Abstract
Niemann-Pick disease type C (NPC) disease is a lipid-storage disorder that is caused by mutations in the genes encoding NPC proteins and results in lysosomal cholesterol accumulation. 2-Hydroxypropyl-β-cyclodextrin (CD) has been shown to reduce lysosomal cholesterol levels and enhance sterol homeostatic responses, but CD’s mechanism of action remains unknown. Recent work provides evidence that CD stimulates lysosomal exocytosis, raising the possibility that lysosomal cholesterol is released in exosomes. However, therapeutic concentrations of CD do not alter total cellular cholesterol, and cholesterol homeostatic responses at the ER are most consistent with increased ER membrane cholesterol. To address these disparate findings, here we used stable isotope labeling to track the movement of lipoprotein cholesterol cargo in response to CD in NPC1-deficient U2OS cells. Although released cholesterol was detectable, it was not associated with extracellular vesicles. Rather, we demonstrate that lysosomal cholesterol trafficks to the plasma membrane (PM), where it exchanges with lipoprotein-bound cholesterol in a CD-dependent manner. We found that in the absence of suitable extracellular cholesterol acceptors, cholesterol exchange is abrogated, cholesterol accumulates in the PM, and reesterification at the ER is increased. These results support a model in which CD promotes intracellular redistribution of lysosomal cholesterol, but not cholesterol exocytosis or efflux, during the restoration of cholesterol homeostatic responses.
Keywords: trafficking, lipoproteins, drug therapy, stable isotope tracers, lysosomal storage disorder, 2-hydroxpropyl-β-cyclodextrin, cellular homeostasis
Cholesterol is an essential component of mammalian cell membranes that plays a major role in tuning membrane fluidity, thickness, and permeability to regulate membrane function and support the needs of specific organelles. Different cellular membranes vary widely in cholesterol content, ranging from the cholesterol-rich plasma membrane (PM) and endosomes to the cholesterol-poor ER and mitochondria (1–3). Due to its hydrophobicity, cholesterol does not transit between membranes through the aqueous phase. Rather, cholesterol transfer is facilitated by lipid-binding proteins or through membrane-fusion events (1). Although a number of proteins have been shown to function in cholesterol movement, the precise time-resolved itinerary of cholesterol trafficking between membranes and the mechanisms of regulation of this trafficking remain to be determined. Moreover, how cells maintain steep gradients of cholesterol concentration across different membranes in the face of rapid and dynamic cholesterol trafficking is not well understood.
Mammalian cells acquire cholesterol through endogenous cholesterol synthesis at the ER or through the uptake of cholesterol and cholesteryl ester-laden lipoprotein particles into the endosomal/lysosomal system. Receptor-mediated endocytosis of LDL by the LDL receptor or acetylated LDL (acLDL) by the scavenger receptor A (SRA) are responsible for cholesterol delivery into the lysosomal compartment. Here, the concerted actions of lysosomal acid lipase (LAL) and the Niemann-Pick disease type C (NPC) proteins NPC1 and NPC2 are critical for the mobilization of LDL cargo. LAL cleaves cholesteryl esters and thereby liberates free cholesterol, which is bound by NPC2, a soluble lysosomal protein. NPC2 transfers cholesterol to NPC1, a transmembrane protein embedded in the limiting lysosomal membrane (4). In the presence of functional LAL, NPC1, and NPC2, cholesterol is efficiently trafficked to the PM and ER as well as other cellular membranes. At the PM, excess cholesterol is effluxed through ABCA1, ABCG1, and SRB1 to apoA1/HDL particles (5). In the ER membrane, cholesterol serves as a critical regulator of sterol homeostasis through the SREBP transcription factors, and excess cholesterol is esterified by the ER-resident protein ACAT for storage in lipid droplets (6).
Interruption of the intralysosomal cholesterol trafficking network (NPC1, NPC2, or LAL) results in abnormal cholesterol homeostasis and lysosomal dysfunction. Mutations in NPC1 or NPC2 cause NPC, a fatal neurodegenerative disorder. Both cholesterol trafficking and homeostatic regulation are disrupted in NPC1-deficient cells, in which the accumulation of free cholesterol in the lysosome is accompanied by an elevated expression of cholesterol uptake and synthesis genes and decreased cholesterol esterification (7, 8). There is currently no U.S. Food and Drug Administration-approved therapy for NPC, but 2-hydroxypropyl-β-cyclodextrin (CD) has shown great promise in animal models and in human clinical trials (9). CD is a cyclic oligosaccharide frequently used as an excipient in drug formulations because of its ability to solubilize hydrophobic molecules. At concentrations >1 mM, CD can extract cholesterol from cultured cells (10). At lower concentrations, in the range of effective doses in vivo, CD enhances cholesterol trafficking from lysosomes without changing total cellular cholesterol, and neither increases in serum cholesterol nor cholesterol excretion are observed (11). In fact, in cell and animal models, CD treatment reduces the expression of SREBP2 gene targets and stimulates cholesterol esterification, consistent with a model in which lysosomal cholesterol is redistributed to the ER membranes (7, 8). While the mechanism of CD action remains unknown, recent studies provide evidence that CD promotes lysosomal exocytosis (2, 12, 13). However, if release from the cells of cholesterol-laden exosomes is responsible for the beneficial effects of CD treatment, this would be predicted to lower cellular cholesterol, a change inconsistent with the observed suppression of SREBP2 gene targets or with enhanced reesterification by ACAT.
To address these disparate findings, we used stable isotope labeling to track the movement of lipoprotein cholesterol cargo in response to CD in NPC1-deficient cells. Our data support a model in which CD promotes the redistribution of lysosomal cholesterol to the PM, where it is exchanged with cholesterol carried by extracellular acceptors, and to the ER, where it directs cholesterol homeostatic responses.
METHODS
Cells and media
U2OS cells expressing the human scavenger receptor A (U2OS-SRA) and U2OS-SRA cells with shNPC1 knockdown (U2OS-SRAshNPC1) were a gift from the Maxfield Laboratory (14). U2OS-SRAshNPC1 cells were transduced with a lentiviral vector for the expression of TMEM192-HA-RFP as previously described (15). Cells were cultured in McCoy’s medium containing 10% FBS, 1.2 g/l sodium bicarbonate, and 1 mg/ml G418. Lipoprotein-deficient medium (LPDM) contained lipoprotein-depleted FBS instead of regular serum. Media depleted of extracellular vesicles were prepared as previously described (16). Unless otherwise specified, experiments were performed using a standard protocol. On day 1, 2 × 105 cells were plated in 6-well dishes and grown overnight. On day 2, growth media were replaced with media containing 10 μM lalistat-1, an inhibitor of LAL (Tocris Bioscience), with 25 μg/ml acLDL or acLDL reconstituted with d7 cholesteryl ester (d7-acLDL), and cells were incubated for an additional 18 h. For reesterification experiments, 36 μg/ml d9 oleate complexed to BSA was included during this period. On day 3, cells were washed twice with PBS and then incubated in fresh growth media containing 500 μM CD (Janssen Pharmaceuticals) or vehicle (H2O) for 6 h. Reconstituted acLDL was prepared as previously described (17). Lipoproteins were obtained from Alfa Aesar or Kalen Biomedical. Human apoA1 was obtained from Millipore Sigma.
