Abstract
Weaning stresses often induce markedly structural and functional changes in the small intestine. However, little data are available on the changes of the morphology, function, and health in the cecum of piglets post-weaning. This study was conducted to measure the effects of weaning on the cecum in piglets. Forty piglets were weaned at 21 d and samples were collected at different time points (days 0, 1, 3, 7, and 14) post-weaning. The crypt depth, crypt width, and total epithelial cell numbers increased on days 7 and 14, compared with days 0, 1, and 3 (P < 0.001). The value of Ki67/total epithelial cells was highest on day 0 compared with all other days (P < 0.05). Besides, a higher concentration of malondialdehyde was observed on day 3 (P < 0.001). The activity of CuZn-superoxide dismutase (P < 0.05) enhanced from day 0 to 1 and the activity of catalase increased from day 1 to 3 (P < 0.001). In addition, days 3, 7, and 14 had greater acetic, propanoic, and butyric acid contents than on day 1 (P < 0.001). The pH and monocarboxylate transporter 1 (MCT1) expression increased from day 0 to 1 and from day 3 to 7 and decreased between days 1 and 3 (P < 0.001). The mRNA expression of solute carrier family 9 member A3 (SLC9A3) decreased on day 1 compared to all other postweaning days (P < 0.05). The abundance of toll-like receptor 4 (TLR4) and interferon-γ (IFN-γ) mRNA expression increased (P < 0.05) during the first 24 h after weaning. The concentration of lipopolysaccharide increased from day 3 to 7, then decreased on day 14 (P < 0.05). The mRNA expression of tumor necrosis factor-α (TNF-α) enhanced from day 7 to 14 (P < 0.05). The abundance of phosphorylated mammalian target of rapamycin protein was lower on day 14 than day 0 (P < 0.05). Taken together, these results show that weaning in piglets influences intestinal morphology, function, and health in the cecum.
Keywords: cell proliferation; cecal function; inflammation, mTOR signaling pathway; weaning stress
Introduction
The main physiological functions of the large intestine include fluid and electrolyte absorption, provision of a controllable route for the excretion of waste products obtained through metabolism and of toxic substances, and provision of a physical barrier against microbial invasion (Williams et al., 2001; Heo et al., 2013). Moreover, the large intestine contains abundant bacterial flora which can ferment nutrients to volatile fatty acids (VFAs), especially in the cecum, which is beneficial to the health of the gut and thus of the host animal (Mortensen and Clausen, 1996; Williams et al., 2001; den Besten et al., 2013). However, it appears that alteration in the structure and functions of the large intestine may be detrimental to the health of the piglets. For example, it has been documented that a combination of alterations in the structure and absorptive function of the large intestine could increase the incidence of postweaning diarrhea in piglets (van Beers-Schreurs et al., 1992; Heo et al., 2013). Besides, a review article reported that simulated halving of the absorption in the large intestine may increase the adverse effects of the activity of enterotoxigenic Escherichia coli in the small intestine of piglets (Nabuurs, 1998).
Weaning is one of the most stressful events in the pig’s life. Weaning stress often results in low feed intake and further induces distinct structural changes in the small intestine, such as villous atrophy and crypt hyperplasia, and reduces the function of the intestinal barrier (Hampson, 1986; Pluske et al., 1997; van Beers-Schreurs et al., 1998; Montagne et al., 2007). It also triggers reductions in digestive and absorptive capacities in the gut, which in turn lead to low feed intake and poor performance (Zong et al., 2018; Chen et al., 2019a). Oxidative stress and inflammatory response may also occur after weaning which is detrimental to the health of the intestine and the animal (Pié et al., 2004; Bomba et al., 2014; Buchet et al., 2017). However, previous researches have focused mainly on the small intestine and studies on the effects of weaning stress on the cecum are rare. This study hypothesizes that weaning may decrease crypt depth and disrupt the function in the cecum, thereby affecting the health of piglets. The present study was conducted to investigate the effects of weaning on lipopolysaccharide (LPS) and VFA concentrations, cytokines and ion channel-related mRNA expression, antioxidant enzymes activity, intestinal morphology, as well as cell proliferation in the cecum of piglets during the 2 wk following weaning.
