Abstract
This tutorial review describes recent developments in catalytic reaction design that involve catalyst-promoted 1,2-metalate shifts as a critical part of the reaction mechanism. Issues pertaining to catalysis, stereoselectivity, and reaction mechanism are discussed.
Introduction
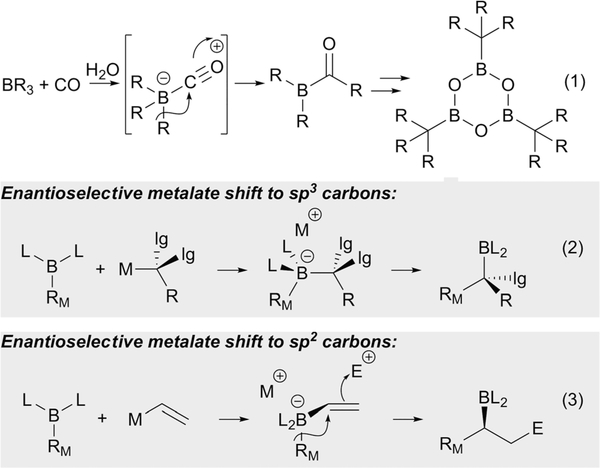
In 1962, M. Hillman at DuPont’s Experimental Station Laboratory described a reaction between trialkylboranes and carbon monoxide wherein coordination of CO to boron was followed by 1,2 migration of an alkyl group from boron to carbon (eqn (1), Scheme 1).1 Notably, the remaining B-alkyl groups also underwent migration - likely facilitated by the presence of water -such that a new isolable organoboron product was generated. This reaction proved to be the first example of what would turn out to be a broad range of organoboron homologation reactions, a class of transformation that establish a new C-C bond while retaining a versatile organoboron functional group in the product. A noteworthy aspect of many such homologation reactions is that they can be conducted in a stereoselective fashion and enable new methods for stereoselective synthesis. A particularly exciting direction surrounds homologation processes that can be facilitated by the influence of an external catalyst, especially when that catalytic entity can be employed to accomplish stereocontrolled transformations. Two such generalized strategies are depicted in Scheme 1. In the first approach (eqn (2)), organoboron compounds are treated with carbanions that bear enantiotopic leaving groups such that the migration step is stereochemistry-determining and can be influenced by an external activator. In the second approach (eqn (3)), a 1,2-metalate shift is facilitated by subjecting alkenylboron-derived ‘ate’ complexes to an external π-activator: if discrimination of the prochiral faces of the reacting alkene can be accomplished, multiple new opportunities for stereoselective synthesis of organoboron compounds can be enabled. Importantly, for all of the processes in Scheme 1, the configuration of the migrating carbon atom (RM) is retained throughout the course of the reaction. This Tutorial Review will cover the above described metalate-shift-based transformations, and will be restricted to those processes that operate in the context of catalytic methods. For the important stereoselective reactions that operate through the use of stoichiometric chiral reagents, the reader is referred to recent reviews.2
Scheme 1.
1,2-Metalate shifts involving organoboron compounds.
Catalytic metalate shifts involving migration to sp3 carbon atoms
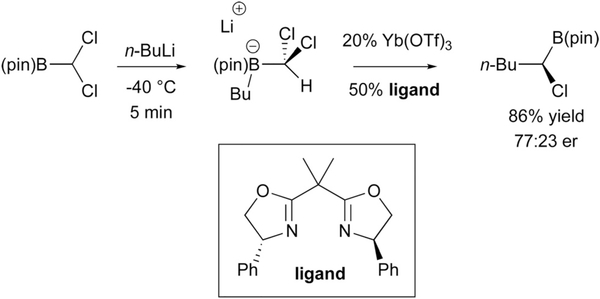
Stereoselective homologation by 1,2-metalate shift to sp3 carbons has been broadly developed, both with chiral diolato ligands on boron3 and through the use of enantiomerically-enriched homologation reagents comprised of organometallic compounds that bear an a leaving group.2 To accomplish enantioselective catalytic migration to an sp3 hybridized carbon atom requires differentiation of two enantiotopic leaving groups on the C(sp3) atom and this is not a trivial task. Hints that such a process might operate can be found in seminal studies by Matteson who found that with chiral pinanediol-derived boronic esters, ZnCl2 was required for high levels of stereoselection during homologation with dichloromethyllithium. It was proposed that Zn2+ engaged in bidentate chelation between a pinacol oxygen and a chloride leaving group.4 In 1997, Jadhav rendered such a process catalytic and enantioselective with the use of an external chiral Lewis acid catalyst that could indeed discriminate between the enantiotopic pro-R and pro-S chloride atoms of an in situ generated (dichloromethyl)-boron ‘ate’ complex (Scheme 2).5 This reaction afforded chiral α-chloroalkylboronic esters which are important intermediates for stereoselective synthesis.6 Of note, the chiral ytterbium salt used for this process could be employed in a substoichiometric fashion. While, there is still ample room for development of this type of process in terms of selectivity and catalyst efficiency, the groundwork has nonetheless been laid by Jadhav demonstrating that these reactions are possible.
Scheme 2.
Catalytic asymmetric homologation by selective halide displacement.
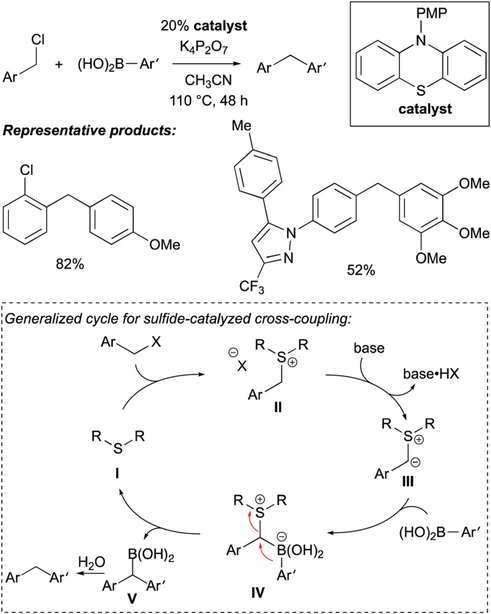
Huang and co-workers developed an organocatalytic cross-coupling reaction between arylboronic acids and benzylic chlorides. This process is catalyzed by organic sulfide compounds and is proposed to occur by a mechanism that involves a 1,2-metalate shift.7 As depicted in Scheme 3, when the benzylic halide substrates were treated with arylboronic acids in the presence of catalytic amounts of a sulfide catalyst and an inorganic base, benzhydryl derivatives were furnished as the reaction product. Influenced by seminal studies by Aggarwal8 on enantioselective homologation of trialkylboranes with chiral sulfur ylides, Huang and co-workers posit that the sulfide catalyst reacts with the benzylic chloride and base to form a sulfur ylide (I → II → III, Scheme 3). After the so-formed ylide coordinates to the three-coordinate boronic acid to give IV, a 1,2-metalate shift establishes a C-C bond and releases the sulfide catalyst. While the immediate product of the reaction is proposed to be a doubly benzylic boronic acid (V) which undergoes rapid protodeboronation and hence loss of a useful functional group, it is plausible that with alternate substrates, a more stable and isolable organoboronic acid derivative might be accessed.
Scheme 3.
Sulfide-catalyzed cross-coupling by a metalate-shift-based pathway.