Lysosomal isolation
U2OS-SRAshNPC1 cells expressing TMEM192-HA-RFP were plated at 12 × 105 in 10 cm dishes loaded with d7-acLDL and treated with CD according to the standard protocol. Lysosomes were isolated as previously described (15) using PierceTM Anti-HA Magnetic Beads (Thermo Fisher Scientific) and eluted from the beads with a 5 min incubation in 50 mM NaOH. Eluates were neutralized immediately with 100 mM Tris (pH 6.8). Cholesterol and cholesteryl ester content were measured in cell lysates, postnuclear supernatants, or elution fractions by LC/MS/MS. For Western blot analysis, lysosomal proteins were eluted from beads in RIPA buffer [50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1X Protease Complete (Roche)], separated on 4% to 12% Bis-Tris gels, transferred to nitrocellulose membranes, and analyzed for protein markers of lysosomes (LAMP1, HEXA), ER (calreticulin), PM (Na/K ATPase), mitochondria (cytochrome oxidase XIV), and nuclei (histone H3). See supplemental Table S1 for antibody sources and dilutions.
Extraction of cholesterol and cholesteryl esters from isolated fractions, cell homogenates, and media
To extract lipids, a portion of the fraction, cell homogenate, or media was added to a 1:1:1:0.5 mixture of chloroform-methanol-water-5M NaCl in glass tubes along with internal standards for cholesterol and cholesteryl ester. Mixtures were vortexed and centrifuged at 1,000 g for 5 min, and the chloroform phase was transferred to a 1.2 ml glass tube, dried under N2, and resuspended in 1:1 methanol-chloroform. Twenty percent of this solution was transferred to a new tube for the derivatization of cholesterols with 0.05 M nicotinic acid, 0.05 M 4-(dimethylamino)pyridine, and 0.05 M 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide for 1 h at 55°C. Lipids were analyzed by LC/MS/MS. Another portion of the fraction or homogenate was used for the determination of protein concentration using BCA assay (Pierce). Conditioned media were collected and centrifuged at 550 g to remove debris and frozen prior to extraction. Lysosomal fractions, extracellular vesicles, and lipoprotein fractions were thawed and then added directly to the extraction mixture. Cells were recovered in PBS by scraping and centrifuged at 2,500 g for 10 min at 4°C and then frozen at −20°C. Pellets were thawed and homogenized with a 25-gauge needle in PBS before extraction.
PM cholesterol and cellular cholesteryl esters
To quantify PM cholesterol and cholesteryl ester, cells were washed three times with 1% BSA in Tris-buffered saline (140 mM NaCl, 3 mM KCl, 25 mM Tris base, pH 7.4), washed twice with PBS, and then fixed for 10 min in 1% glutaraldehyde. Cells were washed twice more with PBS and then incubated in McCoy’s medium containing 2 units/ml cholesterol oxidase and 0.1 units/ml sphingomyelinase for 30 min at 37°C to convert PM cholesterol to cholestenone. Cells were washed again and then incubated in 9:1 methanol-chloroform supplemented with internal standards for cholestenone and cholesteryl ester for 30 min to extract cellular lipids. The lipid extract was transferred to 1.2 ml glass tubes, dried under nitrogen, and resuspended in 1:1 methanol:chloroform. Twenty percent of this solution was transferred to a new tube for the derivatization of cholestenones with 2:1 5 mg/ml O-benzylhydroxylamine hydrochloride/formic acid for 1 h at 55°C, followed by LC/MS/MS analyses. The protein concentration of cellular homogenates prepared in parallel was determined using BCA assay. For lipoprotein-dependent efflux assays, U2OS-SRAshNPC1 were treated in LPDM or LPDM supplemented with 25 μg/ml LDL or 25 μg/ml HDL and harvested as described above, except that cells were plated at 4 × 104 in 24-well dishes.
Extracellular vesicle isolations
For extracellular vesicle (EV) isolations, 12 × 105 cells were plated in each of 5 plates (10 cm) per condition, loaded with 10 μg/ml d7-acLDL in the presence of 10 μM lalistat, and incubated overnight to load the lysosomal compartment. Cells were washed twice with PBS and then incubated in fresh media (depleted of EVs) with or without 500 μM CD for 6 h. EVs were isolated from conditioned media by differential ultracentrifugation as previously described (16). Cholesterol content was quantified in media, EVs, and media following the depletion of EVs by LC/MS/MS. EV proteins were separated on 4% to 12% Bis-Tris precast gels, transferred to PVDF membranes, and probed for nuclear (lamin B1) and EV (CD63) markers.
SiRNA and shRNA knockdowns
For transient knockdowns of SRB1, U2OS-SRAshNPC1 cells were incubated with 25 μg/ml d7-acLDL and 10 μM lalistat in 2.1 ml media that also contained 0.5 ml Opti-MEM/RNAiMax/siRNA. A set of cells was harvested to evaluate protein knockdown by Western blot and baseline d7 cholesteryl oleate content by LC/MS/MS. Cells were then incubated in complete media with or without 500 μM CD for 6 h, and media were collected for the analysis of d7 cholesterol content. For stable knockdown of ABCA1 or ABCG1, shRNA plasmids were obtained from Origene and used to produce lentivirus in HEK 293T cells according to the TransIT-Lenti protocol using TransIT-Lenti transfection reagent and Mission Lentiviral Packaging Mix. U2OS-SRAshNPC1 cells were transduced with viral supernatants (obtained 48–72 h after transfection) and expanded, and the top 10% GFP-expressing cells were isolated by flow cytometry. Protein knockdown was evaluated by Western blot.
Lipoprotein isolation
U2OS-SRAshNPC1 cells were plated and treated according to the standard protocol. Conditioned media were removed from cells and spun at 550 g to remove debris. Cleared media were transferred to a 1.4 ml polycarbonate tube, the density was adjusted to 1.21 g/ml with KBr, and 150 μl 0.9% NaCl was added to the top of the suspension. After centrifugation for 18 h at 259,000 g at 4°C, the top 200 μl were removed as fraction 1, the second 200 μl were removed as fraction 2, and the remaining volume was removed as fraction 3. Cholesterol content was measured for whole media and fractionated media by LC/MS/MS. Proteins were analyzed on NuPage 3% to 12% Bis-Tris gels using NativePage Running Buffer (100 V × 30 min, 150 V × 30 min, and 200 V until the dye front reached the bottom). Gels were fixed and stained using Sypro Ruby according to the manufacturer’s protocol (Thermo Fisher Scientific).
Fluorescent lipoprotein uptake
U2OS-SRAshNPC1 cells were plated at 4 × 104 cells per well in 24-well dishes. After overnight growth, cells were preincubated with 10 μM dynasore (DYN) hydrate (Millipore Sigma) or vehicle (DMSO) in complete media for 30 min. Media were then replaced with LPDM with or without DYN, with or without 25 μg/ml dil-lipoprotein (dil-LDL or dil-HDL; Kalen Biomedical), and with or without 500 μM CD. After 6 h, cells were washed and then lysed in RIPA buffer with protease complete for 30 min at 4°C. The lysate was spun at 16,000 g for 10 min to remove debris. Lysate fluorescence was quantified using a TECAN scanner (excitation/emission: 550/580 nm) in a black flat-bottom 96-well plate. Percentage uptake was calculated as the ratio of cell-associated fluorescence relative to the fluorescence in the media at baseline.
