Abstract
Currently, women with metastatic or recurrent cervical cancer still have very limited treatment options. Despite the rapid advancements in targeted therapies, no targeted therapy was approved for cervical cancer, except for bevacizumab. In the present study, we reported a 52-year-old heavily pre-treated EGFR amplified patient with metastatic cervical squamous cancer who benefited from afatinib with a progression-free survival (PFS) of 5.5 months. The patient was administered with a first-line treatment of chemotherapy and bevacizumab with a PFS of 4.3 months. Subsequently the patient was treated with a second-line regimen of angiogenesis inhibitor apatinib plus chemotherapy and a third-line treatment of pembrolizumab. Genomic profiling revealed significant EGFR amplification in both primary (copy number [CN] =15.9) and metastatic lesions (CN =18). Afatinib monotherapy was then administered as the fourth-line regimen. She achieved partial response (PR) with a PFS of 5.5 months. At disease progression, the CN of EGFR was elevated to 39.9 accompanied by the emergence of PIK3CA amplification (CN =4.2). The patient was treated with everolimus and afatinib and achieved stable disease (SD) after 3 months. To the best of our knowledge, this is the first clinical evidence of an EGFR-amplified metastatic cervical cancer patient benefiting from afatinib as a single agent.
Keywords: afatinib, EGFR amplification, cervical squamous carcinoma, EGFR-tyrosine kinase inhibitor, next-generation sequencing
Introduction
It has been reported that 15% to 61% of women with cervical cancers develop metastatic disease.1 Although early-stage and locally advanced disease can be cured by radical surgery and chemo-radiotherapy, respectively, women with metastatic or non-operable recurrent disease have very limited treatment options. Currently, most metastatic or recurrent cervical cancer patients were treated with palliative chemotherapy with a median survival of 8 to 13 months.2 Despite the rapid advancements in targeted therapies, which revolutionized the treatment of many cancers, no targeted therapy was approved for cervical cancer except for bevacizumab.
Epidermal growth factor receptor (EGFR) has been an attractive target for anticancer therapy due to its involvement in multiple cellular processes thus contributing to the initiation and the development of cancer. Abnormal activation of EGFR (mutation or amplification/overexpression) has been identified in a variety of human tumors,3 including cervical cancer. Although, EGFR mutations are less commonly seen in cervical cancer, EGFR overexpression has been identified in approximately 70% cervical squamous carcinoma but its prognostic and predictive value remain elusive. However, numerous Phase II studies of EGFR inhibitors conducted on metastatic and/or recurrent cervical cancer patients revealed lacked of clinical efficacy. Gefitinib4 and erlotinib5 as single agents as well as cetuximab with or without cytotoxic chemotherapy6,7 showed either minimal activity or no meaningful benefit at all. Afatinib, a second-generation selective and irreversible EGFR-Tyrosine kinase inhibitor (TKI), has demonstrated substantial clinical activity as single agent in non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma.8,9 Here we present a metastatic EGFR-amplified cervical cancer patient benefited afatinib as the fourth-line treatment with a PFS of 5.5 months.
Case Report
A 52-year-old woman presented with irregular vaginal bleeding and was diagnosed with cervical squamous carcinoma with lymph node metastases (LNM) in August 2016. Initial treatment consisted of radical hysterectomy plus pelvic lymph node (LN) dissection and was followed by chemoradiotherapy for 2 months. A chest computed tomography (CT) scan performed 10 months after the completion of the initial chemoradiotherapy revealed multiple metastases in both lungs and the histopathology of the lesion revealed cervical metastatic squamous cell carcinoma. A first-line treatment of chemotherapy (docetaxel and cyclophosphamide) combined with bevacizumab was administered beginning September 20, 2017. After 3 cycles, a repeat chest CT scan indicated stable disease (SD) for pulmonary lesions and LNs on mediastinum, bilateral axillas and right clavicle. The squamous cell carcinoma-related antigen (SCC-Ag) was 0.7ng/mL. The patient remained on the same regiment for another 3 cycles and developed disease progression (PD) according to Response Evaluation Criteria in Solid Tumors 1.1, evident by the enlargement of her lung lesions on January 29, 2018. At PD, her SCC-Ag was elevated to 8.5ng/mL. Subsequently, her treatment was switched to apatinib in combination with chemotherapy (capecitabine+ tegafur) with a PFS of 6.6 months. On August 31 2018, a chest CT revealed enlarged lesions on both lungs, with the largest lesion measuring 47mmx43mm on the left lobe (Figure 1A). The SCC-Ag also increased remarkably to 85ng/mL. The patient was subsequently treated with pembrolizumab and failed to show any response by the end of second cycle.
Figure 1.
