Abstract
Background
Placental protein expression plays a crucial role during pregnancy. We hypothesized that: (1) circulating levels of pregnancy-associated, placenta-related proteins throughout gestation reflect the temporal progression of the uncomplicated, full-term pregnancy, and can effectively estimate gestational ages (GAs); and (2) preeclampsia (PE) is associated with disruptions in these protein levels early in gestation; and can identify impending PE. We also compared gestational profiles of proteins in the human and mouse, using pregnant heme oxygenase-1 (HO-1) heterozygote (Het) mice, a mouse model reflecting PE-like symptoms.
Methods
Serum levels of placenta-related proteins–leptin (LEP), chorionic somatomammotropin hormone like 1 (CSHL1), elabela (ELA), activin A, soluble fms-like tyrosine kinase 1 (sFlt-1), and placental growth factor (PlGF)–were quantified by ELISA in blood serially collected throughout human pregnancies (20 normal subjects with 66 samples, and 20 subjects who developed PE with 61 samples). Multivariate analysis was performed to estimate the GA in normal pregnancy. Mean-squared errors of GA estimations were used to identify impending PE. The human protein profiles were then compared with those in the pregnant HO-1 Het mice.
Results
An elastic net-based gestational dating model was developed (R2 = 0.76) and validated (R2 = 0.61) using serum levels of the 6 proteins measured at various GAs from women with normal uncomplicated pregnancies. In women who developed PE, the model was not (R2 = -0.17) associated with GA. Deviations from the model estimations were observed in women who developed PE (P = 0.01). The model developed with 5 proteins (ELA excluded) performed similarly from sera from normal human (R2 = 0.68) and WT mouse (R2 = 0.85) pregnancies. Disruptions of this model were observed in both human PE-associated (R2 = 0.27) and mouse HO-1 Het (R2 = 0.30) pregnancies. LEP outperformed sFlt-1 and PlGF in differentiating impending PE at early human and late mouse GAs.
Conclusions
Serum placenta-related protein profiles are temporally regulated throughout normal pregnancies and significantly disrupted in women who develop PE. LEP changes earlier than the well-established biomarkers (sFlt-1 and PlGF). There may be evidence of a causative action of HO-1 deficiency in LEP upregulation in a PE-like murine model.
Introduction
Placental protein expression plays a crucial biological role during normal pregnancies. The normal progression of a human pregnancy is associated with a precisely-timed regulation of the expression of maternal and placental proteins [1, 2]. Similarly, the placenta, an endocrine gland unique to pregnancy, secretes hormones that fluctuate with respect to the gestational week of pregnancy. However, these hormones have not been useful in the development of molecular metrics to estimate gestational age (GA) or phenotyping complicated pregnancies prior to overt clinical manifestations of specific pathologic states like preeclampsia (PE) [3, 4].
PE, a pregnancy-related placental vascular disorder affecting 5–8% of all pregnancies [5, 6], is thought to be a multisystem disorder of pregnancy driven by alterations in placental function and resolved by the delivery of the placenta and fetus [7]. Some pregnancy-associated, placenta-related markers have been observed to display different profiles in normal pregnancies compared with pregnancies with PE. Chorionic somatomammotropin hormone like 1 (CSHL1; also called human placental lactogen) is selectively expressed in placental villi with an important role in regulating placental growth. Leptin (LEP) has been suggested to be involved in placental and fetal growth [8]. The relationship between LEP and PE has been demonstrated in a number of studies [9–17]. Circulating levels of activin A, a member of the tumor growth factor protein family, can increase as early as 10–15 weeks of pregnancy in women who subsequently develop PE [18]. Elevated placental levels of angiogenic factors (soluble fms-like tyrosine kinase or sFlt-1) and decreased levels of anti-angiogenic factors (placental growth factor, PlGF) have also been implicated in the pathogenesis of PE [19–25]. As such, the sFlt-1/PlGF ratio has been proposed as an index to identify women with PE [26, 27]. Significant increases in sFlt-1 levels were also observed in sera of pregnant heme oxygenase (HO)-1 heterozygote (Het, HO-1+/-) mice, where the deficiency in HO-1 results in PE-like symptoms [28]. Recent work by Ho et al showed that, in mice, PE is associated with a deficiency in elabela (ELA), a placental hormone that enhances human trophoblast invasiveness in vitro [29].
In this study, we targeted LEP, CSHL1, ELA, activin A, sFlt-1, and PlGF as biomarker candidates for estimating GA and identifying an impending onset of PE. These 6 proteins are not only associated with the placenta and reflect placental growth [8, 30–32]; but also because we found that CSHL1 and PlGF were highly correlated to GA from 10 to 30 weeks of pregnancy by our longitudinal quantitative analysis [2] and that the levels of these proteins differ in women with PE compared with women with normal pregnancies. The predictive value of sFlt-1 and PlGF in PE has been validated by a number of studies [19–27]. Elevations of LEP, CSHL1, and activin A levels were observed early in gestation in women who subsequently develop PE [33–35]. ELA deficiency is associated with PE-like symptoms in mice [29].
We hypothesized that serum levels of placenta-related proteins, LEP, CSHL1, ELA, activin A, sFlt-1, and PlGF are temporally regulated over the course of pregnancy, and profiles of their circulating levels may collectively reflect the normal progression of a term pregnancy. We further hypothesized that disruptions of these profiles in early gestation are associated with placental abnormalities and signal an increased risk of developing PE. We sought to model the longitudinal changes in serum levels of these protein to estimate GA. In addition, we explored whether temporal disruptions in these profiles early in gestation are harbingers of placental pathology and the development of subsequent PE. The model was first developed in human sera and then tested in both human and mouse sera. We chose the HO-1 Het mouse model because during pregnancy these mice display PE-like symptoms, such as elevated diastolic blood pressures and increases in plasma sFlt-1 levels [28]. Furthermore, unlike other mouse models of PE [36, 37], the placentas of these mice have vascular defects, which mimic early-onset PE [28, 38].
Materials and methods
Study design
The study was conducted in three phases: (1) using ELISA methods to characterize the normal pattern of serum placenta-related protein levels; (2) modeling a protein-based GA estimation of normal pregnancies and identifying deviations; and (3) exploration of the protein-based GA estimation with a mouse PE model.
Patients and blood collection
Subjects were selected retrospectively from a cohort of pregnant women who were invited to participate between November 2012 and May 2016 in a prospective longitudinal study sponsored by the March of Dimes. Approval was obtained from the Stanford University Institutional Review Board. Women were eligible for enrollment if they were at least 18 years of age and at their first trimester of a singleton pregnancy. After written informed consent was obtained, blood was collected up to 4 time points, representing the first, second, and third trimesters of pregnancy and 6-weeks postpartum. From this cohort, we analyzed sera from women who had normal uncomplicated pregnancies or who later received a diagnosis of PE. Blood was collected at 1 to 3 time-points prior to a confirmatory diagnosis of PE. GAs were determined by ultrasound measurement.
The diagnosis of PE was made according to the American College of Obstetricians and Gynecologists criteria [39], as follows: a persistent systolic blood pressure ≥ 140 mmHg, or a diastolic blood pressure ≥ 90 mmHg after 20 wks’ GA in a woman with previously normal range of blood pressures in conjunction with one or more of the following: new-onset proteinuria, new-onset thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, or visual or cerebral disturbances in the absence of proteinuria. The PE subgroup in this study included enrolled women who fulfilled the PE diagnostic criteria. The normal subgroup included women who had full-term deliveries and without any complications of pregnancy, or any history of preterm birth or PE.
Animal model study
For the mouse studies, approval was obtained from the Institutional Animal Care and Use Committee at Stanford University. Wild-type (WT) FVB/N male and female mice were purchased from Charles River Laboratories (Wilmington, MA). C57BL/6 HO-1/KO mice were backcrossed with FVB mice to produce an FVB HO-1/KO mouse line as previously described [38]. Mice were mated at 6–10 wks of age. All animals were allowed food and water ad libitum and maintained according to institutional guidelines of Stanford University. Gestational ages were calculated by the presence of a vaginal plug and deemed as E0.5. Mouse line maintenance and genotyping were performed as previously described [28]. Blood was serially collected by submandibular puncture. No anesthesia was used. Mice were then transferred to microtainer tubes containing EDTA. Tubes were then spun at 13,000 x g for 1 minute and then transferred to 1.5 mL microfuge tubes and then stored at -80°C until analysis. Sera were collected from pregnant HO-1 Het or WT dams at 1 to 3 time-points between E7.5 to E18.5.
