Abstract
BACKGROUND:
Direct stenting without pre-dilation or post-dilation has been advocated for saphenous vein graft percutaneous coronary intervention to decrease the incidence of distal embolization, periprocedural myocardial infarction, and target lesion revascularization.
METHODS:
We performed a post hoc analysis of patients enrolled in the DIVA (Drug-Eluting Stents Versus Bare Metal Stents in Saphenous Vein Graft Angioplasty; ) prospective, double-blind, randomized controlled trial. Patients were stratified into stent-only and balloon-stent groups. Primary end point was 12-month incidence of target vessel failure (defined as the composite of cardiac death, target vessel myocardial infarction, or target vessel revascularization). Secondary end points included all-cause death, stent thrombosis, myocardial infarction, and target lesion revascularization during follow-up.
RESULTS:
Of the 575 patients included in this substudy, 185 (32%) patients underwent stent-only percutaneous coronary intervention. Patients in the stent-only versus balloon-stent group had similar baseline characteristics and similar incidence of target vessel failure at 12-months (15% versus 19%; hazard ratio, 1.34 [95% CI, 0.86–2.08]; P=0.19). During long-term follow-up (median of 2.7 years), the incidence of definite stent thrombosis (1% versus 5%; hazard ratio, 9.20 [95% CI, 1.23–68.92]; P=0.0085), the composite of definite or probable stent thrombosis (5% versus 11%; hazard ratio, 2.52 [95% CI, 1.23–5.18]; P=0.009), and target vessel myocardial infarction (8% versus 14%; hazard ratio, 1.92 [95% CI, 1.08–3.40]; P=0.023) was lower in the stent-only group. Multivariable analysis showed that a higher number of years since coronary artery bypass grafting and >1 target saphenous vein graft lesions were associated with increased target vessel failure during entire follow-up, while preintervention Thrombolysis in Myocardial Infarction-3 flow was protective.
CONCLUSIONS:
In patients undergoing percutaneous coronary intervention of de novo saphenous vein graft lesions, there was no difference in target vessel failure at 12 months and long-term follow-up in the stent-only versus the balloon-stent group; however, the incidence of stent thrombosis was lower in the stent-only group, as was target vessel myocardial infarction.
REGISTRATION:
URL: https://www.clinicaltrials.gov. Unique identifier: .
Keywords: angioplasty, dilatation, incidence, myocardial infarction, stents
Percutaneous coronary intervention (PCI) of saphenous vein grafts (SVGs) comprises ≈6% of total PCIs performed in the United States. Whereas pre-dilation and post-dilation are recommended in native coronary PCI1 to facilitate stent delivery and optimize stent apposition and expansion, retrospective studies have shown an association between direct stenting and a lower incidence of periprocedural myocardial infarction or target lesion revascularization in SVG lesions,2–7 possibly due to lower risk for distal embolization.5 Similarly, post-dilatation of SVG lesions could lead to plaque protrusion through the stent struts (cheese-grater effect) leading to distal embolization and periprocedural myocardial infarction, which has been associated with higher mortality.4
We examined the frequency of adjunctive balloon angioplasty during stenting of SVG lesions and associated clinical outcomes in patients enrolled in the DIVA trial (Drug-Eluting Stents Versus Bare Metal Stents in Saphenous Vein Graft Angioplasty).8 We hypothesized that the stent-only group will have better outcomes compared with the balloon-stent group.
METHODS
The design of the DIVA trial has been published.9 Briefly, DIVA was a multicenter, randomized-controlled trial conducted at 25 Veterans Affairs medical centers that randomized patients undergoing SVG PCI to either bare-metal stents or drug-eluting stents. The trial was funded by the Department of Veterans Affairs’ Cooperative Studies Program. Veterans Affairs’ Central Institutional Review Board approval was obtained. The authors declare that all supporting data are available within the article.
Patients
Patients who signed an informed consent were at least 18 years of age and were found to have at least one de novo 50% to 99% stenosis of an SVG with diameter 2.25 to 4.5mm were eligible to be randomized. Intent to use an embolic protection device was required. Patients were excluded if any surgery was planned within 12 months; presented with an ST-segment elevation MI; the culprit SVG stenosis was in the last remaining conduit; had previous PCI of the target SVG within the preceding 12 months; had hemorrhagic diatheses, or refused to receive blood transfusions; required anti-coagulation and were considered high risk of bleeding with triple anticoagulation/antiplatelet therapy; had recent positive pregnancy test, breast-feeding, or possibility of a future pregnancy; had coexisting conditions that limited life expectancy to <12 months; had a history of allergic reaction or significant sensitivity to any drug or metal included in drug-eluting stents; were allergic to clopidogrel and did not present with acute coronary syndrome at sites that used blinded study medication; or participating in another interventional randomized trial. DIVA study did not show any difference in ischemic outcomes between bare-metal stent and drug-eluting stents.8
Assessment of Groups
The balloon-stent group included patients who underwent balloon angioplasty in the SVG target lesion before and/or after (either bare metal or drug-eluting) stent implantation. The stent-only group included patients who did not undergo balloon angioplasty either before or after stent implantation.
PCI was performed using standard techniques per operator preference. The study did not provide any guidance on use of balloon angioplasty.
Follow-Up and Outcomes
Patients were followed every 3 months during the first year and every 6 months thereafter.
