Abstract
Objective:
During the early follicular phase, sleep-related luteinizing hormone (LH) pulse initiation is positively associated with brief awakenings but negatively associated with random eye movement (REM) sleep. The relationship between sleep architecture and LH pulse initiation has not been assessed in other cycle stages or in women with polycystic ovary syndrome (PCOS).
Design and Methods:
We performed concomitant frequent blood sampling (LH pulse analysis) and polysomnography in 8 normal women (cycle day 7-11) and 7 women with PCOS (at least cycle day 7).
Results:
In normal women, the 5 minutes preceding LH pulses contained more wake epochs and fewer REM epochs than the 5 minutes preceding randomly-determined time points (wake: 22.3 vs. 9.1%, p = 0.0111; REM: 4.4 vs. 18.8%, p = 0.0162). However, LH pulse initiation was not related to wake or REM epochs in PCOS; instead, the 5 minutes preceding LH pulses contained more slow wave sleep (SWS) than the 5 minutes before random time points (20.9 vs. 6.7%, p = 0.0089). Compared to normal subjects, women with PCOS exhibited higher REM-associated LH pulse frequency (p = 0.0443) and a lower proportion of wake epochs 0-5 minutes before LH pulses (p = 0.0205).
Conclusions:
Sleep-related inhibition of LH pulse generation during the later follicular phase is normally weakened by brief awakenings and strengthened by REM sleep. In women with PCOS, LH pulse initiation is not appropriately discouraged by REM sleep and may be encouraged by SWS; these abnormalities may contribute to high sleep-related LH pulse frequency in PCOS.
Keywords: Luteinizing hormone, gonadotropin-releasing hormone, sleep, polycystic ovary syndrome
INTRODUCTION
Progesterone is the primary hormonal regulator of day-to-day luteinizing hormone (LH)—and by inference gonadotropin-releasing hormone (GnRH)—pulse frequency in women. Normally-cycling women also demonstrate decreases in LH pulse frequency during sleep, most prominently in the early follicular phase [1-6], but also in the mid- to late follicular phase [7-10]. In contrast, early neuroendocrine puberty is characterized by increases in LH pulse frequency during sleep [11,12]. Since high and low GnRH pulse frequencies favor gonadotrope production of LH and follicle-stimulating hormone (FSH), respectively, sleep-related changes in GnRH pulse frequency have been postulated to contribute to the early follicular prominence of FSH secretion in post-pubertal women [6] and to normal gonadotropin production across puberty in girls [13].
Our understanding of the relationships between GnRH pulse initiation and sleep architecture is incomplete. In normal women studied during the early follicular phase, sleep-related LH pulses were more likely to be associated with brief awakenings compared to rapid eye movement (REM) and slow wave sleep (SWS) [6]. In pubertal subjects, LH pulse initiation was positively related to SWS but uncommon with REM and wake epochs [14,15]. These discordant findings suggest that the relationships between sleep architecture and GnRH pulse initiation may vary according to physiological and/or developmental status. To our knowledge, previous studies have not addressed these relationships in the mid- to late follicular phase, or in different populations such as those with polycystic ovary syndrome (PCOS)—a disorder associated with increased LH (GnRH) pulse frequency [16]. Since sleep architecture may be an important determinant of sleep-related GnRH pulse frequency, we performed concomitant overnight LH pulse assessment and polysomnography (PSG) during the mid- to late follicular phase in normally-cycling, normoandrogenic women and in women with PCOS.
SUBJECTS AND METHODS
The Institutional Review Board at the University of Virginia approved all study procedures, which were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008. The studies were registered with ClinicalTrials.gov (identifiers and ). Each study participant provided full written informed consent after full explanation of the purpose and nature of all study procedures.
Subjects
Fifteen women participated in a total of 20 overnight admissions involving both frequent blood sampling and PSG. Eight subjects were healthy women with regular, predictable menstrual cycles and no evidence of hyperandrogenism. Seven subjects had PCOS, defined as hyperandrogenism (clinical and/or biochemical) and oligomenorrhea (fewer than nine menses per year) without evidence for an alternative etiology. Subject characteristics are included in Table 1.
Table 1.
Summary data
| Normally-cycling, normoandrogenic (n = 8) |
PCOS (n = 7) | p-value | |
|---|---|---|---|
| Age (years) | 19.6 ± 2.7 (18.5 [18-20]) | 26.6 ± 4.1 (27 [24-30]) | 0.0045 |
| Body mass index (kg/m^2) | 22.9 ± 2.7 (22.4 [21.2-23.8]) | 35.2 ± 10.4 (36.7 [26.1-42.6]) | 0.0205 |
| Body fat percentage (%) | 24.8 ± 5.9 (23.2 [22.1-27.5]) | 41.2 ± 15.6 (45.9 [26.0-54.8]) | NS |
| Waist-to-hip ratio | 0.77 ± 0.06 (0.76 [0.74-0.81]) | 0.87 ± 0.09 (0.90 [0.84-0.92]) | 0.0426 |
| Total testosterone (ng/dl) | 26.3 ± 8.1 (25.2 [19.4-32.0]) | 54.1 ± 23.3 (50.4 [49.5-54.0]) | 0.0033 |
| Sex hormone binding globulin (nmol/l) | 51.9 ± 29.6 (52 [29-68]) | 36.1 ± 12.2 (36 [28-41]) | NS |
| Free testosterone (pg/ml) | 3.9 ± 1.7 (3.5 [2.5-5.8]) | 10.4 ± 7.3 (8.6 [7.8-9.8]) | 0.0062 |
| Estradiol during admission (pg/ml) | 98 ± 48 (78.5 [71.5-135.5]) | 109 ± 86 (111 [34-147]) | NS |
| Progesterone during admission (ng/ml) | 0.4 ± 0.1 (0.4 [0.4-0.5]) | 0.5 ± 0.2 (0.5 [0.3-0.5]) | NS |
| Fasting insulin (μIU/ml) | 3.7 ± 3.6 (2.0 [2.0-3.9]) | 18.2 ± 14.8 (14.4 [5.4-31.0]) | 0.0092 |
| Fasting glucose (mg/dl) | 85 ± 6 (87 [81-89]) | 90 ± 12 (90 [87-97]) | NS |
| Sleep-related mean LH (IU/l) | 5.2 ± 3.4 (5.1 [2.4-7.6]) | 7.0 ± 1.4 (7.4 [6.1-7.9]) | NS |
| Sleep-related mean FSH (IU/l) | 3.4 ± 1.4 (3.75 [2.15-4.3]) | 3.6 ± 1.4 (3.5 [1.9-5.2]) | NS |
| Sleep-related LH pulse frequency (pulses/h) * | 0.46 ± 0.15 (0.45 [0.32-0.59]) | 0.65 ± 0.13 (0.65 [0.52-0.76]) | 0.0401 |
| Sleep-related LH pulse amplitude (IU/l) | 3.1 ± 1.8 (2.6 [1.7-4.9]) | 3.5 ± 2.9 (2.4 [2.1-4.1]) | NS |
| Total sleep period (hours) † | 6.7 ± 0.9 (7.0 [6.2-7.4]) | 6.9 ± 0.8 (6.9 [6.3-7.6]) | NS |
| Sleep efficiency (%) ‡ | 78.4 ± 18.9 (82 [72-93]) | 80.0 ± 16.5 (85 [64-96]) | NS |
| Percent wake (%) | 21.6 ± 18.9 (18 [7-28]) | 20.0 ± 16.5 (15 [4-36]) | NS |
| Percent REM sleep (%) | 11.5 ± 5.5 (13 [9-15]) | 15.2 ± 7.9 (19 [11-21]) | NS |
| Percent sleep stage 1+2 (%) | 51.3 ± 13.0 (52 [46-63]) | 53.1 ± 7.0 (53 [47-59]) | NS |
| Percent sleep stage 3 (%) | 15.5 ± 7.3 (16 [10-20]) | 11.7 ± 11.2 (10 [4-19]) | NS |
Data are presented as mean ± standard deviation (median [interquartile range]). The number of subjects is 8 vs. 7 (normal vs. PCOS) for all variables except for body fat percentage and waist-to-hip ratio (8 normal vs. 6 PCOS). Exact Wilcoxon rank sum (two-sample) tests were used to compare variables (normal vs. PCOS); reported p-values are not corrected for multiple comparisons.
