilitating antibody-mediated phagocytosis by neutrophils and monocytes/macrophages or by neutralizing S. aureus toxins and other virulence factors (Spaan et al. 2013b; Foster et al. 2014). It should be noted that antibody-based vaccination strategies targeting capsular polysaccharides 5 and 8 (Shinefield et al. 2002), clumping factor A (ClfA) (Bloom et al. 2005; DeJonge et al. 2007), or a combination of capsular polysaccharides 5 and 8, ClfA plus the manganese ABC transporter (MntC) (Inoue et al. 2018) as well as iron surface determinant B (IsdB) (Fowler et al. 2013) have all failed in clinical trials. In particular, the IsdB vaccine aimed at preventing S. aureus infections following cardiothoracic surgery had the opposite effect, as patients who received the vaccine and developed an invasive S. aureus infection were five times more likely to die than patients who received a placebo vaccine (Fowler et al. 2013). Since the failed IsdB vaccine study was published, increased levels of IsdB antibodies in patients with orthopedic infections was found to correlate with increased mortality (Nishitani et al. 2015). Thus, some antibody-based immune responses may be detrimental to the host.
Protective immunity mediated by antibodies has been suggested by patients who have received commercial preparations of intravenous immunoglobulin (IVIG) in which older studies have indicated that IVIG preparations possess opsonic antibodies against S. aureus (Hiemstra, Brands-Tajouiti and van Furth 1994; Ono et al. 2004). However, more recent studies have indicated that the activity of IVIG preparations against S. aureus infections is more likely due to the high levels of antibodies that neutralize S. aureus secreted toxins, such as PVL and LukAB (Gauduchon et al. 2004; Wood et al. 2017). Indeed, the high titers of antibodies against PVL in IVIG improved survival in a S. aureus rabbit pneumonia model (Diep et al. 2016).
The importance of the antibody response against S. aureus infections is supported by the existence of S. aureus-derived SpA and Sbi, which bind antibodies and prevent immunoglobulin and complement mediated phagocytosis, as mentioned above (Atkins et al. 2008). Interestingly, a study in children found that high natural antibody titers against α-toxin but not PVL correlated with protection against a subsequent S. aureus skin infection (Fritz et al. 2013), providing the rationale for targeting antibody neutralization of S. aureus α-toxin in future vaccination strategies. With relevance to complement activation and C3b-mediated opsonization of S. aureus, humans with loss-of-function mutations in the gene that encodes mannose-binding lectin (MBL) (which activates the alternative complement pathway), suffer from recurrent S. aureus infections (Carlsson et al. 2005). However these studies should be interpreted with caution as humans with primary or secondary immunodeficiencies characterized by selective deficiencies in B cells or antibodies (including agammaglobulinemia) are not highly susceptible to S. aureus infections, and accordingly there are no clinical guideline recommendations to provide coverage for S. aureus infections in these patients (Hoernes, Seger and Reichenbach 2011; Dhalla and Misbah 2015). Rather, patients with deficiencies in B cells or antibodies primarily suffer from infections caused by encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae (Hoernes, Seger and Reichenbach 2011).
T cells in adaptive immunity against S. aureus
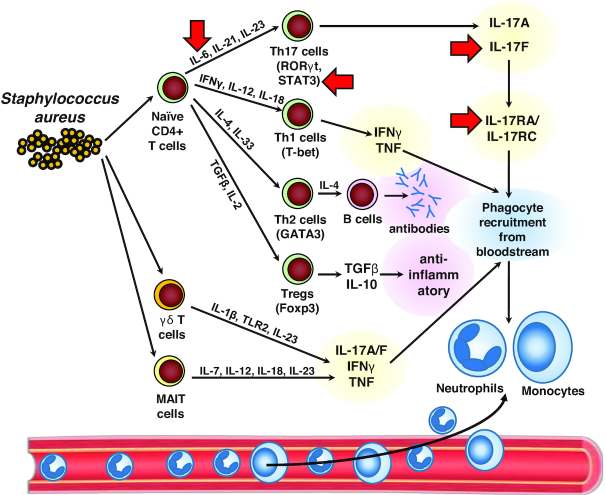
There are several subsets of CD4+ T helper (Th) cells, such as Th1 cells that produce IFNγ, Th17 cells that produce IL-17A, IL-17F and often IL-22, Th22 cells that produce IL-22 (but not IL-17A/F) and T regulatory cells (Tregs) that downregulate immune responses by producing anti-inflammatory cytokines such as TGFβ and IL-10. There is increasing evidence that the CD4+ Th cell responses are critical to human immunity against S. aureus infections (Fig. 2). First, HIV+ patients with low circulating CD4+ Th cell counts are highly susceptible to S. aureus skin and more invasive infections, including bacteremia (Manfredi et al. 1993; Manfredi, Calza and Chiodo 2002; Crum-Cianflone et al. 2010). Notably, the rates of SAB in HIV+ patients is 1960/100 000/year, which is 50 times greater than the rate of SAB in the general population (20–38/100 000/year) (Tong et al. 2015). Recently, the impaired immunity to S. aureus skin and soft tissue infections in HIV+ patients was linked to decreased IFN-γ-producing Th1 cells rather than IL-17-producing Th17 cells (Utay et al. 2016).
Figure 2.
T cells in immunity against S. aureus infections. In response to S. aureus infection, naïve αβ CD4+ T cells can differentiate into different T helper (Th) cell subsets. These include Th17 cells (induced by IL-6, IL-21 and IL-23) that express the transcription factors RORγt (retinoic acid-related-orphan receptor γ) and STAT3 (signal transducer and activator of transcription 3) and produce IL-17A and IL-17F, which activate their receptor comprised of IL-17RA (IL-17 receptor A) and IL-17RC (IL-17 receptor C) to promote phagocyte (neutrophil and monocyte) recruitment from the bloodstream to form an abscess at the site of infection. Similarly, Th1 cells (induced by IFNγ, IL-12 and IL-18) that express the transcription factor T-bet (T-box–containing protein expressed in T cells) and produce IFNγ and TNF, which also promote phagocyte (neutrophil and monocyte) recruitment from the bloodstream to form an abscess at the site of infection. In addition, Th2 cells (induced by IL-4 and IL-33) express the transcription factor GATA3 and promote antibody production by B cells. Finally, Tregs (T regulatory cells) (induced by TGFβ and IL-2) that express the transcription factor FoxP3 (forkhead box P3) downregulate immune responses by producing the anti-inflammatory cytokines TGFβ and IL-10. Unconventional T cells such as γδ T cells (induced by IL-1β, TLR2 and IL-23) and MAIT (mucosa-associated invariant T cells) (induced by IL-7, IL-12, IL-18 and IL-23) produce IL-17A, IL-17F, IFNγ and TNF, which also promote phagocyte recruitment and host defense against S. aureus infections. Red arrows: The specific inflammatory mediators and signaling molecules in which loss-of-function mutations have been identified in humans that result in an increased susceptibility of S. aureus infections.
Second, as mentioned above, patients with the inflammatory skin disease atopic dermatitis have increased skin colonization and superinfection (impetiginization) with S. aureus (Kong et al. 2012; Byrd et al. 2017) and this disease is driven by a Th2 cytokines especially IL-4 and IL-13 in the affected skin of these patients (Weidinger et al. 2018). The Th2 cytokine environment in atopic dermatitis is thought to contribute to a defective skin barrier, decreased HDP expression and enhanced binding of S. aureus to the skin surface (Kim et al. 2019). Notably, the Th2 environment (and specifically IL-4) can increase host keratinocyte expression of fibronectin and fibrinogen receptors on the cell surface, facilitating S. aureus factors such as fibronectin-binding protein (FnBP) and clumping factors (e.g. ClfA) to bind more efficiently to the affected skin (Cho et al.2001a; Cho et al. 2001b). Also in atopic dermatitis, S. aureus produces SAgs such as staphylococcal enterotoxins A-D (e.g. SEA, SEB, SEC and SED) and toxic shock syndrome toxin-1 (TSST-1) that can non-specifically activate T cells by binding to the Vβ chain of the T cell receptor (TCR) and contribute to aberrant skin inflammation (Fig. 3) (Bunikowski et al. 2000; Schlievert et al. 2008; Geoghegan, Irvine and Foster 2018). In addition, S. aureus SAgs appear to preferentially induce Th2 cytokine responses, further contributing to atopic dermatitis pathogenesis (Laouini et al. 2003). In human mast cell cultures, PSMα and δ-toxin have been shown to induce mast cell degranulation, which could contribute to inflammation and itching behavior in humans (Hodille et al. 2016). Consistent with this finding in human mast cells, in an epicutaneous exposure to S. aureus in mice, δ-toxin induced mast cell degranulation and PSMα-mediated release of IL-36α from the keratinocytes that contributed to increased atopic dermatitis-like skin inflammation (Nakamura et al. 2013; Liu et al. 2017; Nakagawa et al. 2017). Therefore, the Th2 cytokine environment can promote S. aureus colonization and superinfection and SAgs and cytolytic toxins of S. aureus also contribute to inducing Th2-mediated skin inflammation.
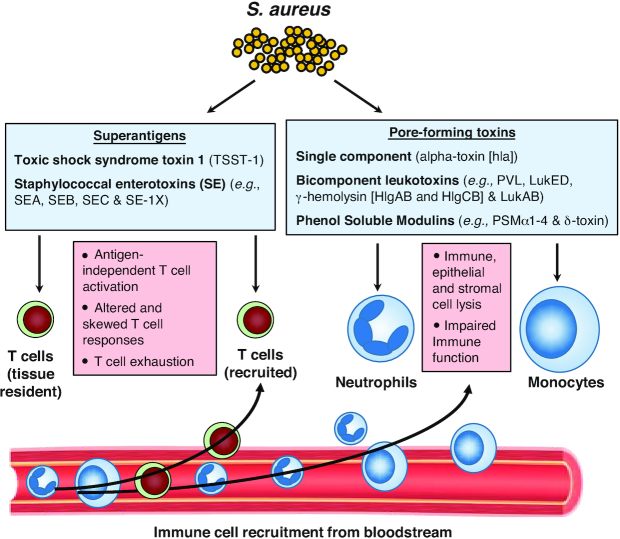
Figure 3.
S. aureus superantigens (SAgs) and pore-forming toxins (PFTs). S. aureus produces SAgs (including Toxic shock syndrome toxin 1 [TSST-1] and Staphylococcal enterotoxins [SE]) that crosslink the Vβ chain of T cell receptors (TCRs) from tissue resident and recruited T cells to MHCII molecules on antigen-presenting cells (APCs), leading to antigen-independent stimulation of T cells and APCs with massive production and release of many different cytokines. The activity of SAgs is a S. aureus immune evasion mechanism of T cell responses as it leads to altered and skewed T cells responses and exhaustion. Staphylococcus aureus also produce single component α-toxin, bicomponent leukocidins (luk) and phenol soluble modulins (PSMs) that result in host cell lysis and inflammatory activation. The activity of PFTs is a S. aureus immune evasion mechanism to counter the host defense activity of epithelial, stromal and immune cells.
Third, human Th17 cells and IL-17A/F responses likely contribute to protective immunity against S. aureus infections, especially against S. aureus skin, mucosal and soft tissue infections (Miller and Cho 2011) (Fig. 2). Th17 cells are induced to differentiate and expand following stimulation of naïve T cells with a combination of cytokines (e.g. IL-6, IL-21 and IL-23, which signal via STAT3, as well as TGFβ and IL-1β) to induce the key transcription factor RORγt (Patel and Kuchroo 2015). With respect to S. aureus skin infections, there have been several primary immunodeficiency disorders with reduced numbers of Th17 cells and/or impaired IL-17A/F responses that are characterized by recurrent S. aureus skin infections (and in some cases, increased S. aureus lung infections), including patients with defects in IL-6 receptor α (IL-6Rα) or antibodies against IL-6 (Puel et al. 2008; Spencer et al. 2019), defects in GP130 (the IL-6 co-receptor) (Schwerd et al. 2017), patients with autosomal dominant hyper-IgE syndrome with dominant-negative mutations in STAT3 (Ma et al. 2008; Milner et al. 2008; Renner et al. 2008) as well as patients with IL-17RA or IL-17F deficiency (Puel et al. 2011; Levy et al. 2016). However, in the patients with specific deficiency in IL-17RA or IL-17F, they primarily suffer from mucocutaneous candidiasis to a much greater extent than S. aureus skin infections (Puel et al. 2011; Levy et al. 2016). The primary mechanism by which IL-17A and IL-17F promote host defense against S. aureus skin infections involves the recruitment of neutrophils to the site of infection as well as enhancing increased expression of HDPs (Minegishi et al. 2009). In normal humans without these genetic diseases, the mechanisms by which S. aureus antigen-specific Th17 cells are generated is an active area of investigation. In humans, IL-1β, IL-6 and IL-23 have been shown to promote the differentiation of S. aureus-specific Th17 cells isolated from human blood (Zielinski et al. 2012). In mice, S. aureus infection of mouse skin triggered Langerhans cells from the epidermis of mouse skin to produce IL-6, IL-1β, and IL-23, which promoted Th17 differentiation (Igyarto et al. 2011). Human langerin (CD207), which is specifically expressed on human Langerhans cells that normally reside in the epidermis, recognizes WTA of S. aureus to promote proinflammatory immune responses (van Dalen et al. 2019). The importance of the Th cell response against S. aureus is further supported by an immune evasion mechanism in which O-acetylation of cell wall S. aureus PGN limits the induction of pro-inflammatory signals that were required for optimal Th17 (and Th1) polarization to provide host defense against a subsequent SAB challenge in mice (Sanchez et al. 2017). A similar mechanism might also occur in humans.
Finally, IFNγ produced by Th1 cells has also been implicated in immunity against various different types of S. aureus infections (Fig. 2). For example, in humans, several studies have also indicated that IFNγ correlated with protection against S. aureus skin infections and bacteremia (Brown et al. 2015, Utay et al. 2016; Uebele et al. 2017; Dillen et al. 2018). Similarly, in mouse models of surgical site infection or bacteremia, IFNγ was found to promote host defense by promoting neutrophil recruitment (McLoughlin et al. 2006; McLoughlin et al. 2008; Lin et al. 2009). Another study in mice found that IFNγ produced by Th1 cells resulted in increased lethality in the setting of vaccine-induced immunity against a SAB infection (Karauzum et al. 2017). Whether Th1 cells cause a similar deleterious response during vaccine-induced immunity against invasive S. aureus infections in humans is not entirely clear but this possibility should be taken into account in future clinical trials of S. aureus vaccines.
Unconventional T cells in immunity against S. aureus
There is emerging evidence that unconventional T cells such as γδ T cells and mucosa-associated invariant T (MAIT) cells contribute to host defense against S. aureus (Fig. 2) (Lalor and McLoughlin 2016; O'Brien and McLoughlin 2019). For example, in mouse models of S. aureus skin or peritonitis infection, a population of Vγ6+Vδ4+ T cells (IMGT nomenclature) expanded in the lymph nodes and trafficked back to the site of infection where they produced IL-17 to promote neutrophil recruitment and bacterial clearance (Murphy et al. 2014; Dillen et al. 2018; Marchitto et al. 2019). In the peritonitis model, the Vγ6+Vδ4+ T cells had memory-like function as they protected against subsequent S. aureus peritonitis challenges (Murphy et al. 2014). Interestingly, in IL-1β-deficient mice, the Vγ6+Vδ4+ T cells that expanded were clonal (expressing a single TCRγδ complementarity determining region [CDR3] amino acid sequence) and induced long-term protection (lasting at least 140 days) against a subsequent S. aureus infection by producing increased TNF and IFNγ (rather than IL-17) and induced neutrophil recruitment mediated clearance (Dillen et al. 2018). Similar to IL-1β-deficient mice, individuals with loss-of-function mutations in IRAK4 who previously suffered recurrent S. aureus skin infections in childhood, there was an expansion of circulating Vδ2+ γδ T cells that produced more TNF and IFNγ than Vδ2+ γδ T cells from normal individuals (Dillen et al. 2018). These results suggest that TNF and IFNγ produced by Vδ2+ γδ T cells might provide protection against S. aureus skin infections (and potentially other sites of infection) in humans. Consistent with this possibility, a prior report found that mice with severe combined immunodeficency (i.e. SCID mice that lack T and B cells), adoptively transferred human Vδ2+ γδ T cells (expanded with pamidronate treatment) were able to protect against lethality during a S. aureus systemic infection (Wang et al. 2001). Taken together, these studies in mice and humans suggest that γδ T cells and their production of IL-17, TNF and/or IFNγ could provide durable and long-term immunity against S. aureus skin and systemic infections.