Lipoprotein cholesterol exchange assay
U2OS-SRAshNPC1 cells were plated and treated as for fluorescent lipoprotein uptake except that lipoproteins were labeled with d7 cholesterol instead of dil. After CD treatment, cellular cholesterol was recovered as described above. Percentage exchange was calculated as the ratio of cell-associated d7 cholesterol relative to the d7 cholesterol in the media at baseline. To prepare lipoproteins with isotope-labeled cholesterol, d7 cholesterol was dried under nitrogen and then incubated with LDL or HDL at a ratio of 15 μg d7 cholesterol to 100 μg protein at a final protein concentration of 2 mg/ml. After overnight incubation at 4°C, lipoprotein mixtures were centrifuged at 10,000 g for 10 min at 4°C. Ninety percent of the supernatant was recovered and diluted to 25 μg/ml in LPDM for the administration to cells.
Mass spectrometry
LC/MS/MS analysis was conducted using a Shimadzu HPLC system coupled to a TSQ Quantum Ultra Plus mass spectrometer (Thermo Fisher Scientific) operating in the positive mode and using selected reaction monitoring. Chromatography for cholesterol and cholestenone was performed on an Eclipse XDB-C18 column (3.0 × 100 mm; Agilent) at 50°C with 1% formic acid in isopropanol-methanol (1:1) as the mobile phase, a flow rate of 0.4 ml/min, and a run time of 4.5 min. Chromatography for cholesteryl esters utilized a BetaSilTM C18 column (100 × 2.1 mm; Thermo Fisher Scientific) at 50°C, with 97% 10 mM ammonium acetate in isopropanol-methanol (1:1) and 3% 10 mM ammonium acetate in acetonitrile-H2O (3:7) as the mobile phase, a flow rate of 0.4 ml/min, and a run time of 11 min. Collision energies for cholesterol, cholestenone, and cholesteryl ester were 22, 30, and 14 V, respectively. Monitored transitions are reported in supplemental Table S2. Additional assay parameters are reported in supplemental Table S3. Data was analyzed using Xcalibur software. Calibration curves were constructed by plotting peak ratios of standard/internal standard versus analyte concentration.
RESULTS
Lysosomal cholesterol is differentially distributed to the media and the ER after CD treatment
U2OS-SRA cells efficiently take up the cholesteryl ester probe reconstituted in acLDL to lysotracker positive compartments (18). In the presence of lalistat, which prevents LAL-mediated ester hydrolysis (19, 20), cargo is retained in the compartment; upon inhibitor washout, cargo is released. We adapted this approach to specifically monitor the NPC1-independent trafficking of lysosomal cholesterol after CD treatment. In NPC1-deficient mice, CD is rapidly cleared from the plasma in 3 h and from the whole body in 6 h (11), and CD is estimated to reach concentrations of 0.1–1 mM in vivo (21, 22). On the basis of these studies, we chose to analyze the effects of CD after 6 h of treatment with 0.5 mM CD. U2OS-SRAshNPC1 cells were incubated with d7-acLDL in the presence of lalistat (Fig. 1). After loading, d7-acLDL and lalistat were removed and replaced with media containing CD. Trafficking of deuterated cholesterol away from the lysosome or to the PM, ER, and culture media was monitored using LC/MS/MS-based biochemical trafficking assays.
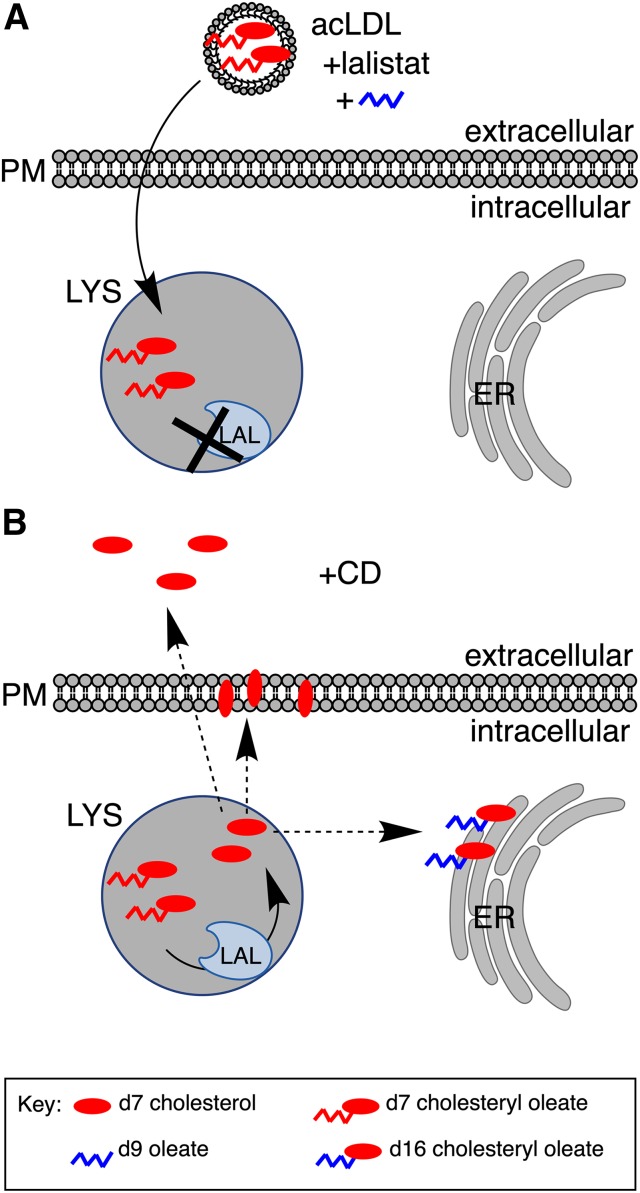
Fig. 1.
Tracing cholesterol cargo. A: U2OS-SRAshNPC1 cells were incubated in medium containing acLDL reconstituted with d7-acLDL (red oval with chain) to load the lysosomal compartment (LYS). Lalistat was included during loading to prevent cleavage of labeled esters by LAL and release from the lysosome. d9 oleate (blue chain) was included during loading to provide cells with labeled oleate to detect the reesterification product. B: Following the removal of acLDL and lalistat, cells were incubated with CD. Washout of lalistat enables the hydrolysis of cholesteryl esters at the lysosome. Upon arrival at the ER, d7 cholesterol was reesterified with d9 oleate to form d16 cholesteryl ester, which can be distinguished from loaded d7 cholesteryl ester. Arrival of cholesterol at the PM was detected by sphingomyelinase and cholesterol oxidase treatment, which converts d7 cholesterol to d7 cholestenone. Efflux of lysosomal cholesterol was monitored by the appearance of d7 cholesterol in the media.