Sleep-related LH pulse frequency was defined for an individual as the number of LH pulses during a sleep period divided by the total sleep period (h).
For subjects with PSG data for two overnight admissions, only the first admission was used for PSG-related summary statistics.
Sleep efficiency is defined here as the percentage of total sleep period occupied by non-wake epochs. To convert metric units to SI units: total testosterone (ng/dl) × 0.0347 (nmol/l); free testosterone (pg/ml) × 3.467 (pmol/l); insulin (μIU/ml) × 7.175 (pmol/l); glucose (mg/dl) × 0.0555 (mmol/l); estradiol × 3.671 (pmol/l); progesterone × 3.18 (nmol/l). Abbreviations: NS, (statistically) nonsignificant (i.e., p > 0.05); PCOS, polycystic ovary syndrome; PSG, polysomnography; REM, random eye movement.
Study procedures
Volunteers underwent detailed screening procedures to exclude significant hormonal and health-related abnormalities, as previously described [9]. Study participants had taken no medications known to affect the reproductive axis in the 90 days prior to study.
Overnight admissions occurred in the context of two ongoing clinical research studies:
is a randomized cross-over study designed to assess the acute effects (on LH pulsatility) of single-dose progesterone vs. placebo in women pretreated with transdermal estradiol (0.2 mg/day) for three days. In this study, overnight sampling and PSG occurred before progesterone or placebo administration (at 0600 h), but transdermal estradiol was continued throughout admissions. Primary analyses in normal women were recently reported [10]. For the current analysis, we only included admissions for which PSG data were available: 12 admissions in 8 normal women, 5 admissions in 4 women with PCOS. Also of note, since the current analysis was restricted to nighttime hours (i.e., before progesterone or placebo administration), sleep-related data from admissions involving later progesterone administration were not excluded from analysis.
is a randomized cross-over study to assess the effect of flutamide vs. placebo pretreatment on wake- and sleep-related LH pulse frequency. Given the potential confounding effect of flutamide, we only analyzed data from admissions preceded by placebo administration and for which PSG data were available (n = 3 PCOS).
Inclusion of PSG in the aforementioned studies related to a pre-planned sub-analysis of the relationships between sleep architecture and LH pulse initiation. Although these studies are ongoing, we are no longer performing PSG in these protocols.
Overnight studies were performed between cycle days 7 and 11 (inclusive) in normally-cycling women, and at least cycle day 7 in PCOS. Plasma progesterone concentrations were confirmed to be less than 1 ng/ml during each overnight study. All admissions involved frequent blood sampling through an indwelling intravenous catheter for later hormone measurement as follows: LH every 10 minutes; progesterone every 30 minutes () or 2 hours (); FSH, estradiol, and testosterone every 2 hours. Frequent blood sampling occurred for 24 hours (2000-2000 h; ) or 15 hours (1500-0700 h; ).
Lights were extinguished, and participants were encouraged to sleep, from 2200 to 0600 h () or from 2300 to 0700 h (). Overnight PSG was performed using conventional techniques, including electroencephalograms, electrooculograms, and submental electromyograms. Sleep stage was visually scored in 30 second epochs by a registered polysomnography technician (HGB) according to the American Academy of Sleep Medicine Scoring of Sleep and Associated Events version 2.1.0 (http://www.aasmnet.org/scoringmanual/v2.1.0/). Sleep stage designations were reviewed in detail and validated by a single physician board-certified in sleep medicine (PMS). Both investigators were blinded to subject group and LH pulse characteristics.
Hormone assays
All hormone assays were performed by the Ligand Assay and Analysis Core (LAAC) of the Center for Research in Reproduction at the University of Virginia. Samples from individual study participants were analyzed in duplicate in the same assay run for each hormone. LH was measured by chemiluminescence (Siemens Healthcare Diagnostics, Los Angeles, CA; LAAC- derived assay characteristics: sensitivity 0.1 IU/liter; intra-assay coefficient of variation [CV] 3.3%; interassay CV 5.8%). Other LAAC assay procedures and characteristics have been reported previously [9,17].
Data and statistical analysis
As our primary analysis, we compared sleep stages during and preceding LH pulses vs. randomly-determined time points, as described by Hall and colleagues [6]. Our a priori hypothesis for both study groups: compared to random time points, LH pulse onsets are (a) more likely to occur during and to be preceded by wake epochs and (b) less likely to occur during and to be preceded by REM epochs.
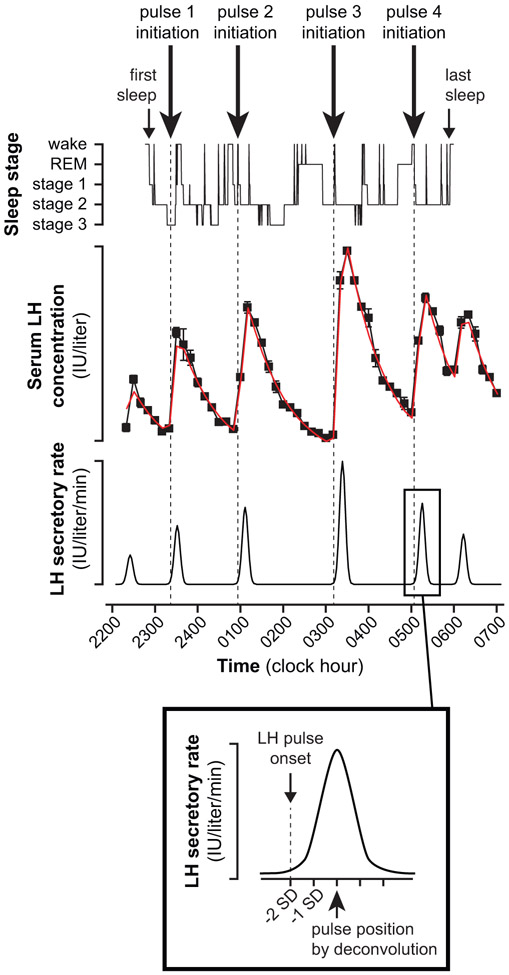
Each subject’s PSG records were synchronized to her LH time series (Figure 1). For each LH concentration time series, we employed a computerized data reduction protocol (StdCurve) to establish statistically accurate estimates for experimental LH measurement error, and pulsatile LH secretion was characterized using deconvolution (AutoDecon) [10,18]. The temporal location of LH pulse initiation was assigned as two secretory standard deviations (SD) before the time of peak LH secretion rate (Figure 1). To limit false positives, we excluded AutoDecon-identified pulses that did not achieve a 20% increment between the preceding nadir and at least 2 points at the peak. We also excluded pulses occurring before sleep initiation, after final sleep termination, or that did not have at least one non-wake epoch in the preceding 15 minutes.