Materials and Methods
The experimental design and procedures in this study were reviewed and approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
Animals
Forty piglets (Duroc × Landrace × Yorkshire, gilts and barrows in half) were weaned at 21 d of age (day 0) and assigned to five groups based on similar initial body weight (6.55 ± 0.15 kg). And then one group of piglets of day 0 was euthanized for sampling (see Sample collection section for specific methods). The other four groups of piglets were housed individually and had free access to water and a corn–soybean meal diet, during the experimental period, containing 30.14% corn, 30% extruded corn, 9% soybean meal (44% CP), 9% whey, 7% fish meal (63% CP), 5% plasma protein powder, 0.45% l-Lys HCl (98%), 0.20% DL-Met, 0.14% l-Thr, 0.02% l-Trp, 3% glucose, 3.8% soybean oil, 1.05% limestone, 0.10% choline chloride (50%), 0.05% antioxidants, 0.50% citric acid, 0.10% salt, and 0.45% vitamin–mineral premix (Zhou et al., 2019).
Sample collection
One group per time-point (days 0, 1, 3, 7, and 14 after weaning) were randomly selected to euthanize with Zoletil (15 mg tiletamine/kg BW, 15 mg zolazepam/kg BW, intramuscular injection) and bled by exsanguinations, and then cecal samples were collected. In brief, the abdomen of slaughtered piglets was opened, and the intestines separated from the mesentery. The boundaries between the ileum, cecum, and colon were ligatured to prevent chyme flow into other parts of the intestine. The cecal digesta pH was measured and then collected and immediately frozen in liquid nitrogen and stored at −80 °C until required for LPS and VFA concentration analyses. The cecum was collected and flushed with saline to remove the chyme. Approximately 1 cm full thickness segment of the middle cecum was immediately frozen in liquid nitrogen and stored at −80 °C until the antioxidative physiological, mRNA, and protein abundance analyses. Adjacent segment was fixed in 4% neutral-buffered formalin for subsequent morphology and immunohistochemistry analysis.
Intestinal morphology analysis
Formalin-fixed cecum samples were dehydrated and embedded in paraffin wax. Segments of cross-sections were cut at 5-μm thickness and stained with hematoxylin and eosin. No fewer than 30 images were acquired with a microscope, using an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, UK). Image-pro Plus 6.0 software was used to determine the crypt depth and width of the cecum was calculated. At least, 30 well-oriented intact crypts were blindly examined in each piglets’ cecum. The mean value of crypt depth and width in the cecum was then calculated for each piglet (Zong et al., 2018).
Immunohistochemistry
The enterocyte proliferation was evaluated via Ki67 immunohistochemistry, which was measured using the methods described in the previous study (Wang et al., 2019). In brief, 5 μm tissue sections were cut from paraffin-embedded samples, which were firstly deparaffinized in xylene and then rehydrated in a descending ethanol series. The samples were incubated with 3% hydrogen peroxide (H2O2) to inhibit endogenous peroxidase. Antigen retrieval was performed by boiling twice in a sodium citrate buffer (0.01 M, pH 6.0). A 5% bovine serum albumin (Boster Biological Technology Co. Ltd, Wuhan, China) was used to block nonspecific binding. After overnight incubation with primary antibody: anti-Ki-67 (Abcam, ab15580; 1:600 dilution), sections were treated with goat anti-rabbit Immunoglobulin G (IgG) secondary antibody (ZSGB-BIO, Beijing, China). Each step, except the blocking step, was followed by three 5-min washes in phosphate-buffered saline (PBS). Positive cells were visualized with diaminobenzidine Kit (ZSGB-BIO, Beijing, China). Crypt images were captured using a microscope, using an image processing and analysis system (Version 1, Leica Imaging Systems Ltd., Cambridge, UK). The positive stained cells of Ki67 and total epithelial cells for at least 30 crypts of each sample were counted using Image-Pro Plus 6.0 software (Zorn et al., 2011) and the ratio of Ki67 cells to the total epithelial cells was calculated. The total epithelial cells were determined by counting cell nuclear (hematoxylin stained) in the crypt that counted Ki67-positive cells.
Antioxidative physiological analyses
The frozen cecum (about 100 mg) samples were pulverized in liquid nitrogen and homogenized in saline, then centrifuged at 3,000 rpm for 10 min at 4 °C to acquire the supernatant. The activities of total superoxide dismutase (T-SOD), CuZn-SOD, Mn-SOD, and catalase (CAT) and the concentration of malondialdehyde (MDA) were analyzed using assay kits following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The protein abundance of the supernatant for each sample was determined with a bicinchoninic acid (BCA) assay kit (Beyotime Biotechnology, Shanghai, China).
