Abstract
Introduction
Prognostic nutritional index (PNI) reflects the nutritional and immunologic status of the patients. The clinical application of PNI is already well-known in various kinds of solid tumors. However, there is no study investigating the relationship between PNI and oncological outcome of the resected ampulla of Vater (AoV) cancer.
Materials and methods
From January 2005 to December 2012, the medical records of patients who underwent pancreaticoduodenectomy for pathologically confirmed AoV cancer were retrospectively reviewed. Long-term oncological outcomes were compared according to the preoperative PNI value.
Result
A total of 118 patients were enrolled in this study. The preoperative PNI was 46.13±6.63, while the mean disease-free survival was 43.88 months and the mean disease-specific survival was 55.3 months. In the multivariate Cox analysis, initial CA19-9 (p = 0.0399), lymphovascular invasion (p = 0.0031), AJCC 8th N-stage (p = 0.0018), and preoperative PNI (p = 0.0081) were identified as significant prognostic factors for resected AoV cancer. The disease-specific survival was better in the high preoperative PNI group (≤48.85: 40.77 months vs. >48.85: 68.05 months, p = 0.0015). A highly accurate nomogram was developed based on four clinical components to predict the 1, 3, and 5-year disease-specific survival probability (C-index 0.8169, 0.8426, and 0.8233, respectively).
Conclusion
In resected AoV cancer, preoperative PNI can play a significant role as an independent prognostic factor for predicting disease-specific survival.
Introduction
Primary ampulla of Vater (AoV) cancer only occurs in 4 to 6 cases per million population, but it is responsible for 20% of all tumor-related obstructions of the common bile duct. The incidence of this cancer has increased over the last 30 years [1, 2]. Patients undergoing pancreaticoduodenectomy (PD) for AoV cancer have a 5-year disease-free survival of approximately 65%, and the 5-year disease-specific survival varies from 33.3% to 59.9% [3–6]. These results indicate a better prognosis than that in other types of periampullary cancers. As far as recent studies are concerned, the independent factors deciding AoV cancer outcomes are AJCC T/N staging [3–6], R-status [3, 4], tumor differentiation [1, 3–5], pathological tumor size [1, 5], Different histopathologic [7], perineural invasion [8], tumor budding [9] and extranodal extension of nodal metastasis [10].
Prognostic nutritional index (PNI) is an indicator of the nutritional and immunologic status of the patients [11, 12]. Recently, multiple studies have demonstrated its correlation with postoperative complications and cancer outcomes in various kinds of solid organ cancer, such as gastric cancer [13], small cell lung cancer [14], non-small cell lung cancer [15], ovarian cancer [12], pancreatic cancer [16, 17], colorectal cancer [18], hepatocellular carcinoma [19–21], esophageal cancer [22], and renal cell carcinoma [23]. However, there is no specific study investigating the potential relationships between PNI and AoV cancer. Therefore, in this study, we investigated the potential oncological impact of preoperative PNI on resected AoV cancer.
Materials and methods
Patients
This was a retrospective study involving patients who underwent PD at Severance Hospital, Seoul, Korea, between January 2005 and December 2012. Only patients with pathologically confirmed AoV adenocarcinoma were enrolled in our study. The medical records and demographic characteristics of the patients were retrospectively reviewed from the electrical medical record (EMR). All data were fully anonymized before assessment and were kept on saving materials under restricted access for only authorized clinicians. The present study has waived the requirement for informed consent because of minimal risk (level I) and approved by the institutional review board (IRB) of Severance Hospital at Yonsei University College of Medicine (IRB no. 4-2019-0379).
Preoperative and intraoperative measurements
Data on initial CA 19–9, initial total bilirubin, preoperative total bilirubin, albumin, lymphocyte count, and liver functions were collected and each PNI was calculated from the preoperative results [albumin (g/dL) × 10 + preoperative lymphocytic count × 0.005] [11]. In our study, adjusted preoperative CA 19–9 (serum CA 19–9 divided by serum total bilirubin) were applied as CA 19–9 level could be elevated from biliary obstruction, which could be helpful to reduce bias and to estimate true value of CA 19–9 [24–26].
Radiological tumor size and preoperative biliary drainage procedure were checked as each of them was known as associated with postoperative surgical outcome [27, 28]. The operation type, operation time, intraoperative estimated blood loss, and transfusion history were reviewed as covariates.
More than 90% of the patients had the operation in 6wks from the first diagnosis, and the average time from diagnosis to the operation was 18.5 days. During this period patients went further cancer evaluation and were treated for preoperative general conditions like jaundice or cholangitis.
Pathological and postoperative outcomes
Data regarding the pathological tumor size, total number of retrieved lymph nodes, number of metastatic lymph nodes, AJCC 8th TNM, perineural invasion, lymphovascular invasion, tumor differentiation, tumor gross type, R-status, and histological types were collected and examined by pathologists. The details of postoperative complications severe than Clavien-Dindo grade IIIa, postoperative pancreatic fistula (POPF) [29, 30], and adjuvant chemotherapy were collected. Adjuvant chemotherapy was selectively done under clinician’s decision in patients with advanced stages like AJCC 8th T stage higher than T2, positive lymph nodes, R1 resection, or positive perineural invasion. Long-term oncological outcomes were investigated, including disease-free survival (the duration after the pancreaticoduodenectomy to the date of diagnosis of recurrent AoV cancer) and disease-specific survival (the duration from the pancreaticoduodenectomy to the time of death from AoV cancer).
Statistical analyses
The continuous variables were expressed as the mean ± standard deviation, and the categorical variables were expressed as the frequency (%). Student’s t-test was performed with the continuous variables which were normally distributed, and Mann-Whitney U test used for the continuous variables which were not normally distributed. Chi-square test or Fisher’s extract test was used for the categorical variables.
To evaluate oncologic outcomes and survival analysis, the selection of statistically significant variables (p<0.05) was done, following univariate Cox regression test. These variables underwent multivariate Cox regression analysis to evaluate oncologic outcomes. Backward elimination used for final multivariate Cox regression results. Also Kaplan Meier survival analysis and log-rank test methods were used for survival analysis. [31–33]
For evaluating the discrimination of the predictive model, Harrell’s c-index was used for the nomogram model. The c-index and 95% confidence interval (CI) were shown after 10,000 times of bootstrap resampling. The proximity between the estimated and actual value was visually inspected with a calibration plot. The goodness of fit test was performed with GND (Greenwood-Nam-D’Agostino) test [34]. The cut-off value of PNI and CA 19–9 were calculated based on the Contal and O’quigley’s method [35–37]. SPSS Statistics version 23 was used for the analyses.