Figure 1. Example of aligned LH and PSG data.
In deconvolution, the rate of LH secretion from the anterior pituitary during a pulse is assumed to exhibit a Gaussian pattern; and deconvolution results include the pulse position—the center of the underlying secretory event (i.e., time of peak LH secretion rate)—and a secretory standard deviation (SD). The precise timing of sleep-associated LH pulse initiation (denoted by dashed vertical lines) was defined as two secretory SDs before pulse position.
For every sleep-related LH pulse identified, we determined 3 random time points for the same admission. Random numbers were generated using the RANDBETWEEN function of the Excel® spreadsheet program (Microsoft Corporation, Redmond, WA), with the lowest possible whole number corresponding to the time of initial “lights out,” and a first decimal place allowing placement into one of two 30-second sleep epochs per clock minute. The following random points were excluded: those occurring before first sleep initiation or after final sleep termination, those without at least one non-wake epoch in the 15 minutes prior, and those located within 15 minutes of an LH pulse.
In keeping with the analysis of Hall et al. [6], we combined sleep stages 1 and 2, yielding four sleep stages: wake, REM, non-REM (NREM) stages 1+2, and NREM stage 3. We identified the instantaneous sleep stage corresponding to each LH pulse and random time point. For each subject group, we tested whether pulses and random points were proportionally partitioned across the four sleep stages using Fisher’s exact testing for a 2 (pulse vs. random) x 4 (wake, REM, NREM 1+2, NREM 3) contingency table. We then tested whether pulses and random points were differentially associated with each sleep stage via chi-square or Fisher’s exact tests for a 2 (pulse vs. random) x 2 (sleep stage A vs. not sleep stage A) contingency table.
We then identified the proportion of each sleep stage occurring in three 5-minute time blocks (0-5, 5-10, and 10-15 minutes) preceding each pulse and random point. For each time block, we compared (a) the proportion of each sleep stage preceding LH pulses to (b) the proportion of the corresponding sleep stage preceding random points using Wilcoxon rank sum (two-sample) tests.
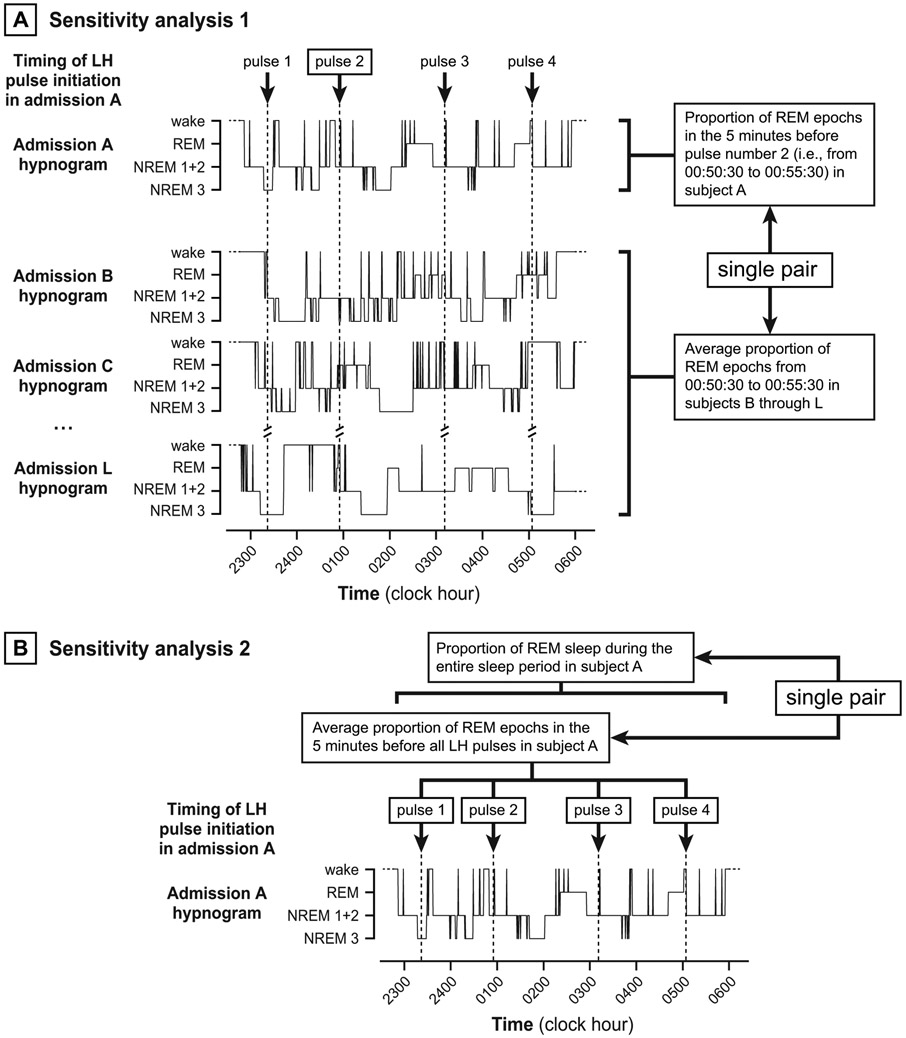
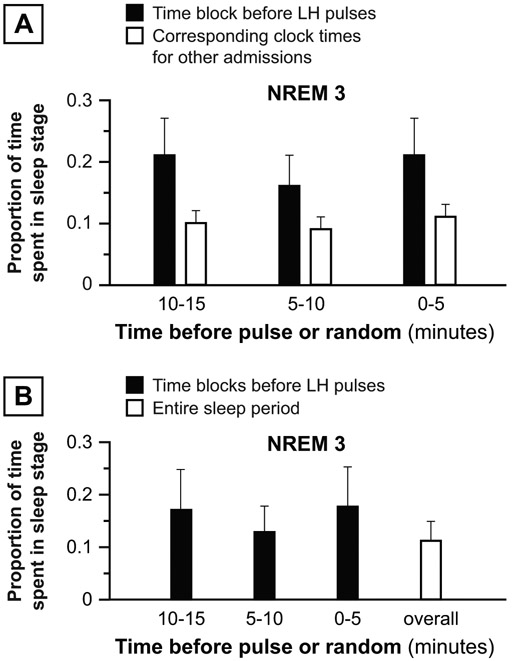
We performed two sensitivity analyses to explore the robustness of positive findings from our primary analyses. In the first, we paired (a) sleep stage proportions before each LH pulse to (b) average corresponding sleep stage proportions during the same clock times for all other admissions (Figure 2A). We restricted this analysis to clock times after which all subjects had initiated sleep and before which no subject had finally terminated sleep. These paired data were analyzed for consistent differences using Wilcoxon signed rank tests. In the second sensitivity analysis, we paired (a) average sleep stage proportions before all LH pulses during an individual admission to (b) corresponding sleep stage proportions from first to last sleep during the same admission (Figure 2B). These paired data were analyzed using Wilcoxon signed rank tests.
