Abstract
Inhaled ground level ozone (O3) has well described adverse health effects, which may be augmented in susceptible populations. While conditions, such as pre-existing respiratory disease, have been identified as factors enhancing susceptibility to O3-induced health effects, the potential for chemical interactions in the lung to sensitize populations to pollutant-induced responses has not yet been studied. In the airways, inhaled O3 reacts with lipids, such as cholesterol, to generate reactive and electrophilic oxysterol species, capable of causing cellular dysfunction and inflammation. The enzyme regulating the final step of cholesterol biosynthesis, 7-dehydrocholesterol reductase (DHCR7), converts 7-dehydrocholesterol (7-DHC) to cholesterol. Inhibition of DHCR7 increases the levels of 7-DHC, which is much more susceptible to oxidation than cholesterol. Chemical analysis established the capacity for a variety of small molecule antipsychotic drugs, like Aripiprazole (APZ), to inhibit DHCR7 and elevate circulating 7-DHC. Our results show that APZ and the known DHCR7 inhibitor, AY9944, increase 7-DHC levels in airway epithelial cells and potentiate O3-induced IL-6 and IL-8 expression and cytokine release. Targeted immune-related gene array analysis demonstrates that APZ significantly modified O3-induced expression of 16 genes, causing dysregulation in expression of genes associated with leukocyte recruitment and inflammatory response. Additionally, we find that APZ increases O3-induced IL-6 and IL-8 expression in human nasal epithelial cells from male but not female donors. Overall, the evidence we provide describes a novel molecular mechanism by which chemicals, such as APZ, that perturb cholesterol biosynthesis affect O3-induced biological responses.
Graphical Abstract
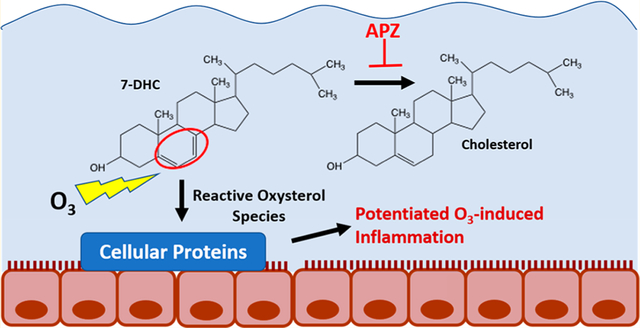
INTRODUCTION
Chemical modification of cholesterol biosynthesis has been of long-standing clinical interest. We recently screened a small library of pharmacologically active compounds for their ability to inhibit the enzyme 7-dehydrocholesterol reductase (DHCR7), the last step in cholesterol biosynthesis, resulting in the accumulation of 7-dehydrocholesterol (7-DHC) (Figure 1A). Drugs such as aripiprazole, trazodone, nefazodone, perospirone, haloperidol, and buspirone increased 7-DHC levels at 1 μM, similar to known DHCR7 inhibitors, such as AY9944.1 While genetic mutations of DHCR7 and accumulation of 7-DHC are hallmarks of the disease Smith-Lemli-Optiz syndrome (SLOS), biological outcomes of pharmacologically inhibiting DHCR7 are unknown.
Figure 1.
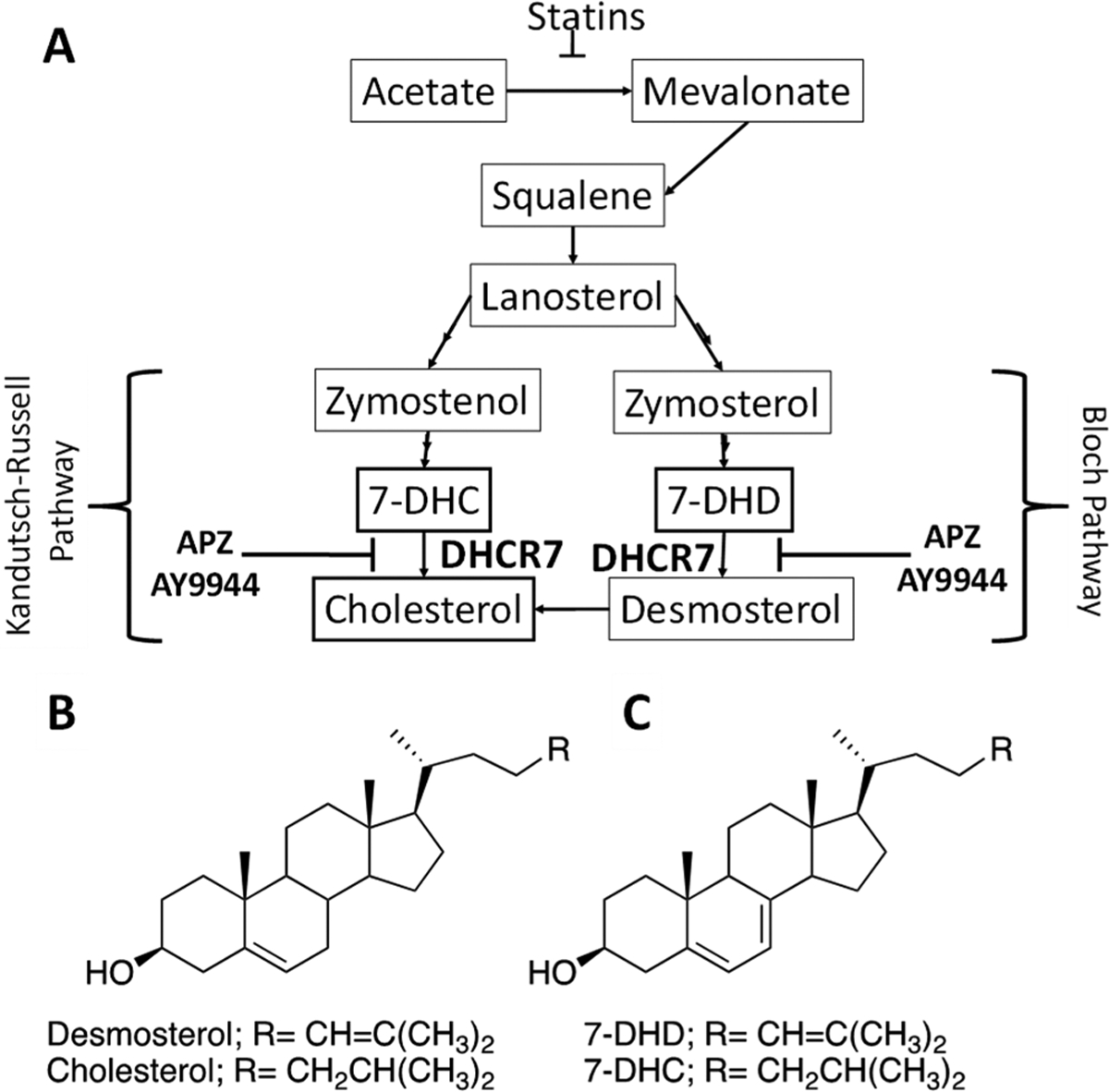
APZ inhibition of cholesterol synthesis pathway, 7-DHC and cholesterol structures. (A) Cholesterol synthesis pathway and impact of APZ and AY9944 on DHCR7. (B) Chemical structure of cholesterol and (C) 7-DHC.
7-DHC contains conjugated double bonds in the sterol B-ring (Figure 1C), making it highly susceptible to oxidation and lipid peroxidation chain propagation.2 Ozone (O3) is a highly reactive oxidant gas, rapidly reacting with organic molecules in human tissue, including lipids. O3 reacts with pulmonary surfactant in epithelial cell membranes to produce lipid peroxides and oxidant species.3 We and others have demonstrated that in the airways, O3-induced oxidation of cholesterol leads to the formation of electrophilic oxysterols (oxidized cholesterols), which in turn are capable of forming protein adducts, perturbing normal cellular signaling, and increasing inflammation.4–6 Because of the two double bonds in the B-ring, the oxidation potential of 7-DHC is greater than that of cholesterol. Therefore, chemical interactions that alter cholesterol biosynthesis and increase 7-DHC levels could potentially modulate O3-induced lipid peroxidation and inflammation in the lung.