In addition to γδ T cells, mouse and human MAIT cells are abundant T cells in the liver (up to 40% of resident cells), mucosal sites such as the lung and gut and represent up to 10% of circulating T cells (Downey, Kaplonek and Seeberger 2019; Godfrey et al. 2019). MAIT cells possess a restricted set of Vβ TCR chains and recognize vitamin B2 (riboflavin) derivatives presented by the MHC-I related protein, MR1. Importantly, MAIT cells are a substantial source of IL-17, TNF and IFNγ in inflammatory, autoimmune and infectious diseases, suggesting a potential role in host defense against S. aureus, especially at mucosal sites (Fig. 2) (Dias et al. 2018). A previous report found that mouse and human MAIT cells are uniquely hyper-responsive to S. aureus SAgs (especially SEB) and induced a cytokine storm with high production of IFNγ, TNF and IL-2 to a much greater extent than conventional T cells, NK T cells or γδ T cells (Shaler et al. 2017). Interestingly, the SAg-stimulated MAIT cells acquired a molecular signature of exhaustion, which rendered them anergic and unable to mediate effective host defense (Shaler et al. 2017). Indeed, ICU patients that showed MAIT cell exhaustion were then more prone to infections from other bacteria during their ICU stay (Grimaldi et al. 2014; Kim and Oldham 2019). Therefore, S. aureus SAgs provide an immune evasion mechanism against the protective immune responses of MAIT cells.
Cystic fibrosis: a mucosal immunodeficiency disease with an increased susceptibility to S. aureus infection
Cystic fibrosis (CF) is an autosomal recessive disease with mutations in the transmembrane conductance regulator (CFTR) gene. The gene product CFTR forms a channel for chloride and water, and mutations of CFTR in CF leads to improper movement of water across the lung epithelium, leading to the production to thick mucus and frequent pulmonary infections usually caused by S. aureus and Pseudomonas aeruginosa. In CF patients, S. aureus lung infections are associated with decreased survival (Dasenbrook et al. 2010; Pillarisetti et al. 2011; Junge et al. 2016). There is emerging evidence that CFTR dysfunction in CF leads to a primary defect in lung mucosal immunity that is associated with multiple impairments in neutrophil function (Table 2) (Cohen and Prince 2012). For example, the lungs of CF patients have increased levels of IL-8 and TNF that promote excessive neutrophil recruitment and activation (Bonfield et al. 1995; Schuster, Haarmann and Wahn 1995; Berger 2002). In addition, neutrophil-derived reactive oxygen species (ROS) damage the lung epithelium because they are not neutralized by host antioxidants (such as glutathione and thiocyanate), which are normally transported to the epithelial-lining by functional CFTR channels (Gao et al. 1999; Xu et al. 2009). Neutrophil-derived proteases in lungs of CF patients can also cleave macrophage surface receptors that impairs their phagocytic and bacterial killing capabilities (Vandivier et al. 2002). The lungs of CF patients also have Th17 cell dysfunction with overproduction of IL-17, which also increases neutrophilic inflammation and tissue damage (McAllister et al. 2005; Decraene et al. 2010; Tan et al. 2011). Finally, there are increased neutrophil extracellular traps (NETs) in the lungs of CF patients that contain proteases and HDPs, which facilitate bacterial killing but also exacerbate tissue injury (Law and Gray 2017; Gray et al. 2018). Taken together, the aberrant neutrophil function in CF patients contributes to the increased susceptibility to chronic S. aureus infections.
The predisposition to S. aureus infections that is associated with the impaired mucosal immunity in the lungs of CF patients is supported by the identification of S. aureus adaptation mechanisms that promote bacterial survival and persistent infections. In the chronically infected lungs of CF patients, S. aureus strains have been isolated that have a mucoid phenotype that resist neutrophil-mediated killing (Schwartbeck et al. 2016; Lennartz et al. 2019). Staphylococcus aureus strains isolated from lungs of CF patients also develop a small colony variant (SCV) phenotype (with defective electron transport) that permits survival within biofilms and host respiratory epithelial cells, shielding the S. aureus bacteria from antibiotics and immune defenses (Kahl et al. 1998; von Eiff, Peters and Becker 2006; Besier et al. 2007; Mitchell et al. 2011; Akil and Muhlebach 2018). Of note, neutrophil uptake of SCVs is reduced compared with normal S. aureus strains (Ruotsalainen et al. 2008). Most recently, whole genome sequencing of S. aureus isolates from CF patients revealed that S. aureus undergoes substantial metabolic adaptation to generate ATP, produce biofilms and evade immune responses in this unique airway environment (Gabryszewski et al. 2019).
Conclusions based on the findings in humans susceptible to S. aureus infections
In summary, a pattern of emerges wherein increased incidence and severity of S. aureus infections occurs in humans who have specific impairments in immune cell function. These protective immune cell types mainly include cells that function in innate immunity such as keratinocytes, neutrophils and monocytes/macrophages. In addition, adaptive immune cells such as T cells can influence the balance between protective (e.g. Th1 and Th17 cells) and deleterious (e.g. Th2 cell) cytokine immune responses. Although B cell antibody responses might contribute to host defense against S. aureus infections through opsonization or neutralizing S. aureus toxins, it is striking that patients with primary or secondary immunodeficiencies with defective antibody production are not characterized by an increased susceptibility to S. aureus infections. Hence, the evidence to develop of vaccines based upon opsonic antibodies is not fully supported by the clinical findings in humans with an increased susceptibility to S. aureus infections.
DIFFERENTIAL CYTOKINE LEVELS CORRELATE WITH CLINICAL OUTCOME IN S. aureus BACTEREMIA
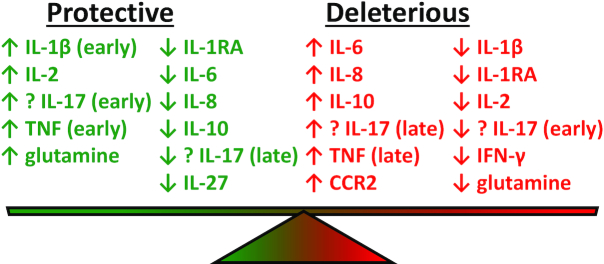
The serum levels cytokines in patients with invasive S. aureus infections are summarized in Table 3 and Fig. 4. Nine of the ten studies examined patients with SAB, and most of the studies measured cytokine levels early (day of first positive culture) and later in the course (3–4 days after the first culture) (Rose et al. 2012; Fowler et al. 2013; McNeely et al. 2014; McNicholas et al. 2014; Minejima et al. 2016; Chantratita et al. 2017; Rose et al. 2017; Greenberg et al. 2018; Guimaraes et al. 2019; Volk et al. 2019). Since patients can be enrolled into a clinical study at different times during the infectious course with variable antibiotic treatment and because blood samples are not drawn at the exactly the same time, there is inherent variation among the results of the different studies. Nevertheless, several trends have become apparent. Survival and/or a less complicated course of SAB infection correlated with early rises in the pro-inflammatory cytokines IL-1β, IL-2, IL-6, TNF, glutamine and decreased levels of IL-IRA, IL-6, IL-8, IL-10 and IL-27. Although reported in only one study, the correlation of low IL-27 levels with improved outcome (Guimaraes et al. 2019) is compatible with the low IL-10 levels as IL-27 induces IL-10 production by CD4+ T cells (Yoshida and Hunter 2015). Conversely, a more severe and complicated course (including persistent bacteremia and death) correlated with high early levels of IL-6, IL-8, IL-10 and CCR2, high late levels of TNF and low levels of IL-1β, IL-1RA, IL-2, IFNγ and glutamine.
Table 3.
Serum cytokines levels and correlations with clinical outcomes in SAB.
| Study | Type of infection | Survival or less complicated course | Death or complicated course |
|---|---|---|---|
| (Soderquist, Sundqvist and Vikerfors 1992) | 65 patients with SAB | ↓ IL-6 | ↑ IL-6 (persistent); ↑ IL-8 (trend) |
| (Rose et al. 2012); (Rose et al. 2017); (Volk et al. 2019) | 59 patients with SAB113; 133 patients with SAB; 59 patients with SAB, respectively |
↑ IL-1β and ↓ IL-10 (days 0, 3 and 7) | ↓ IL-1β; ↑ IL-10 (days 0, 3 and 7) |
| (Fowler et al. 2013); (McNeely et al. 2014) | IsdB vaccine trial: invasive S. aureus infections after cardiothoracic surgery | ↑ preoperative IL-2 and IL-17A | ↓ (undetectable) preoperative IL-2 and IL-17A |
| (McNicholas et al. 2014) | 61 patients with SAB | ↓ IL-6 (day 1) | ↑ IL-6 |
| (Minejima et al. 2016) | 196 patients with SAB | ↓ TNF and IL-10 (day 1) | ↑ TNF, IL-6, IL-8 and IL-10 (day 3) |
| (Chantratita et al. 2017) | 327 patients with SAB | ↓ IL-6 and IL-8 | ↑ IL-6 and IL-8 |
| (Scott et al. 2018) | 168 patients with SAB | ↑ glutamine (increases IL-1β) | ↓ glutamine (Gls2 genetic variant) |
| (Greenberg et al. 2018) | 95 patients with SAB with flow cytometry in 28 patients | ↓ IL-6 and IL-17A (days 2–4); ↓ IL-6 (days 6–9); ↓ Th17/Treg ratio (day 6) | ↑ IL-17A (day 2) and IL6 (days 6–9); ↑ Th17/Th1 ratio; ↑ Th17/Treg ratio |
| (Guimaraes et al. 2019) | 156 patients with SAB | ↓ IL-10, IL-1RA, IL6, IL-27 (days 1–3) | ↑ IL-6, IL-8, IL-10, CCL2 (↑ mortality); ↑ IL-17A (persistent bacteremia) |
Figure 4.
Serum cytokines levels in patients with S. aureus bacteremia (SAB) and their correlation with clinical outcome. ↑ (up arrow) = relatively increased cytokine level. ↓ (down arrow) = relatively decreased cytokine level. Green text = protective clinical outcome. Red text = deleterious clinical outcome. Early = the cytokine level within the first 3 days following the diagnosis of SAB. Late = the cytokine level after the first 3 days following the diagnosis of SAB. IL = interleukin. IL-1RA = interleukin-1 receptor antagonist. TNF = tumor necrosis factor. IFN-γ = interferon γ. CCR2 = C-C chemokine receptor type 2.
Regarding the specific role of T cell subsets and cytokines, the low serum levels of IL-2 (which induces proliferation and activation of both pro-inflammatory and anti-inflammatory T cell subsets) in patients who received the IsdB vaccine were associated with mortality when they developed an invasive S. aureus infection (McNeely et al. 2014). Therefore, the low levels of IL-2 might have predisposed the patients to a poor outcome following vaccination, suggesting that the baseline and post-vaccination cytokine levels of patients should be evaluated in future vaccine trials. Another study found that an increased neutrophil count-to-lymphocyte count ratio along with increased Th17 cells relative to Th1 cells or Tregs were each independently associated with increased mortality during an S. aureus SAB infection (Greenberg et al. 2018). The serum levels of IL-17A and how they correlate with disease outcome following a SAB infection are somewhat controversial but most studies have suggested that early high levels of IL-17A and late lower levels of IL-17A are associated with a better outcome whereas high late levels of IL-17A and early lower levels of IL-17A are associated with a more severe or complicated course (McNeely et al. 2014; Greenberg et al. 2018; Guimaraes et al. 2019). As mentioned above in human and mouse studies, IL-17 responses likely play a more important role in host defense against S. aureus skin infections rather than host defense against bacteremia (Cho et al. 2010; Puel et al. 2011; Montgomery et al. 2014; Chan et al. 2015; Marchitto et al. 2019).
The most dramatic conclusions that can be drawn from these studies is that a more complicated clinical course and death from S. aureus SAB relates to profound cytokine imbalances. With SAB, monocytes/macrophages likely contribute to host defense against the S. aureus infection, yet levels of IL-1β were typically not increased (Rose et al. 2012), which was clearly to the detriment of these patients. However, in SAB patients treated with antibiotics, high levels of IL-10 but not IL-1β predicted mortality (Volk et al. 2019). In ex vivo blood samples, blockade of IL-1β with a synthetic IL-1RA (Anakinra) resulted in enhanced S. aureus killing, supporting a host defense role for IL-1β in bacterial clearance (Volk et al. 2019). Similarly, blockade of IL-1β with Anakinra worsened S. aureus pneumonia by slowing bacterial clearance (Labrousse et al. 2014). Interestingly, S. aureus isolates that developed decreased susceptibility to vancomycin, the primary agent to treat MRSA, reduced NF-κB activation and TNF and IL-1β expression ex vivo (Howden et al. 2008). This same phenomenon was displayed in a mouse sepsis model where reduced vancomycin susceptibility attenuated IL-6 responses resulting in higher organism burdens and persistent infection (Cameron et al. 2017). Regarding TNF, another important pro-inflammatory cytokine, a single report found that persistently elevated TNF levels later in the course of S. aureus SAB predicted a worse outcome (Minejima et al. 2016). Collectively, these results suggest that there are significant innate host–pathogen interactions, and it is critical to have a pro-inflammatory cytokine response early on during the bacteremia (e.g. IL-1β, IL-2, IL-6, TNF and glutamine). However, persistently high levels of some of these same cytokines (e.g. IL-6 and TNF) as well as IL-8, IL-10, IL-17 and CCR2 are detrimental, as they might contribute to systemic inflammatory response syndrome and death.
ROLE OF S. aureus ANTI-TOXIN ANTIBODIES IN REDUCING DISEASE SEVERITY
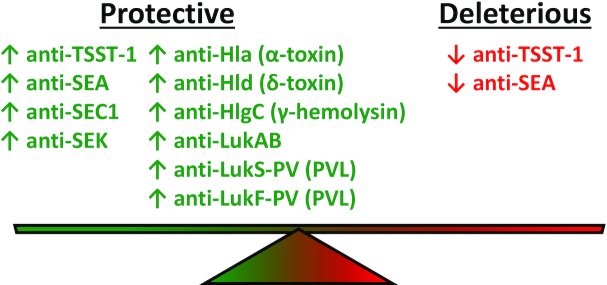
Data from human subjects predisposed to S. aureus infections (Table 2, Figs. 1 and 2) and the serum cytokine levels from clinical studies of patients with SAB (Table 3) suggest that innate immune cells, T cells and differential cytokine levels contribute to protection against S. aureus infections. However, given that S. aureus toxins have profound cytolytic and proinflammatory effects on cells and cause tissue damage and injury during S. aureus infections, it is important to consider their pathogenic role during infection as well as the therapeutic potential of toxin neutralization. In this section, the role of the S. aureus SAg TSST-1 as well as S. aureus PFTs that damage host membranes often resulting in lysis, including (i) α-toxin and (ii) bicomponent leukotoxins, such as PVL, LukED, HlgAB and HlgCB (that comprise γ-hemolysin) and LukAB (Aman and Adhikari 2014; Spaan, van Strijp and Torres 2017) in human S. aureus infections will be reviewed. The role of SAgs and PFTs in increasing the severity of S. aureus infections is summarized in Table 4 and Fig. 3. In addition, the role of cytokine levels in SAB and how they correlate with disease severity and clinical outcome is summarized in Table 3 and Fig. 4. Lastly, the impact of anti-S. aureus toxin neutralizing antibodies on the disease severity of various S. aureus infections are summarized in Table 5 and Fig. 5.