The primary end point of the DIVA trial and this substudy was the 12-month incidence of target vessel failure (TVF), defined as the composite of cardiac death, target vessel myocardial infarction (MI), or target vessel revascularization (TVR). Secondary end points included the 12-month incidence of all-cause death, death due to cardiac or unknown cause, MI during follow-up, MI due to target SVG (or indeterminate vessel) during follow-up, MI due to nontarget vessel, any revascularization or target vessel revascularization (PCI or CABG [coronary artery bypass grafting]), target lesion revascularization, nontarget vessel revascularization, target lesion failure (composite of cardiac or unknown death, target vessel, or target lesion revascularization), stent thrombosis, stroke, postprocedural bleeding, and patient-oriented composite end point (any death, any MI, or TVR).
Definitions of the study end points have been published.9 Stent thrombosis was defined using Academic Research Consortium as definite, probable, or possible. Periprocedural MI was defined as >3× upper limit of normal CK-MB increase in patients with normal baseline CK-MB and >50% increase in patients with elevated baseline CK-MB.
Statistical Analysis
For this post hoc analysis of DIVA data, we stratified patients into stent-only and balloon-stent groups. The primary end point was the 12-month incidence of TVF. Nominal variables were presented as percentages and compared using the χ2 test and continuous variables were presented as mean (SD) or median (interquartile range) and compared using the t-test or Wilcoxon rank-sum test, as appropriate. Cumulative incidence curves and log-rank tests were used to compare the 2 groups on the incidence of the primary and secondary clinical outcomes. Statistical analyses were performed using SAS 9.2 (SAS institute, Cary, NC).
In addition to the univariate models, Cox proportional hazards models were used to assess the effect of balloon usage on TVF in the presence of other covariates assessed at baseline (age, diabetes mellitus, number of target lesions, years since most recent CABG, indication for PCI, CHF, pre-PCI Thrombolysis in Myocardial Infarction-flow, presence of thrombus, and use of embolic protection device). A 2-sided P value of 0.05 from the log-rank test was used as the level of significance for the primary outcome. As a preplanned secondary analysis, we performed similar analyses as above to compare time to TVF based on all follow-up data.
RESULTS
Patients
After screening 3482 patients with prior CABG, 597 patients undergoing PCI of a de novo 50% to 99% SVG stenosis were randomly assigned to receive either bare-metal stent or drug-eluting stents between January 2012 and December 2015. After excluding 22 patients (17 patients had lesions which did not qualify for inclusion, 4 did not undergo PCI, and 1 subject who had failed PCI), 575 patients who received a stent in the target SVG were included in the present study, of whom 185 (32%) underwent stent-only PCI and 390 (68%) underwent balloon-stent SVG PCI.
The baseline demographic, angiographic, and procedural characteristics were similar in the stent-only and balloon-stent groups (Tables 1 and 2). The target SVG lesion location was more frequently in the SVG body in the stent-only group. In the balloon-stent group, a second balloon was used in 156 patients and was larger than the first balloon. Compared with the stent only group, the incidence of periprocedural MI was numerically higher in the balloon-stent group.
Table 1.
Baseline Clinical and Demographic Characteristics of the Study Patients
| Stent-Only (n=185) | Balloon-Stent (n=390) | P Value | |
|---|---|---|---|
| Age | 68.48±7.35 | 68.65±7.66 | 0.91 |
| Men, n (%) | 183 (99%) | 390 (100%) | 0.10 |
| Diabetes mellitus, n (%) | 111 (60%) | 234 (60%) | 1.00 |
| Current smoking, n (%) | 46 (25%) | 83 (21%) | 0.34 |
| Hypertension, n (%) | 179 (97%) | 374 (96%) | 0.62 |
| Hyperlipidemia | 183 (99%) | 376 (96%) | 0.09 |
| Indication for PCI, n (%) | |||
| Stable angina | 71 (38%) | 146 (37%) | 0.95 |
| Unstable angina | 57 (31%) | 120 (31%) | |
| NSTEMI | 41 (22%) | 94 (24%) | |
| Other | 16 (9%) | 30 (8%) | |
| Number of diseased coronary vessels, n (%) | |||
| None | 0 (0%) | 1 (0%) | 0.95 |
| 1-vessel | 3 (2%) | 8 (2%) | |
| 2-vessel | 18 (10%) | 36 (9%) | |
| 3-vessel | 161 (87%) | 334 (86%) | |
| Other | 3 (2%) | 10 (3%) | |
| Prior MI, n (%) | 94 (51%) | 210 (54%) | 0.50 |
| LVEF, % | 49.92±14.82 | 49.31±12.70 | 0.39 |
| Congestive heart failure, n (%) | 67 (36%) | 137 (35%) | 0.80 |
| Body mass index (kg/m2) | 30.67±5.89 | 30.44±5.31 | 0.91 |
| PreIndex PCI estimated GFR (mean) | 77.13±24.87 | 76.96±23.86 | 0.84 |
| History of atrial fibrillation, n (%) | 35 (19%) | 70 (18%) | 0.78 |
| Peripheral arterial disease, n (%) | 33 (18%) | 70 (18%) | 0.97 |
GFR indicates glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; and PCI, percutaneous coronary intervention.
Table 2.