ELISAs
Serum placental proteins from pregnant women or mice were measured using species-specific commercial kits: LEP (R&D System Inc., MN, USA); CSHL1 (Mybiosource, San Diego, CA, USA); ELA (Peninsula Laboratories International, Inc., San Carlos, CA, USA); activin A (R&D System Inc.); sFlt-1 (R&D System Inc.,); and PlGF (R&D System Inc.).
Statistical analyses
Patient demographic data were analyzed using the “Epidemiological Calculator” (R epicalc package). Hypothesis testing was performed using Mann-Whitney U-tests (two-tailed). Samples collected ≥ 30 weeks of gestation or having any of the placenta-related protein measurements out of limits on the standard curves were excluded from the cohort for modeling. An elastic net (EN) algorithm [40] was applied for estimating GAs using the ELISA data. EN is a statistical learning technique that has been used for GA estimation [2, 41], biomarker discovery [42, 43], and risk prediction [44]. The input of the model was the ELISA data of each analyte, and the output was the GA at sample collection. The model was trained and validated with the women with normal pregnancies, and then tested in women who developed PE. The detail of the modeling procedure is shown in S1 File.
The mean squared error (MSE) of the GA model was used to separate PE patients from women with normal pregnancies. One MSE was calculated for each woman by comparing the observed GA with the model-predicted GA over the associated longitudinal samples. MSE values of normal women and PE women were compared, and receiver operating characteristic (ROC) curves and Mann-Whitney U-tests were calculated to test the performance of MSE in classifying women.
The EN model was then adjusted using 5 analytes as inputs (ELA was excluded see below). The model performance was assessed by R2. The role of each analyte in differentiating complicated from normal pregnancies was explored by analyzing the distribution of the concentrations at different GAs. Comparisons were made between the human and mouse to identify the common behaviors in proteins that were associated with the outcome of PE. Loess regression, Mann-Whitney U-tests, and fold changes were used for the analyses.
Results
Samples
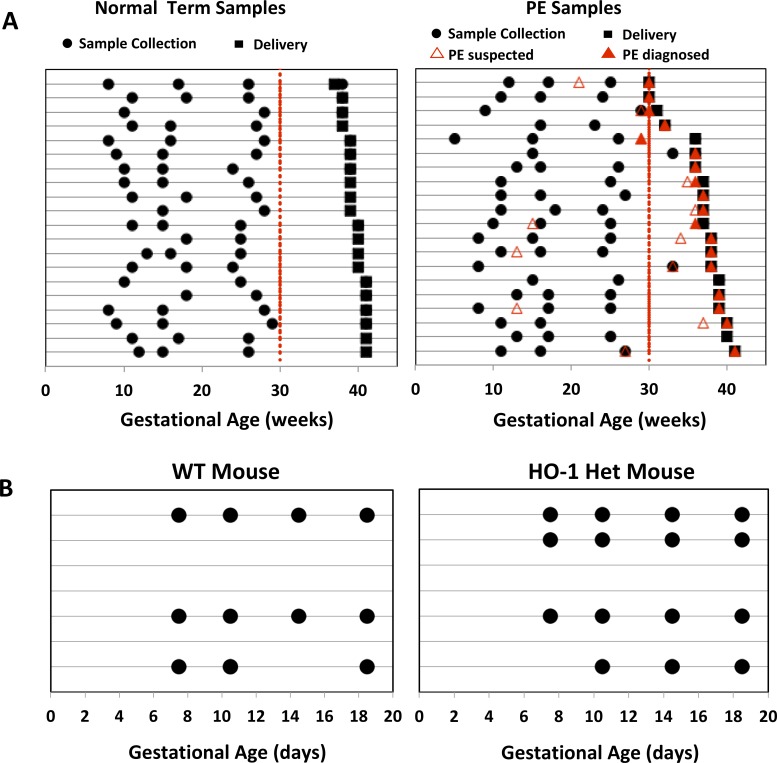
The demographics of the forty pregnant women (20 term pregnancies, 20 with PE) in our study cohort are listed in Table 1. Sample collection times for each woman are shown on Fig 1A. 10 women (4 normal pregnancies, 6 with PE) were excluded from the EN-based modeling because samples were either not collected before 30 weeks of gestation or had at least 1 protein candidate that was out of limits on its standard curve. The latter was done because outliers on the standard curve might cause distortion of our continuous regression analysis. A total of 30 women (16 normal pregnancies, 14 with PE) were therefore used for these analyses. Our training cohort included 10 patients who delivered at term (≥ 37 weeks’ GA). An independent cohort of 6 women who delivered at term and 14 women diagnosed with PE were subsequently enrolled for the validation study of normal pregnancy and then tested on women who developed PE.
Table 1. Subject demographics.
| Characteristic | Overall Normal (n = 20) | PE (n = 20) |
|---|---|---|
| Race, n (%) | ||
| White | 20 (100) | 9 (45) |
| Asian | 0 (0) | 5 (25) |
| African-American | 0 (0) | 1 (5) |
| Other | 0 (0) | 5 (25) |
| Age, mean (SD), years | 31.9 (4.8) | 31.8 (6.0) |
| GA at delivery, mean (SD), weeks | 39.5 (1.2) | 36.7 (3.3) |
| Early-onset PE (Diagnosed < 34 weeks’ GA), n (%) | NA | 5 (25) |
| Diagnosed with severe PE, n (%) | NA | 10 (50) |
Fig 1.
(A) Serial blood sampling from each normal term and PE subject at different GAs. Times of sample collections, infant deliveries, suspected PE, and confirmatory PE diagnoses of individual women (denoted by each row) are represented by black circles, black squares, red unfilled triangles, and red-filled triangles, respectively. (B) Serial blood collection from each pregnant WT (left) and HO-1 Het (right) mouse at different GAs. Sample collection days and individual mice are represented by filled circles and lines, respectively.
The approach was also tested with serum samples collected longitudinally from pregnant WT (n = 3 with 11 samples) and HO-1 Het (n = 4 with 15 samples) mice (Fig 1B). Blood samples were collected at E7.5, E10.5, E14.5, and E18.5.
A placenta-related, protein-based GA estimation of human pregnancy
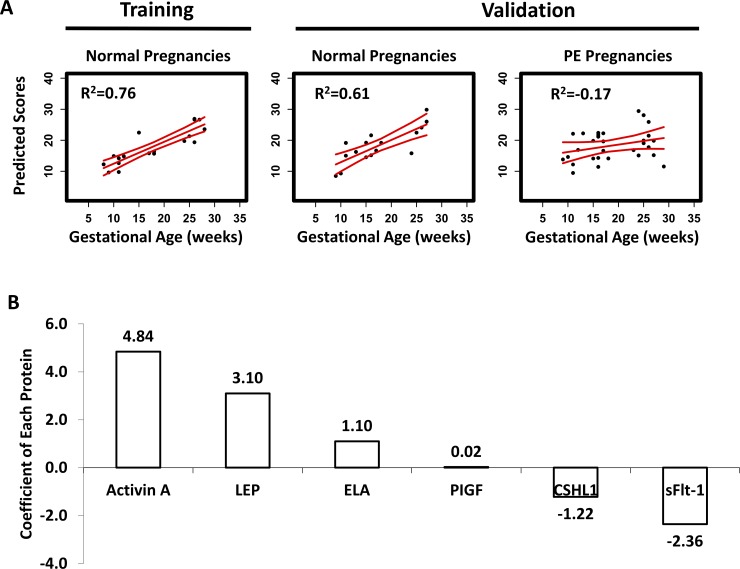
We hypothesized that levels of circulating placenta-related proteins throughout pregnancy reflect the temporal progression of a normal human term pregnancy, and thus can effectively estimate GA. Using an EN algorithm, we developed a 6-protein model using a training cohort of 10 pregnant women that was strongly associated with GA at the time of sampling (R2 = 0.76, P = 2x10-7, Fig 2A, left panel). The EN model was prospectively tested using sera serially collected from 6 additional normal, full-term pregnant women. The EN model was found to predict GA at time of sampling in this independent normal cohort (R2 = 0.61, P = 2x10-4, Fig 2A, middle panel). EN model coefficients of each protein in the model are shown in Fig 2B. Together, the analyses confirmed that there is a highly-regulated temporal pattern of expression of these 6 proteins in sera over the course of pregnancy (Fig 3).
