Abstract
Pancreatic ductal adenocarcinoma behaves aggressively, with surgically resectable disease having the best chance of long-term survival. Recurrence after surgery and adjuvant therapy is commonly due to distant metastatic disease and is typically managed with systemic therapies, not surgery. We present a rare case of an isolated gastric metastasis due to endoscopic ultrasound-guided with fine-needle aspiration (EUS-FNA) needle tract seeding that was managed surgically. Treatment was informed by input from a mutlidisciplinary team of medical, surgical, and radiation oncologists, radiologists, and pathologists. Rising carbohydrate antigen (CA)19-9 levels suggested disease recurrence, but the tumor’s unusual location and slow growth made diagnosing the cause difficult, resulting in the late identification of the tumor. Palliative resection was performed, rending the patient with no evidence of disease followed by normalized CA19-9 levels. This case highlights the importance of multidisciplinary decision-making in detecting and treating the uncommon but significant tumor seeding with EUS-FNA biopsies in pancreatic ductal adenocarcinoma.
Keywords: tumor seeding, needle tract implantation, endoscopic ultrasound-guided fine-needle aspiration, pancreatic cancer, carbohydrate antigen 19-9, endoscopy, cancer detection, metastasis, multidisciplinary tumor board
Introduction
Pancreatic ductal adenocarcinoma is known for its aggressive behavior, with a 5-year overall survival rate of 5%.1 Curative resection is only possible for 15% to 20% of patients at diagnosis due to the extent of the disease, and 5-year survival rates are only 30% for patients during the earliest stage of disease after resection.2,3 Data suggest that factors associated with recurrence and survival after surgery include tumor size, tumor extension beyond the pancreas, lymph node metastases at the time of resection, preoperative carbohydrate antigen 19-9 (CA19-9) levels, and tumor grade.4,5 Recurrence due to needle tract seeding with tumor cells after a biopsy is rare in patients with pancreatic cancer, although biopsies for solid and cystic pancreatic lesions are commonly performed for definitive diagnoses and treatment planning. These biopsies are performed via percutaneous or endoscopic ultrasound-guided with fine-needle aspirations (EUS-FNAs). Cancer recurrence related to tumor seeding after a biopsy is important to diagnose, as these lesions may be amenable to surgical management. An institutional review of 73 patients reported a 1.4% incidence rate of needle tract seeding after percutaneous-FNA biopsies in patients with pancreatic cancer.6 Reports of needle tract seeding after EUS-FNA biopsies of pancreatic body or tail lesions are limited to case reports, and the level of incidence is unknown.7-18 Data are too sparse to determine whether this phenomenon is an indication of the malignant potential of these tumors or a technical flaw and whether overall survival is more affected in patients with needle tract seeding than in those without it.
We describe a case of a patient with postoperative elevation of the biochemical marker CA19-9 without an identifiable site of recurrence. Although many such cases can be attributed to the peritoneal spread of disease or widespread metastases, this case was a late identification of an isolated gastric metastasis with features suggestive of needle tract tumor seeding following EUS-FNA. We discuss the diagnostic challenges and treatment considerations for patients with this unique type of pancreatic cancer metastasis.
Methods
Patient Case Report
A 61-year-old male with epigastric pain was diagnosed with a pancreatic mass on imaging after failing empiric proton pump inhibitor therapy. A computed tomography (CT) scan identified a 3.7 cm × 2 cm mass in the body of the pancreas. Endoscopic ultrasound-guided with fine-needle aspiration of the mass was performed using 3 passes of a 25-gauge FNA needle. This was diagnostic and consistent with adenocarcinoma (Figure 1). Completion of staging determined this lesion to be a cT3N0M0 stage IIA pancreatic adenocarcinoma.