Angiographic Characteristics of the Study Patients
| Stent-Only (n=185 Subjects and 248 Lesions) | Balloon-Stent (n=390 Subjects and 440 Lesions) | P Value | |
|---|---|---|---|
| Target graft recipient vessel–subject, n (%) | |||
| LAD/diagonal | 39 (21%) | 86 (22%) | 0.51 |
| Circumflex/OM | 69 (37%) | 161 (41%) | |
| RCA/PDA | 77 (42%) | 143 (37%) | |
| Target graft recipient vessel–lesion, n (%) | |||
| LAD/diagonal | 51 (21%) | 100 (23%) | 0.23 |
| Circumflex/OM | 95 (38%) | 188 (43%) | |
| RCA/PDA | 102 (41%) | 152 (35%) | |
| BMS–subject, n (%) | 91 (49%) | 201 (52%) | 0.60 |
| BMS–lesion, n (%) | 121 (49%) | 232 (53%) | 0.32 |
| DES–subject, n (%) | 94 (51%) | 191 (49%) | 0.68 |
| First generation | 11 (6%) | 12 (3%) | 0.10 |
| Second generation | 86 (46%) | 179 (46%) | 0.89 |
| DES–lesion, n (%) | 127 (51%) | 210 (48%) | 0.38 |
| First generation | 15 (6%) | 12 (3%) | 0.0312 |
| Second generation | 115 (46%) | 198 (45%) | 0.73 |
| Embolic protection device used–subject, n (%) | 135 (73%) | 275 (71%) | 0.54 |
| Embolic protection device–lesion, n (%) | 178 (72%) | 309 (70%) | 0.67 |
| SVG target lesion location–subject, n (%) | |||
| Ostial | 37 (20%) | 104 (27%) | 0.0011 |
| Body | 139 (75%) | 237 (61%) | |
| Anastomosis | 9 (5%) | 49 (13%) | |
| SVG target lesion location–lesion, n (%) | |||
| Ostial | 38 (17%) | 118 (25%) | 0.0003 |
| Body | 176 (78%) | 297 (64%) | |
| Anastomosis | 10 (4%) | 49 (11%) | |
| Pre-stenting target lesion TIMI-flow–subject, n (%) | |||
| 0 | 1 (1%) | 4 (1%) | 1.00 |
| 1 | 4 (2%) | 16 (4%) | 0.24 |
| 2 | 22 (12%) | 66 (17%) | 0.12 |
| 3 | 160 (86%) | 309 (79%) | 0.0361 |
| Pre-stenting target lesion TIMI-flow–lesion, n (%) | |||
| 0 | 1 (0%) | 4 (1%) | 0.17 |
| 1 | 7 (3%) | 17 (4%) | |
| 2 | 26 (10%) | 69 (16%) | |
| 3 | 214 (86%) | 350 (80%) | |
| Post-stenting target lesion TIMI-flow–subject, n (%) | |||
| 0 | 1 (1%) | 1 (0%) | 0.54 |
| 1 | 0 (0%) | 0 (0%) | N/A |
| 2 | 4 (2%) | 6 (2%) | 0.73 |
| 3 | 182 (98%) | 385 (99%) | 0.72 |
| Post-stenting target lesion TIMI-flow–lesion, n (%) | |||
| 0 | 1 (0%) | 0 (0%) | 0.39 |
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 4 (2%) | 6 (1%) | |
| 3 | 243 (98%) | 434 (99%) | |
| Lesion severity, mean % (SD) | |||
| Pre-PCI | 80.71 (10.91) | 86.13 (10.02) | <0.0001 |
| Post-PCI | 0.38 (1.60) | 0.84 (2.96) | 0.19 |
| Thrombus present–subject, n (%) | 27 (15%) | 55 (14%) | 0.87 |
| Thrombus present–lesion, n (%) | 31 (13%) | 58 (13%) | 0.80 |
| Arterial access, n (%) | |||
| Femoral | 167 (90%) | 362 (93%) | 0.46 |
| Radial | 17 (9%) | 25 (6%) | |
| Other | 1 (1%) | 3 (1%) | |
| Target lesion stent diameter, mm | 3.41±0.52 | 3.39±0.53 | 0.68 |
| Target lesion first balloon diameter, mm–437 lesions in 390 subjects | N/A | 2.75±0.79 | N/A |
| Target lesion second balloon diameter, if used, mm–171 lesions in 156 subjects | N/A | 3.36±0.78 | N/A |
| Total length of stents in target lesions per patient, mm | 25.46±16.92 | 27.47±19.36 | 0.19 |
| Number of balloons used in target lesions per subject | N/A | 1.72±1.05 | N/A |
| Anticoagulant, n (%) | |||
| Heparin | 105 (57%) | 228 (58%) | 0.70 |
| Bivalirudin | 88 (48%) | 163 (42%) | 0.19 |
| GP2b3a | 32 (17%) | 52 (13%) | 0.21 |
| Highest ACT | 312.03±73.04 | 303.60±87.01 | 0.23 |
| Angiographic success–subject, n (%) | 185 (100%) | 388 (99%) | 1.00 |
| Angiographic success–lesion, n (%) | 247 (100%) | 438 (100%) | 1.00 |
| Any procedural complication, n (%) | 6 (3%) | 25 (6%) | 0.12 |
| No reflow, n (%) | 5 (3%) | 14 (4%) | 0.58 |
| Periprocedural MI, n (%) | 5 (3%) | 23 (6%) | 0.10 |
ACT indicates activated clotting time; BMS, bare metal stent; DES, drug-eluting stent; LAD, left anterior descending; MI, myocardial infarction; N/A, not any; OM, obtuse marginal branch; PCI, percutaneous coronary intervention; RCA/PDA, right coronary artery or posterior descending artery; SVG, saphenous vein graft; and TIMI, Thrombolysis in Myocardial Infarction.
