Abstract
Several studies have indicated that the use of antihypertensive medications may influence the incidence of bladder/kidney cancer, with some scholars refuting any such association. Hence, a systematic review is needed to verify this linkage. we comprehensively searched PubMed, Embase, Web of Science, and the Cochrane Library for original studies reporting a relationship between antihypertensive medications and risk of bladder/kidney cancer. We included 31 articles comprising 3,352,264 participants. We found a significant association between the risk of kidney cancer and any antihypertensive medications use (relative risk (RR) = 1.45, 95% CI 1.20-1.75), as well as angiotensin-converting enzyme inhibitors (RR = 1.24, 95% CI 1.04-1.48), angiotensin II receptor blockers (ARB) (RR = 1.29, 95% CI:1.22-1.37), beta-blockers (RR = 1.36, 95% CI 1.11-1.66), calcium-channel blockers (RR = 1.65, 95% CI 1.54-1.78) and diuretics (RR = 1.34, 95% CI 1.19-1.51). In case of bladder cancer, a statistical significance was observed with the use of ARB (RR = 1.07, 95% CI 1.03-1.11) but not with the other antihypertensive medications. There was a linear association between the duration of antihypertensive medications and the risk of kidney cancer (P = 0.061 for a non-linear trend) and the pooled RR for the per year increase in antihypertensive medications duration of use was 1.02 (95% CI: 1.01-1.02). Our results indicate that there is a significant association between each class of antihypertensive medications and the risk of kidney cancer, and this trend presented as a positive linear association. Furthermore, the use of ARB has been linked to the risk of bladder cancer.
Keywords: antihypertensive medications, kidney cancer, bladder cancer, meta-analysis, risk
INTRODUCTION
Hypertension is a highly prevalent chronic disease worldwide in the elderly, necessitating the long-term use of various antihypertensive medications to prevent cardiovascular morbidity and mortality. However, several studies have demonstrated the potential risks of antihypertensive medications including orthostatic hypotension, falls, cognitive decline, dementia, fractures, diabetes, and cancer [1].
Preclinical experimental studies have indicated that antihypertensive medications, such as angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), calcium-channel blockers (CCB) and beta-blockers (BB), can facilitate or interfere with tumor cell proliferation, migration, and apoptosis, as well as angiogenesis [2–4]. For example, it was observed that angiotensin II type I receptor (Ang II AT1R) was highly expressed in bladder cancer cells of high-stage and/or high-grade tumors and Ang II AT1R signaling could induce the expression of the vascular endothelial growth factor (VEGF) [5]. Moreover, ACEIs and ARBs have demonstrated anti-angiogenetic effects, reducing VEGF expression in bladder malignancies [4]. Notably, telmisartan played a potent anti-proliferative role in urological cancer cells through the peroxisome proliferator-activated receptor gamma (PPAR-γ) [6]. However, the thiazide diuretic treatment in rats could result in degenerative changes including cell apoptosis and tumor cell markers in the distal tubule [7].
A parallel, randomized, double-blind, controlled, clinical trial assessing the effects of candesartan observed an unexpected phenomenon. Reportedly, the candesartan group (3803 patients) displayed a higher cancer mortality than patients treated with the placebo (3796 patients) (2.3% vs 1.6%, p=0.038) [8]. Furthermore, several meta-analyses demonstrated that antihypertensive medications usage may influence the incidence of cancer [9–13]. In 2010, a meta-analysis based on nine randomized trials demonstrated that, compared to the placebo or comparator agents, ARB therapy indicated a modestly increased risk of new cancer occurrence, with a significant association observed in lung cancer, but not prostate and breast cancer, among the solid organ cancers examined [9]. However, a network meta-analysis refuted the relative risk increase between cancer or cancer-related death and the use of most antihypertensive medications classes [10].
Notably, the morbidity and mortality of kidney and bladder cancers are increasing, and the prognosis remains unfavorable. According to the 2018 Global cancer statistics, it was estimated that 549,393 individuals were newly diagnosed with bladder cancer, with 199,922 patients’ deaths reported from the disease. Additionally, kidney cancer reportedly accounted for approximately 2.2% of all new cancer cases and 175,018 deaths worldwide in 2018 [14]. Therefore, there is an urgent need to elucidate relevant mechanisms and risk factors. In previous epidemiologic studies, several potential risk factors for bladder/kidney cancer have been investigated including age, obesity, cigarette smoking, a family history of bladder or kidney cancer, exposure to certain chemicals, and sex, with men indicating a higher incidence of both cancers compared to women [15, 16]. However, with the emergence of observational data, the association between antihypertensive medications and the risk of kidney/bladder cancer is more controversial. Therefore, we conducted this review to evaluate the existence of an association between these factors.
RESULTS
Characteristics and quality of studies
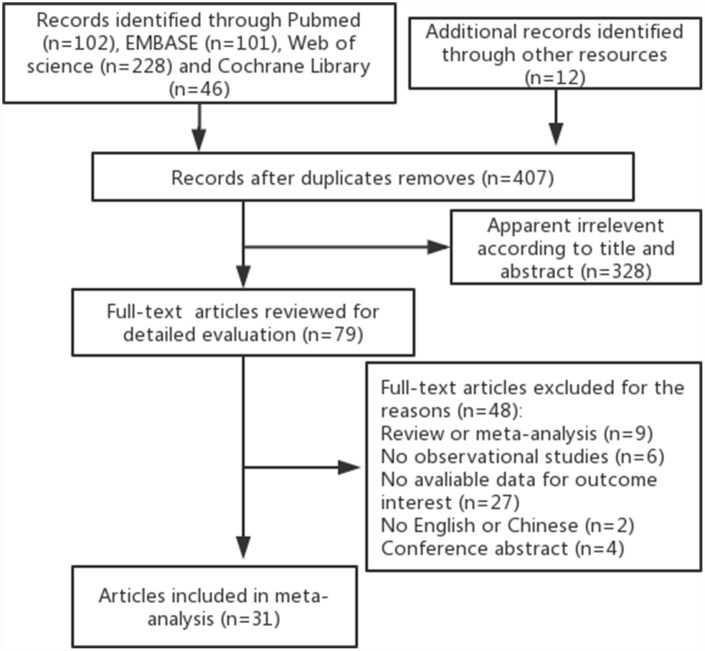
As illustrated in Figure 1, our initial search identified 407 potentially relevant citations from online databases and reference lists, from which 328 were excluded after screening the titles and abstracts. Ultimately, 31 articles met the inclusion criteria and were finally included in this meta-analysis [17–47]. This selection consisted of 18 articles designed as case-control studies and 13 articles designed as cohort studies, with approximately 3,352,264 participants. Moreover, these articles were regarded as independent studies since the role of antihypertensive medications in the risk of bladder/kidney cancer was assessed according to the different antihypertensive medications classes (ACEI, ARB, BB, CCB, diuretics or any antihypertensive medications), cancer sites (kidney, bladder), and gender. The characteristics of the articles are listed in Table 1. Data collection for antihypertensive medications use was not consistent across studies, with most using questionnaires or prescription database reviews. Cancer cases were ascertained by cancer registries or medical records from the included studies. Most studies controlled potential confounding factors (age, gender, BMI, smoking, hypertension) by matching or adjustments; however, inconsistencies among the adjustments in each study were observed. The scores of the Newcastle-Ottawa Scale (NOS) quality assessment ranged from 6 to 8 and are listed in Table 1.
