ABSTRACT
Background
Postruminal supply of Met during the periparturient period enhances production efficiency (feed conversion to milk) in dairy cows partly through alleviation of oxidant and inflammatory status. Whether alterations in hepatic 1-carbon metabolism (major contributor of antioxidants) and/or energy metabolism contribute to these beneficial effects is unknown.
Objectives
To investigate alterations in hepatic 1-carbon and energy metabolism and associations with plasma amino acids (AAs) and production efficiency in response to enhanced postruminal supply of Met.
Methods
Holstein cows (n = 30 per group) were fed during the last 28 d of pregnancy a control diet (CON) or the control plus ethylcellulose rumen-protected Met (MET; 0.9 g/kg of dry matter intake). Plasma (n = 15 per group) and liver tissue (n = 10 per group) were collected throughout the periparturient period to evaluate AA profiles, activity of the tricarboxylic acid cycle, and 1-carbon metabolism via mRNA abundance, enzyme activity, and targeted metabolomics.
Results
Cows in the MET group had greater overall (27%, P = 0.027) plasma Met concentrations, but had similar total plasma AA concentrations. Although mRNA abundance of 1-carbon metabolism enzymes did not differ, hepatic activity of cystathionine β-synthase (CBS) (51.2 compared with 44.4 mmol/h/mg protein; P = 0.032) and concentration (19%, P = 0.048) of the cellular antioxidant glutathione were greater overall in the MET group. mRNA abundance of aconitase 2 and fumarate hydratase was greater overall (P = 0.049), and phosphoenolpyruvate carboxykinase 1 tended (P = 0.093) to be greater overall in cows fed MET. There was a tendency (P ≤ 0.093) for greater overall hepatic concentrations of malic acid, α-ketoglutaric acid, and isocitric acid in cows fed MET.
Conclusions
Greater activity of CBS in response to enhanced postruminal supply of Met likely contributes to alleviating oxidant status by increasing concentrations of glutathione. Hence, transsulfuration plays an important role in the observed improvements in production efficiency of dairy cows during the periparturient period.
Keywords: amino acids, 1-carbon metabolism, lactation; methyl donors; nutrition
Introduction
The periparturient or “transition” period (3 wk prepartum through 3 wk postpartum) is a critical stage in the life cycle of dairy cows (1, 2). Failure to adequately meet its challenges can compromise production, induce metabolic disorders, and increase rates of removal from the herd (3). Most notably, dairy cows experience an increase in nutrient demands around parturition to sustain fetal growth and lactation that is coupled with a gradual decrease in voluntary feed intake as parturition approaches. An important outcome of such events is a state of negative nutrient balance among which the decreased supply of amino acids (AAs) reaching the small intestine can compromise milk synthesis. Despite the marked negative metabolizable protein balance (i.e., microbial plus rumen-undegradable protein reaching the small intestine), the boost in production efficiency in cows fed rumen-protected Met (i.e., to prevent microbial degradation in the rumen) during the periparturient period highlights the pivotal role of postruminal indispensable AA availability (4–6). Benefits of postruminal Met supply go beyond production and encompass better health status in part due to a reduction in oxidant status and inflammatory state (5, 7–10).
In addition to their essential role as nutrients, Met, choline, and folic acid are key components of 1-carbon metabolism, contributing to transmethylation and transsulfuration reactions (11). Conversion of Met to S-adenosylmethionine (SAM)—the principal methyl donor—is the first step in transmethylation and is followed by metabolism of SAM to homocysteine via the intermediate S-adenosylhomocysteine (12). Homocysteine can then either be recycled back to Met in a folate/choline-dependent manner through 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) and betaine-homocysteine S-methyltransferase (BHMT), or can be rapidly converted to cystathionine via cystathionine β-synthase (CBS) followed by various steps resulting in synthesis of taurine (Tau) and glutathione as 2 major end-products (13). These compounds are considered major intracellular antioxidants. Recent data from dairy cows underscore that key enzymes in 1-carbon metabolism are responsive to postruminal supply of Met and choline (14–16).
The increase in hepatic activity of BHMT, MTR, and CBS after parturition in sheep and dairy cows underscored the importance of 1-carbon metabolism during the transition into lactation (14, 17). Liver and plasma glutathione and Tau data from dairy cows suggest that supply of Met or choline has a positive effect on production of these vital antioxidants (8,15, 18, 19). As such, these compounds contribute to alleviating oxidative stress and inflammation while benefitting the immune system of the cow (8, 9). The 1-carbon metabolism is also tightly linked with cellular energy status (20), helping in part explain improvements in cow energy metabolism following supplementation with rumen-protected Met (5, 19).
The main objective of the present study was to investigate alterations in hepatic 1-carbon and energy metabolism, and associations with plasma AA and production efficiency in response to enhanced postruminal supply of Met. To achieve this, a systems approach combining mRNA abundance, activity of BHMT, MTR, and CBS, and targeted metabolomics in liver tissue from a subset of cows in a larger study was used (4).
Methods
Animals, experimental design, and treatments
The Institutional Animal Care and Use Committee (IACUC) at University of Illinois Urbana (IACUC protocol #14,270) approved all experimental procedures. Details of the experimental design have been reported previously (4). Briefly, multiparous Holstein cows were used in a randomized, complete block design experiment with 30 cows per treatment. Cows were blocked by expected day of parturition, and blocks were balanced within each of the 2 experimental groups by parity, previous 305-d milk yield, and body condition score (BCS). The BCS used to block cows was measured at 30 d before parturition using the scale 1 to 5 [1 = thin, 5 = fat, scale in 0.25 increments (21)]. Cows were fed the experimental treatments consisting of a basal control [CON; n = 30; 1.47 Mcal/kg dry matter (DM)] diet with no added Met or the basal diet plus ethylcellulose rumen-protected Met (MET; Mepron; Evonik Nutrition & Care GmbH; n = 30) from −28 to 30 d relative to parturition. The Met product was supplied (top-dressed) at a rate of 0.9 and 1.0 g/kg DM on the total mixed diet during the prepartum and postpartum period, respectively. This supply of Met was based on recent experiments demonstrating a benefit of achieving a Lys:Met ratio close to 2.8:1 in terms of production performance and health (5, 6). Mepron is a commercial rumen-protected source of dl-Met that resists ruminal degradation through an ethylcellulose film coating. Pellets measure 1.8 × 3 mm and contain 85% dl-Met. Mepron contains an equimolar mixture of the d- and l-isomers, and a minimum of 75 ± 3% of the ingested d-Met is transformed into l-Met by the dairy cow (22). The intestinal digestibility coefficient and ruminal bypass value of Mepron are 90% (23) and 80% (24), respectively; therefore, per 10 g of Mepron, the cows received 6.1 g Met available for absorption. During the preliminary period (from −45 to −29 d relative to the expected parturition day) all cows received the same diet containing 1.33 Mcal/kg DM with no added Met. Treatment diets were mixed daily in a tumble-mixer and fed once daily (at 13:00). The ingredients and chemical compositions of the diets are reported in Table 1. Diets were formulated to meet cows’ predicted requirements for energy, protein, minerals, and vitamins (25).