In our previously published screen of drugs affecting 7-DHC levels, aripiprazole (APZ) was particularly potent in inhibiting DHCR7 and increasing 7-DHC levels.1,7 In 2014, APZ (sold as Abilify), which is often prescribed for off-label use as an antidepressant or for anxiety, accounted for 8 billion dollars in sales and was ranked second in the U.S. for top selling prescription drugs.8 APZ is a piperazine and quinolone derivative that acts as a mixed regulator of serotonin and dopamine receptors. However, whether compounds, such as APZ, known to modify cholesterol biosynthesis and lead to the accumulation of reactive cholesterol precursors, such as 7-DHC, could potentially exacerbate O3-induced inflammation presents a clinically important knowledge gap.
According to the World Health Organization (WHO), total global air pollution increased 8% between 2011 and 2016, with more than 80% of people living in developed urban settings exceeding WHO air quality guidelines.9 Concurrently, the development of small-molecule pharmaceutical drugs has increased exponentially over the last 20 years, with antidepressant/antipsychotic use doubling from 6.8% to 13% between 1999 and 2012.10,11 Notably, 59% of the United States population are taking one or more pharmaceutical drugs. Combined, the pervasive issue of global air pollution and the steady increase of pharmaceutical drug use provide an ever-growing opportunity for biochemical interaction between pharmaceutical drugs and pollutant-induced adverse health effects. Despite the capacity for drugs to biochemically alter the targets for pollutants and potentially exacerbate injury, little research has been conducted on the potential human health implications of such an interaction.
The likely concurrent prevalence of APZ treatment and elevated O3 exposure further illuminate the need to understand the potential adverse health outcomes associated with the chemical interaction between oxidant air pollutants and pharmacologically active compounds. Our study is designed to elucidate the molecular mechanism by which cholesterol-modifying chemicals, such as APZ, modify O3-induced biological responses in human airway epithelial cells.
MATERIALS AND METHODS
Cell Culture.
16HBE14o (16HBE) cells, a SV-40 transformed human bronchial epithelial cell line, were a gift from Dr. D. C. Gruenert (University of California San Francisco, San Francisco, CA). For experiments performed at the air–liquid interface (ALI), 16HBE cells were plated on fibronectin-coated (LHC Basal Medium [Life Technologies, Carlsbad, CA], 0.01% bovine serum albumin (BSA) [Sigma-Aldrich, St. Louis, MO], 1% Vitricol [Advanced Bio Matrix, San Diego, CA], and 1% human fibronectin [BD Biosciences, San Jose, CA]) 0.4 μm Transwell plates (Costar, Corning, NY) and grown submerged in minimal essential media (MEM) (Gibco, Thermo Fisher, Gaithersburg, MD) with 10% fetal bovine serum (FBS) (Gibco), 1% penicillin–streptomycin (Gibco), and 1% l-glutamine (Gibco) until confluent for 6 days. For experiments performed with submerged cultures, 16HBEs were plated on fibronectin-coated 12- or 24-well plates and given 10% FBS in MEM. For both types of in vitro experiments, the serum concentration was reduced to 2% FBS, apical media was removed (if at ALI), and the cells were grown for an additional day before treatment and subsequent challenges.
Human nasal epithelial cells were obtained and cultured as described by us before.12 Briefly, superficial scrape biopsies were obtained from male and female healthy volunteers, expanded to passage 2 in in PneumaCult-Ex Plus Medium (Stemcell 05040) supplemented with hydrocortisone (0.48 μg/mL), penicillin (100U/ml), streptomycin (100ug/mL), amphotericin B (0.25ug/mL), and then plated on 0.4 μm Transwell plates and cultured in the same media until confluency. Similar to 16HBE cells, apical media were removed prior to exposure to air or O3.
Treatment of 16HBE Cells with APZ and AY9944.
APZ (Sigma-Aldrich) and AY9944 (Sigma-Aldrich) were reconstituted in dimethyl sulfoxide (DMSO) to create 10 mM stock solutions. Cells at ALI were supplemented daily with APZ or AY9944 at the indicated concentrations in the basolateral compartment for 1, 3, or 6 days prior to O3 exposure. For the experiments shown in Figure 5, cells were supplemented in tissue culture plates prior to stimulation with Tumor Necrosis Factor alpha (TNFα) for 4 h.
Figure 5.
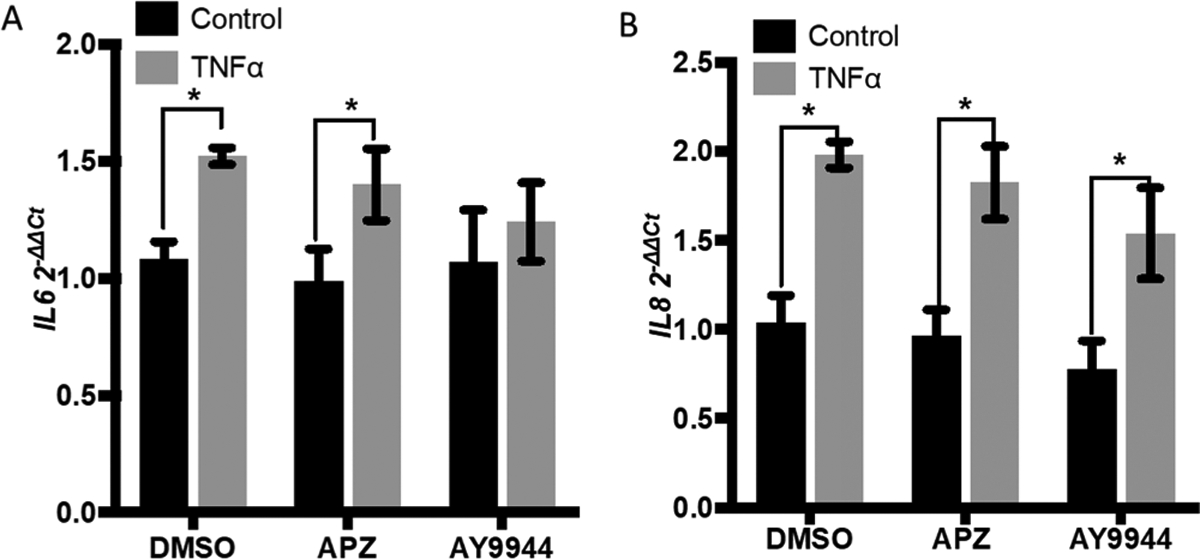
Stimulation with TNFa does not incite APZ-induced IL-6 and IL-8 expression Treatment with 1 μM APZ or AY9944 for 3 d followed by 20 ng/mL TNFa for 4 h did not potentiate any significant change in (A) IL-6 and (B) IL-8 expression compared to the respective DMSO controls. n = 4. Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and Fishers LSD post hoc test. *p < 0.05.
LC–MS (SRM) Measurement of 7-DHC and Cholesterol.
The extracted sterols were derivatized with 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD). The PTAD-derivatized sterols (10 μL injection) were analyzed on an ultraperformance liquid chromatography (UPLC) C18 column (Acquity UPLC BEH C18, 1.7 μm, 2.1 mm × 50 mm) with 100% MeOH (0.1% v/v acetic acid) mobile phase at a flow rate of 500 μL/min and runtime of 1.2 min. A TSQ Quantum Ultra mass spectrometer (ThermoFisher) was used for mass spectrometry (MS) detections, and data were acquired with a Finnigan Xcalibur software package. Selected reaction monitoring (SRM) of the PTAD derivatives was acquired in the positive ion mode using atmospheric pressure chemical ionization (APCI). MS parameters were optimized for the 7-DHC-PTAD adduct and were as follows: auxiliary nitrogen gas pressure at 55 psi and sheath gas pressure at 60 psi; discharge current at 22 μA and vaporizer temperature at 342 °C. Collision-induced dissociation13 was optimized at 12 eV under 1.0 mTorr of argon. The SRM transition of precursor to product ion included m/z 560 → 365 for 7-DHC-PTAD adduct and a pseudo-SRM transition of 369 → 369 to monitor Chol since Chol does not react with PTAD.