Figure 1.
Flow chart of selection process of observational studies.
Table 1. Characteristics of the articles included in the meta-analysis.
| Author, yr [Ref] | Location | Study period | Age (yr) | No.of cases / participants | Class of medication (reference group) | Outcome | Type of study | Adjustment for covariates | QS |
| Assimes TL 2008 | Canada | 1980-2003 | 71.8 | 11,697/77,887 | BB, CCB, RASIs (thiazide diuretics) | risk of kidney cancer | case-control | age, all measured comorbid conditions, and exposure to all other classes of antihypertensive not of interest | 7 |
| Chow WH 1995 | USA | 1988-1990 | 64(20–79) | 151/842 | Diuretics, AHT (no use) | risk of renal cell cancer | case-control | age, sex, smoking, BMI, hypertension | 8 |
| Chuang YW 2017 | China | 2005-2011 | 71 | 32,167/64,334 | ACEI, ARB, CCB, Diuretics (no use) | risk of bladder cancer and kidney cancer | case-control | age, sex, diabetes, COPD, stroke, coronary arterial disease, related comorbidities such as benign prostate hyperplasia, CKD, urinary stones, and urinary tract infection | 7 |
| Colt JS 2017 | USA | 2002-2007 | 20–79 | 1,217/2,452 | ACEI, CCB, Diuretics (no use) | risk of renal cell cancer | case-control | age, race, sex, region, education, smoking status, body mass index, family history of cancer, and self-reported extent of hypertension control | 7 |
| Finkle WD 1993 | USA | 1980-1989 | 59.6 | 191/382 | Diuretics (no use) | risk of renal cell cancer | case-control | hypertension, smoking, obesity | 6 |
| Guercio V 2019 | Italy | 2003-2014 | 66.5 | 690/1355 | CCB (no use) | risk of bladder cancer | case-control | age, sex, study centre, year of interview, education, tobacco smoking and diabetes | 7 |
| Hiatt RA 1994 | USA | 1964-1989 | 50.7 | 257/514 | Diuretics (no use) | risk of renal cell cancer | case-control | smoking, BMI, hypertension, history of kidney infection | 7 |
| Hole DJ 1998 | UK | 1980-1995 | 52 | 2,297/5,207 | CCB (no use) | risk of bladder cancer and kidney cancer | case-control | age, sex, year of observation, smoking habit | 7 |
| Jiang X 2010 | USA | 1987-1996 | 25–64 | 1,585/3,170 | Diuretics, AHT (no use) | risk of bladder cancer | case-control | smoking, education, lifetime use of ‘non-steroidal anti-inflammatory drugs (NSAIDs), intake of carotenoids (quintiles), ever held a high-risk occupation | 7 |
| Kreiger N 1993 | Canada | 1986- | 25–69 | 518/1,899 | Diuretics (no use) | risk of renal cell cancer | case-control | age, smoking, hypertension, combined Quetelet index | 7 |
| McCrediM 1992 | Australia | 1989-1991 | 20–79 | 636/1,159 | AHT, Diuretics, BB (no use) | risk of renal cell cancer | case-control | age, sex, smoking, obesity, hypertension, terms for diuretics and potassium supplements, method of interview | 6 |
| McLaughli JK 1995 | Australia | 1989-1991 | 20–79 | 1,732/4,041 | Diuretics, AHT (no use) | risk of renal cell cancer | case-control | age. sex, BMI, smoking, hypertension, center | 8 |
| Mellemgaard A 1994 | Denmark | 1989-1992 | 20–79 | 368/764 | AHT, ACEI, BB, CCB, Diuretics (no use) | risk of renal cell cance | case-control | age, smoking, socioeconomic status, BMI, hypertension | 8 |
| RosenberL 1998 | USA | 1983-1996 | 40–69 | 9,513/16,005 | ACEI, BB, CCB (no use) | risk of kidney and bladder cancer | case-control | age, physician visits 2 years previously | 7 |
| Shapiro JA 1999 | USA | 1980-1995 | 18–84 | 238/854 | ACEI, BB, CCB, Diuretics (no use) | risk of renal cell cancer | case-control | age, BMI | 7 |
| Weinman S 1994 | USA | 1960-1991 | 63(W)/ 64(M) | 206/498 | AHT, BB, Diuretics (no use) | risk of renal cell cancer | case-control | age, sex, date of entry into the Health plan, number of months in the Health plan | 7 |
| Yu MC 1986 | USA | 1975-1979 | 46.1 | 160/320 | Diuretics (no use) | risk of renal cell cancer | case-control | sex, birth date (within 5 yr), race, and neighborhood of residence at time of diagnosis. | 7 |
| Yuan JM 1998 | USA | 1986-1994 | 58.8 | 1,204/2,408 | Diuretics (no use) | risk of renal cell cancer | case-control | education, BMI, hypertension | 8 |
| Braun S 1998 | Israel | 1990-1993 | 59.8 | 43/11,575 | CCB (no use) | risk of bladder and kidney cancer | cohort | age, sex, smoking | 6 |
| Chang PY 2015 | China | 2000-2011 | 54.6 | 70/24,238 | BB (no use) | risk of bladder and kidney cancer | cohort | age, sex, CCI score, hypertension, angina pectoris, aroxysmal supraventricular tachycardia, hypertensive renal disease, essential tremor, anxiety, thyrotoxicosis, migraine and medication of statins, metformin, aspirin, a-blockers, other b-blockers. | 8 |
| Flaherty KT 2005 | USA | 1976-2000 | 42.4(W)/ 54 (M) | 265/167,144 | Diuretics (no use) | risk of renal cell cancer | cohort | age, hypertension, BMI | 7 |
| Fraser GE 1990 | USA | 1977-1982 | 72.3 | 14/34,198 | AHT (no use) | risk of renal cancer | cohort | age, sex | 7 |
| Fryzek JP 2005 | Denmark | 1989-2002 | 62(30–85) | 330/113,298 | ACEI, ARB, BB, CCB, Diuretics (no use, BB) | risk of renal cell cancer | cohort | age, sex, calendar period | 7 |
| Heath CW 1997 | USA | 1982-1989 | >=30 | 335/998,904 | Diuretics, AHT (no use) | risk of renal cell cancer | cohort | age, race, educabon, smoking, BMI, acetaminophen use, history of urologic disease, and asbestos exposure. | 7 |
| MackenzieTA 2016 | USA | 2006-2012 | 75.1(P)/ 76.7(I) | 4433/1,161,443 (P), 320,090(I) | ACEI, ARB (no use) | risk of bladder cancer | cohort | age, gender, race, low-income subsidy, alcohol, chronic obstructive lung disease and/or tobacco use, obesity, diabetes complications and Charlson comorbidities | 8 |
| Prineas RJ 1997 | USA | 1986-1993 | 55–69 | 62/35,192 | Diuretics (no use) | risk of renal cell cancer | cohort | age, maximum weight, WHR, uncertainty about blood transfusion history | 7 |
| Schouten LJ 2005 | Netherlands | 1986-1997 | 61.9 | 337/4,774 | AHT, BB, Diuretics (no use) | risk of renal cell cancer | cohort | age, sex, BMI, smoking | 7 |
| Setiawan VW 2007 | USA | 1993-2002 | 59 | 347/161,126 | Diuretics (no use) | risk of renal cell cancer | cohort | BMI, smoking (status and pack-years of smoking), alcohol drinking, hypertension, and physical activity. | 8 |
| Sugiura R 2012 | Japan | 2001-2004 | 65 | 1,024/2,049 | ARB (non ARB standarzed AHT) | risk of bladder and kidney cancer | cohort | age, sex, co-morbidities, pharmacotherapy. | 7 |
| Tseng CH 2011 | China | 2003-2005 | NR | 589/998,947 | ACEI, Diuretics (no use, BB) | risk of bladder cancer | cohort | NR | 7 |
| Weikert S 2008 | Europe | 1992-2004 | 25–70 | 250/296,638 | AHT (no use) | risk of renal cell cancer | cohort | sex, body mass index, education, duration of smoking, smoking status, systolic blood pressure | 8 |
QS: NOS quality assessment; AHT: antihypertensive medications; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; CCB: calcium-channel blockers; BB: beta-blockers; RASIs: renin angiotensin system inhibitors; BMI: body mass index; M:men, W: women; P; Prevalent cohort; I: Incident cohor.