TABLE 1.
Ingredient and chemical composition of diets fed during preliminary (from day −45 to −29 relative to parturition) and treatment (last 28 d of pregnancy) periods1
| Preliminary period | Treatment period | ||
|---|---|---|---|
| Item | Control | Met | |
| Ingredient, g/kg/DM | |||
| Alfalfa haylage | — | 65.5 | 65.5 |
| Corn silage | 347 | 266 | 266 |
| Wheat straw | 33.7 | 265 | 265 |
| Corn grain, ground, dry | — | 126 | 126 |
| Molasses, beet sugar | — | 40.3 | 40.3 |
| Soybean hulls | 157 | 34.6 | 34.6 |
| Soybean meal, 48% CP | 120 | 78.3 | 78.3 |
| Expeller soybean meal2 | — | 58.0 | 58.0 |
| Protein supplement3 | — | 7.80 | 7.80 |
| Urea | 4.60 | 5.90 | 5.90 |
| Soychlor4 | — | 12.3 | 12.3 |
| Salt | 4.00 | — | — |
| Dicalcium phosphate | 5.00 | 5.20 | 5.20 |
| Magnesium sulfate | 19.0 | 20.8 | 20.8 |
| Mineral vitamin mix5 | 4.00 | 1.70 | 1.70 |
| Vitamin A6 | — | 0.30 | 0.30 |
| Vitamin D7 | — | 0.30 | 0.30 |
| Vitamin E8 | 4.00 | 6.00 | 6.00 |
| Biotin9 | — | 7.00 | 7.00 |
| Monensin10 | 0.10 | — | — |
| Rumen-protected Met11 | — | — | 0.90 |
| Chemical composition, g/kg/DM | |||
| Crude protein | 139 | 156 | 157 |
| Neutral detergent fiber | 545 | 408 | 407 |
| Acid detergent fiber | 369 | 275 | 274 |
| Nonfiber carbohydrate | 247 | 349 | 349 |
| Ether extract | 18.1 | 23.2 | 23.3 |
CP, crude protein; DM, dry matter.
SoyPlus, West Central Soy.
ProvAAl AAdvantage, Perdue AgriBusiness.
West Central Soy.
Composition in kg−1 DM: 50 Mg, 100 S, 75 K, 2.0 Fe, 3.0 Zn, 3.0 Mn, 5 Cu, 0.03 I, 0.04 Co, and 0.15 Se per kg DM. Vitamins in milligrams per kilogram DM: retinol 220, cholecalciferol 66, and α-tocopherol 770.
Contained 0.9 g/kg retinol.
Contained 125 g/kg cholecalciferol.
Contained 29.5 g/kg α-tocopherol.
ADM Animal Nutrition.
Rumensin, Elanco Animal Health.
Mepron, Evonik Nutrition & Care GmbH.
Blood collection
Per IACUC guidelines, subsets of 15 cows per treatment were used for blood sampling (4). To ensure a homogeneous representation and avoid confounding factors, selection of cows from each group took into account parity (3.6 ± 1.3 lactations), body weight (BW; mean: 812 ± 86 kg), and BCS (range: 3.50 to 3.70) at −30 d (i.e., prior to assignment to CON or MET), and previous 305-d milk yield (mean: 10,612 ± 1755 kg). Only cows that were free of clinical disease throughout the study and had the full set of samples were used for analyses. Samples were collected from the coccygeal vein before the meal at −14, 1, 7, 21, and 30 d relative to expected parturition. Samples were collected into evacuated tubes (BD Vacutainer; BD and Co) containing lithium heparin. After collection, samples were placed on ice until centrifugation (∼40 min). Plasma was obtained by centrifugation (2000 × g; 30 min; 4°C). Aliquots of plasma were frozen (−80°C) until further analysis.
Liver tissue collection
Per IACUC guidelines, a subset of 10 cows per treatment were used for liver biopsies (4). Selection of cows for biopsy followed the same approach as described above for blood samples (3.5 ± 1.4 lactations; BCS: 3.67 to 3.68; BW: 782 ± 86 kg; 10,918 ± 1078 kg milk). Actual days receiving diets prepartum for these cows was 28 ± 3 d, and all had the full set of biopsies. Tissue (1–2 g) was obtained via puncture biopsy. Samples were collected under local anesthesia at approximately 08:00 on days −10, 10, and 30 relative to parturition. Liver was frozen immediately in liquid nitrogen and stored (−80°C) until further analysis.
Laboratory analyses
AAs
Plasma AA concentrations were analyzed according to established protocols (26). Briefly, samples were first oxidized at 0°C for 16 h with performic acid followed by an incubation with sodium sulfite for 30 min in an ice bath to remove excess performic acid. This step allowed quantification of Met and Cys. Hydrolysis was performed with hydrochloric acid at 110°C for 24 h. Amino acid profiling was performed with a Biochrom 30+ (Biochrom Ltd) analyzer.
RNA isolation, cDNA synthesis, and qPCR
Total RNA was isolated from 50 mg of liver tissue using the miRNeasy kit (Qiagen) following the manufacturer's protocols. Samples were treated on-column with DNaseI (Qiagen); quantification was assessed using the NanoDrop ND-1000 (NanoDrop Technologies), and RNA quality was measured using an Agilent 2100 Bioanalyzer (Agilent). All samples had an RNA integrity number factor >7. The qPCR was performed as described previously (19). Primer sequence information is available in Supplemental Table 1. Performance of qPCR reactions is available in Supplemental Table 2.