Clinical responses to afatinib treatment. (A) Prior to afatinib treatment; (B) The patient achieved partial response (PR) after one month of afatinib treatment. (C) The PR was confirmed 3 months after afatinib treatment. (D) Progressive disease (PD) was detected 5.5 months after the afatinib treatment. The red circles indicate the lung lesion.
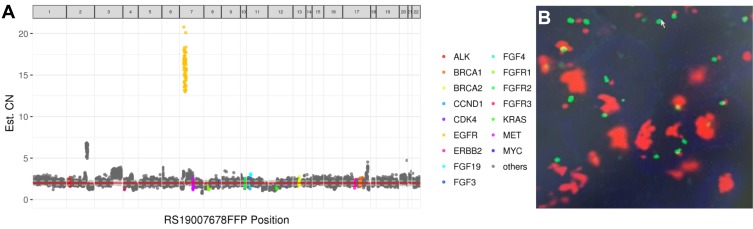
Both primary cervical lesion and metastatic lung lesion were sent for genomic profiling using a next generation sequencing (NGS) panel consisting of 520 genes in October 2018. The results showed EGFR amplification in both primary (CN=15.9, Figure 2A) and metastatic lesions (CN=18). Additionally, two missense mutations (p.Glu542Lys and p.Glu545Lys) in PIK3CA were also detected. Fluorescence in situ hybridization (FISH) was performed on the metastatic pulmonary specimen to confirm EGFR amplification (Figure 2B). The patient was administered with afatinib starting from October 16, 2018 and achieved partial response (PR) on November 12, 2018 based on the significant shrinkage of the left pulmonary lesion (Figure 1B). Another CT scan was performed in January 2018 and the patient remained as PR (Figure 1C). The patient developed PD with enlarged pulmonary lesions (Figure 1D) and LNs on mediastinum and left pulmonary hulim on April 1, 2019 with a PFS of 5.5 months. A biopsy of the left lung lesion was performed and revealed the retention of EGFR amplification with a very high copy number (CN=39.9) and the emergence of PIK3CA amplification (CN=4.2). Everolimus was added to the regimen of afatinib and the patient achieved SD after 3 months of treatment.
Figure 2.
Demonstration of EGFR amplification detected in primary and metastatic lesions. (A) NGS detected an EGFR copy number (CN) of 15.9 in primary cervical lesion. The 520 oncogenic genes of the panel are displayed according to their positions on chromosomes (horizontal axis). Capture domains of each gene are divided into several continuous bins. The CN (vertical axis) of each bin is calculated and represented by each spot in the diagram. The CNs of key genes are highlighted in color. The final CN of each gene is calculated as the average of CNs of all its bins. (B) Fluorescence in situ hybridization (FISH) confirmation of the EGFR amplification in metastatic lung lesion.
Discussion
This study presented the first clinical evidence of EGFR-amplified metastatic cervical squamous carcinoma patient benefited from afatinib as a single agent. Over-expression of EGFR has been frequently seen in cervical squamous carcinoma,5,10 whereas the EGFR amplification is identified only in 10–20% of cervical squamous cancers.10–12 Several studies have evaluated the efficacies of EGFR inhibitors in advanced cervical cancer but yielded unfavorable results. A single arm phase II trial of erlotinib in recurrent cervical squamous carcinomas showed zero objective response rate (ORR) with 16% of patients achieving SD.5 The median PFS and OS were 1.87 months and 4.96 months, respectively. The degree of EGFR expression was not taken into consideration in this. Similar results were observed in another study evaluating gefitinib as second- or third-line treatment in recurrent or metastatic cervical carcinomas. A median PFS of 37 days and a median OS of 107 days with zero objective responses were observed. Six (20%) patients experienced SD.4 EGFR expression was determined by immunohistochemistry (IHC) in this study. All the patients with SD had staining intensity of 2+ or 3+. In contrast, patients with PD had ranged from 0 to 3+, indicating a potential correlation between EGFR expression level and clinical response. Another single arm study conducted in Asian advanced cervical cancer patients showed an ORR of 10% with one patient achieving complete response (CR) and another one achieving PR (2/20). The observed PFS and OS were 4 months and 5 months, respectively.13 The EGFR status was not evaluated in the study. A potential factor contributing to the failure of above studies might be the lack of biomarker to stratify patients.
EGFR mutation status which is widely used as the biomarker to predict response to anti-EGFR therapies in a variety of cancers, controversies exist regarding whether EGFR amplification/overexpression or the degree of amplification/overexpression serve as reliable predictive markers for anti-EGFR agents.3 One of the explanations for these inconsistent results is the lack of unified testing method for EGFR amplification or expression,14,15 thus making it difficult to compare among studies. In our case, EGFR was significantly amplified in both the primary (CN=15.9) and the metastatic lesion (CN=18), potentially responsible for the observed clinical benefits. The EGFR amplification was assessed by NGS and the reported high copy number was calculated and normalized by the tumor cell proportion, which more reliably represents of overall genomic condition in tumor lesion.
