Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide usually diagnosed in the advanced stage. In this study, the serum concentration of tumor endothelial marker 1 (TEM1) was measured and correlated with clinicopathological features to evaluate whether TEM1 might serve as a biomarker for early CRC diagnosis, progression, and prognosis. The concentration of TEM1 was measured in the serum samples of 45 patients with CRC and 35 healthy individuals using enzyme-linked immunosorbent assay test. The mean serum concentration of TEM1 was significantly higher in the patients with CRC compared to the healthy individuals (1.31 ± 0.16 vs 0.92 ± 0.90 ng/mL; P < .001). The mean concentration of TEM1 significantly increased in the patients having CRC with early stage (stage I + II) compared to noncancer control individuals (stage I + II vs control 1.21 ± 0.13 ng/mL: 0.92 ± 0.90 ng/mL; P < .001). The TEM1 concentration in blood serum also showed a significant association with the development of T stages (P < .001), N stages (P < .001), and M stages (P = .006). The TEM1 sensitivity and specificity in CRC detection are higher than routinely used blood markers (carcinoembryonic antigen [CEA] and carbohydrate antigen [Ca 19-9]). Patients with high TEM1 concentration (≥1.055 ng/mL) had a worse overall survival rate compared to the patients having CRC with low TEM1 concentration (<1.055 ng/mL). In conclusion, TEM1 can act as a potential diagnostic, progression, and prognostic serum biomarker for patients with CRC; TEM1 might be a good supplement for commonly used markers CEA and Ca 19-9.
Keywords: tumor endothelial marker 1, TEM1, endosialin, CD248, colorectal cancer, biomarker
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world with approximately 1.4 million new cases and almost 700 000 associated deaths reported per year.1 The incidence, morbidity, and mortality rates of CRC are expected to increase due to population aging and profound adverse effects of many life style-related factors.2 Nowadays, numerous national programs aim to improve early detection of CRC, before the promotion/progression stages for successful outcomes.3 Despite the continuous improvement in diagnostic methods (ie, colonoscopy, flexible sigmoidoscopy, stool-based tests, blood markers), the National Cancer Institute’ figures indicate a significant number of CRC cases diagnosed in later stages (III and IV).4 Most commonly used serum markers for diagnosis and monitoring of CRC are carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (Ca 19-9). However, these markers show insufficient sensitivity, especially at early stages of CRC.5 Therefore, finding sensitive and specific, noninvasive, serum biomarkers is a prerequisite for early CRC diagnosis.
Tumor growth is dependent on the complex and multistage angiogenesis. New blood vessels formation is required for logarithmic tumor development and invasion.6 Tumor blood vessels are characterized with abnormal morphology and function compared to normal blood vessels.7 Except for disorganized structure, abnormal basement membrane, and increased leakiness, tumor vascular cells express unique protein named tumor endothelial markers (TEMs). As a 20-fold higher expression of TEMs has been reported in human tumor vasculature, it was suggested TEMs could discriminate between tumor and nontumor vascular cells.8,9
Tumor endothelial marker 1 (TEM1, endosialin, CD 248) is transmembrane glycoprotein which expression is significantly higher in tumor vasculature (5-, 10-, and 20-fold) compared to the vessels of healthy tissues.10 TEM1 gene switch off resulted in tumor vascularization disturbances, growth, invasion, and metastasis reduction of human CRC cells.11 Tissue overexpression of TEM1 was found in patients with CRC compared to healthy controls as well as in rectal cancer tissues when comparing tumor, lymph nodes, metastasis (TNM) stage I with other stages: TNM II + III + IV.12,13 Although the biology of TEM1 is intensely studied, including clinical studies with anti-TEM1 molecules,9,14-16 there is no data about the serum TEM1 concentration and its potential usefulness in CRC detection, prediction of progression, and prognosis.
In this study, we aimed to measure the concentration of TEM1 in blood serum and to evaluate TEM1 as a marker for assisting early CRC diagnosis and predicting progression, and prognosis in patients with CRC.