Figure 2. Depiction of sensitivity analyses.
Panel A. In the first sensitivity analysis, we paired (a) sleep stage proportions before each LH pulse to (b) average sleep stage proportions during the same time block for all other admissions. For each group (normal, PCOS), paired data were analyzed for consistent differences using Wilcoxon signed rank tests. Panel B. In the second sensitivity analysis, we paired (a) the average proportion of sleep stages before all LH pulses during an admission to (b) the proportion of sleep stages occupying the entire sleep period (same admission). For each group (normal, PCOS), paired data were analyzed for consistent differences using Wilcoxon signed rank tests.
Lastly, we assessed differences between the normal and PCOS groups. We used exact Wilcoxon rank sum (two-sample) tests to compare (normal vs. PCOS) estimated LH pulse frequencies for each sleep stage, calculated for each admission as the number of sleep-related LH pulses instantaneously associated with a sleep stage divided by the total time (in hours) spent in that sleep stage. We also used exact Wilcoxon rank sum (two-sample) tests to compare (normal vs. PCOS) “normalized” sleep stage proportions before LH pulses (i.e., differences between [a] average sleep stage proportion 0-5 minutes before all sleep-related LH pulses in an admission and [b] corresponding sleep stage proportions for the entire sleep period). For subjects with two overnight admissions, results were calculated for each admission and averaged.
All statistical analyses involved nonparametric tests, which are based on ranks of observations and require no assumptions about underlying data distribution. All hypothesis tests were two-sided, conducted at the 0.05 level of significance, and performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). We did not correct for multiple comparisons because, in most cases, related comparisons were not independent. For example, changes in the percentage of one sleep stage cannot occur independently of changes in the percentage of other sleep stages; and sleep stage epochs in close temporal proximity are commonly correlated. Instead, we provide precise p-values when less than 0.05.
RESULTS
Normally-cycling, normoandrogenic women
Overnight admissions in normal women occurred on cycle days 9.6 ± 1.6 (10 [8.5-11]), expressed as mean ± SD (median [interquartile range]). The number of sleep-associated pulses per admission was 3.3 ± 1.2 (3 [2-4]). Total sleep period (time from first to last sleep), sleep efficiency (percentage of total sleep period occupied by non-wake epochs), and proportions of time spent in each sleep stage (time in sleep stage divided by total sleep period) are shown in Table 1.
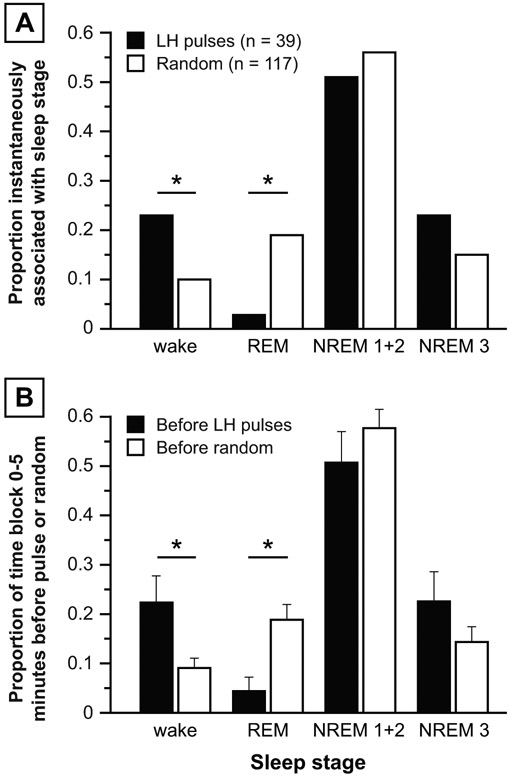
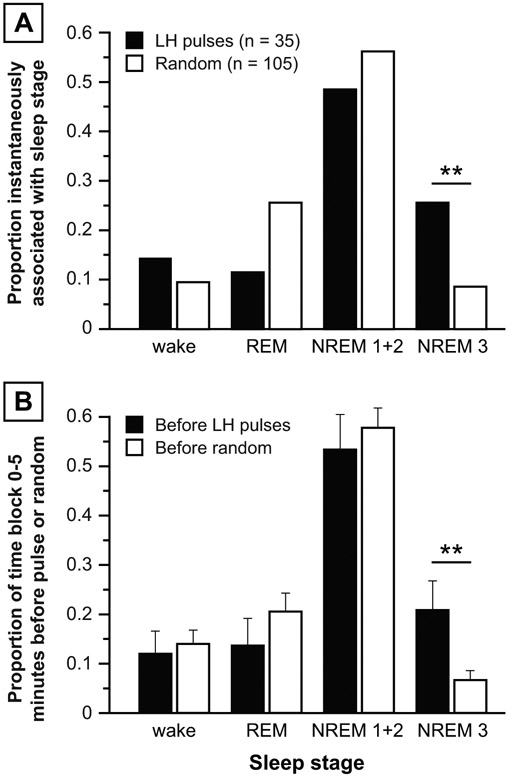
Pulses and random time points were not proportionally partitioned across the four sleep stages (p = 0.0132). Compared to random time points, LH pulses were more likely to be initiated during wake epochs (9 of 39 pulses vs. 12 of 117 random points; p = 0.0422) but less likely to be initiated during REM epochs (1 of 39 pulses vs. 22 of 117 random points; p = 0.0164) (Figure 3A). No differences were observed for NREM 1+2 or NREM 3.
Figure 3: Primary analyses, normal women.
Panel A: Proportion of LH pulses (solid bars) vs. random time points (open bars) instantaneously associated with various sleep stages, calculated as the number of LH pulses (or random time points) instantaneously associated with a sleep stage divided by the total number of LH pulses (or random time points). Panel B: Proportion of time frame 0-5 minutes before LH pulses (solid bars) vs. random time points (open bars) occupied by each sleep stage. Data are expressed as mean ± standard error of the mean. * p < 0.05.
Compared to the 5 minutes preceding randomly-determined time points, the 5 minutes preceding LH pulse initiation contained a higher percentage of wake epochs (22.3 vs. 9.1%; p = 0.0111) and a lower percentage of REM epochs (4.4 vs. 18.8%; p = 0.0162), with no significant differences for NREM 1+2 or NREM 3 (Figure 3B). No sleep stage differences were demonstrable in the 5-10 or 10-15 minutes before LH pulses vs. random time points.