Measurement of VFA and LPS concentrations
The VFA concentration was measured using gas chromatography according to the procedure of a previous study (Zhou et al., 2019). Briefly, the cecal digesta samples were homogenized and added 5-mL distilled water to 1 g samples in an airtight tube. After being shaken mechanically and centrifuged at 15,000 rpm for 15 min, a mixture of supernatant fluid and 25% metaphosphoric acid solution (3.6:0.4 mL) was allowed to stand overnight. Afterward, the mixed solution was centrifuged at 15,000 rpm for 10 min and filtered through a membrane filter (pore size 0.45 μm). The VFA concentration was determined via gas chromatography (Agilent Technologies 7890B GC System, AGILENT, USA) and DB-FFAP column (30 m × 250 μm× 0.25 μm). Nitrogen was the carrier gas with a flow rate of 0.8 mL/min. Oven temperature, detector temperature, and injector temperature were 220, 280, and 250 °C, respectively.
The concentration of LPS was measured by Porcine Lipopolysaccharides ELISA Kit (E07L0268, BlueGene Biotech Co., Ltd, Shanghai, China) according to the manufacturer’s instructions. Briefly, after thawing, the cecal digesta were centrifuged at 3,000 rpm and 4 °C for 15 min, and then the supernatant was collected for measuring LPS concentrations.
Extraction of RNA and real-time quantitative Polymerase Chain Reaction (PCR)
The cecum tissue (about 1 cm in length) of each piglet was pulverized in liquid nitrogen. Then the pulverized sample (about 100 mg) was added into RNase-free microfuge tube that contained 1 mL RNAiso Plus (TaKaRa, Dalian, China) and then treated with DNase I (TaKaRa, Dalian, China) to remove trace DNA; 1 μg of RNA was reverse-transcribed (RT) to cDNA using an RT reagent Kit (TaKaRa, Dalian, China). Primer 5.0 (Premier Biosoft International, Palo Alto, California, USA) was used to design primers. The primer sequences for target genes and β-actin are shown in Table 1. The cDNAs were diluted (1:5) with sterile double-distilled (dd) water (H2O) before they were used in real-time quantitative PCR reactions. Each PCR reaction was performed in triplicate, as previously described in the study of Yang et al. (2013). Each involved a total volume of 10 μL containing 5 μL of SYBR Green mix (TaKaRa, Dalian, China), 1 μL of cDNA, 0.3 μL each of forward and reverse primers, and 3.4 μL of dd H2O. The β-actin was used as an internal control to normalize the expression of those target genes. The relative expression abundance of target genes was calculated using the 2−ΔΔCt method (Chen et al., 2019b).
Table 1.
Primers used for real-time PCR analysis
| Genes | Primers | Sequences (5´-3´) | Size |
|---|---|---|---|
| FFAR2 | forward | ACCTTACGTGTTGCTCCTCA | 164 bp |
| reverse | CGCTCTTCTTCAGTTTCCCG | ||
| FFAR3 | forward | TCACGGCCTACATCCTCATC | 196 bp |
| reverse | GTGATCTTCAAGGGCAGCAG | ||
| MCT1 | forward | GATGGGCGGTAGTAGTTGGA | 227 bp |
| reverse | GCAGCCGCCAATAATCATGA | ||
| SMCT1 | forward | TTGAGCACCGTGTCCTCTAG | 220 bp |
| reverse | GTCCACCAACCATGCCAAAT | ||
| TNF-α | forward | ACAGGCCAGCTCCCTCTTAT | 102 bp |
| reverse | CCTCGCCCTCCTGAATAAAT | ||
| IFN-γ | forward | CCATTCAAAGGAGCATGGAT | 146 bp |
| reverse | GAGTTCACTGATGGCTTTGC | ||
| IL-1β | forward | CCTGGACCTTGGTTCTCT | 123 bp |
| reverse | GGATTCTTCATCGGCTTCT | ||
| TLR4 | forward | CATTCCTCACCCAGTCTTCGTC | 169 bp |
| reverse | AGTGCGTCAGTTCTCACCTTCC | ||
| SLC9A3 | forward | CGACATGATCATCCGGACCAG | 266 bp |
| reverse | ACAGGTGGAAGCCGATCTT | ||
| SLC26A3 | forward | TGAAAAGTGGGTTTCAGGCCC | 197 bp |
| reverse | CCCGCCGCATATGTTACTCA | ||
| KCNMA1 | forward | TGGATGACCTCCGTGAAAG | 148 bp |
| reverse | GGCAGGATTCTATTGGGTTT | ||
| β-actin | forward | AGTTGAAGGTGGTCTCGTGG | 215 bp |
| reverse | TGCGGGACATCAAGGAGAAG |
Western blotting analysis