Fig 2.
(A) The results of the EN model developed with serial sampling analyses of 6 placenta-related proteins, dating GAs in normal term pregnancies for both training and validation cohorts, and the results with the patients who developed PE. R2 was calculated as 1-RSS/TSS, where RSS is a residual sum of squares, and TSS is a total sum of squares. (B) Coefficients of each protein analyte in the EN model. Positive and negative values indicate positive and negative correlations, respectively, between GA and the serum protein concentrations. Predicted scores in (A) were calculated based on the coefficients of the 6 proteins shown in (B).
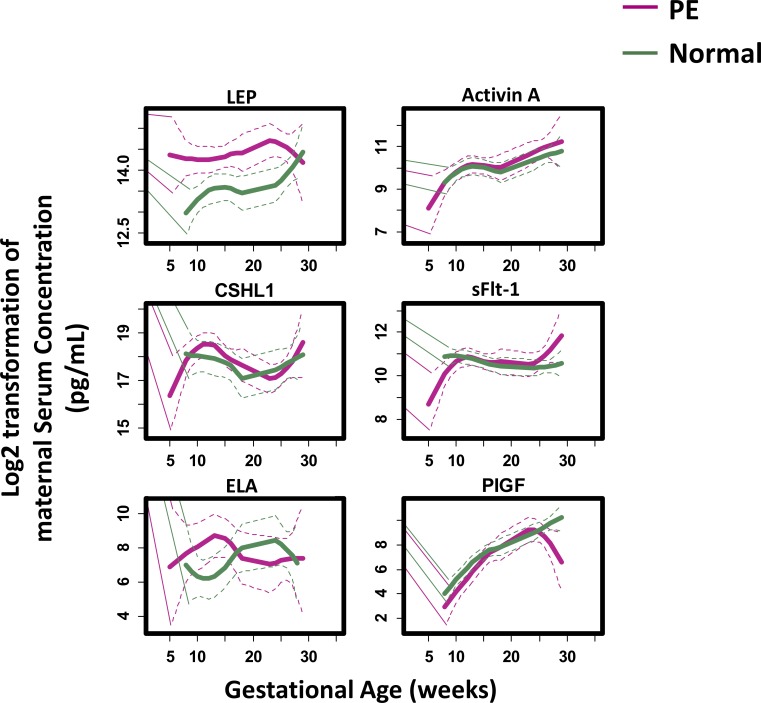
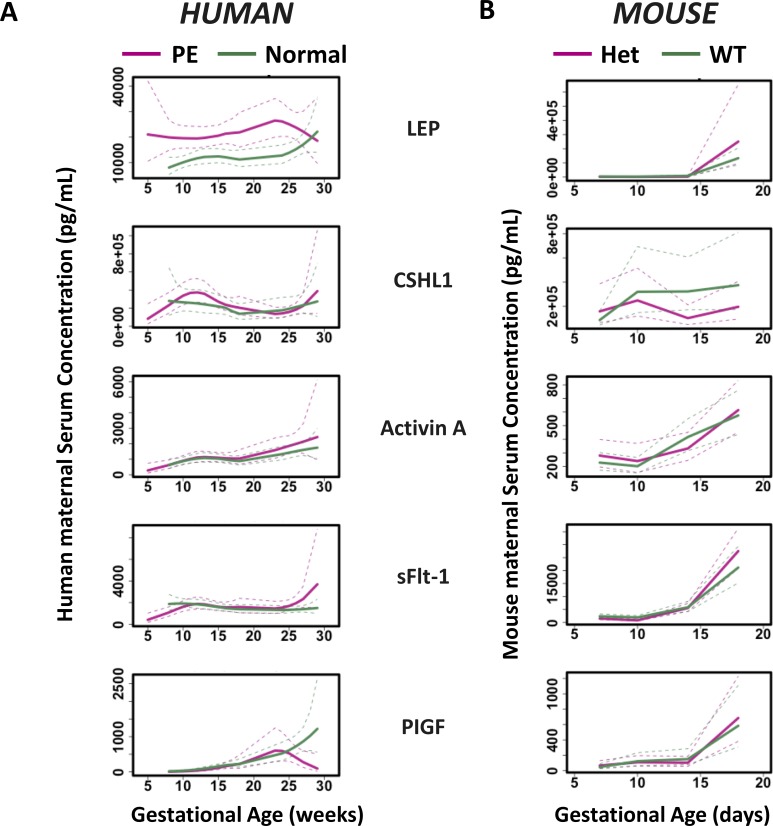
Fig 3. Maternal serum concentrations of the 6 studied placenta-related proteins plotted as a function of GA.
Loess smooth function was applied to demonstrate the trend of the proteins in normal term (green line) and PE (red line) pregnancies, respectively. Color-matched dotted lines show the 95% confidence interval for each cohort.
The placenta-related, protein-based GA estimation malfunctions in PE
Based on the above findings, we hypothesized that our EN model can identify abnormal phenotypes, such as in PE, that may have an attendant disrupted placenta-related protein profile. In contrast to the normal cohort (training R2 = 0.76 and testing R2 = 0.61, Fig 2A), the EN model did not predict GA at time of sampling and yielded random data predictions in the PE cohort (Fig 2A, right panel, R2 = -0.17, P = 0.2). These findings suggest that the protein-based GA estimation (and profile) is disrupted in PE.
The pathogenesis of PE is complex and progresses from an asymptomatic stage, characterized by undetected placental abnormalities during the first trimester to a symptomatic stage with proteinuria and hypertension in late gestation [45]. Our analyses revealed unique longitudinal expression patterns of serum protein levels of specific biomarkers (Fig 3). Specifically, LEP, CSHL1, and ELA levels at approximately 10 weeks of gestation differentiated women with PE from women with uncomplicated, full-term pregnancies, indicating that the pathogenesis of PE may arise very early in gestation. In addition, differences in activin A levels begin to appear around 20 weeks of gestation and in sFlt-1 and PlGF after 25 weeks. Examination of the profiles of the levels of these proteins revealed significant gestational windows (5–9, 10–14, 15–25, 26–33, and 27–38 weeks’ GA, Table 2, S1 Fig, and S1 Table) specific for each biomarker. These findings are in line with our longitudinal biomarker trending analyses (Fig 3). Since there was a positive association [8] between maternal serum LEP concentrations and body mass index (BMI) (and consequently, gestational weight gain) during pregnancy, we normalized serum LEP levels by BMI and found similar results (S2 Fig). Taken together, these data indicate that alterations in the profiles of serum levels of LEP, CSHL1, and ELA begin much earlier in GA than the changes in sFlt-1 (increase) and PlGF (decrease) at late GA.
Table 2. Comparisons of the serum levels of each protein between normal and PE pregnancies.