In Vitro O3 Exposure and Real-Time qPCR.
Cultured 16HBE cells at ALI were exposed to either filtered air or 0.4 ppm of O3 for 4 h in exposure chambers operated by the U.S. Environmental Protection Agency (EPA) to mimic the 8 h average exposure of an individual. This dose of O3 is shown to have minimal cytotoxicity and maximal innate immune response in our 16HBE cells.14 For qPCR analysis, at 1 h post exposure, RNA was collected in lysis buffer provided by the Pure Link RNA Mini Kit (Life Technologies, Carlsbad, CA) prepared with 1% 2-mercaptoethanol (Sigma-Aldrich). Total RNA was isolated from the 16HBE lysates using the RNA kit listed above. cDNA preparation and real-time qPCR were performed as previously described.15,16 The β-actin primer was purchased from Applied Biosystems, Foster City, CA. Human IL-8:5′-FAM-CCTTGGCAAAACTGCACCTTCAC-TAMRA-3′ (probe), 5′-TTGGCAGCCTTCCTGATTTC-3′ (sense), and 5′-TATGCACTGACATCTAAGTTCTTTAGCA-3′ (antisense) and IL-6:5′-FAM-CCAGCATCA-GTCCCAAGAAGGCAACT-TAMRA-3′ (probe), 5′-TATGAAGTTCCTCTCTGCAAGAGA-3′ (sense), and 5′-TAGGGAAGGCCGTGGTT-3′ (antisense) were prepared in-house. Expression was determined by the ΔΔCt method using β-actin for normalization as previously described.17,18 The fold change in gene expression was then calculated as 2−ΔΔCt.
NanoString Gene Expression Analysis.
16HBE RNA isolated as described above was analyzed for gene expression via Nanostring (Seattle, WA) nCounter PanCancer Immune Profiling Panel. Nanostring gene expression data were normalized to the geometric mean of stable housekeeping genes and to positive and negative control genes, and analyzed using the nSolver software.19
Protein Concentration Analysis.
Basolateral supernatant from 16HBE cell samples collected as described above was analyzed using ELISA cytokine analysis BD OptEIA human IL-8 ELISA kit (San Jose, CA).
Ozonization of Sterols in Solution.
O3 was generated using compressed gas, oxidizing, N.O.S. (oxygen, nitrous oxide) (UN3156, Airgas USA, Radnor, PA) flowing through an ozone generator (AquaZone, Red Sea, Huston, TX) and bubbled through Hanks’ balanced salt solution with calcium and magnesium (HBSS++) (Life Technologies) containing either 20 μM cholesterol (Sigma-Aldrich), 7-DHC (Sigma-Aldrich), or DMSO for 30 min. According to measurements taken using the indigo method, O3 was dissolved into solution at approximately 0.3 mg/L.20,21 Solutions were immediately applied to submerged 16HBE cultures for 1 h, and cells were analyzed for expression of Interleukin-6 (IL-6) and Interleukin-8 (IL-8). As a control, air exposed solutions containing DMSO, cholesterol, or 7-DHC were directly applied to cells for 1 h.
For studies of comparative sterol-ozone reactivity, an NMR tube was charged with equimolar (30 mM) cholesterol and 7-DHC in 1 mL of 2:1 CDCl3:MeOD with 10 μL of DMF-d7 internal standard. After a T0 1H NMR spectrum was acquired, a minimal flow of ozone was admitted to the tube for 20 s above the solvent, and the tube was sealed, vigorously vortexed, and let stand 1 h at which time an NMR spectrum (T1) was obtained. A third spectrum (T2) was obtained after additional vortexing and a 5 h period, and a final spectrum (T3) was acquired after vortexing and a 48 h wait. Quantitative data for consumption of cholesterol and 7-DHC were acquired by integration of the vinyl signals of cholesterol at 5.3 δ and those of 7-DHC at 5.4 and 5.5 δ. 1H signals of DMF at 2.8 and 2.95 δ were used as an internal standard for comparison with the sterol vinylic protons.
Statistical Analysis.
All in vitro data were performed in at least three separate experiments, each with multiple technical replicates. Data shown are mean ± SEM. Figure 2A was analyzed using one-way ANOVA. Data in Figures 2, 3, 5, 6C–F, and 7 were analyzed using a two-way ANOVA and Fisher’s LSD post hoc test. The ELISA data in Figure 4 and gene expression data in Figure 6 were analyzed by a paired two-tailed t-test to isolate APZ-induced modifications of the respective end points and reduce the effects of confounders such as variability of O3-induced responses. Figure 6 was generated using the nSolver software, both using an average Pearson’s correlation and log2 fold change. Inclusion in the heatmap was determined by relative count |fold change (FC)| greater than 1.5 and p less than 0.05, compared to DMSO/Air.22 Table 1 shows analysis of log2 fold change from the DMSO/Air group and an unpaired two tailed student’s t-test.
Figure 2.

APZ and AY9944 increase the 7-DHC/cholesterol ratio in airway epithelial cells LC–MS (SRM) analysis revealed an increase in the (A) ratio of 7-DHC to cholesterol in 16HBE cells grown at ALI in 16HBE cells treated with IJJM APZ or AY9944 for 1 or 3 days. (B) Cholesterol concentration alone was significantly reduced in the 3 day treatment, while (C) 7-DHC concentration significantly increased over 1 or 3 days treatment compared to the respective DMSO control treatment. (D) XH NMR spectral monitoring of the reaction of (1:1) cholesterol:7-DHC with a limited amount of ozone. Protons H3 and H6 are monitored for cholesterol, H3′, H6′, and H7′ for 7-DHC. Spectrum T0 is for the starting 1:1 mixture of the sterols; T1 is taken 1 h after ozone exposure; T2, 6 h; and T3, 48 h later. Over the course of the experiment, the integrated areas of the relevant protons indicated that cholesterol decreased to 90% of its T0 value, while the 7-DHC decrease was to 40% of its T0 value. Integrated areas are compared to a DMF internal standard. n = 3. Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and Fishers LSD post hoc test. *p < 0.05, **p < 0.01.
Figure 3.

APZ and AY9944 potentiate O3-induced IL-6 and IL-8 expression 16HBE cells grown at ALI treated in the basolateral compartment with lfiM APZ or AY9944 for 3 days prior to 0.4 03 exposure yielded enhanced expression of (A) IL-6 and (C) IL-8 for APZ and (B) IL-6 (D) IL-8 for AY9944 with significant increases in both markers compared to the DMSO control. n = 3–5. Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and Fishers LSD post hoc test. *p < 0.05, **p <0.01.
Figure 6.