Antihypertensive medications and the risk of bladder cancer
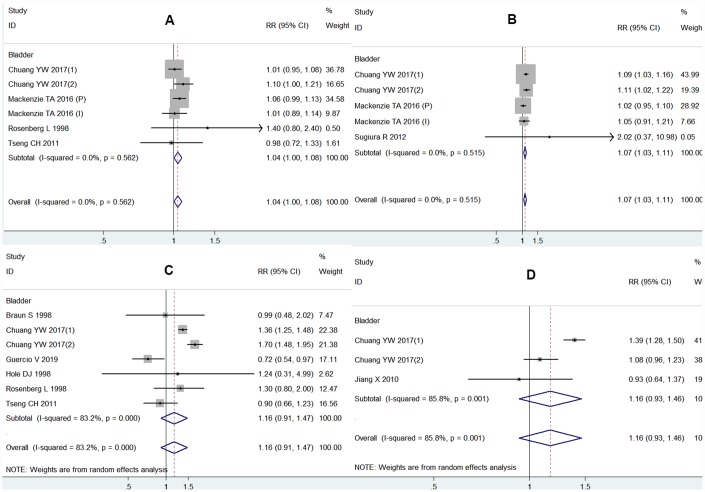
As shown in Figure 2, the outcomes based on five studies indicated that ARB use was associated with an increased risk of bladder cancer (relative risk (RR) = 1.07, 95% Confidence Interval (CI) 1.03-1.11) with little heterogeneity (I2 = 0.0%), with no statistical significance demonstrated for other antihypertensive medications usage. Two studies adjusted for hypertension recorded that a significant association existed with ARB therapy and the risk of bladder cancer (RR = 1.10, 95% CI 1.04-1.15). However, two studies adjusted for smoking demonstrated no relevant association between ARB therapy and cancer risks (RR = 1.03, 95% CI 0.96-1.10). Moreover, after adjusting for hypertension, the results of CCB or diuretic therapy shifted from no statistical significance to statistically significant for bladder cancer (Table 2).
Figure 2.
Forest plot of association between using each class of antihypertensive medications and bladder cancer risk: (A) ACEI and bladder cancer risk; (B) ARB and bladder cancer risk; (C) CCB and bladder cancer risk; (D) diuretics and bladder cancer risk.
Table 2. The results of the association between the each class of antihypertensive medications and bladder cancer risk.
| Comparison | ACEI vs nonuse | ARB vs nonuse | ||||||
| Category | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) |
| Bladder | 6 | 1.04 (1.00,1.08) | 0.0 | 0.562 | 5 | 1.07 (1.03, 1.11) | 0.0 | 0.515 |
| Adjustment of individual estimates for hypertension | ||||||||
| Yes | 2 | 1.04 (0.98, 1.09) | 52.9 | 0.145 | 2 | 1.10 (1.04, 1.15) | 0.0 | 0.740 |
| No | 4 | 1.05 (0.99, 1.11) | 0.0 | 0.636 | 3 | 1.03 (0.96, 1.10) | 0.0 | 0.691 |
| Adjustment of individual estimates for smoking | ||||||||
| Yes | 2 | 1.05 (0.99, 1.11) | 0.0 | 0.5 | 2 | 1.03 (0.96, 1.10) | 0.0 | 0.723 |
| No | 4 | 1.04 (0.99, 1.09) | 11.7 | 0.334 | 3 | 1.10 (1.04, 1.15) | 0.0 | 0.737 |
| Comparison | CCB vs nonuse | Diuretics vs nonuse | ||||||
| Category | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) |
| Bladder | 7 | 1.16 (0.91, 1.47) | 83.2 | 0.000 | 3 | 1.16 (0.93, 1.46) | 85.8 | 0.001 |
| Adjustment of individual estimates for hypertension | ||||||||
| Yes | 2 | 1.51 (1.21, 1.88) | 86.3 | 0.007 | 2 | 1.23 (1.96, 1.58) | 91.2 | 0.001 |
| No | 5 | 0.90 (0.73, 1.13) | 19.1 | 0.293 | 1 | 0.93 (0.64, 1.36) | - | - |
| Adjustment of individual estimates for smoking | ||||||||
| Yes | 3 | 0.77 (0.59, 1.00) | 0.0 | 0.570 | 1 | 0.93 (0.64, 1.36) | - | - |
| No | 4 | 1.33 (1.07, 1.65) | 81.1 | 0.001 | 2 | 1.23 (1.96, 1.58) | 91.2 | 0.001 |
ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; CCB: calcium-channel blockers; BB: beta-blockers; (h): heterogeneity; n: number of study.
Antihypertensive medications and kidney cancer risk
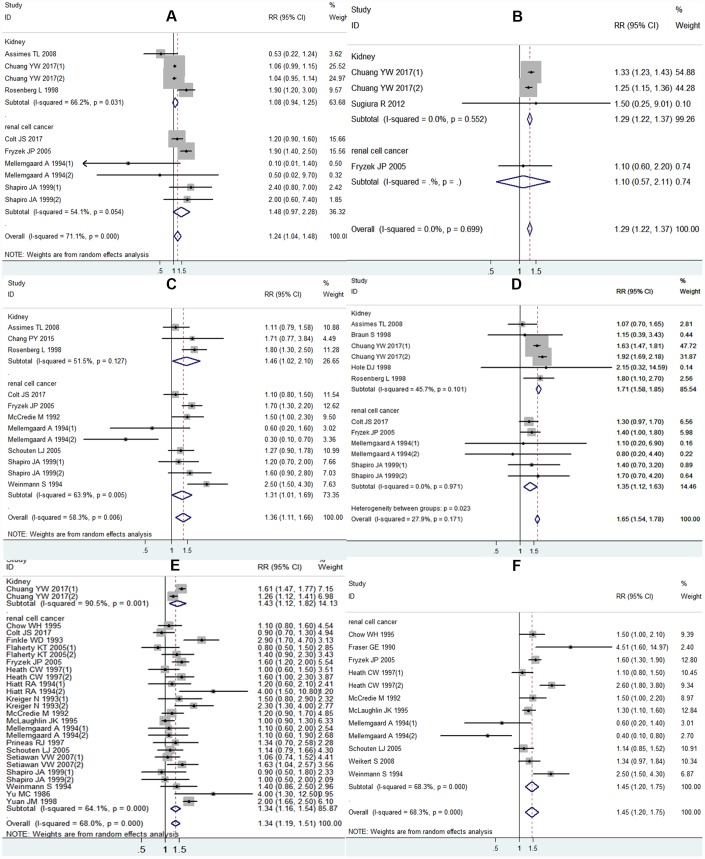
As shown in Figure 3A, ten studies reported the association between ACEI and the risk of kidney cancer. We observed a significant overall effect size estimate for ACEI therapy and the risk of kidney cancer in the pooled RR (RR = 1.24, 95% CI 1.04-1.48). An obvious heterogeneity existed among the pooled RR studies (I2 = 71.1%). No association between the risk of kidney cancer and ACEI use was observed upon evaluating the studies grouped according to gender. Moreover, the statistical significance disappeared after adjusting for hypertension or smoking (Table 3).