Mann-Whitney U-test P-value was calculated. *0.005 < P < 0.05. **P < 0.005.
| 5–9 weeks’ GA |
10–14 weeks’ GA | 15–25 weeks’ GA | 26–33 weeks’ GA | 27–38 weeks’ GA | |
|---|---|---|---|---|---|
| LEP | 0.02* | 0.02* | 3x10-6** | 0.3 | 0.5 |
| CSHL1 | 0.4 | 0.01* | 0.3 | 0.7 | 0.9 |
| ELA | 0.9 | 0.03* | 0.9 | 0.8 | 0.4 |
| Activin A | 0.5 | 0.5 | 0.8 | 0.04* | 0.2 |
| sFlt-1 | 0.2 | 0.3 | 0.8 | 0.02* | 3x10-3** |
| PlGF | 0.6 | 0.9 | 0.5 | 0.3 | 0.01* |
Disruption of the protein-based GA estimation identifies impending PE
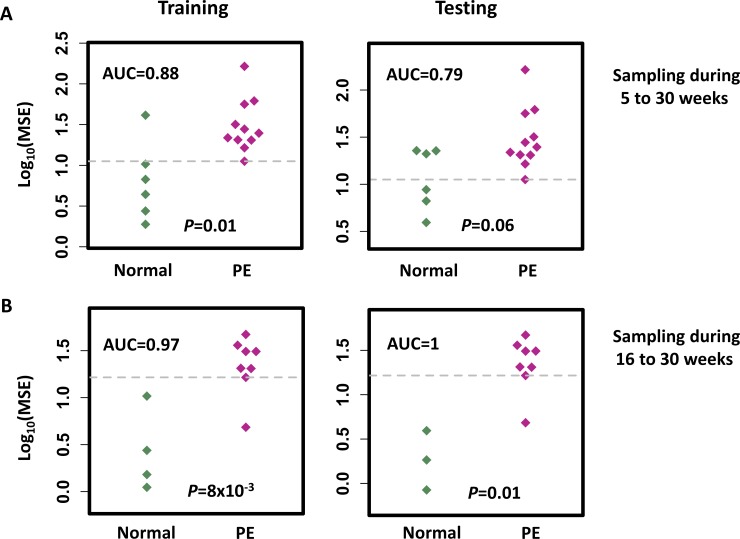
Our placenta-related protein-based GA estimation characterized the gestational progression of normal term pregnancies. Significant random disruptions of this normal “term” model were observed in women with PE. Logarithm-transformed MSE of our EN estimations were utilized to define the binary classifications to identify risk for impending PE (Fig 4). For samples collected at 5–30 weeks’ GA, the MSE metric differentiated normal women from those who developed PE (Mann-Whitney U-test P = 0.01 on the training cohort, and P = 0.06 on the testing cohort) with areas under the curve (AUCs) of 0.88 on the training and 0.79 on the testing cohorts. An optimized cutoff value calculated on the training data yielded a positive predictive value (PPV) of 0.79 with a sensitivity of 1.00, and a negative predictive value (NPV) of 1.00 with a specificity of 0.50 on the testing data. In contrast, in the 16–30 weeks of gestation window, performance was improved: Mann-Whitney U-test (P = 8x10-3 on the training and P = 0.01 on the testing) and AUC of 0.97 on the training and 1 on the testing data, PPV of 1.00 with a sensitivity of 0.88, and NPV of 0.75 with a specificity of 1.00 on the testing data. These results may be more sensitive than using a single biomarker on the testing data in a window of 16–30 weeks of gestation (with AUCs of 0.53 for LEP; 0.76 for CHSL1; 0.58 for ELA; 0.53 for activin A; 0.65 for sFlt-1; and 0.65 for PlGF). Thus, our results demonstrate that significant disruptions in the protein-based GA estimation can be used to identify a woman’s risk for developing impending PE.
Fig 4. The mean squared error (MSE) of the EN model in estimating the GA of normal and PE patients in the training and testing cohorts, respectively.
Mann-Whitney U-test P-value was calculated to measure the difference in MSE between the normal and PE patients. The cut-off point (grey dotted line) shows the maximum value of the sum square of the sensitivity and 1-specificity on classification of the training cohort of normal and PE women at blood sampling during (A) 5–30 and (B) 16–30 weeks of gestation.
Our EN model was also further tested to see if the different PE subtypes could be identified [46, 47]. For samples collected at 5–30 weeks’ GA, the MSE metric had an AUC of 0.82 (0.90 for training and 0.73 for testing) and 0.85 (0.86 for training and 0.83 for testing) for differentiating normal women from those who developed early-onset PE or late-onset PE, respectively, and an AUC of 0.80 (0.86 for training and 0.74 for testing) and 0.90 (0.92 for training and 0.88 for testing) for differentiating normal women from those who developed mild or severe PE, respectively. For samples collected at 16–30 weeks’ GA, the MSE metric had an AUC of 0.95 (0.92 for training and 1 for testing) and 1 for differentiating normal women from those who developed early-onset or late-onset PE, respectively, and an AUC of 0.97 (0.95 for training and 1 for testing) and 1 for differentiating normal women from those who developed mild or severe PE, respectively. There was no significant differences (P > 0.05) in performance with regard to differentiating women who develop early- or late-onset PE, or those who develop mild or severe PE.
A placenta-related, protein-based GA estimation with reduced number of features
Due to the lack of robustness of the mouse ELA ELISA assay, we tested the performance of our EN-based model excluding ELA. The model had an R2 of 0.72 and 0.61 on the training and testing cohorts, respectively. Disruptions in protein profiles were observed at < 30 weeks of gestation in women who developed PE (R2 = 0.27). Similar to the 6-protein model, the 5-protein model was still able to estimate GA during normal pregnancies.
Comparative analysis of serological GA estimation between the human and a mouse model
We hypothesized that similar temporal placenta-related protein expression patterns should be conserved in mouse pregnancies, therefore, we explored our EN-based 5-protein model to normal (n = 3 with 12 samples) and pregnant HO-1 Het mice (n = 4 with 15 samples), a mouse model reflecting PE-like symptoms. Model coefficients were adjusted to establish the link between GA (in days) and the targeted serum protein levels. The model had an R2 of 0.85 (P = 2 x 10–4) for pregnant WT, while the R2 was reduced to 0.30 (P = 0.07) for HO-1 Het mice with PE-like symptoms. Fold changes of protein levels of HO-1 Het over normal pregnancies were calculated and then compared between the human and mouse in early (human: 5–26 weeks; mouse: 7.5–14.5 days) and late (human: 27–38 weeks; mouse: 18.5 days) gestations separately. The largest fold change was observed in LEP at late gestation of mice (Fig 5). Unlike mice, LEP levels were elevated in women with PE in early gestation (Fig 3, S1 Fig, and Table 2). Fold changes of sFlt-1 and PlGF in mice increased from early to late gestation. The temporal patterns of sFlt-1 in mice were similar to those in human, which decreased in early gestation and increased in late gestation of complicated pregnancies (Fig 5). In contrast, PlGF significantly decreased in women with PE after 27 weeks’ GA, but not in HO-1 Het mice at late gestation.
Fig 5.
Loess smoothing of the serum levels of the 5 placenta-related proteins in (A) human and (B) mice as a function of GA for both normal and (A) PE or (B) HO-1 Het samples. The x axis represents GA, and the y axis represents serum concentrations. Red curves represent patterns of PE women and HO-1 Het mouse. Green curves represent patterns of normal pregnant women and WT mouse. Dotted lines represent 95% confidence intervals.
Discussion
The placenta plays a key role in fetal development, where cell communication occurs to support nutrition acquisition, immune adaption, and other functions of maternal-fetal interaction [48, 49]. Placental proteins are expressed in a time-dependent manner and cross-talk with other organs, such as the thyroid, pituitary, and ovary, and are necessary to ensure normal fetal development. Characterization of the temporal patterns of circulating placental proteins may serve as a basis for understanding the biology underlying both normal and pathological pregnancies. Our results support our hypothesis that multivariate modeling of the levels of circulating placental-secreted proteins, LEP, CSHL1, ELA, activin A, sFlt-1, and PlGF can be used to estimate GA during the course of a normal pregnancy, but not in women who develop PE. The longitudinal placental-related protein profile in sera was also observed in pregnant WT mice, but not in pregnant HO-1 Het mice.