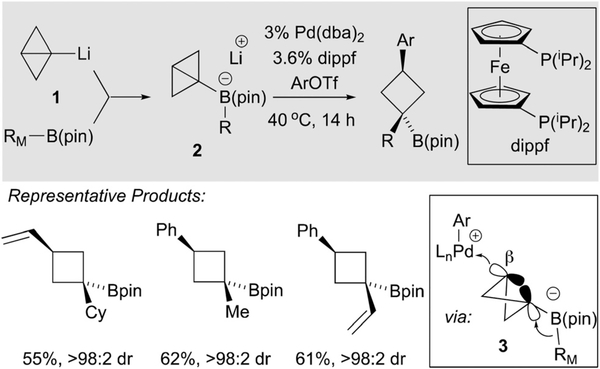
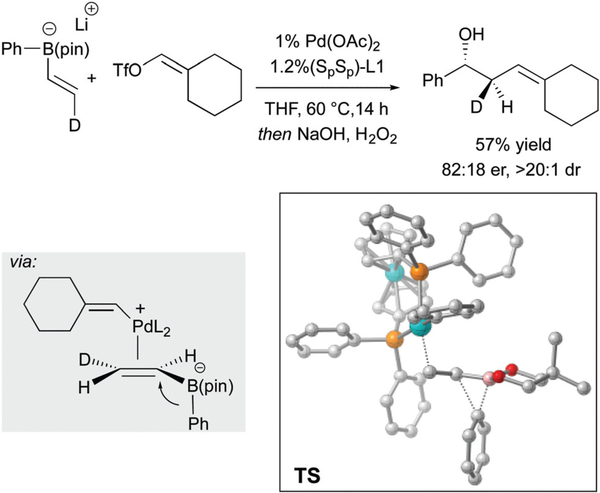
A recent report by Aggarwal describes a very different catalytic metalate shift that involves migration of a carbon from boron to an attached sp3-hybridized carbon atom.9 As depicted in Scheme 4, when borate complex 2 - a structure that is prepared from readily available organolithium 1 - was treated with 3 mol% of a palladium complex and organotriflate electrophiles, efficient conversion to cyclobutanes occurs. This process is made possible by the use of the bicyclo [1.1.0] butane motif that bears a ring strain of approximately 66 kcal mol−1. Importantly, the team found that triflate electrophiles were essential for the formation of cationic palladium that is a needed to facilitate the 1,2 migration reaction. On the basis of the stereochemical outcome of this process, it appears that subsequent to oxidative addition, an intermediate cationic Pd complex reacts at the β-carbon of the strained bicycle (3, Scheme 4). Unlike most transmetalation reactions of organoboronates, the α carbon in the bicycle of 12 is rendered inaccessible by the boron ligand framework. Instead, the authors propose Pd approaches from the backside of the β-carbon, taking advantage of the p-character at the atoms in the strained ring. While this pathway is plausible, it is none the less remarkable that direct transmetalation of the migrating group, resulting in Suzuki-Miyaura coupling of the RM group is not a competing side reaction.
Scheme 4.
Catalytic ring-opening of strained bicycles by 1,2-metalate shift.
Catalytic metalate shifts involving migration to sp and sp2 carbons
Background
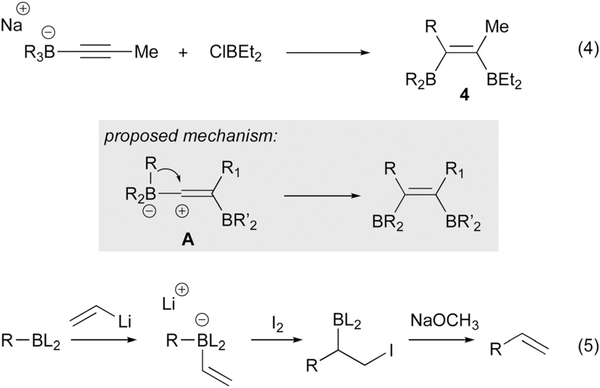
Association of alkenyl- and alkynylboron derived ‘ate’ complexes with external π-acidic electrophiles can prompt a 1,2-migration from boron to the adjacent unsaturated carbon atom. This mode of activation was first observed by Binger and Köster with main group electrophiles, specifically in the context of a chlorodiethylborane-induced rearrangement of alkynylborates (eqn (4), Scheme 5) that was postulated to occur by the mechanism depicted in A.10 Upon treatment of the vicinal diboron product 4 with acetic acid, the cis-alkene was isolated. This outcome secured the configurational assignment of organoboron 4 and suggests a metalate shift by anti addition of the electrophile and the migrating group across the olefin, a pathway that is followed by the preponderance of metalate rearrangements. Metalate shifts in alkenylborates were first proposed by Zweifel11 in iodine-promoted rearrangements of trialkylborane-derived substrates. These olefinations were expanded upon by Matteson12 and Evans13 for boronic ester derivatives. In these processes (eqn (5)), subsequent to iodine-prompted rearrangement, β-elimination generates an alkene-containing product. In addition to the examples in Scheme 5, a broad range of other main group electrophiles have been shown to promote the 1,2-metalate shift with unsaturated organoboron compounds.14
Scheme 5.
Early stoichiometric electrophile-induced metalate shifts involving unsaturated boron ‘ate’ complexes.
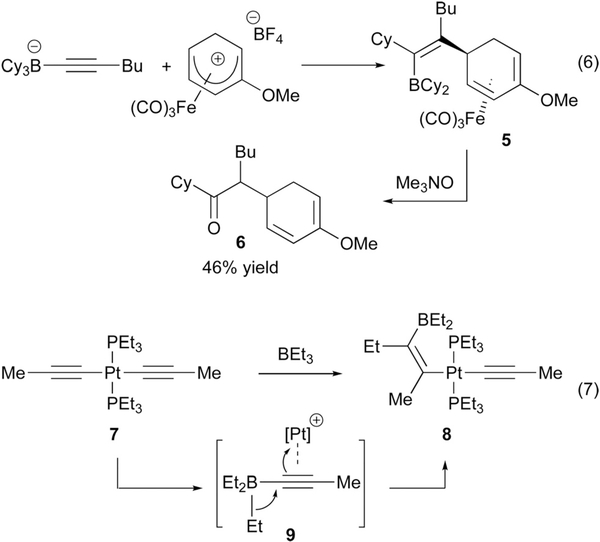
Catalytic reactions that involve 1,2-metalate shifts of alkenyl- and alkynylborates most often employ transition-metal-based complexes. With transition metal promotion, metalate rearrangements have been observed to occur through both inner sphere (direct binding of the metal to the alkene) and outer sphere (nucleophilic attack of the ‘ate’ complex on a metal-bound ligand) pathways. Precedent for the outer sphere path was provided by Pelter (eqn (6), Scheme 6) who found that an alkynylborate would react with a cationic ƞ5-dienyl iron complex by addition anti to the iron center, providing compounds such as 5 (Scheme 6).15 Treatment of 5 with excess trimethylamine N-oxide served to both oxidize the alkenylboron to a ketone as well as oxidatively decomplex iron from the diene, yielding 6. Precedent for inner sphere activation was first obtained by Wrackmeyer (eqn (7)), who found that treatment of platinum bis(acetylide) complex 7 with triethylborane resulted in formation of platinaborylalkene 8.16 This unusual rearrangement was proposed to occur by transmetalation of an acetylide from Pt to BEt3 to form an ‘ate’ complex, followed by activation of generated ‘ate’ complex by platinum cation (9); subsequent metalate shift generates the product alkenylplatinum complex.
Scheme 6.
Stoichiometric metalate shifts involving outer sphere and inner sphere addition to transition metal complexes.