Frozen samples of the cecal segment were powdered under liquid nitrogen and lysed in ice-cold radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Shanghai, China) with the protease inhibitor phenylmethanesulfonyl fluoride (Beyotime Biotechnology, Shanghai, China). The sample was then centrifuged at 12,000 × g for 10 min at 4 °C. A BCA (Beyotime Biotechnology, Shanghai, China) was used to determine the protein concentration in the supernatant fluid. All samples were adjusted to an equal protein concentration and diluted with 5 × loading buffer (Beyotime Biotechnology, Shanghai, China), and then heated in boiling water for 10 min. The soluble proteins were first separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat milk in tris-buffered saline mixed with 0.05% Tween-20 for 1 h and incubated with primary antibodies overnight, followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Enhanced chemiluminescence (Applygen Technologies Inc., Beijing, China) was used to detect the bound antibodies. Antibodies used in this study were as followed: β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; SC-47778; 1:3,000 dilution), phospho-mammalian target of rapamycin (mTOR; Cell Signaling Technology, Ser2448; 1:1,000 dilution), and mTOR (Cell Signaling Technology Inc., Danvers, MA, USA; 7C10; 1:1,000 dilution). The intensity of the protein bands was semi-quantified using the AlphaImager 2200 software (Alpha Innotech Corporation, CA, USA), with normalization of mTOR and phospho-mTOR protein against β-actin.
Statistical analysis
Animals were blocked in a completely randomized design and data were analyzed using one-way ANOVA with SPSS software (version 22.0; IBM Corp., Chicago, IL, USA). The piglet (N = 40) was the experimental unit. All data were normally distributed and we conducted homogeneity test of variance. Differences among the groups were estimated using the Duncan’s multiple comparisons, and values were expressed as means with SEM. Differences with P < 0.05 were considered statistically significant.
Results
Changes in cecal growth performance, morphology, and cell proliferation
Piglets final BW was greater (P < 0.001) on days 7 and 14 compared with day 0. average daily gain (ADG) was increased from days 3, 7, and 14 than day 1(P < 0.001). average daily feed intake (ADFI) was greater with the increase in postweaning time (P < 0.001; Zhou et al., 2019). The crypt depth and width, as well as the number of total epithelial cells, were significantly higher on days 7 and 14, compared with days 0, 1, and 3 (P < 0.001; Table 2). The crypt depth was significantly lower on day 3 than on day 0 (P < 0.001). A significant decrease in crypt width occurred from day 0 to 1 (P < 0.001). The ratio of Ki67 cells to total epithelial cells was highest on day 0 than all other days (P < 0.05).
Table 2.
Intestinal morphology and proliferation and total epithelial cell numbers in the cecum of piglets at days 0, 1, 3, 7, and 14 post-weaning1
| Item | Day post-weaning | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| Crypt depth, μm | 305.70b | 260.51bc | 256.52c | 383.73a | 362.29a | 11.04 | <0.001 |
| Crypt width, μm | 59.69b | 52.66c | 53.10bc | 69.73a | 67.07a | 1.52 | <0.001 |
| Total epithelial cells | 185.19b | 172.43b | 203.70b | 277.00a | 244.78a | 9.91 | <0.001 |
| Ki67/epithelial cells, % | 16.72a | 9.92b | 9.44b | 9.50b | 10.05b | 0.78 | 0.042 |
1Values are presented as means with SEM (n = 8).
a,b,cValues carrying different superscripts are significant statistical differences (P < 0.05).
Changes in cecal VFA receptors, transports, and ion channel-related genes mRNA abundance
The expression of monocarboxylate transporter 1 (MCT1) increased from day 0 to 1 or day 3 to 7 and decreased from day 1 to 3 or day 7 to 14 (P < 0.001, Table 3). Weaning had no significant effect on free fatty acid receptor (FFAR2), FFAR3, and sodium-coupled monocarboxylate transporter 1 (SMCT1) mRNA abundance. The mRNA abundance of solute carrier family 9 member A3 (SLC9A3) decreased on day 1 compared with all other postweaning days (P < 0.05). There was no significant effect on SLC26A3 and potassium calcium-activated channel subfamily M alpha 1 (KCNMA1) mRNA expression after weaning.
Table 3.