Study Outcomes
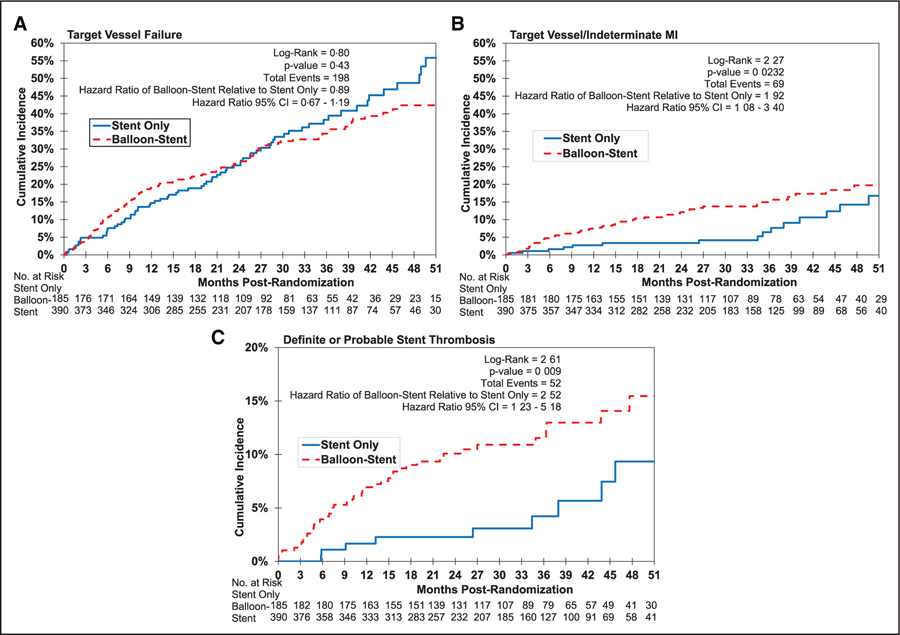
The 12-month incidence of TVF was 15% in the stent-only group and 19% in the balloon-stent group (hazard ratio for balloon-stent group, 1.34 [95% CI, 0.86–2.08]; P=0.19; Table 3). The incidence of target vessel myocardial infarction and definite or probable stent thrombosis was higher in the balloon-stent group (Figure).
Table 3.
Clinical Events at 12-Month Follow-Up for the Study Patients
| Stent-Only (N=185) | Balloon-Stent (N=390) | Balloon and Stent HR (95% CI) | Likelihood Ratio P Value* | |
|---|---|---|---|---|
| 12 mo | ||||
| TVF | 27 (15%) | 74 (19%) | 1.34 (0.86–2.08) | 0.19 |
| Death from any cause | 14 (8%) | 29 (7%) | 1.00 (0.53–1.89) | 1.00 |
| Cardiac or unknown cause of death (component of TVF) | 10 (5%) | 22 (6%) | 1.06 (0.50–2.23) | 0.88 |
| Cardiac death | 9 (5%) | 17 (4%) | 0.91 (0.41–2.04) | 0.82 |
| Noncardiac death | 4 (2%) | 7 (2%) | 0.85 (0.25–2.91) | 0.80 |
| Unknown cause of death | 1 (1%) | 5 (1%) | 2.40 (0.28–20.52) | 0.41 |
| Any MI during follow-up | 15 (8%) | 42 (11%) | 1.36 (0.75–2.45) | 0.31 |
| Target vessel MI during follow-up (component of TVF) | 5 (3%) | 29 (7%) | 2.86 (1.11–7.38) | 0.0232 |
| Non-target vessel MI during follow-up | 11 (6%) | 15 (4%) | 0.65 (0.30–1.41) | 0.27 |
| Any revascularization | 31 (17%) | 71 (18%) | 1.10 (0.72–1.68) | 0.65 |
| Target vessel revascularization (component of TVF) | 17 (9%) | 44 (11%) | 1.26 (0.72–2.21) | 0.41 |
| Target lesion revascularization | 12 (6%) | 35 (9%) | 1.44 (0.75–2.77) | 0.28 |
| Patient-oriented composite end point | 37 (20%) | 85 (22%) | 1.11 (0.75–1.63) | 0.61 |
| Target lesion failure | 22 (12%) | 68 (17%) | 1.53 (0.94–2.47) | 0.08 |
| Definite stent thrombosis | 1 (1%) | 12 (3%) | 5.92 (0.77–45.50) | 0.05 |
| Definite or probable stent thrombosis | 3 (2%) | 26 (7%) | 4.30 (1.30–14.20) | 0.0091 |
| Stroke | 0 (0%) | 5 (1%) | N/A | 0.18* |
| Postprocedural bleed | 0 (0%) | 2 (1%) | N/A | 1.00* |
| TVF or SVG occlusion that did not receive intervention | 27 (15%) | 75 (19%) | 1.36 (0.88–2.11) | 0.17 |
TVF is a composite of cardiac death, target vessel MI, and target vessel revascularization. Patient-oriented composite end point includes all cause death, any MI, and target vessel revascularization. Target lesion failure is a composite of cardiac or unknown death, target vessel MI, and TLR. HR indicates hazard ratio; MI, myocardial infarcation; N/A, not any; SVG, saphenous vein graft; and TVF, target vessel failure.