Metabolomics analysis
Approximately 50 mg of frozen tissue was extracted by a 2-step protocol (27), which included methanol/chloroform/water extraction. Briefly, liver was homogenized in 4 mL methanol and 0.85 mL water per gram of tissue. Afterwards, the homogenate was removed and combined with 4 mL chloroform and 2 mL water per gram of tissue. Samples were then left to partition on ice for 10 min, and centrifuged for 5 min at 2000 × g at 4°C to separate the polar and nonpolar layer. Targeted metabolomics (LC/MS/MS) of the polar layer was performed to quantify metabolites related to the 1-carbon metabolism, the transsulfuration pathway, and tricarboxylic acid (TCA) cycle. Samples were analyzed with the 5500 QTRAP LC/MS/MS system (Sciex). The 1200 series HPLC system (Agilent Technologies) includes a degasser, an autosampler, and a binary pump. The LC separation was performed on a Phenomenex C18(2) column (4.6 × 150 mm, 5 μm) with mobile phase A (10 mM ammonium formate) and mobile phase B (methanol). The flow rate was 0.4 mL/min. The linear gradient was as follows: 0–1 min, 95% A; 8 min, 50% A; 15–18 min, 0% A; 18.1–26 min, 95% A. The autosampler was set at 10°C. The injection volume was 10 μL. Mass spectra were acquired under both positive (ion spray voltage +5500 V) and negative (ion spray voltage −4500 V) electrospray ionization. The source temperature was 500°C. The curtain gas, ion source gas 1, and ion source gas 2 were 35 psi, 65 psi, and 55 psi, respectively. Multiple reaction monitoring was used for quantitation (Supplemental Table 3). Software Analyst 1.6.2 (AB Sciex Pte. Ltd, Singapore)was used for data acquisition and analysis. Before statistical analysis, the AUC was normalized by the protein concentration of the samples.
Enzyme activity assays
Detailed protocols for evaluating activity of BHMT, MTR, and CBS were originally published by our group (14) and subsequently used in experiments with bovine placenta (28), cow liver (15, 16), and calf liver (29).
Statistical analysis
The data were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute) according to the following model:
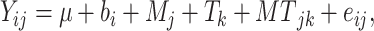 |
(1) |
where Yij = dependent, continuous variable; μ = overall mean; bi = random effect of block (i = 1 to 7); Mj = fixed effect of treatment (j = control compared with Met); Tk = fixed effect of time (d); MTjk = interaction between treatment and time; and eij = residual error. First-order autoregressive was the covariate structure used for repeated measures analysis because it resulted in the lowest Bayesian information criterion for most of the variables measured. For mRNA abundance, the data were log-2 transformed before analysis. Normality of the residuals was checked with normal probability and box plots, and homogeneity of variances with plots of residuals compared with predicted values. Data are presented as least-squares means with pooled SEMs. Significance was declared at a Tukey-adjusted P < 0.050 and tendencies at 0.050 ≤ P ≤ 0.100.
Results
Plasma AAs
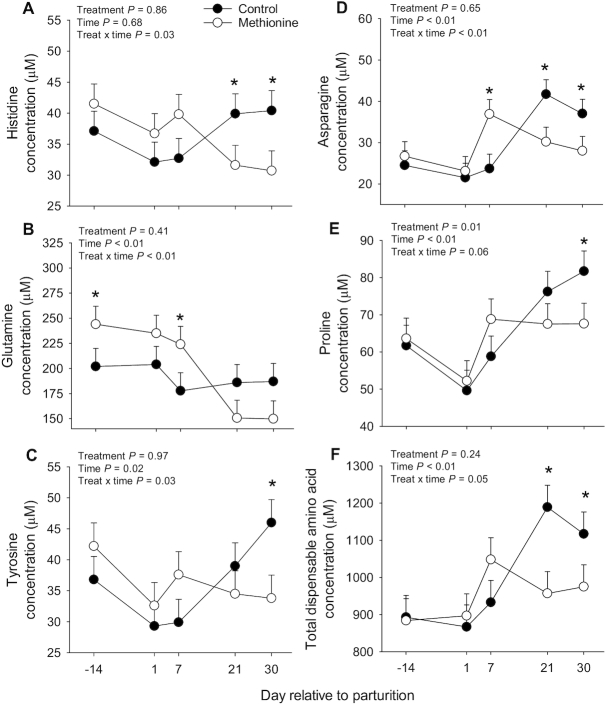
With the exception of His (Figure 1) and Tau, concentrations of all AAs and other nitrogen-containing molecules were altered by time around parturition (Time, P < 0.050; Table 2). Cows fed MET had an overall greater plasma concentration of Met (P = 0.027), which also led to a greater percentage of Met relative to total AA content in plasma. Cows fed MET also had lower overall (Treat, P < 0.050; Table 2) concentrations of Tau and α-aminobutyric acid, and tended to have lower overall 3-methylhistidine (Treat, P = 0.050), Gly (Treat, P = 0.095), and 1-methylhistidine (Treat, P = 0.094; Table 2). A treatment by time interaction was observed for His, Asn, Gln, Tyr, and total dispensable AA (Treat × Time, P < 0.050; Figure 2), and Pro tended to have a Treat × Time effect (P = 0.064, Figure 2). Cows fed MET had greater prepartum concentrations of Gln (Figure 2). In contrast, concentrations of His, Asn, Pro, Tyr, and total dispensable AAs were lower in cows fed MET, especially at 21 and 30 d postpartum (Figure 2).
FIGURE 1.