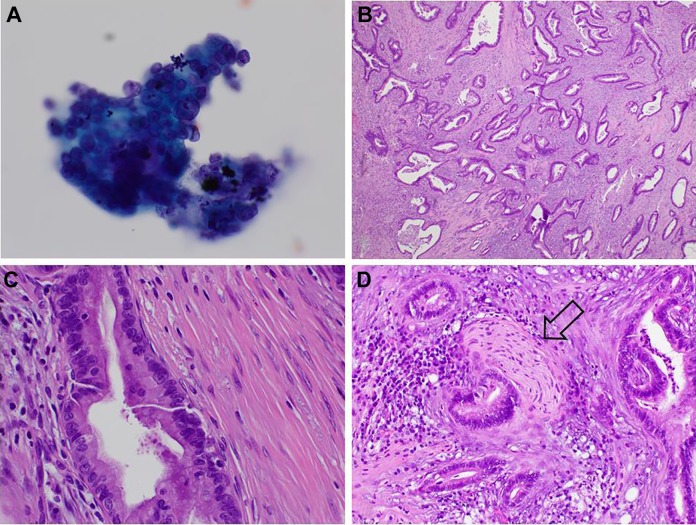
Figure 1.
Pancreatic adenocarcinoma. A, Endoscopic ultrasound-guided with fine-needle aspiration of pancreatic mass; high magnification shows tridimensional cluster of malignant cells with marked variation in nuclear size that are positive for malignancy and compatible with adenocarcinoma (Pap-stained smear, original magnification ×600). B, Histological examination of the pancreatectomy specimen in low magnification showing medium-sized glands with haphazard growth embedded in dense desmoplastic stroma, all characteristic findings of pancreatic adenocarcinoma (hematoxylin-eosin, original magnification ×40). C, Higher magnification showing glands composed of malignant cells with marked variation in nuclear size, disorderly arrangement of nuclei, irregular nuclear membranes, and mitosis (hematoxylin-eosin, original magnification ×400). D, Adenocarcinoma wrapping a nerve; perineural invasion is another common feature in this tumor (hematoxylin-eosin, original magnification ×200).
The patient underwent upfront surgery with a distal pancreatectomy and splenectomy. Final pathology confirmed an invasive well-differentiated pancreatic ductal adenocarcinoma with pathological state pT3N0M0 per American Joint Committee on Cancer (AJCC) seventh edition criteria. The tumor had invaded beyond the pancreatic capsule but did not involve adjacent structures. Perineural invasion was identified. Regional nodes were negative, and margins of resection were free of tumor cells.
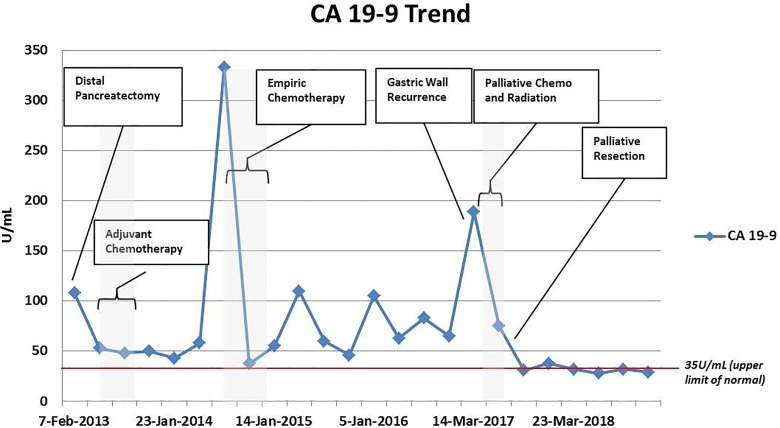
Postoperatively, the patient was followed using surveillance imaging, and CA19-9 levels were monitored (Figure 2). He completed adjuvant gemcitabine and nab-paclitaxel in the context of a clinical trial. His CA19-9 levels, which were initially recorded at 108 U/mL, decreased postoperatively but never normalized; they fluctuated in the 50 to 80 U/mL range and then spiked to a level of 332 U/mL 17 months after surgery and 11 months after finishing adjuvant chemotherapy. This prompted a restaging effort that included CT and positron emission tomography (PET) scans, which showed no evidence of disease. The multidisciplinary tumor board recommended initiation of empiric chemotherapy again with gemcitabine and nab-paclitaxel for a presumed diagnosis of occult metastatic disease. The patient continued to respond to chemotherapy with decreasing CA19-9 levels and had no evidence of disease on imaging studies at 3-month intervals. The patient elected to stop chemotherapy approximately 20 months after surgery due to intolerance of this treatment. Thereafter, he continued to have no evidence of disease on imaging despite fluctuations in his CA19-9 levels, which never normalized.