Table 4.
Summary of worldwide studies on the impact of S. aureus toxins on disease severity. The bolded text indicates the type of S. aureus infection.
| Disease | Study details | Comment | Reference |
|---|---|---|---|
| S. aureus Bacteremia (SAB) | |||
| Increased disease severity | 80 patients with SAB (19 with septic shock and 61 with bacteremia only) (retrospective) | SEA-positive strains highly correlated with sepsis. | (Ferry et al. 2005) |
| 22 pediatric patients with MRSA bacteremia (retrospective) | PVL-positive isolates were associated with vancomycin treatment failure. | (Welsh et al. 2010) | |
| Possible impact on disease severity | 266 patients colonized with S. aureus and 46 of these patients that developed (retrospective) | PVL-positive strains correlated with a decrease time to develop SAB, but was not associated with infections at other sites, length of hospital stay or mortality. | (Blaine et al. 2010) |
| No impact on disease severity | 230 patients (141 MSSA and 80 MRSA) in North America and Europe (prospective) | PVL-positive strains had better outcome and less persistent bacteremia; Patients with USA300 PVL-positive strains were more likely to be intravenous drug users (IVDU). | (Lalani et al. 2008) |
| 113 adult patients (retrospective) | PVL-positive isolates were not associated with a relapse of infection | (Welsh et al. 2011) | |
| Pneumonia | |||
| Increased disease severity | 52 patients (retrospective and prospective) | PVL-positive (16 patients) in France with more rapid hemorrhagic, necrotizing pneumonia in otherwise healthy children and young adults often with preceding influenza compared with PVL-negative cases (36 patients). | (Gillet et al. 2002) |
| 14 adolescent patients with severe (septic) community-acquired infections (retrospective) | Pulmonary and/or bone involvement was found in 13 of 14 cases. 100% were caused by PVL-positive isolates and these were associated with 21% mortality. | (Gonzalez et al. 2005b) | |
| 113 pediatric patients with community-acquired MRSA or MSSA with pulmonary involvement (prospective) | PVL-positive infections had abnormal findings on pulmonary imaging in 64% of cases compared with PVL-negative cases of only 9% | (Gonzalez et al. 2005a) | |
| 17 cases S. aureus community-acquired pneumonia with influenza-type symptoms (case series) | PVL, staphylococcal enterotoxins or TSST-1 were found in all infecting isolates. However, PVL was the only toxin found in 85% of these isolates. 71% had laboratory evidence of influenza infection. Overall, there was mortality of 29%. | (Hageman et al. 2006) | |
| 10 cases of severe MRSA community-acquired pneumonia associated with an influenza-like illness (case series) | 100% of the MRSA isolates were PVL-positive and there was a high mortality (60%) with 60% laboratory-confirmed influenza. | (Pogue et al. 2007) | |
| 50 patients (retrospective) | All PVL-positive patients in France. Airway bleeding, erythroderma and leukopenia were associated with fatal outcome from necrotizing pneumonia. | (Gillet et al. 2007) | |
| 40 patients with newly acquired MRSA lung isolates (all children with cystic fibrosis) (prospective) | Cystic fibrosis patients with MRSA isolates that were PVL-positive were more likely to have invasive lung infections, including lung abscesses. | (Elizur et al. 2007) | |
| 51 cases of community-acquired S. aureus pneumonia ± influenza-like illness (case series) | 79% of isolates were due to MRSA. Of the 17 MRSA and 1 MSSA isolates examined for PVL genes, all but one were PVL-positive. Overall, there was a high (51%) mortality and an associated influenza-like infection in 47%. | (Kallen et al. 2009) | |
| 114 patients (retrospective) | Previous PVL-positive skin infection (furuncle) in the Netherlands was associated with less death and severity of PVL-positive pneumonia. | (Rasigade et al. 2011) | |
| 133 patients (retrospective) | All PVL-positive S. aureus community-acquired pneumonia patients (104 MSSA and 29 MRSA) in France with high lethality of 39% of all PVL-positive cases regardless of the presence or absence of methicillin resistance. | (Sicot et al. 2013) | |
| 10 cases with MRSA community-acquired pneumonia (case series) | PVL-positive in 80% of cases and there was 20% mortality, 70% empyema and 22.5-day length of hospital stay. | (Toro et al. 2014) | |
| 50 patients (all children with cystic fibrosis) (prospective) | In cystic fibrosis patients, LukAB, α-toxin and PVL antigen titers were all increased if S. aureus was detected at the time of the pulmonary exacerbation. | (Chadha et al. 2016) | |
| 152 patients (all children) (prospective) | PVL-positive S. aureus pneumonia in Spain was associated with invasive infection leading to death or admission to intensive care due to hemodynamic instability or respiratory failure compared with PVL-negative cases, irrespective of MSSA or MRSA. | (Gijon et al. 2016) | |
| 100 patients (observational, retrospective study) | Hospital-acquired and ventilator-associated pneumonia due to MRSA were compared in China. PVL-positive infections had a shorter interval between diagnosis and death than PVL-negative infections. | (Zhang et al. 2016) | |
| Possible impact on disease severity | 22 patients (prospective, case-control study) | Trend towards more severe infection with requirement of intensive care unit admission and longer duration of hospital stay with PVL-positive versus than PVL-negative cases. PVL-positive cases were also younger in age. | (Wehrhahn et al. 2010) |
| 117 patients (all children) (retrospective) | Most infections of community-acquired S. aureus pneumonia were due to USA300 MRSA strains that were PVL-positive in 95.5%. 88% improved with treatment, 5% recurred, 6% respiratory sequelae and 1% mortality. | (Carrillo-Marquez et al. 2011) | |
| No impact on disease severity | 55 patients (all children) (retrospective) | Community-acquired S. aureus pneumonia in China. PVL-positive strains (not USA300) were not associated with more severe or necrotic disease. | (Geng et al. 2010) |
| 30 patients (retrospective) | Hospital-acquired S. aureus pneumonia in Singapore. Only 5% of cases were PVL-positive (not USA300). | (Hsu et al. 2005) | |
| 34 patients (all children with cystic fibrosis) (prospective) | In cystic fibrosis patients, isolation of PVL-positive MRSA strains were not associated with pulmonary exacerbation, including necrotizing pneumonia or lung abscesses. | (Glikman et al. 2008) | |
| 12 patients (prospective, observational) | Community-acquired pneumonia in Thailand (not USA300) with higher all-cause mortality associated with MRSA but PVL-positive strains had lower all-cause mortality compared with PVL-negative strains. | (Nickerson et al. 2009) | |
| 109 patients (retrospective, observational) | Hospital-acquired pneumonia/ventilator-associated pneumonia infected with MRSA in U.S.A. (33% USA300) in which the clinical outcome was not influenced by the presence or absence of PVL (mortality was 10% in both). | (Peyrani et al. 2011) | |
| 287 S. aureus isolates from patients with S. aureus hospital-acquired pneumonia (retrospective) | PVL and 30 other virulence genes were screened, and there was no correlation with clinical outcomes with the presence of any of the 30 genes, including PVL, α-toxin, δ-toxin. | (Sharma-Kuinkel et al. 2012) | |
| Skin Infection | |||
| Increased disease severity | 98 pediatric patients (prospective) | Exfoliative toxin b (ETB)-positive strains were associated with more severe impetigo. | (Koning et al. 2003) |
| Enrolled 59 skin and soft tissue infections from children with gentamicin-susceptible MRSA in Australia (prospective) | PVL-positive in 86% of skin and soft tissue isolates. PVL-positive and PVL-negative strains had no difference in length of hospital stay. 40% of PVL-positive strains whereas only 13% of PVL-negative strains required surgery. | (Gubbay et al. 2008) | |
| 204 skin and soft tissue infections (96 PVL-positive and 98 PVL-negative) (prospective) | PVL-positive isolates caused more abscesses (73% versus 27%) and surgical intervention (81% versus 53%) versus PVL-negative isolates. | (Jahamy et al. 2008) | |
| 384 MRSA isolates and 192 matches MSSA isolates | PVL-positive strains were more commonly associated with furunculosis (59% versus 10%) and required surgical treatment (67% versus 44%) versus PVL-negative strains. | (Munckhof et al. 2008) | |
| 57 patients with S. aureus skin abscesses (prospective) | Primary skin abscesses are mainily caused by PVL-positive S. aureus strains as PVL-positive strains were detected in 38 of 41 primary infections and only 2 of 16 secndary infections. | (del Giudice et al. 2009) | |
| 526 of CA-MRSA isolates from a Finland population study (retrospective) | PVL-positive strains were more commonly associated with an infection (90% verus 52%) and surgery (57% versus 32%) versus PVL-negative strains. | (Kanerva et al. 2009) | |
| 522 patients. International study. (retrospective) | 83% USA300 and 89% PVL-positive strains. PVL-positive strains were more likely to be in young patients, from North America and presented with larger abscesses. | (Bae et al. 2009) | |
| 134 MSSA isolates from paitents in New Zealand (retrospective) | PVL-positive strains were associated with younger age, had a community onset infection and skin and soft tissue infections required surgical treatment more often (60% versus 28%) versus PVL-negative strains. | (Muttaiyah et al. 2010) | |
| 239 CA-MRSA isolates collected in Australia (prospective) | PVL-positive strains were associated with community-acquired disease, younger age, presentation with sepsis and presence of an abscess (50% versus 7%) compared with PVL-negative strains. | (Tong et al. 2010) | |
| 25 patients with furuncles versus 30 patients with infected dermatitis (HIV-positive and HIV-negative patients) (prospective) | PVL-positive isolates were found in 96% of S. aureus isolates from HIV-positive patients versus only 10% of S. aureus isolates from infected dermatitis. | (Baba-Moussa et al. 2011) | |
| 101 S. aureus skin and soft tissue infection isolates (retrospective) | PVL-positive strains were MRSA (77%) and MSSA (36%). Incision and drainage was higher for PVL-positive than PVL-negative MSSA strains (81% versus 57%). | (Kaltsas et al. 2011) | |
| 473 patients with S. aureus skin and soft tissue infections. International study. (retrospective) | PVL-positive strains were associated with larger abscess size. Cure rates of PVL-positive and PVL-negative strains were similar. | (Tong et al. 2012) | |
| 96 S. aureus isolates from skin and soft tissue infections and bacteremia infections (adults and children) (retrospective) | Expression levels of the genes (lukS-PV mRNA) for PVL were higher among skin and soft tissue isolates versus with blood isolates, community-acquired versus hospital-acquired isolates and children versus adults. | (Yu et al. 2013) | |
| 10 patients from Japan with CA-MRSA PVL-positive infection (case report) | PVL-positive CA-MRSA strains in Japan and 8 of the 10 cases involved severe skin infections. | (Nakaminami et al. 2017) | |
| No impact on disease severity | 207 S. aureus isolates collected from ulcerative upper-extremity infections in Greece | PVL-positive strains increased over the 4 year study but no increase in hospitalizations during that time period (most isolates belonged to the ST-80 clone) | (Dailiana et al. 2008) |
| 90 isolates from FAST II trial of S. aureus skin infections | High prevelance of PVL-positive strains, but PVL were more associated with a cure than strains from patients that failed or had an inderminant outcome. | (Campbell et al. 2008) | |
| Pyomyositis | |||
| Increased disease severity | 24 patients with pyomyositis and myositis (all children in Houston, TX) (retrospective) | PVL-positive strains required more surgical draining procedures (81%) versus PVL-negative strains (38%). | (Pannaraj et al. 2006) |
| Nasal and pharyngeal swabs from 141 patients with HIV and 206 healthy controls from patients in Sub-Saharan Africa (retrospective) | PVL-positive strain colonization were more commonly seen in HIV positive patients and had more frequent skin and soft tissue infections and patients with PVL-negative strain colonization. | (Kraef et al. 2015) | |
| 101 patients with pyomyositis versus 417 children with asymptomatic S. aureus nasal carriage (all children from Cambodia) (retrospective) | The presence of a PVL-positive S. aureus strain increased the odds of developing pyomyositis by 130-fold. | (Young et al. 2019) | |
| Osteomyelitis | |||
| Increased disease severity | 100 patients with S. aureus nasal colonization and 86 patients with S. aureus infection from the Democratic Republic of the Congo (prospective) | PVL-positive strains (and strains that were positive for β-hemolysin) were more commonly associated with skin and soft tissue infections and recurrent disease than PVL-negative strains. | (Lebughe et al. 2017) |
| 59 patients with musculoskeletal infections (all children) (retrospective) | PVL-positive strains had more complications than PVL-negative strains. | (Martinez-Aguilar et al. 2004) | |
| 89 patients with osteomyelitis (all children) (prospective) | PVL-positive isolates (66%) associated with higher erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels and were more likely to have positive blood cultures and concomitant myositis or pyomyositis versus PVL-negative isolates. | (Bocchini et al. 2006) | |
| 14 patients with PVL-positive strains versus 14 PVL-negative strains with osteomyelitis and septic arthritis infections (retrospective) | PVL-positive bone and joint infections were more severe infections with sepsis, more deep-seated infections, prolonged treatment and longer hospital stays. | (Dohin et al. 2007) | |
| 98 patients (all children) (prospective) | PVL-positive (87.1%) of total isolates and 85% (68/81) of PVL-positive cases (all USA300) versus 47% (8/17) of PVL-negative cases required surgical intervention. | (Abdel-Haq et al. 2009) | |
| 139 S. aureus isolates from ostemyelitis infections (retrospective) | lukSF-PV, bbp, sei genes were associated with longer duration of osteomyelitis and more serious inflammatory responses. | (Jiang et al. 2017) | |
| Studies with inclusion of different types of S. aureus infections | |||
| Increased disease severity | 346 isolates from skin infections, septicemia and symptomatic nasal carriers (prospective) | 58 isolates were PVL-positive and 86% of these were associated with skin infections (primarily furuncles). PVL-positive strains were not associated with septicemia or nasal carriage. | Prevost et al. 1995) |
| 172 PVL-positive strains collected from of different types of S. aureus infections (retrospective) | PVL-positive strains were associated in 93% of skin infections (furunculosis, cellulitis and cutaneous abscesses) and in 85% of severe necrotizing pneumonia. No association of PVL-positive strains with endocarditis, mediastinitis, hospital-acquired pneumonia, urinary tract infection, enterocolitis or toxic shock syndrome. | (Lina et al. 1999) | |
| 1321 hospitalized patients with various infections community-associated and hospital-associated MRSA and MSSA strains (retrospective) | The presence of PVL-positive strains was associated with double the odds of sepsis. | (Tong et al. 2009) | |
| 78 intensive care unit (ICU) patients with different types of S. aureus infections (case control study) | The detection of plasma SAgs (SEA, SEB, SEC or TSST-1) were found in 42% of patients with septic shock and 31% of patients with sepsis but without shock. | (Azuma et al. 2004) | |
| Possible impact on disease severity | 173 cancer patients with different MRSA invasive infections | There was no difference in response to treatment (including in neutropenic patients) between infections caused PVL-positive and PVL-negative strains. | (Campo et al. 2011) |
| No impact on disease severity | 162 MSSA isolates from patients with skin and soft tissue infections (SSTI), hospital-aquired pneumonia and infective endocarditis (IE) (retrospective) | There was no associations between PSMα1–4 and clincial outcome among any of the different infections. Isolates from SSTI had highest levels of PSMα1–4 as compared with IE. PSMα1–4-positive strains had larger SSTI lesions. | (Qi et al. 2016) |
| Decreased disease severity | 270 patients with different types of invasive S. aureus infections in Thailand (prospective) | PVL-positive strains were associated with less mortality (11% versus 39%). Mortality was associated with older patients, underlying cardiac disease, repiratory infection. Patients that had one or more abscesses as the presenting source of infection were associated with survival. | (Nickerson et al. 2009) |
Table 5.