The mRNA expression of VFA receptors and transports and ion channel-related genes in the cecum of piglets at days 0, 1, 3, 7, and 14 post-weaning1
| Item | Day post-weaning | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| FFAR2 | 1.05 | 1.84 | 1.17 | 1.42 | 1.30 | 0.12 | 0.764 |
| FFAR3 | 1.15 | 1.85 | 1.93 | 1.13 | 1.38 | 0.14 | 0.183 |
| MCT1 | 0.75bc | 1.46a | 0.45c | 1.04ab | 0.56c | 0.09 | <0.001 |
| SMCT1 | 0.49 | 1.52 | 3.80 | 0.88 | 1.66 | 0.43 | 0.122 |
| SLC9A3 | 0.77a | 0.37b | 0.99a | 0.87a | 1.07a | 0.07 | 0.011 |
| SLC26A3 | 0.65 | 0.69 | 0.62 | 1.05 | 1.07 | 0.08 | 0.139 |
| KCNMA1 | 1.05 | 0.85 | 0.98 | 0.75 | 1.02 | 0.05 | 0.257 |
1 Values are presented as means with SEM (n = 8).
a,b,cValues carrying different superscripts are significant statistical differences (P < 0.05).
Changes in cecal pH and VFA concentration
The pH increased from day 0 to 1 or day 3 to 7 and decreased between days 1 and 3 (P < 0.001). The levers of acetic, propanoic, and butyric acid contents declined from day 0 to 1 and increased from day 1 to 3 (P < 0.001, Table 4). And days 3, 7, and 14 had greater acetic, propanoic, and butyric acid concentrations than on day 1 (P < 0.001). Besides, the concentrations of isobutyric, pentanoic, and isopentanoic acid decreased from day 0 to 1 and from day 1 to 3 (P < 0.001). Total VFA concentration in day 1 was less than other days, and its concentration was greater in days 0, 3, and 14 than day 7 (P < 0.001).
Table 4.
The pH and the concentration of cecal contents in piglets at days 0, 1, 3, 7, and 14 post-weaning1
| VFA, μg/g | Day post-weaning | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| pH | 6.12b | 7.02a | 5.60d | 5.97bc | 5.74cd | 0.09 | <0.001 |
| Acetic acid | 3860.96a | 1,947.72c | 4,112.46a | 3,017.75b | 3,606.91ab | 165.76 | <0.001 |
| Propanoic acid | 1,765.37c | 1,025.27d | 2,517.96a | 1,977.40bc | 2,378.20ab | 114.60 | <0.001 |
| Butyric acid | 811.94b | 385.64d | 598.68c | 683.10bc | 1,117.02a | 49.35 | <0.001 |
| Isobutyric acid | 275.94a | 175.33b | 64.27c | 36.56c | 76.66c | 18.03 | <0.001 |
| Pentanoic acid | 370.28a | 218.17b | 114.95c | 113.55c | 179.66bc | 18.49 | <0.001 |
| Isopentanoic acid | 418.89a | 339.50b | 79.22d | 81.07d | 153.25c | 24.57 | <0.001 |
| Total VFA | 7,128.85a | 3,720.02c | 7,479.50a | 5,893.76b | 7,324.16a | 289.56 | <0.001 |
1 Values are presented as means with SEM (n = 8).
a–dValues carrying different superscripts are significant statistical differences (P < 0.05).
Changes in cecal SOD and CAT activities and MDA concentration
The concentration of MDA was highest on day 3 of postweaning (P < 0.001, Table 5). A significant increase in MDA concentration occurred from day 1 to 3 (P < 0.001). Besides, the activity of CuZn-SOD enhanced during the first 24 h post-weaning (P< 0.05) and the activities of T-SOD and CuZn-SOD were higher on day 1 than on days 3 and 7 (P < 0.05). In addition, the activity of CAT increased from day 1 to 3 and was lower on days 0 and 1 than on days 3, 7, and 14 (P < 0.001).
Table 5.
The antioxidant indices in the cecum of piglets at days 0, 1, 3, 7, and 14 post-weaning1
| Item | Day post-weaning | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| MDA, nmol/mg prot | 0.36b | 0.29b | 0.57a | 0.20c | 0.29b | 0.02 | <0.001 |
| CAT, U/ mg prot | 1.75cd | 1.15d | 2.60ab | 2.15bc | 2.87a | 0.13 | <0.001 |
| T-SOD, U/ mg prot | 128.17abc | 151.93a | 121.77bc | 110.18c | 141.35ab | 4.41 | 0.020 |
| CuZn-SOD, U/ mg prot | 100.11b | 120.01a | 100.81b | 88.10b | 104.10ab | 3.00 | 0.015 |
| Mn-SOD, U/ mg prot | 28.06ab | 31.92ab | 20.97b | 22.08b | 37.24a | 1.80 | 0.014 |
1 Values are presented as means with SEM (n = 8).
a–dValues carrying different superscripts are significant statistical differences (P < 0.05).