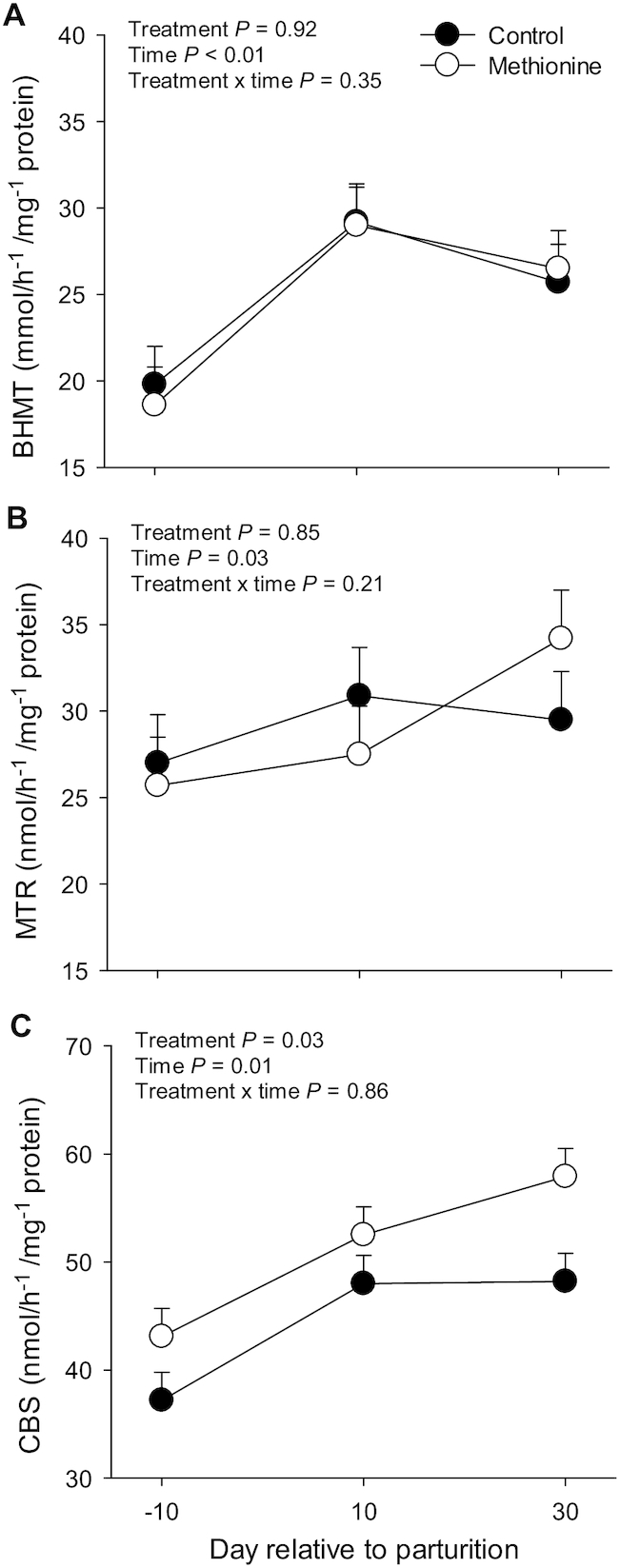
Activity of BHMT (A), MTR (B), and CBS (C) in liver tissue from Holstein cows individually fed a control diet or the control plus rumen-protected Met (0.9 g/kg dry matter intake) during the 28 d before parturition. Values are means ± pooled SEMs, n = 10 per treatment group. BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase.
TABLE 2.
Blood plasma amino acid concentrations in Holstein cows individually fed a control diet or the control plus rumen-protected Met (0.9 g/kg dry matter intake) during the last 28 d before parturition. Temporal concentrations of His, Gln, Tyr, Asn, and total dispensable amino acids (Treat × Time, P < 0.05) and Pro (Treat × Time, P = 0.064) are reported in Figure 11
| Treatments | P value | ||||
|---|---|---|---|---|---|
| Compound | Control | Met | Treat | Time | Treat × Time |
| Indispensable amino acids, μM | |||||
| Ile | 100 ± 5.8 | 107 ± 5.8 | 0.417 | 0.007 | 0.607 |
| Leu | 135 ± 6.5 | 140 ± 6.5 | 0.651 | 0.016 | 0.963 |
| Lys | 54.6 ± 2.4 | 58.8 ± 2.4 | 0.692 | <0.001 | 0.643 |
| Met | 9.43 ± 0.76 | 12.0 ± 0.76 | 0.027 | 0.441 | 0.750 |
| Phe | 39.9 ± 1.2 | 39.7 ± 1.2 | 0.794 | 0.141 | 0.511 |
| Thr | 60.9 ± 2.7 | 58.7 ± 2.7 | 0.717 | 0.022 | 0.140 |
| Val | 213 ± 11.9 | 230 ± 11.9 | 0.339 | 0.001 | 0.937 |
| Total | 652 ± 25 | 679 ± 25 | 0.344 | 0.006 | 0.101 |
| Met as % of total | 1.54 ± 0.13 | 1.88 ± 0.13 | 0.114 | 0.003 | 0.983 |
| Dispensable amino acids, μM | |||||
| Ala | 182 ± 8.8 | 178 ± 8.8 | 0.750 | 0.017 | 0.070 |
| Arg | 50.9 ± 2.0 | 49.7 ± 2.0 | 0.680 | <0.001 | 0.218 |
| Asp | 9.59 ± 0.76 | 9.88 ± 0.76 | 0.793 | 0.007 | 0.842 |
| Glu | 39.1 ± 2.0 | 41.2 ± 2.0 | 0.483 | 0.003 | 0.623 |
| Gly | 297 ± 17.4 | 254 ± 17.4 | 0.095 | <0.001 | 0.195 |
| Ser | 67.3 ± 3.2 | 63.9 ± 3.2 | 0.472 | 0.022 | 0.150 |
| Tau | 29.9 ± 0.6 | 24.7 ± 0.6 | <0.001 | 0.980 | 0.512 |
| BCAA, μM | 448 ± 24 | 477 ± 24 | 0.424 | 0.003 | 0.903 |
| Total indispensable + dispensable, μM | 1652 ± 43 | 1631 ± 43 | 0.747 | 0.002 | 0.190 |
| Met as % of total | 0.58 ± 0.04 | 0.75 ± 0.04 | 0.014 | 0.011 | 0.932 |
| Lys as % of total | 5.76 ± 0.22 | 5.84 ± 0.22 | 0.811 | <0.001 | 0.626 |
| Other compounds, μM | |||||
| α-Aminobutyric acid | 16.6 ± 1.1 | 14.3 ± 1.1 | 0.053 | <0.001 | 0.252 |
| Ornithine | 19.1 ± 1.7 | 19.4 ± 1.7 | 0.884 | 0.007 | 0.434 |
| 1-Methylhistidine | 14.0 ± 0.9 | 12.0 ± 0.9 | 0.094 | <0.001 | 0.353 |
| 3-Methylhistidine | 5.57 ± 0.49 | 4.06 ± 0.49 | 0.050 | <0.001 | 0.506 |
Values are means ± pooled SEMs, n = 10/treatment group. BCAA, branched-chain amino acid.
FIGURE 2.