Material and Methods
Patients and Collection of Blood Samples
The study population included a total of 45 adult patients (64% men, 36% women; mean age: 68.83 ± 10.99, range 44-84 years; mean body mass index [BMI]: 27.16 ± 4.01, range 21.30-35.49 kg/m2) admitted between January 2014 and December 2014 to the General, Oncological and Minimally Invasive Surgery Department of the 1 Military Clinical Hospital with the Outpatient Clinic in Lublin, Poland. Patients with CRC were diagnosed due to clinical records. The CRC cases were confirmed by histopathologic tests provided in Pathology Department of our hospital. Tumor staging was classified according to the American Joint Committee on Cancer Staging, TNM staging classification. The patients never received preoperative radiotherapy, chemotherapy, or chemoradiotherapy. Basic clinical characteristic of patients with CRC was summarized in Table 1.
Table 1.
Clinical Characteristics of Patients With Colorectal Cancer Enrolled in the Study.
| Characteristics | CRC Group, n (%) |
|---|---|
| Tumor site | |
| Colon | 23 (51) |
| Rectum | 22 (49) |
| Tumor size, cm | |
| <5.0 | 23 (51) |
| ≥5.0 | 22 (49) |
| TNM stage | |
| I + II | 26 (58) |
| III + IV | 19 (42) |
| Depth of invasion (T stage) | |
| T1 | 5 (11) |
| T2 | 10 (22) |
| T3 | 18 (40) |
| T4 | 12 (27) |
| Lymph node metastasis (N stage) | |
| Absent (N0) | 26 (58) |
| Present (N1+2) | 19 (42) |
| Distant metastasis (M stage) | |
| Absent (M0) | 40 (89) |
| Present (M1) | 5 (11) |
| Lymphovascular invasion | |
| Absent | 26 (58) |
| Present | 19 (42) |
Abbreviations: CRC, colorectal cancer; TNM, tumor, lymph nodes, metastasis.
The follow-up data of all the patients with respect to survival status were acquired from the 6-month, 12-month, 18-month, 24-month, 30-month, and 36-month postoperative telephone calls or visits.
The control cohort included 35 healthy volunteers with no clinical evidence of CRC (54% men, 46% women; mean age 64 ± 8.76, range: 43-80 years; mean BMI 26.86 ± 3.76, range: 21.86-33.27 kg/m2). These individuals were free from any chronic or acute diseases during the study period and recruited from the Outpatient Clinic of our hospital. Healthy blood donors matched in terms of age and BMI with the patients with CRC.
All the patients were informed about the aim of the study and signed written consent forms before the study procedures. The Ethical Committee of the Medical University of Lublin in Poland approved the study (decision no. KE-0254/240/2008). The whole research was conducted in accordance with the approved guidelines.
Study Parameters
Measurement of TEM1 (CD248) concentration in blood serum
Blood was collected into commercially available anticoagulant-treated tubes (ethylenediaminetetraacetic acid) from each patient before the surgery. Cells were removed from serum by centrifugation for 10 minutes at 1000 × g using a refrigerated centrifuge (2-8°C). The resulting supernatant (plasma) was immediately transferred into a clean polypropylene tubes. The samples were stored at −80°C. Concentration of TEM1 in serum samples was quantified by sandwich enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (MyBiosource, catalog no. MBS912792). The samples were centrifuged again after thawing before the assay. The 100 µL of standard and sample were added into appropriate wells, covered with the adhesive strip, and incubated for 2 hours at 37°C. After incubation, the liquid was removed of each well. In next step, 100 µL of biotin-antibody were addend into wells, covered with a new adhesive strip, and incubated for 1 hour at 37°C. Plate washes were performed with an automatic washer TriNEST (Perkin Elmer, Waltham, MA, USA). Then 100 µL of horseradish peroxidase-avidin were added into wells, covered with a new adhesive strip, and incubated for 1 hour at 37°C. After incubation, plate was automatically washed, 90 µL of TMB substrate was added and incubated for 15 minutes at 37°C. In the last step, 50 µL of stop solution was added into wells. The optical density was read at 450 nm wavelength using a microplate reader Victor (Perkin Elmer). The standard curve was created by reducing the data using computer software capable of generating a 4 parameter logistic curve fit. The detection range for the assay was 0.312 to 20 ng/mL and sensitivity limits were less than 0.078 ng/mL for TEM1 (CD248).