Early diagnosis of PE remains a challenge in clinical settings. The traditional diagnosis of PE is based on the presence of maternal hypertension and proteinuria [50]. sFlt-1 and PlGF are well-established PE biomarkers [51] with clinical prognostic utility in the management of PE. The ratio of sFlt-1 and PlGF has been shown to effectively differentiate PE from normal term pregnancies, but only after 25 weeks of gestation [27]. Previous transcriptomic [52–58] and proteomic [2, 59–63] profiling of normal and complicated pregnancies have identified disease-specific expression patterns and signaling networks, which suggest candidate biomarkers for possible early clinical diagnoses and for offering new biological insights. Our findings suggest that a composite placental-related protein panel from serial blood collection (for MSE calculations) may provide a diagnostic test to assess PE earlier (~10 weeks of gestation) than previously suggested by sFlt-1 and PlGF (after 25 weeks of gestation as observed in this study). The diagnostic performance of the panel was similar among the different types of PE (early-onset vs. late-onset, mild vs. severe). Compared to a recent proteomics study where different models were predictive at different GA windows [64], our model with 6 proteins works across 5–30 wks of GA, which is more useful when exact gestational age is unknown. Therefore, this model may offer a new investigational approach towards the understanding of placental biology during pregnancy as well as guiding innovative methods for PE diagnosis.
Our findings of serum protein levels during a normal pregnancy are consistent with those from previous studies. They are in line with the ranges reported in healthy pregnancies and have similar patterns during the pregnancy as previous results [65–73]. We found that LEP increased continuously during the first and second trimesters. Activin A remained stable between 10–20 weeks of gestation and increased late in the second trimester. sFlt-1 levels were also unchanged before 30 weeks, while PlGF progressively rose over pregnancy. We further integrated the quantitative trending information of each individual protein into a continuous regression model that expressed GA as a linear combination of the levels of proteins.
An important finding of the study is that women who develop PE had serum LEP levels much higher than normal pregnant women in early gestation (<25 weeks). It is consistent with findings from other studies [74, 75], which indicated that LEP can serve as an early biomarker of PE. It is intriguing that the serum LEP levels increased weeks or months prior to the onset of PE (P = 3x10-6 at 15–25 weeks, while the onset dates of PE were 29 weeks or later in our study), suggesting that LEP itself may be involved in the early pathogenesis of the disease. Given that LEP is a master regulator of energy expenditure, the observations hint that placental insufficiency as a consequence of an energy imbalance may be a precursor to PE that is manifested as hypertension in mid to late gestation. Furthermore, since LEP is a placenta-related protein, its early increase observed in women who develop PE may support the hypothesis that early-onset PE is a placenta-based disease [46, 47]. LEP may be associated with placental vasculature defects, or even cause these changes.
Data from our HO-1 Het mouse studies suggest an association between placental HO-1 and the development of PE observed in humans [76] and provide a window of the mechanism of how a deficiency in HO-1 may affect the placental vascular network and leads to PE-like symptoms as mediated by increases in sFlt-1 [38]. Disruptions in placental vascular formation and spiral artery remodeling has been implicated as a causative factor of PE. Previous studies have shown that HO-1 expression is reduced in placentas of women with PE [76], and that HO-1 is involved in placental vascular network development [77]. The role of HO-1 in placental malfunction was further evaluated with HO-1 Het mice [38], revealing that a deficiency of maternal HO-1 results in an impaired placental vascular network and spiral artery malformation, and causes PE-like symptoms such as high diastolic blood pressures and elevated sFlt-1 levels [28].
Our HO-1 Het mouse study provided additional insights into the PE in terms of placental protein changes, especially relating to the role of LEP in its pathophysiology. Here, we observed that: serum LEP, CSHL1, Activin A, sFlt-1, and PlGF levels were highly correlated with GA in normal WT mice during pregnancy; In addition, this correlation was disrupted in pregnant HO-1 Het mice; Finally, both serum sFlt-1 and LEP levels were higher in pregnant HO-1 Het mice compared with normal pregnant WT mice. These findings were consistent with what we observed in human sera, and demonstrated that our EN model developed using human sera for identifying impending PE early in gestation could be duplicated in mice. Furthermore, the characterization of temporal pattern of LEP expression in mice may help us understand the pathophysiology of PE. The main action of LEP is in the maternal interface regulating angiogenesis, growth, and immunomodulation in the placenta during the early stages of pregnancy [78–84]. Although a dysregulation of LEP levels has been found to correlate with the pathogenesis of various pregnancy disorders [85], including PE, the exact mechanism of action of LEP and its upstream regulation remain unknown. Our characterization of serum placental protein profiles in the pregnant HO-1 Het mouse provides evidence of an association between HO-1 deficiency and an upregulation of LEP in this PE-like murine model. Taken together with the significant correlation of LEP levels at < 25 weeks’ GA and impending PE in our human pregnancy cohort may indicate a mechanistic role of LEP and HO-1 in the pathogenesis of PE, and lead to the identification of novel treatment strategies, such as pravastatin [86].
We also note that placenta-related proteins have distinct temporal patterns between human and rodent pregnancies. PlGF is significantly down-regulated after 27 weeks’ GA in humans with complicated pregnancies while the down-regulation was not observed in mice. For LEP, its maximum differentiating power is found at early gestation (< 25 weeks) for humans but not at early gestation in mice. The differences in placenta-related protein expression profiles between humans and mice may be explained by their differences in placental structures (e.g. a choriovitelline placenta is initially present in mice but absent in human; trophoblast cell invasion is restricted in mice but deep in human) and different placental endocrine functions [87–89].
This study has several limitations. First, the sample sizes for our human cohorts were small, and our population lacked racial heterogeneity. Second, the time intervals of blood collections between two serial samples varied (3–31 weeks for normal, and 3–25 weeks for PE). Most samples were collected in the first or second trimester. Only 12 normal and 9 PE patients had samples collected in the third trimester. Third, serum concentrations of LEP can be influenced by maternal status [90, 91]. We addressed this through the normalization to maternal BMI (S2 Fig) and found the temporal pattern in LEP remained. Fourth, variations in circulating protein levels could be due to the contributions from other tissues besides the placenta. Meta-analysis of PE and GA-matched uncomplicated pregnancy-associated placental gene expression patterns, including the targeted analytes of this study, has revealed similar expression trending along the gestations and differentiation between normal women and those who develop PE [92]. Fifth, ELA was not included in the rodent analyses due to the lack of the robustness of the mouse ELISA assay. Sixth, missing clinical information, such as intrauterine growth restriction, might limit our insights into the pathophysiology of PE. Future study with comprehensive clinical data plus placenta-related proteins like PlGF may give better results in PE assessment [93]. Finally, although the expression profiles of our candidate proteins may have clinical utility to assess impending PE, the robustness of this model can be greatly improved with more frequent blood collection times and a larger sample cohort.
Conclusions
Longitudinal EN analysis of the circulating pregnancy-associated, placenta-related protein expression throughout pregnancy revealed patterns of the normal temporal progression of human gestation that can estimate GA. The elevated MSE of the EN metric, quantifying the disruptions of the estimation, offers a potential approach to identify impending PE. The protein markers in sera shared by the human and mouse and their significant associations with GA are conserved. In addition, PE-related patterns found in human are preserved in normal and HO-1 Het pregnant mice. This provides direct evidence of the causative action of HO-1 deficiency in the upregulation of LEP in a PE-like murine model and may reveal a new therapeutic target, such as HO-1, to prevent placental vascular disorders [86]. All of these demonstrate that the exploration of the temporal expression patterns of the placenta-related proteins in rodent models can be used to study the biology of human pregnancy disorders such as PE.
Supporting information
Mann-Whitney U-test P-values are shown.
(PDF)
Maternal serum concentrations of (A) LEP and (B) LEP normalized to body mass index (BMI) (pg/mL/kg/m2) shown as a function of GA in normal term (red line) and PE (green line) pregnancies. Loess smooth function was applied. Color-coded dotted lines: show the 90% confidence interval for each cohort.
(PDF)
(PDF)
Signed differences in mean (PE minus normal; pg/mL) were calculated.
(PDF)
Acknowledgments
The authors thank colleagues at the Stanford University Pediatric Proteomics group and the March of Dimes Prematurity Research Center at Stanford University for critical discussions.