P value is based on χ2 goodness-of-fit test.
Figure.
Kaplan-Meier curves during the entire duration of follow-up. A, Target vessel failure; B, target vessel/indeterminate myocardial infarction (MI); C, definite or probable stent thrombosis.
The incidence of TVF during the entire duration of follow-up was 38% in the stent-only group versus 33% in the balloon-stent group (hazard ratio, 0.89 [95% CI, 0.67–1.19]; P=0.43; Table 4). The incidence of definite and definite/probable stent thrombosis and the incidence of target vessel myocardial infarction was higher in the balloon-stent group (Table 4). Medication usage was similar in the 2 study groups (Table 5).
Table 4.
Clinical Events During the Entire Duration of Follow-Up (Median, 2.7 Years) of the Study Patients
| Stent-Only (N=185) | Balloon-Stent (N=390) | Balloon and Stent HR (95% CI) | Likelihood Ratio P Value* | |
|---|---|---|---|---|
| Entire study | ||||
| TVF | 71 (38%) | 127 (33%) | 0.89(0.67–1.19) | 0.43 |
| Death from any cause | 38 (21%) | 62 (16%) | 0.84 (0.56–1.26) | 0.39 |
| Cardiac or unknown cause of death (component of TVF) | 31 (17%) | 45 (12%) | 0.74 (0.47–1.17) | 0.20 |
| Cardiac death | 16 (9%) | 27 (7%) | 0.85 (0.46–1.57) | 0.60 |
| Noncardiac death | 7 (4%) | 17 (4%) | 1.27 (0.53–3.06) | 0.60 |
| Unknown cause of death | 15 (8%) | 18 (5%) | 0.63 (0.32–1.25) | 0.18 |
| Any MI during follow-up | 31 (17%) | 77 (20%) | 1.30 (0.86–1.98) | 0.21 |
| Target vessel MI during follow-up (component of TVF) | 15 (8%) | 54 (14%) | 1.92 (1.08–3.40) | 0.0232 |
| Non-Target vessel MI during follow-up | 20 (11%) | 34 (9%) | 0.86 (0.50–1.50) | 0.59 |
| Any revascularization | 73 (39%) | 120 (31%) | 0.78 (0.59–1.05) | 0.10 |
| Target vessel revascularization (component of TVF) | 44 (24%) | 72 (18%) | 0.80 (0.55–1.17) | 0.25 |
| Target lesion revascularization | 27 (15%) | 54 (14%) | 1.02 (0.64–1.61) | 0.95 |
| Patient-oriented composite end point | 84 (45%) | 155 (40%) | 0.91 (0.70–1.19) | 0.48 |
| Target lesion failure | 59 (32%) | 115 (29%) | 1.01 (0.74–1.38) | 0.96 |
| Definite stent thrombosis | 1 (1%) | 18 (5%) | 9.20 (1.23–68.92) | 0.0085 |
| Definite or probable stent thrombosis | 9 (5%) | 43 (11%) | 2.52 (1.23–5.18) | 0.009 |
| Stroke | 4 (2%) | 18 (5%) | 2.37 (0.80–7.02) | 0.11 |
| TVF or SVG occlusion that did not receive intervention | 73 (39%) | 134 (34%) | 0.91 (0.68–1.21) | 0.51 |
TVF is a composite of cardiac death, target vessel MI, and target vessel revascularization. Patient-oriented composite end point includes all cause death, any MI, and target vessel revascularization. HR indicates hazard ratio; MI, myocardial infarction; SVG, saphenous vein graft; and TVF, target vessel failure.
P value is based on χ2 goodness-of-fit test.
Table 5.