Measurements of CEA and Ca 19-9 levels in blood serum
The evaluation of serum markers (CEA and Ca 19-9) was performed within 1 hour after venipuncture. The serum markers CEA and Ca 19-9 were assessed using a Cobas 6000 biochemistry analyzer (Roche Diagnostic, North America). The normal values for the CEA marker are less than 3.8 ng/mL (20-39 years of age) and 5.0 ng/mL (40-69 years of age) for nonsmokers and less than 5.5 ng/mL (20-39 years of age) and 6.5 ng/mL (40-69 years of age) for smokers, while the normal value for the Ca 19-9 marker is 0.6-39.00 ng/ml.
Statistical Analysis
Statistical analysis was performed with the SPSS software (SPSS 15.0, Chicago, Illinois) and XLSTAT 2017; Data Analysis and Statistical Solution for Microsoft Excel (Addinsoft, Paris, France, 2017). The results were expressed as mean ± standard deviation (SD). The Mann-Whitney U test was used to assess the difference in the TEM1 and blood marker (CEA and Ca 19-9) levels between the patients with CRC and control group. Within the CRC group, the Kruskal-Wallis test was used to compare the TEM1 concentration across 4 groups of the depth of invasion (T1, T2, T3, T4), while the TEM1 concentration across 2 groups of the lymph nodes (N0, N1+2) and distal metastases (M0, M1) was compared with the Mann-Whitney U test. Receiver-operating characteristics (ROC) curve analysis was performed to identify cutoff values of studied parameters. Survival curves were estimated using the Kaplan-Meier method, and the differences in survival distribution were evaluated by the long-rank test. Differences of P < .05 were considered to be significant.
Results
Concentration of TEM1 in Blood Serum of the Patients With CRC and Control Group
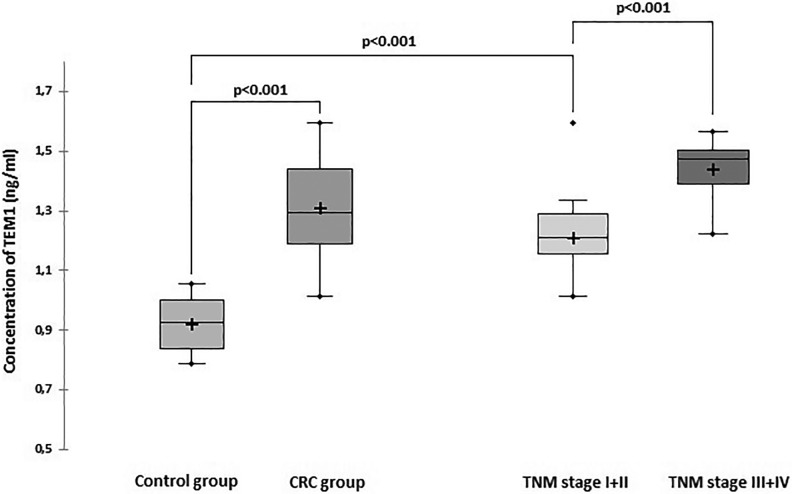
In the patients with CRC, the TEM1 values ranged from 1.01 to 1.59 ng/mL. The serum TEM1 mean concentration was significantly higher in the patients with CRC compared to the healthy individuals (1.31 ± 0.16 vs 0.92 ± 0.90 ng/mL; P < .001; Figure 1). To evaluate whether the serum TEM1 concentration is useful for early detection of CRC, the TEM1 level in blood serum from early stage (I + II) patients was compared with those of normal controls. The mean concentration of TEM1 significantly increased in the patients having CRC with early stage (stage I + II) compared to the noncancer control individuals (stage I + II vs control 1.21± 0.13 ng/mL: 0.92 ± 0.90 ng/mL; P < .001).
Figure 1.
Tumor endothelial marker 1 (TEM1) concentration in blood serum of the CRC and control groups. CRC indicates colorectal cancer.