Results
General characteristics of the patients
A total of 118 patients were included in the study. Table 1 describes the demographic characteristics of the patients (Table 1). There were 64 males and 54 females with a mean age of 61.1 ± 10.2 years. The mean follow-up period was of 53.3 ± 34.3 months. A total of 69 patients (58.5%) survived, 49 patients (41.5%) died, and 77 patients (65.3%) received postoperative adjuvant chemotherapy.
Table 1. Demographic characteristics of the patients.
| Total | N = 118 (%) |
| Gender | |
| Male | N = 64 (54.2) |
| Female | N = 54 (45.8) |
| Age (Year) | 61.13 ± 10.23 |
| Follow-up (Month) | 53.3 ± 34.31 |
| Initial CA 19–9 (U/mL) (Adjusted) | 499.41 ± 2147.24 |
| Initial T.bil (mg/dL) | 4.45 ± 5.40 |
| Pre-OP T.bil (mg/dL) | 1.70 ± 1.78 |
| Pre-OP CEA (ng/mL) | 2.44 ± 2.36 |
| Pre-OP Bile drainage | N = 70 (59.3%) |
| PTBD | N = 12 (17.1%), |
| ENBD | N = 5 (7.1%), |
| ERBD | N = 51 (72.9%) |
| Dual manner* | 2 (2.9%) |
| OP method | |
| Open | N = 109 (92.4%) |
| Laparoscopic | N = 9 (7.6%) |
| Subtype | |
| Pancreatobiliary type | N = 63 (53.4%) |
| Intestinal type | N = 55 (46.6%) |
| Pathological T-stage | |
| Tis | N = 2 (1.7%) |
| 1a/1b | N = 7/30 (5.9%/25.4%) |
| 2 | N = 32 (27.1%) |
| 3a/3b | N = 19/28 (16.1%/23.7%) |
| Pathological N-stage | |
| N0 | N = 76 (64.4%) |
| N1 | N = 23 (19.5%) |
| N2 | N = 19 (16.1%) |
| Survival | |
| Survival | N = 69 (58.5%) |
| Death | N = 49 (41.5%) |
| Adjuvant Chemotherapy | |
| Yes | N = 77 (65.3%) |
| No | N = 41 (34.7%) |
CA 19–9, carbohydrate antigen 19–9; OP, operation; CEA, carcinoembryonic antigen; PTBD, percutaneous transhepatic biliary drainage; ENBD, endoscopic nasobiliary drainage; ERBD, endoscopic retrograde bile drainage
*PTBD followed by ERBD, ERBD followed by ENBD each.
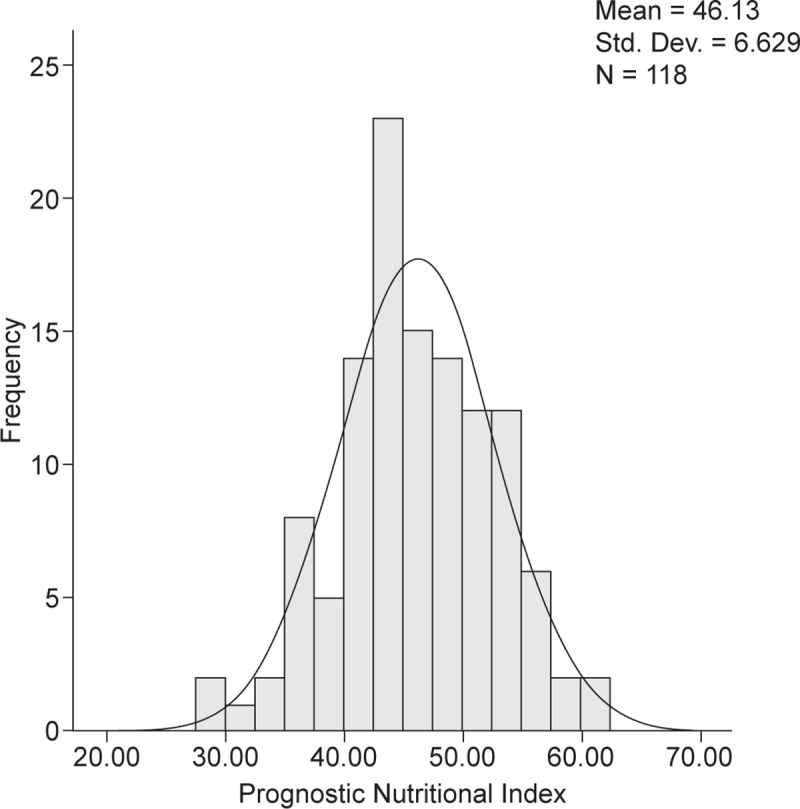
Fig 1 describes the distribution of preoperative PNI in the resected AoV cancer. The PNI was 46.13 ± 6.63 (median, 45.8) (Fig 1).
Fig 1. Distribution of the preoperative PNI in resected AoV cancer.
Survival analysis in resected AoV cancer
The mean disease-free survival was found as 43.88 months, (95% CI, 38.49−49.27) and the mean disease-specific survival was 55.3 months (95% CI, 50.53−60.05). In disease-specific survival, Table 2 shows the baseline characteristics and univariable Cox regression analysis for predicting cancer-related death in the resected AoV cancer (Table 2). The left side of the table shows baseline characteristics. Student’s t-test was done in continuous variables which fit normal distribution and Mann-Whitney U test was done in continuous variables which didn’t fit normal distribution. Categorical variables were analyzed with Chi-square test or Fisher’s exact test (Table 2, Left side of the table). Univariable Cox regression analysis was done in these variables (Table 2, Right side of the table).
Table 2. Baseline characteristics and univariate Cox regression analysis for predicting cancer-related death in resected AoV cancer.
(Left column: Baseline characteristics, Right column: univariate Cox regression analysis).