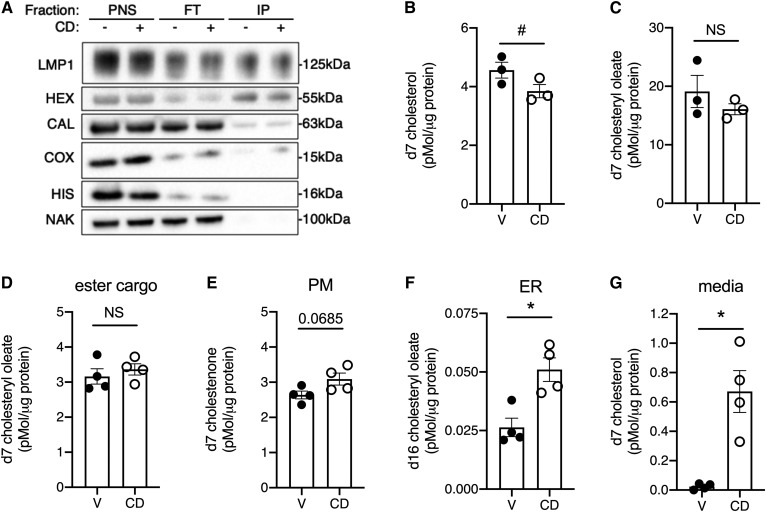
Although previous studies have used filipin staining to demonstrate that total lysosomal cholesterol is decreased by CD treatment (8), these studies have not metabolically traced lipoprotein-derived cholesterol. We used U2OS-SRAshNPC1 cells expressing TMEM192-RFP-HA, a tagged lysosomal protein, to quantify changes in lysosomal cholesterol cargo derived from endocytosed lipoproteins. Lysosomes from U2OS-SRAshNPC1-TMEM192-RFP-HA cells contained LAMP1 and HEXA, membrane-bound and soluble lysosomal proteins, respectively (Fig. 2A). The isolated lysosomal fraction was depleted of contaminating membranes from the nucleus and PM, as indicated by histone H3 and Na/K ATPase markers, respectively. While substantially depleted, the ER marker calreticulin and mitochondria marker cytochrome oxidase XIV could still be detected in the isolated fractions. The lysosome-enriched fraction isolated from cells treated with CD had less d7 cholesterol relative to the vehicle-treated control (Fig. 2B). D7 cholesteryl oleate associated with the enriched fraction did not differ significantly between CD and vehicle-treated cells (Fig. 2C). Some LAMP1-containing membranes were not immunoisolated and were detected in the flow through. These LAMP+ membranes could represent lysosome-related organelles that do not contain TMEM197 or HEXA but may harbor cholesterol.
Fig. 2.
Lysosomal cholesterol is differentially distributed to the media and the ER after CD treatment. U2OS-SRAshNPC1 cells that express epitope-tagged lysosomal protein TMEM192 (A–C) or U2OS-SRAshNPC1 cells (D–G) were incubated overnight with d7-acLDL in the presence of lalistat and then treated for 6 h in media with CD or vehicle before the immunoisolation of intact lysosomes (A–C) or analysis of other compartments (D–G). A: Immunoblots of PNS, FT, and IPs for LMP, HEX, CAL, COX, HIS, and NAK. B, C: LC/MS/MS quantification of lysosomal d7 cholesterol (B) and d7 cholesteryl oleate (C) normalized to lysosomal protein. D–G: d7 cholesterol species quantified by LC/MS/MS in treated cells that also received d9 oleate. Remaining d7 cholesteryl ester cargo (D), PM d7 cholestenone (E), ER reesterification product d16 cholesteryl oleate (F), and media-associated d7-cholesterol (G), each normalized to cellular protein. Means ± SEs (n = 3–4). #P < 0.05 by paired t-test (B, C); *P < 0.05 by unpaired t-test (D–G). CAL, calreticulin; COX, cytochrome c oxidase IV; FT, flow through; HEX, hexaminidase; HIS, histone 3; IP, immunoisolated lysosome; LMP, LAMP1; NAK, NaK ATPase; PNS, postnuclear supernatant.
To trace the fate of lipoprotein cholesterol cargo to other cellular compartments, we analyzed cholesterol movement in U2OS-SRAshNPC1 cells. Similar to our findings in the lysosomes, total cellular d7 cholesteryl oleate was unchanged by CD treatment (Fig. 2D), suggesting LAL activity was not affected. To measure PM cholesterol, cells were fixed and treated with cholesterol oxidase and sphingomyelinase to oxidize d7 cholesterol to d7 cholestenone. Under vehicle-treated conditions, d7 cholesterol trafficked to the PM (Fig. 2E). There was a trend for increased trafficking of lysosomal cholesterol to the PM following CD treatment, but this did not reach significance. To assess reesterification, d9 oleate was included during loading to provide substrate for ACAT-mediated reesterification of d7 cholesterol to d16 cholesteryl ester. CD administration resulted in a 2-fold increase in the formation of d16 cholesteryl esters from cholesterol cargo originating in the lysosome (Fig. 2F). We also analyzed d7 cholesterol content of conditioned media to assess efflux. Most strikingly, compared with the vehicle treatment, CD treatment increased the efflux of d7 cholesterol to the culture medium 28-fold (Fig. 2G). Overall, the magnitude of d7 cholesterol arriving at the ER was small compared with the d7 cholesterol arriving at the PM and into the media.
Released d7 cholesterol is not detected in isolated extracellular vesicles
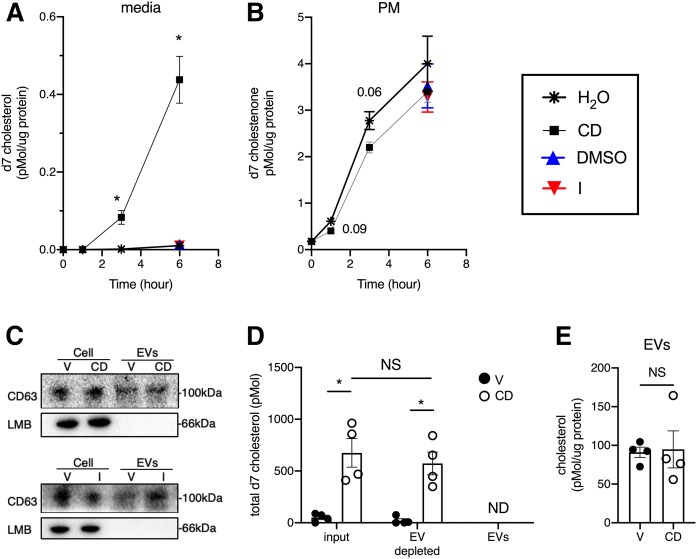
To further characterize the mechanism of release of d7 cholesterol into the culture medium, we performed a time course. D7 cholesterol was detectable in the culture medium as early as 3 h after lalistat washout and CD treatment. Between 3 and 6 h, d7 cholesterol in the media increased ∼5-fold (Fig. 3A). The appearance of lysosomal cholesterol in the PM preceded its appearance in the media (Fig. 3B). Similar levels of PM d7 cholesterol were measured after ionomycin or DMSO vehicle treatment. In contrast to CD, ionomycin did not promote the release of the cholesterol into the media over 6 h. These data show that the distribution of d7 cholesterol from the lysosome to the PM is an order of magnitude greater and occurs more rapidly than distribution into the media.