Relationship Between Serum TEM1 Concentration and the Clinicopathological Features of the Patients With CRC
The mean TEM1 concentration was significantly higher in the patients having CRC with the advanced stage (stage III + IV) compared to the patients with the early stage (stage I + II) group (1.44 ± 0.89 vs 1.21 ± 0.13 ng/mL, respectively; P < .001; Figure 1).
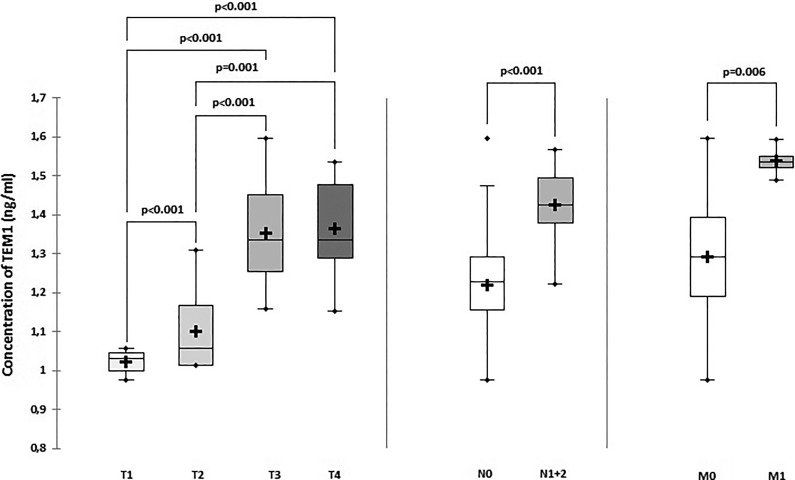
The Kruskal-Wallis analysis revealed that the TEM1 concentration was T stage dependent and increased following the development of T stages (P < .001). The mean ± SD levels of TEM1 in T1, T2, T3, and T4 stages was 1.02 ± 0.04, 1.07 ± 0.72, 1.35 ± 0.12, 1.38 ± 0.13, respectively. Significant differences between stages have been proved by the Mann-Whitney test (P < .001; Figure 2). The TEM1 concentration in blood serum also showed a significant association with the development of N stages (P < .001). The TEM1 level was higher in the M1 subgroup compared to the M0 subgroup (P = .006).
Figure 2.
Tumor endothelial marker 1 (TEM1) concentration in blood serum of the patients with CRC dependent on the TNM staging classification. CRC indicates colorectal cancer; TNM, tumor, lymph nodes, metastasis.
An increase of the TEM1 serum concentration was noted in the patients with lymphovascular invasion in tumor tissue compared to those without lymphovascular invasion (1.38 ± 0.12 vs 1.25±0.17; P = .006). No significant association was noted between the TEM1 level in blood serum and tumor location (colon vs rectum = 1.32 ± 0.18 vs 1.29 ± 0.15 ng/mL; P = .540) and between TEM1 level and tumor size (tumor <5 cm vs tumor ≥5 cm = 1.28 ± 0.18 vs 1.33 ± 0.14; P = .382).
The serum TEM1 concentration was not impacted by the age and BMI in the patients with CRC (for age <60 vs ≥60 years = 1.33 ± 0.17 vs 1.30 ± 0.16; P = .704; for BMI normal vs obese = 1.29 ± 0.16 ng/mL vs 1.41 ± 0.15 ng/mL; P = .072).
Performance of TEM1 in the Detection of Patients With CRC
To evaluate the value of TEM1 in clinical application, we contrasted TEM1 to biomarkers CEA and Ca 19-9. The mean values of CEA and Ca 19-9 were significantly higher in the patients with CRC compared to the controls (Table 2).
Table 2.
Mean Values of Serum Markers of the Study Groups.
| CRC Group | Control Group | P Value | |
|---|---|---|---|
| CEA | 10.99 ± 7.03 | 4.17 ± 1.80 | <.001 |
| Ca 19-9 | 14.68 ± 7.91 | 8.38 ± 7.00 | <.001 |
Abbreviations: Ca 19-9, carbohydrate antigen; CEA, carcinoembryonic antigen; CRC, colorectal cancer.