Medication Use During Follow-Up of the Study Patients
| Medications During Follow-Up | Stent-Only (n=185) | Balloon-Stent (n=390) | P Value |
|---|---|---|---|
| Patients with a 12-mo visit, n (%) | 164 (89%) | 345 (88%) | 0.95 |
| Aspirin at 12 mo, n (%) | 154 (94%) | 319 (92%) | 0.46 |
| P2Y12 inhibitor at 12 mo, n (%) | 146 (89%) | 305 (88%) | 0.76 |
| Clopidogrel at 12 mo, n (%) | 137 (84%) | 283 (82%) | 0.62 |
| Ticlopidine at 12 mo, n (%) | 0 (0%) | 1 (0%) | 1.00 |
| Prasugrel at 12 mo, n (%) | 7 (4%) | 13 (4%) | 0.78 |
| Other at 12 mo, n (%) | 2 (1%) | 9 (3%) | 0.52 |
| Statin at 12 mo, n (%) | 154 (94%) | 319 (92%) | 0.46 |
| Number of subjects who had a 24-month visit, n (%) | 134 (72%) | 257 (66%) | 0.12 |
| Aspirin at 24 mo, n (%) | 125 (93%) | 229 (89%) | 0.18 |
| P2Y12 inhibitor at 24 mo, n (%) | 75 (56%) | 151 (59%) | 0.60 |
| Clopidogrel at 24 mo, n (%) | 71 (53%) | 136 (53%) | 0.99 |
| Ticlopidine at 24 mo, n (%) | 0 (0%) | 0 (0%) | N/A |
| Prasugrel at 24 mo, n (%) | 2 (1%) | 7 (3%) | 0.72 |
| Other at 24 mo, n (%) | 2 (1%) | 8 (3%) | 0.50 |
| Statin at 24 mo, n (%) | 124 (93%) | 234 (91%) | 0.62 |
| Number of subjects who had a 36-mo visit, n (%) | 91 (49%) | 164 (42%) | 0.11 |
| Aspirin at 36 mo, n (%) | 81 (89%) | 132 (80%) | 0.13 |
| P2Y12 inhibitor at 36 mo, n (%) | 47 (52%) | 68 (41%) | 0.15 |
| Clopidogrel at 36 mo, n (%) | 41 (45%) | 60 (37%) | 0.23 |
| Ticlopidine at 36 mo, n (%) | 0 (0%) | 0 (0%) | N/A |
| Prasugrel at 36 mo, n (%) | 2 (2%) | 6 (4%) | 0.71 |
| Other at 36 mo, n (%) | 4 (4%) | 2 (1%) | 0.19 |
| Statin at 36 mo, n (%) | 81 (89%) | 144 (88%) | 0.85 |
N/A indicates not any.
Multivariable analysis showed that adding group identifier (stent-only versus balloon-stent) to the proportional hazard model with selected participant and graft characteristics did not significantly increase the ability to predict TVF during the entire follow-up period, though number of years since CABG and >1 target SVG lesion were associated with increased TVF during entire follow-up, while preintervention Thrombolysis in Myocardial Infarction-3 flow was protective. Using this model, adjunctive balloon angioplasty, presence of thrombus, or use of embolic protection device were not associated with TVF during the entire follow-up period (Table 6).
Table 6.
Multivariate Analysis for Time to TVF at Entire Follow-Up
| Characteristic | HR (95% CI) | P Value |
|---|---|---|
| Balloon-stent balloon use | 0.84 (0.63–1.13) | 0.24 |
| Age | 1.00 (0.98–1.02) | 0.78 |
| Diabetes mellitus | 1.09 (0.81–1.46) | 0.57 |
| >1 target lesions | 1.60 (1.12–2.29) | 0.0097 |
| Years since CABG | 1.03 (1.00–1.05) | 0.0382 |
| History of CHF | 1.26 (0.94–1.69) | 0.12 |
| PCI for ACS | 1.17 (0.87–1.58) | 0.30 |
| Presence of thrombus | 0.83 (0.55–1.25) | 0.37 |
| Baseline TIMI-3 flow | 0.56 (0.40–0.78) | 0.0006 |
| EPD used | 0.99 (0.72–1.36) | 0.96 |
TVF is a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization. ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; CHF, congestive heart failure; EPD, embolic protection device; HR, hazard ratio; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; and TVF, target vessel failure.
DISCUSSION
The main finding of our study is that in patients undergoing PCI of de novo SVG lesions, use of adjunctive balloon angioplasty (performed in two thirds of the study patients) was associated with higher incidence of stent thrombosis and target vessel MI during follow-up as compared with the stent-only group.
There are several potential explanations for the higher incidence of ST in the stent-balloon group. First, lesions treated with adjunctive balloon angioplasty were more likely to be located at the aortic or distal anastomosis, which may be more difficult to expand, as aorto-ostial lesions are more likely to be calcified10 and both aorto-ostial and distal anastomotic lesions are affected by postsurgical fibrotic changes.11 In contrast, SVG body lesions more commonly include softer, friable plaque and might require less lesion preparation with predilatation.12 Second, balloon angioplasty may lead to distal embolization, which can lead to myocardial injury in the supplied myocardium and possibly impair subsequent myocardial flow. Indeed, there was a trend for higher incidence of periprocedural myocardial infarction in the balloon stent group4 (Table 2). Third, balloon angioplasty may cause more extensive SVG wall injury, potentially triggering formation of neointima and neoatherosclerosis that could form the nidus for subsequent stent thrombosis and/or restenosis. Although not observed in our study, a prior retrospective study showed higher 12-month incidence of target lesion revascularization at 1 year with the use of balloon angioplasty as compared with direct stenting during SVG PCI (34% versus 21%; P=0.02).5 Fourth, there may be unmeasured differences in lesion composition and overall PCI approach in the stent-only versus stent-balloon groups that contribute to subsequent outcomes. The Kaplan-Meier curves for stent thrombosis continued to separate after 12 months of follow-up (Figure), suggesting that the factors underlying the aforementioned differences have a long-lasting impact, hence are unlikely to be purely related to differences in periprocedural complications.
While our study supports stenting without pre-or post-dilation in SVG PCI, many SVG lesions may still require either predilation to create a channel in a severely stenosed SVG to deploy an embolic protection device distally or post-dilation to optimize stenting result. Compared with native vessel PCI, a lower incidence of calcification (reported in <20% of SVG lesions) further decreases the need for predilation compared with native vessel PCI.13 Finally, slight stent under-sizing may have no adverse impact in short- or long-term outcomes and may actually be beneficial in SVG PCI,14 although what constitutes an acceptable result remains to be determined. Use of intravascular imaging could facilitate assessment of SVG lesions and guide PCI.13 Compared with SVG-PCI, native coronary PCI has demonstrated lower rates of TVF and therefore should be preferred when feasible.15
Our study has limitations. It is a post hoc analysis of a prospective randomized-controlled trial, with all associated limitations. Data on the timing of balloon angioplasty (before or after stent placement or both) were not available. As is typical in VA studies, nearly all study participants were men, limiting extrapolation of the results to women, although the majority of patients undergoing PCI after CABG are men.