We also observed PIK3CA mutations concomitant with the EGFR amplification in our case which is consistent with the finding that PIK3CA mutation acquisition is related to EGFR expression in cervical squamous cell carcinoma.16 The addition of everolimus, an inhibitor of the PI3K-AKT-mTOR pathway, to afatinib resulted in SD. Our observation supports the suggestion that patients with EGFR overexpression and PIK3CA alterations might benefit from combination treatment.16
Collectively, our case highlights the clinical benefit of afatinib in cervical squamous carcinoma harboring a high copy number of EGFR. Further studies on efficacies of anti-EGFR-based regimens in larger cohorts of advanced cervical cancer patients are necessary to evaluate the degree of EGFR amplification and clinical outcomes.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of The Sun Yat-sen University and National Research Committee and with the 1964 Helsinki Declaration and its later amendments. The institutional approval was not required to publish the case details.
Patient Informed Consent
Written informed consent was obtained from the patient described in this report to have the case details and any accompanying images published.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
Disclosure
Lin Shao, Han Han-Zhang, Fan Yang, Yang Wang, and Jing Liu are employed by Burning Rock Biotech. The authors report no other conflicts of interest in this work.
References
- 1.Vora C, Gupta S. Targeted therapy in cervical cancer. ESMO Open. 2018;3(Suppl 1):e000462. doi: 10.1136/esmoopen-2018-000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43. doi: 10.3802/jgo.2016.27.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato S, Okamura R, Mareboina M, et al. Revisiting epidermal growth factor receptor (EGFR) amplification as a target for anti-EGFR therapy: analysis of cell-free circulating tumor DNA in patients with advanced malignancies. JCO Precis Oncol. 2019;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goncalves A, Fabbro M, Lhomme C, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol Oncol. 2008;108(1):42–46. doi: 10.1016/j.ygyno.2007.07.057 [DOI] [PubMed] [Google Scholar]
- 5.Schilder RJ, Sill MW, Lee YC, Mannel R. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int J Gynecol Cancer. 2009;19(5):929–933. doi: 10.1111/IGC.0b013e3181a83467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley J, Sill MW, Birrer M, et al. Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group Study. Gynecol Oncol. 2011;121(2):303–308. doi: 10.1016/j.ygyno.2011.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santin AD, Sill MW, McMeekin DS, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 2011;122(3):495–500. doi: 10.1016/j.ygyno.2011.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised Phase 3 trial. Lancet Oncol. 2015;16(5):583–594. doi: 10.1016/S1470-2045(15)70124-5 [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Tang Y, Cheng X, Ji J, Zhang J, Zhou X. EGFR protein expression and gene amplification in squamous intraepithelial lesions and squamous cell carcinomas of the cervix. Int J Clin Exp Pathol. 2014;7(2):733–741. [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378–384. doi: 10.1038/nature21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida K, Nakayama K, Rahman MT, et al. EGFR gene amplification is related to adverse clinical outcomes in cervical squamous cell carcinoma, making the EGFR pathway a novel therapeutic target. Br J Cancer. 2011;105(3):420–427. doi: 10.1038/bjc.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma DN, Rath GK, Julka PK, Gandhi AK, Jagadesan P, Kumar S. Role of gefitinib in patients with recurrent or metastatic cervical carcinoma ineligible or refractory to systemic chemotherapy: first study from Asia. Int J Gynecol Cancer. 2013;23(4):705–709. doi: 10.1097/IGC.0b013e31828b1699 [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Zhang Y, Tang H, He J. EGFR gene copy number as a predictive/biomarker for patients with non-small-cell lung cancer receiving tyrosine kinase inhibitor treatment: a systematic review and meta-analysis. J Investig Med. 2017;65(1):72–81. doi: 10.1136/jim-2016-000252 [DOI] [PubMed] [Google Scholar]
- 15.Tian WJ, Huang ML, Qin QF, Chen Q, Fang K, Wang PL. Prognostic impact of epidermal growth factor receptor overexpression in patients with cervical cancer: a meta-analysis. PLoS One. 2016;11(7):e0158787. doi: 10.1371/journal.pone.0158787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bumrungthai S, Munjal K, Nandekar S, et al. Epidermal growth factor receptor pathway mutation and expression profiles in cervical squamous cell carcinoma: therapeutic implications. J Transl Med. 2015;13:244. doi: 10.1186/s12967-015-0611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]