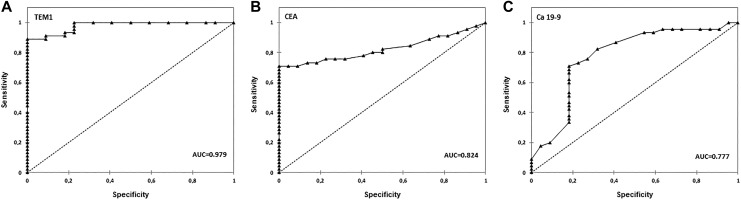
To further detect the diagnostic value of TEM1, ROC curves were developed (Figure 3). Tumor endothelial marker 1 predicted the diagnosis of the patients having CRC with an area under the curve (AUC) of 0.979 (95% confidence interval [CI]: 0.955-1.000) at a cutoff point of 1.055 ng/mL. This cutoff point provided 88.9% sensitivity and 90.9% specificity. The cutoff point of CEA was 6.0 ng/mL (AUC: 0.824 [95% CI: 0.726-0.922]), sensitivity 73.3%, and 86.4% specificity; Ca 19-9 predicted the diagnosis of patients having CRC with an AUC of 0.777 (95% CI: 0.645-0.908) at a cutoff point of 7.98 ng/mL. This cutoff point was characterized with 71.1% sensitivity and 81.8% specificity.
Figure 3.
Receiver-operating characteristics (ROC) curve analysis of TEM1 (A), CEA (B), and Ca 19-9 (C). Ca 19-9 indicates carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; TEM1, tumor endothelial marker 1.
Relationship of TEM1 Concentration With Overall Survival of Patients With CRC
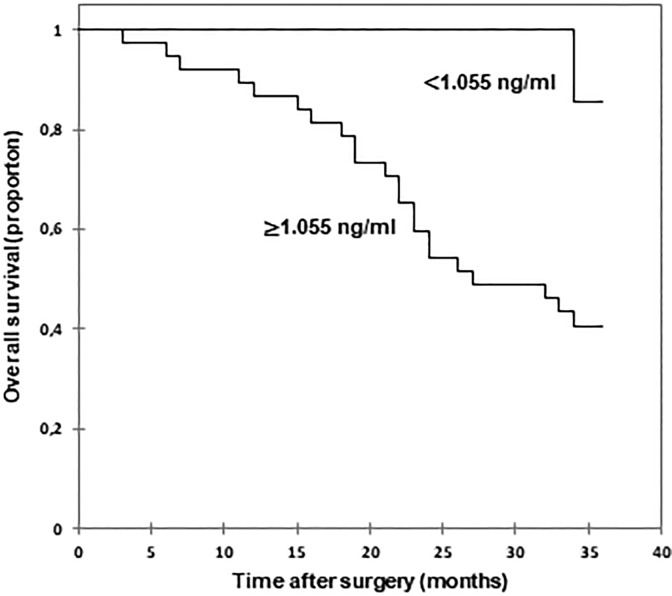
Patients with high TEM1 concentration (≥1.055 ng/mL) had worse overall survival (OS) rate compared to patients with CRC of low TEM1 concentration (<1.055 ng/mL; Figure 4). The postoperative mean OS time of patients with low serum TEM1 concentration cannot be estimated for over 50% patients survived. The OS for patients with high TEM1 concentration was 25.68 months (95% CI: 22.61-28.74).
Figure 4.
Kaplan-Meier survival curves of patients with CRC according to serum TEM1 concentration. CRC indicates colorectal cancer; TEM1, tumor endothelial marker 1.