Catalytic allylic substitutions involving metalate shifts
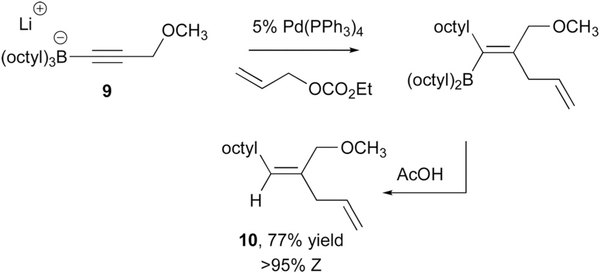
Reactions that occur by outer sphere attack of ‘ate’ complexes on metal-bound ligands comprise the first examples of catalysed reactions involving metalate shifts. In 1990, the Deng laboratory found that treatment of lithium trialkylalkynylborates such as 9 (Scheme 7) with allyl carbonate and 5 mol% Pd(PPh3)4 resulted in the stereoselective formation of borylated skipped dienes; these compounds could be subjected to protodeborylation with acetic acid to furnish the hydrocarbon 10.17 Generally, this process was found to occur with very high levels of stereocontrol, providing products derived from anti addition of the migrating group and Pd(allyl) complex across the alkyne.
Scheme 7.
Catalytic synthesis of skipped dienes by addition of an alkynylborate to a Pd(allyl) complex with concomitant metalate shift.
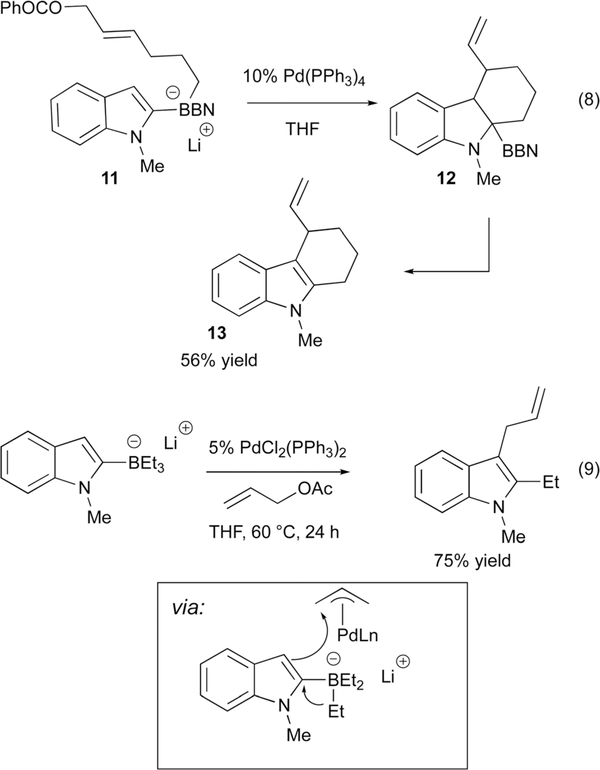
Beginning in 1991, Ishikura reported a series of studies involving indole-derived ‘ate’ complexes.18 In one example (Scheme 8), the transition metal catalyzed 1,2 metalate rearrangement from indole derived borate complex 11 onto a tethered Pd(allyl) complex provides ring-fused product 13 by way of 12. The proposed mechanism suggests initial oxidative addition of Pd(0) to generate a palladium π-allyl complex, which then induces a chemoselective 1,2 migration reaction. Notably, the primary alkyl group undergoes migration in preference to the 9-BBN carbon atoms. Also of note is that the migration reaction itself serves to temporarily dearomatize the indole nucleus providing an added barrier to the metalate shift, yet the reaction still proceeds efficiently. Subsequent to rearrangement, the alkyl BBN undergoes a deborylative re-aromatization process that likely requires an oxidant. In addition to the intramolecular examples represented by eqn (8), intermolecular addition to allyl acetate (eqn (9)) and addition to epoxy dienes,19 as well as and propargylic carbonates,20 were also developed.
Scheme 8.
Ishikura’s Pd-catalyzed addition of indole borates to allyl electrophiles.
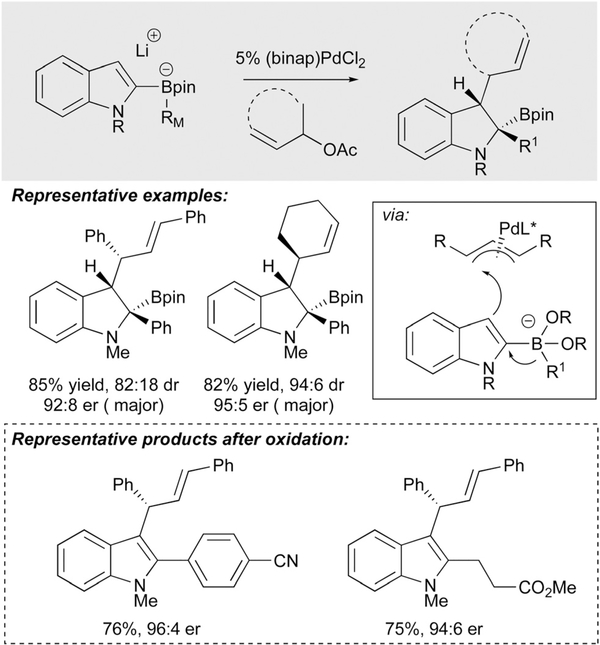
With precedent established for catalytic addition of indole-derived borates to Pd(allyl) complexes, Ready studied an asymmetric version of this reaction.21 Generation of three contiguous stereogenic centres in indoles and indolines is a desired but challenging one-step synthetic transformation. The three component coupling developed by Ready allows enantio-, regio- and diastereoselective palladium-catalyzed C-C bond formation at C-2 and C-3 of the indole in a single flask process (Scheme 9). This method provides a powerful inroad to pharmaceutically relevant motifs from a simple lithiated indole, a substituted allyl acetate electrophile, and an organoboron nucleophile. While the initial indoline product contains a boronic ester, oxidation provides a convenient route to the substituted indole derivative. The Ready group further applied this method to the synthesis of indolines with quaternary stereocenters at C3.21
Scheme 9.
Ready’s catalytic asymmetric coupling of indolylborates and allyl acetates.
Reaction of indolylborates with activated cyclopropanes
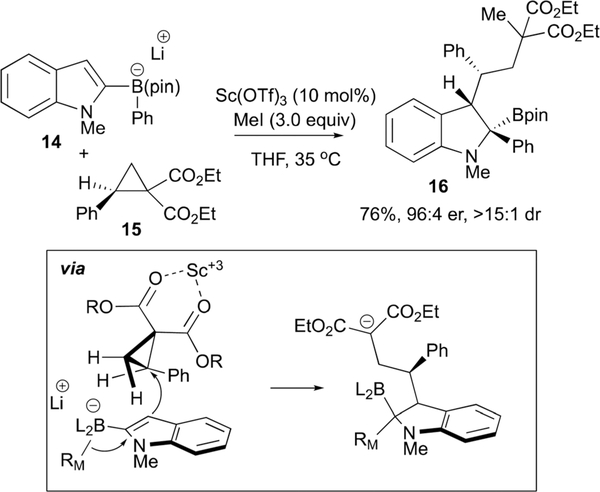
Studer expanded the scope of electrophiles that can activate 2-indolylboron ‘ate’ complexes to include donor-acceptor cyclopropanes.22 As depicted in Scheme 10, treatment of phenyl-substituted cyclopropane 15 with 10 mol% Sc(OTf)3 is sufficient to activate the strained ring for nucleophilic attack by indolyl- borate 14, thereby generating a substituted indoline containing a malonate anion; reaction of this species with alkyl halides delivers the reaction product 16 and likely facilitates turnover of the catalyst. Of note, this protocol allows the formation of three new C-C bonds in a highly diastereoselective fashion. By using a chiral donor-acceptor cyclopropane, the stereochemical course of the process could be followed and allowed a plausible mechanism to be proposed (inset, Scheme 10) that suggests the ring opening is stereospecific: the 1,2 migration occurs in an anti fashion with respect to the cyclopropane attack, and the cyclopropane substituent (Ph) is directed away from the large boron framework (Scheme 10). While stereoinduction with chiral substrates allows for construction of non-racemic products with this process, use of achiral cyclopropanes in conjunction with chiral Lewis acid catalysts would appear to be a plausible and exciting strategy for absolute stereochemical control in this reaction.