Fig. 3.
Released d7 cholesterol is not detected in isolated EVs. U2OS-SRAshNPC1 cells were loaded overnight with d7-acLDL in the presence of lalistat and then treated for 6 h in media with 500 μM CD, 5 μM ionomycin, or vehicle (H2O for CD and DMSO for ionomycin). A: LC/MS/MS quantification of d7 cholesterol associated with the media over time normalized to cellular protein. B: PM d7 cholestenone over time normalized to cellular protein. C–E: EVs were isolated from conditioned media at 6 h by differential centrifugation. Immunoblot analysis of total cell lysates (cells) and EVs for exosomal marker CD63 and nuclear marker LMB (C). Total d7 cholesterol in 27 ml conditioned media (input), 27 ml EV-depleted media, and EVs (from 27 ml conditioned media) at 6 h (D). Total EV cholesterol normalized to EV protein (E). Means ± SEs (n = 3). *P < 0.05 by unpaired t-test. LMB, lamin B; V, vehicle.
A recent report that CD induces lysosomal exocytosis in HeLa cells (13) provides a potential mechanism for the appearance of lysosomal cholesterol in the culture medium. To test whether d7 cholesterol arriving in the media was contained within membrane-bound structures released from the cells, we used differential centrifugation to isolate EVs from conditioned media from U2OS-SRAshNPC1 cells loaded with deuterated cholesterol and then treated with CD. The EV fraction was positive for the exosome marker CD63 but negative for nuclear marker laminin B by Western blot (Fig. 3C, top). Similar results were seen with EVs isolated from conditioned media from cells treated with ionomycin (Fig. 3C, bottom). While d7 cholesterol was readily detected in whole media and EV-depleted media from CD-treated cells, d7 cholesterol was not detected in EVs. Although EVs contained d0 cholesterol, the amount did not differ significantly between EVs isolated from the media of vehicle- and CD-treated cells (Fig. 3E). Together, these data indicate that EV release does not significantly contribute to the d7 cholesterol pool detected in the culture media.
CD-induced accumulation of cholesterol in the media is largely independent of canonical cell-surface cholesterol transporters
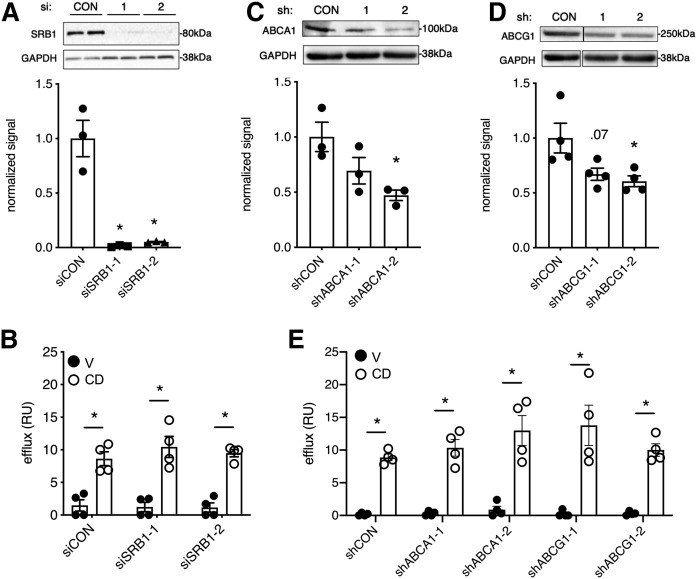
We next considered alternate mechanisms for the release of lysosomal cholesterol following CD treatment. SRB1, ABCA1, and ABCG1 are cell-surface sterol transport proteins. To test their contributions to CD-mediated accumulation of cholesterol in the media, we knocked down these proteins by siRNA or shRNA and quantified the delivery of d7 lysosome-derived cholesterol into the media. Two independent SRB1 siRNAs (siSRB1-1 and siSRB1-2) robustly suppressed SRB1 protein expression (>95%), as shown by Western blot (Fig. 4A). Despite this suppression, CD-induced cholesterol accumulation in the media was not significantly different between siSRB1 and control siRNA-treated cells (Fig. 4B). To test the effects of ABCA1 and ABCG1 protein depletion, stable shRNA knockdown cells were generated using target-specific (shABCA1-1 or shABCA1-2 and shABCG1-1 or shABCG1-2) or control shRNAs. Using two different shRNAs for ABCA1, we isolated cell populations with 31% and 53% knockdown (Fig. 4C); for shABCG1, cell populations with 33% and 39% knockdown were isolated (Fig. 4D). Neither knockdown of ABCA1 nor knockdown of ABCG1 impaired d7 cholesterol accumulation in the media (Fig. 4E). Thus, the CD-induced release of lysosomal cholesterol into the media is independent of SRBI and occurs even with substantial ABCA1 or ABCG1 knockdown.
Fig. 4.
CD-induced accumulation of cholesterol in the media is largely independent of canonical cell-surface cholesterol transporters. U2OS-SRAshNPC1 cells were treated with control or SRB1-targeting siRNAs (A, B) or with control or ABCA1- or ABCG1-targeting shRNAs (C–E). After loading with d7-acLDL in the presence of lalistat, cells were treated for 6 h with CD or vehicle, and effluxed d7 cholesterol was quantified by LC/MS/MS. A: Representative immunoblot for SRB1 with GAPDH loading control (above) and quantification for n = 3 (below). B: Moles released d7 cholesterol relative to moles d7 cholesteryl ester loaded expressed as efflux (relative units). C, D: Representative immunoblots for ABCA1 (C) and ABCG1 (D) with GAPDH loading control (above) and quantification for n = 3 (below). E: Relative efflux. Means ± SEs (n = 3). *P < 0.05 for siRNA or shRNA versus control by unpaired t-test. siRNA or shRNA versus control by two-way ANOVA was not significant for vehicle and CD conditions. CON, control; RU, relative unit; V, vehicle.