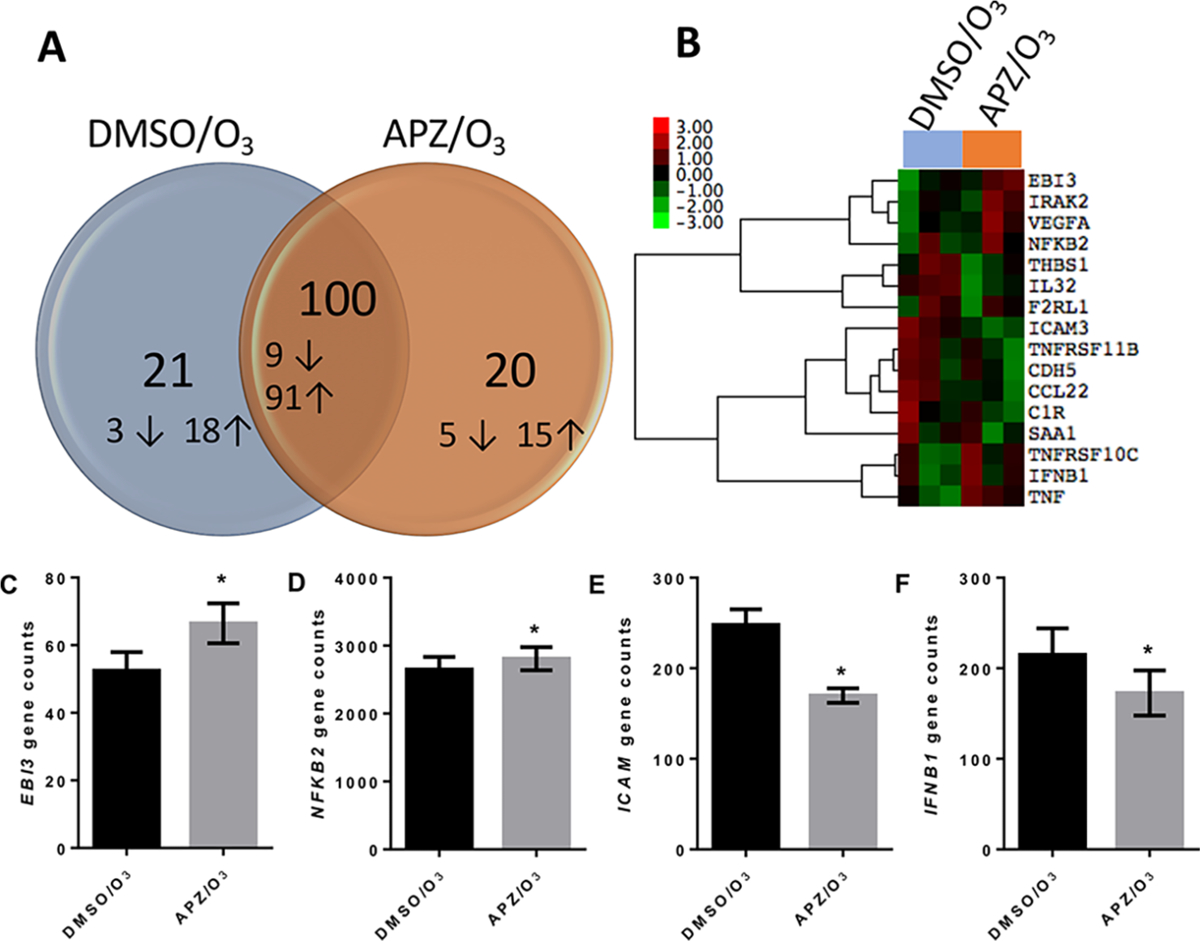
Nanostring analysis of gene expression in 16HBE cells treated with APZ. Cells were treated with DMSO and orl |iM APZ for 3 d prior to 0.4 ppm O3 exposure for 4 h. RNA was collected 1 h postexposure and analyzed for gene expression profiles. (A) Venn diagram of the number of genes significantly changed in each group compared to the DMSO/air control and (B) unbiased heat map of gene expression for cells exposed to O3. Genes were included in the heat map if the average relative gene count I fold change (FC)I was >1.5 and p < 0.05 compared to DMSO/air. Log2 fold change is plotted in the heat map. (C–F) Distinct gene expression profiles for EBB, NFKB2, ICAM, and IFNBl. n = 3. Genes in (A–F) were identified using a paired, two tailed t-test comparing O3 exposed groups (p < 0.05).
Figure 7.
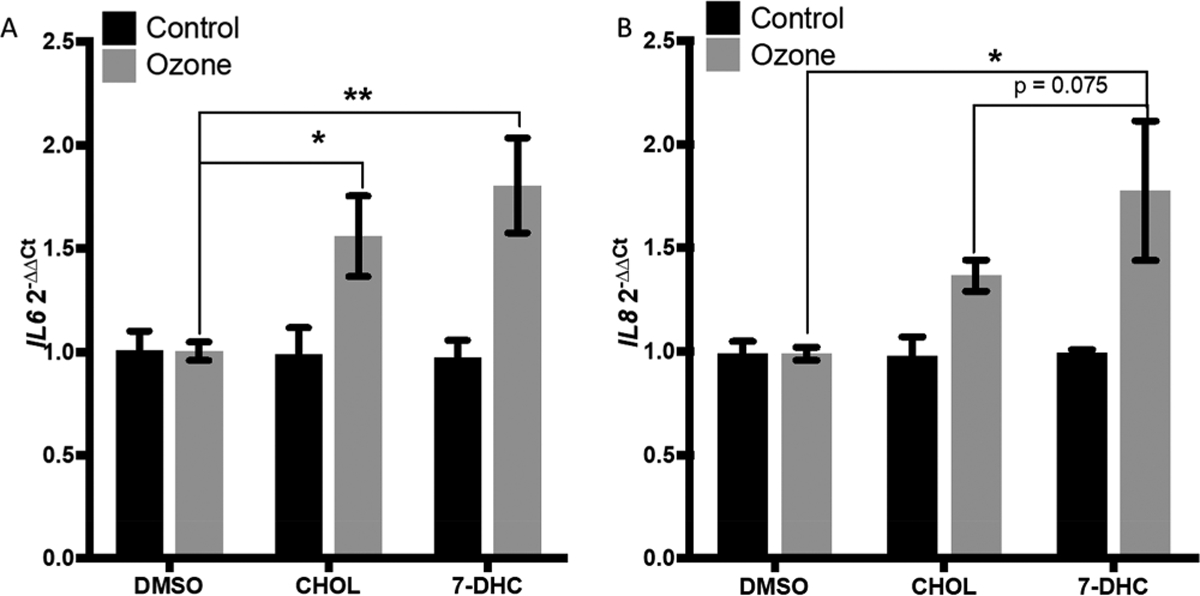
Ozonized 7-DHC increased IL-6 and IL-8 expression to a greater extent than ozonized cholesterol 20 μM 7-DHC, cholesterol, and the DMSO vehicle were ozonized in buffer for 30 min and immediately added to submerged cells without ozonization as a control. (A) IL-6 and (B) IL-8 expression was significantly increased in the 7-DHC ozonization sample as compared to the control. n = 4. Data are presented as mean ± SEM. Statistical analysis was performed using a two-way ANOVA and Fishers LSD post hoc test. *p < 0.05, **p < 0.01.
Figure 4.
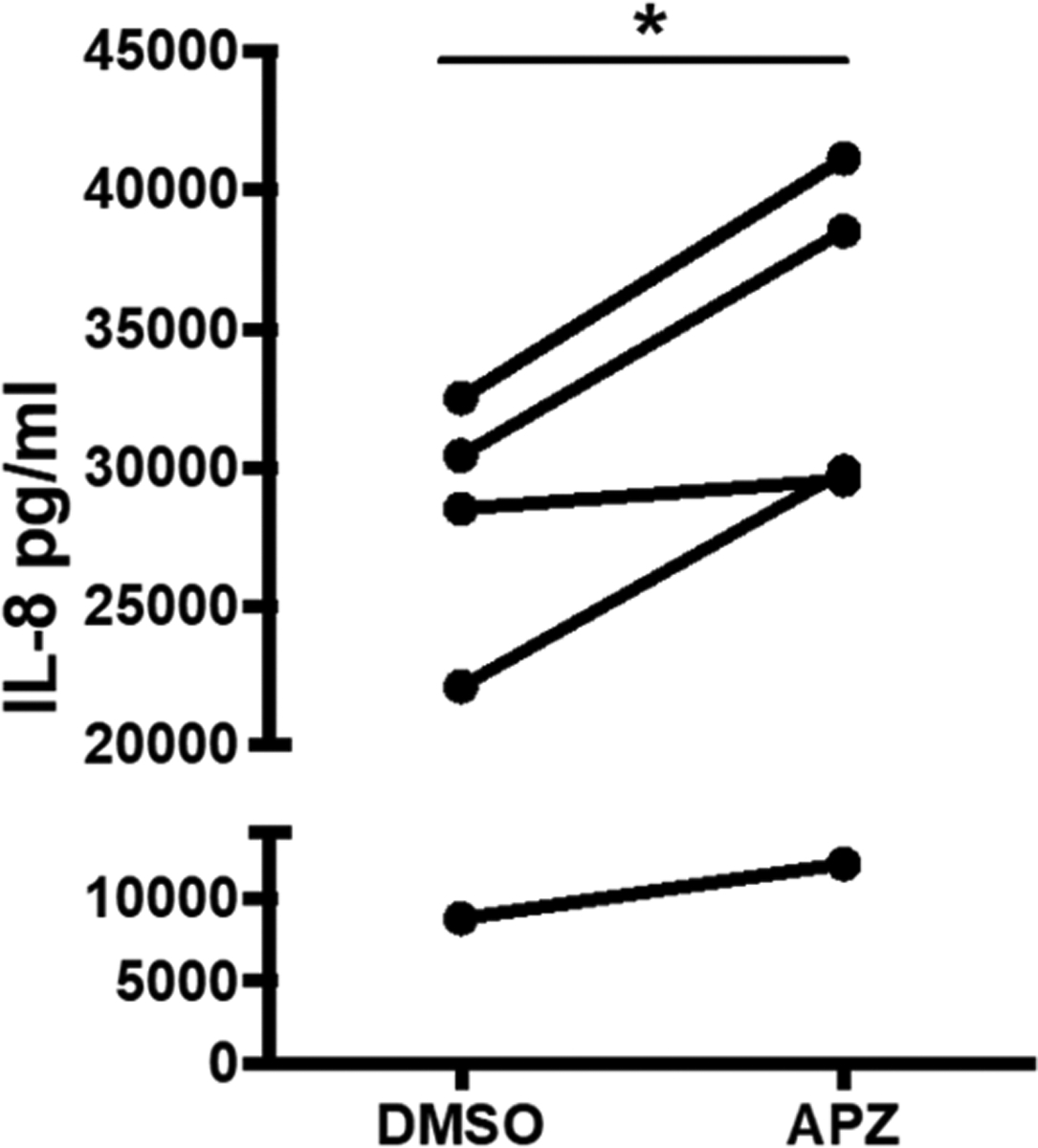
APZ facilitates O3-induced IL-8 protein release treatment with 1 μM APZ significantly increases the concentration of IL-8 cytokine in O3 exposed 16HBE cells compared to DMSO vehicle treated cells. n = 5. Data presented as mean ± SEM. Statistical analysis was performed using a paired two-tailed t test. *p < 0.05.