Abbreviations
- PE
Preeclampsia
- CSHL1
Chorionic somatomammotropin hormone like 1
- LEP
Leptin
- GA
Gestational age
- sFlt-1
Soluble fms-like tyrosine kinase
- PlGF
Placental growth factor
- HO-1
Heme oxygenase-1
- ELA
Elabela
- WT
Wild-type
- EN
Elastic net
- MSE
Mean squared error
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- PPV
Positive predictive value
- NPV
Negative predictive value
- BMI
Body mass index
Data Availability
We uploaded a dataset of ELISA results to our group’s public website at Stanford University (http://translationalmedicine.stanford.edu/data/PE_2020_1/ELISA/S2.file.xlsx).
Funding Statement
This work was supported in part by the Stanford University Spark Spectrum Pilot Program and the March of the Dimes Prematurity Research Center at Stanford University, and Stanford Child Health Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Landek-Salgado MA, Gutenberg A, Lupi I, Kimura H, Mariotti S, Rose NR, et al. Pregnancy, postpartum autoimmune thyroiditis, and autoimmune hypophysitis: intimate relationships. Autoimmun Rev. 2010;9(3):153–7. Epub 2009/06/23. 10.1016/j.autrev.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghaeepour N, Lehallier B, Baca Q, Ganio EA, Wong RJ, Ghaemi MS, et al. A Proteomic Clock of Human Pregnancy. American journal of obstetrics and gynecology. 2017. 10.1016/j.ajog.2017.12.208 . [DOI] [PubMed] [Google Scholar]
- 3.Dugoff L, Hobbins JC, Malone FD, Vidaver J, Sullivan L, Canick JA, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstetrics and gynecology. 2005;106(2):260–7. Epub 2005/08/02. 10.1097/01.AOG.0000172419.37410.eb . [DOI] [PubMed] [Google Scholar]
- 4.Yefet E, Kuzmin O, Schwartz N, Basson F, Nachum Z. Predictive Value of Second-Trimester Biomarkers and Maternal Features for Adverse Pregnancy Outcomes. Fetal diagnosis and therapy. 2017. Epub 2017/04/11. 10.1159/000458409 . [DOI] [PubMed] [Google Scholar]
- 5.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstetrics and gynecology. 2009;113(5):1075–81. Epub 2009/04/23. 10.1097/AOG.0b013e3181a09fc0 . [DOI] [PubMed] [Google Scholar]
- 6.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics and gynecology. 2001;97(4):533–8. Epub 2001/03/29. 10.1016/s0029-7844(00)01223-0 . [DOI] [PubMed] [Google Scholar]
- 7.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–69. Epub 2011/06/22. 10.1161/CIRCULATIONAHA.109.853127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samano R, Martinez-Rojano H, Chico-Barba G, Godinez-Martinez E, Sanchez-Jimenez B, Montiel-Ojeda D, et al. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients. 2017;9(10). Epub 2017/09/28. 10.3390/nu9101067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muy-Rivera M, Ning Y, Frederic IO, Vadachkoria S, Luthy DA, Williams MA. Leptin, soluble leptin receptor and leptin gene polymorphism in relation to preeclampsia risk. Physiological research / Academia Scientiarum Bohemoslovaca. 2005;54(2):167–74. . [PubMed] [Google Scholar]
- 10.Ouyang Y, Chen H, Chen H. Reduced plasma adiponectin and elevated leptin in pre-eclampsia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2007;98(2):110–4. 10.1016/j.ijgo.2007.04.021 . [DOI] [PubMed] [Google Scholar]
- 11.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. Journal of reproductive immunology. 2005;65(1):65–75. 10.1016/j.jri.2004.09.004 . [DOI] [PubMed] [Google Scholar]
- 12.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. American journal of obstetrics and gynecology. 2005;193(3 Pt 2):979–83. 10.1016/j.ajog.2005.06.041 . [DOI] [PubMed] [Google Scholar]
- 13.Tommaselli GA, Pighetti M, Nasti A, D'Elia A, Guida M, Di Carlo C, et al. Serum leptin levels and uterine Doppler flow velocimetry at 20 weeks' gestation as markers for the development of pre-eclampsia. Gynecol Endocrinol. 2004;19(3):160–5. 10.1080/09513590400007267 . [DOI] [PubMed] [Google Scholar]
- 14.Ozkan S, Erel CT, Madazli R, Aydinli K. Serum leptin levels in hypertensive disorder of pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 2005;120(2):158–63. 10.1016/j.ejogrb.2004.02.046 . [DOI] [PubMed] [Google Scholar]
- 15.Kocyigit Y, Bayhan G, Atamer A, Atamer Y. Serum levels of leptin, insulin-like growth factor-I and insulin-like growth factor binding protein-3 in women with pre-eclampsia, and their relationship to insulin resistance. Gynecol Endocrinol. 2004;18(6):341–8. 10.1080/09513590410001704975 . [DOI] [PubMed] [Google Scholar]
- 16.Teppa RJ, Ness RB, Crombleholme WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism: clinical and experimental. 2000;49(8):1043–8. 10.1053/meta.2000.7707 . [DOI] [PubMed] [Google Scholar]
- 17.Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H, et al. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. The Journal of clinical endocrinology and metabolism. 1998;83(9):3225–9. 10.1210/jcem.83.9.5117 . [DOI] [PubMed] [Google Scholar]
- 18.Muttukrishna S, North RA, Morris J, Schellenberg JC, Taylor RS, Asselin J, et al. Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum Reprod. 2000;15(7):1640–5. Epub 2000/06/30. . [DOI] [PubMed] [Google Scholar]
- 19.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. The Journal of clinical endocrinology and metabolism. 2005;90(8):4895–903. Epub 2005/05/12. 10.1210/jc.2004-1955 . [DOI] [PubMed] [Google Scholar]
- 20.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111(5):649–58. Epub 2003/03/06. 10.1172/JCI17189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf M, Shah A, Lam C, Martinez A, Smirnakis KV, Epstein FH, et al. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. American journal of obstetrics and gynecology. 2005;193(1):16–22. Epub 2005/07/16. 10.1016/j.ajog.2005.03.016 . [DOI] [PubMed] [Google Scholar]
- 22.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26(7):563–73. Epub 2005/07/05. 10.1016/j.placenta.2004.09.001 . [DOI] [PubMed] [Google Scholar]
- 23.Taylor AP, Rodriguez M, Adams K, Goldenberg DM, Blumenthal RD. Altered tumor vessel maturation and proliferation in placenta growth factor-producing tumors: potential relationship to post-therapy tumor angiogenesis and recurrence. International journal of cancer Journal international du cancer. 2003;105(2):158–64. Epub 2003/04/04. 10.1002/ijc.11059 . [DOI] [PubMed] [Google Scholar]
- 24.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. American journal of obstetrics and gynecology. 2001;184(6):1267–72. 10.1067/mob.2001.113129 . [DOI] [PubMed] [Google Scholar]
- 25.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. American journal of obstetrics and gynecology. 1998;179(6 Pt 1):1539–44. 10.1016/s0002-9378(98)70021-3 . [DOI] [PubMed] [Google Scholar]
- 26.Stepan H, Schaarschmidt W, Jank A, Verlohren S, Kratzsch J. [Use of angiogenic factors (sFlt-1/PlGF ratio) to confirm the diagnosis of preeclampsia in clinical routine: first experience]. Zeitschrift fur Geburtshilfe und Neonatologie. 2010;214(6):234–8. Epub 2011/01/06. 10.1055/s-0030-1262827 . [DOI] [PubMed] [Google Scholar]
- 27.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. American journal of obstetrics and gynecology. 2010;202(2):161 e1–e11. Epub 2009/10/24. 10.1016/j.ajog.2009.09.016 . [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Wong RJ, Kalish FS, Nayak NR, Stevenson DK. Effect of heme oxygenase-1 deficiency on placental development. Placenta. 2009;30(10):861–8. 10.1016/j.placenta.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho L, van Dijk M, Chye STJ, Messerschmidt DM, Chng SC, Ong S, et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science. 2017;357(6352):707–13. Epub 2017/07/01. 10.1126/science.aam6607 . [DOI] [PubMed] [Google Scholar]