Figure 3.
Forest plot of association between using each class of antihypertensive medications and kidney cancer risk: (A) ACEI and kidney cancer risk; (B) ARB and kidney cancer risk; (C) BB and kidney cancer risk; (D) CCB and kidney cancer risk; (E) diuretics and kidney cancer risk; (F) any antihypertensive medications and kidney cancer risk.
Table 3. The results of the association between the each class of antihypertensive medications and kidney cancer risk.
| Comparison | ACEI vs nonuse | ARB vs nonuse | BB vs nonuse | |||||||||
| Category | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) |
| Kidney | 4 | 1.08 (0.94, 1.25) | 66.2 | 0.031 | 3 | 1.29 (1.22, 1.37) | 0.0 | 0.552 | 3 | 1.46 (1.02, 2.10) | 51.5 | 0.127 |
| Renal cell | 6 | 1.48 (0.97, 2.28) | 54.1 | 0.054 | 1 | 1.10 (0.57, 2.11) | - | - | 9 | 1.31 (1.01, 1.69) | 63.9 | 0.005 |
| All kidney | 10 | 1.24 (1.04, 1.48) | 71.1 | 0.000 | 4 | 1.29 (1.22, 1.37) | 0.0 | 0.699 | 12 | 1.36 (1.11, 1.66) | 58.3 | 0.006 |
| Gender | ||||||||||||
| Women | 3 | 1.04 (0.95, 1.14) | 0.0 | 0.534 | 1 | 1.25 (1.15, 1.36) | - | - | 2 | 0.73 (0.14, 3.74) | 88.2 | 0.004 |
| Men | 3 | 1.03 (0.38, 2.80) | 64.8 | 0.058 | 1 | 1.33 (1.23, 1.43) | - | - | 2 | 0.99 (0.54, 1.82) | 26.5 | 0.243 |
| All | 4 | 1.39 (0.93, 2.07) | 73.8 | 0.010 | 2 | 1.14 (0.62, 2.10) | 0.0 | 0.750 | 8 | 1.48 (1.23, 1.77) | 46.9 | 0.068 |
| Adjustment of individual estimates for hypertension | ||||||||||||
| Yes | 5 | 1.06 (0.99, 1.13) | 13.0 | 0.331 | 2 | 1.29 (1.22, 1.37) | 14.0 | 0.281 | 5 | 1.00 (0.63, 1.58) | 64.9 | 0.023 |
| No | 5 | 1.62 (1.07, 2.44) | 50.8 | 0.087 | 2 | 1.14 (0.62, 2.10) | 0.0 | 0.750 | 7 | 1.52 (1.26, 1.84) | 41.1 | 0.117 |
| Adjustment of individual estimates for smoking | ||||||||||||
| Yes | 4 | 1.10 (0.33, 3.71) | 50.2 | 0.111 | 4 | 0.92 (0.53. 1.60) | 71.9 | 0.014 | ||||
| No | 6 | 1.22 (1.03, 1.45) | 79.5 | 0.000 | 4 | 1.29 (1.22, 1.37) | 0.0 | 0.699 | 8 | 1.49 (1.22, 1.82) | 46.7 | 0.069 |
| Comparison | CCB vs nonuse | Diuretics vs nonuse | Any antihypertensive medications vs nonuse | |||||||||
| Category | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) | n | RR 95% CI | I2(%) | P(h) |
| Kidney | 6 | 1.71 (1.58, 1.85) | 45.7 | 0.101 | 2 | 1.43 (1.12, 1.82) | 90.5 | 0.001 | ||||
| Renal cell | 6 | 1.35 (1.12, 1.63) | 0.0 | 0.971 | 25 | 1.34 (1.16, 1.54) | 64.1 | 0.000 | 12 | 1.45 (1.20, 1.75) | 68.3 | 0.000 |
| All kidney | 12 | 1.65 (1.54, 1.78) | 27.9 | 0.171 | 27 | 1.34 (1.19, 1.51) | 68.0 | 0.000 | ||||
| Gender | ||||||||||||
| Women | 3 | 1.90 (1.68, 2.16) | 0.0 | 0.525 | 10 | 1.58 (1.27, 1.97) | 56.4 | 0.014 | 2 | 1.09 (0.17, 6.79) | 90.9 | 0.001 |
| Men | 3 | 1.62 (1.46, 1.80) | 0.0 | 0.845 | 8 | 1.16 (0.92, 1.48) | 60.6 | 0.013 | 2 | 0.97 (0.61, 1.57) | 25.9 | 0.245 |
| All | 6 | 1.35 (1.15, 1.60) | 0.0 | 0.678 | 9 | 1.31 (1.04, 1.64) | 79.1 | 0.000 | 8 | 1.48 (1.26, 1.73) | 47.4 | 0.065 |
| Adjustment of individual estimates for hypertension | ||||||||||||
| Yes | 5 | 1.70 (1.57, 1.83) | 54.5 | 0.066 | 20 | 1.35 (1.17, 1.54) | 74.0 | 0.000 | 8 | 1.34 (1.05, 1.70) | 68.3 | 0.002 |
| No | 7 | 1.40 (1.15, 1.70) | 0.0 | 0.782 | 7 | 1.34 (1.07, 1.68) | 27.3 | 0.220 | 4 | 1.74 (1.19, 2.53) | 72.9 | 0.011 |
| Adjustment of individual estimates for smoking | ||||||||||||
| Yes | 4 | 1.15 (0.55, 2.40) | 0.0 | 0.890 | 15 | 1.33 (1.13, 1.58) | 55.9 | 0.004 | 10 | 1.36 (1.09, 1.69) | 67.7 | 0.001 |
| No | 8 | 1.66 (1.54, 1.78) | 48.9 | 0.057 | 12 | 1.36 (1.16, 1.60) | 72.9 | 0.000 | 2 | 1.86 (1.23, 2.82) | 59.1 | 0.118 |
ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; CCB: calcium-channel blockers; BB: beta-blockers; (h): heterogeneity; n: number of study.
Four studies reported a connection between ARB therapy and the risk of kidney cancer, with a significant result detected (RR = 1.29, 95% CI: 1.22-1.37) without heterogeneity (I2 = 0.0%), as shown in Figure 3B. For studies adjusted for hypertension, the pooled RR was significant (Table 3).
As illustrated in Figure 3C, 12 studies reported an association between BB use and the risk of kidney cancer. We observed an increased risk of kidney cancer with BB therapy (RR = 1.36, 95% CI 1.11-1.66). However, no association between BB therapy and risk for kidney cancer was observed when the RR was pooled based on an adjustment for hypertension or smoking (Table 3).