Plasma concentrations of amino acids (A–E) and total dispensable amino acids (F) from Holstein cows individually fed a control diet or the control plus rumen-protected Met (0.9 g/kg dry matter intake) during the last 28 d before parturition. Data reported had a significant treatment × time (Treat × Time P < 0.050) effect or tended (Pro, P = 0.064) to have a significant interaction effect. Values are means ± pooled SEMs, n = 10 per treatment group. *Means differ (Treat × Time P < 0.050).
Hepatic enzyme activity, metabolomics, and mRNA abundance
Compared with prepartum levels, activity of BHMT, MTR, and CBS increased postpartum (Time, P < 0.050; Figure 1). Met supplementation did not affect (Treatment, P > 0.050) activity of BHMT and MTR, whereas it led to an overall greater (Treatment, P = 0.032; Figure 1) activity of CBS throughout the periparturient period.
Liver tissue concentrations of Met and Tau (Treat, P = 0.080 and 0.101; Table 3) and malic, α-ketoglutaric, and isocitric acids tended (Treat, P = 0.093, 0.084, and 0.063) to be greater in cows fed MET. In contrast, concentration of glutathione was greater overall (Treat, P = 0.048) in cows fed MET.
TABLE 3.
Metabolite concentrations (AUC/mg protein) in liver tissue from Holstein cows individually fed a control diet or the control plus rumen-protected Met (0.9 g/kg dry matter intake) during the last 28 d before parturition1
| Treatment | P value | ||||
|---|---|---|---|---|---|
| Item | Control | Met | Treat | Time | Treat × Time |
| TCA cycle, AUC/mg protein | |||||
| Acetyl-CoA | 7.14 ± 0.28 | 7.48 ± 0.28 | 0.442 | 0.685 | 0.813 |
| Succinyl-CoA | 7.29 ± 0.18 | 7.29 ± 0.18 | 0.891 | 0.998 | 0.499 |
| Malic acid | 12.8 ± 0.08 | 13.7 ± 0.08 | 0.093 | 0.764 | 0.716 |
| α-Ketoglutaric acid | 10.21 ± 0.04 | 10.37 ± 0.04 | 0.084 | 0.375 | 0.526 |
| Isocitric acid | 5.87 ± 0.08 | 6.22 ± 0.08 | 0.063 | 0.084 | 0.827 |
| Met and folic acid cycle, AUC/mg protein | |||||
| Glycine | 11.31 ± 0.05 | 11.21 ± 0.05 | 0.221 | 0.178 | 0.994 |
| N-methylglycine | 8.09 ± 0.07 | 8.17 ± 0.07 | 0.184 | 0.123 | 0.516 |
| N,N-dimethylglycine | 11.2 ± 0.16 | 11.2 ± 0.16 | 0.822 | 0.404 | 0.324 |
| Betaine | 9.24 ± 0.08 | 9.28 ± 0.08 | 0.814 | 0.002 | 0.403 |
| Met | 11.1 ± 0.06 | 11.3 ± 0.06 | 0.080 | 0.323 | 0.994 |
| S-5′-adenosylmethionine | 10.5 ± 0.21 | 10.3 ± 0.21 | 0.492 | 0.034 | 0.482 |
| S-5′-adenosylhomocysteine | ND | ND | |||
| 5-Methyltetrahydrofolic acid | 7.85 ± 0.28 | 8.03 ± 0.28 | 0.564 | 0.010 | 0.510 |
| Tetrahydrofolic acid | ND | ND | |||
| Folic acid | ND | ND | |||
| Vitamin B-12 | ND | ND | |||
| Adenosine | 9.21 ± 0.21 | 9.29 ± 0.21 | 0.822 | 0.301 | 0.674 |
| Transsulfuration pathway, AUC/mg protein | |||||
| Serine | 11.83 ± 0.11 | 11.89 ± 0.11 | 0.743 | 0.630 | 0.202 |
| Hypotaurine | 9.38 ± 0.15 | 9.15 ± 0.15 | 0.284 | 0.181 | 0.443 |
| Cysteine | 9.24 ± 0.09 | 9.27 ± 0.09 | 0.390 | 0.253 | 0.564 |
| Homocysteine | ND | ND | |||
| Vitamin B-6 | ND | ND | |||
| Cystathionine | 9.35 ± 0.14 | 9.36 ± 0.14 | 0.961 | 0.934 | 0.245 |
| Glutathione | 6.96 ± 0.46 | 8.31 ± 0.46 | 0.048 | 0.403 | 0.436 |
| Taurine | 9.74 ± 0.08 | 9.99 ± 0.08 | 0.101 | 0.305 | 0.891 |
| Cysteinesulfinic acid | 7.07 ± 0.28 | 6.83 ± 0.28 | 0.564 | 0.274 | 0.638 |
Values are means ± pooled SEMs, n = 10 per treatment group. ND, not detected; TCA, tricarboxylic acid.
The abundance of aconitase 2 (ACO2) and fumarate hydratase (FH) mRNA in liver tissue was greater (Treat, P = 0.045 and 0.041; Table 4) in cows fed MET. Furthermore, methionine adenosyltransferase 1A (MAT1A) and phosphoenolpyruvate carboxykinase 1 (PCK1) mRNA tended (Treat, P = 0.064 and 0.093; Table 4) to be greater and CBS mRNA tended to be lower (Treat, P = 0.073) in cows fed MET.
TABLE 4.