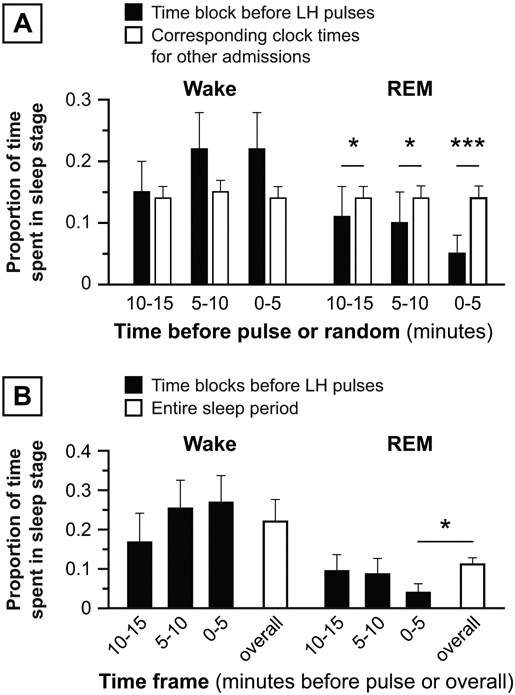
In the first sensitivity analysis, the proportion of REM sleep was significantly lower 0-5, 5-10, and 10-15 minutes before LH pulses compared to the proportion of REM sleep in corresponding clock times for all other subjects (5 vs. 14% [p = 0.0003], 10 vs. 14% [p = 0.0184], and 11 vs. 14% [p = 0.0171], respectively) (Figure 4A). In the second sensitivity analysis, the average proportion of REM sleep in the 0-5 minutes before sleep-related LH pulses during an admission was less than the overall proportion of REM during the same sleep periods (4 vs. 11% [p = 0.0161]) (Figure 4B). Differences in wake proportions were not statistically significant for either sensitivity analysis.
Figure 4: Sensitivity analyses, normal women.
Panel A: First sensitivity analysis: Proportion of sleep stage before LH pulse initiation (solid bars) vs. average proportion of the same sleep stage occurring at the same clock times in other admissions in normal subjects (open bars). Panel B: Second sensitivity analysis: Average proportion of sleep stage before all LH pulses in an admission (solid bars) vs. proportion of the same sleep stage during the same admission’s sleep period. Data are presented as mean ± standard error of the mean. * p < 0.05; *** p < 0.001.
Women with PCOS
Overnight admissions occurred on cycle days 41.6 ± 36.5 (33.5 [12-61]). The number of sleep-associated pulses per admission was 4.4 ± 1.1 (4.5 [3.5-5]). Total sleep period, sleep efficiency, and proportions of time spent in each sleep stage are shown in Table 1.
LH pulses and random time points were not proportionally partitioned across the four sleep stages (p = 0.0301). Compared to random time points, LH pulses were more likely to be initiated during NREM 3 epochs (9 of 35 pulses vs. 9 of 105 random points; p = 0.0087), with no differences for wake, REM, or NREM 1+2 (Figure 5A). Although Figure 5A suggests that, compared to random time points, LH pulses were less likely to occur during REM epochs (4 of 35 pulses vs. 27 of 105 random points), the difference was not significant (p = 0.1005 by Fisher’s exact test).
Figure 5: Primary analyses, women with PCOS.
Panel A: Proportion of LH pulses (solid bars) vs. random time points (open bars) instantaneously associated with various sleep stages. Panel B: Proportion of time frame 0-5 minutes before LH pulses (solid bars) vs. random time points (open bars) occupied by each sleep stage. Data are presented as mean ± standard error of the mean. ** p < 0.01.
Compared to the 5 minutes before randomly-determined time points, the 5 minutes preceding LH pulse initiation contained a higher percentage of NREM 3 (20.9 vs. 6.7%; p = 0.0089) (Figure 5B). Similar differences were demonstrable in the 10-15-minute time block (19.4 vs. 7.1%; p = 0.0392). No differences were observed during any time frames for wake, REM, or NREM 1+2 sleep. In sensitivity analyses, NREM 3 was not demonstrably more common before LH pulses (Figure 6).
Figure 6: Sensitivity analyses, women with PCOS.
Panel A: First sensitivity analysis: Proportion of sleep stage before LH pulse initiation (solid bars) vs. average proportion of the same sleep stage occurring at the same clock times in other subjects with PCOS (open bars). Panel B: Second sensitivity analysis: Average proportion of sleep stage before all LH pulses in an admission (solid bars) vs. proportion of the same sleep stage during the same admission’s sleep period. Data are presented as mean ± standard error of the mean.
Normal women vs. women with PCOS
Compared to normal women, women in the PCOS group were older and demonstrated higher BMI, higher total and free testosterone, and higher fasting insulin (Table 1). Overnight admissions also occurred on later cycle days in women with PCOS (41.6 ± 36.5 vs. 9.6 ± 1.6; p = 0.0171).
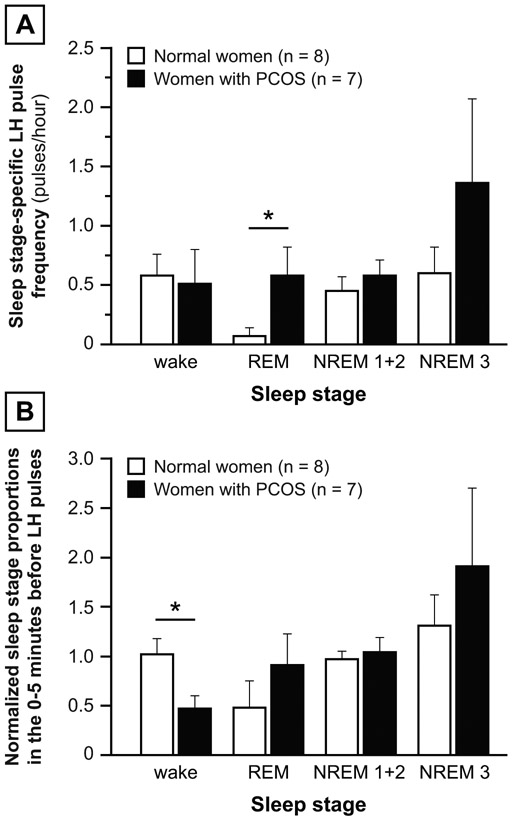
Sleep-related LH pulse frequency was higher in PCOS compared to controls (0.65 ± 0.13 vs. 0.46 ± 0.15 pulses/hour; p = 0.0401). As shown in Figure 7A, REM-associated LH pulse frequency was higher in women with PCOS compared to normal women (p = 0.0443), but other sleep stage-associated LH pulse frequencies were similar. The normalized proportion of wake epochs 0-5 minutes before LH pulses was lower in women with PCOS compared to normal women (p = 0.0205; Figure 7B), but no other group differences were demonstrable.
Figure 7: Normal vs. PCOS.