| Variables | Survival N = 77 | Death N = 41 | p-value | HR | Lower | Upper | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 62 (54 −67) | 66 (57 −70) | 0.0747 | 1.031 | 0.996 | 1.067 | 0.0835 | |||
| Gender | Male | 40 (51.95) | 24 (58.54) | 0.4940 | 1 (ref) | |||||
| Female | 37 (48.05) | 17 (41.46) | 0.745 | 0.400 | 1.386 | 0.3526 | ||||
| BMI (kg/m2) | 23.44±2.91 | 22.95±2.5 | 0.3689 | 0.941 | 0.844 | 1.050 | 0.2766 | |||
| Initial CA19-9 (U/mL) (cut-off)** (Adj.) $ $ | <53.19 | 55 (71.43) | 17 (42.46) | 0.0015* | 1 (ref) | |||||
| ≥53.19 | 22 (28.57) | 24(58.54) | 1.954 | 10.31 | 3.701 | 0.0399* | ||||
| Initial total bilirubin (mg/dL) | 1.50 (0.60 −4.10) | 5.30 (1.60 −10.60) | 0.0003* | 1.079 | 1.041 | 1.118 | < .0001* | |||
| PreOP total bilirubin (mg/dL) | 0.80 (0.50 −1.80) | 1.60 (0.90 −2.70) | 0.0028* | 1.140 | 1.006 | 1.292 | 0.0394* | |||
| Radiologic tumor size (mm) | 20 (14 −25) | 20 (15 −25) | 0.4808 | 0.997 | 0.966 | 1.029 | 0.8511 | |||
| PreOP-PNI (cut-off)** | PNI ≤48.85 | 45 (58.44) | 35 (85.37) | 0.0029* | 1 (ref) | |||||
| PNI >48.85 | 32 (41.56) | 6 (14.63) | 0.270 | 0.113 | 0.643 | 0.0031* | ||||
| PreOP-biliary drainage | No | 36 (46.75) | 12 (29.27) | 0.0656 | 1 (ref) | |||||
| Yes | 41 (53.25) | 29 (70.73) | 2.031 | 1.036 | 3.983 | 0.0392* | ||||
| Operation method | Open | 69 (89.61) | 40 (97.56) | 0.1595 | 1 (ref) | |||||
| Lapa | 8 (10.39) | 1 (2.44) | 0.226 | 0.031 | 1.648 | 0.1425 | ||||
| Operation time (min) | 390 (328 −460) | 409 (362 −487) | 0.3268 | 1.002 | 0.999 | 1.004 | 0.2501 | |||
| Estimated blood loss (ml) | 400 (200 −700) | 500 (200 −900) | 0.3865 | 1.000 | 1.000 | 1.001 | 0.1483 | |||
| Transfusion | No | 63 (81.82) | 32 (78.05) | 0.6226 | 1 (ref) | |||||
| Yes | 14 (18.18) | 9 (21.95) | 1.034 | 0.493 | 2.167 | 0.9295 | ||||
| Number of total retrieved LNs | 19 (11 −29) | 19 (11 −28) | 0.9459 | 1.001 | 0.979 | 1.022 | 0.9559 | |||
| Number of positive LNs | 0 (0 −0) | 2 (0 −4) | <0.0001* | 1.212 | 1.129 | 1.302 | < .0001* | |||
| Pathologic tumor size (mm) | 20 (15 −27) | 20 (17 −25) | 0.6931 | 0.995 | 0.966 | 1.024 | 0.7211 | |||
| Complication | No | 30 (38.96) | 11 (26.83) | 0.1876 | 1 (ref) | |||||
| Yes | 47 (61.04) | 30 (73.17) | 1.619 | 0.811 | 3.231 | 0.1719 | ||||
| POPF | No | 45 (58.44) | 24 (58.54) | 0.0841 | 1 (ref) | |||||
| Grade A | 22 (28.57) | 6 (14.63) | 0.554 | 0.226 | 1.356 | 0.1958 | ||||
| Grade B | 10 (12.99) | 10 (24.39) | 1.811 | 0.865 | 3.792 | 0.1151 | ||||
| Grade C | 0 (0) | 1 (2.44) | 2.133 | 0.288 | 15.787 | 0.4583 | ||||
| R-status | R0 | 75 (97.4) | 39 (95.12) | 0.6092 | 1 (ref) | |||||
| R1 | 2 (2.6) | 2 (4.88) | 2.175 | 0.522 | 9.059 | 0.2856 | ||||
| Perineural invasion | No | 71 (92.21) | 27 (65.85) | 0.0003* | 1 (ref) | |||||
| Yes | 6 (7.79) | 14 (34.15) | 3.141 | 1.640 | 6.017 | 0.0006* | ||||
| Lymphovascular invasion | No | 66 (85.71) | 25 (60.98) | 0.0023* | 1 (ref) | |||||
| Yes | 11 (14.29) | 16 (39.02) | 3.758 | 1.993 | 7.088 | < .0001* | ||||
| Subtype | PB | 34 (44.16) | 29 (70.73) | 0.0059* | 1 (ref) | |||||
| Int. | 43 (55.84) | 12 (29.27) | 0.341 | 0.173 | 0.671 | 0.0018* | ||||
| Gross type | Polypoid | 51 (66.23) | 27 (65.85) | 0.8411 | 1 (ref) | |||||
| Ulcerative | 14 (18.18) | 7 (17.07) | 0.922 | 0.403 | 2.110 | 0.8481$ | ||||
| Mixed | 2 (2.6) | 0 (0) | 0.918 | 0.052 | 16.154 | 0.9531$ | ||||
| Unknown | 10 (12.99) | 7 (17.07) | 1.369 | 0.599 | 3.132 | 0.4565$ | ||||
| Tumor grade | Well | 34 (44.16) | 8 (19.51) | 0.0176* | 1 (ref) | |||||
| Moderate | 40 (51.95) | 30 (73.17) | 2.733 | 1.249 | 5.978 | 0.0118* | ||||
| Poor | 3 (3.9) | 3 (7.32) | 7.286 | 1.900 | 27.942 | 0.0038* | ||||
| T-stage (AJCC 8th) | IA | 8 (10.39) | 1 (2.44) | 0.0217* | 1 (ref) | |||||
| IB | 25 (32.47) | 5 (12.2) | 1.743 | 0.204 | 14.920 | 0.6121 | ||||
| II | 20 (25.97) | 12 (29.27) | 4.469 | 0.581 | 34.382 | 0.1504 | ||||
| IIIA | 11 (14.29) | 8 (19.51) | 5.288 | 0.661 | 42.321 | 0.1166 | ||||
| IIIB | 13 (16.88) | 15 (36.59) | 8.274 | 1.090 | 62.793 | 0.0410* | ||||
| N-stage (AJCC 8th) | N0 | 60 (77.92) | 16 (39.02) | 0.0001* | 1 (ref) | |||||
| N1 | 9 (11.69) | 14 (34.15) | 4.335 | 2.099 | 8.954 | < .0001* | ||||
| N2 | 8 (10.39) | 11 (26.83) | 7.770 | 3.518 | 17.159 | < .0001* | ||||
| Postop-adjuvant chemotherapy | No | 52 (67.53) | 16 (39.02) | 0.0028* | 1 (ref) | |||||
| Yes | 25 (32.47) | 25 (60.98) | 2.303 | 1.227 | 4.324 | 0.0094* | ||||
| Recurrence | No | 62 (80.52) | 7 (17.07) | < .0001* | 1 (ref) | |||||
| Yes | 15 (19.48) | 34 (82.93) | 14.558 | 6.244 | 33.943 | < .0001* | ||||
HR, hazard ratio; BMI, body mass index; PreOP, preoperative; LN, lymph node; PNI, prognostic nutritional index; POPF, postoperative pancreatic fistula; PB, pancreatobiliary type; Int., intestinal type.