Messenger RNA abundance (log-2 scale) in liver tissue from Holstein cows individually fed a control diet or the control plus rumen-protected Met (0.9 g/kg dry matter intake) during the last 28 d before parturition1
| Treatment | P value | ||||
|---|---|---|---|---|---|
| Item | Control | Met | Treat | Time | Treat × Time |
| TCA cycle, log-2 | |||||
| CS | 2.24 ± 0.08 | 2.11 ± 0.08 | 0.342 | 0.003 | 0.416 |
| ACO2 | 4.74 ± 0.10 | 4.92 ± 0.10 | 0.045 | 0.005 | 0.367 |
| IDH1 | 2.99 ± 0.07 | 3.07 ± 0.07 | 0.496 | 0.012 | 0.202 |
| OGDH | 1.13 ± 0.09 | 1.04 ± 0.09 | 0.124 | 0.222 | 0.433 |
| SUCLA2 | 2.99 ± 0.07 | 3.07 ± 0.07 | 0.325 | 0.006 | 0.205 |
| SDHA | 1.13 ± 0.13 | 1.14 ± 0.13 | 0.186 | 0.173 | 0.346 |
| FH | 1.89 ± 0.11 | 2.12 ± 0.11 | 0.041 | 0.007 | 0.164 |
| MDH1 | 0.89 ± 0.16 | 0.87 ± 0.16 | 0.267 | 0.030 | 0.371 |
| Met and folic acid cycle, log-2 | |||||
| BHMT | 1.99 ± 0.07 | 1.07 ± 0.07 | 0.493 | 0.004 | 0.201 |
| MTR | 1.13 ± 0.13 | 1.04 ± 0.13 | 0.125 | 0.724 | 0.550 |
| MAT1A | 1.28 ± 0.12 | 1.49 ± 0.12 | 0.064 | 0.063 | 0.488 |
| GNMT | 0.98 ± 0.15 | 1.01 ± 0.15 | 0.236 | 0.232 | 0.323 |
| AHCY | 1.14 ± 0.11 | 1.15 ± 0.11 | 0.423 | 0.214 | 0.412 |
| Transsulfuration pathway, log-2 | |||||
| CBS | 0.74 ± 0.07 | 0.73 ± 0.07 | 0.073 | 0.006 | 0.491 |
| CTH | 1.58 ± 0.05 | 1.64 ± 0.05 | 0.395 | 0.008 | 0.135 |
| CDO | 0.13 ± 0.09 | 0.12 ± 0.09 | 0.466 | 0.010 | 0.416 |
| GSR | 0.62 ± 0.06 | 0.66 ± 0.06 | 0.514 | 0.004 | 0.583 |
| GPX1 | 0.34 ± 0.07 | 0.35 ± 0.07 | 0.887 | 0.024 | 0.317 |
| Gluconeogenesis, log-2 | |||||
| PC | 2.11 ± 0.07 | 2.14 ± 0.07 | 0.425 | 0.003 | 0.453 |
| PCK1 | 2.09 ± 0.11 | 2.29 ± 0.11 | 0.093 | 0.001 | 0.218 |
Values are means ± pooled SEMs, n = 10 per treatment group. ACO2, aconitase 2; AHCY, adenosylhomocysteinase; BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; CDO, cysteine dioxygenase; CS, citrate synthase; CTH, cystathionine γ-lyase; FH, fumarate hydratase; GNMT, glycine N-methyltransferase; GPX1, glutathione peroxidase 1; GSR, glutathione-disulfide reductase; IDH1, isocitrate dehydrogenase (NADP(+)) 1; MAT1A, methionine adenosyltransferase 1A; MDH1, malate dehydrogenase 1; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; ND, not detected; OGDH, oxoglutarate dehydrogenase; PC, pyruvate carboxylase; PCK1, phosphoenolpyruvate carboxykinase 1; SDHA, succinate dehydrogenase complex flavoprotein subunit A; SUCLA2, succinate-CoA ligase ADP-forming β-subunit; TCA, tricarboxylic acid.
Discussion
Previous studies have demonstrated the ability of “protection” technologies to deliver target nutrients—for example, Met (18, 24, 30, 31)—to the dairy cow directly, bypassing the rumen environment. The 27% increase in plasma Met concentration in the study confirmed the rumen-bypass ability of the ethylcellulose protection technology of the supplemented MET product. Although similar to findings in older studies (24, 30, 31), the greater systemic availability of Met coupled with lower circulating concentrations of other indispensable AAs, dispensable AAs (e.g., Ala, Asn, Gly, His, Pro, Tyr), and total AAs (indispensable + dispensable) in MET cows contrasts with a recent study in which periparturient dairy cows were fed a polymer-protected Met product (18). We speculate that feeding rumen-protected Met improved the balance and utilization of plasma AAs, following nutritional theories that postruminal increases of the most-limiting AAs (e.g., Lys, Met) lead to a decrease of other AAs in plasma because of an increase in synthesis of milk protein (32, 33). The greater milk protein production of cows fed MET supports this theory (4). Furthermore, the improved AA profile and the increase in overall DM intake observed in the cows used in the present study (4) reduced the need of MET cows to tap into their protein reserves (e.g., muscle mass), as indicated by the tendency for lower circulating concentrations of biomarkers of muscle mobilization (e.g., 1- and 3-methylhistidine).
Despite a statistical tendency for greater concentrations in cows fed MET, the hepatic concentration of Met during the experimental period did not follow the same increment observed in the plasma. As first utilizer of the blood flow from the gastrointestinal tract, the liver either deaminates the excess AAs to synthesize other dispensable AAs, channels them through metabolic pathways for synthesis of other metabolites, or allows them to pass untouched into the systemic circulation. Because blood urea (4) and ornithine concentrations remained constant between MET and CON cows, the additional Met supplied by rumen-protected Met did not seem to have been used for deamination purposes. In fact, the greater CBS enzyme activity suggests the channeling of Met into the Met cycle and the transsulfuration pathways. Despite the lower plasma concentration of α-aminobutyric acid (AABA)—a marker of flow into the transsulfuration pathway (34)—in cows fed MET, the greater concentrations of glutathione—also observed recently in a similar study (8)—and tendency for Tau support the CBS activity data.
Both Tau and glutathione are major players in the cellular antioxidant systems, and could have helped cows fed MET handle the pro-oxidant status characteristic of the periparturient period (35). In agreement with this idea, analyses in blood and liver tissue from the bigger cohort of cows in this study proved that these animals underwent a period of oxidative stress [e.g., postpartum increase of reactive oxygen metabolites (ROMs) and decrease of reduced glutathione] (8). However, cows fed MET, probably thanks to the increased hepatic synthesis of glutathione and Tau, displayed a better antioxidant capacity (e.g., ferric reducing antioxidant power), lower ROM, and greater reduced glutathione concentrations (8). Furthermore, the greater concentration of AABA in the CON cows might have been due to liver damage from oxidative stress (36).