Panel A: LH pulse frequency for a given sleep stage was calculated as the number of LH pulses instantaneously associated with the sleep stage divided by the percentage of total sleep period (in hours) occupied by that sleep stage. For women studied during two overnight admissions, sleep stage-associated LH pulse frequencies were calculated for both admissions and results averaged. Panel B: “Normalized” sleep stage proportions in the 0-5 minutes before LH pulses, defined as the average sleep stage proportion 0-5 minutes before all sleep-related LH pulses in an admission divided by the corresponding sleep stage proportion for the sleep period. Both panels: For subjects with two overnight admissions, results were calculated for each admission and averaged. Data are presented as mean ± standard error of the mean. * p < 0.05.
Post hoc analyses
Since normal and PCOS groups differed in a number of ways, we performed simple and partial correlation procedures to assess whether specific variables associated with the PCOS group in our study (i.e., age, BMI, testosterone, insulin, cycle day) correlated with (a) REM-associated LH pulse frequency or (b) normalized wake epochs 0-5 minutes before LH pulses. For subjects with two overnight admissions, admission-specific results were averaged prior to analysis; accordingly, all correlation analyses involved 15 observations (8 normal women, 7 PCOS).
REM-associated LH pulse frequency was positively correlated with total testosterone (rs = 0.65; p = 0.0092), free testosterone (rs = 0.57; p = 0.0280), and fasting insulin (rs = 0.62; p = 0.0143), but not age, BMI, or cycle day. After correcting for all other PCOS group-related variables (partial correlation), REM-associated LH pulse frequency was independently predicted by total testosterone (rs = 0.84; p = 0.0014), fasting insulin (rs = 0.62; p = 0.0432), and, although not reaching statistical significance, free testosterone (rs = 0.57; p = 0.0660). Age, BMI, and cycle day were not independent predictors of REM-associated LH pulse frequency.
The normalized proportion of wake epochs 0-5 minutes before LH pulses was negatively correlated with total testosterone (rs = −0.53; p = 0.0428), free testosterone (rs = −0.63; p = 0.0127), BMI (rs = −0.55; p = 0.0323), and fasting insulin (rs = −0.58; p = 0.0237), but not age or cycle day. However, none of the PCOS group-related variables was an independent predictor of the normalized proportion of wake epochs 0-5 minutes before LH pulses.
Lastly, one subject with PCOS had obstructive sleep apnea, and we repeated analyses after exclusion of this subject. These analyses produced very similar results. For example, compared to the 5 minutes before randomly-determined time points, the 5 minutes preceding LH pulses contained a higher percentage of NREM 3 epochs in PCOS: 19.3% before pulses and 4.6% before random points (p = 0.0085). Also, REM-associated LH pulse frequency was higher in women with PCOS compared to normal women: 0.678 ± 0.262 vs. 0.071 ± 0.071, respectively (p = 0.0430). These analyses are described in detail in Supplemental Materials.
DISCUSSION
Our primary analyses suggested that, in normal women assessed in the mid- to late follicular phase, LH pulses were (a) more likely than randomly-determined time points to occur during and to be preceded by wake epochs and (b) less likely than randomly-determined time points to occur during and to be preceded by REM epochs. In addition to providing new information about the later follicular phase, our analysis helps to corroborate findings in the early follicular phase [6]. Our sensitivity analyses suggested that the negative relationship between REM sleep and LH pulses was more robust than the positive relationship between brief awakenings and LH pulses. However, our decision to exclude LH pulses without at least one non-wake sleep epoch in the preceding 15 minutes could have partially masked a relationship between wake epochs and LH pulses.
Although we modeled our analysis after that of Hall et al. [6], our a priori plan involved minor methodological differences. Hall and colleagues used a validated modification of the Santen and Bardin pulse detection method, assigning LH pulse onset as the first time point after the nadir preceding the pulse (i.e., onsets were constrained to 10-minute intervals), and assigning associated sleep stage as the predominant sleep stage in the 10 minutes preceding the pulse. We employed multi-parameter deconvolution, which permitted assignment of (estimated) pulse onsets to individual 30-second sleep epochs. Also, when assigning sleep stages to time blocks, we represented each sleep stage in accordance with the proportion of time occupied by that sleep stage instead of employing the predominant (simple majority) sleep stage. We used the American Academy of Sleep Medicine Scoring of Sleep and Associated Events version 2.1.0 instead of the Rechtschaffen and Kales criteria; and we employed conservative nonparametric analyses instead of parametric statistical procedures (ANOVA). Despite these minor methodological differences, we observed remarkable concordance with the results of Hall and colleagues [6]; this increases our confidence in both sets of results.
To our knowledge, this is the first study to investigate the relationship between sleep architecture and LH (GnRH) pulse initiation in PCOS. Our primary analyses suggest that relationships are altered in PCOS, with LH pulses more likely than randomly-determined time points to occur during and to be preceded by slow wave sleep (SWS). Our analyses also suggest that REM sleep does not inhibit GnRH pulse initiation as effectively in PCOS compared to normal. For example, the negative relationship between LH pulses and REM sleep was highly robust in normal women, but no demonstrable relationship was observed in PCOS despite a similar number of analyzed pulses in each group (39 normal vs. 35 PCOS). Moreover, in direct group comparisons, women with PCOS exhibited higher REM-associated LH pulse frequency compared to normal women. Importantly, these putative abnormalities—SWS encouragement of pulse initiation and failure of REM sleep to inhibit pulse initiation—may contribute to high sleep-related GnRH pulse frequency in PCOS, which would be expected to enhance LH release, thus contributing to ovarian hyperandrogenemia and ovulatory dysfunction.
While the PCOS and control groups differed with regard to age, BMI, testosterone, insulin, and cycle day of study, post hoc analyses suggested that apparent differences in REM-associated LH pulse frequency related specifically to differences in testosterone and insulin concentrations. Of interest, results in our relatively young adult controls (aged 19.6 ± 2.7 years) were remarkably consistent with those in the older group of normal women (aged 28.6 ± 3.9 years) studied by Hall et al. [6]; this suggests that the relationship between sleep stages and LH pulse initiation does not change substantially across the third decade of life. Also, although obesity is associated with lower mean LH concentrations and lower LH pulse amplitude—largely a reflection of lower LH responses to GnRH stimulation and shorter gonadotropin half-lives—and obesity per se is not associated with alterations in LH pulse frequency [19-23]. Similarly, human data regarding the potential influence of insulin per se on LH release are inconclusive [24]. Although these findings imply that obesity and hyperinsulinemia are unlikely to affect the relationship between sleep stages and LH pulse initiation, published data do not directly address these possibilities.