Figure 2.
Carbohydrate antigen 19-9 (CA19-9) trend demonstrating the time course of treatment for this patient with pancreatic cancer recurrence due to tumor seeding after an endoscopic ultrasound-guided with fine-needle aspiration biopsy of a mass in the body of the pancreas.
Three and a half years after resection, the patient was found to have a hypodense 2 cm posterior gastric wall mass with a lipomatous appearance. An 18F-fluorodeoxyglucose PET/CT scan demonstrated hypermetabolism of the mass with a standardized uptake value of 7.4. An endoscopic biopsy of the mass showed a focal area of adenocarcinoma involving the gastric wall at the lower portion of the lamina propria and muscularis mucosa level; there was no evidence of a superficial mucosal precursor lesion of gastric origin (Figure 3). The adenocarcinoma had a morphology and immunohistochemical (IHC) profile similar to the patient’s previous pancreatic adenocarcinoma (Figure 4). These findings were consistent with pancreatic adenocarcinoma metastatic to the gastric wall.
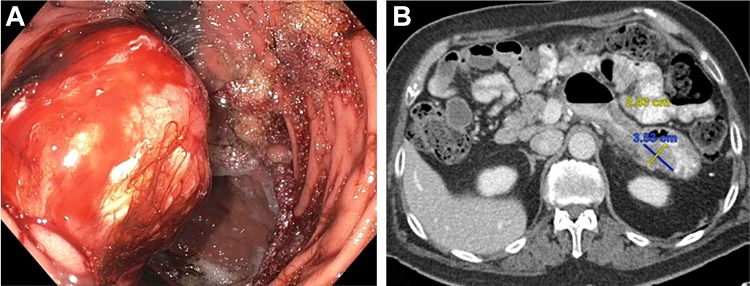
Figure 3.
A, Esophagogastroduodenoscopy demonstrating a large ulcerated, noncircumferential mass with evidence of bleeding found on the posterior wall of the gastric body. B, Axial computed tomography showing this hypoenhancing lesion correlating with the endoscopic description of the mass.
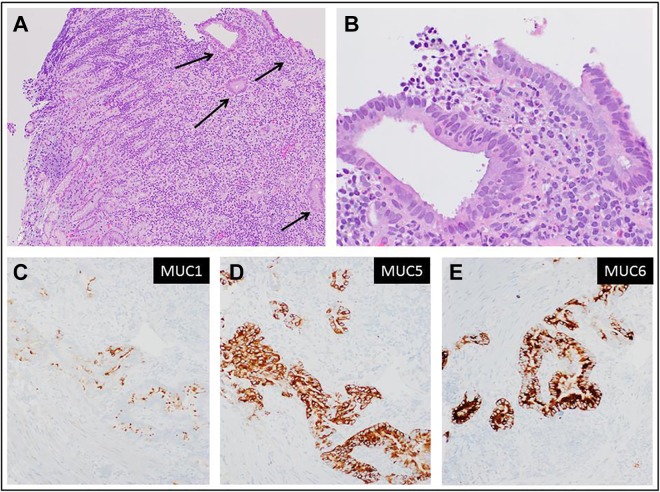
Figure 4.
Metastatic/recurrent pancreatic adenocarcinoma to gastric wall. A, Gastric biopsy; low magnification showing a fragment of gastric mucosa with unremarkable superficial gastric epithelium and underlying lamina propria. At the far right, malignant glands invade the lower part of the lamina propria (hematoxylin-eosin, original magnification ×40). B, Higher magnification showing medium-sized glands with haphazard growth, composed of malignant cells with marked variation in nuclear size, disorderly arrangement of nuclei, irregular nuclear membranes (hematoxylin-eosin, original magnification ×400). C-E, Immunohistochemical stain for mucin-1, mucin 5, and mucin 6, respectively, highlighting the malignant glands (original magnification ×200).