Despite supplemental Met potentially inducing greater flux through the transsulfuration pathway, the greater hepatic concentration of Tau in cows fed MET was not maintained at the systemic level, because plasma concentrations were greater in CON cows. As for other AAs, we speculate that the greater supply of an indispensable AA (i.e., Met) might have increased its utilization in the cow's tissues. During the transition period dairy cows experience metabolic dysfunction due to physiological adaptations associated with the high requirements of the lactating mammary gland (2). At least in nonruminants, Tau not only has fundamental roles as an antioxidant and anti-inflammatory metabolite, but it also participates in energy metabolism, specifically by strengthening it under normal conditions (e.g., enhancing electron transport chain activity and protecting the mitochondria against excessive superoxide generation), or by directly or indirectly reversing histological abnormalities and improving metabolic activities under stressful or pathological conditions (37). At the systemic level, the observed lower concentrations of circulating nonesterified fatty acids in the current (4) and another study using ethylcellulose rumen-protected Met (38) suggest a better energy metabolism status through the periparturient period. At the level of the liver, the mRNA abundance of genes (e.g., ACO2, FH) and intermediates (e.g., malic, α-ketoglutaric, and isocitric acids) of metabolic pathways in cows fed MET also underscored better energy metabolism. Because dairy cows rely heavily on hepatic gluconeogenesis to supply peripheral tissues with metabolic fuels, the tendency for greater abundance of PCK1 mRNA offers additional support for better energy status in cows fed MET (2).
The greater activity of CBS in response to feeding rumen-protected Met supports the notion that increased postruminal availability of this indispensable AA around parturition can alter hepatic flux through the folic acid and Met cycles and the transsulfuration pathway. As such, it can contribute to alleviating oxidant status by increasing concentrations of glutathione and Tau, while improving energy metabolism. Together, these adaptations help explain the consistent improvements in production, health, and immunological status of periparturient dairy cows fed rumen-protected Met (5,6,9, 38–40).
To overcome some limitations of the current study, future research should encompass measurements of protein abundance for MTR, BHMT, and CBS, to allow a more complete view of how they are regulated as a function of supply of Met or other methyl donors (e.g., choline, folic acid). Along with the protein data, absolute quantification of hepatic metabolites via the use of purified standards would help better address potential changes in flux as related to Met supply (or other nutrients such as choline and folic acid). Such data also would allow for better comparisons among published studies of 1-carbon metabolism in dairy cows. Overall, a combination of in vivo (tissue) and in vitro (primary hepatocytes) experiments would be helpful in terms of enhancing our understanding of physiological mechanisms that respond to changes in the supply of methyl donors.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JJL and CP: designed research; FB: conducted the animal experiment, collected biological samples, and performed analyses; RRCSY and MV-R: performed analyses; Y-XP: contributed reagents; MV-R, FB, and JJL: wrote the manuscript; MV-R and JJL: interpreted the data; and all authors: read and approved the final manuscript.
Notes
Author disclosures: MV-R, FB, RRCSY, Y-XP, and JJL no conflict of interests. CP is an employee of Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany), which had a role in the study design and provided financial support to cover costs of animal use, data collection, and sample analysis.
Supplemental Tables 1, 2, and 3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AA, amino acid; AABA, α-aminobutyric acid; ACO2, aconitase 2; BCS, body condition score; BHMT, betaine-homocysteine S-methyltransferase; BW, body weight; CBS, cystathionine β-synthase; CON, control group; DM, dry matter; FH, fumarate hydratase; IACUC, Institutional Animal Care and Use Committee; MET, Met-supplemented group; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; PCK1, phosphoenolpyruvate carboxykinase 1; ROM, reactive oxygen metabolite; SAM, S-adenosylmethionine; Tau, taurine; TCA, tricarboxylic acid.
References
- 1. Bell AW. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci. 1995;73(9):2804–19. [DOI] [PubMed] [Google Scholar]
- 2. Drackley JK. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: the final frontier?. J Dairy Sci. 1999;82(11):2259–73. [DOI] [PubMed] [Google Scholar]
- 3. Curtis CR, Erb HN, Sniffen CJ, Smith RD, Kronfeld DS. Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. J Dairy Sci. 1985;68(9):2347–60. [DOI] [PubMed] [Google Scholar]
- 4. Batistel F, Arroyo JM, Bellingeri A, Wang L, Saremi B, Parys C, Trevisi E, Cardoso FC, Loor JJ. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci. 2017;100(9):7455–67. [DOI] [PubMed] [Google Scholar]
- 5. Osorio JS, Ji P, Drackley JK, Luchini D, Loor JJ. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J Dairy Sci. 2013;96(10):6248–63. [DOI] [PubMed] [Google Scholar]
- 6. Zhou Z, Vailati-Riboni M, Trevisi E, Drackley JK, Luchini DN, Loor JJ. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J Dairy Sci. 2016;99(11):8716–32. [DOI] [PubMed] [Google Scholar]
- 7. Osorio JS, Trevisi E, Ji P, Drackley JK, Luchini D, Bertoni G, Loor JJ. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J Dairy Sci. 2014;97(12):7437–50. [DOI] [PubMed] [Google Scholar]
- 8. Batistel F, Arroyo JM, Garces CIM, Trevisi E, Parys C, Ballou MA, Cardoso FC, Loor JJ. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci. 2018;101(1):480–90. [DOI] [PubMed] [Google Scholar]
- 9. Zhou Z, Bulgari O, Vailati-Riboni M, Trevisi E, Ballou MA, Cardoso FC, Luchini DN, Loor JJ. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J Dairy Sci. 2016;99(11):8956–69. [DOI] [PubMed] [Google Scholar]
- 10. Zhou Z, Ferdous F, Montagner P, Luchini DN, Correa MN, Loor JJ. Methionine and choline supply during the peripartal period alter polymorphonuclear leukocyte immune response and immunometabolic gene expression in Holstein cows. J Dairy Sci. 2018;101(11):10374–82. [DOI] [PubMed] [Google Scholar]