CD-induced cholesterol accumulation in the media depends on serum lipoproteins
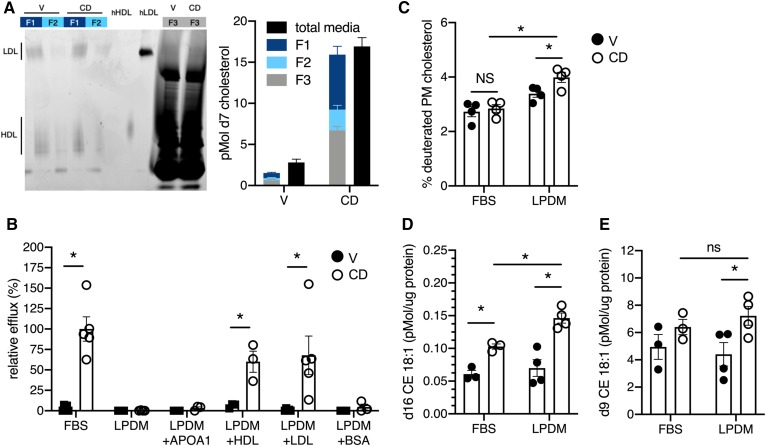
Most cholesterol found in serum is associated with serum lipoproteins. To test whether extracellular lipoproteins play a role in the CD-induced release of lysosomal cholesterol into the media, the lipoprotein fraction was isolated from conditioned medium after CD treatment. LDL and HDL are the major lipoproteins present in FBS (23). In line with this, lipoprotein fractions 1 and 2 from conditioned culture medium of cells treated with CD or vehichle contained two dominant species that comigrated with either human LDL (Fig. 5A, lanes 1–4, upper band) or human HDL (Fig. 5A, lanes 1–4, lower band) on a native PAGE gel. Following CD treatment, the lipoprotein fractions contained >50% of the d7 cholesterol found in the medium (Fig. 5A, graph, right). When cells were treated with CD in LPDM, d7 cholesterol was no longer detected in the medium (Fig. 5B). Supplementation of LPDM with 25 μg/ml HDL or LDL restored d7 cholesterol release. Equivalent concentrations of APOA1 or BSA did not support cholesterol release, suggesting that CD-mediated accumulation in the media requires lipidated lipoproteins. Although the proportion of lysosomal cholesterol in the PM was not significantly different between vehicle- and CD-treated cells when delivered in medium containing FBS (Fig. 2E), lysosome-derived cholesterol accumulated in the PM when lipoproteins were absent in the media (Fig. 5C). Reesterification of lysosome-derived cholesterol, detected as d16 cholesteryl ester, was enhanced in the absence of lipoproteins (Fig. 5D). This was likely due to the increased delivery of d7 cholesterol to the ER for esterification rather than increased overall esterification, as the d9-oleate pool (esterification product of nonlabeled cholesterol) was not significantly different between FBS and LPDM conditions (Fig. 5E). Together these findings support a model in which CD enhances the trafficking of lysosomal cholesterol to the PM, where it is released into the media if lipoproteins are present, or further trafficked to the ER, where it is esterified.
Fig. 5.
CD-induced cholesterol accumulation in the media depends on serum lipoproteins. U2OS-SRAshNPC1 cells were loaded overnight with d7-acLDL in the presence of lalistat and then treated with CD or vehicle for 6 h in media of differing compositions. A: After treatment in media containing FBS, lipoproteins were isolated from conditioned media and assayed for d7 cholesterol content. Sypro Ruby-stained native PAGE gel analysis (left) and d7 cholesterol content (right) of conditioned media and its lipoprotein fractions (F1 and F2) and remaining material (F3) after lipoprotein isolation. Pure hLDL and hHDL are shown as markers. B: Media-associated d7 cholesterol quantified after 6 h of treatment in FBS, LPDM, or LPDM supplemented with 25 μg/ml APOA1, HDL, LDL, or BSA. Efflux reported relative to efflux with FBS. C: PM-localized lysosomal cholesterol after 6 h of CD treatment in FBS or LPDM reported as a percentage of the total cholestenone pool that was deuterated. D: Reesterification of lysosomal cholesterol after 6 h of CD treatment in FBS or LPDM. E: Esterification of nondeuterated cholesterol (d9 CE 18:1) after 6 h of CD treatment in FBS or LPDM. Means ± SEs (n = 3–5). *P < 0.05 by unpaired t-test or two-way ANOVA for comparisons indicated. hHDL, human HDL; hLDL, human LDL; V, vehicle.
Bidirectional cholesterol exchange occurs at the PM
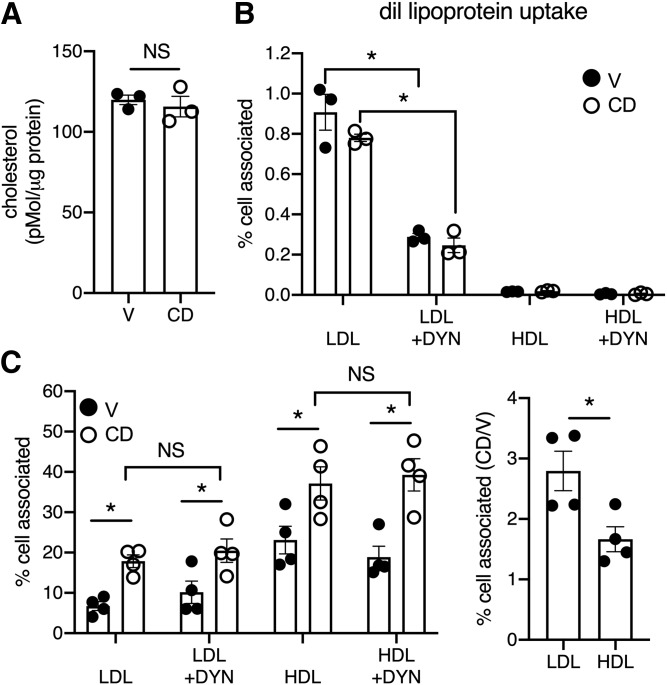
Despite the release of lysosomal cholesterol from the cell, CD treatment in complete media did not alter total cellular cholesterol (Fig. 6A). Thus, we incubated cells with diI-labeled lipoproteins to test whether lysosome-derived cholesterol release was matched by the reuptake of lipoprotein cholesterol from the media during CD treatment. Only 1% per milligram of protein of the diI-LDL was taken up under both vehicle and CD conditions (Fig. 6B). This was blunted by the dynamin inhibitor DYN, consistent with a mechanism of receptor-mediated endocytosis. The uptake of HDL was negligible. On the basis of these observations we hypothesized that in the presence of CD the release of cholesterol from the PM to lipoproteins in the media occurs as an exchange with lipoprotein cholesterol. To test this hypothesis, we treated cells with CD in media containing lipoproteins carrying deuterated cholesterol. CD treatment increased cell-associated deuterated cholesterol in the presence of LDL or HDL donors (Fig. 6C, left). Suppression of the cellular uptake of lipoproteins with DYN did not inhibit the CD-dependent transfer of LDL- or HDL-derived cholesterol (Fig. 6C, left). CD increased the transfer of cholesterol from LDL to cells 2.8-fold more than the vehicle (Fig. 6C, right). The transfer of cholesterol from HDL to the cells was less efficient. Together, our data support a model in which CD facilitates the exchange of lysosome-derived d7 with lipoprotein acceptors in the media, with LDL providing the more efficient exchange.
Fig. 6.
Bidirectional cholesterol exchange occurs at the PM. A: Total cellular cholesterol in U2OS-SRAshNPC1 cells treated for 6 h with CD or vehicle quantified by LC/MS/MS. B: Cellular uptake of diI-labeled LDL or HDL after 6 h with CD or vehicle and in the presence or absence of DYN to inhibit dynamin activity. Data expressed by the ratio of cell-associated fluorescence relative to fluorescence in the media at baseline per milligram of total protein. C: Cells were incubated with d7 cholesterol-loaded LDL or HDL for 6 h in the presence or absence of CD and in the presence or absence of DYN. The graph on the left reports the ratio of cell-associated d7 cholesterol relative to d7 cholesterol in the media at baseline. The graph on the right reports cell-associated cholesterol for each lipoprotein incubation in the presence of CD relative to vehicle (no DYN). Means ± SEs (n = 3–5). *P < 0.05 by unpaired t-test or two-way ANOVA for comparisons indicated. V, vehicle.