*p-value <0.05
$Using firth bias correction for the estimation of 95% CI [38].
$ $Adj. = Adjusted
Among the preoperative factors, adjusted initial CA19-9 of ≥53.19 (p = 0.0015), initial/preoperative total bilirubin (p = 0.0003, p = 0.0028 respectively), and preoperative PNI of ≤48.85 (p = 0.0029) were noted as significant variables. The number of positive metastatic lymph node was found as a significant variable among the intraoperative factor in predicting the survival (p<0.0001). Among the postoperative factors, it was found that perineural invasion (p = 0.0003), lymphovascular invasion (p = 0.0023), subtype of tumor (p = 0.0059), tumor grade (p = 0.0176), AJCC 8th T/N-stage (p = 0.0217, p = 0.0001 each), postoperative adjuvant chemotherapy (p = 0.0028), and recurrence (p = <0.0001) were significant [1, 3–6].
Preoperative PNI as an independent prognostic factor
Multivariate Cox analysis was used to predict the significant prognostic factors in resected AoV cancer (Table 3). Adjusted initial CA 19–9 [HR = 1.954 (95% CI, 1.031−3.701), p = 0.0399], lymphovascular invasion [HR = 2.775 (95% CI, 1.412−5.452), p = 0.0031], AJCC 8th N stage [N-Stage 1: HR = 3.282 (95% CI, 1.553−6.932), p = 0.0018; N-Stage 2: HR = 4.978 (95% CI, 2.122−11.676), p = 0.0002, respectively], and PreOP-PNI [HR = 0.300 (95% CI, 0.123−0.732), p = 0.0081] were identified as important factors for disease-specific survival in resected AoV cancer.
Table 3. Multivariate Cox analysis for predicting the disease-specific survival in resected AoV cancer.
| Variables | Death (0: survival, 1: death) | ||||
|---|---|---|---|---|---|
| HR | Lower | Upper | p-value | ||
| Initial CA19 (U/mL) (Adjusted) | 0: CA19 <53.19 | 1 (ref) | |||
| 1: CA19 ≥53.19 | 1.954 | 1.031 | 3.701 | 0.0399 | |
| Lymphovascular invasion | 0: No | 1 (ref) | |||
| 1: Yes | 2.775 | 1.412 | 5.452 | 0.0031 | |
| AJCC8_Nstage | 0: No | 1 (ref) | |||
| 1: N1 | 3.282 | 1.553 | 6.932 | 0.0018 | |
| 2: N2 | 4.978 | 2.122 | 11.676 | 0.0002 | |
| PreOP-PNI | 0: PNI ≤48.85 | 1 (ref) | |||
| 1: PNI >48.85 | 0.300 | 0.123 | 0.732 | 0.0081 | |
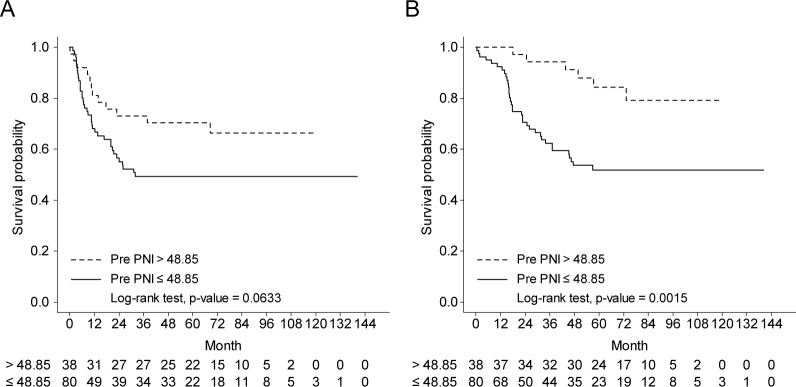
It was estimated that the disease-free survival was different according to the preoperative PNI with a marginal significance [≤48.85: 21.75 months (95% CI, 19.03−24.4) vs. >48.85: 51.88 months (95% CI, 43.10−60.66), p = 0.0633, Fig 2A]. However, significant difference showed in disease-specific survival according to preoperative PNI [≤48.85: 40.77 months (95% CI, 36.28−45.26), vs. <48.85: 68.05 months (95% CI, 63.02−73.06) p = 0.0015, Fig 2B].
Fig 2. Long term oncological outcomes according to the preoperative PNI in resected AoV cancer.
(A) Disease-free survival. (B) Disease-specific survival.
Survival analyses at PNI high/low group under stratification was done to evaluate if PNI correlates with disease status and to exclude bias from our study (Table 4.). However it showed no difference of important factors for disease-specific survival in resected AoV cancer even after stratify with high/low PNI group (Initial CA19-9, Lymphovascular invasion, AJCC 8th N stage at common) (Table 5.).
Table 4. Univariate Cox analysis for predicting the disease-specific survival in resected AoV cancer.
(PNI group stratification).