Discussion
Variety of methods used in CRC diagnosis are not sufficient, due to a lack of sensitivity as well as specificity.4,5,17 Currently, measuring serum molecules improving diagnosis using protein or genetic biomarkers pretend to be exploited in the clinics for cancer detection, monitoring, and prognosis.18 Many cell surface proteins have been found in systemic circulation, for example, Ca-125, HE4, and mesothelin. These molecules are used as biomarkers in detection and monitoring of patients with cancer, for example, in ovarian cancer, mesothelioma.19 Similarly, based on the lability of TEM1 (endosialin, CD248) to trypsin, it is suggested that TEM1 is soluble protein which can be cleaved and detected in the blood.16
Here, we investigated TEM1 as a novel serum biomarker for early cancer detection and progression, and as a prognostic factor in patients with CRC. We proved that TEM1 is simply to detect in blood serum using a common type of ELISA assays. TEM1 demonstrated higher levels in the serum of the patients with CRC than healthy individuals. Higher levels of circulating TEM1 observed in the serum of the patients with CRC can be associated with increased expression of TEM1 in tumor tissue and may be interrelated to the role of TEM1 in tumor angiogenesis.9,20 Previously, TEM1 expression was studied in about 50 human cell lines and clinical samples21 and a consistent upregulation of TEM-1 expression was found in tumor blood vessels (5-, 10- or even 20-fold higher) compared to the vessels of healthy tissues, therefore its value in identifying vasculature in malignant tissues has been suggested.10,22
In our study, the level of TEM1 in the serum was specific to early CRC stages (I + II) compared to noncancer individuals. Moreover, we evidenced that TEM1 sensitivity and specificity in CRC detection are higher than routinely used blood markers (CEA, CA 19-9). In this context, TEM1 is emerging as potential disease biomarker for CRC diagnosis.
In this study, the difference in TEM1 levels between an early cancer stage (I + II) and advantage cancer stage (III + IV) was found. Further, TEM1 concentration in serum was strongly associated with the TNM staging classification, which suggests that the TEM1 serum concentration may be potentially useful as a biomarker for cancer progression.
The TEM1 concentration was associated with the prognosis of the patients with CRC. We observed that the high-TEM1 group (≥1.055 ng/mL) had significantly lower OS rate than the low-TEM1 group (<1.055 ng/mL). This evidence suggests that high TEM1 serum concentration can function as an indicator of poor prognosis of the patients with CRC. High TEM1 levels were associated with patients having advanced disease (stage III + IV, patients with T3 and T4 stages, patients with present lymph node and distal metastases). Usually, the CRC advance results in a large and rapidly increasing number of cancer deaths.23,24
There are a few limitations in this study that could be addressed in future research. The present study is a retrospective observational study and included a limited number of patients with CRC (n = 45). All the patients with CRC were diagnosed with cancer by colonoscopy and were scheduled for the surgery and thus are recognized as a homogenous sample. The mean time from the first CRC detection (colonoscopy) to the surgical treatment and blood sample collections was 14 days. Moreover, the patients were treated surgically before any chemotherapy or radiotherapy was delivered. The validity of the study depends upon the comparability of the studied and the control group, thus the control group met the same exclusion criteria as the CRC patients and were matched according to the demographic data. The preliminary character of the research included the collection of the blood samples in one-time point, before the resection of cancer. In the future, we consider the collection and blood’ analysis before the surgery and during the follow-up period. In the present study, the assessment of the disease-free survival time was impossible due to much controversial information from the patients and their families. They could not declare the detection of the onset/recurrent of cancer. This is why the statistical analysis did not describe disease-free survival time. The 36 months follow-up for patients with CRC might also be seen in the light of some limitations. This time allowed to obtain the data from all the patients. It will be ideal to have longer, that is, 60 months of observation however, it could remain an unrealistic goal and decrease the statistical analysis due to the loss of the patients. In conclusion, to the best of our knowledge, this is the first study that suggests that TEM1 can act as a potential diagnostic, progression, and prognostic serum biomarker for patients with CRC; TEM1 might be a good supplement for commonly used markers CEA and CA 19-9. We expect that our results will provide the basis for further studies on the TEM1 biology and importance for CRC detection, progression, and prognosis.
Acknowledgments
Authors would like to thank Prof Anna Torres, MD, PhD, for the agreement to perform the analytical analyses of the studied parameters in the Laboratory of the Biostructure, Chair and Department of Human Anatomy, Medical University of Lublin, Lublin, Poland.