- 11. Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tavares CD, Sharabi K, Dominy JE, Lee Y, Isasa M, Orozco JM, Jedrychowski MP, Kamenecka TM, Griffin PR, Gygi SP et al.. The methionine transamination pathway controls hepatic glucose metabolism through regulation of the GCN5 acetyltransferase and the PGC-1alpha transcriptional coactivator. J Biol Chem. 2016;291(20):10635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalhan SC, Marczewski SE. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev Endocr Metab Disord. 2012;13(2):109–19. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Z, Garrow TA, Dong X, Luchini DN, Loor JJ. Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J Nutr. 2017;147(1):11–9. [DOI] [PubMed] [Google Scholar]
- 15. Coleman DN, Alharthi A, Lopreiato V, Trevisi E, Miura M, Pan YX, Loor JJ. Choline supply during negative nutrient balance alters hepatic cystathionine beta-synthase, intermediates of the methionine cycle and transsulfuration pathway, and liver function in Holstein cows. J Dairy Sci. 2019;102(9):8319–31. [DOI] [PubMed] [Google Scholar]
- 16. Coleman DN, Vailati-Riboni M, Elolimy AA, Cardoso FC, Rodriguez-Zas SL, Miura M, Pan YX, Loor JJ. Hepatic betaine-homocysteine methyltransferase and methionine synthase activity and intermediates of the methionine cycle are altered by choline supply during negative energy balance in Holstein cows. J Dairy Sci. 2019;102(9):8305–18. [DOI] [PubMed] [Google Scholar]
- 17. Snoswell AM, Xue GP. Methyl group metabolism in sheep. Comp Biochem Physiol B. 1987;88(2):383–94. [DOI] [PubMed] [Google Scholar]
- 18. Zhou Z, Vailati-Riboni M, Luchini DN, Loor JJ. Methionine and choline supply during the periparturient period alter plasma amino acid and one-carbon metabolism profiles to various extents: potential role in hepatic metabolism and antioxidant status. Nutrients. 2016;9(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osorio JS, Ji P, Drackley JK, Luchini D, Loor JJ. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways. J Dairy Sci. 2014;97(12):7451–64. [DOI] [PubMed] [Google Scholar]
- 20. Luciano-Mateo F, Hernandez-Aguilera A, Cabre N, Camps J, Fernandez-Arroyo S, Lopez-Miranda J, Menendez JA, Joven J. Nutrients in energy and one-carbon metabolism: learning from metformin users. Nutrients. 2017;9(2):E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wildman EE, Jones GM, Wagner PE, Boman RL, Troutt HF, Lesch TN. A dairy-cow body condition scoring system and its relationship to selected production characteristics. J Dairy Sci. 1982;65(3):495–501. [Google Scholar]
- 22. Lapierre H, Holtrop G, Calder AG, Renaud J, Lobley GE. Is D-methionine bioavailable to the dairy cow?. J Dairy Sci. 2012;95(1):353–62. [DOI] [PubMed] [Google Scholar]
- 23. Schwab CG. Protected proteins and amino acids for ruminants. In: Wallace RJ, Chesson A, editors. Biotechnology in animal feeds and animal feeding. Weinheim: VCH Verlagsgesellschaft mbH; 1995:115–41. [Google Scholar]
- 24. Overton TR, LaCount DW, Cicela TM, Clark JH. Evaluation of a ruminally protected methionine product for lactating dairy cows. J Dairy Sci. 1996;79(4):631–8. [DOI] [PubMed] [Google Scholar]
- 25. NRC. Nutrient requirements of dairy cattle, 7th rev. ed. National Academies Press; 2001. [PubMed] [Google Scholar]
- 26. AOAC. Official methods of analysis. Arlington (VA): Association of Official Analytical Chemists; 1995. [Google Scholar]
- 27. Wu H, Southam AD, Hines A, Viant MR. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal Biochem. 2008;372(2):204–12. [DOI] [PubMed] [Google Scholar]
- 28. Batistel F, Alharthi AS, Yambao RRC, Elolimy AA, Pan YX, Parys C, Loor JJ. Methionine supply during late-gestation triggers offspring sex-specific divergent changes in metabolic and epigenetic signatures in bovine placenta. J Nutr. 2019;149(1):6–17. [DOI] [PubMed] [Google Scholar]
- 29. Alharthi AS, Coleman DN, Liang Y, Batistel F, Elolimy AA, Yambao RC, Abdel-Hamied E, Pan YX, Parys C, Alhidary IA et al.. Hepatic 1-carbon metabolism enzyme activity, intermediate metabolites, and growth in neonatal Holstein dairy calves are altered by maternal supply of methionine during late pregnancy. J Dairy Sci. 2019; 102(11):10291–303. [DOI] [PubMed] [Google Scholar]
- 30. Blum JW, Bruckmaier RM, Jans F. Rumen-protected methionine fed to dairy cows: bioavailability and effects on plasma amino acid pattern and plasma metabolite and insulin concentrations. J Dairy Sci. 1999;82(9):1991–8. [DOI] [PubMed] [Google Scholar]
- 31. Yang WR, Sun H, Wang QY, Liu FX, Yang ZB. Effects of rumen-protected methionine on dairy performance and amino acid metabolism in lactating cows. Am J Anim Vet Sci. 2010;5(1):1–7. [Google Scholar]
- 32. Nimrick K, Hatfield EE, Kaminski J, Owens FN. Qualitative assessment of supplemental amino acid needs for growing lambs fed urea as the sole nitrogen source. J Nutr. 1970;100:1293–300. [DOI] [PubMed] [Google Scholar]
- 33. Bull LS, Vandersall JH. Sulfur source for in vitro cellulose digestion and in vivo ration utilization, nitrogen metabolism, and sulfur balance. J Dairy Sci. 1973;56(1):106–12. [DOI] [PubMed] [Google Scholar]
- 34. Stabler SP, Sekhar J, Allen RH, O'Neill HC, White CW. alpha-Lipoic acid induces elevated S-adenosylhomocysteine and depletes S-adenosylmethionine. Free Radical Bio Med. 2009;47(8):1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sordillo LM, Mavangira V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim Prod Sci. 2014;54(9):1204–14. [Google Scholar]
- 36. Effros RM. Alpha aminobutyric acid, an alternative measure of hepatic injury in sepsis?. Transl Res. 2011;158(6):326–7. [DOI] [PubMed] [Google Scholar]
- 37. Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, He S, Li J, Yin Y, Wen CY et al.. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. 2019;63(2):1800536. [DOI] [PubMed] [Google Scholar]
- 38. Sun F, Cao Y, Cai C, Li S, Yu C, Yao J. Regulation of nutritional metabolism in transition dairy cows: energy homeostasis and health in response to post-ruminal choline and methionine. PLoS One. 2016;11(8):e0160659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toledo MZ, Baez GM, Garcia-Guerra A, Lobos NE, Guenther JN, Trevisol E, Luchini D, Shaver RD, Wiltbank MC. Effect of feeding rumen-protected methionine on productive and reproductive performance of dairy cows. PLoS One. 2017;12(12):e0189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee C, Lobos NE, Weiss WP. Effects of supplementing rumen-protected lysine and methionine during prepartum and postpartum periods on performance of dairy cows. J Dairy Sci [Internet] 2019. doi: 10.3168/jds.2019-17125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.