Scheme 10.
Studer’s catalytic stereoselective coupling of indolylborates and cyclopropanes by way of a metalate shift.
Catalytic activation of alkenylboronates by sulfenium ion transfer
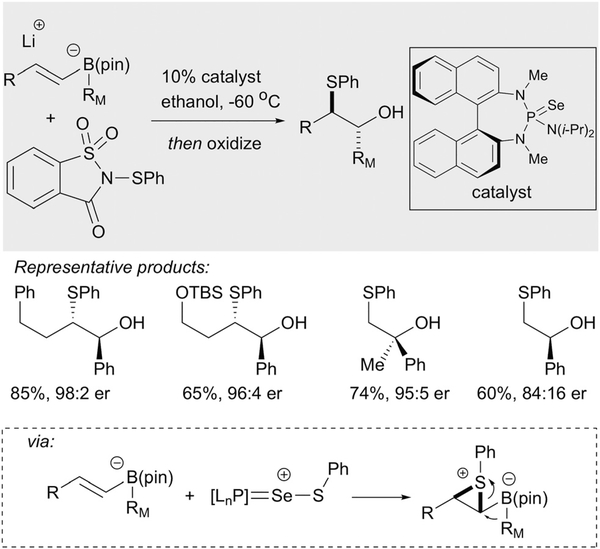
Recently, Denmark established an enantio- and diastereoselective Lewis base-catalyzed carbosulfenylation of alkenyl-boron compounds.23 In a creative reaction design, these authors first generated a series of prochiral alkenylboron-derived ‘ate’ complexes and then subjected them to catalytic stereoselective sulfenium ion transfer using chiral selenium-based catalysts the group had developed for sulfenofunctionalization reactions.24 In the context of alkenylboronates, reaction of the olefin with a cationic donor-acceptor complex results in selective formation of a thiiranium ion which undergoes stereospecific opening by a 1,2-metalate shift. This rearrangement applies to mono-di- and trisubstituted alkenylboron substrates and affords products with vicinal stereogenic centers in high levels of enantioselectivity (Scheme 11). Other than oxidation, the authors also develop an array of reactions that apply to the β-borylthioether products.
Scheme 11.
Denmark’s catalytic asymmetric metalate rearrangement through sulfenium ion catalysis.
Activation of alkynyltrialkylboronates with Pd complexes
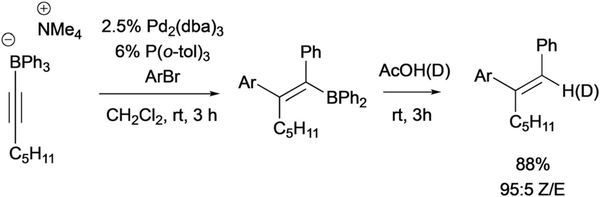
In 2007, Murakami developed the first system wherein a transition metal complex appeared to activate an unsaturated boronate for 1,2-metalate rearrangement by direct bonding between a metal and the substrate.25 In the example depicted in Scheme 12, an (aryl)Pd(II) complex, generated by oxidative addition between Pd(0) and an aryl halide, induced rearrangement of an alkynyl-trialkylborate, ultimately furnishing a trisubstituted (Z)-alkenylboron as the product. A variety of aryl migrating groups, and aryl bromide electrophiles were competent in the reaction, whereas aryl chlorides, and triflates were found to be poor substrates. Of note, the organoborane products underwent efficient protodeboronation to give trisubstituted alkenes in excellent yield and diastereoselectivity. This reaction was further developed to engage alkynyltrialkylborates with electrophilic ammonium salts, 2-bromo-pyridine-N-oxide, and allyl electrophiles.26
Scheme 12.
Murakami’s Pd-catalyzed coupling of alkynylborates and aryl bromides.
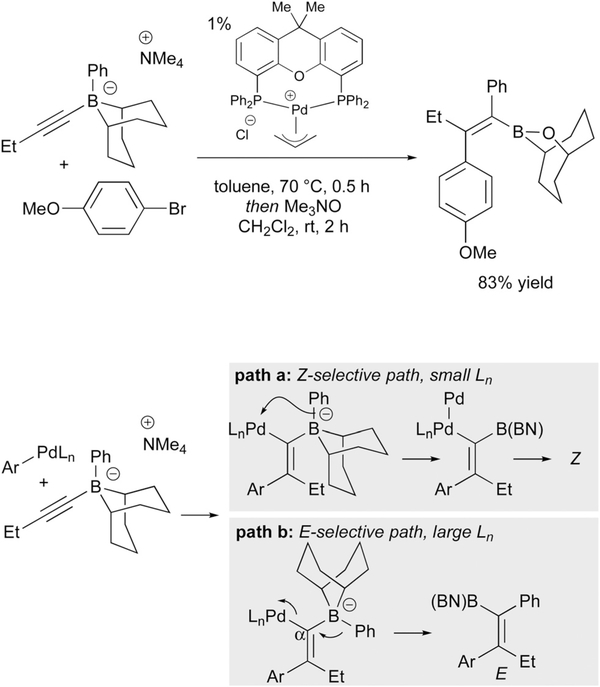
Later, the Murakami group reported the E-selective synthesis of trisubstituted alkenyl-9-BBN derivatives via a (xantphos)-palladium-catalyzed reaction of alkynylborates and aryl halides (Scheme 13).27 Mechanistically, the trans-addition product was proposed to arise via the mechanism in Scheme 13 (path B). Unlike other reactions involving a metal-catalyzed metalate shift, in this process a cationic (aryl)palladium complex is proposed to undergo carbopalladation of the alkyne. Subsequently, the aryl group migrates from boron to the α-carbon (path b) and reductively displaces Pd with inversion at the sp2 carbon, thereby giving net anti addition of the electrophile and migrating group across the alkyne. The stereochemical outcome was largely dependent on the ligand: with less encumbered tri(o-tolyl)phosphine, 1,3-migration of the phenyl group from boron to palladium (path a) thereby delivering the Z isomer depicted in Scheme 12. In contrast, with xantphos, a bulky bidentate phosphine ligand, transmetallation is inhibited and 1,2-migration of the phenyl group onto the α carbon occurs, resulting in the E product. In terms of the migrating groups, electron rich and electron poor arenes could participate. Electron rich and electron deficient aryl halide electrophiles were also competent in the reaction. A wide range of functional groups such thiophenyl, silyl ether, ester, and phthalimide were tolerated in the reaction.
Scheme 13.
Murakami’s Pd-catalyzed coupling of alkynyl-9-BBN ‘ate’ complexes with aryl bromides, and the role of ligand.