A total of 12 studies reported a connection between CCB therapy and the risk of kidney cancer. A significant association between CCB and the risk of kidney cancer was established, according to the pooled RR (RR = 1.65, 95% CI 1.54-1.78), as shown in Figure 3D. A modest heterogeneity existed among these studies (I2 = 27.9%). A significant association was observed in the gender subgroup and adjustments for hypertension, but not smoking (Table 3).
Twenty-seven studies evaluated the association between the use of diuretics and the risk of kidney cancer. We detected an increased risk of kidney cancer on comparing the use of diuretics versus nonusers in the pooled RR (RR = 1.34, 95% CI 1.19-1.51), as shown in Figure 3E. Notably, in the subgroup analyses, the association was significant with the adjustment for hypertension and smoking. The pooled RR stratified according to gender demonstrated a significant association for women but not for men (Table 3).
As shown in Figure 3F, 12 studies reported that all antihypertensive medications classes were related to the risk of kidney cancer. As reported, there was an increased risk for kidney cancer (RR = 1.45, 95% CI 1.20-1.75), with some heterogeneity (I2 = 68.3%). According to the gender subgroups, antihypertensive medications use in men and women was not associated with the risk of kidney cancer. Regardless of whether the study had been adjusted for hypertension or smoking, a significant relationship between antihypertensive medications use and kidney cancer risk was observed (Table 3).
Dose-response association between the duration of antihypertensive medications therapy and the risk of kidney cancer
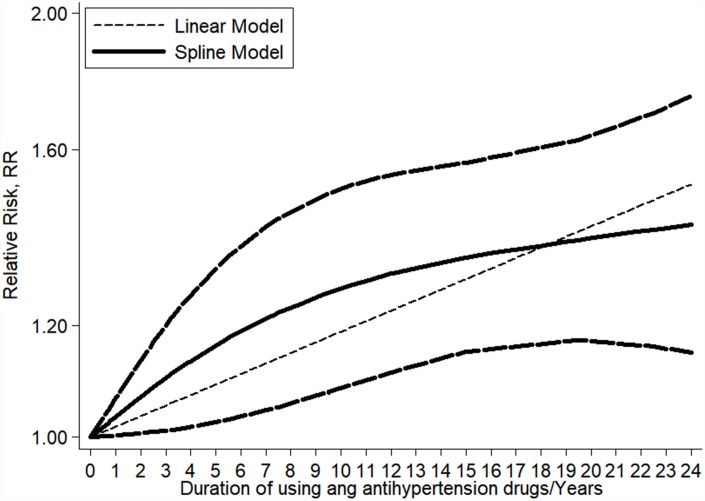
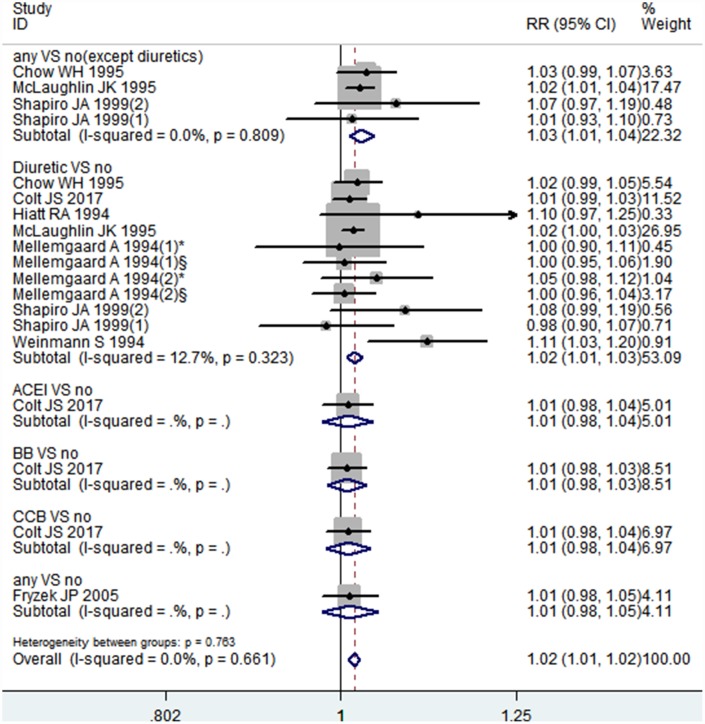
We included eight articles in our dose-response analysis [18, 20, 23, 28, 29, 31, 32, 39]. As shown in Figure 4, the results indicated that there was a linear association between the duration of antihypertensive medications therapy and the risk of kidney cancer (P = 0.061 for a non-linear trend). The pooled RR for each year of increasing antihypertensive medications use was 1.02 (95% CI: 1.01-1.02), with little heterogeneity among studies (I2 = 0.0%, P = 0.661) (Figure 5). Particularly, the per year increase in diuretics therapy was associated with a 2% higher incidence of kidney cancer (RR = 1.02, 95% CI: 1.01-1.03). However, a small number of studies researched the relationship between the duration of ACEI, ARB, BB, or CCB use and the risk of kidney cancer, necessitating relevant studies to assess these results.
Figure 4.
Linear dose–response meta-analysis between the duration of antihypertensive medications use and kidney cancer risk.
Figure 5.
Forest plot of association between per 1-year increment of using antihypertensive medications and kidney cancer risk.
Publication bias and sensitivity analysis
Begg’s funnel plot and Egger’s test were performed and found no evidence of publication bias in the analysis, as shown in Supplementary Figures 1 and 2. A sensitivity analysis on the risk of kidney cancer was performed as shown in Supplementary Figure 3. The pooled RR remained statistically significant, indicating that our results are stable. As the number of bladder cancer investigations were limited, a sensitivity analysis was omitted.
DISCUSSION
The present systematic review with meta-analysis indicates that the risk of bladder cancer is related to ARB, but not with other antihypertensive medications classes. Additionally, we note that ACEI, ARB, BB, CBB, diuretics and all antihypertensive medications classes are associated with a risk of kidney cancer. The results from the dose-response analysis provided evidence that with the prolonged use of antihypertensive medications, the risk of kidney cancer increases.
Notably, the mechanism of association between the risk of bladder/kidney cancer and antihypertensive medications therapy remains unclear. In vitro studies have suggested that ARB increased the risk of cancer by promoting cellular proliferation, angiogenesis, and tumor progression [48]. In contrast, other studies have reported that ACEI and ARB have a possible antitumor effect by reducing angiogenesis in bladder malignancies and renal cell carcinoma [4, 6]. Furthermore, investigators have also reported the antitumor effect of CCB, implicated in the regulation of cell proliferation and calcium influx [49]. In addition, it has been long hypothesized that diuretics have a low-grade carcinogenic effect by targeting the renal tubular cell [43]. For example, it has been reported that rodents developed nephropathy and renal adenomas after diuretic treatment [50]. Preclinical studies have corroborated that various antihypertensive medications classes have effects on cancer cells or in animal models; however, the exact mechanism is unknown. In order to evaluate the existence of a relationship between antihypertensive medications and bladder/kidney cancer risk, we performed this meta-analysis from the clinical point of view.