Anti-Staphylococcus aureus toxin antibodies associated with reduced disease severity.
| Study | Study Design | Anti-toxin antibodies and clinical outcome |
|---|---|---|
| (Bergdoll et al. 1981) (Stolz et al. 1985) | 181 cases of tampon-associated TSS (toxic shock syndrome). (retrospective) | Gradual and low rate (9.5%) developed acute anti-TSST-1 antibodies and many had sustained anti-TSST-1 titers 1 year after TSS (62.7%). Women with anti-TSST-1 antibodies had less TSS and fewer deaths. |
| (Christensson, Hedstrom and Kronvall 1983) | 119 patients with S. aureus sepsis versus 22 patients with non-S. aureus sepsis. (comparative study) | Patients that survived sepsis had higher anti-Hla (α-toxin) Abs compared with the non-S. aureus sepsis group. |
| (Bonventre et al. 1984) | 38 women with TSS versus 70 women without history of TSS. (retrospective) | Low anti-TSST-1 antibody titers were associated with development of TSS. |
| (Ruotsalainen et al. 2008) | 430 patients with SAB in which 44 were intravenous drug users (IVDU) and 44 non-IVDU compared. 98% of isolates were PVL-positive. (retrospective) | IVDU developed high titers of anti-α-toxin antibodies that were associated with protection against endocarditis as these patients had less endocarditis (44%) compared with the patients that developed endocarditis (6%). |
| (Jacobsson et al. 2010) | 150 patients with invasive S. aureus infections versus 115 controls. (prospective) | Antibodies against teichoic acid, SEA, and lipase had 3–4 fold reduced mortality. Anti-Hla antibodies had no significant effect |
| (Rasigade et al. 2011) | 114 cases necrotizing pneumonia. (retrospective) | Death and severity factors (need for mechanical ventilation and inotropic support) was less frequent in patients with prior PVL-associated infection (furuncle) than in those without, suggesting that pre-existing immunity to PVL might protect against a subsequent PVL-positive S. aureus pneumonia. |
| (Adhikari et al. 2012) | 100 patients with SAB (27 developed sepsis versus 73 who did not develop sepsis) (prospective) | High antibody titers against α-toxin (Hla), Hld, PVL, SEC-1 and PSM-α3 antibodies had fewer deaths from sepsis. |
| (Fritz et al. 2013) | 235 children with skin infections. (prospective) | Anti-α-toxin (Hla) antibodies but not anti-PVL antibodies protected from S. aureus skin reinfections and persisted for the 12 month study. |
| (Adhikari et al. 2015) | 100 patients with SAB (63 without sepsis and 27 with sepsis). (prospective) | Higher titers of anti-LukS, LukF-PV, HlgC, LukE and LukAB were associated with less sepsis and death. |
| (Yu et al. 2016) | 25 patients with MRSA pneumonia following an influenza infection, 22 patients with MSSA pneumonia following an influenza infection and 13 control patients infected with influenza only. (prospective) | 9 deaths in patients with MRSA pneumonia following an influenza infection compared with no deaths in patients with MSSA pneumonia following an influenza infection or influenza infection alone. Anti-Hla antibodies produced by patients protected mice in a murine model of MRSA pneumonia. |
| (Ghasemzadeh-Moghaddam et al. 2018) | 27 patients with SAB infection (ST239) versus 31 non-infected controls. (prospective) | Patients with SAB all developed high titers of anti-SEA antibodies. |
| (Sharma-Kuinkel et al. 2019) | 50 patients with S. aureus bacteremic pneumonia compared with matched controls with S. aureus bacteremia or gram-negative bacteremia. (retrospective) | Patients with S. aureus bacteremia pneumonia had higher IgG titers against α-toxin. Levels of IgG titers against α-toxin and IgM titers against ClfA, FnbpA and SdrC were higher in patients with a clinical cure than treatment failures. |
Figure 5.
Serum antibody titers against S. aureus superantigens (SAgs) and pore-forming toxins (PFTs) in patients with S. aureus bacteremia (SAB) and their correlation with clinical outcome. ↑ (up arrow) = relatively increased antibody titers. ↓ (down arrow) = relatively decreased antibody titers. Green text = protective clinical outcome. Red text = deleterious clinical outcome. TSST-1 = Toxic shock syndrome toxin 1. SE = Staphylococcal enterotoxin. Hl = hemolysin. PVL = Panton-Valentine leukocidin.
TSST-1 is a S. aureus SAg that crosslinks the Vβ chain of TCRs to MHCII molecules on APCs in the absence of antigen, thus leading to nonspecific stimulation of T cells and APCs with massive production and release of many different cytokines (Fig. 3) (Spaulding et al. 2013; Stach, Herrera and Schlievert 2014). In general, SAgs have activity against 30%–70% of an individual's αβ T cell repertoire, providing an immune evasion mechanism to counter S. aureus antigen-specific T cell responses by inducing non-specific inflammation (Spaulding et al. 2013; Stach, Herrera and Schlievert 2014). Over 25 years ago, antibody levels against S. aureus TSST-1 were found to correlate with protection and fewer deaths from tampon-associated toxic shock syndrome (TSS) (Bonventre et al. 1984; Stolz et al. 1985). In addition, nearly half of non-menstrual associated S. aureus-induced TSS has been associated with the activity of TSST-1 (Davis et al. 1980; Schlievert 1986). On a population level, 80% of individuals in the USA develop antibodies against TSST-1 early in life and anti-TSST-1 titers plateau by age 40 whereas 20% of individuals do not develop antibodies against TSST-1 (Vergeront et al. 1983). Thus, there is a relatively large subpopulation of individuals that will be susceptible to developing TSS.
S. aureus α-toxin is a single component small β-barrel PFT that recognizes its receptor ADAM10 that is expressed on the cell membrane of a variety of human epithelial, endothelial and immune cells, such as neutrophils, monocytes/macrophages, T cells and platelets (Fig. 3) (Berube and Bubeck Wardenburg 2013). Antibodies against α-toxin have been associated with protection against human S. aureus skin infections (Table 4) (Fritz et al. 2013). However, natural high antibody titers against α-toxin are generated following S. aureus invasive infections, including SAB and pneumonia (Holtfreter, Kolata and Broker 2010; Yu et al. 2016; Sharma-Kuinkel et al. 2019). In addition, lower anti-α-toxin antibody titers were associated with a poor prognosis in SAB (Adhikari et al. 2012), suggesting that neutralizing α-toxin might have a therapeutic benefit in SAB. No correlation between toxin gene presence and outcome was identified in patients with hospital associated pneumonia (HAP) (Sharma-Kuinkel et al. 2019).
The role of PVL (which is comprised of components LukS-PV and LukF-PV) (Fig. 3) in contributing to S. aureus disease severity and anti-PVL antibodies reducing severity has been controversial. As shown in Table 4, clinical studies are summarized that have found that the presence of PVL-positive isolates have had divergent impact on disease severity. With respect to SAB, several studies have linked a more severe outcome to the presence of PVL-positive S. aureus strains. For example, in pediatric patients, vancomycin associated treatment failures of SAB were associated PVL-positive isolates (Welsh et al. 2010). One study found that the expression of PVL in SAB was not associated with infections at other sites, length of hospital stay or mortality but the presence of PVL-positive isolates in colonized patients decreased the time to develop SAB (Blaine et al. 2010). On the contrary, other studies have found the PVL-positive strains were associated with a better clinical outcome with a less likelihood of developing persistent SAB (Lalani et al. 2008). In cancer patients, the presence of PVL-positive strains had no difference in response to treatment, even in cases of neutropenia (Campo et al. 2011). There are probably several reasons for different outcomes. First, S. aureus has multiple virulence factors and finding a single factor that produces a worse outcome in all studies seems unlikely. Second, the human populations being compared are quite heterogeneous geographically and in terms of age. Third, murine models for studying PVL pathogenicity are not representative of human S. aureus infections because PVL has no activity against murine cells (Bubeck Wardenburg et al. 2008; Diep et al. 2010; Loffler et al. 2010). Fourth PVL-positive strains are more associated with community-derived S. aureus infections, which may be overall more susceptible to multiple antibiotics and this likely affected the clinical outcome (David and Daum 2010). Fifth, in many cases with PVL-positive SAB, these patients were much younger than the patients with PVL-negative strains, and this would bias the outcomes against PVL being important as older age is the most important predictor of outcome with SAB (van Hal et al. 2012). PVL has activity against human (and rabbit) cells because PVL binds to its receptors C5aR and C5L2 in these species (Spaan et al. 2013b) and PVL activity requires the presence of human CD45 (Tromp et al. 2018). Furthermore, the primary receptor for the binding of PVL is C5aR, which explains why PVL targets neutrophils, monocytes and macrophages but not lymphocytes (Spaan et al. 2013b). Overall, there is evidence from human data that PVL plays a role in the pathogenesis of SAB, pneumonia, osteomyelitis and skin and soft tissue infections (including tropical pyomyositis).
Severe S. aureus pneumonia has been linked to the expression of PVL (Gillet et al. 2002). These were young French patients most of whom had a preceding viral (e.g. influenza) infection that deteriorated into a hemorrhagic pneumonia with very high, early mortality (Table 4). Further studies from across the globe reported similar events (Zhang et al. 2009; Gijon et al. 2016; Zhang et al. 2016). Interestingly, a study of 114 cases found that the patients with pre-existing immunity to PVL (with history of a prior PVL-positive S. aureus infection) were associated with less mortality from PVL-positive S. aureus pneumonia than patients without a prior history of a PVL-positive S. aureus infection (Rasigade et al. 2011), suggesting that anti-PVL immunity resulted in protection from severe infection and death. However, it is likely that many of these cases were previously reported in a prior study (Gillet et al. 2002). The presence of PVL had a trend towards increased severity of pneumonia in a small study (22 patients) in which the deaths that occurred in the younger patients who were infected with PVL-positive strains (Wehrhahn et al. 2010). In another large series of 117 children with community-acquired pneumonia, the patients had unusually severe infections (Carrillo-Marquez et al. 2011). However, these infections were dominated by the highly virulent USA300 strain, making it difficult to implicate PVL as the only contributor to disease severity. The clinical data for a role of PVL has also been replicated in a humanized mouse model of S. aureus pneumonia and in rabbit models of S. aureus pneumonia (Diep et al. 2010; Diep et al. 2016; Prince et al. 2017). However, in contrast to these findings, in other clinical studies, an association between PVL-positive S. aureus strains and disease severity was not found (Nickerson et al. 2009; Chen et al. 2010; Li et al. 2011; Peyrani et al. 2011). Thus, while many reports suggest that PVL-positive S. aureus strains were associated with more severe cases of pneumonia, this was not always the case, suggesting that PVL is one of multiple factors that contribute to the severity of S. aureus pneumonia.
In patients with osteomyelitis, the largest series of 139 S. aureus osteomyelitis isolates revealed that the presence of pvl, bbp and sei genes were associated with longer osteomyelitis duration and more serious inflammatory responses (Jiang et al. 2017) (Table 4). This was consistent with earlier reports in which strains possessing the pvl gene were associated with more complications, increased numbers of sepsis, longer hospital stays and increased need for surgery (Martinez-Aguilar et al. 2004; Dohin et al. 2007).
Regarding skin and soft tissue infections, the largest study of 473 patients with skin and soft tissue infections demonstrated that PVL-positive S. aureus strains were associated with larger abscesses (Tong et al. 2012). While some of the increase in abscess size might be dependent on the virulence of the S. aureus strain, as these infections were reported during the USA300 epidemic, it still suggests that PVL-positive strains produced more severe disease. However, the association of more severe skin infections has been reported in other locations in China and Japan where USA300 was not the predominant strain (Yu et al. 2013; Nakaminami et al. 2017). Taken together, 10 of 11 studies found that patients with PVL-positive strains had larger abscesses and required more surgical intervention (Table 4). Notably, more of the PVL-positive strain cases were in younger patients that sought medical attention sooner than PVL-negative cases, hence it is not surprising that the presence of PVL was not associated with a worse outcome. In African and Cambodian patients with tropical pyomyositis, there is unequivocal evidence that the pathogenesis of this severe and invasive soft tissue infection is linked to PVL (Pannaraj et al. 2006; Kraef et al. 2015; Young et al. 2019). These studies also found that β-hemolysin (Hlb) and PVL had synergistic activity in the tropical pyomyositis disease severity (Lebughe et al. 2017). Finally, in a humanized mouse model of S. aureus skin infection that possessed human immune cells (especially human neutrophils), PVL was shown to contribute to dermonecrosis and tissue damage (Tseng et al. 2015). In summary, the collective data indicate that the presence of PVL is associated with increased disease severity in SAB and S. aureus infections of the lung, bone, skin and muscle infections.
As shown in Table 5 and Fig. 5, higher natural antibody levels against α-toxin, δ-hemolysin (Hld), PVL, staphylococcal enterotoxin C-1 (SEC-1) and PSM-α3 might protect against sepsis in patients with SAB (Adhikari et al. 2012). Also, antibodies against some two-component toxins (LukS-PV, LukF-PV, HlgC, LukE, LukAB) had reduced severity of disease (Adhikari et al. 2015). This occurred in both S. aureus pneumonia and SAB (Rasigade et al. 2011; Adhikari et al. 2012; Adhikari et al. 2015). Similar results are found with antibodies against α-toxin in S. aureus skin infections (Fritz et al. 2013). Moreover, β-hemolysin seems to be synergistic with PVL for tropical pyomyositis (Lebughe et al. 2017). Therefore, neutralizing a single S. aureus toxin might provide some protection against the activity of another toxin.
Of note, PVL and other two-component, PFTs, such as LukAB and other PFTs, synergize with PVL to trigger inflammasome activation and IL-1β release (Holzinger et al. 2012; Perret et al. 2012; Melehani et al. 2015). After S. aureus infections in humans, antibodies also develop against LukAB and LukED (Thomsen et al. 2017; Wood et al. 2017; Tromp et al. 2018; Wood et al. 2019), which neutralize toxin activities against human cells in vitro, but their protective value in human S. aureus infections in vivo have not yet been assessed (Thomsen et al. 2017; Wood et al. 2017; Tromp et al. 2018; Wood et al. 2019). Similarly, it is unclear whether antibodies directed against γ-hemolysins (HlgAB and HlgCB) are associated with improved clinical outcomes following human S. aureus infections.
HYPOTHESIS FOR FUTURE TARGETS FOR VACCINE THERAPY: TARGETING NEUTRALIZATION OF S. aureus TOXINS WILL LEAD TO IMPROVED OUTCOMES
There are three primary lines of evidence that converge to create the following hypothesis for future vaccine efforts against S. aureus invasive infections: Targeting neutralization of S. aureus toxins will lead to improved clinical outcomes. (i) An increased incidence of S. aureus infections occurs in patients with defects in the phagocytic cells, especially neutrophils and monocytes/macrophages, which are highly sensitive to the activity of S. aureus PFTs, as well as specific T cell responses, which are impacted by the activity of S. aureus SAgs. (ii) The cytokine profile of patients with SAB suggest that differential serum cytokines (especially T cell-derived cytokines) are associated with better or worse clinical outcomes. (iii) The distinct and synergistic effects of PFTs on host cells and presence of high titers of serum antibodies against PFTs and SAgs are associated with improved clinical outcomes. These three lines of evidence form the basis for a hypothesis in which neutralizing the S. aureus SAgs and PFTs to inhibit the function of these critical S. aureus virulence factors would thereby allow the combined host immune response with adjunctive antibiotic therapy to have enhanced activity in promoting bacterial clearance and improving clinical outcomes. This hypothesis is evidence-based on the aforementioned data from studies of S. aureus infections in human subjects and the compounded knowledge that every S. aureus vaccine attempted in human trials that focused solely on the generation of antibodies to facilitate opsonophagocytosis either lacked efficacy or resulted in increased mortality.