- 30.Mannik J, Vaas P, Rull K, Teesalu P, Laan M. Differential placental expression profile of human Growth Hormone/Chorionic Somatomammotropin genes in pregnancies with pre-eclampsia and gestational diabetes mellitus. Mol Cell Endocrinol. 2012;355(1):180–7. 10.1016/j.mce.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mylonas I, Schiessl B, Jeschke U, Vogl J, Makrigiannakis A, Kuhn C, et al. Expression of inhibin/activin subunits alpha (-alpha), beta A (-beta (A)) and beta B (-beta (B)) in placental tissue of normal and intrauterine growth restricted (IUGR) pregnancies. J Mol Histol. 2006;37(1–2):43–52. 10.1007/s10735-006-9029-6 . [DOI] [PubMed] [Google Scholar]
- 32.Palmer KR, Tong S, Kaitu'u-Lino TJ. Placental-specific sFLT-1: role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis. Molecular human reproduction. 2017;23(2):69–78. 10.1093/molehr/gaw077 . [DOI] [PubMed] [Google Scholar]
- 33.Spencer K, Cowans NJ, Nicolaides KH. Maternal serum inhibin-A and activin-A levels in the first trimester of pregnancies developing pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32(5):622–6. 10.1002/uog.6212 . [DOI] [PubMed] [Google Scholar]
- 34.Taylor BD, Ness RB, Olsen J, Hougaard DM, Skogstrand K, Roberts JM, et al. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension. 2015;65(3):594–9. 10.1161/HYPERTENSIONAHA.114.03979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaiman D, Mondon F, Garces-Duran A, Mignot TM, Robert B, Rebourcet R, et al. Hypoxia-activated genes from early placenta are elevated in preeclampsia, but not in Intra-Uterine Growth Retardation. BMC Genomics. 2005;6:111 10.1186/1471-2164-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2 Pt 2):337–42. 10.1161/hy02t2.102904 . [DOI] [PubMed] [Google Scholar]
- 37.Bytautiene E, Lu F, Tamayo EH, Hankins GD, Longo M, Kublickiene K, et al. Long-term maternal cardiovascular function in a mouse model of sFlt-1-induced preeclampsia. Am J Physiol Heart Circ Physiol. 2010;298(1):H189–93. 10.1152/ajpheart.00792.2009 . [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Azuma J, Kalish F, Wong RJ, Stevenson DK. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol Reprod. 2011;85(5):1005–12. 10.1095/biolreprod.111.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulletins—Obstetrics ACoP. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstetrics and gynecology. 2002;99(1):159–67. 10.1016/s0029-7844(01)01747-1 . [DOI] [PubMed] [Google Scholar]
- 40.Zou H, Hastie T. Regularization and variable selection via elastic net. J R Stat Soc B Methodol. 2005;67:301–20. [Google Scholar]
- 41.Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15). 10.1126/sciimmunol.aan2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laing EE, Moller-Levet CS, Dijk DJ, Archer SN. Identifying and validating blood mRNA biomarkers for acute and chronic insufficient sleep in humans: a machine learning approach. Sleep. 2019;42(1). 10.1093/sleep/zsy186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen L, Kim S, Qi Y, Inlow M, Swaminathan S, Nho K, et al. Identifying Neuroimaging and Proteomic Biomarkers for MCI and AD via the Elastic Net. Multimodal Brain Image Anal (2011). 2011;7012:27–34. 10.1007/978-3-642-24446-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das J, Gayvert KM, Bunea F, Wegkamp MH, Yu H. ENCAPP: elastic-net-based prognosis prediction and biomarker discovery for human cancers. BMC Genomics. 2015;16:263 10.1186/s12864-015-1465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30 Suppl A:S32–7. 10.1016/j.placenta.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. 10.1097/OGX.0b013e3182331028 . [DOI] [PubMed] [Google Scholar]
- 47.Robillard P-Y. Maternal and placental preeclampsia. Physiopathological and geographical differences. Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health. 2017;7:59 10.1016/j.preghy.2016.10.013. [DOI] [Google Scholar]
- 48.Pavlicev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome research. 2017;27(3):349–61. 10.1101/gr.207597.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140066 10.1098/rstb.2014.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gynecologists ACoOa. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstetrics and gynecology. 2002;99(1):159–67. Epub 2005/09/24. 10.1016/s0029-7844(01)01747-1 . [DOI] [PubMed] [Google Scholar]
- 51.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350(7):672–83. Epub 2004/02/07. 10.1056/NEJMoa031884 . [DOI] [PubMed] [Google Scholar]
- 52.Lapaire O, Grill S, Lalevee S, Kolla V, Hosli I, Hahn S. Microarray screening for novel preeclampsia biomarker candidates. Fetal diagnosis and therapy. 2012;31(3):147–53. 10.1159/000337325 . [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa H, Pryor-Koishi K, Kato T, Kowa H, Kurahashi H, Udagawa Y. Microarray analysis of differentially expressed fetal genes in placenta tissue derived from early and late onset severe preeclampsia. Placenta. 2007;28:487–97. 10.1016/j.placenta.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 54.Loset M, Mundal SB, Johnson MP, Fenstad MH, Freed KA, Lian IA, et al. A transcriptional profile of the decidua in preeclampsia. American journal of obstetrics and gynecology. 2011;204(1):84 e1-27. Epub 2010/10/12. 10.1016/j.ajog.2010.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson A, Loset M, Mundal SB, Johnson MP, Freed KA, Fenstad MH, et al. Partial correlation network analyses to detect altered gene interactions in human disease: using preeclampsia as a model. Human genetics. 2011;129(1):25–34. Epub 2010/10/12. 10.1007/s00439-010-0893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30(5):424–33. Epub 2009/03/03. 10.1016/j.placenta.2009.01.012 . [DOI] [PubMed] [Google Scholar]
- 57.Tsai S, Hardison NE, James AH, Motsinger-Reif AA, Bischoff SR, Thames BH, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32(2):175–82. Epub 2010/12/25. 10.1016/j.placenta.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–62. Epub 2008/09/27. 10.1210/en.2008-0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolla V, Jeno P, Moes S, Lapaire O, Hoesli I, Hahn S. Quantitative proteomic (iTRAQ) analysis of 1st trimester maternal plasma samples in pregnancies at risk for preeclampsia. Journal of biomedicine & biotechnology. 2012;2012:305964 Epub 2012/05/10. 10.1155/2012/305964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mary S, Patil GV, Kulkarni AV, Kulkarni MJ, Joshi SR, Mehendale SS, et al. Dynamic proteome in enigmatic preeclampsia: an account of molecular mechanisms and biomarker discovery. Proteomics Clinical applications. 2012;6(1–2):79–90. Epub 2012/03/27. 10.1002/prca.201100089 . [DOI] [PubMed] [Google Scholar]
- 61.Carty DM, Siwy J, Brennand JE, Zurbig P, Mullen W, Franke J, et al. Urinary proteomics for prediction of preeclampsia. Hypertension. 2011;57(3):561–9. 10.1161/HYPERTENSIONAHA.110.164285 . [DOI] [PubMed] [Google Scholar]
- 62.Erez O, Romero R, Maymon E, Chaemsaithong P, Done B, Pacora P, et al. The prediction of late-onset preeclampsia: Results from a longitudinal proteomics study. PloS one. 2017;12(7):e0181468 10.1371/journal.pone.0181468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Erez O, Maymon E, Chaemsaithong P, Xu Z, Pacora P, et al. The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study. American journal of obstetrics and gynecology. 2017;217(1):67 e1- e21. 10.1016/j.ajog.2017.02.037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarca AL, Romero R, Benshalom-Tirosh N, Than NG, Gudicha DW, Done B, et al. The prediction of early preeclampsia: Results from a longitudinal proteomics study. PloS one. 2019;14(6):e0217273 10.1371/journal.pone.0217273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosimann B, Amylidi-Mohr S, Holand K, Surbek D, Risch L, Raio L. Importance of Timing First-Trimester Placental Growth Factor and Use of Serial First-Trimester Placental Growth Factor Measurements in Screening for Preeclampsia. Fetal diagnosis and therapy. 2017;42(2):111–6. 10.1159/000455946 . [DOI] [PubMed] [Google Scholar]