| Variables | Low group N = 80 | High group N = 38 | p-value | HR | Lower | Upper | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 62 (56–69) | 59 (51–66) | 0.034* | 1.019 | 0.985 | 1.055 | 0.279 | |||
| Gender | Male | 47 (58.8) | 17 (44.7) | 0.219 | 1 (ref) | |||||
| Female | 33 (41.3) | 21 (55.3) | 0.883 | 0.504 | 1.548 | 0.664 | ||||
| BMI (kg/m2) | 22.86±2.65 | 24.14±2.85 | 0.018* | 0.973 | 0.870 | 1.089 | 0.639 | |||
| Initial CA19-9 (U/mL) (cut-off)** (Adj.) $ $ | <53.19 | 42(52.5) | 30(78.9) | 0.011* | 1 (ref) | |||||
| ≥53.19 | 38(47.5) | 8(21.1) | 2.643 | 1.503 | 4.649 | 0.001* | ||||
| Initial total bilirubin (mg/dL) | 5.52 (1.67–9.37) | 2.21 (1.31–3.11) | <0.001* | 1.062 | 1.023 | 1.102 | 0.002* | |||
| PreOP total bilirubin (mg/dL) | 2.05 (1.00–3.10) | 0.97 (0.67–1.27) | 0.001* | 1.035 | 0.938 | 1.227 | 0.304 | |||
| Radiologic tumor size (mm) | 21 (16–26) | 20 (15–26) | 0.568 | 0.995 | 0.963 | 1.028 | 0.761 | |||
| PreOP-biliary drainage | No | 24(30.0) | 24(63.2) | 0.001* | 1 (ref) | |||||
| Yes | 56(70.0) | 14(36.8) | 1.453 | 0.812 | 2.598 | 0.208 | ||||
| Operation method | Open | 77(96.3) | 32(84.2) | 0.030* | 1 (ref) | |||||
| Lapa | 3(3.8) | 6(15.8) | 0.183 | 0.025 | 1.323 | 0.092 | ||||
| Operation time (min) | 406 (356–457) | 423 (347–500) | 0.584 | 1.002 | 0.999 | 1.004 | 0.183 | |||
| Estimated blood loss (ml) | 550 (256–844) | 538 (263–813) | 0.906 | 1.000 | 1.000 | 1.001 | 0.137 | |||
| Transfusion | No | 62(77.5) | 33(86.8) | 0.343 | 1 (ref) | |||||
| Yes | 18(22.5) | 5(13.2) | 1.017 | 0.520 | 1.991 | 0.960 | ||||
| Number of total retrieved LNs | 23 (15–31) | 20 (9–31) | 0.235 | 1.000 | 0.978 | 1.023 | 0.971 | |||
| Number of positive LNs | 2 (1–4) | 0 (0–0) | 0.132 | 1.183 | 1.101 | 1.271 | <0.001* | |||
| Pathologic tumor size (mm) | 23 (18–28) | 22 (15–29) | 0.768 | 0.992 | 0.963 | 1.021 | 0.584 | |||
| Complication | No | 32(40.0) | 9(23.7) | 0.125 | 1 (ref) | |||||
| Yes | 48(60.0) | 29(76.3) | 1.128 | 0.626 | 2.032 | 0.688 | ||||
| POPF | No | 53(66.3) | 16(42.1) | <0.001* | 1 (ref) | |||||
| Grade A | 9(11.3) | 19(50.0) | 0.817 | 0.398 | 1.677 | 0.582 | ||||
| Grade B | 18(22.5) | 2(5.3) | 1.379 | 0.652 | 2.916 | 0.400 | ||||
| Grade C | 0(0) | 1(2.6) | 3.635 | 0.487 | 27.127 | 0.208 | ||||
| R-status | R0 | 76(95.0) | 38(100.0) | 0.304 | 1 (ref) | |||||
| R1 | 4(5.0) | 0(0) | 2.727 | 0.842 | 8.834 | 0.094 | ||||
| Perineural invasion | No | 64(80.0) | 34(89.5) | 0.308 | 1 (ref) | |||||
| Yes | 16(20.0) | 4(10.5) | 2.584 | 1.403 | 4.762 | 0.002* | ||||
| Lymphovascular invasion | No | 59(73.8) | 32(84.2) | 0.303 | 1 (ref) | |||||
| Yes | 21(26.2) | 6(15.8) | 2.531 | 1.386 | 4.621 | 0.003* | ||||
| Subtype | PB | 49(61.2) | 14(36.8) | 0.022* | 1 (ref) | |||||
| Int. | 31(38.8) | 24(63.2) | 0.226 | 0.115 | 0.444 | <0.001* | ||||
| Gross type | Polypoid | 48(60.0) | 30(79.0) | 0.255 | 1 (ref) | |||||
| Ulcerative | 17(21.3) | 4(10.5) | 2.200 | 0.828 | 5.846 | 0.114 $ | ||||
| Mixed | 2(2.5) | 0(0) | 2.000 | 0.120 | 33.270 | 0.629 $ | ||||
| Unknown | 13(16.2) | 4(10.5) | 3.667 | 1.220 | 11.021 | 0.021 $ | ||||
| Tumor grade | Well | 26(32.5) | 16(42.1) | 0.535 | 1 (ref) | |||||
| Moderate | 50(62.5) | 20(52.6) | 3.333 | 1.547 | 7.181 | 0.002* | ||||
| Poor | 4(5.0) | 2(5.3) | 9.910 | 32.05 | 30.639 | <0.001* | ||||
| T-stage (AJCC 8th) | IA | 4(5.0) | 5(13.2) | 0.120 | 1 (ref) | |||||
| IB | 18(22.5) | 12(31.6) | 15786.3 | 0.000 | 1.246 E+75 | 0.908 | ||||
| II | 20(25.0) | 12(31.6) | 27052.2 | 0.000 | 2.134 E+75 | 0.902 | ||||
| IIIA | 16(20.0) | 3(7.8) | 48475.2 | 0.000 | 3.825 E+75 | 0.897 | ||||
| IIIB | 22(27.5) | 6(15.8) | 89926.6 | 0.000 | 7.092 E+75 | 0.891 | ||||
| N-stage (AJCC 8th) | N0 | 49(61.2) | 27(71.1) | 0.247 | 1 (ref) | |||||
| N1 | 15(18.8) | 8(21.1) | 4.281 | 2.141 | 8.563 | <0.001* | ||||
| N2 | 16(20.0) | 3(7.8) | 9.764 | 4.879 | 19.540 | <0.001* | ||||
| Postop-adjuvant chemotherapy | No | 42(52.5) | 27(71.1) | 0.087 | 1 (ref) | |||||
| Yes | 38(47.5) | 11(28.9) | 2.502 | 1.406 | 4.453 | 0.002* | ||||
HR, hazard ratio; BMI, body mass index; PreOP, preoperative; LN, lymph node; PNI, prognostic nutritional index; POPF, postoperative pancreatic fistula; PB, pancreatobiliary type; Int., intestinal type.; E+, exponential
*p-value <0.05
$Using firth bias correction for the estimation of 95% CI [38].
$ $Adj. = Adjusted
Table 5. Multivariate Cox analysis for predicting the disease-specific survival in resected AoV cancer (PNI group stratification).
| Variables | Death (0: survival, 1: death) | ||||
|---|---|---|---|---|---|
| HR | Lower | Upper | p-value | ||
| Initial CA19 (Adjusted) (U/mL) | 0: CA19 <53.19 | 1 (ref) | |||
| 1: CA19 ≥53.19 | 2.058 | 1.073 | 3.937 | 0.030 | |
| Lymphovascular invasion | 0: No | 1 (ref) | |||
| 1: Yes | 2.703 | 1.372 | 5.319 | 0.004 | |
| AJCC8_Nstage | 0: No | 1 (ref) | |||
| 1: N1 | 3.341 | 1.584 | 7.047 | 0.002 | |
| 2: N2 | 4.828 | 2.062 | 11.308 | <0.001 | |
Statistical analysis of disease free-survival is noted on S1 and S2 File. S2 File is the analysis of disease free survival after PNI stratification. S1 File showed that adjusted CA 19–9, preoperative T.bilirubin positive lymph node number and subtype of adenocarcinoma were relevant with oncologic survival. S2 File showed adjusted initial CA 19–9, the subtype of adenocarcinoma, and AJCC 8th N stage had statistical significance to disease free survival after PNI stratification.