Table 1.
DHCR7 Inhibiting Pharmaceutical Drugsa
| drug [common names] | U.S. sales rank 2014 | Z-score | drug type |
|---|---|---|---|
| Metoprolol [Lopressor, Metolar] | 6 | 63 | β-blocker |
| Trazodone [Desyrel, Oleptro] | 28 | 120 | antidepressant |
| Buspirone [Buspar] | 95 | 110 | antianxiety |
| Oxybutynin [Ditropan, Lyrinel] | 107 | 30 | antimuscarinic |
| Donepezil [Aricept] | 108 | 80 | cholinesterase inhibitor |
| Aripiprazole [Abilify] | 119 | 200 | antipsychotic |
| Risperidone [Risperdal] | 128 | 10 | antipsychotic |
| Nebivolol [Bystolic, Nebilet] | 141 | 234 | β-blocker |
Selected pharmaceutical drugs from the 2014 top 200 selling drugs in U.S. Drugs are organized by sales rank and Z-score for DHCR7 inhibitory effect in Neuro2A cells treated with l μM concentration for 24 h (24). Data gathered by Dr. Phillip Wages of Vanderbilt University.
RESULTS
APZ and AY9944 Increase 7-DHC Levels in Human Airway Epithelial Cells.
We treated 16HBE cells with increasing concentrations of APZ or the known DHCR7 antagonist AY9944 over several time points. No morphological changes were observed at any concentration tested, and our 1 μM APZ treatment regimen showed no overt cytotoxicity after 1, 3, or 6 days of treatment (data not shown). Although the 1-day treatment regimen of APZ increased the 7-DHC:cholesterol ratio, the 3-day 1 μM APZ treatment regimen yielded the greatest increase in 7-DHC:cholesterol to an approximate 0.6 ratio as compared to a negligible ratio at baseline (Figure 2A). Similarly, the known DHCR7 antagonist AY9944 also altered the 7-DHC:cholesterol ratio in an almost identical manner, significantly increasing the 7-DHC:cholesterol ratio to approximately 0.7 (Figure 2A). These changes in 7-DHC:cholesterol ratio were caused by effects of APZ and AY9944 on both cholesterol and 7-DHC levels. Specifically, 3-day treatment regimen with APZ and AY9944 decreased cholesterol levels (Figure 2B) while significantly increasing 7-DHC levels (Figure 2C). Notably, exposure to O3 had no additional effects on 7-DHC:cholesterol ratio, cholesterol levels, or 7-DHC levels. To determine whether 7-DHC has greater rectivity toward O3, we conducted competition assays followed by NMR spectral monitoring. Figure 2D shows that in an equimolar mixture of cholesterol:7-DHC, 7-DHC is consumed much faster and to a greater extent than cholesterol (compare peaks 3, 6 for cholesterol with peaks 3′, 6′, and 7′ for 7-DHC).
APZ Treatment Causes O3-Specific Increases in Proinflammatory Cytokine Levels.
As shown in previous studies,23 exposure to O3 causes an inflammatory response in 16HBE cells marked by significant increases in the gene expression of pro-inflammatory cytokines IL-6 and IL-8 (Figure 3A–D). APZ treatment with 1 μM for 3 days prior to O3 exposure significantly potentiated IL-6 and IL-8 expression 1.5- to 2-times higher when compared to vehicle (0.01% DMSO) treated cells exposed to O3 (Figure 3A,C). Similarly, treatment with the DHCR7 inhibitor AY9944 at 1 μM over 3 days increased pro-inflammatory IL6 and IL8 expression in O3 exposed 16HBE cells (Figure 3B,D). Moreover, APZ pretreatment significantly increased the O3-induced levels of pro-inflammatory cytokine IL-8 in 16HBE cells compared to DMSO vehicle treatment (Figure 4). Interestingly, the APZ-induced potentiation of inflammatory gene expression appears to be O3-specific as 16HBE cells challenged with another stimulant, TNFα, did not exhibit the same APZ-exacerbated expression of IL-6 and IL-8 (Figure 5A,B).
APZ-Exacerbated Expression of IL-6 and IL-8 (Figure 5A,B). Nanostring Gene Expression Analysis of APZ and O3.
Using the NanoString nCounter PanCancer Immune Profiling Panel, 16HBEs treated for 3 days with 1 μM of APZ or DMSO and exposed to air or O3 were examined for 730 genes, of which 487 were detectable at baseline in our DMSO/Air exposed cells. Together, the O3 exposed groups (DMSO/O3 and APZ/O3) showed significantly altered expression and exhibited a fold change of at least ±1.5 in 144 genes as compared to DMSO/air control cells (Supporting Table 1). Of these 144 genes, 21 and 20 genes were uniquely changed in DMSO/O3 and APZ/O3 exposed groups, respectively, and 100 genes were significantly changed in both O3 exposed groups (Figure 6A). Only nine genes were significantly altered in the APZ/Air group compared to the DMSO/Air vehicle (see Supporting Table 1). When comparing DMSO/O3 and APZ/O3 exposed groups, APZ treatment significantly modified the expression of 16 genes (Figure 6B). Within this group, Epstein–Barr virus-induced gene 3 (EBI3) and nuclear factor kappa-B 2 NFKB2 (Figure 6C,D) were robustly increased by APZ, while intercellular adhesion molecule-1 (ICAM) and interferon beta 1 (IFNB1) (Figure 6E,F) were among the genes whose expression decreased following APZ treatment.
Ozonized 7-DHC Increases Expression of Inflammatory Cytokines.
O3 was bubbled through HBSS++ buffer with vehicle DMSO, 20 μM cholesterol, or 20 μM 7-DHC for 30 min. Buffer solutions were immediately added to submerged 16HBE cultures for 1 h, and cells were subsequently analyzed for IL-6 and IL-8 expression. IL-6 expression was significantly enhanced in cells treated with buffer containing ozonized cholesterol or ozonized 7-DHC. However, only buffer containing ozonized 7-DHC significantly increased IL-8 expression, which was enhanced, albeit not statistically different, as compared to ozonized cholesterol (p = 0.075) (Figure 7A,B). No significant changes in IL-6 or IL-8 expression were observed between the DMSO and sterol group cells which received the control treatment.