DISCUSSION
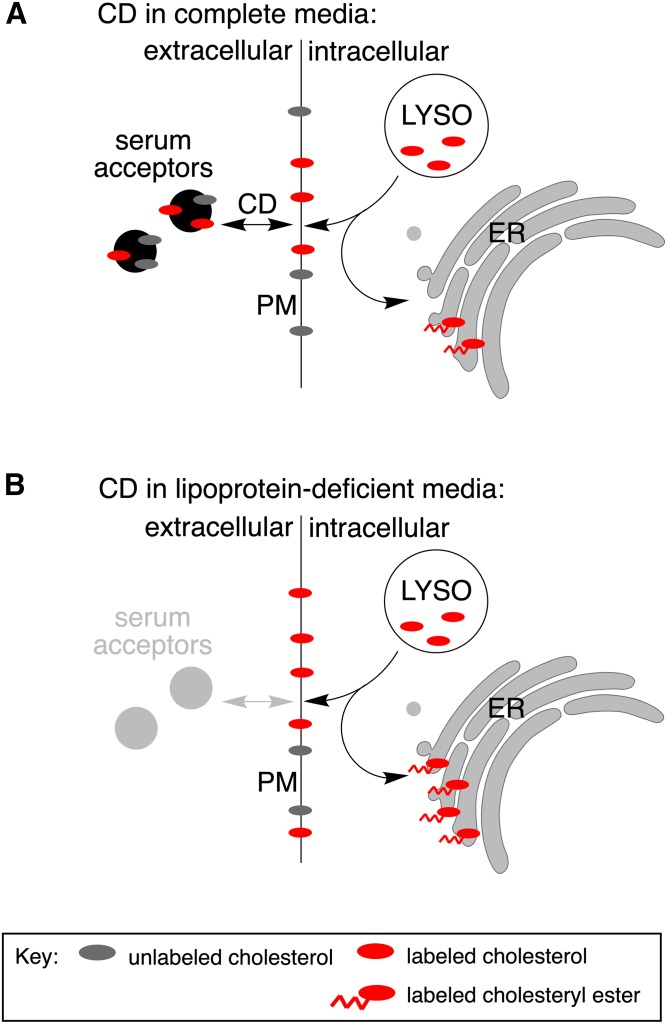
The mechanism by which CD redistributes cholesterol from the lysosomal compartment in the setting of NPC1 deficiency is not well understood. To specifically investigate the lysosomal cholesterol pool, we loaded lysosomes with isotopically labeled cholesteryl esters in reconstituted lipoproteins prior to CD treatment and tracked the itinerary of the labeled cholesterol pool. We observed increased esterification and release of lysosome-derived cholesterol into the media. The cholesterol released into the media was largely associated with lipoprotein particles, but the release was independent of SRBI and did not decrease with ABCA1 and ABCG1 knockdown. In the absence of lipidated lipoproteins, a release did not occur, and lysosome-derived cholesterol accumulated in the PM. In the presence of lipoproteins, total cellular cholesterol was maintained through the bidirectional movement of cholesterol between lipoproteins and the cell surface. Together these data support a model by which CD enhances the trafficking of lysosomal cholesterol to the PM, where it is available for exchange with extracellular lipoproteins or routed internally for esterification (Fig. 7).
Fig. 7.
Model for CD-mediated cholesterol trafficking. CD promotes the redistribution of the lysosomal cholesterol pool. A: In the presence of lipoproteins, lysosomal cholesterol is trafficked to the PM, where CD promotes exchange with serum lipoproteins (acceptors). Lysosomal cholesterol is also trafficked to the ER, directly or indirectly by way of the PM, and is reesterified. B: In the absence of suitable acceptors in the media, lysosomal cholesterol is not effluxed but rather accumulates in the PM. Arrival of the cholesterol at the ER and reesterification are enhanced under lipoprotein-deficient conditions.
There is evidence that CD exerts its effects on cholesterol homeostasis by stimulating lysosomal exocytosis, a hypothesis supported by the localization of lysosomal markers with the PM and release of a lysosomal lipid, lysobisphosphatidic acid, into the media in NPC1-deficient cells treated with CD (2, 12, 13). Furthermore, TRPML1, a lysosomal ion channel that has been implicated in lysosomal exocytosis, autophagy, and vesicular trafficking (24–26), is required for CD-mediated correction of lysosomal cholesterol accumulation in NPC1-deificent cells (13). Relocalization of lysosomes to the PM in a TPRML1-dependent matter could also provide a mechanism by which CD increases the trafficking of lysosomal cholesterol to the PM. However, these studies did not directly quantify the movement of bulk or lysosome-derived cholesterol out of the cells.
Our data do not support a model in which lysosomal cholesterol is directly released from the cell in exosomes. Although exosomes are rich in total cholesterol, d7 cholesterol that originated from the lysosome could not be detected in isolated EVs. It is possible that U2OS-SRAshNPC1 cells do not secrete robust amounts of EVs relative to previously studied cell lines. However, even the pharmacologic stimulation of lysosomal exocytosis with ionomycin did not increase the release of lysosomal cholesterol into the media. On the other hand, in the presence of CD, we observed d7 cholesterol movement from the PM to extracellular lipoproteins that was balanced by the delivery of lipoprotein cholesterol to the cells. These actions are consistent with a large body of literature on the cholesterol solubilization properties of CD (27). While our tracing shows that lysosomal cholesterol accumulates in the media following CD treatment, the mechanism is one of exchange and not net efflux, and it is inconsequential with respect to overall cholesterol trafficking to the ER. The exchange mechanism is consistent with observations in vivo that CD treatment does not decrease total cellular cholesterol or increase cellular cholesterol clearance (11). Taken together, these data indicate that CD facilitates cholesterol exchange but does not support net efflux to CD as a functional pathway.
The arrival and reesterification of lysosomal cholesterol at the ER is the signature of CD-mediated restoration of cholesterol homeostatic responses. It was recently reported that, in normal cells, lysosomal cholesterol transits to the PM before trafficking to the ER (28). Our finding that the treatment with CD in LPDM, which lacks suitable extracellular cholesterol acceptors, leads to significant PM accumulation of lysosomal d7 cholesterol supports a similar lysosome to PM itinerary for the movement of cholesterol in NPC1-deficient cells. The increase in the CD-stimulated esterification of d7 cholesterol in LPDM compared with full media likely reflects the enrichment of d7 cholesterol within the overall PM pool, providing more d7 substrate for esterification (d16 cholesteryl esters), despite the absence of changes in overall esterification (d9 cholesteryl esters).