Given these results, the patient was restarted on chemotherapy with gemcitabine and nab-paclitaxel, which the patient had responded to during prior cycles. Other chemotherapy regimens were considered, but it was believed that this patient would not be tolerant of them. The first course was complicated by tumor bleeding, which required palliative radiation treatment. After 2 cycles of chemotherapy, restaging showed tumor response without any other sites of metastatic disease. Subsequently, the patient underwent a palliative wedge gastrectomy that revealed a 2.5-cm tumor that was confined to the muscularis mucosae and involved the underlying adipose tissue. Surgical margins were free of tumor. Histological examination confirmed the morphological and IHC findings from the gastric biopsy.
Follow-up surveillance showed the patient achieved normalization of his CA19-9 levels, and adjuvant chemotherapy with gemcitabine and nab-paclitaxel was then completed; surveillance is ongoing. Currently, the patient has no evidence of disease, 6 years after the initial resection of his stage IIA well-differentiated pancreatic adenocarcinoma.
Discussion
Occult recurrence of pancreatic ductal adenocarcinoma can be suspected when a significant elevation in CA19-9 levels is detected on surveillance after resection and when there is no evidence of other causes, such as pancreatitis or biliary obstruction.19 Our case highlights the challenges associated with treating patients whose CA19-9 levels are elevated but show no evidence of evaluable disease on surveillance with cross-sectional imaging. Prior studies have shown that elevations of CA19-9 levels frequently precede radiographic recurrence by more than 6 months in patients with pancreatic cancer.19 However, our patient did not demonstrate any radiographic recurrence for a prolonged period of follow-up (>2 years). Postoperative surveillance of patients with pancreatic cancer at our institution includes the use of serum biomarkers and cross-sectional imaging every 3 months for the first 2 years and then every 6 months for the next 3 years and annually thereafter. If additional information is needed to clarify indeterminate findings during the surveillance, a PET scan may be used, which has been shown to increase the diagnostic yield of metastatic or recurrent disease.15 All patients with concern for disease recurrence are presented at our institution’s multidisciplinary tumor board for joint evaluation by pancreatic cancer subspecialists representing medical, surgical, and radiation oncology as well as diagnostic radiology and pathology. In our workup paradigm for patients suspected to have disease recurrence, endoscopy is reserved to evaluate suspicious sites of disease noted on imaging. As discussed in a recent review of tumor seeding with EUS-FNA biopsies, greater suspicion of the potential occurrence of needle track seeding may lead to additional endoscopic evaluations to rule out this seeding at the transgastric FNA site.20 Although many studies describe the presence of a mass lesion protruding into the gastric lumen, most lesions are submucosal. Such lesions may be difficult to recognize via gastroscopy alone until they are large enough to be seen on cross-sectional imaging. Endoscopic ultrasound may be useful in earlier identification of these lesions if suspicion is high, although the pretest probability is likely too low to be cost-effective in most cases.