Developing nomogram to predict cancer-specific survival
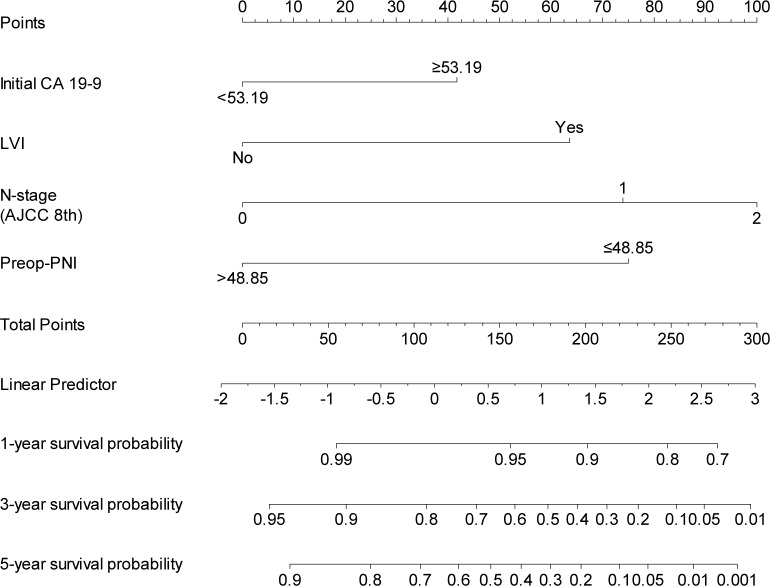
Based on the significant independent variables, such as adjusted initial CA 19–9, lymphovascular invasion, AJCC N-stage, and preoperative PNI, a nomogram for predicting the 1, 3, and 5-year survival probability in resected AoV cancer was developed (Fig 3).
Fig 3. Nomogram to predict the disease-specific survival in resected AoV cancer.
Model performance and calibration
The performance of the nomogram was assessed with Harrell’s C-index (Table 6). The c-index and 95% CI were shown after 10,000 times of bootstrap resampling. Every single average C-index was noted to be >0.80 with a narrow confidence interval suggesting that the currently developed nomogram model was highly predictive.
Table 6. Model performance.
| Overall | 1-year | 3-year | 5-year | ||||
|---|---|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | C-index | 95% CI | C-index | 95% CI |
| 0.8171 | 0.7558−0.8737 | 0.8169 | 0.6531−0.9643 | 0.8426 | 0.7773−0.9024 | 0.8233 | 0.7622−0.8812 |
C-index: <0.5 (very poor model), 0.5 (no better than random change), 0.7−0.8 (good model), >0.8 (strong model), 1 (perfectly predicts a certain outcome).
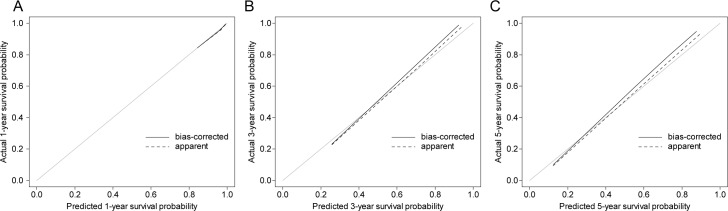
The calibration plot was made by comparing the predictive value with the real value. Considering that approximation with the 45-degree oblique dotted line estimates better results, the present calibration plot suggests that our nomogram has an acceptable accuracy in predicting the survival in resected AoV cancer (Fig 4).
Fig 4.
Calibration plot (A) predicted the 1-year survival probability, (B) predicted the 3-year survival probability, and (C) predicted the 5-year survival probability.
Discussion
In cancer patients, it is well known that the nutritional status is a conclusive independent factor for the postoperative outcomes [12]. In addition, nutrition correlates with general immunological functions and internal metabolisms. One commonly used indicator for nutrition is the PNI, which is calculated by using two clinical variables: preoperative albumin and lymphocytic count in the blood [11]. Recently, multiple studies have shown that preoperative PNI is a good predictive factor for estimating cancer outcome after cancer surgery [11], such as gastric cancer [13], esophageal cancer [22], hepatocellular cancer [19–21], pancreatic cancer [16, 17], colorectal cancer [18], renal cell carcinoma [23], non-small cell lung cancer [15], and small cell lung cancer [14].
TNM staging, recurrence, pathological tumor size, and tumor differentiation are the factors for predicting the postoperative oncological outcome of resected AoV cancer [1, 3–6]. Till now, no study has reported the potential oncological impact of preoperative PNI in resected AoV cancer. In this study, it has been successfully demonstrated that there is a potential association between the preoperative PNI and the long-term oncological outcome in resected AoV cancer. In this study, in the univariate analysis, the adjusted initial CA19-9 of ≥53.19, initial/preoperative total bilirubin, preoperative PNI of ≤48.85, number of positive metastatic lymph nodes, perineural invasion, lymphovascular invasion, subtype of the tumor, tumor grade, AJCC 8th T/N-stage, postoperative adjuvant chemotherapy, and recurrence were identified as significant variables to predict cancer-related survival, concurrent to previous studies [3–6]. The subsequent multivariate Cox analysis found that preoperative PNI can predict the long-term survival [HR = 0.300 (95% CI, 0.123−0.732), p = 0.0081] along with other well-known clinical parameters, such as adjusted initial CA 19–9 (p = 0.0399), lymphovascular invasion (p = 0.0031), and AJCC 8th N staging (p<0.05). Although there were no significant differences in the disease-free survival [Preop-PNI of ≤48.85: 21.75 months (95% CI, 19.03−24.4) vs. preop-PNI of >48.85: 51.88 months (95% CI, 43.10−60.66), p = 0.0633], it was found that the higher Preop-PNI group showed a significant positive oncological impact on the disease-specific survival in resected AoV cancer [40.77 months (95% CI, 36.28−45.26] vs. 68.04 months (95% CI, 63.02−73.06), p = 0.0015].
These results display that the potentiality of PNI is not inferior to that of the well-known predictive factors. Further, PNI could play a role as an independent factor influencing the overall survival. Without pathological confirmation, we can simply calculate PNI from the basic laboratory results and can predict only with the imaging study. This can be helpful for preoperative risk assessment and diagnosis of the hazardous group. Though it had no effect on the disease-free survival, patients who had a higher PNI of 48.85 had a significant benefit on the disease-specific survival in resected AoV cancer with an HR of 0.300 (p = 0.0081). In addition, we’ve done stratified survival analyses to evaluate if preOP PNI indicates the advanced disease stage. In multivariate Cox analysis, there was no difference of statistically significant variables in disease-specific survival in resected AoV cancer (Adjusted initial CA19-9 HR 0.486, p = 0.030; Lymphovascular invasion HR 2.703, p = 0.004; AJCC 8th N stage N1-HR 3.341, p = 0.002 / N2-HR 4.828, p<0.001).