CONCLUSIONS
Adjunctive balloon angioplasty during SVG PCI is associated with higher incidence of stent thrombosis and myocardial infarction, favoring a primary stenting without postdilation approach.
WHAT IS KNOWN
Compared with direct stenting, adjunctive balloon angioplasty during stenting of saphenous vein grafts leads to an increase in distal embolization and periprocedural myocardial infarction.
WHAT THE STUDY ADDS
This substudy from a large, multicenter randomized controlled trial demonstrated that when compared with stenting alone, adjunctive balloon angioplasty during stenting of saphenous vein grafts leads to an increase in stent thrombosis and an increase in target vessel–related myocardial infarction.
Acknowledgments
Sources of Funding
DIVA trial (Drug-Eluting Stents Versus Bare Metal Stents in Saphenous Vein Graft Angioplasty) was funded by the Department of Veterans Affairs’ Cooperative Studies Program.
Disclosures
Dr Latif receives Speaker honoraria from Abbott Vascular, Inc. Dr Bhatt discloses the following relationships–Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. Dr Shunk is consultant for PercAssist, Inc, TransAortic Medical, and Terumo, and receives research support from Cardiovascular Systems, Inc and Svelte Medical, Inc. Dr Kinlay is Consultant on DSMB (Colorado Prevention Center). Dr Banerjee receives Research and educational grants from Boston Scientific and Merck; and consulting and speaking honoraria from Medtronic, Cardiovascular Systems, Inc, and Gore. Dr Brilakis receives consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, InfraRedx, Medtronic, and Teleflex; research support from Regeneron and Siemens. He is shareholder for MHI Ventures. Other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- DIVA
Drug-Eluting Stents Versus Bare Metal Stents in Saphenous Vein Graft Angioplasty
- EPD
embolic protection device
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- PMI
periprocedural myocardial infarction
- SVG
saphenous vein graft
Contributor Information
Faisal Latif, VA Medical Center, Oklahoma City, University of Oklahoma, Oklahoma City.
Lauren Uyeda, VA Cooperative Studies Program Coordinating Center, Mountain View, CA.
Robert Edson, VA Cooperative Studies Program Coordinating Center, Mountain View, CA.
Deepak L. Bhatt, VA Boston Healthcare System, MA, Brigham and Women’s Hospital Heart & Vascular Center, Boston, MA, Harvard Medical School, Boston, MA.
Steven Goldman, University of Arizona Sarver Heart Center, Tucson.
David R. Holmes, Jr, Mayo Clinic, Rochester, MN.
Sunil V. Rao, Durham VA Medical Center, NC.
Kendrick Shunk, San Francisco VA Medical Center, CA.
Kul Aggarwal, Harry S Truman VA Hospital, Columbia, MO, University of Missouri Healthcare, Columbia.
Barry Uretsky, Central Arkansas Veterans Health System, Little Rock, University of Arkansas for Medical Sciences, Little Rock.
Islam Bolad, Indiana University School of Medicine, Indianapolis, Roudebush VA Medical Center, Indianapolis, Indiana.
Khaled Ziada, University of Kentucky, Lexington.
Edward McFalls, VA Medical Center, Minneapolis, MN, University of Minnesota, Minneapolis.
Anand Irimpen, Southeast Louisiana Veterans Health Care System, New Orleans, Tulane University Heart and Vascular Institute, New Orleans, LA.
Huu Tam Truong, VA Loma Linda Medical Center, CA.
Scott Kinlay, VA Boston Healthcare System, MA.
Vasilios Papademetriou, VA Medical center Washington DC, Georgetown University, Washington DC.
Raghava S. Velagaleti, VA Boston Healthcare System, MA.
Bavana V. Rangan, Minneapolis Heart Institute Foundation, MN.
Kreton Mavromatis, Atlanta VA Healthcare System, GA, Emory University, Atlanta, GA.
Mei-Chiung Shih, VA Cooperative Studies Program Coordinating Center, Mountain View, CA.
Subhash Banerjee, Dallas VA Medical Center, TX, University of Texas Southwestern Medical Center, Dallas.
Emmanouil S. Brilakis, Minneapolis Heart Institute Foundation, MN, Minneapolis Heart Institute, MN, Abbott Northwestern Hospital, Minneapolis, MN.