DATA FROM HUMANS THAT TARGETING NEUTRALIZATION OF S. aureus TOXINS LEAD TO IMPROVED OUTCOMES
There have been recent studies that have attempted the neutralization of S. aureus toxins in human trials. For example, a relatively small trial evaluated the adjunctive efficacy of an anti-α-toxin monoclonal antibody (mAb) (AR-301, Aridis Pharmaceuticals) in which the mAb was administered within 36 hours of the diagnosis of hospital-acquired bacterial pneumonia (HABP), ventilator-associated pneumonia (VAP) or community-acquired pneumonia (CAP) (Francois et al. 2018). In 13 of the 48 enrolled subjects, only six had pneumonia attributable to MRSA. However, a post-hoc analysis found that patients who received AR-301 had shorter ventilation duration and better and faster microbiologic eradication at day 28. These findings will need to be confirmed with a larger clinical trial.
More recently, another clinical trial used a multivalent anti-toxin monoclonal antibody (ASN100, Arsanis, Inc.), which had activity in neutralizing five different S. aureus PFTs (α-toxin, PVL, LukED, HlgAB and HlgCB) due to shared epitopes among these toxins, in preventing S. aureus VAP in intensive care unit (ICU) patients (Magyarics et al. 2019a). The trial was ended in futility, as it did not reach its primary endpoint of a 50% reduction in occurrence of S. aureus pneumonia in the ASN100 arm when compared to placebo. A major limitation in this trial was that S. aureus VAP is very difficult to diagnose because colonization of the endotracheal tube is only 30%–60% predictive of having S. aureus VAP (Nair and Niederman 2015; Fan et al. 2016). In addition, although patients in this study might have had infiltrates in the lungs by chest X-ray, these infiltrates could have been from other causes than S. aureus pneumonia such as congestive heart failure, pulmonary hemorrhage, or lung infections from a variety of other pathogens (especially gram-negative bacteria). Detailed information of this trial have not been published, but since mixed infections with more than one bacterial pathogen are common in VAP and if this was the case in the ASM100 trial, this might explain why this clinical trial did not reach the success goal of 50% efficacy. Hence, this may have been the correct vaccine approach for targeting multiple S. aureus PFTs, but the incorrect disease for which to test it. In addition, the half-life of this mAb in the ASN100 vaccine was only 3 weeks, which might not have been enough time for the antibodies to have a beneficial effect in S. aureus VAP (Magyarics et al. 2012b).
Another recent clinical trial evaluated a mAb against S. aureus α-toxin (suvratoxumab, AstraZeneca) (SAATELLITE clinical trial) to prevent VAP by S. aureus in ICU patients (François et al. 2019). Although the results of this trial should be interpreted with caution as they did not reach statistical significance, there was an 18% incidence of pneumonia with the anti-α-toxin mAb versus 26% with placebo ( P = 0.166). Of note, the anti-α-toxin mAb was more effective with lower organism burden in patients < 65 years old, when no previous anti- S. aureus antibiotic was administered, and when optimal VAP prevention care guidelines were used. A total of 2% of patients developed an allergic reaction to the suvratoxumab treatment. It should also be mentioned that this anti-α-toxin mAb was engineered to have a much longer half-life of 80–112 days (Yu et al. 2017), which could have contributed to its better efficacy than the study with ASN100. Taken together, while this trial did not reach statistical significance, it does provide evidence for a toxin-neutralization approach in a S. aureus vaccine, even with all of the caveats involved in difficulty diagnosing and treating S. aureus VAP (Nair and Niederman 2015; Fan et al. 2016).
In addition to these passive mAb-based vaccines, there are also several active vaccines that involve targeting the neutralization of S. aureus toxins that are in various stages of human clinical trials. These include IBT-V02 (a heptavalent vaccine targeted against α-toxin, PVL, SEA, SEB and TSST-1) (Integrated Biotherapeutics) (Aman 2018), a four component S. aureus vaccine directed against α-toxin as well as EsxAB (a fusion protein of 2 ESAT-6-like secreted virulence factors EsxA and EsxB), FhuD2 (a lipoprotein involved in iron uptake) and Csa1A (a putative lipoprotein) (4C-Staph, GlaxoSmithKline) (Bagnoli et al. 2015; Mancini et al. 2016), a toxoid vaccine against α-toxin, PVL as well as capsular polysaccharides 5 and 8 and teichoic acids (PentaStaph, Nabi Pharmaceuticals) and a S. aureus vaccine directed against α-toxin as well as fibronectin-binding protein A (FnBPA) (National Natural Science Foundation of China) (Yeaman et al. 2014; Redi et al. 2018). Whether these multivalent vaccines are effective in improving patient outcomes of S. aureus infections will be critical to demonstrating the viability of these approaches in humans.
Consistent with the approach of targeting PFTs, rabbit polyclonal antibodies against LukAB, α-toxin, and PVL when combined together nearly completely inhibited their cytolytic effect against human monocytes and a human monocytic cell line (THP-1 cells) (Kailasan et al. 2019). Similarly, antibodies generated against a fusion toxin protein comprised of SEA, SEB and TSST-1 (TBA225) neutralized the activity of these toxins and provided a therapeutic benefit against S. aureus toxic shock in a mouse model (Venkatasubramaniam et al. 2019).
Finally, it should also be mentioned that in addition to vaccines, recently identified centyrins, which are small protein scaffolds derived from the fibronectin type III–binding domain of the human protein tenascin-C, have activity in neutralizing PVL, LukAB, LukED, HlgAB and HlgCB (Chan et al. 2019). These centyrins represent an alternative to antibody-based approaches to inhibit the activity of S. aureus PFTs and they have proven effective in mouse models of systemic S. aureus infection but they have yet to be evaluated in human S. aureus infections (Chan et al. 2019).
GENETIC DETERMINANTS OF S. aureus INFECTIONS IN HUMANS
The data reviewed thus far concerning outcomes of S. aureus human infections have concentrated upon host immunodeficiencies and the balance between toxins and anti-toxins; however there is a third major factor in determining outcome and that is the specific genetic make-up of the host genes for the receptors for S. aureus toxins and non-toxin virulence factors. By this we mean that people may have varying response to the same staphylococcal toxin if they had mutations in the host receptors for staphylococcal toxins. Support for a host genetic susceptibility to S. aureus colonization and disease comes from the variable susceptibility to colonization as supported by epidemiological data and the differential ability to neutralize a multitude of S. aureus virulence factors ranging from adhesion molecules to necrotizing toxins. Furthermore, variable clinical outcome has been observed with genetic defects in neutrophils and T cells despite being infected with genotypically identical virulent strains. Genes associated with susceptibility to colonization are different from the ones for susceptibility to the disease processes. However, the repertoire and relative contribution of host genes governing the immune responses particularly the delicate balance between proinflammatory versus anti-inflammatory mediators (e.g. interleukins and cytokines) has not been adequately investigated partly because host genetic susceptibility to S. aureus diseases is complex as it is likely to involve genes for a cascade of receptors involved in multiple stages in a disease process including colonization, transmission through the epidermidis or bloodstream infection, and genetically controlled innate and adaptive immune responses (Shukla, Rose and Schrodi 2015). Indeed, different degrees of resistances and susceptibility to S. aureus infections has also been reported in different strains of mice and are driven by virulence profile of the different mouse strains (von Kockritz-Blickwede et al. 2008; Nippe et al. 2011). Preclinical murine models of S. aureus infections have suggested that the susceptibility to infection is increased if there are defects in certain chemokines, interleukins and TLRs (Miller and Cho 2011; Kim, Missiakas and Schneewind 2014; Montgomery, David and Daum 2015).
Patients with SAB experience a wide spectrum of disease severity, duration and clinical outcomes. SAB could be of heterogeneous phenotype, certainly it is the case with respect to its resolution, short-term bacteremia versus prolonged bacteremia, for example (Rose et al. 2012; Rose et al. 2017). Variation in the activity or expression of C5aR has been postulated to be a cause of variable susceptibility of humans to PVL (Spaan et al. 2013b). This heterogeneity suggests the role of individual variation in host defense mechanisms, inflammatory responses, and cytokine signaling. These variable host immune defenses are expected to be driven, in part, by the variations in host genetic susceptibility and pathogen's virulence profile. However, the relative contributions of host genetics, antibody responses to major staphylococcal toxins and cytokine signaling are poorly understood, especially as it relates to therapeutic outcome (Shukla, Rose and Schrodi 2015).
Furthermore, antibiotics might elicit changes in host immune responses, so understanding the role of traditional therapies on the host immune signature would help provide more precision antibiotic therapy at the host level. As previously discussed, despite several new antibiotics developed against S. aureus over the last two decades, the mortality rate remains persistently high. Therefore, new advances in precision medicine and human SAB biomarkers to combat this issue could markedly improve survival rates. Little is known about the relationships involved among host genetic susceptibility and innate and adaptive immune responses to SAB. Relative contributions of each of these factors are expected to vary across individual hosts. There is a lack of systematic studies into the mechanisms by which host genetic susceptibilities drive the inadequate, and in some cases, dysregulated host immune responses that affect the clearance of SAB. In other words, how subtle host genetic variations impacting immune function that could potentially be responsible for variable protective versus deleterious host responses is incompletely understood and requires further investigation.
The only Genome-wide associated study (GWAS) in humans to date to have identified a genetic variant associated with susceptibility to S. aureus infection involved a case:control (1:10 distribution) study of ∼50,000 subjects (DeLorenze et al. 2016). Of these 50 000 subjects, ∼4,700 had S. aureus infection, and ∼ 47,000 of matched controls had no S. aureus infection. Two imputed single nucleotide polymorphisms (SNPs) (P < 5 × 10−8) in the HLA class II region (near HLA-DRB1–0401 and HLA-DRB1–0402) were associated with increased risk for S. aureus infection at a Genome-wide level of significance (DeLorenze et al. 2016). Using an Admixture mapping among African Americans with S. aureus bacteremia, this same region in the European HLA class region II was again identified at a genome wide level of significance to be associated with susceptibility to S. aureus (Cyr et al. 2017). Polymorphisms in HLA-DR, including the genetic variants identified in the DeLorenze paper were associated with the increased susceptibility to S. aureus infection (DeLorenze et al. 2016) and were shown to influence the host response to staphylococcal toxic shock toxin in a transgenic mouse model (Krogman et al. 2017). Two previous GWAS that had yielded putative host susceptibility genes (e.g. CDON [which encodes a member of the immunoglobulin family], NMRK2 [which encodes an integrin binding molecule] and DAPK3 [which encodes a serine/threonine kinase]) for S. aureus infections needs to be followed up with targeted gene study approach (Nelson et al. 2014; Ye et al. 2014). A GWAS approach has its inherent weakness in identifying true controls that are not susceptible to S. aureus infections. A more direct interrogation of known infection- and inflammation-associated genes involved in the pathogenesis of invasive S. aureus infections should be explored in concert with immunological markers.
FUTURE PERSPECTIVES
In the future, it will be important to further study and confirm the specific antibody titers against S. aureus SAgs and PFTs that correlate with better or worse outcomes following S. aureus SAB and other invasive infections. For SAB, in particular, it would be ideal to have correlative data between the cytokine levels anti-toxin antibody titers to build on the existing data (Tables 3 and 4 and Figs. 4 and 5). Nevertheless, there are clinical data and human in vitro studies showing that S. aureus SAgs and PFTs greatly impact immune function, and there are data suggesting that neutralization of these toxins is associated with better outcomes (Table 5 and Fig. 5). There are a myriad of studies showing that staphylococcal toxins directly alter neutrophil, macrophage and T cell functions and that anti-toxins can reverse these effects as described herein. Therefore, it is reasonable to hypothesize that a multivalent anti-toxin vaccine might reduce the immune dysfunction and improve outcomes in S. aureus SAB and other invasive infections.
CONCLUSION
It should be emphasized that none of the failed S. aureus vaccine trials that targeted the generation of opsonic antibodies had definitive clinical data that clearly supported the role of targeting opsonophagocytosis as a mechanism to improve outcomes against S. aureus in human infections. In contrast, prior to developing vaccines against Neisseria meningitidis meningitis, Streptococcus pneumoniae pneumonia or Haemophilus influenzae infections (e.g. meningitis, pneumonia, pericarditis and bacteremia), it was well-established that opsonic antibodies against surface antigens of these bacteria were indeed protective in humans. In addition, these vaccines were more specifically directed against an infection in an organ system in which the protection or beneficial effect could be accurately assessed. Thus, it is perhaps not surprising, in retrospect, to find that clinical trials against many staphylococcal surface antigens (e.g. IsdB, ClfA, capsular polysaccharides 5 and 8, MntC, etc.) failed (Fowler and Proctor 2014).
In this comprehensive review of the scientific evidence based on the study of S. aureus infections in humans, we believe that several conclusions can be reached. First, there are protective and detrimental immune responses in humans that correlate with clinical outcomes. Second, S. aureus toxins, especially SAgs and PFTs, disrupt host innate and adaptive immune responses that are important in protective immunity. Third, specific naturally-generated or vaccine-induced anti-S. aureus toxin antibodies are associated with improved clinical outcomes. Fourth, while the preponderance of studies suggest that toxins make S. aureus infections more severe and anti-toxin antibodies reduce the severity, the correlation is not 100%. Of course, this is to be expected because there is likely genetic variability amongst humans in terms of responses to toxins and in the expression of the various toxins by the infecting S. aureus strain. Hence, the complexity of interactions between S. aureus and the host will be significant when considering multiple toxins, multiple genes involved in the human response, and variable levels of antitoxin antibody production. In addition, the potential protective role of antitoxin antibodies in conjunction with traditional antibiotic therapy is not well understood. This does not mean that an effective vaccine cannot be developed; however, it strongly emphasizes that the efficacy of a multivalent anti-toxin vaccine will have some variability in comparative outcomes as not every control subject will respond in the same way. Finally, the host response and toxins are likely different among the anatomical sites of infection and future vaccine effects must take these tissue-specific responses into account. Ultimately, many factors from human data should be considered in the future development an effective anti-S. aureus toxin vaccine along with measured and reasonable expectations to provide a better therapeutic approach to combat invasive S. aureus infections.
ACKNOWLEDGEMENTS
Funding: This work was supported by the grants R01AR073665 (LSM), R01AR069502 (LSM), R01AI068804 (VGF), R01AI132627 (WER and RAP) and R21AI144060 (WER) from the USA National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the USA NIH.
Contributor Information
Lloyd S Miller, Immunology, Janssen Research and Development, 1400 McKean Road, Spring House, PA, 19477, USA; Department of Dermatology, Johns Hopkins University School of Medicine, 1550 Orleans Street, Cancer Research Building 2, Suite 209, Baltimore, MD, 21231, USA; Department of Medicine, Division of Infectious Diseases, Johns Hopkins University School of Medicine, 1830 East Monument Street, Baltimore, MD, 21287, USA; Department of Orthopaedic Surgery, Johns Hopkins University School of Medicine, 601 North Caroline Street, Baltimore, MD, 21287, USA; Department of Materials Science and Engineering, Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218, USA.
Vance G Fowler, Jr, Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, 315 Trent Drive, Hanes House, Durham, NC, 27710, USA; Duke Clinical Research Institute, Duke University Medical Center, 40 Duke Medicine Circle, Durham, NC, 27710, USA.
Sanjay K Shukla, Center for Precision Medicine Research, Marshfield Clinic Research Institute, 1000 North Oak Avenue, Marshfield, WI, 54449, USA; Computation and Informatics in Biology and Medicine, University of Wisconsin, 425 Henry Mall, Room 3445, Madison, WI, 53706, USA.
Warren E Rose, Department of Medicine, University of Wisconsin-Madison School of Medicine and Public Health, 1685 Highland Avenue, 5158 Medical Foundation Centennial Building, Madison, WI, 53705, USA; Pharmacy Practice Division, University of Wisconsin-Madison, 777 Highland Avenue, 4123 Rennebohm Hall, Madison, WI, 53705 USA.
Richard A Proctor, Department of Medicine, University of Wisconsin-Madison School of Medicine and Public Health, 1685 Highland Avenue, 5158 Medical Foundation Centennial Building, Madison, WI, 53705, USA; Department of Medical Microbiology and Immunology, University of Wisconsin-Madison School of Medicine and Public Health, 1550 Linden Drive, Microbial Sciences Building, Room 1334, Madison, WI, 53705, USA.