In addition, preoperative a PNI-based nomogram was developed to calculate the postoperative long-term oncological outcomes in resected AoV cancer. Predicting the power assessed with Harrell’s C-index showed PNI-based nomogram works well, and the survival probability at 1-year, 3-year, and 5-year showed the C-index of >0.80 with a short 95% CI range.
As mentioned before, the most well-known prognostic factors for resected AoV cancer are mostly determined based on pathological examination after surgical excision. However, surgeons or clinicians can easily calculate the preoperative PNI from routine blood laboratory tests. Therefore, it is anticipated that the present study can be helpful in predicting the postoperative long-term oncological outcomes clinically prior to the surgical approach. It suggests that the oncological outcomes can be modulated by surgeons or clinicians before surgery. Unlike other prognostic factors, such as adjusted initial CA 19–9, lymphovascular invasion, and N-stage, preoperative PNI is thought to be affected by patient’s general condition and nutritional status, which can be improved by appropriate preoperative management, such as nutritional support or conservative management for improving the general condition.
Although patients with AoV cancers have a typical characteristic that presents a history of obstructive jaundice, our results showed the growing type of tumor did not have much effect on preoperative PNI by chronic loss of appetite with slow-growing. (fast-growing pancreatobiliary type PNI-median 44.9, slow-growing intestinal type PNI-median 47.5, p = 0.032)
Albumin, prealbumin, and transferrin are well known prognostic factors in various kinds of solid cancer [39–46]. Albumin can be routinely checked and the impact of albumin is thought to be incorporated in the concept of PNI (albumin (g/dL)×10 + preoperative lymphocytic count×0.005). In this study, PNI is currently known as a better factor in reflecting prognosis than albumin. However, our center does not routinely check prealbumin and transferrin. Therefore, the potential oncologic impact of these factors in managing AoV cancer needs to be further investigated in near future.
Even though the exact mechanism how PNI affects cancer outcomes is not understood yet, nowadays researchers are focusing on immunity and nutritional factor [47–50]. It is hypothesized that patients with high PNI may have the appropriate general conditions, as result, they can be easily presumed to have better compliance at adjuvant treatment, which could make difference in long term oncologic outcomes. The mechanism of PNI and the way to improve preoperative PNI are potential topics to be investigated in the near future by our further studies.
Our study has several limitations. It had a retrospective study design and a limited number of patients were included. The nomogram developed also needs external validation. Further study is necessary to reconfirm the potential association between preoperative PNI and long-term oncological outcomes based on a large study population. In summary, the present study showed that preoperative PNI was an independent prognostic factor for predicting the long-term oncological outcomes in resected AoV cancer. This is the first study to show the potential oncological impact of preoperative PNI in resected AoV cancer, suggesting that improving the preoperative PNI can result in a positive oncological impact in resected AoV cancer.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100(7):598–605. Epub 2009/08/22. 10.1002/jso.21374 . [DOI] [PubMed] [Google Scholar]
- 2.Palazzo L. Staging of ampullary carcinoma by endoscopic ultrasonography. Endoscopy. 1998;30 Suppl 1:A128–31. Epub 1998/10/09. 10.1055/s-2007-1001493 . [DOI] [PubMed] [Google Scholar]
- 3.Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the Ampulla of Vater: Experience With Local or Radical Resection in 171 Consecutively Treated Patients. JAMA Surgery. 1999;134(5):526–32. 10.1001/archsurg.134.5.526 [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg. 2011;100(2):92–8. Epub 2011/07/09. 10.1177/145749691110000205 . [DOI] [PubMed] [Google Scholar]
- 5.Junrungsee S, Kittivarakul E, Ko-iam W, Lapisatepun W, Sandhu T, Chotirosniramit A. Prognostic Factors and Survival of Patients with Carcinoma of the Ampulla of Vater after Pancreaticoduodenectomy. Asian Pac J Cancer Prev. 2017;18(1):225–9. Epub 2017/02/28. 10.22034/APJCP.2017.18.1.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomiyasu S, Oda E, Tanaka H, Ishikawa S, Sugita H, Arita T, et al. Prognostic factor of carcinoma of the ampulla of vater after surgery. Journal of Clinical Oncology. 2015;33(3_suppl):270–. 10.1200/jco.2015.33.3_suppl.270 [DOI] [Google Scholar]
- 7.Zhou Y, Li D, Wu L, Si X. The histopathologic type predicts survival of patients with ampullary carcinoma after resection: A meta-analysis. Pancreatology. 2017;17(2):273–8. Epub 2017/01/31. 10.1016/j.pan.2017.01.007 . [DOI] [PubMed] [Google Scholar]
- 8.Luchini C, Veronese N, Nottegar A, Riva G, Pilati C, Mafficini A, et al. Perineural Invasion is a Strong Prognostic Moderator in Ampulla of Vater Carcinoma: A Meta-analysis. Pancreas. 2019;48(1):70–6. Epub 2018/11/20. 10.1097/MPA.0000000000001194 . [DOI] [PubMed] [Google Scholar]
- 9.Ohike N, Coban I, Kim GE, Basturk O, Tajiri T, Krasinskas A, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol. 2010;34(10):1417–24. Epub 2010/09/28. 10.1097/PAS.0b013e3181f0b05a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, et al. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016;28(2):205–9. Epub 2015/11/14. 10.1097/MEG.0000000000000520 . [DOI] [PubMed] [Google Scholar]
- 11.Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai zasshi. 1984;85(9):1001–5. . [PubMed] [Google Scholar]
- 12.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1537–49. Epub 2014/06/01. 10.1007/s00432-014-1714-3 . [DOI] [PubMed] [Google Scholar]
- 13.Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–54. Epub 2013/03/07. 10.1245/s10434-013-2926-5 . [DOI] [PubMed] [Google Scholar]
- 14.Hong S, Zhou T, Fang W, Xue C, Hu Z, Qin T, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389–97. Epub 2014/12/21. 10.1007/s13277-014-2973-y . [DOI] [PubMed] [Google Scholar]
- 15.Mori S, Usami N, Fukumoto K, Mizuno T, Kuroda H, Sakakura N, et al. The Significance of the Prognostic Nutritional Index in Patients with Completely Resected Non-Small Cell Lung Cancer. PLoS One. 2015;10(9):e0136897 Epub 2015/09/12. 10.1371/journal.pone.0136897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–74. Epub 2010/10/21. 10.1002/bjs.7305 . [DOI] [PubMed] [Google Scholar]
- 17.Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41(11):1508–14. Epub 2015/09/08. 10.1016/j.ejso.2015.07.022 . [DOI] [PubMed] [Google Scholar]