APZ Increases O3-Induced Expression of Inflammatory Cytokines in Primary Human Nasal Cells from Males but Not Females.
Human nasal epithelial cells were obtained from male and female donors and cultured on Transwells as described by us before.12 Upon confluency, cells were treateded with 1 μM APZ from the basolateral side for 3 days prior to O3 exposure, which potentiated IL-6 and IL-8 expression in cells from males, but not females (Figure 8A,B).
Figure 8.
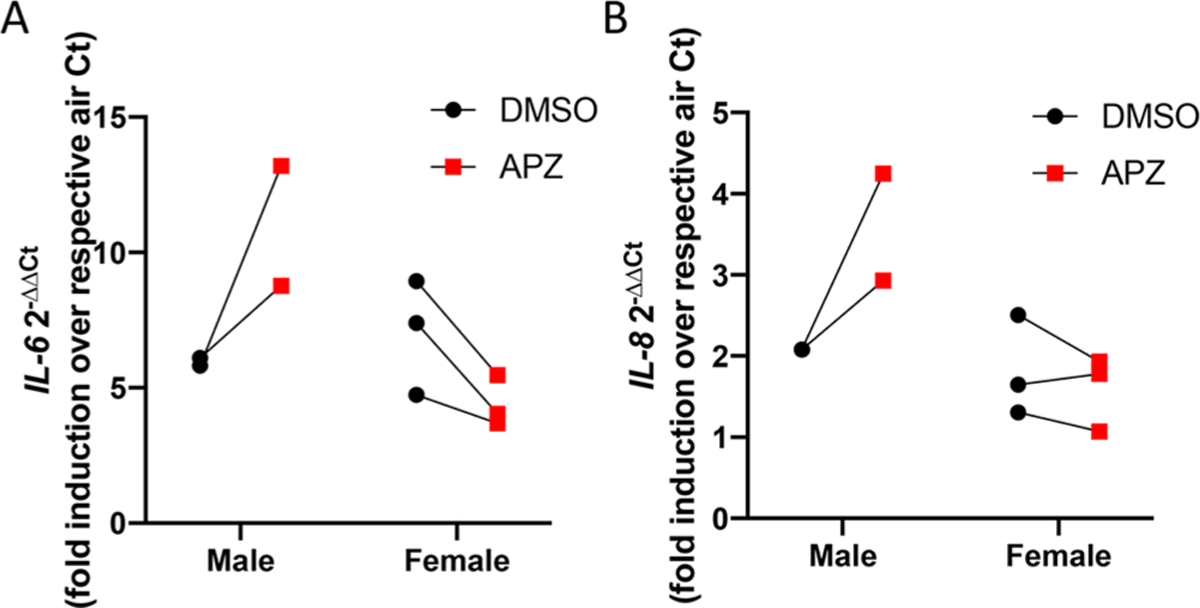
APZ increases O3-induced IL-6 and IL-8 gene expression in a sex-specific manner in primary human nasal epithelial cells. Human nasal epithelial cells grown at ALI were treated in the basolateral compartment with l|iM APZ for 3 days prior to 0.4 ppm O3 exposure, and RNA was collected 1 h postexposure. Treatment with APZ significantly enhanced expression of (A) IL-6 and (B) IL-8 in nasal epithelial cells from males, but not females as compared to the DMSO control. n = 2–3. Data are presented as individual data points.
Theoretical Framework and Discussion.
The increasing concurrent use of chemically active pharmaceutical drugs and potential for exposure to environmental pollutants in the developed world highlight the need to study their possible molecular interactions and combined biological effects. O3 is a highly reactive pollutant gas, which rapidly interacts with biomolecules present in the respiratory tract including cholesterol. The precursor of cholesterol biosynthesis is 7-DHC, which is highly susceptible to ozonization because of the conjugated double bonds on the sterol B-ring. Whether chemicals that modify cholesterol biosynthesis at the level of DHCR7 and consequently increase the levels of 7-DHC affect O3-induced biological responses has never been examined. To fill this knowledge gap, we explored the impact of chemicals with DHCR7 inhibitory activity on O3-induced inflammatory responses in human airway epithelial cells. We observed that the drug APZ and the known DHCR7 inhibitor AY9944 decreased cholesterol levels, elevated 7-DHC levels, and consequently increase the 7-DHC:cholesterol ratio. These treatments also increased O3-induced pro-inflammatory cytokine expression and increased the expression of numerous other genes associated with pro-inflammatory signaling pathways and decreased expression of genes associated with intercellular cytokine/chemokine responses. We also demonstrate that direct ozonization of 7-DHC elicited a greater effect on IL-8 expression in exposed epithelial cells than ozonized cholesterol. Taken together, these data provide a basis for chemically induced exacerbation of biological responses caused by exposure to O3, which could have significant public health implications.
As illustrated in Figure 1C and Figure 7, 7-DHC represents a unique target for ozonization as the additional double bond at C7 makes the molecule oxidatively unstable, allowing the molecule to be highly reactive toward free radical oxidation and one of the best chain-carrying molecules studied to date.24–26 Competition studies also indicated that 7-DHC was consumed to a much greater extent than cholesterol when an equimolar mixture of the two sterols was exposed to a limiting supply of O3 (Figure 2D). Thus, over 60% of 7-DHC was oxidized when the headspace of a solution of the two sterols was sparged with a slow O3 stream for 20 s and the sealed solution of the O3-sterol mixture was let stand for 2 days. During this time, minimal disappearance of cholesterol was observed. Although the airways are not a significant contributor to the production of endogenous cholesterol, perturbations to systemic cholesterol homeostasis and an increase in available 7-DHC in the lung could lead to the creation of oxysterol species with unique activities following interaction with oxidants, such as O3. The oxidized lipids formed due to 7-DHC ozonizaton are different than those formed due to cholesterol ozonization and have the potential to be more electrophilic and oxidatively reactive, capable of more rapidly modifying available proteins and propagating lipid peroxidation.25,27 The product mixture from 7-DHC ozonization is, however, extremely complex and the identification of primary oxysterol products formed during cellular APZ/O3 exposures is hampered by the highly unstable character of these oxysterols (Dr. Ned Porter; personal communication), which is a limitation of this study. To directly link 7-DHC ozonization products with enhanced inflammatory gene expression seen in airway epithelial cells, we conducted experiments stimulating cells with ozonized sterol mixtures derived from either 7-DHC or cholesterol. As shown in Figure 7, the data demonstrate that the ozonized 7-DHC products enhance IL-8 expressions to a greater extent than ozonized cholesterol products. These data suggest that the more reactive oxysterol species derived from the ozonization of 7-DHC cause greater inflammatory responses in epithelial cells as compared to oxysterol species derived from ozonization of cholesterol.
Our previous studies have demonstrated that APZ inhibits DHCR7, leading to increased levels of 7-DHC.1 We aimed to link the potential for APZ to inhibit DHCR7 and increase the ratio of 7-DHC:cholesterol in airway epithelial cells as the primary mechanism for the potentiated O3 effect, by confirming our observations using a commercially available DHCR7 inhibitor. We show here that APZ significantly increases levels of 7-DHC in human airway epithelial cells to a similar extent as the DHCR7 inhibitor AY9944. As expected, AY9944 increased the 7-DHC:cholesterol ratio and potentiated O3 induced inflammatory effects in 16HBE cells. The comparable effects of AY9944 and APZ on increasing 7-DHC levels support our hypothesis that APZ inhibition of DHCR7 and increased abundance of 7-DHC mediate the potentiation of O3 induced inflammation. Interestingly, IL-6 and IL-8 expression following stimulation with TNF-α was not affected by APZ (Figure 5), indicating that the effects of APZ-induced enhancement of IL-6 and IL-8 expression are not generally applicable to all pro-inflammatory stimuli, but more specific to oxidants, such as O3.