Activation of alkenylboronic ester-derived ‘ate’ complexes with Pd: conjunctive cross-coupling
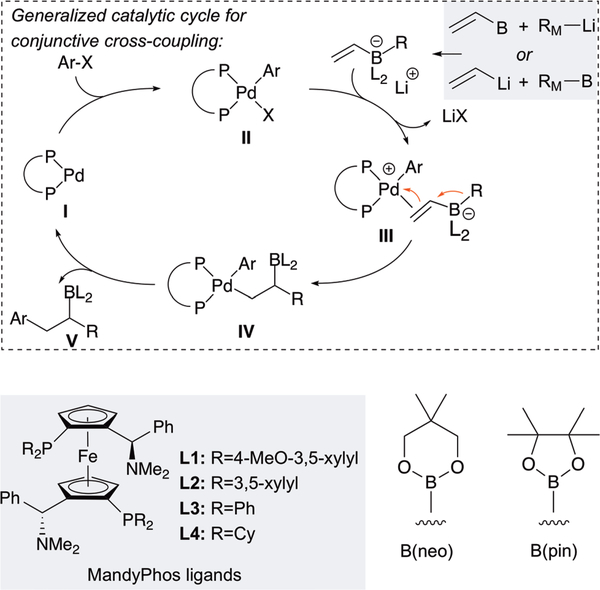
In 2015, the Morken group began investigating the use of transition metal complexes to activate alkenylboron-derived ‘ate’ complexes. These studies would lead to the first examples of catalysis of metalate shifts involving direct activation of the π system by metal-π bonding, and also provide the first examples of enantioselective processes. Mechanistically, it was considered that after oxidative addition of a transition metal to an electrophile (I → II, Scheme 14), if the metal complex could activate the alkenylboronate for a 1,2-metalate shift (III → IV), reductive elimination would generate C-C coupled product V. The overall process has been termed “conjunctive cross-coupling” to indicate the merger of two ostensibly nucleophilic reagents (an organoboron and an organolithium), during the course of the reaction. In regards to catalysis, to facilitate olefin binding to complex II, aryl triflates were employed as electrophiles and this choice would prove to be critical: in the absence of specially-chosen additives, as little as 1 mol% halide arising from either the catalyst, the electrophile, or during the synthesis of ‘ate’ complexes, leads to significant inhibition of the reaction. A similarly critical choice was the decision to employ wide bite-angle bidentate ligands for palladium: this selection was made in an effort to speed reductive elimination relative to β-hydrogen elimination from intermediate IV, and so far, only these ligands, particularly the MandyPhos28 class (Scheme 14), reliably deliver the conjunctive coupling product.
Scheme 14.
Mechanistic hypothesis for catalytic conjunctive cross-coupling by metal-induced metalate rearrangement.
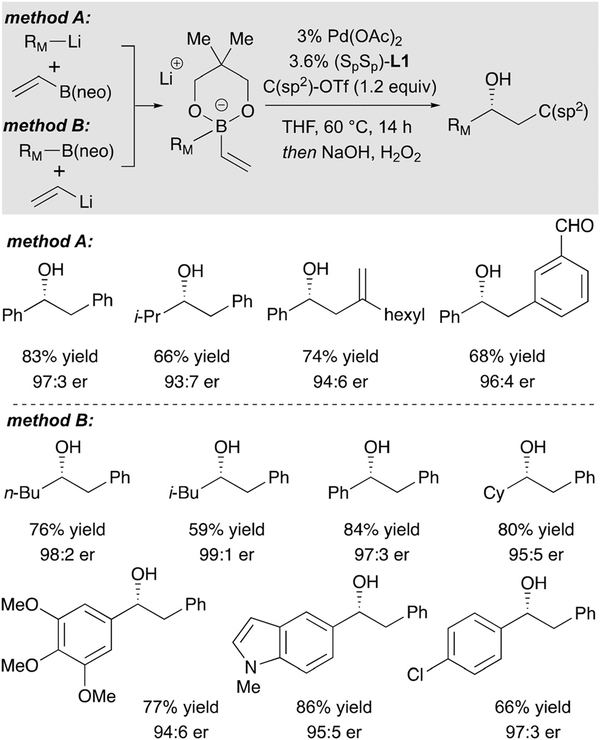
Initial experiments employed neopentyl glycol-derived boronic esters and a complex prepared from Pd(OAc)2 and MandyPhos as catalyst (Scheme 15).29 Cross-coupling with a range of aryl and alkenyl triflates were found to furnish chiral secondary boronic esters in good yield and excellent enantioselectivity. Of note, both aromatic and aliphatic groups underwent efficient migration in these reactions and it is also notable that the reaction can accommodate sensitive functional groups, such as an aldehyde, in the electrophilic partner. A last noteworthy feature in regards to synthesis utility is that similar reaction outcomes are observed whether the ‘ate’ complex was prepared from organolithium reagents and vinyl boron compounds (method A) or from organoboron reagents and vinyllithium (method B).
Scheme 15.
Catalytic enantioselective conjunctive cross-coupling.
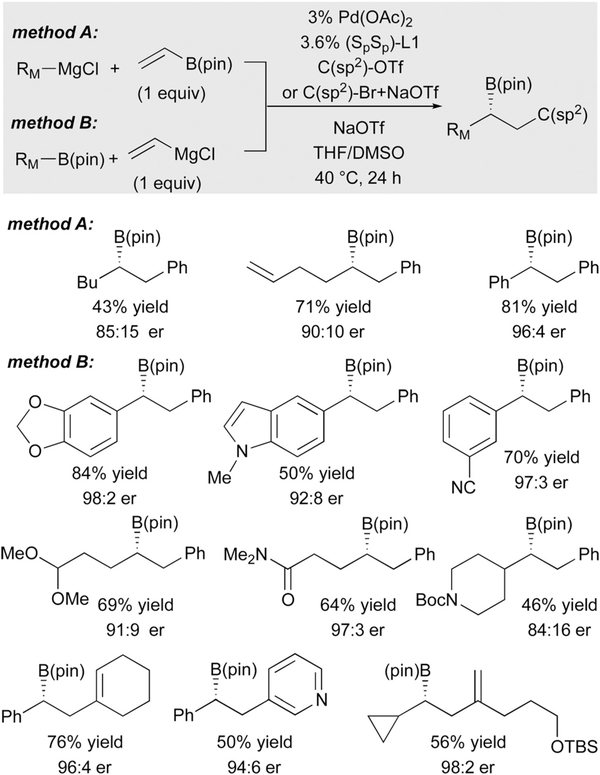
As alluded to above, halide inhibition is a significant problem in the palladium-catalyzed conjunctive coupling process. Indeed, when the organolithium reagents employed in Scheme 15 were prepared from halide precursors, they required recrystallization to remove halide contaminants. The origin of this problem was attributed to halide’s ability to outcompete the ‘ate’ complex for binding to Pd(II). To address this issue, and to enable the use of Grignard reagents in place of organolithium compounds, NaOTf was employed as an additive.30 Of note, this compound was found to not only remove halide from the reaction milieu (sequestration is likely due to both strong NaCl ionic bonding and the insolubility of NaCl in the reaction solvent), but NaOTf also facilitates formation of ‘ate’ complexes from the Grignard reagents and organoboron compounds. Through the use of this additive and the use of DMSO as solvent (DMSO appears to stabilize the ‘ate’ complex in the reaction medium), a broad range of practical conjunctive coupling reactions were enabled (Scheme 16). Of note, an impressive array of useful functionality can be accommodated on the migrating group, with amides, carbamates, esters, acetals, and nitriles surviving the reaction intact.
Scheme 16.
Catalytic conjunctive cross-coupling employing Grignard reagents.
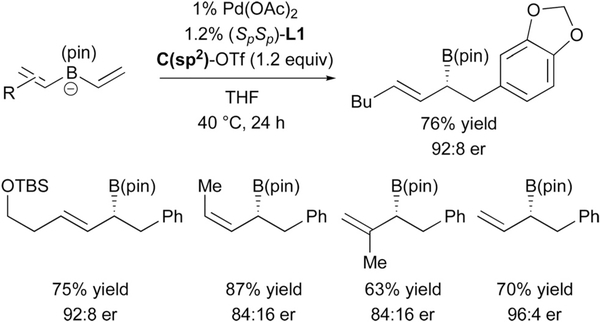
While initial experiments employed alkyl or aryl groups as migrating ligands during the metalate rearrangement, when an alkenyl group migrates, the product of conjunctive coupling is a chiral allylboronic ester (Scheme 17).31 These types of compounds are versatile reagents in organic synthesis as they may be oxidized to allylic alcohols and amines, or they may undergo stereoselective allylation reactions. However, a challenge can be envisioned during conjunctive coupling with an alkenyl migrating group due to chemoselectivity: which alkene is activated and which undergoes migration? As depicted in Scheme 17, the Pd(MandyPhos) complex appears to activate the non-substituted alkene in preference to the substituted alkene for a variety of different substrates and therefore provides a catalytic route to a number of substituted allylboronic esters by conjunctive coupling.