Conflicts of interest. LSM is a full-time employee of Janssen Research and Development and may own Johnson & Johnson stock and stock options. L.S.M has also received grant support from AstraZeneca/MedImmune, Pfizer, Boerhinger Ingelheim, Regeneron Pharmaceuticals, Sun Pharma/DUSA Pharmaceuticals and Moderna Therapeutics, is a shareholder of Noveome Biotherapeutics, is a paid consultant for Armirall and Janssen Research and Development, which are developing therapeutics against infections (including S. aureus and other pathogens). VGF has received grant/research support from: AstraZeneca/MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Genentech, Regeneron and BasileaPaid. Is a paid consultant for Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, AstraZeneca/MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny and is a member of Merck Co-Chair V710 Vaccine and has received educational fees from Green Cross, Cubist, Cerexa, Durata, Theravance and Debiopharm, which are developing therapeutics against infections (including S. aureus and other pathogens). W.E.R. has received grant support from Merck. S.K.S. has no conflicts of interest to disclose. L.S.M., V.G.F., and RAP are paid consultants and on the scientific advisory board of Integrated Biotherapeutics, which is developing therapeutics against infections (including S. aureus and other pathogens).
REFERENCES
- Abdel-Haq N, Al-Tatari H, Chearskul Pet al.. Methicillin-resistant Staphylococcus aureus (MRSA) in hospitalized children: correlation of molecular analysis with clinical presentation and antibiotic susceptibility testing (ABST) results. Eur J Clin Microbiol Infect Dis. 2009;28:547–51. [DOI] [PubMed] [Google Scholar]
- Adhikari RP, Kort T, Shulenin Set al.. Antibodies to S. aureus LukS-PV Attenuated Subunit Vaccine Neutralize a Broad Spectrum of Canonical and Non-Canonical Bicomponent Leukotoxin Pairs. PLoS One. 2015;10:e0137874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari RP, Ajao AO, Aman MJet al.. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis. 2012;206:915–23. [DOI] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer Ket al.. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7. [DOI] [PubMed] [Google Scholar]
- Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2018;53:S64–74. [DOI] [PubMed] [Google Scholar]
- Alonzo F 3rd, Kozhaya L, Rawlings SAet al.. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MJ. Integrated BioTherapeutics. Hum Vaccin Immunother. 2018;14:1308–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MJ, Adhikari RP. Staphylococcal bicomponent pore-forming toxins: targets for prophylaxis and immunotherapy. Toxins (Basel). 2014;6:950–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdahl H, Haapasalo K, Tan Let al.. Staphylococcal protein Ecb impairs complement receptor-1 mediated recognition of opsonized bacteria. PLoS One. 2017;12:e0172675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T, Sullivan KE. Infections in patients with inherited defects in phagocytic function. Clin Microbiol Rev. 2003;16:597–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarian F, van Sorge NM, Sangvik Met al.. A Staphylococcus aureus TIR domain protein virulence factor blocks TLR2-mediated NF-kappaB signaling. J Innate Immun. 2014;6:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins KL, Burman JD, Chamberlain ESet al.. S. aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol Immunol. 2008;45:1600–11. [DOI] [PubMed] [Google Scholar]
- Azuma K, Koike K, Kobayashi Tet al.. Detection of circulating superantigens in an intensive care unit population. Int J Infect Dis. 2004;8:292–8. [DOI] [PubMed] [Google Scholar]
- Baba-Moussa L, Sina H, Scheftel JMet al.. Staphylococcal Panton-Valentine leucocidin as a major virulence factor associated to furuncles. PLoS One. 2011;6:e25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae IG, Tonthat GT, Stryjewski MEet al.. Presence of genes encoding the panton-valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol. 2009;47:3952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli F, Fontana MR, Soldaini Eet al.. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci USA. 2015;112:3680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoel BW, Vos R, Bouman Tet al.. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J Mol Med (Berl). 2012;90:1109–20. [DOI] [PubMed] [Google Scholar]
- Bergdoll MS, Crass BA, Reiser RFet al.. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–21. [DOI] [PubMed] [Google Scholar]
- Berger M. Inflammatory mediators in cystic fibrosis lung disease. Allergy Asthma Proc. 2002;23:19–25. [PubMed] [Google Scholar]
- Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel). 2013;5:1140–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besier S, Smaczny C, von Mallinckrodt Cet al.. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol. 2007;45:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine KP, Tuohy MJ, Wilson Det al.. Progression to bacteremia in critical care patients colonized with methicillin-resistant Staphylococcus aureus expressing Panton-Valentine leukocidin. Diagn Microbiol Infect Dis. 2010;68:28–33. [DOI] [PubMed] [Google Scholar]
- Bloom B, Schelonka R, Kueser Tet al.. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr Infect Dis J. 2005;24:858–66. [DOI] [PubMed] [Google Scholar]
- Bocchini CE, Hulten KG, Mason EO Jr.et al.. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–40. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Panuska JR, Konstan MWet al.. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. [DOI] [PubMed] [Google Scholar]
- Bonventre PF, Linnemann C, Weckbach LSet al.. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984;150:662–6. [DOI] [PubMed] [Google Scholar]
- Bouma G, Ancliff PJ, Thrasher AJet al.. Recent advances in the understanding of genetic defects of neutrophil number and function. Br J Haematol. 2010;151:312–26. [DOI] [PubMed] [Google Scholar]
- Bouma G, Doffinger R, Patel SYet al.. Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol. 2009;147:153–6. [DOI] [PubMed] [Google Scholar]
- Braff MH, Zaiou M, Fierer Jet al.. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AF, Murphy AG, Lalor SJet al.. Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection. PLoS Pathog. 2015;11:e1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto Met al.. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikowski R, Mielke ME, Skarabis Het al.. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105:814–9. [DOI] [PubMed] [Google Scholar]
- Burman JD, Leung E, Atkins KLet al.. Interaction of human complement with Sbi, a staphylococcal immunoglobulin-binding protein: indications of a novel mechanism of complement evasion by Staphylococcus aureus. J Biol Chem. 2008;283:17579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Deming C, Cassidy SKBet al.. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017;9:eaal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DR, Lin YH, Trouillet-Assant Set al.. Vancomycin-intermediate Staphylococcus aureus isolates are attenuated for virulence when compared with susceptible progenitors. Clin Microbiol Infect. 2017;23:767–73. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Deshmukh HS, Nelson CLet al.. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M, Hachem R, Jiang Yet al.. Panton Valentine Leukocidin exotoxin has no effect on the outcome of cancer patients with methicillin-resistant Staphylococcus aureus (MRSA) infections. Medicine (Baltimore). 2011;90:312–8. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Sjoholm AG, Eriksson Let al.. Deficiency of the mannan-binding lectin pathway of complement and poor outcome in cystic fibrosis: bacterial colonization may be decisive for a relationship. Clin Exp Immunol. 2005;139:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Marquez MA, Hulten KG, Hammerman Wet al.. Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children's Hospital. Pediatr Infect Dis J. 2011;30:545–50. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–91. [DOI] [PubMed] [Google Scholar]
- Chadha AD, Thomsen IP, Jimenez-Truque Net al.. Host response to Staphylococcus aureus cytotoxins in children with cystic fibrosis. J Cyst Fibros. 2016;15:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LC, Chaili S, Filler SGet al.. Nonredundant Roles of Interleukin-17A (IL-17A) and IL-22 in Murine Host Defense against Cutaneous and Hematogenous Infection Due to Methicillin-Resistant Staphylococcus aureus. Infect Immun. 2015;83:4427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R, Buckley PT, O'Malley Aet al.. Identification of biologic agents to neutralize the bicomponent leukocidins of Staphylococcus aureus. Sci Transl Med. 2019;11:eaat0882. [DOI] [PubMed] [Google Scholar]
- Chantratita N, Tandhavanant S, Seal Set al.. TLR4 genetic variation is associated with inflammatory responses in Gram-positive sepsis. Clin Microbiol Infect. 2017;23:47 e41–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Wang JL, Chen THet al.. Differences between methicillin-resistant Staphylococcus aureus bacteremic isolates harboring type IV and type V staphylococcal cassette chromosome mec genes based on prior patient healthcare exposure. Eur J Clin Microbiol Infect Dis. 2010;29:1539–46. [DOI] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NCet al.. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. JClinInvest. 2010;120:1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Strickland I, Boguniewicz Met al.. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol. 2001a;108:269–74. [DOI] [PubMed] [Google Scholar]
- Cho SH, Strickland I, Tomkinson Aet al.. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Invest Dermatol. 2001b;116:658–63. [DOI] [PubMed] [Google Scholar]
- Chonchol M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial. 2006;19:291–6. [DOI] [PubMed] [Google Scholar]
- Christensson B, Hedstrom SA, Kronvall G. Antibody response to alpha- and betahemolysin from Staphylococcus aureus in patients with staphylococcal infections and in normals. Acta Pathol Microbiol Immunol Scand B. 1983;91:351–6. [DOI] [PubMed] [Google Scholar]
- Clarke SR, Mohamed R, Bian Let al.. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. [DOI] [PubMed] [Google Scholar]
- Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RR, Gao X, Allen ICet al.. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4:e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone NF, Grandits G, Echols Set al.. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr. 2010;54:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DD, Allen AS, Du GJet al.. Evaluating genetic susceptibility to Staphylococcus aureus bacteremia in African Americans using admixture mapping. Genes Immun. 2017;18:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailiana ZH, Rigopoulos N, Varitimidis SEet al.. Clinical and epidemiological features of upper-extremity infections caused by Staphylococcus aureus carrying the PVL gene: a four-year study in Greece. Med Sci Monit. 2008;14:CR511–514. [PubMed] [Google Scholar]
- Dasenbrook EC, Checkley W, Merlo CAet al.. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–92. [DOI] [PubMed] [Google Scholar]
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Chesney PJ, Wand PJet al.. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–35. [DOI] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt APet al.. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decraene A, Willems-Widyastuti A, Kasran Aet al.. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable Cystic Fibrosis patients. Respir Res. 2010;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJonge M, Burchfield D, Bloom Bet al.. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–5. [DOI] [PubMed] [Google Scholar]
- del Giudice P, Blanc V, de Rougemont Aet al.. Primary skin abscesses are mainly caused by Panton-Valentine leukocidin-positive Staphylococcus aureus strains. Dermatology. 2009;219:299–302. [DOI] [PubMed] [Google Scholar]
- DeLorenze GN, Nelson CL, Scott WKet al.. Polymorphisms in HLA Class II Genes Are Associated With Susceptibility to Staphylococcus aureus Infection in a White Population. J Infect Dis. 2016;213:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppermann C, Kubes P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun. 2018;24:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2015;15:505–13. [DOI] [PubMed] [Google Scholar]
- Dias J, Boulouis C, Sobkowiak MJet al.. Factors influencing functional heterogeneity in human mucosa-associated invariant T cells. Front Immunol. 2018;9:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Chan L, Tattevin Pet al.. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA. 2010;107:5587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Le VT, Badiou Cet al.. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med. 2016;8:357ra124. [DOI] [PubMed] [Google Scholar]
- Dillen CA, Pinsker BL, Marusina AIet al.. Clonally expanded gammadelta T cells protect against Staphylococcus aureus skin reinfection. J Clin Invest. 2018;128:1026–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohin B, Gillet Y, Kohler Ret al.. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J. 2007;26:1042–8. [DOI] [PubMed] [Google Scholar]
- Downey AM, Kaplonek P, Seeberger PH. MAIT cells as attractive vaccine targets. FEBS Lett. 2019;593:1627–40. [DOI] [PubMed] [Google Scholar]
- DuMont AL, Yoong P, Day CJet al.. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci USA. 2013;110:10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont AL, Nygaard TK, Watkins RLet al.. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizur A, Orscheln RC, Ferkol TWet al.. Panton-Valentine Leukocidin-positive methicillin-resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest. 2007;131:1718–25. [DOI] [PubMed] [Google Scholar]
- Fan Y, Gao F, Wu Yet al.. Does ventilator-associated event surveillance detect ventilator-associated pneumonia in intensive care units? A systematic review and meta-analysis. Crit Care. 2016;20:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VKet al.. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VG, Allen KB, Moreira EDet al.. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. [DOI] [PubMed] [Google Scholar]
- Fowler VG Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014;20 Suppl 5:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GRet al.. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–8. [DOI] [PubMed] [Google Scholar]
- Francois B, Mercier E, Gonzalez Cet al.. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 2018;44:1787–96. [DOI] [PubMed] [Google Scholar]
- François B, GarciaSanchez M, Eggimann Pet al.. Efficacy and Safety Profile of Suvratoxumab, a Novel Anti-Staphylococcus aureus Monoclonal Antibody: Results of the SAATELLITE Study in Mechanically Ventilated Intensive Care Unit Patients. 29th Meeting of the European Society of Microbiology and Infectious Diseases (ECCMID) Abstract: L0013. 2019. [Google Scholar]
- Fritz SA, Tiemann KM, Hogan PGet al.. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis. 2013;56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryszewski SJ, Wong Fok Lung T, Annavajhala MKet al.. Metabolic Adaptation in Methicillin-Resistant Staphylococcus aureus Pneumonia. Am J Respir Cell Mol Biol. 2019;61:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Kim KJ, Yankaskas JRet al.. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol. 1999;277:L113–118. [DOI] [PubMed] [Google Scholar]
- Gauduchon V, Cozon G, Vandenesch Fet al.. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis. 2004;189:346–53. [DOI] [PubMed] [Google Scholar]
- Geng W, Yang Y, Wu Det al.. Community-acquired, methicillin-resistant Staphylococcus aureus isolated from children with community-onset pneumonia in China. Pediatr Pulmonol. 2010;45:387–94. [DOI] [PubMed] [Google Scholar]
- Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26:484–97. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh-Moghaddam H, van Wamel W, van Belkum Aet al.. Humoral immune consequences of Staphylococcus aureus ST239-associated bacteremia. Eur J Clin Microbiol Infect Dis. 2018;37:255–63. [DOI] [PubMed] [Google Scholar]
- Giersing BK, Dastgheyb SS, Modjarrad Ket al.. Status of vaccine research and development of vaccines for Staphylococcus aureus. Vaccine. 2016;34:2962–6. [DOI] [PubMed] [Google Scholar]
- Gijon M, Bellusci M, Petraitiene Bet al.. Factors associated with severity in invasive community-acquired Staphylococcus aureus infections in children: a prospective European multicentre study. Clin Microbiol Infect. 2016;22:643. [DOI] [PubMed] [Google Scholar]
- Gillet Y, Vanhems P, Lina Get al.. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis. 2007;45:315–21. [DOI] [PubMed] [Google Scholar]
- Gillet Y, Issartel B, Vanhems Pet al.. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. [DOI] [PubMed] [Google Scholar]
- Glikman D, Siegel JD, David MZet al.. Complex molecular epidemiology of methicillin-resistant staphylococcus aureus isolates from children with cystic fibrosis in the era of epidemic community-associated methicillin-resistant S aureus. Chest. 2008;133:1381–7. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Koay HF, McCluskey Jet al.. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20:1110–28. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barca E, Carratala J, Mykietiuk Aet al.. Predisposing factors and outcome of Staphylococcus aureus bacteremia in neutropenic patients with cancer. Eur J Clin Microbiol Infect Dis. 2001;20:117–9. [DOI] [PubMed] [Google Scholar]
- Gonzalez BE, Hulten KG, Dishop MKet al.. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005a;41:583–90. [DOI] [PubMed] [Google Scholar]
- Gonzalez BE, Martinez-Aguilar G, Hulten KGet al.. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005b;115:642–8. [DOI] [PubMed] [Google Scholar]
- Gray RD, Hardisty G, Regan KHet al.. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, Hrusch CL, Jaffery MRet al.. Distinct T-helper cell responses to Staphylococcus aureus bacteremia reflect immunologic comorbidities and correlate with mortality. Crit Care. 2018;22:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D, Le Bourhis L, Sauneuf Bet al.. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40:192–201. [DOI] [PubMed] [Google Scholar]
- Gubbay JB, Gosbell IB, Barbagiannakos Tet al.. Clinical features, epidemiology, antimicrobial resistance, and exotoxin genes (including that of Panton-Valentine leukocidin) of gentamicin-susceptible methicillin-resistant Staphylococcus aureus (GS-MRSA) isolated at a paediatric teaching hospital in New South Wales, Australia. Pathology. 2008;40:64–71. [DOI] [PubMed] [Google Scholar]