The observed association between the risk of bladder/ kidney cancer and antihypertensive medications therapy can be explained by factors such as obesity, smoking, alcohol consumption, and hypertension. Hypertension has been documented as a general risk factor for cancer, particularly for renal cell carcinoma [51]. This could be attributed to the common risk factors such as smoking, diabetes, obesity, and alcohol consumption shared between hypertension and cancer. However, the persisting question for decades has been whether the association between antihypertensive medications therapy and the risk of bladder/kidney cancer is independent of hypertension. Several studies support this hypothesis. For example, one study indicated that, even with an adjustment for the use of other antihypertensive medications classes and the time from a hypertension diagnosis to the end of the study, the risk trend for papillary renal cell cancer persisted during the use of diuretics among participants with a history of hypertension [20]. In the subjects with normal blood pressure, diuretic use has also been associated with renal cell carcinoma [21, 33]. However, in studies adjusted for hypertension, no association was observed between antihypertensive medications and the risk of bladder/kidney cancer [28, 29]. Moreover, our result demonstrated a dose-response relationship, reporting an increased risk of kidney cancer risk with the length of exposure to antihypertensive medications. This could indicate that long-time antihypertensive medications therapy is a risk factor for renal cell carcinoma; however, this could simply reflect the increasing risks associated with the duration and severity of hypertension. To resolve this dispute, we conducted subgroup analyses with adjustments for individual estimates of hypertension/smoking. In this meta-analysis, we observed that ARB usage remained a significant risk factor for bladder cancer even after adjusting for hypertension. Notably, the significant association disappeared after adjusting for smoking. In the kidney cancer subgroup, the pooled RRs remain significant with diuretics when adjusted for hypertension and smoking. However, no statistical significance was observed when adjusted for the presence of hypertension or smoking in ACEI, ARB, BB, and CCB therapy. The rationale behind the pooled RRs decreasing or approaching insignificance when adjusted for hypertension or smoking is presumed as follows. First, there are no associations between ARB and bladder cancer risk, and between ACEI or ARB or BB or CCB and the risk of kidney cancer, with significant results attributed to the unadjusted risk factors such as smoking and hypertension. On the other hand, the number of studies adjusted for hypertension or smoking were minimal and the results lacked statistical power. Therefore, it is difficult to ascertain whether ACEI, ARB, BB, and CCB confer an additional risk in bladder/kidney cancer beyond smoking or hypertension.
After adjusting for hypertension and smoking, the RR for diuretics and the risk of kidney cancer was still significant, indicating that diuretics are a risk factor for kidney cancer. Our pooled data also observed that women demonstrate a significant risk of kidney cancer associated with diuretics, but men do not. Several studies have attempted to explain the differential effects of diuretics between sexes. Previously, studies have indicated that women are at a higher diuretic-associated cancer risk than men [42, 44]. The proposed explanation suggested that women were prescribed diuretics more frequently than men, with a higher chemical burden on the tubular cells resulting in carcinogenicity [52]. Additionally, there is a higher estrogen exposure in women, which has been known to enhance the density of the thiazide sensitive NaCl transporter in the distal tubule [53].
This research has several limitations. First, this is a meta-analysis based on observational studies, which are inherently prone to several types of bias including selection bias, detection bias, recall bias, publication bias, and confounding bias. Second, only a small number of studies with data on bladder cancer risk and gender subgroup analysis were included. Consequently, the statistical power in the analyses is insufficient and the results should be interpreted with caution. Third, significant heterogeneity was observed among the studies. Although a sensitivity analysis and subgroup analysis were employed, the heterogeneity persisted.
In conclusion, our meta-analysis suggests that antihypertensive medications therapy, including ACEI, ARB, BB, CCB, and diuretics, is consistently associated with the risk of kidney cancer but not bladder cancer, except for ARB. The longer the duration of antihypertensive medications therapy, the higher the risk of kidney cancer, presenting a positive linear trend. Although our results indicate that the use of antihypertensive medications can slightly increase the risk of kidney cancer, hypertensive patients should continue to stabilize blood pressure with antihypertensive medications to reduce the morbidity and mortality associated with cardiovascular events, while simultaneously undergoing kidney and bladder cancer screening.
MATERIALS AND METHODS
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidance [54].
Data sources and search strategy
Two investigators independently searched the literature in PubMed, Embase, Web of Science, and Cochrane Library databases from inception to July 2019 using the following text and corresponding range of medication names: “urinary tract cancer or kidney cancer or renal cell cancer or urinary bladder cancer or urethra cancer or kidney carcinoma or renal cell carcinoma or bladder carcinoma or urinary tract carcinoma or urethra carcinoma” combined with “antihypertensive medications or angiotensin-converting enzyme inhibitors or angiotensin receptor blockers or beta-blockers or calcium-channel blockers or diuretics.” Additionally, a manual search of the references cited in relevant original and review articles was conducted.
Selection criteria
The eligible studies were required to meet all of the following inclusion criteria: (1) assessing the association between exposure to antihypertensive medications (ACEI, ARB, BB, CCB, diuretics) and urinary bladder neoplasms and kidney cancer (renal cell carcinomas) risk, (2) original case-control study, nested case-control study, and cohort study, (3) reporting the odds ratio (OR) or RR with corresponding 95% confidence intervals (CI). Renal pelvis and ureter cancers were excluded as they were mostly of transitional cell type and had an etiology comparable to bladder cancer than renal cell cancer. When multiple studies included overlapping data, the latest and most complete study was included. Published letters, editorials, abstracts, reviews, case reports, and expert opinions were not included. The discrepancies between two investigators were resolved through discussion or in consultation with a third reviewer.
Data extraction and quality assessment
For each included study, the following baseline characteristics were extracted and recorded: first author’s name, publication date, study design, source of participants, study period, age, number of participants, class of medication exposure, assessment of outcome, estimated effect size (OR, RR), corresponding 95% CI, and adjustments for confounders. The risk estimates, adjusted by multiple factors, were preferably extracted from each eligible study. Moreover, to investigate the dose-response relationship, we extracted the cumulative duration outcomes observed with any class of antihypertensive medications therapy. To assess the quality of the included studies, NOS was used and NOS score > 6 was regarded as a high-quality study.
Statistical analysis
We used an RR with 95% CI to estimate the associations between each class of antihypertensive medications and bladder/kidney cancer risk. In order to explore whether gender, smoking, and hypertension affected this association, subgroup analyses were performed.
We used generalized least squares trend regression models to perform dose-response analyses and investigate the trend between the duration of antihypertensive medications therapy and cancer risk [55, 56]. The restricted cubic spline model with 3 knots at 25%, 50% and 75% percentiles of the whole distribution was conducted to explore the potential non-linear dose-response association. The null hypothesis that the coefficient of the second spline was equal to zero was tested to calculate the P-value of non-linearity [57, 58]. A pooled risk estimate was calculated for a standardized increment with the duration of antihypertensive medications therapy. This analysis used data from the RR and 95% CI, number of cases, number of overall participants, and median or mean duration of antihypertensive medications therapy (in years) for each group.