Tumor seeding from the EUS-FNA biopsy, as opposed to hematogenous spread of disease, was suspected when the gastrectomy specimen showed an infiltrative tumor with a morphology and IHC profile matching that of the primary tumor. Although there are no universal criteria to differentiate a recurrence due to hematogenous metastasis from a recurrence due to tumor seeding, the clinical and pathological characteristics of this patient’s tumor suggested that the cause was tumor seeding. Lesions from tumor seeding and the primary tumor are expected to have similar IHC profiles, differentiations, and morphologies, and metastatic tumors tend to demonstrate a higher grade, less differentiation, and potential variations in IHC staining.21 Interestingly, the hematogenous spread of pancreatic cancer to the stomach is a rare phenomenon, with one autopsy study demonstrating only 2 lesions of pancreatic origin in 347 patients with gastric metastases from all cancer types.22
Prior reports have noted tumor seeding of pancreatic cancer after EUS-FNA biopsies, with recurrence being identified from 3 to 48 months after the initial procedure. Although limited to 12 published case reports on pancreatic adenocarcinoma, data show variability in the time frame during which this disease may manifest and highlight the rarity of this phenomenon.7-18 Notably, this finding was only reported for patients with pancreatic cancer in the body and tail of the gland as opposed to the head, wherein the needle tract is resected within the pancreaticoduodenectomy specimen. A population-based review of patients with pancreatic cancer treated with curative intent surgery suggested that overall survival rates were not affected by the low rate of tumor seeding with EUS-FNA biopsies; patients who had biopsies had equivalent if not better rates of survival than those who did not.23 The authors of this study concluded that the risk of tumor seeding should not limit the use of EUS-FNA when tissue diagnosis is indicated. Despite the lack of prospective data to inform this practice, a recent commentary on the diagnostic use of EUS-FNA for pancreatic cancer concurred that EUS-FNA biopsies should be used to diagnose pancreatic cancer if the information obtained could change the treatment of patients.24 However, the recommendation to use EUS-FNA biopsies for pancreatic adenocarcinoma did not extend to cholangiocarcinoma, as tumor seeding and peritoneal spread are more common in this disease after FNA biopsies.25
The usefulness of preoperative tissue for diagnosing resectable solid pancreatic tumors has been challenged by others in the literature.26 At our institution, biopsies are used to confirm the histologic diagnosis of this disease. Having this diagnosis, we are able to inform a patient and their support system about the patient’s prognosis and to advise them on available treatment options. With this information, patients are brought into the decision-making process before the surgery. Biopsies can also influence decisions regarding neoadjuvant therapy; however, at the time of our patient’s presentation, neoadjuvant therapies were not routinely used in the management of these lesions.
Another factor that may have added complexity to our patient’s case is the low tumor grade. Although not a component of the most recent American Joint Committee on Cancer eighth edition TNM staging, tumor grades have been shown in multiple studies to have a prognostic value for patients with pancreatic adenocarcinoma.27,28 The low grade of this patient’s tumor may have contributed to the slow growth of the gastric metastasis and led to the difficulty in identifying this site earlier in his postoperative course.
Conclusion
Needle tract tumor seeding following EUS-FNA in pancreatic cancer is a rare phenomenon that is unique to tumors in the body and tail of the pancreas, considering that the needle tract site is not typically removed during surgical resection of those tumors. The incidence rate of this seeding is low but not well-defined, as published data are limited to case reports and literature reviews. For patients who have undergone EUS-FNA for body or tail lesions in the pancreas before resection, tumor seeding can be considered a source of recurrence when no evidence of disseminated disease is seen on staging. In addition, if this is the only site of recurrence, these cases should be considered for surgical management.