Because sensitivity analyses did not corroborate the relationship between SWS and LH pulses in PCOS, and since no SWS-related differences were evident between groups (PCOS vs. normal), we believe that this finding requires further confirmation. Regardless, it is of interest that a similar relationship between SWS and LH pulse initiation has been described during puberty, a developmental phase marked by sleep-related increases in LH pulse frequency [13]. Early reports suggested that sleep-related pulses during puberty occur primarily during non-REM sleep [11,12]; and recent detailed work by Shaw and colleagues [14,15] disclosed a strong relationship between SWS and LH pulse initiation. Thus, instead of a negative relationship between SWS and LH pulse initiation observed in adult women during the early follicular phase, a positive relationship may exist in both early puberty and PCOS. Similarly, instead of a positive relationship between wake epochs and LH pulse initiation observed in adult women in the early and later follicular phases, no relationship or a negative relationship may exist in PCOS and early puberty, respectively. The negative relationship between REM sleep and LH pulse initiation is the most consistent finding in studies of normal adult and pubertal subjects, but it was not formally demonstrable in our subjects with PCOS.
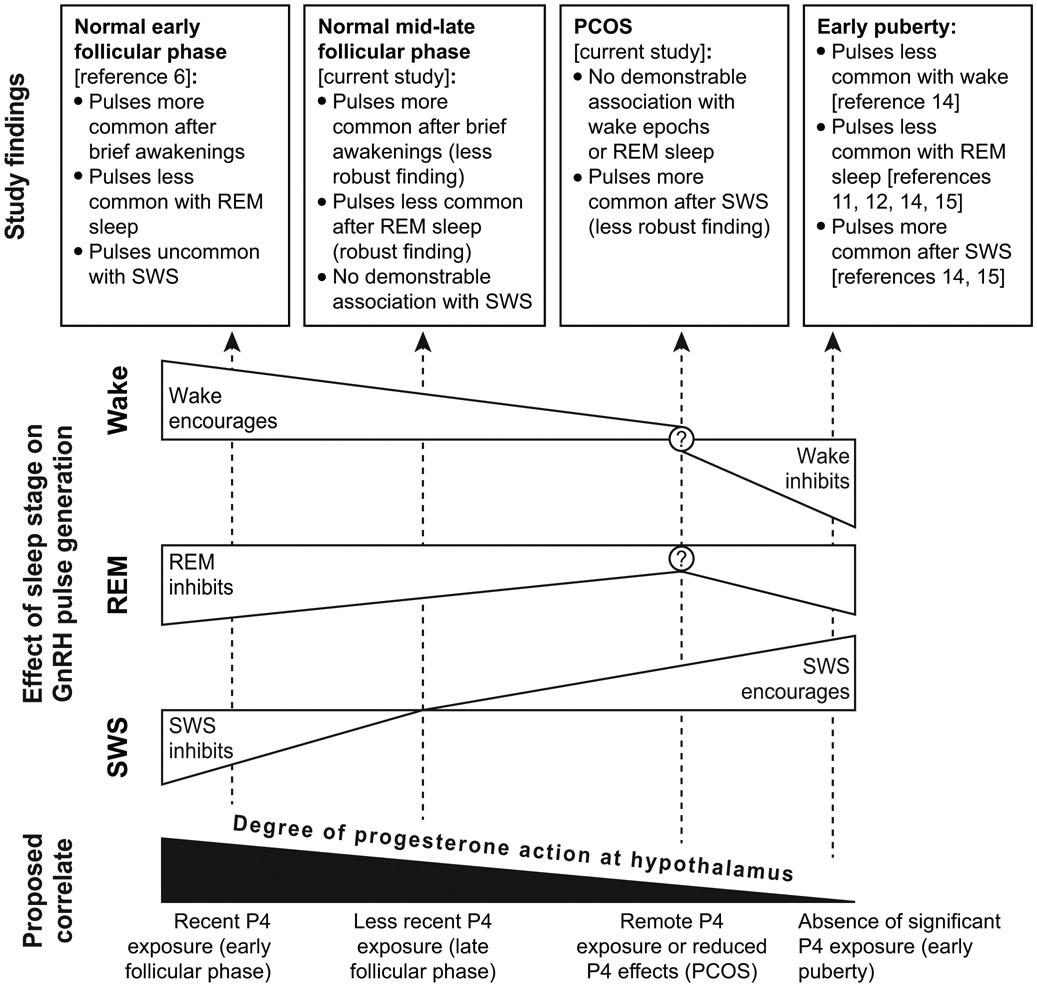
Although the reasons for these discordant findings are unclear, it is tempting to speculate that characteristics common to both puberty and PCOS (e.g., relative hyperandrogenemia, insulin resistance/hyperinsulinemia) could influence the interaction between sleep architecture and GnRH pulse initiation. Interactions between sleep stages and GnRH pulse initiation could also be impacted by recent progesterone exposure, which differed among normal women in the early follicular phase [6], our normal women in the mid- to late follicular phase (cycle days 9.6 ± 1.6), our women with PCOS (cycle days 41.6 ± 36.5), and early pubertal subjects (presumably minimal prior progesterone exposure) [14,15]. Further supporting this supposition, the negative feedback effects of progesterone on GnRH pulse frequency are mediated by hypothalamic opioids [25,26], and one study suggested that naloxone (an opioid-receptor antagonist) prevented the sleep-associated decrease in LH pulse frequency during the early follicular phase [3]. Our group has also published data suggesting that the increase in nocturnal LH pulse frequency across the follicular phase may reflect the gradual loss of the restraining effects of progesterone [27]; and a study of recently menarcheal girls assessed during the early to mid-follicular phase (cycle days 1-9)—to date published in abstract form only [28]—suggested that sleep-related LH pulse frequency was inversely related to degree of recent progesterone exposure. We speculate that lingering effects of recent progesterone action may influence sleep-related LH pulse frequency by altering the relationship between sleep architecture and LH pulse initiation (Figure 8). Note that relative hyperandrogenemia could also play a role in this regard since androgens antagonize the negative feedback effects of progesterone on the GnRH pulse generator [29,30]; and a potential role of hyperandrogenemia is provisionally supported in our data set by an independent association between testosterone and REM-associated LH pulse frequency. Alternatively, it remains possible that hyperandrogenemia more directly mitigates the ability of REM sleep to inhibit GnRH pulses. Although study involved androgen receptor blockade, the paucity of PCOS subjects with PSG data after flutamide administration (n = 2) precludes meaningful analysis.
Figure 8. Hypothetical model.
We speculate that progesterone action—including lingering effects of recent progesterone exposure—strengthens (a) the positive relationship between wake epochs and GnRH pulse initiation, (b) the negative relationship between REM sleep and GnRH pulse initiation, and (c) the negative relationship between slow wave sleep (SWS) and GnRH pulse initiation. In contrast, the absence of substantial progesterone action may be associated with partial or full reversal of these relationships. Instead of a negative relationship between SWS and GnRH pulse initiation, as observed in the early follicular phase (adults), a positive relationship between SWS and GnRH pulse initiation is observed in PCOS and early puberty. Similarly, instead of the positive relationship between wake epochs and GnRH pulse initiation observed in the early and late follicular phases (adults), no relationship or a negative relationship is observed in PCOS and early puberty, respectively. Other factors could also play important roles in the relationship between sleep architecture and GnRH pulse initiation. For example, hyperandrogenemia may antagonize the effects of progesterone on these relationships.