Scheme 17.
Catalytic asymmetric coupling of bis(alkenyl)borates provides allylboronic esters.
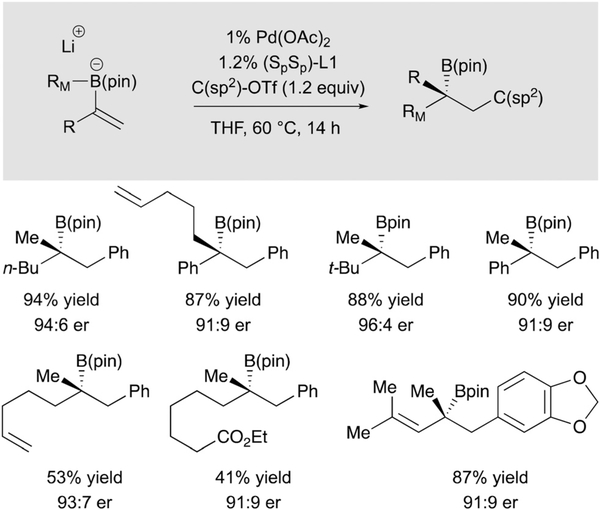
Tertiary boronic esters are precursors to tertiary alcohols and amines. Moreover, through the use of C-C bond-forming homologation reactions, tertiary boronic esters can provide access to compounds with quaternary centers. Extending the conjunctive coupling reaction to construction of these important motifs entails the use of α-substituted alkenylboronic esters as substrates. With such substitution, the direct Suzuki-Miyaura reaction becomes a competing reaction pathway. However, use of boronic ester bearing a pinacoldiolato group led to a significant suppression of the direct cross-coupling products and allowed production of tertiary boronic ester-containing conjunctive coupling products in good yield and enantioselectivity (Scheme 18).32
Scheme 18.
Synthesis of tertiary organoboronic esters by conjunctive cross-coupling of α-substituted vinylboron ‘ate’ complexes.
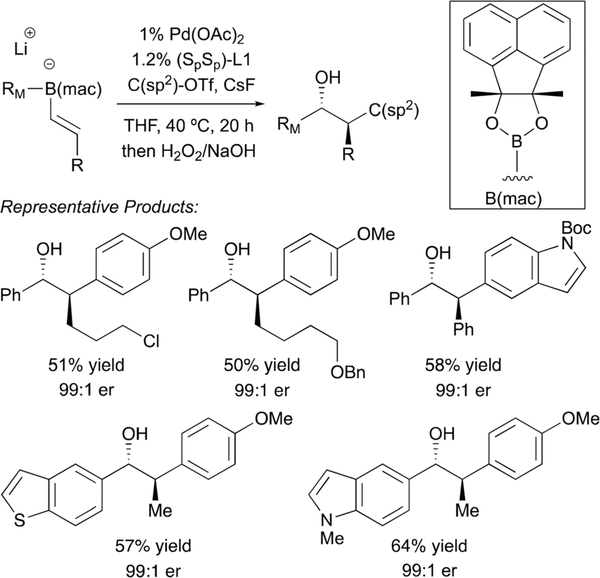
A recent advance from the Morken laboratory involves reactions of β-substituted alkenylboronic esters (Scheme 19).33 Conjunctive coupling with these substrates provides reaction products bearing vicinal stereogenic centers. In the development phase of this process, it was found that with either neopentyl glycol or pinacol boronic ester derivatives, the predominant product arose from Suzuki-Miyaura reaction. This outcome is not surprising since C-Pd bond formation during the metalate shift is now impeded by additional substitution at the β-carbon; in contrast, the rate of transmetalation leading to direct Suzuki-Miyaura products is likely unaltered by the additional substitution. On the basis of the hypothesis that a more encumbered boron ligand might tilt chemoselectivity back towards the metalate-shift-based pathway by precluding access of Pd to the α-carbon, a number of different encumbered ligands were investigated. A practical solution was found in the use of the “mac” ligand (Scheme 19). This motif is readily prepared in a single step by methylation of acenaphthoquinone. As depicted in Scheme 19, conjunctive-coupling with B(mac) derived substrates proceeds in useful yields and with outstanding levels of diastereo- and enantioselectivity.
Scheme 19.
Conjunctive coupling of β-substituted alkenylboronates.
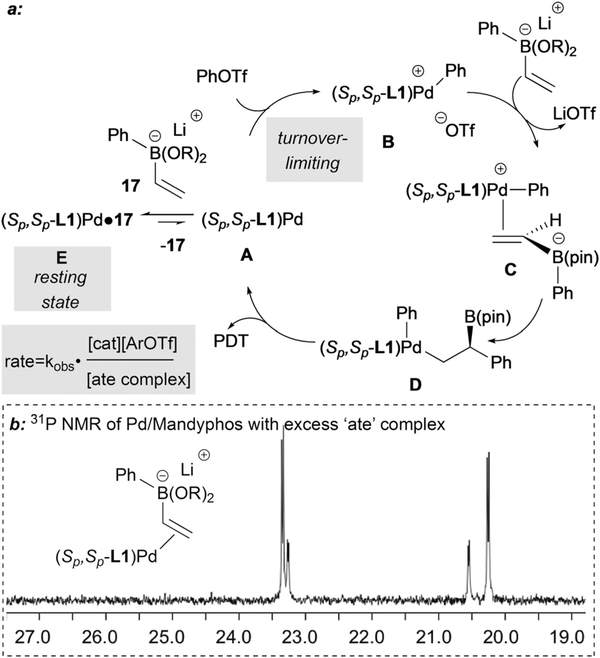
The mechanism of the Pd-catalyzed conjunctive-coupling with alkenylboronic ester derivatives has been studied with a variety of techniques.32 Kinetic analysis was conducted by in situ NMR and revealed that the reaction is overall zero-order. Analysis of individual components showed the reaction to be first order in [electrophile] and [catalyst] but inverse order in [‘ate’ complex]. This observation is most accommodated by a mechanism (Scheme 20a) where the oxidative addition (A → B) is turnover-limiting, while Pd(0) is removed from the catalytic cycle by formation of a complex with the boronate (A → E). Thus the electrophile and the ate complex compete for Pd(0), and the net result is a zero-order process. Of note, for the inhibition by ‘ate’ complex to have an order of [ate]−1 and perfectly balance a first-order oxidative addition, the equilibrium between A and E must strongly favor E, making E the resting state of the reaction. 31P NMR analysis of Pd complexes in the absence of electrophile are consistent with the formation of E (Scheme 20b, two diastereomeric complexes are observed), and it is worth noting that this is the sole species detected by 31P NMR throughout the entire course of the catalytic reaction.
Scheme 20.
(a) Kinetic analysis of conjunctive coupling reveals reaction 1 rate law. (b) Observation of the catalyst resting state supports kinetic analysis.
The stereochemical course of conjunctive-coupling reactions is consistent with a mechanism involving a Pd-promoted 1,2- metalate shift. As depicted in Scheme 21, when deuterium-labelled substrate is employed in these reactions, a single diastereoisomer of reaction product is obtained.29 The configurational assignment of the reaction product is consistent with anti addition of the migrating group and the electrophile across the alkene. Note that this stereochemical pathway is also observed in the reactions of β-substituted substrates described above in Scheme 19. Along with this evidence, DFT analysis32 of the reaction shows that the 1,2-metalate shift step itself has a barrier of only 5.0 kcal mol−1 and is exergonic by 28.2 kcal mol−1.
Scheme 21.