- 66.Schubring C, Englaro P, Siebler T, Blum WF, Demirakca T, Kratzsch J, et al. Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Horm Res. 1998;50(5):276–83. 10.1159/000023290 . [DOI] [PubMed] [Google Scholar]
- 67.Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf). 1997;47(1):101–6. 10.1046/j.1365-2265.1997.2441017.x . [DOI] [PubMed] [Google Scholar]
- 68.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34(3):205–11. 10.1016/j.placenta.2012.11.035 . [DOI] [PubMed] [Google Scholar]
- 69.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertension in pregnancy: official journal of the International Society for the Study of Hypertension in Pregnancy. 2004;23(1):101–11. 10.1081/PRG-120028286 . [DOI] [PubMed] [Google Scholar]
- 70.Hirashima C, Ohkuchi A, Arai F, Takahashi K, Suzuki H, Watanabe T, et al. Establishing reference values for both total soluble Fms-like tyrosine kinase 1 and free placental growth factor in pregnant women. Hypertens Res. 2005;28(9):727–32. 10.1291/hypres.28.727 . [DOI] [PubMed] [Google Scholar]
- 71.Tsiakkas A, Duvdevani N, Wright A, Wright D, Nicolaides KH. Serum soluble fms-like tyrosine kinase-1 in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol. 2015;45(5):584–90. 10.1002/uog.14817 . [DOI] [PubMed] [Google Scholar]
- 72.Lage M, Garcia-Mayor RV, Tome MA, Cordido F, Valle-Inclan F, Considine RV, et al. Serum leptin levels in women throughout pregnancy and the postpartum period and in women suffering spontaneous abortion. Clin Endocrinol (Oxf). 1999;50(2):211–6. 10.1046/j.1365-2265.1999.00637.x . [DOI] [PubMed] [Google Scholar]
- 73.Muttukrishna S, Fowler PA, George L, Groome NP, Knight PG. Changes in peripheral serum levels of total activin A during the human menstrual cycle and pregnancy. The Journal of clinical endocrinology and metabolism. 1996;81(9):3328–34. 10.1210/jcem.81.9.8784092 . [DOI] [PubMed] [Google Scholar]
- 74.Ning Y, Williams MA, Muy-Rivera M, Leisenring WM, Luthy DA. Relationship of maternal plasma leptin and risk of pre-eclampsia: a prospective study. J Matern Fetal Neonatal Med. 2004;15(3):186–92. 10.1080/14767050410001668293 . [DOI] [PubMed] [Google Scholar]
- 75.Anim-Nyame N, Sooranna SR, Steer PJ, Johnson MR. Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum Reprod. 2000;15(9):2033–6. 10.1093/humrep/15.9.2033 . [DOI] [PubMed] [Google Scholar]
- 76.Bainbridge SA, Smith GN. HO in pregnancy. Free radical biology & medicine. 2005;38(8):979–88. 10.1016/j.freeradbiomed.2004.11.002 . [DOI] [PubMed] [Google Scholar]
- 77.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, et al. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2008;10(10):1767–812. 10.1089/ars.2008.2043 . [DOI] [PubMed] [Google Scholar]
- 78.Magarinos MP, Sanchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007;76(2):203–10. 10.1095/biolreprod.106.051391 . [DOI] [PubMed] [Google Scholar]
- 79.Perez-Perez A, Toro AR, Vilarino-Garcia T, Guadix P, Maymo JL, Duenas JL, et al. Leptin reduces apoptosis triggered by high temperature in human placental villous explants: The role of the p53 pathway. Placenta. 2016;42:106–13. 10.1016/j.placenta.2016.03.009 . [DOI] [PubMed] [Google Scholar]
- 80.Toro AR, Perez-Perez A, Corrales Gutierrez I, Sanchez-Margalet V, Varone CL. Mechanisms involved in p53 downregulation by leptin in trophoblastic cells. Placenta. 2015;36(11):1266–75. 10.1016/j.placenta.2015.08.017 . [DOI] [PubMed] [Google Scholar]
- 81.Toro AR, Maymo JL, Ibarbalz FM, Perez-Perez A, Maskin B, Faletti AG, et al. Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PloS one. 2014;9(6):e99187 10.1371/journal.pone.0099187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Perez A, Maymo J, Gambino Y, Duenas JL, Goberna R, Varone C, et al. Leptin stimulates protein synthesis-activating translation machinery in human trophoblastic cells. Biol Reprod. 2009;81(5):826–32. 10.1095/biolreprod.109.076513 . [DOI] [PubMed] [Google Scholar]
- 83.Perez-Perez A, Maymo J, Duenas JL, Goberna R, Calvo JC, Varone C, et al. Leptin prevents apoptosis of trophoblastic cells by activation of MAPK pathway. Arch Biochem Biophys. 2008;477(2):390–5. 10.1016/j.abb.2008.06.015 . [DOI] [PubMed] [Google Scholar]
- 84.Hoggard N, Haggarty P, Thomas L, Lea RG. Leptin expression in placental and fetal tissues: does leptin have a functional role? Biochem Soc Trans. 2001;29(Pt 2):57–63. 10.1042/0300-5127:0290057 . [DOI] [PubMed] [Google Scholar]
- 85.Perez-Perez A, Toro A, Vilarino-Garcia T, Maymo J, Guadix P, Duenas JL, et al. Leptin action in normal and pathological pregnancies. J Cell Mol Med. 2018;22(2):716–27. 10.1111/jcmm.13369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsur A, Kalish F, Burgess J, Nayak NR, Zhao H, Casey KM, et al. Pravastatin improves fetal survival in mice with a partial deficiency of heme oxygenase-1. Placenta. 2019;75:1–8. 10.1016/j.placenta.2018.11.001 . [DOI] [PubMed] [Google Scholar]
- 87.Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whiteley K, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279 10.1038/msb.2009.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malassine A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Human reproduction update. 2003;9(6):531–9. 10.1093/humupd/dmg043 . [DOI] [PubMed] [Google Scholar]
- 89.Soncin F, Khater M, To C, Pizzo D, Farah O, Wakeland A, et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development. 2018;145(2). 10.1242/dev.156273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shaarawy M, el-Mallah SY. Leptin and gestational weight gain: relation of maternal and cord blood leptin to birth weight. J Soc Gynecol Investig. 1999;6(2):70–3. 10.1016/s1071-5576(99)00003-9 . [DOI] [PubMed] [Google Scholar]
- 91.Karakosta P, Georgiou V, Fthenou E, Papadopoulou E, Roumeliotaki T, Margioris A, et al. Maternal weight status, cord blood leptin and fetal growth: a prospective mother-child cohort study (Rhea study). Paediatric and perinatal epidemiology. 2013;27(5):461–71. 10.1111/ppe.12074 . [DOI] [PubMed] [Google Scholar]
- 92.Liu LY, Yang T, Ji J, Wen Q, Morgan AA, Jin B, et al. Integrating multiple 'omics' analyses identifies serological protein biomarkers for preeclampsia. BMC Med. 2013;11:236 10.1186/1741-7015-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wright D, Tan MY, O'Gorman N, Poon LC, Syngelaki A, Wright A, et al. Predictive performance of the competing risk model in screening for preeclampsia. American journal of obstetrics and gynecology. 2019;220(2):199 e1–e13. 10.1016/j.ajog.2018.11.1087 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mann-Whitney U-test P-values are shown.
(PDF)
Maternal serum concentrations of (A) LEP and (B) LEP normalized to body mass index (BMI) (pg/mL/kg/m2) shown as a function of GA in normal term (red line) and PE (green line) pregnancies. Loess smooth function was applied. Color-coded dotted lines: show the 90% confidence interval for each cohort.
(PDF)
(PDF)
Signed differences in mean (PE minus normal; pg/mL) were calculated.
(PDF)
Data Availability Statement
We uploaded a dataset of ELISA results to our group’s public website at Stanford University (http://translationalmedicine.stanford.edu/data/PE_2020_1/ELISA/S2.file.xlsx).