Heterogeneity across the eligible studies was assessed using Cochran’s Q test and I2 statistic. The criterion of a P-value < 0.05 or I2 > 50% indicated significant heterogeneity [59, 60]. If significant heterogeneity was detected, a random-effects model was used, otherwise, a fixed-effects model was employed [61]. Publication bias was examined with Begg’s and Egger’s regression tests [62]. Sensitivity analyses were conducted to determine the effect of each study and the stability of the meta-analysis results. Statistical analyses were performed using STATA software (version 12.0; STATA Corp LP, College Station, TX)
Supplementary Material
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitors
- ARB
angiotensin II receptor blockers
- CCB
calcium-channel blockers
- BB
beta-blockers
- Ang II AT1R
angiotensin II type I receptor
- VEGF
vascular endothelial growth factor
- BMI
body mass index
Footnotes
AUTHOR CONTRIBUTION: Yuxiu Xie and Peng Xu designed the study; Zhijun Dai managed the study; Men Wang, Yi Zheng, Tian Tian and Si Yang extracted the data; Yujiao Deng, Ying Wu and Zhen Zhai performed the analyses; Yuxiu Xie, Peng Xu, Qian Hao interpreted the evidence and wrote the manuscript; Qian Hao, Dingli Song, Dai Zhang and Zhijun Dai revised the article. All authors agreed to be accountable for the work.
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest.
FUNDING: This study was supported by the National Natural Science Foundation of China (No.81471670) and the Key research and development plan, Shaanxi Province, China (2017ZDXM-SF-066).
REFERENCES
- 1.Butt DA, Harvey PJ. Benefits and risks of antihypertensive medications in the elderly. J Intern Med. 2015; 278:599–626. 10.1111/joim.12446 [DOI] [PubMed] [Google Scholar]
- 2.Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, Zanelotti A, Adamoli L, Colleoni M, Viale G, Goldhirsch A, Gandini S. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013; 140:567–75. 10.1007/s10549-013-2654-3 [DOI] [PubMed] [Google Scholar]
- 3.Imai N, Hashimoto T, Kihara M, Yoshida S, Kawana I, Yazawa T, Kitamura H, Umemura S. Roles for host and tumor angiotensin II type 1 receptor in tumor growth and tumor-associated angiogenesis. Lab Invest. 2007; 87:189–98. 10.1038/labinvest.3700504 [DOI] [PubMed] [Google Scholar]
- 4.Tanaka N, Miyajima A, Kosaka T, Shirotake S, Hasegawa M, Kikuchi E, Oya M. Cis-dichlorodiammineplatinum upregulates angiotensin II type 1 receptors through reactive oxygen species generation and enhances VEGF production in bladder cancer. Mol Cancer Ther. 2010; 9:2982–92. 10.1158/1535-7163.MCT-10-0535 [DOI] [PubMed] [Google Scholar]
- 5.Shirotake S, Miyajima A, Kosaka T, Tanaka N, Maeda T, Kikuchi E, Oya M. Angiotensin II type 1 receptor expression and microvessel density in human bladder cancer. Urology. 2011; 77:1009.e19–25. 10.1016/j.urology.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama M, Funao K, Kuratsukuri K, Tanaka T, Kawahito Y, Sano H, Chargui J, Touraine JL, Yoshimura N, Yoshimura R. Telmisartan inhibits human urological cancer cell growth through early apoptosis. Exp Ther Med. 2010; 1:301–06. 10.3892/etm_00000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loffing J, Loffing-Cueni D, Hegyi I, Kaplan MR, Hebert SC, Le Hir M, Kaissling B. Thiazide treatment of rats provokes apoptosis in distal tubule cells. Kidney Int. 1996; 50:1180–90. 10.1038/ki.1996.426 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S, and CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003; 362:759–66. 10.1016/S0140-6736(03)14282-1 [DOI] [PubMed] [Google Scholar]
- 9.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010; 11:627–36. 10.1016/S1470-2045(10)70106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011; 12:65–82. 10.1016/S1470-2045(10)70260-6 [DOI] [PubMed] [Google Scholar]
- 11.Rotshild V, Azoulay L, Zarifeh M, Masarwa R, Hirsh-Raccah B, Perlman A, Muszkat M, Matok I. The Risk for Lung Cancer Incidence with Calcium Channel Blockers: A Systematic Review and Meta-Analysis of Observational Studies. Drug Saf. 2018; 41:555–64. 10.1007/s40264-018-0644-4 [DOI] [PubMed] [Google Scholar]
- 12.Song T, Choi CH, Kim MK, Kim ML, Yun BS, Seong SJ. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: a meta-analysis. Eur J Cancer Prev. 2017; 26:78–85. 10.1097/CEJ.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 13.Zhao YT, Li PY, Zhang JQ, Wang L, Yi Z. Angiotensin II receptor blockers and cancer risk a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016; 95:e3600. 10.1097/MD.0000000000003600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 15.Cumberbatch MG, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, Kiemeney L, Lotan Y, Pang K, Silverman DT, Znaor A, Catto JW. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur Urol. 2018; 74:784–95. 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 16.Dhote R, Thiounn N, Debré B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am. 2004; 31:237–47. 10.1016/j.ucl.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Assimes TL, Elstein E, Langleben A, Suissa S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf. 2008; 17:1039–49. 10.1002/pds.1656 [DOI] [PubMed] [Google Scholar]
- 18.Chow WH, McLaughlin JK, Mandel JS, Wacholder S, Niwa S, Fraumeni JF Jr. Risk of renal cell cancer in relation to diuretics, antihypertensive drugs, and hypertension. Cancer Epidemiol Biomarkers Prev. 1995; 4:327–31. [PubMed] [Google Scholar]
- 19.Chuang YW, Yu MC, Huang ST, Yang CK, Chen CH, Lo YC, Lin CL, Shu KH, Yu TM, Kao CH. Spironolactone and the risk of urinary tract cancer in patients with hypertension: a nationwide population-based retrospective case-control study. J Hypertens. 2017; 35:170–77. 10.1097/HJH.0000000000001130 [DOI] [PubMed] [Google Scholar]
- 20.Colt JS, Hofmann JN, Schwartz K, Chow WH, Graubard BI, Davis F, Ruterbusch J, Berndt S, Purdue MP. Antihypertensive medication use and risk of renal cell carcinoma. Cancer Causes Control. 2017; 28:289–97. 10.1007/s10552-017-0857-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkle WD, McLaughlin JK, Rasgon SA, Yeoh HH, Low JE. Increased risk of renal cell cancer among women using diuretics in the United States. Cancer Causes Control. 1993; 4:555–58. 10.1007/BF00052431 [DOI] [PubMed] [Google Scholar]
- 22.Guercio V, Turati F, Bosetti C, Polesel J, Serraino D, Montella M, Libra M, Galfano A, La Vecchia C, Tavani A. Bladder cancer risk in users of selected drugs for cardiovascular disease prevention. Eur J Cancer Prev. 2019; 28:76–80. 10.1097/CEJ.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 23.Hiatt RA, Tolan K, Quesenberry CP Jr. Renal cell carcinoma and thiazide use: a historical, case-control study (California, USA). Cancer Causes Control. 1994; 5:319–25. 10.1007/BF01804982 [DOI] [PubMed] [Google Scholar]
- 24.Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Robertson JW, Lever AF. Cancer risk of hypertensive patients taking calcium antagonists. J Hypertens. 1998; 16:119–24. 10.1097/00004872-199816010-00017 [DOI] [PubMed] [Google Scholar]