- 18.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–92. Epub 2013/07/26. 10.1007/s00268-013-2156-9 . [DOI] [PubMed] [Google Scholar]
- 19.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106(8):1439–45. Epub 2012/03/22. 10.1038/bjc.2012.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39(6):1501–9. Epub 2015/02/12. 10.1007/s00268-015-2982-z . [DOI] [PubMed] [Google Scholar]
- 21.Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. 2015;22(13):4138–48. Epub 2015/03/25. 10.1245/s10434-015-4516-1 . [DOI] [PubMed] [Google Scholar]
- 22.Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396–400. Epub 2002/07/09. 10.1053/ejso.2002.1257 . [DOI] [PubMed] [Google Scholar]
- 23.Hofbauer SL, Pantuck AJ, de Martino M, Lucca I, Haitel A, Shariat SF, et al. The preoperative prognostic nutritional index is an independent predictor of survival in patients with renal cell carcinoma. Urol Oncol. 2015;33(2):68 e1–7. Epub 2014/09/23. 10.1016/j.urolonc.2014.08.005 . [DOI] [PubMed] [Google Scholar]
- 24.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Sasaki K, Furukawa H, et al. Serum CA19-9 is a Significant Predictor among Preoperative Parameters for Early Recurrence after Resection of Pancreatic Adenocarcinoma. Journal of Gastrointestinal Surgery. 2012;16(5):977–85. 10.1007/s11605-012-1859-9 [DOI] [PubMed] [Google Scholar]
- 25.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. The American Journal of Surgery. 2009;198(3):333–9. 10.1016/j.amjsurg.2008.12.031 [DOI] [PubMed] [Google Scholar]
- 26.Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, et al. The Use of Adjusted Preoperative CA 19–9 to Predict the Recurrence of Resectable Pancreatic Cancer. Journal of Surgical Research. 2007;140(1):31–5. 10.1016/j.jss.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 27.Lillemoe KD. Preoperative biliary drainage and surgical outcome. Annals of surgery. 1999;230(2):143–4. 10.1097/00000658-199908000-00002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Liu H, Li Z, Yang C, Sun Y, Wang C. Long-term prognosis of surgical treatment for early ampullary cancers and implications for local ampullectomy. BMC surgery. 2015;15:32–. 10.1186/s12893-015-0019-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. Epub 2004/07/27. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. 10.1016/j.surg.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 31.Núñez E, Steyerberg EW, Núñez J. Regression Modeling Strategies. Revista Española de Cardiología (English Edition). 2011;64(6):501–7. 10.1016/j.rec.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Simon R, Altman DG. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994;69(6):979–85. 10.1038/bjc.1994.192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steyerberg EW, Eijkemans MJ, Harrell FE Jr., Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Medical decision making: an international journal of the Society for Medical Decision Making. 2001;21(1):45–56. Epub 2001/02/24. 10.1177/0272989x0102100106 . [DOI] [PubMed] [Google Scholar]
- 34.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Statistics in medicine. 2015;34(10):1659–80. Epub 2015/02/11. 10.1002/sim.6428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers J, Mandrekar J, editors. Cutpoint Determination Methods in Survival Analysis using SAS®: Updated% FINDCUT macro. Proc SAS Glob Forum; 2015.
- 36.Mandrekar J, Mandrekar S, Cha S, editors. Cutpoint determination methods in survival analysis using SAS®. Proceedings of the 28th SAS Users Group International Conference (SUGI); 2003.
- 37.Klein JP, Wu J-TJHoS. Discretizing a continuous covariate in survival studies. 2003;23:27–42. [Google Scholar]
- 38.Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: A SAS macro for proportional and nonproportional subdistribution hazards regression. Computer Methods and Programs in Biomedicine. 2015;118(2):218–33. 10.1016/j.cmpb.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni X-C, Xu J, Yi Y, Fu Y-P, Cai X-Y, Liu G, et al. Inflammation–nutrition score predicts prognosis of patients with resectable hepatocellular carcinoma. 2019;24(7):825–35. 10.1007/s10147-019-01402-4 [DOI] [PubMed] [Google Scholar]
- 40.González-Trejo S, Carrillo JF, Carmona-Herrera DD, Baz-Gutiérrez P, Herrera-Goepfert R, Núñez G, et al. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: A retrospective cohort study. Medicine (Baltimore). 2017;96(15):e6610–e. 10.1097/MD.0000000000006610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Huang D. The value of the systematic inflammation-based Glasgow Prognostic Score in patients with gastric cancer: A literature review. 2014;10(4):799–804. 10.4103/0973-1482.146054 [DOI] [PubMed] [Google Scholar]
- 42.Kaya V, Yildirim M, Demirpence O, Yildiz M, Yalcin AYJAPJCP. Prognostic significance of basic laboratory methods in non-small-cell-lung cancer. 2013;14(9):5473–6. [DOI] [PubMed] [Google Scholar]
- 43.Aydın Y, Kaplan I, Gundogdu B, Albayrak B, Turkyilmaz A, Eroglu AJTJTCS. Prognostic importance of serum CRP, prealbumin, and transferrin levels in patients with advanced stage esophageal cancer. 2011;19(3):384–90. [Google Scholar]
- 44.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutrition Journal. 2010;9(1):69 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crumley ABC, Stuart RC, McKernan M, McMillan DCJWJoS. Is Hypoalbuminemia an Independent Prognostic Factor in Patients with Gastric Cancer? 2010;34(10):2393–8. 10.1007/s00268-010-0641-y [DOI] [PubMed] [Google Scholar]
- 46.Milano G, Cooper EH, Goligher JC, Giles GR, Neville AM. Serum Prealbumin, Retinol-Binding Protein, Transferrin, and Albumin Levels in Patients With Large Bowel Cancer2. JNCI: Journal of the National Cancer Institute. 1978;61(3):687–91. 10.1093/jnci/61.3.687%JJNCI Journal of the National Cancer Institute. [DOI] [PubMed] [Google Scholar]
- 47.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki MJWJoS. Prognostic Nutritional Index Predicts Postoperative Outcome in Colorectal Cancer. 2013;37(11):2688–92. 10.1007/s00268-013-2156-9 [DOI] [PubMed] [Google Scholar]
- 48.Cheung K, Lee SS, Raman M. Prevalence and Mechanisms of Malnutrition in Patients With Advanced Liver Disease, and Nutrition Management Strategies. Clinical Gastroenterology and Hepatology. 2012;10(2):117–25. 10.1016/j.cgh.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 49.Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World Journal of Surgical Oncology. 2016;14(1):170 10.1186/s12957-016-0920-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng J-F, Chen Q-X. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag. 2014;10:1–7. Epub 2013/12/16. 10.2147/TCRM.S56159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.