Although it is important to emphasize the capacity for APZ to potentiate canonically O3 induced markers of inflammation like IL-6 and IL-8, further attention is needed to characterize the breadth of stimulated changes in gene expression. Data shown in Figure 6B–D and Supporting Table 1 indicate that other genes, such as EBI3 and NFKB2, are also enhanced in cells treated with APZ prior to O3 exposure. EBI3 is a subunit of the heterodimeric cytokines IL-27 and IL-35, which have multiple immune regulatory effects, including T cell differentiation.28,29 NFKB2 encodes the NF-κB p100/p52 subunit, a part of the noncanonical NF-κB signaling pathway, shown to increase sensitivity to LPS-induced lung injury.30 APZ-induced increases in NFKB2 or EBI3 expression could further intensify O3-associated inflammatory responses in the human airway and may contribute to sustained adverse health outcomes associated with pollutant exposure. Furthermore, our gene expression array data suggest that APZ treatment reduced O3-induced expression of certain genes such as ICAM3 and IFNB, which have immune regulatory functions (Figure 6E,F). IFN-β has been shown to be essential in responses to pathogen and viral infection, suggesting that APZ-induced suppression of IFNB expression could enhance susceptibility to viral infections, which we have previously demonstrated.12 In addition, ICAM3 is an adhesion molecule important during leukocyte trafficking, and decreased expression in the context of O3 exposure could impair the recruitment and migration of lymphocytes, impairing critical host defense responses.31 Overall, these data suggest that individuals taking Abilify could be susceptible to dynamic and broad modifications in inflammatory and immune responses triggered by inhalation of oxidants such as O3.
APZ and similar small molecule drugs commonly prescribed for depression are widely used in the US with nearly 10 million prescriptions filled annually.8,32 While APZ is designated an antipsychotic, only 2.5% of the U.S. population are diagnosed as such. More commonly, APZ is prescribed in combination with other drugs to treat depression, tic disorders, and irritability associated with autism.33 Circulating levels can reach up to 0.7 μM in patients treated for severe bipolar and schizophrenic disorder, thus making the use of 1 μM in our in vitro system within pharmacologically relevant doses.34,35 At this concentration, our data demonstrate that APZ treatment enhances the already well-described markers of O3-induced inflammation in human airway epithelial cells.23,36,37 In Figure 3, APZ treatment regimens at 1 μM over 3 days significantly increased O3-induced IL-6 and IL-8 expression almost two-fold over the vehicle-treated cell response to O3. Similarly, we observe that APZ pretreatment significantly increases the already heightened concentrations of IL-8 in 16HBE cells exposed to O3 (Figure 4). Elevated expression and concentrations of cytokines such as IL-8 in the human airway is a hallmark of many inflammatory diseases, and our observation that APZ can amplify these biological responses suggests that it may predispose individuals to O3-induced adverse health effects.44,45 Interestingly, the ablity of APZ to enhance O3-induced IL-6 and IL-8 expression in human nasal epithelial cells was sex dependent (Figure 8). We have recently demonstrated that at baseline the nasal mucosa of males is more “pro-inflammatory”.38,39 In contrast, rodent studies have shown that female mice have greater O3-induced pro-inflammatory responses in the lung as compared to male mice.40–43 O3-induced IL-6 and IL-8 expression did not differ between nasal epithelial cells from males and females, but the ability of APZ to increase this response did, suggesting a sex-dependent difference in APZ metabolism or ability to modify cholesterol synthesis.
Overall, our research findings support the hypothesis that APZ-induced inhibition of DHCR7 and subsequently elevated 7-DHC levels potentiate O3-induced biological responses in human airway epithelial cells. These findings also describe a previously unknown interaction between chemically active pharmaceuticals and environmental pollutants, which could have broader implications beyond the effects described here. We believe our findings are indicative of a larger number of potential interactions that have yet to be uncovered and potentially represent a new field of study. Aside from APZ, different classes of therapeutic drugs have the potential for cholesterol modification including other small molecule antidepressants and β-blockers (Table 1).1 Broadly, we propose additional comprehensive screening studies to look at widely prescribed chemicals known to inhibit DHCR7 (Table 1) and their influence on O3-induced biological responses.
Supplementary Material
Funding
The following funding sources were used to conduct this research: R01-ES028269, R21-ES024666, T32-ES007126, and T32-LM012420. This research was funded in part by US EPA Cooperative Agreement CR83578501 but has not been subjected to review and does not necessarily reflect EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
ABBREVIATIONS
- 7-DHC
7-dehydrocholesterol
- ALI
air–liquid interface
- APZ
aripiprazole
- DHCR7
7-dehydrocholesterol reductase
- EBI3
Epstein–Barr virus induced gene 3
- HBE
human bronchial epithelial cells
- ICAM
intercellular adhesion molecule
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- INF-γ
Interferon-γ
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- O3
ozone
- oxysterol
oxidized cholesterol
- TNF
tumor necrosis factor
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.9b00149.
Gene expression changes summarized in the text (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Kim HY, Korade Z, Tallman KA, Liu W, Weaver CD, Mirnics K, and Porter NA (2016) Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem. Res. Toxicol 29 (5), 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Xu L, Davis TA, and Porter NA (2009) Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc 131 (36), 13037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Pulfer MK, and Murphy RC (2004) Formation of Biologically Active Oxysterols during Ozonolysis of Cholesterol Present in Lung Surfactant. J. Biol. Chem 279 (25), 26331–26338. [DOI] [PubMed] [Google Scholar]
- (4).Speen AM, Kim HH, Bauer RN, Meyer M, Gowdy KM, Fessler MB, Duncan KE, Liu W, Porter NA, and Jaspers I (2016) Ozone-derived Oxysterols Affect Liver X Receptor (LXR) Signaling: A POTENTIAL ROLE FOR LIPID-PROTEIN ADDUCTS. J. Biol. Chem 291 (48), 25192–25206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pryor WA (1994) Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radical Biol. Med 17 (5), 451–65. [DOI] [PubMed] [Google Scholar]
- (6).Windsor K, Genaro-Mattos TC, Miyamoto S, Stec DF, Kim HY, Tallman KA, and Porter NA (2014) Assay of protein and peptide adducts of cholesterol ozonolysis products by hydro-phobic and click enrichment methods. Chem. Res. Toxicol 27 (10), 1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hall P, Michels V, Gavrilov D, Matern D, Oglesbee D, Raymond K, Rinaldo P, and Tortorelli S (2013) Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab 110 (1–2), 176–8. [DOI] [PubMed] [Google Scholar]
- (8).Lindsley CW (2015) 2014 prescription medications in the United States: tremendous growth, specialty/orphan drug expansion, and dispensed prescriptions continue to increase. ACS Chem. Neurosci 6 (6), 811–2. [DOI] [PubMed] [Google Scholar]
- (9).(2016) Ambient air pollution: A global assessment of exposure and burden of disease in WHO Library Cataloguing-in-Publication Data, WHO. [Google Scholar]
- (10).Kantor ED, Rehm CD, Haas JS, Chan AT, and Giovannucci EL (2015) Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. Jama 314 (17), 1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Pratt La Fau-Brody DJ, Brody Dj Fau-Gu Q, and Gu Q (2017) Antidepressant Use Among Persons Aged 12 and Over :United States, 2011–2014 in NCHS Data Brief No. 283, NCHS. [PubMed] [Google Scholar]