Limitations of our study include relatively small numbers of subjects, potentially affecting statistical power. This may explain why our sensitivity analyses did not formally support all relationships suggested by primary analyses, despite similar data trends. An additional potential limitation is that some study participants were pretreated with estradiol () while others were not (). However, estradiol pretreatment resulted in serum estradiol concentrations that were compatible with values in the mid- to late follicular phase. We believe that all other procedural differences ( vs. ) were minor and unlikely to represent confounders in this analysis. Also, our normal and PCOS groups differed in ways that may limit precise interpretation of apparent group differences. Although post hoc analyses suggested that group differences in primary endpoints are best explained by differences in testosterone and insulin concentrations, the other group differences (age, BMI, cycle day) remain important potential confounders. Lastly, our study was observational in nature (i.e., we did not perform an intervention), but it provided new information regarding sleep stage-LH pulse relationships in a different cycle stage and in a different subject population.
In conclusion, we provide data suggesting that sleep-related inhibition of LH pulse secretion during the mid- to late follicular phase is normally weakened by brief awakenings and strengthened by REM sleep—similar to previously-reported findings in the early follicular phase. In contrast, our data in PCOS suggest that REM does not appropriately inhibit LH pulse initiation; instead, sleep-related LH pulse initiation may be associated with slow wave sleep in PCOS—similar to findings in pubertal subjects. Although these letter findings require confirmation, such abnormalities may contribute to high sleep-related LH pulse frequency—and thus ovarian hyperandrogenemia and ovulatory dysfunction—in PCOS.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Lauren Lockhart and Anne Gabel for subject recruitment, study scheduling, and assistance with data management; the nurses and staff of the Clinical Research Unit at the University of Virginia for implementation of these sampling protocols; certified polysomnography technicians Gene Bush and Heather Bonner who performed polysomnography in these studies; and the Center for Research in Reproduction Ligand Assay and Analysis Core Laboratory for performance of all assays. Current affiliations: Christine Lu – Icahn School of Medicine at Mount Sinai, New York, NY; Eleanor G. Hutchens – Augusta Health Diabetes and Endocrinology Clinic, Fishersville, VA.
FUNDING
This work was supported by National Institutes of Health (NIH) Grants R01 HD058671 (CRM, PMS) and R01 HD058671-02S1 (CRM); the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54 HD28934 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (currently known as the National Centers for Translational Research in Reproduction and Infertility and funded via a P50 mechanism) (CRM); NIH DP3 DK106907 (LSF); and NIH Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant T32 DK07646 (EGH).
REFERENCES
- 1.Soules MR, Steiner RA, Cohen NL, Bremner WJ, Clifton DK. Nocturnal slowing of pulsatile luteinizing hormone secretion in women during the follicular phase of the menstrual cycle. J Clin Endocrinol Metab 1985; 61: 43–49. [DOI] [PubMed] [Google Scholar]
- 2.Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 1986; 62: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 3.Rossmanith WG, Yen SS. Sleep-associated decrease in luteinizing hormone pulse frequency during the early follicular phase of the menstrual cycle: evidence for an opioidergic mechanism. J Clin Endocrinol Metab 1987; 65: 715–718. [DOI] [PubMed] [Google Scholar]
- 4.Rossmanith WG, Liu CH, Laughlin GA, Mortola JF, Suh BY, Yen SS. Relative changes in LH pulsatility during the menstrual cycle: using data from hypogonadal women as a reference point. Clin Endocrinol (Oxf) 1990; 32: 647–660. [DOI] [PubMed] [Google Scholar]
- 5.Rossmanith WG, Lauritzen C. The luteinizing hormone pulsatile secretion: diurnal excursions in normally cycling and postmenopausal women. Gynecol Endocrinol 1991; 5: 249–265. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 2005; 90: 2050–2055. [DOI] [PubMed] [Google Scholar]
- 7.Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab 1994; 78: 910–915. [DOI] [PubMed] [Google Scholar]
- 8.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 2003; 88: 297–311. [DOI] [PubMed] [Google Scholar]
- 9.McCartney CR, Blank SK, Marshall JC. Progesterone acutely increases LH pulse amplitude but does not acutely influence nocturnal LH pulse frequency slowing during the late follicular phase in women. Am J Physiol Endocrinol Metab 2007; 292: E900–906. [DOI] [PubMed] [Google Scholar]
- 10.Hutchens EG, Ramsey KA, Howard LC, Abshire MY, Patrie JT, McCartney CR. Progesterone has rapid positive feedback actions on LH release but fails to reduce LH pulse frequency within 12 h in estradiol-pretreated women. Physiol Rep 2016. 4(16) pii: e12891. doi: 10.14814/phy2.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 1972; 287: 582–586. [DOI] [PubMed] [Google Scholar]
- 12.Kapen S, Boyar RM, Finkelstein JW, Hellman L, Weitzman ED. Effect of sleep-wake cycle reversal on luteinizing hormone secretory pattern in puberty. J Clin Endocrinol Metab 1974; 39: 293–299. [DOI] [PubMed] [Google Scholar]
- 13.McCartney CR. Maturation of sleep-wake gonadotrophin-releasing hormone secretion across puberty in girls: potential mechanisms and relevance to the pathogenesis of polycystic ovary syndrome. J Neuroendocrinol 2010; 22: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw ND, Butler JP, McKinney SM, Nelson SA, Ellenbogen JM, Hall JE. Insights into puberty: the relationship between sleep stages and pulsatile LH secretion. J Clin Endocrinol Metab 2012; 97: E2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw ND, Butler JP, Nemati S, Kangarloo T, Ghassemi M, Malhotra A, Hall JE. Accumulated deep sleep is a powerful predictor of LH pulse onset in pubertal children. J Clin Endocrinol Metab 2015; 100: 1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 2012; 77: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 2009; 94: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem 2008; 381: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 1997; 82: 2248–2256. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 1997; 82: 3728–3733. [DOI] [PubMed] [Google Scholar]
- 21.Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab 2006; 91: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 22.Srouji SS, Pagan YL, D'Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab 2007; 92:1347–1352. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 2007; 92: 2468–2473. [DOI] [PubMed] [Google Scholar]
- 24.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33: 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metab 1985; 60: 34–36. [DOI] [PubMed] [Google Scholar]
- 26.Gindoff PR, Jewelewicz R, Hembree W, Wardlaw S, Ferin M. Sustained effects of opioid antagonism during the normal human luteal phase. J Clin Endocrinol Metab 1988; 66: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 27.McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC. Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab 2002; 87: 2194–2200. [DOI] [PubMed] [Google Scholar]
- 28.Shaw ND, Kangarloo T, Sluss PM, Adams JM, Chandler DW, Zava DT, Hall JE. Progesterone mediates sleep-specific slowing of LH pulses during the early follicular phase in early post-menarchal girls. In: Proceedings of the 98th Annual Meeting of the Endocrine Society Boston, MA: 2016. Abstract SAT-163, presented April 2, 2016; http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2016.RE.3.SAT-163 (accessed 1/4/2017). [Google Scholar]
- 29.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 2000; 85: 4047–4052. [DOI] [PubMed] [Google Scholar]
- 30.Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 2006; 147: 1474–1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.