Authors’ Note: ŁP contributed to study design; ŁP and PW contributed to analytical procedures; LP contributed to statistical analyses, graphs, and results interpretation; LP wrote the manuscript. All the patients were informed about the aim of the study and signed written consent forms before the study procedures. The Ethical Committee of the Medical University of Lublin in Poland approved the study (decision no. KE-0254/240/2008). The whole research was conducted in accordance with the approved guidelines.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Medical University of Lublin (grant no. MNmb 230).
ORCID iD: Łukasz Pietrzyk, MD, PhD  https://orcid.org/0000-0003-2931-5391
https://orcid.org/0000-0003-2931-5391
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed November 25, 2018. [Google Scholar]
- 2. Pietrzyk Ł, Torres A, Maciejewski R, Torres K. Obesity and obese-related chronic low-grade inflammation in promotion of colorectal cancer development. Asian Pac J Cancer Prev. 2015;16(1):4161–4168. [DOI] [PubMed] [Google Scholar]
- 3. Ganepola GA, Nizin J, Rutledge JR, Chang DH. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. World J Gastrointest Oncol. 2014;6(4):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Cancer Institute. SEER Stat Facts Sheets: Colon and Rectum Cancer. 2018. http://seer.cancer.gov/statfacts/html/colorect.html/. Accessed November 25, 2018.
- 5. Yamashita K, Watanabe M. Clinical signicance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009;100(5):195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Folkman J, Kerbel R. Role of angiogenesis in tumor growth and metastasis clinical translation of angiogenesis inhibitors. Semin Oncol. 2002;29(4):15–18. [DOI] [PubMed] [Google Scholar]
- 7. Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–111. [DOI] [PubMed] [Google Scholar]
- 8. Carson-Walter EB, Watkins DN, Nanda A, et al. Cell surface tumour endothelial markers are conserved in mice and humans. Cancer Res. 2001;61(2):6649–6655. [PubMed] [Google Scholar]
- 9. Pietrzyk Ł. Biomarkers discovery for colorectal cancer: a review on tumor endothelial markers as perspective candidates. Dis Markers. 2016;2016:4912405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–1202. [DOI] [PubMed] [Google Scholar]
- 11. Nanda A, Karim B, Peng Z, et al. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci USA. 2006;103(9):3351–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rmali KA, Puntis MC, Jiang WG. Prognostic values of tumor endothelial markers in patients with colorectal cancer . World J Gastroenterol. 2005;11(9):1283–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang ZY, Zhang H, Adell G, Sun XF. Endosialin expression in relation to clinicopathological and biological variables in rectal cancers with a Swedish clinical trial of preoperative radiotherapy. BMC Cancer. 2011;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Hu J, Wang Y, et al. Tumour endothelial marker 1/endosialin-mediated targeting of human sarcoma. Eur J Cancer. 2018;90:111–121. [DOI] [PubMed] [Google Scholar]
- 15. Lange SE, Zheleznyak A, Studer M, et al. Development of 89Zr-ontuxizumab for in vivo TEM-1/endosialin PET applications. Oncotarget. 2016;7(11):13082–13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’shannessy DJ, Smith MF, Somers EB, et al. Novel antibody probes for the characterization of endosialin/TEM-1. Oncotarget. 2016;7(2):69420–69435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds LM, Bissett IP, Consedine NS. Emotional predictors of bowel screening: the avoidance-promoting role of fear, embarrassment, and disgust. BMC Cancer. 2018;18(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fei W, Chen L, Chen J, et al. THBS2 are serum biomarkers for diagnosis of colorectal cancer. Oncotarget. 2017;8(54):92254–92264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Felder M, Kapur A, Gonzalez-Bosquet J, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bagley RG. Endosialin: from vascular target to biomarker for human sarcomas. Biomark Med. 2009;3(5):589–604. [DOI] [PubMed] [Google Scholar]
- 21. Rouleau C, Curiel M, Weber W, et al. Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res. 2008;14(22):7223–7236. [DOI] [PubMed] [Google Scholar]
- 22. Facciponte JG, Ugel S, De Sanctis F, et al. Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature. Clin Invest. 2014;12(4):1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dienstmann R, Mason MJ, Sinicrope FA, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28(5):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr. 2018;37(4):1279–1285. [DOI] [PubMed] [Google Scholar]