- Guimaraes AO, Cao Y, Hong Ket al.. A Prognostic Model of Persistent Bacteremia and Mortality in Complicated Staphylococcus aureus Bloodstream Infection. Clin Infect Dis. 2019;68:1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman JC, Uyeki TM, Francis JSet al.. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair PS, Wagner SM, Friederich PTet al.. Complement regulator C4BP binds to Staphylococcus aureus and decreases opsonization. Mol Immunol. 2012;50:253–61. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers Eet al.. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. [DOI] [PubMed] [Google Scholar]
- Harris TA, Gattu S, Propheter DCet al.. Resistin-like Molecule alpha Provides Vitamin-A-Dependent Antimicrobial Protection in the Skin. Cell Host Microbe. 2019;25:777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn L, Prajsnar TK, Klapholz Cet al.. Innate immunity. A Spaetzle-like role for nerve growth factor beta in vertebrate immunity to Staphylococcus aureus. Science. 2014;346:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann I, Rath S, Ziesemer Set al.. Staphylococcus aureus hemolysin A disrupts cell-matrix adhesions in human airway epithelial cells. Am J Respir Cell Mol Biol. 2015;52:14–24. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS, Brands-Tajouiti J, van Furth R. Comparison of antibody activity against various microorganisms in intravenous immunoglobulin preparations determined by ELISA and opsonic assay. J Lab Clin Med. 1994;123:241–6. [PubMed] [Google Scholar]
- Higgins J, Loughman A, van Kessel KPet al.. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol Lett. 2006;258:290–6. [DOI] [PubMed] [Google Scholar]
- Hodille E, Cuerq C, Badiou Cet al.. Delta Hemolysin and Phenol-Soluble Modulins, but Not Alpha Hemolysin or Panton-Valentine Leukocidin, Induce Mast Cell Activation. Front Cell Infect Microbiol. 2016;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoernes M, Seger R, Reichenbach J. Modern management of primary B-cell immunodeficiencies. Pediatr Allergy Immunol. 2011;22:758–69. [DOI] [PubMed] [Google Scholar]
- Holland TL, Arnold C, Fowler VG Jr.. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus - The anti-S. aureus antibody response. Int J Med Microbiol. 2010;300:176–92. [DOI] [PubMed] [Google Scholar]
- Holzinger D, Gieldon L, Mysore Vet al.. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol. 2012;92:1069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden BP, Smith DJ, Mansell Aet al.. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC Microbiol. 2008;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LY, Koh TH, Kurup Aet al.. High incidence of Panton-Valentine leukocidin-producing Staphylococcus aureus in a tertiary care public hospital in Singapore. Clin Infect Dis. 2005;40:486–9. [DOI] [PubMed] [Google Scholar]
- Igyarto BZ, Haley K, Ortner Det al.. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshima I, Inoshima N, Wilke GAet al.. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Yonemura T, Baber Jet al.. Safety, tolerability, and immunogenicity of a novel 4-antigen Staphylococcus aureus vaccine (SA4Ag) in healthy Japanese adults. Hum Vaccin Immunother. 2018;14:2682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel L, Wang Y, Bulek Ket al.. Human adaptive immunity rescues an inborn error of innate immunity. Cell. 2017;168:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson G, Colque-Navarro P, Gustafsson Eet al.. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2010;29:715–25. [DOI] [PubMed] [Google Scholar]
- Jahamy H, Ganga R, Al Raiy Bet al.. Staphylococcus aureus skin/soft-tissue infections: the impact of SCCmec type and Panton-Valentine leukocidin. Scand J Infect Dis. 2008;40:601–6. [DOI] [PubMed] [Google Scholar]
- Janela B, Patel AA, Lau MCet al.. A subset of type I conventional dendritic cells controls cutaneous bacterial infections through VEGFalpha-mediated recruitment of neutrophils. Immunity. 2019;50:1069–83. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang Y, Feng Zet al.. Panton-valentine leucocidin (PVL) as a potential indicator for prevalence, duration, and severity of Staphylococcus aureus osteomyelitis. Front Microbiol. 2017;8:2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Bokarewa M, Foster Tet al.. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172:1169–76. [DOI] [PubMed] [Google Scholar]
- Junge S, Gorlich D, den Reijer Met al.. Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcus aureus. PLoS One. 2016;11:e0166220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M, Peters W, Carr Det al.. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol. 2003;111:813–7. [DOI] [PubMed] [Google Scholar]
- Kahl B, Herrmann M, Everding ASet al.. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177:1023–9. [DOI] [PubMed] [Google Scholar]
- Kailasan S, Kort T, Mukherjee Iet al.. Rational Design of Toxoid Vaccine Candidates for Staphylococcus aureus Leukocidin AB (LukAB). Toxins (Basel). 2019;11:E339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen AJ, Brunkard J, Moore Zet al.. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53:358–65. [DOI] [PubMed] [Google Scholar]
- Kaltsas A, Guh A, Mediavilla JRet al.. Frequency of panton-valentine leukocidin-producing methicillin-sensitive Staphylococcus strains in patients with complicated skin and soft tissue infection in bronx, new york. J Clin Microbiol. 2011;49:2992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva M, Salmenlinna S, Vuopio-Varkila Jet al.. Community-associated methicillin-resistant Staphylococcus aureus isolated in Finland in 2004 to 2006. J Clin Microbiol. 2009;47:2655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karauzum H, Haudenschild CC, Moore INet al.. Lethal CD4 T Cell Responses Induced by Vaccination Against Staphylococcus aureus Bacteremia. J Infect Dis. 2017;215:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolos MH, Horsburgh MJ, Ingham Eet al.. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149:2749–58. [DOI] [PubMed] [Google Scholar]
- Kim EY, Oldham WM. Innate T cells in the intensive care unit. Mol Immunol. 2019;105:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim BE, Ahn Ket al.. Interactions between atopic dermatitis and Staphylococcus aureus infection: clinical implications. Allergy Asthma Immunol Res. 2019;11:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming Cet al.. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning S, van Belkum A, Snijders Set al.. Severity of nonbullous Staphylococcus aureus impetigo in children is associated with strains harboring genetic markers for exfoliative toxin B, Panton-Valentine leukocidin, and the multidrug resistance plasmid pSK41. J Clin Microbiol. 2003;41:3017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Hatfield K, Baggs Jet al.. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep. 2019;68:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraef C, Alabi AS, Peters Get al.. Co-detection of Panton-Valentine leukocidin encoding genes and cotrimoxazole resistance in Staphylococcus aureus in Gabon: implications for HIV-patients' care. Front Microbiol. 2015;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg Met al.. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogman A, Tilahun A, David CSet al.. HLA-DR polymorphisms influence in vivo responses to staphylococcal toxic shock syndrome toxin-1 in a transgenic mouse model. HLA. 2017;89:20–28. [DOI] [PubMed] [Google Scholar]
- Kuipers A, Stapels DA, Weerwind LTet al.. The Staphylococcus aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis. Microbiology. 2016;162:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ray P, Kanwar Met al.. A comparative analysis of antibody repertoire against Staphylococcus aureus antigens in patients with deep-seated versus superficial staphylococcal infections. Int J Med Sci. 2005;2:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse D, Perret M, Hayez Det al.. Kineret(R)/IL-1ra blocks the IL-1/IL-8 inflammatory cascade during recombinant Panton Valentine Leukocidin-triggered pneumonia but not during S. aureus infection. PLoS One. 2014;9:e97546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Villaruz AE, Li Met al.. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol. 2007;63:497–506. [DOI] [PubMed] [Google Scholar]
- Lai Y, Li D, Li Cet al.. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Finn A. Neutrophil disorders and their management. J Clin Pathol. 2001;54:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani T, Federspiel JJ, Boucher HWet al.. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol. 2008;46:2890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor SJ, McLoughlin RM. Memory gammadelta T Cells-Newly Appreciated Protagonists in Infection and Immunity. Trends Immunol. 2016;37:690–702. [DOI] [PubMed] [Google Scholar]
- Laouini D, Kawamoto S, Yalcindag Aet al.. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–7. [DOI] [PubMed] [Google Scholar]
- Law SM, Gray RD. Neutrophil extracellular traps and the dysfunctional innate immune response of cystic fibrosis lung disease: a review. J Inflamm (Lond). 2017;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebughe M, Phaku P, Niemann Set al.. The Impact of the Staphylococcus aureus Virulome on Infection in a Developing Country: A Cohort Study. Front Microbiol. 2017;8:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz FE, Schwartbeck B, Dubbers Aet al.. The prevalence of Staphylococcus aureus with mucoid phenotype in the airways of patients with cystic fibrosis-A prospective study. Int J Med Microbiol. 2019;309:283–7. [DOI] [PubMed] [Google Scholar]
- Levy R, Okada S, Beziat Vet al.. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci USA. 2016;113:E8277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DZ, Chen YS, Yang JPet al.. Preliminary molecular epidemiology of the Staphylococcus aureus in lower respiratory tract infections: a multicenter study in China. Chin Med J (Engl). 2011;124:687–92. [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu Xet al.. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina G, Piemont Y, Godail-Gamot Fet al.. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. [DOI] [PubMed] [Google Scholar]
- Liu CI, Liu GY, Song Yet al.. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319:1391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JTet al.. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Archer NK, Dillen CAet al.. Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses. Cell Host Microbe. 2017;22:653–66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Mazhar M, Miller LS. Immune and inflammatory reponses to Staphylococcus aureus skin infections. Curr Dermatol Rep. 2018;7:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler B, Hussain M, Grundmeier Met al.. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson Net al.. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyarics Z, Provost K, Adi Net al.. Results of a phase 2, randomized, double-blind, placebo-controlled study to determine the safety and efficacy of a single dose of the monoclonal antibody combination ASN100 for the PREVENTION of Staphylococcus aureus pneumonia in endotracheal heavily colonized, mechanically ventilated subjects. 29th Meeting of the European Society of Microbiology and Infectious Diseases (ECCMID) Abstract: L0011. 2019a. [Google Scholar]
- Magyarics Z, Leslie F, Bartko Jet al.. Randomized, double-blind, placebo-controlled, single-ascending-dose study of the penetration of a monoclonal antibody combination (ASN100) targeting Staphylococcus aureus cytotoxins in the lung epithelial lining fluid of healthy volunteers. Antimicrob Agents Chemother. 2019b;63:e00350–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F, Monaci E, Lofano Get al.. One Dose of Staphylococcus aureus 4C-Staph vaccine formulated with a novel TLR7-dependent adjuvant rapidly protects mice through antibodies, effector CD4+ T cells, and IL-17A. PLoS One. 2016;11:e0147767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. J Cutan Pathol. 2002;29:168–72. [DOI] [PubMed] [Google Scholar]
- Manfredi R, Costigliola P, Ricchi Eet al.. Sepsis-bacteraemia and other infections due to non-opportunistic bacterial pathogens in a consecutive series of 788 patients hospitalized for HIV infection. Clin Ter. 1993;143:279–90. [PubMed] [Google Scholar]
- Marchitto MC, Dillen CA, Liu Het al.. Clonal Vgamma6(+)Vdelta4(+) T cells promote IL-17-mediated immunity against Staphylococcus aureus skin infection. Proc Natl Acad Sci USA. 2019;116:10917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton Ket al.. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. [DOI] [PubMed] [Google Scholar]
- Martinez-Aguilar G, Avalos-Mishaan A, Hulten Ket al.. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23:701–6. [DOI] [PubMed] [Google Scholar]
- Marzec NS, Bessesen MT. Risk and outcomes of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia among patients admitted with and without MRSA nares colonization. Am J Infect Control. 2016;44:405–8. [DOI] [PubMed] [Google Scholar]
- McAllister F, Henry A, Kreindler JLet al.. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin RM, Lee JC, Kasper DLet al.. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J Immunol. 2008;181:1323–32. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Solinga RM, Rich Jet al.. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci USA. 2006;103:10408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely TB, Shah NA, Fridman Aet al.. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother. 2014;10:3513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas S, Talento AF, O'Gorman Jet al.. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis. 2014;14:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melehani JH, James DB, DuMont ALet al.. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS Pathog. 2015;11:e1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel M, Voelcker V, Kollisch Get al.. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121:1389–96. [DOI] [PubMed] [Google Scholar]
- Menzies BE, Kenoyer A. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect Immun. 2006;74:6847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LHet al.. Inflammasome-mediated production of IL-1beta Is required for neutrophil recruitment against Staphylococcus aureus in vivo. JImmunol. 2007;179:6933–42. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence Aet al.. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Nagasawa Met al.. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minejima E, Bensman J, She RCet al.. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med. 2016;44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas D, Schneewind O. Staphylococcus aureus vaccines: deviating from the carol. J Exp Med. 2016;213:1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, Grondin G, Bilodeau Get al.. Infection of polarized airway epithelial cells by normal and small-colony variant strains of Staphylococcus aureus is increased in cells with abnormal cystic fibrosis transmembrane conductance regulator function and is influenced by NF-kappaB. Infect Immun. 2011;79:3541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N, Wang MY, Le Huec JCet al.. Vaccine development to prevent Staphylococcus aureus surgical-site infections. Br J Surg. 2017;104:e41–54. [DOI] [PubMed] [Google Scholar]
- Montgomery CP, David MZ, Daum RS. Host factors that contribute to recurrent staphylococcal skin infection. Curr Opin Infect Dis. 2015;28:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery CP, Daniels M, Zhao Fet al.. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun. 2014;82:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Anstett MA, Muller P, Albrecht Tet al.. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One. 2010;5:e13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munckhof WJ, Nimmo GR, Carney Jet al.. Methicillin-susceptible, non-multiresistant methicillin-resistant and multiresistant methicillin-resistant Staphylococcus aureus infections: a clinical, epidemiological and microbiological comparative study. Eur J Clin Microbiol Infect Dis. 2008;27:355–64. [DOI] [PubMed] [Google Scholar]
- Munoz-Planillo R, Franchi L, Miller LSet al.. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AG, O'Keeffe KM, Lalor SJet al.. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol. 2014;192:3697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttaiyah S, Coombs G, Pandey Set al.. Incidence, risk factors, and outcomes of Panton-Valentine leukocidin-positive methicillin-susceptible Staphylococcus aureus infections in Auckland, New Zealand. J Clin Microbiol. 2010;48:3470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Matsumoto M, Katayama Yet al.. Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 2017;22:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaminami H, Ito A, Sakanashi Det al.. Genetic diversity of pvl-positive community-onset methicillin-resistant Staphylococcus aureus isolated at a university hospital in Japan. J Infect Chemother. 2017;23:856–8. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KBet al.. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar K, Seifert H, Rieg Set al.. Survival following Staphylococcus aureus bloodstream infection: A prospective multinational cohort study assessing the impact of place of care. J Infect. 2018;77:516–25. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Pelak K, Podgoreanu MVet al.. A genome-wide association study of variants associated with acquisition of Staphylococcus aureus bacteremia in a healthcare setting. BMC Infect Dis. 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson EK, Wuthiekanun V, Wongsuvan Get al.. Factors predicting and reducing mortality in patients with invasive Staphylococcus aureus disease in a developing country. PLoS One. 2009;4:e6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nippe N, Varga G, Holzinger Det al.. Subcutaneous infection with S. aureus in mice reveals association of resistance with influx of neutrophils and Th2 response. J Invest Dermatol. 2011;131:125–32. [DOI] [PubMed] [Google Scholar]
- Nishitani K, Beck CA, Rosenberg AFet al.. A diagnostic serum antibody test for patients with Staphylococcus aureus osteomyelitis. Clin Orthop Relat Res. 2015;473:2735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurjadi D, Kain M, Marcinek Pet al.. Ratio of T-Helper Type 1 (Th1) to Th17 cytokines in whole blood is associated with human beta-defensin 3 expression in skin and persistent Staphylococcus aureus nasal carriage. J Infect Dis. 2016;214:1744–51. [DOI] [PubMed] [Google Scholar]