- 25.Jiang X, Castelao JE, Yuan JM, Groshen S, Stern MC, Conti DV, Cortessis VK, Coetzee GA, Pike MC, Gago-Dominguez M. Hypertension, diuretics and antihypertensives in relation to bladder cancer. Carcinogenesis. 2010; 31:1964–71. 10.1093/carcin/bgq173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control. 1993; 4:101–10. 10.1007/BF00053150 [DOI] [PubMed] [Google Scholar]
- 27.McCredie M, Stewart JH. Risk factors for kidney cancer in New South Wales, Australia. II. Urologic disease, hypertension, obesity, and hormonal factors. Cancer Causes Control. 1992; 3:323–31. 10.1007/BF00146885 [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin JK, Chow WH, Mandel JS, Mellemgaard A, McCredie M, Lindblad P, Schlehofer B, Pommer W, Niwa S, Adami HO. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995; 63:216–21. 10.1002/ijc.2910630212 [DOI] [PubMed] [Google Scholar]
- 29.Mellemgaard A, Niwa S, Mehl ES, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark: role of medication and medical history. Int J Epidemiol. 1994; 23:923–30. 10.1093/ije/23.5.923 [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, Warshauer ME, Shapiro S. Calcium channel blockers and the risk of cancer. JAMA. 1998; 279:1000–04. 10.1001/jama.279.13.1000 [DOI] [PubMed] [Google Scholar]
- 31.Shapiro JA, Williams MA, Weiss NS, Stergachis A, LaCroix AZ, Barlow WE. Hypertension, antihypertensive medication use, and risk of renal cell carcinoma. Am J Epidemiol. 1999; 149:521–30. 10.1093/oxfordjournals.aje.a009848 [DOI] [PubMed] [Google Scholar]
- 32.Weinmann S, Glass AG, Weiss NS, Psaty BM, Siscovick DS, White E. Use of diuretics and other antihypertensive medications in relation to the risk of renal cell cancer. Am J Epidemiol. 1994; 140:792–804. 10.1093/oxfordjournals.aje.a117328 [DOI] [PubMed] [Google Scholar]
- 33.Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst. 1986; 77:351–6. 10.1093/jnci/77.2.351 [DOI] [PubMed] [Google Scholar]
- 34.Yuan JM, Castelao JE, Gago-Dominguez M, Ross RK, Yu MC. Hypertension, obesity and their medications in relation to renal cell carcinoma. Br J Cancer. 1998; 77:1508–13. 10.1038/bjc.1998.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun S, Boyko V, Behar S, Reicher-Reiss H, Laniado S, Kaplinsky E, Goldbourt U, and Benzafibrate Infarction Prevention (BIP) Study Research Group. Calcium channel blocking agents and risk of cancer in patients with coronary heart disease. J Am Coll Cardiol. 1998; 31:804–08. 10.1016/S0735-1097(98)00008-4 [DOI] [PubMed] [Google Scholar]
- 36.Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, Kao CH. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine (Baltimore). 2015; 94:e1097. 10.1097/MD.0000000000001097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaherty KT, Fuchs CS, Colditz GA, Stampfer MJ, Speizer FE, Willett WC, Curhan GC. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States). Cancer Causes Control. 2005; 16:1099–106. 10.1007/s10552-005-0349-8 [DOI] [PubMed] [Google Scholar]
- 38.Fraser GE, Phillips RL, Beeson WL. Hypertension, antihypertensive medication and risk of renal carcinoma in California Seventh-Day Adventists. Int J Epidemiol. 1990; 19:832–38. 10.1093/ije/19.4.832 [DOI] [PubMed] [Google Scholar]
- 39.Fryzek JP, Poulsen AH, Johnsen SP, McLaughlin JK, Sørensen HT, Friis S. A cohort study of antihypertensive treatments and risk of renal cell cancer. Br J Cancer. 2005; 92:1302–06. 10.1038/sj.bjc.6602490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath CW Jr, Lally CA, Calle EE, McLaughlin JK, Thun MJ. Hypertension, diuretics, and antihypertensive medications as possible risk factors for renal cell cancer. Am J Epidemiol. 1997; 145:607–13. 10.1093/oxfordjournals.aje.a009157 [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie TA, Zaha R, Smith J, Karagas MR, Morden NE. Diabetes Pharmacotherapies and Bladder Cancer: A Medicare Epidemiologic Study. Diabetes Ther. 2016; 7:61–73. 10.1007/s13300-016-0152-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prineas RJ, Folsom AR, Zhang ZM, Sellers TA, Potter J. Nutrition and other risk factors for renal cell carcinoma in postmenopausal women. Epidemiology. 1997; 8:31–36. 10.1097/00001648-199701000-00005 [DOI] [PubMed] [Google Scholar]
- 43.Schouten LJ, van Dijk BA, Oosterwijk E, Hulsbergen-van de Kaa CA, Kiemeney LA, Goldbohm RA, Schalken JA, van den Brandt PA. Hypertension, antihypertensives and mutations in the Von Hippel-Lindau gene in renal cell carcinoma: results from the Netherlands Cohort Study. J Hypertens. 2005; 23:1997–2004. 10.1097/01.hjh.0000186023.74245.48 [DOI] [PubMed] [Google Scholar]
- 44.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007; 166:932–40. 10.1093/aje/kwm170 [DOI] [PubMed] [Google Scholar]
- 45.Sugiura R, Ogawa H, Oka T, Koyanagi R, Hagiwara N, and HIJ-CREATE Investigators. Candesartan-based therapy and risk of cancer in patients with systemic hypertension (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] substudy). Am J Cardiol. 2012; 109:576–80. 10.1016/j.amjcard.2011.09.050 [DOI] [PubMed] [Google Scholar]
- 46.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011; 54:2009–15. 10.1007/s00125-011-2171-z [DOI] [PubMed] [Google Scholar]
- 47.Weikert S, Boeing H, Pischon T, Weikert C, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Trichopoulou A, Mountokalakis T, Trichopoulos D, Sieri S, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008; 167:438–46. 10.1093/aje/kwm321 [DOI] [PubMed] [Google Scholar]
- 48.Walther T, Menrad A, Orzechowski HD, Siemeister G, Paul M, Schirner M. Differential regulation of in vivo angiogenesis by angiotensin II receptors. FASEB J. 2003; 17:2061–67. 10.1096/fj.03-0129com [DOI] [PubMed] [Google Scholar]
- 49.Kunert-Radek J, Stepien H, Radek A, Lyson K, Pawlikowski M. Inhibitory effect of calcium channel blockers on proliferation of human glioma cells in vitro. Acta Neurol Scand. 1989; 79:166–69. 10.1111/j.1600-0404.1989.tb03731.x [DOI] [PubMed] [Google Scholar]
- 50.Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. 1999; 83:1090–93. [DOI] [PubMed] [Google Scholar]
- 51.Chow WH, Gridley G, Fraumeni JF Jr, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000; 343:1305–11. 10.1056/NEJM200011023431804 [DOI] [PubMed] [Google Scholar]
- 52.Messerli FH. Diuretic therapy and renal cell carcinoma—another controversy? Eur Heart J. 1999; 20:1441–42. 10.1053/euhj.1999.1534 [DOI] [PubMed] [Google Scholar]
- 53.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest. 1998; 101:1661–69. 10.1172/JCI601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, and PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 55.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993; 4:218–28. 10.1097/00001648-199305000-00005 [DOI] [PubMed] [Google Scholar]
- 56.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135:1301–09. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 57.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989; 8:551–61. 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 58.Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988; 80:1198–202. 10.1093/jnci/80.15.1198 [DOI] [PubMed] [Google Scholar]
- 59.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950; 37:256–66. 10.1093/biomet/37.3-4.256 [DOI] [PubMed] [Google Scholar]
- 60.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 62.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.