- (12).Kesic MJ, Simmons SO, Bauer R, and Jaspers I (2011) Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radical Biol. Med 51 (2), 444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Koman PD, and Mancuso P (2017) Ozone Exposure, Cardiopulmonary Health, and Obesity: A Substantive Review. Chem. Res. Toxicol 30 (7), 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Muller L, Brighton LE, and Jaspers I (2013) Ozone exposed epithelial cells modify cocultured natural killer cells. Am. J. Physiol Lung Cell Mol. Physiol 304 (5), L332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jaspers I, Zhang W, Fraser A, Samet JM, and Reed W (2001) Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am. J. Respir. Cell Mol. Biol 24 (6), 769–77. [DOI] [PubMed] [Google Scholar]
- (16).Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, and Madden MC (2005) Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol. Sci 85 (2), 990–1002. [DOI] [PubMed] [Google Scholar]
- (17).Bauer RN, Brighton LE, Mueller L, Xiang Z, Rager JE, Fry RC, Peden DB, and Jaspers I (2012) Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J. Allergy Clin. Immunol 130 (4), 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Horvath KM, Brighton LE, Zhang W, Carson JL, and Jaspers I (2011) Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am. J. Respir. Cell Mol. Biol 45 (2), 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wang H, Horbinski C, Wu H, Liu Y, Sheng S, Liu J, Weiss H, Stromberg AJ, and Wang C (2016) NanoStringDiff: a novel statistical method for differential expression analysis based on NanoString nCounter data. Nucleic Acids Res 44 (20), No. e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hart EJ, Sehested K, and Holoman J (1983) Molar absorptivities of ultraviolet and visible bands of ozone in aqueous solutions. Anal. Chem 55 (1), 46–49. [Google Scholar]
- (21).Bader H (1982) Determination of Ozone In Water By The Indigo Method: A Submitted Standard Method. Ozone: Sci. Eng 4(4), 169–176. [Google Scholar]
- (22).Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES, Lee KY, Luo Y, Sun YA, Willey JC, Setterquist RA, Fischer GM, Tong W, Dragan YP, Dix DJ, Frueh FW, Goodsaid FM, Herman D, Jensen RV, Johnson CD, Lobenhofer EK, Puri RK, Schrf U, Thierry-Mieg J, Wang C, Wilson M, Wolber PK, Zhang L, Amur S, Bao W, Barbacioru CC, Lucas AB, Bertholet V, Boysen C, Bromley B, Brown D, Brunner A, Canales R, Cao XM, Cebula TA, Chen JJ, Cheng J, Chu TM, Chudin E, Corson J, Corton JC, Croner LJ, Davies C, Davison TS, Delenstarr G, Deng X, Dorris D, Eklund AC, Fan XH, Fang H, Fulmer-Smentek S, Fuscoe JC, Gallagher K, Ge W, Guo L, Guo X, Hager J, Haje PK, Han J, Han T, Harbottle HC, Harris SC, Hatchwell E, Hauser CA, Hester S, Hong H, Hurban P, Jackson SA, Ji H, Knight CR, Kuo WP, LeClerc JE, Levy S, Li QZ, Liu C, Liu Y, Lombardi MJ, Ma Y, Magnuson SR, Maqsodi B, McDaniel T, Mei N, Myklebost O, Ning B, Novoradovskaya N, Orr MS, Osborn TW, Papallo A, Patterson TA, Perkins RG, Peters EH, Peterson R, Philips KL, Pine PS, Pusztai L, Qian F, Ren H, Rosen M, Rosenzweig BA, Samaha RR, Schena M, Schroth GP, Shchegrova S, Smith DD, Staedtler F, Su Z, Sun H, Szallasi Z, Tezak Z, Thierry-Mieg D, Thompson KL, Tikhonova I, Turpaz Y, Vallanat B, Van C, Walker SJ, Wang SJ, Wang Y, Wolfinger R, Wong A, Wu J, Xiao C, Xie Q, Xu J, Yang W, Zhang L, Zhong S, Zong Y, and Slikker W Jr. (2006) The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat. Biotechnol 24 (9), 1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jaspers I, Flescher E, and Chen LC (1997) Ozone-induced IL-8 expression and transcription factor binding in respiratory epithelial cells. Am. J. Physiol 272 (3), L504–11. [DOI] [PubMed] [Google Scholar]
- (24).Xu L, Korade Z, Rosado DA Jr., Mirnics K, and Porter NA (2013) Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res 54 (4), 1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Xu L, Korade Z, and Porter NA (2010) Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc 132 (7), 2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Korade Z, Xu L, Shelton R, and Porter NA (2010) Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res 51 (11), 3259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pfeffer BA, Xu L, Porter NA, Rao SR, and Fliesler SJ (2016) Differential cytotoxic effects of 7-dehydrocholesterol-derived oxysterols on cultured retina-derived cells: Dependence on sterol structure, cell type, and density. Exp. Eye Res 145, 297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, and Weiner HL (2012) IFN-γ Limits Th9-Mediated Autoimmune Inflammation through Dendritic Cell Modulation of IL-27. J. Immunol 189, 5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gao P, Su Z, Lv X, and Zhang J (2017) Interluekin-35 in Asthma and Its Potential as an Effective Therapeutic Agent. Mediators Inflammation 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Saxon JA, Cheng D-S, Han W, Polosukhin VV, McLoed AG, Richmond BW, Gleaves LA, Tanjore H, Sherrill TP, Barham W, Yull FE, and Blackwell TS (2016) p52 Overexpression Increases Epithelial Apoptosis, Enhances Lung Injury, and Reduces Survival after Lipopolysaccharide Treatment. J. Immunol 196 (4), 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Tang MLK, and Fiscus LC (2001) Important Roles for L-Selectin and ICAM-1 in the Development of Allergic Airway Inflammation in Asthma. Pulm. Pharmacol. Ther 14 (3), 203–210. [DOI] [PubMed] [Google Scholar]
- (32).Rhee TG, Mohamed S, and Rosenheck RA (2018) Antipsychotic Prescriptions Among Adults With Major Depressive Disorder in Office-Based Outpatient Settings: National Trends From 2006 to 2015. J. Clin. Psychiatry 79 (2), 17m11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Greenaway M, and Elbe D (2009) Focus on aripiprazole: a review of its use in child and adolescent psychiatry. J. Can. Acad. Child Adolesc Psychiatry 18 (3), 250–60. [PMC free article] [PubMed] [Google Scholar]
- (34).Kirschbaum KM, Hiemke C, and Schmitt U (2009) Rotarod impairment: catalepsy-like screening test for antipsychotic side effects. Int. J. Neurosci 119 (10), 1509–22. [DOI] [PubMed] [Google Scholar]
- (35).Kirschbaum KM, Muller MJ, Zernig G, Saria A, Mobascher A, Malevani J, and Hiemke C (2005) Therapeutic monitoring of aripiprazole by HPLC with column-switching and spectrophotometric detection. Clin. Chem 51 (9), 1718–21. [DOI] [PubMed] [Google Scholar]
- (36).Devlin RB, McKinnon KP, Noah T, Becker S, and Koren HS (1994) Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am.J. Physiol 266 (6), L612–9. [DOI] [PubMed] [Google Scholar]
- (37).Jaspers I, Flescher E, and Chen LC (1997) Respiratory epithelial cells display polarity in their release of the chemokine IL-8 after exposure to ozone. Inflammation Res. 46, S173–4. [DOI] [PubMed] [Google Scholar]
- (38).Rebuli ME, Speen AM, Clapp PW, and Jaspers I (2017) Novel applications for a noninvasive sampling method of the nasal mucosa. Am. J. Physiol Lung Cell Mol. Physiol 312 (2), L288–l296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Rebuli ME, Speen AM, Martin EM, Addo KA, Pawlak EA, Glista-Baker E, Robinette C, Zhou H, Noah TL, and Jaspers I (2019) Wood Smoke Exposure Alters Human Inflammatory Responses to Viral Infection in a Sex-Specific Manner. A Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med 199 (8), 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cabello N, Mishra V, Sinha U, DiAngelo SL, Chroneos ZC, Ekpa NA, Cooper TK, Caruso CR, and Silveyra P (2015) Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol Lung Cell Mol. Physiol 309 (10), L1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fuentes N, Roy A, Mishra V, Cabello N, and Silveyra P (2018) Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ 9 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Mishra V, DiAngelo SL, and Silveyra P (2016) Sex-specific IL-6-associated signaling activation in ozone-induced lung inflammation. Biol. Sex Differ 7, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Noutsios GT, Thorenoor N, Zhang X, Phelps DS, Umstead TM, Durrani F, and Floros J (2019) Major Effect of Oxidative Stress on the Male, but Not Female, SP-A1 Type II Cell miRNome. Front. Immunol 10, 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hellermann GR, Nagy SB, Kong X, Lockey RF, and Mohapatra SS (2002) Mechanism of cigarette smoke condensate-induced acute inflammatory response in human bronchial epithelial cells. Respir. Res 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yi G, Liang M, Li M, Fang X, Liu J, Lai Y, Chen J, Yao W, Feng X, Hu L, Lin C, Zhou X, and Liu Z (2018) A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflammation Res. 67 (6), 539–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