Stereochemical and DFT analysis of conjunctive coupling. Ligand on Pd for DFT is dppf.
Conjunctive cross-coupling with Ni-based catalysts
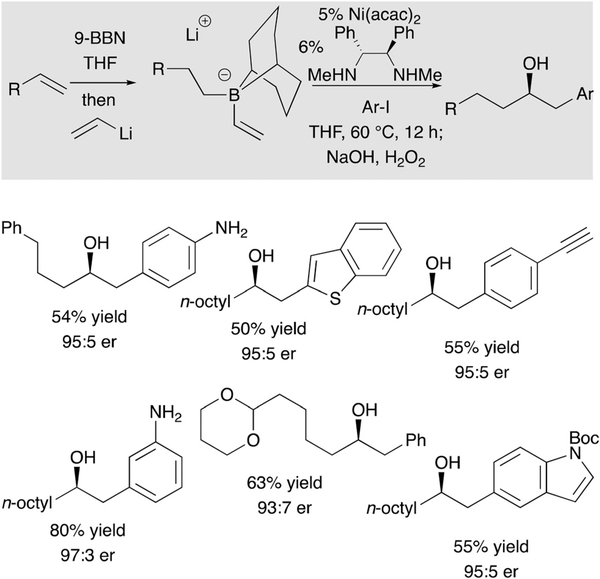
While conjunctive coupling with Pd-based catalysts is efficient across a range of alkenylboronic ester-derived substrates, it is restricted to C(sp2) electrophiles. To expand the scope of this reaction to a broader array of electrophilic partners, Ni-based catalysts have been examined in conjunctive couplings. To initiate these studies, couplings of aryl halide electrophiles were examined.34 While these electrophiles would not undergo coupling with boronic ester-derived ‘ate’ complexes and Ni catalysis, as depicted in Scheme 22, it was found that 9-BBN-derived ‘ate’ complexes were competent coupling partners and provide a complementary path to conjunctive coupling products relative to Pd catalysis. In the presence of a chiral diamine ligand, these processes could also be accomplished with excellent levels of enantioselection. The underlying reason for the influence of the boron ligand in this system is not clearly understood and, indeed, boronic ester derivatives were later found to be compatible in Ni catalysis (vide infra) with alternate electrophiles and Ni ligands.
Scheme 22.
Conjunctive coupling with Ni-based catalysts.
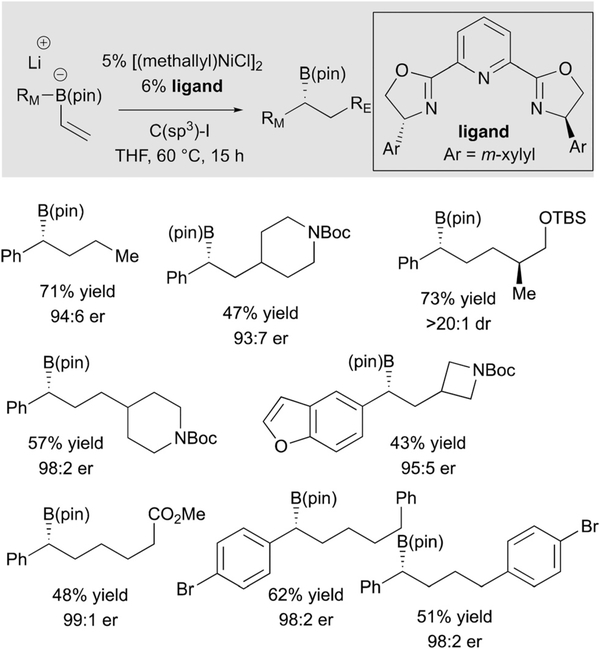
With the ability to employ Ni catalysts as activators for metalate shifts, efforts towards development of a coupling that employ C(sp3) electrophiles were undertaken.35 After some experimentation, it was determined that Ni(pybox) complexes could effectively couple B(pin)-derived ‘ate’ complexes and alkyl electrophiles. As noted in Scheme 23, the electrophile can bear a number of different functional groups, or none at all, and high levels of stereoselection can be achieved.
Scheme 23.
Ni-catalyzed conjunctive coupling with C(sp3) electrophiles.
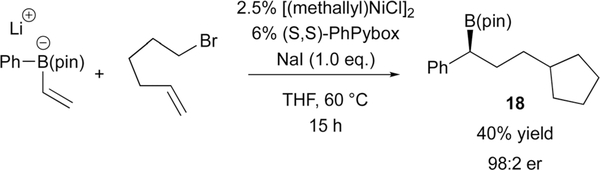
Isotope labelling experiments conducted on the systems depicted in both Schemes 22 and 23 show that anti addition of migrating group and the electrophile to the alkene occurs. This feature is analogous to the Pd-based process. However, it should be noted that prior to engaging in the metalate shift, the Ni-based reaction of C(sp3) electrophiles appears to follow a different course, with oxidative addition occurring by a radical based process. For example, the electrophile 5-bromo-1-hexene undergoes cyclization prior to engaging in cross-coupling to give 18 (Scheme 24), thereby suggesting the intermediacy of carbon-centered radicals in the Ni-catalyzed process. In line with this hypothesis, it was found that iodomethylcyclopropane undergoes ring-opening during coupling, and the coupling reaction of other C(sp3) halides is subject to inhibition by the radical scavenger TEMPO.
Scheme 24.
Ni-catalyzed conjunctive coupling with C(sp3) electrophiles.
Vinylidenation of organoboronic esters enabled by a catalytic metalate shift
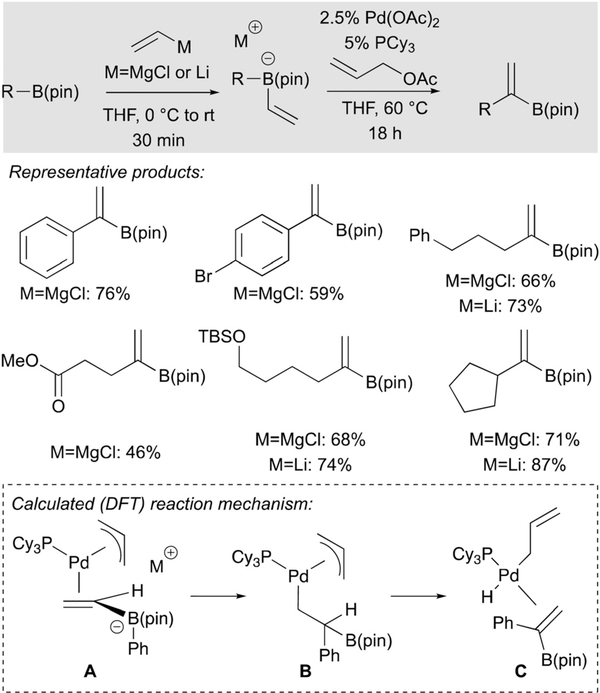
Palladium-catalysed reaction of some alkenylboron ‘ate’ complexes with allyl acetate produces new alkenylboronic esters by a process that appears to entail metal-activated 1,2-boronate rearrangement.36 This process is depicted in Scheme 24, where it can be seen that aryl and alkyl boronic esters undergo “vinylidenation” when their derived ‘ate’ complex is subjected to catalytic Pd(OAc)2/PCy3 and allyl acetate. Mechanistically, this reaction is considered to be initiated by activation of the ‘ate’ complex upon binding to (π-allyl)Pd complex A (Scheme 25). This inner sphere pathway is in contradistinction to the outer sphere addition to Pd(allyl) complexes developed by Ishikura and Ready (Schemes 8 and 9), and for the process in Scheme 25 was found to require the use of a monodentate ligand structure which provides ready access to a coordinatively unsaturated Pd center requisite for alkene binding. The metalate shift step is calculated to occur with an 11.5 kcal mol−1 activation barrier; subsequent β-hydrogen elimination (ΔΔG≠ = 20.8 kcal mol−1) and reductive elimination are expected to produce propene as well as the vinylidenation product.