REFERENCES
- 1.Brilakis ES, Wang TY, Rao SV, Banerjee S, Goldman S, Shunk K, Kar B, Holmes DR Jr, Dai D, Chin CT, et al. Frequency and predictors of drug-eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American college of cardiology national cardiovascular data cathPCI registry. JACC Cardiovasc Interv. 2010;3:1068–1073. doi: 10.1016/j.jcin.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci D, Valenti R, Migliorini A, Moschi G, Bolognese L, Cerisano G, Buonamici P, Santoro GM. Direct infarct artery stenting without predilation and no-reflow in patients with acute myocardial infarction. Am Heart J. 2001;142:684–690. doi: 10.1067/mhj.2001.117778 [DOI] [PubMed] [Google Scholar]
- 3.Stys T, Lawson WE, Liuzzo JP, Hanif B, Bragg L, Cohn PF. Direct coronary stenting without balloon or device pretreatment: acute success and long-term results. Catheter Cardiovasc Interv. 2001;54:158–163. doi: 10.1002/ccd.1258 [DOI] [PubMed] [Google Scholar]
- 4.Hong MK, Mehran R, Dangas G, Mintz GS, Lansky AJ, Pichard AD, Kent KM, Satler LF, Stone GW, Leon MB. Creatine kinase-MB enzyme elevation following successful saphenous vein graft intervention is associated with late mortality. Circulation. 1999;100:2400–2405. doi: 10.1161/01.cir.100.24.2400 [DOI] [PubMed] [Google Scholar]
- 5.Leborgne L, Cheneau E, Pichard A, Ajani A, Pakala R, Yazdi H, Satler L, Kent K, Suddath WO, Pinnow E, et al. Effect of direct stenting on clinical outcome in patients treated with percutaneous coronary intervention on saphenous vein graft. Am Heart J. 2003;146:501–506. doi: 10.1016/S0002-8703(03)00309-0 [DOI] [PubMed] [Google Scholar]
- 6.Nageh T, Thomas MR, Sherwood RA, Harris BM, Jewitt DE, Wainwright RJ. Direct stenting may limit myocardial injury during percutaneous coronary intervention. J Invasive Cardiol. 2003;15:115–118. [PubMed] [Google Scholar]
- 7.Lozano I, López-Palop R, Pinar E, Saura D, Fuertes J, Rondán J, Suárez E, Valdés M, Morís C. [Direct stenting in saphenous vein grafts. Immediate and long-term results]. Rev Esp Cardiol. 2005;58:270–277. [PubMed] [Google Scholar]
- 8.Brilakis ES, Edson R, Bhatt DL, Goldman S, Holmes DR Jr, Rao SV, Shunk K, Rangan BV, Mavromatis K, Ramanathan K, et al. ; DIVA Trial Investigators. Drug-eluting stents versus bare-metal stents in saphenous vein grafts: a double-blind, randomised trial. Lancet. 2018;391:1997–2007. doi: 10.1016/S0140-6736(18)30801-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brilakis ES, Banerjee S, Edson R, Shunk K, Goldman S, Holmes DR Jr, Bhatt DL, Rao SV, Smith MW, Sather M, et al. Rationale and design of the drug-eluting stents vs bare-metal stents in Saphenous Vein Graft Angioplasty (DIVA) Trial. Clin Cardiol. 2017;40:946–954. doi: 10.1002/clc.22763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong YJ, Jeong MH, Ahn Y, Kang JC, Mintz GS, Kim SW, Lee SY, Kim SY, Pichard AD, Satler LF, et al. Impact of lesion location on intravascular ultrasound findings and short-term and five-year long-term clinical outcome after percutaneous coronary intervention for saphenous vein graft lesions. Int J Cardiol. 2013;167:29–33. doi: 10.1016/j.ijcard.2011.11.078 [DOI] [PubMed] [Google Scholar]
- 11.Thomas WJ, Cowley MJ, Vetrovec GW, Malloy W, Goudreau E. Effectiveness of rotational atherectomy in aortocoronary saphenous vein grafts. Am J Cardiol. 2000;86:88–91. doi: 10.1016/s0002-9149(00)00834-1 [DOI] [PubMed] [Google Scholar]
- 12.Sano K, Mintz GS, Carlier SG, Fujii K, Yasuda T, Kimura M, Costa JR Jr, Costa RA, Lui J, Weisz G, et al. Intravascular ultrasonic differences between aorto-ostial and shaft narrowing in saphenous veins used as aortocoronary bypass grafts. Am J Cardiol. 2006;97:1463–1466. doi: 10.1016/j.amjcard.2005.11.080 [DOI] [PubMed] [Google Scholar]
- 13.Redfors B, Genereux P, Witzenbichler B, McAndrew T, Diamond J, Huang X, Maehara A, Weisz G, Mehran R, Kirtane AJ, et al. Percutaneous coronary intervention of saphenous vein graft. Circ Cardiovasc Interv. 2017;10: e004953. doi: 10.1161/CIRCINTERVENTIONS [DOI] [PubMed] [Google Scholar]
- 14.Hong YJ, Pichard AD, Mintz GS, Kim SW, Lee SY, Kim SY, Ahn Y, Jeong MH, Satler LF, Kent KM, et al. Outcome of undersized drug-eluting stents for percutaneous coronary intervention of saphenous vein graft lesions. Am J Cardiol. 2010;105:179–185. doi: 10.1016/j.amjcard.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Brilakis ES, O’Donnell CI, Penny W, Armstrong EJ, Tsai T, Maddox TM, Plomondon ME, Banerjee S, Rao SV, Garcia S, et al. Percutaneous coronary intervention in native coronary arteries versus bypass grafts in patients with prior coronary artery bypass graft surgery: insights from the veterans affairs clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. 2016;9:884–893. doi: 10.1016/j.jcin.2016.01.034 [DOI] [PubMed] [Google Scholar]