For this study, we directly monitored the movement of lysosomal cholesterol cargo after CD treatment. Prior studies have relied on filipin staining to quantify residual lysosomal cholesterol or radiolabeled oleates to monitor total cholesterol esterification. The former is uninformative with respect to the postlysosomal trafficking of cholesterol, and the latter is indirect because it quantifies all cholesterol esterification rather than the esterification of molecules originating from the lysosome. Moreover, these approaches are confounded by the asynchronous export of cholesterol from the lysosome. By contrast, our stable isotopic approach allowed for specific loading of the lysosomal compartment with labeled cargo and the synchronous release of labeled cholesterol through the use of a reversible LAL inhibitor. Using sensitive identification of deuterated products by MS and selective enzymatic-modification approaches (e.g., treatment with cholesterol oxidase), we followed the postlysosomal trafficking of the pool of cholesterol that accumulates aberrantly in lysosomes in NPC1. These techniques provide powerful tools with which to more precisely delineate the itinerary of cholesterol and to investigate mechanisms that underlie its postlysosomal trafficking.
Footnotes
Abbreviations:
- acLDL
- acetylated LDL
- CD
- 2-hydroxypropyl-β-cyclodextrin
- DYN
- dynasore
- d7-acLDL
- acetylated LDL reconstituted with d7 cholesteryl ester
- EV
- extracellular vesicle
- LAL
- lysosomal acid lipase
- LPDM
- lipoprotein-deficient media
- NPC
- Niemann-Pick disease type C
- PM
- plasma membrane
- SRA
- scavenger receptor A
- U2OS-SRA
- U2OS cells expressing the human scavenger receptor A
- U2OS-SRAshNPC1
- U2OS-SRA cells with shNPC1 knockdown
This work was supported by National Institutes of Health Grants R01 HL067773 (D.S.O., J.E.S.) and F31 HL142167-01 (M.F.). The content is solely the responsibility of the authors and does not necessary reflect the views of the National Institutes of Health. D.S.O. works for and holds equity in Casma Therapeutics, a company whose goal is to develop therapeutics that target the autophagy pathway for treatment of diseases that include lysosomal storage diseases. The other authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Wüstner D., and Solanko K.. 2015. How cholesterol interacts with proteins and lipids during its intracellular transport. Biochim. Biophys. Acta. 1848: 1908–1926. [DOI] [PubMed] [Google Scholar]
- 2.Chen F. W., Li C., and Ioannou Y. A.. 2010. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS One. 5: e15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das A., Brown M. S., Anderson D. D., Goldstein J. L., and Radhakrishnan A.. 2014. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife. 3: e02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deffieu M. S., and Pfeffer S. R.. 2011. Niemann-Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc. Natl. Acad. Sci. USA. 108: 18932–18936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips M. C. 2014. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289: 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein J. L., DeBose-Boyd R. A., and Brown M. S.. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 7.Abi-Mosleh L., Infante R. E., Radhakrishnan A., Goldstein J. L., and Brown M. S.. 2009. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA. 106: 19316–19321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peake K. B., and Vance J. E.. 2012. Normalization of cholesterol homeostasis by 2-hydroxypropyl-beta-cyclodextrin in neurons and glia from Niemann-Pick C1 (NPC1)-deficient mice. J. Biol. Chem. 287: 9290–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Turley S. D., Burns D. K., Miller A. M., Repa J. J., and Dietschy J. M.. 2009. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA. 106: 2377–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian A. E., Haynes M. P., Phillips M. C., and Rothblat G. H.. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38: 2264–2272. [PubMed] [Google Scholar]
- 11.Taylor A. M., Liu B., Mari Y., Liu B., and Repa J. J.. 2012. Cyclodextrin mediates rapid changes in lipid balance in Npc1−/− mice without carrying cholesterol through the bloodstream. J. Lipid Res. 53: 2331–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demais V., Barthelemy A., Perraut M., Ungerer N., Keime C., Reibel S., and Pfrieger F. W.. 2016. Reversal of pathologic lipid accumulation in NPC1-deficient neurons by drug-promoted release of LAMP1-coated lamellar inclusions. J. Neurosci. 36: 8012–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vacca F., Vossio S., Mercier V., Moreau D., Johnson S., Scott C. C., Montoya J. P., Moniatte M., and Gruenberg J.. 2019. Cyclodextrin triggers MCOLN1-dependent endo-lysosome secretion in Niemann-Pick type C cells. J. Lipid Res. 60: 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipalia N. H., Subramanian K., Mao S., Ralph H., Hutt D. M., Scott S. M., Balch W. E., and Maxfield F. R.. 2017. Histone deacetylase inhibitors correct the cholesterol storage defect in most Niemann-Pick C1 mutant cells. J. Lipid Res. 58: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Remaileh M., Wyant G. A., Kim C., Laqtom N. N., Abbasi M., Chan S. H., Freinkman E., and Sabatini D. M.. 2017. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science. 358: 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Théry C., Amigorena S., Raposo G., and Clayton A.. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol . 30: 3.22.1-3–3.22.29. [DOI] [PubMed] [Google Scholar]
- 17.Krieger M. 1986. Reconstitution of the hydrophobic core of low-density lipoprotein. Methods Enzymol. 128: 608–613. [DOI] [PubMed] [Google Scholar]
- 18.Feltes M., Moores S., Gale S. E., Krishnan K., Mydock-McGrane L., Covey D. F., Ory D. S., and Schaffer J. E.. 2019. Synthesis and characterization of diazirine alkyne probes for the study of intracellular cholesterol trafficking. J. Lipid Res. 60: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pipalia N. H., Huang A., Ralph H., Rujoi M., and Maxfield F. R.. 2006. Automated microscopy screening for compounds that partially revert cholesterol accumulation in Niemann-Pick C cells. J. Lipid Res. 47: 284–301. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum A. I., Cosner C. C., Mariani C. J., Maxfield F. R., Wiest O., and Helquist P.. 2010. Thiadiazole carbamates: potent inhibitors of lysosomal acid lipase and potential Niemann-Pick type C disease therapeutics. J. Med. Chem. 53: 5281–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai S., Dulcey A. E., Hu X., Wassif C. A., Porter F. D., Austin C. P., Ory D. S., Marugan J., and Zheng W.. 2017. Methyl-beta-cyclodextrin restores impaired autophagy flux in Niemann-Pick C1-deficient cells through activation of AMPK. Autophagy. 13: 1435–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ory D. S., Ottinger E. A., Farhat N. Y., King K. A., Jiang X., Weissfeld L., Berry-Kravis E., Davidson C. D., Bianconi S., Keener L. A., et al. 2017. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet. 390: 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forte T. M., Bell-Quint J. J., and Cheng F.. 1981. Lipoproteins of fetal and newborn calves and adult steer: a study of developmental changes. Lipids. 16: 240–245. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Cheng X., Yu L., Yang J., Calvo R., Patnaik S., Hu X., Gao Q., Yang M., Lawas M., et al. 2016. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 7: 12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatachalam K., Wong C. O., and Zhu M. X.. 2015. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 58: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaPlante J. M., Sun M., Falardeau J., Dai D., Brown E. M., Slaugenhaupt S. A., and Vassilev P. M.. 2006. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol. Genet. Metab. 89: 339–348. [DOI] [PubMed] [Google Scholar]
- 27.Davidson C. D., Fishman Y. I., Puskas I., Szeman J., Sohajda T., McCauliff L. A., Sikora J., Storch J., Vanier M. T., Szente L., et al. 2016. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 3: 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Infante R. E., and Radhakrishnan A.. 2017. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife. 6: e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]