Acknowledgments
The authors thank Paul Fletcher and Daley Drucker (Moffitt Cancer Center) for editorial assistance. They were not compensated beyond their regular salaries.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Luke D. Rothermel, MD, MPH  https://orcid.org/0000-0001-5700-8028
https://orcid.org/0000-0001-5700-8028
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2. Barugola G, Partelli S, Marcucci S, et al. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16(12):3316–3322. [DOI] [PubMed] [Google Scholar]
- 3. Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265(1):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishio K, Kimura K, Amano R, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol. 2017;15(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helm J, Centeno BA, Coppola D, et al. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115(18):4080–4089. [DOI] [PubMed] [Google Scholar]
- 6. Kosugi C, Furuse J, Ishii H, et al. Needle tract implantation of hepatocellular carcinoma and pancreatic carcinoma after ultrasound-guided percutaneous puncture: clinical and pathologic characteristics and the treatment of needle tract implantation. World J Surg. 2004;28(1):29–32. [DOI] [PubMed] [Google Scholar]
- 7. Paquin SC, Gariepy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61(4):610–611. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed K, Sussman JJ, Wang J, Schmulewitz N. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc. 2011;74(1):231–233. [DOI] [PubMed] [Google Scholar]
- 9. Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74(4):933–935. [DOI] [PubMed] [Google Scholar]
- 10. Katanuma A, Maguchi H, Hashigo S, et al. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44(suppl 2 UCTN):E160–E161. [DOI] [PubMed] [Google Scholar]
- 11. Anderson B, Singh J, Jafri SF. Tumor seeding following endoscopic ultrasonography-guided fine-needle aspiration of a celiac lymph node. Dig Endosc. 2013;25(3):344–345. [DOI] [PubMed] [Google Scholar]
- 12. Sakurada A, Hayashi T, Ono M, et al. A case of curatively resected gastric wall implantation of pancreatic cancer caused by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2015;47(suppl 1 UCTN):E198–E199. [DOI] [PubMed] [Google Scholar]
- 13. Minaga K, Kitano M, Yamashita Y. Surgically resected needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration in pancreatic cancer. J Hepatobiliary Pancreat Sci. 2015;22(9):708–709. [DOI] [PubMed] [Google Scholar]
- 14. Tomonari A, Katanuma A, Matsumori T, et al. Resected tumor seeding in stomach wall due to endoscopic ultrasonography-guided fine needle aspiration of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21(27):8458–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kita E, Yamaguchi T, Sudo K. A case of needle tract seeding after EUS-guided FNA in pancreatic cancer, detected by serial positron emission tomography/CT. Gastrointest Endosc. 2016;84(5):869–870. [DOI] [PubMed] [Google Scholar]
- 16. Yamabe A, Irisawa A, Shibukawa G, et al. Rare condition of needle tract seeding after EUS-guided FNA for intraductal papillary mucinous carcinoma. Endosc Int Open. 2016;4(7): E756–E758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minaga K, Kitano M, Enoki E, Kashida H, Kudo M. Needle-tract seeding on the proximal gastric wall after EUS-guided fine-needle aspiration of a pancreatic mass. Am J Gastroenterol. 2016;111(11):1515. [DOI] [PubMed] [Google Scholar]
- 18. Iida T, Adachi T, Ohe Y, et al. Re-recurrence after distal gastrectomy for recurrence caused by needle tract seeding during endoscopic ultrasound-guided fine-needle aspiration of a pancreatic adenocarcinoma. Endoscopy. 2016;48(suppl 01):E304–E305. [DOI] [PubMed] [Google Scholar]
- 19. Rieser CJ, Zenati M, Hamad A, et al. CA19-9 on postoperative surveillance in pancreatic ductal adenocarcinoma: predicting recurrence and changing prognosis over time. Ann Surg Oncol. 2018;25(12):3483–3491. [DOI] [PubMed] [Google Scholar]
- 20. Minaga K, Takenaka M, Katanuma A, et al. Needle Tract Seeding: an overlooked rare complication of endoscopic ultrasound-guided fine-needle aspiration. Oncology. 2017;93(suppl 1):107–112. [DOI] [PubMed] [Google Scholar]
- 21. Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133(3):413–422. [DOI] [PubMed] [Google Scholar]
- 22. Oda Kondo H, Yamao T, Saito D, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33(6):507–510. [DOI] [PubMed] [Google Scholar]
- 23. Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64(7):1105–1110. [DOI] [PubMed] [Google Scholar]
- 24. Gleeson FC, Lee JH, Dewitt JM. Tumor seeding associated with selected gastrointestinal endoscopic interventions. Clin Gastroenterol Hepatol. 2018;16(9):1385–1388. [DOI] [PubMed] [Google Scholar]
- 25. Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13(5):356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartwig W, Schneider L, Diener MK, Bergmann F, Buchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96(1):5–20. [DOI] [PubMed] [Google Scholar]
- 27. Rochefort MM, Ankeny JS, Kadera BE, et al. Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Ann Surg Oncol. 2013;20(13):4322–4329. [DOI] [PubMed] [Google Scholar]
- 28. Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23(30):7529–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]