- Nygaard TK, Pallister KB, DuMont ALet al.. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7:e36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien EC, McLoughlin RM. Considering the ‘Alternatives’ for next-generation anti-Staphylococcus aureus vaccine development. Trends Mol Med. 2019;25:171–84. [DOI] [PubMed] [Google Scholar]
- Ommori R, Ouji N, Mizuno Fet al.. Selective induction of antimicrobial peptides from keratinocytes by staphylococcal bacteria. Microb Pathog. 2013;56:35–39. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt Cet al.. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. [DOI] [PubMed] [Google Scholar]
- Ono Y, Ito T, Watanabe Tet al.. Opsonic activity assessment of human intravenous immunoglobulin preparations against drug-resistant bacteria. J Infect Chemother. 2004;10:234–8. [DOI] [PubMed] [Google Scholar]
- Palmqvist N, Patti JM, Tarkowski Aet al.. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect. 2004;6:188–95. [DOI] [PubMed] [Google Scholar]
- Pannaraj PS, Hulten KG, Gonzalez BEet al.. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953–60. [DOI] [PubMed] [Google Scholar]
- Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43:1040–51. [DOI] [PubMed] [Google Scholar]
- Perret M, Badiou C, Lina Get al.. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol. 2012;14:1019–36. [DOI] [PubMed] [Google Scholar]
- Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol. 2013;11:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, Otto M, Jack RWet al.. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–10. [DOI] [PubMed] [Google Scholar]
- Peschel A, Jack RW, Otto Met al.. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrani P, Allen M, Wiemken TLet al.. Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis. 2011;53:766–71. [DOI] [PubMed] [Google Scholar]
- Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev. 2011;24:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, von Bernuth H, Ghandil Pet al.. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore). 2010;89:403–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillarisetti N, Williamson E, Linnane Bet al.. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. [DOI] [PubMed] [Google Scholar]
- Pogue M, Burton S, Kreyling Pet al.. Centers for disease control and prevention (CDC). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006—January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325–9. [PubMed] [Google Scholar]
- Potaczek DP, Nastalek M, Okumura Ket al.. An association of TLR2-16934A >T polymorphism and severity/phenotype of atopic dermatitis. J Eur Acad Dermatol Venereol. 2011;25:715–21. [DOI] [PubMed] [Google Scholar]
- Powers ME, Kim HK, Wang Yet al.. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers ME, Becker RE, Sailer Aet al.. Synergistic Action of Staphylococcus aureus alpha-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell Host Microbe. 2015;17:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost G, Couppie P, Prevost Pet al.. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J Med Microbiol. 1995;421:1237–45., . [DOI] [PubMed] [Google Scholar]
- Prince A, Wang H, Kitur Ket al.. Humanized Mice Exhibit Increased Susceptibility to Staphylococcus aureus Pneumonia. J Infect Dis. 2017;215:1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012a;54:1179–86. [DOI] [PubMed] [Google Scholar]
- Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012a;30:2921–7. [DOI] [PubMed] [Google Scholar]
- Proctor RA. Recent developments for Staphylococcus aureus vaccines: clinical and basic science challenges. Eur Cell Mater. 2015;30:315–26. [DOI] [PubMed] [Google Scholar]
- Proctor RA. Immunity to Staphylococcus aureus: Implications for Vaccine Development. Microbiol Spectr. 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Picard C, Lorrot Met al.. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647–54. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante Jet al.. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi R, Joo HS, Sharma-Kuinkel Bet al.. Increased in vitro phenol-soluble modulin production is associated with soft tissue infection source in clinical isolates of methicillin-susceptible Staphylococcus aureus. J Infect. 2016;72:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel SM, Paller AS. Bacterial colonization, overgrowth, and superinfection in atopic dermatitis. Clin Dermatol. 2018;36:641–7. [DOI] [PubMed] [Google Scholar]
- Rasigade JP, Sicot N, Laurent Fet al.. A history of Panton-Valentine leukocidin (PVL)-associated infection protects against death in PVL-associated pneumonia. Vaccine. 2011;29:4185–6. [DOI] [PubMed] [Google Scholar]
- Redi D, Raffaelli CS, Rossetti Bet al.. Staphylococcus aureus vaccine preclinical and clinical development: current state of the art. New Microbiol. 2018;41:208–13. [PubMed] [Google Scholar]
- Renner ED, Rylaarsdam S, Anover-Sombke Set al.. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Robles T, Alonzo F 3rd, Kozhaya Let al.. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe. 2013;14:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg S, Steffen H, Seeber Set al.. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174:8003–10. [DOI] [PubMed] [Google Scholar]
- Rooijakkers SH, van Wamel WJ, Ruyken Met al.. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7:476–84. [DOI] [PubMed] [Google Scholar]
- Rose WE, Eickhoff JC, Shukla SKet al.. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose WE, Shukla SK, Berti ADet al.. Increased Endovascular Staphylococcus aureus Inoculum Is the Link Between Elevated Serum Interleukin 10 Concentrations and Mortality in Patients With Bacteremia. Clin Infect Dis. 2017;64:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SA, Simanski M, Rademacher Fet al.. The pattern recognition receptor NOD2 mediates Staphylococcus aureus-induced IL-17C expression in keratinocytes. J Invest Dermatol. 2014;134:374–80. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen E, Karden-Lilja M, Kuusela Pet al.. Methicillin-sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J Infect. 2008;56:249–56. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014;12:585–91. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Kolar SL, Muller Set al.. O-Acetylation of peptidoglycan limits helper T cell priming and permits Staphylococcus aureus reinfection. Cell Host Microbe. 2017;22:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda ABet al.. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert PM. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;1:1149–50. [DOI] [PubMed] [Google Scholar]
- Schlievert PM, Case LC, Strandberg KLet al.. Superantigen profile of Staphylococcus aureus isolates from patients with steroid-resistant atopic dermatitis. Clin Infect Dis. 2008;46:1562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A, Haarmann A, Wahn V. Cytokines in neutrophil-dominated airway inflammation in patients with cystic fibrosis. Eur Arch Otorhinolaryngol. 1995;252 Suppl 1:S59–60. [DOI] [PubMed] [Google Scholar]
- Schwartbeck B, Birtel J, Treffon Jet al.. Dynamic in vivo mutations within the ica operon during persistence of Staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog. 2016;12:e1006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerd T, Twigg SRF, Aschenbrenner Det al.. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med. 2017;214:2547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WK, Medie FM, Ruffin Fet al.. Human genetic variation in GLS2 is associated with development of complicated Staphylococcus aureus bacteremia. PLos Genet. 2018;14:e1007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilie ES, Bubeck Wardenburg J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin Cell Dev Biol. 2017;72:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaler CR, Choi J, Rudak PTet al.. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 2017;15:e2001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Kuinkel BK, Ahn SH, Rude THet al.. Presence of genes encoding panton-valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J Clin Microbiol. 2012;50:848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Kuinkel BK, Tkaczyk C, Bonnell Jet al.. Associations of pathogen-specific and host-specific characteristics with disease outcome in patients with Staphylococcus aureus bacteremic pneumonia. Clin Transl Immunology. 2019;8:e01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinefield H, Black S, Fattom Aet al.. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. [DOI] [PubMed] [Google Scholar]
- Shukla SK, Rose W, Schrodi SJ. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends Microbiol. 2015;23:529–36. [DOI] [PubMed] [Google Scholar]
- Sicot N, Khanafer N, Meyssonnier Vet al.. Methicillin resistance is not a predictor of severity in community-acquired Staphylococcus aureus necrotizing pneumonia–results of a prospective observational study. Clin Microbiol Infect. 2013;19:E142–148. [DOI] [PubMed] [Google Scholar]
- Sieprawska-Lupa M, Mydel P, Krawczyk Ket al.. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanski M, Glaser R, Koten Bet al.. Staphylococcus aureus subverts cutaneous defense by D-alanylation of teichoic acids. Exp Dermatol. 2013;22:294–6. [DOI] [PubMed] [Google Scholar]
- Smit J, Sogaard M, Schonheyder HCet al.. Diabetes and risk of community-acquired Staphylococcus aureus bacteremia: a population-based case-control study. Eur J Endocrinol. 2016;174:631–9. [DOI] [PubMed] [Google Scholar]
- Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936-1955). Lancet. 1960;1:1318–22. [PubMed] [Google Scholar]
- Soderquist B, Sundqvist KG, Vikerfors T. Kinetics of serum levels of interleukin-6 in Staphylococcus aureus septicemia. Scand J Infect Dis. 1992;24:607–12. [DOI] [PubMed] [Google Scholar]
- Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15:435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan AN, Henry T, van Rooijen WJMet al.. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013a;13:584–94. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland Ret al.. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol. 2013b;67:629–50. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Vrieling M, Wallet Pet al.. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun. 2014;5:5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding AR, Salgado-Pabon W, Kohler PLet al.. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013;26:422–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012;34:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Kostel Bal S, Egner Wet al.. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. 2019;216:1986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach CS, Herrera A, Schlievert PM. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 2014;59:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz SJ, Davis JP, Vergeront JMet al.. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis. 1985;151:883–9. [DOI] [PubMed] [Google Scholar]
- Tan HL, Regamey N, Brown Set al.. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen IP, Sapparapu G, James DBAet al.. Monoclonal Antibodies Against the Staphylococcus aureus Bicomponent Leukotoxin AB Isolated Following Invasive Human Infection Reveal Diverse Binding and Modes of Action. J Infect Dis. 2017;215:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A, Tong SY, Zhang Yet al.. Panton-Valentine leukocidin is not the primary determinant of outcome for Staphylococcus aureus skin infections: evaluation from the CANVAS studies. PLoS One. 2012;7:e37212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger Eet al.. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Lilliebridge RA, Bishop EJet al.. Clinical correlates of Panton-Valentine leukocidin (PVL), PVL isoforms, and clonal complex in the Staphylococcus aureus population of Northern Australia. J Infect Dis. 2010;202:760–9. [DOI] [PubMed] [Google Scholar]
- Tong SY, Bishop EJ, Lilliebridge RAet al.. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J Infect Dis. 2009;199:1461–70. [DOI] [PubMed] [Google Scholar]
- Toro CM, Janvier J, Zhang Ket al.. Community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia without evidence of antecedent viral upper respiratory infection. Can J Infect Dis Med Microbiol. 2014;25:e76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp AT, Van Gent M, Abrial Pet al.. Human CD45 is an F-component-specific receptor for the staphylococcal toxin Panton-Valentine leukocidin. Nat Microbiol. 2018;3:708–17. [DOI] [PubMed] [Google Scholar]
- Tseng CW, Biancotti JC, Berg BLet al.. Increased susceptibility of humanized NSG mice to Panton-Valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog. 2015;11:e1005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele J, Stein C, Nguyen MTet al.. Antigen delivery to dendritic cells shapes human CD4+ and CD8+ T cell memory responses to Staphylococcus aureus. PLoS Pathog. 2017;13:e1006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utay NS, Roque A, Timmer JKet al.. MRSA Infections in HIV-Infected People Are Associated with Decreased MRSA-Specific Th1 Immunity. PLoS Pathog. 2016;12:e1005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalen R, De La Cruz Diaz JS, Rumpret Met al.. Langerhans cells sense Staphylococcus aureus wall teichoic acid through langerin to induce inflammatory responses. MBio. 2019;10:e00330–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–71. [DOI] [PubMed] [Google Scholar]
- van Hal SJ, Jensen SO, Vaska VLet al.. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier RW, Fadok VA, Hoffmann PRet al.. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramaniam A, Adhikari RP, Kort Tet al.. TBA225, a fusion toxoid vaccine for protection and broad neutralization of staphylococcal superantigens. Sci Rep. 2019;9:3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergeront JM, Stolz SJ, Crass BAet al.. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J Infect Dis. 1983;148:692–8. [DOI] [PubMed] [Google Scholar]
- Volk CF, Burgdorf S, Edwardson Get al.. IL-1beta and IL-10 host responses in patients with Staphylococcus aureus bacteremia determined by antimicrobial therapy. Clin Infect Dis. 2019, DOI: 10.1093/cid/ciz686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Puel Aet al.. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol. 2012;42:3126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Zet al.. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Peters G, Becker K. The small colony variant (SCV) concept – the role of staphylococcal SCVs in persistent infections. Injury. 2006;37 Suppl 2:S26–33. [DOI] [PubMed] [Google Scholar]
- von Eiff C, Becker K, Machka Ket al.. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. [DOI] [PubMed] [Google Scholar]
- von Kockritz-Blickwede M, Rohde M, Oehmcke Set al.. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kamath A, Das Het al.. Antibacterial effect of human V gamma 2 V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrhahn MC, Robinson JO, Pearson JCet al.. Clinical and laboratory features of invasive community-onset methicillin-resistant Staphylococcus aureus infection: a prospective case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:1025–33. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Beck LA, Bieber Tet al.. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. [DOI] [PubMed] [Google Scholar]
- Welsh KJ, Abbott AN, Lewis EMet al.. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J Clin Microbiol. 2010;48:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KJ, Skrobarcek KA, Abbott ANet al.. Predictors of relapse of methicillin-resistant Staphylococcus aureus bacteremia after treatment with vancomycin. J Clin Microbiol. 2011;49:3669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JB, Jones LS, Soper NRet al.. Commercial intravenous immunoglobulin preparations contain functional neutralizing antibodies against the Staphylococcus aureus leukocidin LukAB (LukGH). Antimicrob Agents Chemother. 2017;61:e00968–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JB, Jones LS, Soper NRet al.. Serologic detection of antibodies targeting the leukocidin LukAB strongly predicts Staphylococcus aureus in children with invasive infection. J Pediatric Infect Dis Soc. 2019;8:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA. 2009;106:20515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SNet al.. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. [DOI] [PubMed] [Google Scholar]
- Ye Z, Vasco DA, Carter TCet al.. Genome wide association study of SNP-, gene-, and pathway-based approaches to identify genes influencing susceptibility to Staphylococcus aureus infections. Front Genet. 2014;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR, Filler SG, Schmidt CSet al.. Applying convergent immunity to innovative vaccines targeting Staphylococcus aureus. Front Immunol. 2014;5:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–43. [DOI] [PubMed] [Google Scholar]
- Young BC, Earle SG, Soeng Set al.. Panton-Valentine leucocidin is the key determinant of Staphylococcus aureus pyomyositis in a bacterial GWAS. Elife. 2019;8:e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Liu Y, Xu Yet al.. Expression of Panton-Valentine leukocidin mRNA among Staphylococcus aureus isolates associates with specific clinical presentations. PLoS One. 2013;8:e83368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KO, Randolph AG, Agan AAet al.. Staphylococcus aureus alpha-toxin response distinguishes respiratory virus-methicillin-resistant S. aureus coinfection in children. J Infect Dis. 2016;214:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XQ, Robbie GJ, Wu Yet al.. Safety, Tolerability, and Pharmacokinetics of MEDI4893, an Investigational, Extended-Half-Life, Anti-Staphylococcus aureus Alpha-Toxin Human Monoclonal Antibody, in Healthy Adults. Antimicrob Agents Chemother. 2017;61:e01020–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Guo L, Chu Xet al.. Presence of the Panton-Valentine Leukocidin Genes in Methicillin-Resistant Staphylococcus aureus Is Associated with Severity and Clinical Outcome of Hospital-Acquired Pneumonia in a Single Center Study in China. PLoS One. 2016;11:e0156704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LJ, Guerrero-Juarez CF, Hata Tet al.. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shen X, Zhang Het al.. Molecular epidemiological analysis of methicillin-resistant Staphylococcus aureus isolates from Chinese pediatric patients. Eur J Clin Microbiol Infect Dis. 2009;28:861–4. [DOI] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner Det al.. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–8. [DOI] [PubMed] [Google Scholar]