Scheme 25.
A metalate shift in the course of Pd-catalyzed vinylidenation of boronic esters.
Conclusions
Catalysis of 1,2-metalate shift reactions is an exciting new forefront in synthetic organoboron chemistry and reaction design. These transformations can be expected to provide useful new strategies for construction of chiral organic building blocks for use in asymmetric synthesis. The examples charted so far suggest that a broad array of electrophilic reagents can be activated catalytically and employed in new reaction design. Given the significant progress made in the relatively short time that these reactions have been under study suggests that development of a broad array of new chemical reactions based on 1,2 metalate shifts is imminent.
Key learning points.
Metalate shifts to both sp2 and sp3 can be accelerated by the addition of external Lewis acids.
Metalate shifts to sp and sp2 carbons can be promoted by addition to ligands that are associated with metal complexes.
Direct binding of metal complexes to alkenylboronates can activate the boron reagent for a metalate shift.
Catalytic reactions involving 1,2-metalate shifts can be conducted enantioselectively.
Acknowledgements
We would like to thank the many co-workers in the Morken laboratory who have contributed to the development of the concepts described in this review. We would also like to thank the NIH for support of this program (NIGMS R35GM127140).
Biography

Sheila Namirembe
Sheila Namirembe received her BA in Chemistry from the College of the Holy Cross in 2015. Under the supervision of professor André K. Isaacs, she worked on copper catalyzed reactions that engaged ketenimines with nucleophiles to generate synthetically useful compounds. She is currently a PhD candidate at Boston College working under the mentorship of Professor James P. Morken. Her thesis studies are focused on the development of new catalytic, enantio- and diastereoselective reactions for the synthesis of boron-containing compounds and their broader utility in the total synthesis of biologically active natural products.

James P. Morken
James P. Morken was born in Concord, California and obtained his BS in 1989 from UC Santa Barbara working with Prof. Bruce Rickborn. He obtained his PhD from Boston College in 1995 with Prof. Amir Hoveyda and was an NSF Postdoctoral Fellow with Stuart Schreiber at Harvard University. In 1997, he became an Assistant Professor at the University of North Carolina at Chapel Hill. He was promoted to Associate Professor in 2002 and in 2006 joined the faculty of Boston College. In 2014, he was named the Louise and James Vanderslice and Family Chaired Professor of Chemistry at Boston College.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Hillman MED, J. Am. Chem. Soc, 1962, 84, 4715–4720. [Google Scholar]
- 2.Leonori D and Aggarwal VK, Acc. Chem. Res, 2014, 47, 3174–3183. [DOI] [PubMed] [Google Scholar]
- 3.Matteson DS and Majumdar D, J. Am. Chem. Soc, 1980, 102, 7590–7591. [Google Scholar]
- 4.For a discussion: Matteson DS, Tetrahedron, 1998, 54, 10555–10607. [Google Scholar]
- 5.Jadhav PK and Man H-W, J. Am. Chem. Soc, 1997,119, 846–847. [Google Scholar]
- 6.Matteson DS, Chem. Rev, 1989, 89, 1535–1551. [Google Scholar]
- 7.He Z, Song F, Sun H and Huang Y, J. Am. Chem. Soc, 2018, 140, 2693–2699. [DOI] [PubMed] [Google Scholar]
- 8.Fang GY, Wallner OA, Di Blasio N, Ginesta X, Harvey JN and Aggarwal VK, J. Am. Chem. Soc, 2007, 129, 14632–14639. [DOI] [PubMed] [Google Scholar]
- 9.Fawcett A, Biberger T and Aggarwal VK, Nat. Chem, 2019, 11, 117–122. [DOI] [PubMed] [Google Scholar]
- 10.Binger P and Köster R, Tetrahedron Lett, 1965, 6, 1901–1906. [Google Scholar]
- 11.Zweifel G, Arzoumanian H and Whitney CC, J. Am. Chem. Soc, 1967, 89, 3652–3653. [Google Scholar]
- 12.Matteson DS and Jesthi PK, J. Organomet. Chem, 1976, 110, 25–37. [Google Scholar]
- 13.Evans DA, Crawford TC, Thomas RC and Walker JA, J. Org. Chem, 1976, 41, 3947–3953. [DOI] [PubMed] [Google Scholar]
- 14.Negishi E-I and Idacavage MJ, Org. React, 1985, 33,1–246. [Google Scholar]
- 15.Pelter A and Gould KJ, J. Chem. Soc., Chem. Commun, 1974, 1029–1030. [Google Scholar]
- 16.Sebald A and Wrackmeyer B, J. Chem. Soc., Chem. Commun, 1983, 309–310. [Google Scholar]
- 17.Chan Y, Li NS and Deng M-Z, Tetrahedron Lett, 1990, 31, 2405–2406. [Google Scholar]
- 18.Ishikura M and Terashima M, J. Chem. Soc., Chem. Commun, 1991, 1219–1221. [Google Scholar]
- 19.Ishikura M and Kato H, Tetrahedron, 2002, 58, 9827–9838. [Google Scholar]
- 20.Ishikura M, Matsuzaki Y, Agata I and Katagiri N, Tetrahedron, 1998, 54, 13929–13942. [Google Scholar]
- 21.Panda S and Ready JM, J. Am. Chem. Soc, 2017, 139, 6038–6041; S. Panda and J. M. Ready, J. Am. Chem. Soc., 2018, 140, 13242–13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Daniliuc CG and Studer A, Angew. Chem., Int. Ed, 2018, 57, 40534057. [DOI] [PubMed] [Google Scholar]
- 23.Tao Z, Robb KA, Panger JL and Denmark SE, J. Am. Chem. Soc, 2018, 140, 15621–15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.For an overview: Denmark SE, Hartmann E, Kornfilt DJP and Wang H, Nat. Chem, 2014, 6, 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida N, Miura T and Murakami M, Chem. Commun, 2007, 4381–4383. [DOI] [PubMed] [Google Scholar]
- 26.Ishida N, Narumi M and Murakami M, Org. Lett, 2008, 10, 12879; I. Naoki, S. Tatsuo, S. Shota, M. Tomoya and M. Murakami, Bull. Chem. Soc. Jpn., 2010, 83, 1380–1385; N. Ishia, W. Ikemoto, M. Narumi and M. Murakami, Org. Lett., 2011, 13, 3008–3011. [DOI] [PubMed] [Google Scholar]
- 27.Ishida N, Shimamoto Y and Murakami M, Org. Lett, 2009, 11, 5434–5437. [DOI] [PubMed] [Google Scholar]
- 28.Almena Perea JJ, Lotz M and Knochel P, Tetrahedron: Asymmetry, 1999, 10, 375–384. [Google Scholar]
- 29.Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP and Morken JP, Science, 2016, 351, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovinger GJ, Aparece MD and Morken JP, J. Am. Chem. Soc, 2017, 139, 3153–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelstein EK, Namirembe S and Morken JP, J. Am. Chem. Soc, 2017, 139, 5027–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myhill JA, Zhang L, Lovinger GJ and Morken JP, Angew. Chem., Int. Ed, 2018, 130, 12799–12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myhill JA, Wilhelmsen CA, Zhang K and Morken JP, J. Am. Chem. Soc, 2018, 140, 15181–15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chierchia M, Law C and Morken JP, Angew. Chem., Int. Ed, 2017, 56, 11870–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovinger GJ and Morken JP, J. Am. Chem. Soc, 2017,139, 17293–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aparece MD, Gao C, Lovinger GJ and Morken JP, Angew. Chem., Int. Ed, 2018, 58, 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]