Changes in cecal LPS concentration and TLR4, TNF-α, IFN-γ, and IL-1β mRNA abundance
The concentration of LPS in cereal contents and toll-like receptor 4 (TLR4) in cecal tissue mRNA abundance were higher on days 1, 3, 7, and 14 compared to day 0 (Table 6). The concentration of LPS increased from day 3 to 7 and decreased from day 7 to 14 (P < 0.05). Besides, a significant increase in TLR4 and IFN-γmRNA expression occurred during the first 24 h postweaning (P < 0.05). The mRNA abundance of IFN-γ decreased from day 1 to 3 (P < 0.05). In addition, a significant increase in TNF-α mRNA expression occurred on day 14 compared with day 7 (P < 0.05). However, the mRNA expression of IL-1β did not change significantly post-weaning.
Table 6.
The concentration of LPS and mRNA abundance of TLR4, TNF-α, IFN-γ, and IL-1β in the cecum of piglets at days 0, 1, 3, 7, and 14 post-weaning1
| Item | Day post-weaning | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| LPS, ng/mL | 77.48b | 96.88ab | 78.63b | 115.60a | 87.01b | 3.94 | 0.015 |
| TLR4 | 0.70b | 1.34a | 0.93ab | 1.17a | 1.37a | 0.07 | 0.010 |
| TNF-α | 0.55b | 0.69b | 1.12b | 0.73b | 1.81a | 0.13 | 0.003 |
| IFN-γ | 0.77b | 1.37a | 0.52b | 1.02ab | 0.76b | 0.09 | 0.035 |
| IL-1β | 0.97 | 1.72 | 0.62 | 1.12 | 2.49 | 0.23 | 0.091 |
1 Values are presented as means with SEM (n = 8).
a,bValues carrying different superscripts are significant statistical differences (P < 0.05).
Expression of protein abundance of mTOR and p-mTOR
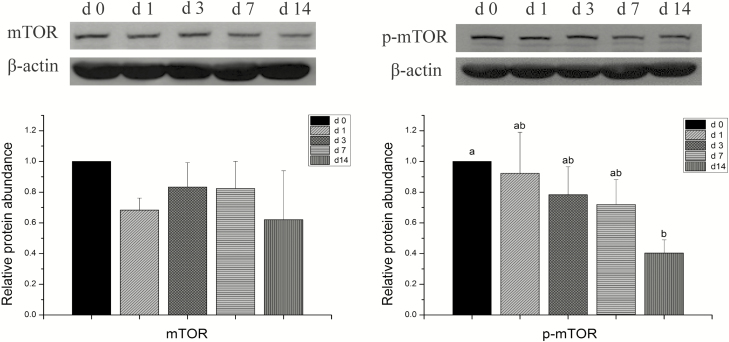
No significant differences were observed in the protein abundance of mTOR in the cecum after weaning (Figure 1). The abundance of p-mTOR protein decreased gradually post-weaning and the p-mTOR amount was significantly lower on day 14 compared with the abundance on day 0 (P < 0.05).
Figure 1.
Effects of weaning on protein abundance of mammalian target of rapamycin (mTOR) signaling pathway of the cecum in piglets. The expression of proteins was measured using Western blotting and β-actin was used as an internal control to normalize abundance. Column data are presented as mean, and the bars represent SEM, n = 3 (phosphorylated mammalian target of rapamycin, p-mTOR). a,bSuperscripts above column in different letters are significant statistical differences (P < 0.05).
Discussion
Our results indicate that changes in the total VFA concentration were opposite to the changes in pH. It was also found that when the total VFA content increased, the pH decreased, and vice versa. In addition, change in mRNA expression of VFA transporter MCT1 was approximately consistent with the change in pH. Similarly, Kolver and de Veth (2002) found a negative correlation between rumen VFA concentration and rumen pH. When the pH increased, the mRNA expression of MCT1 also increased (Neolaka et al., 2017). MCT1, as a transporter of VFA, is directly involved in the transport of VFA across the cecal epithelium toward the blood side (Kirat and Kato, 2006). Therefore, mRNA expression of MCT1 increased within 24 h after weaning, which may contribute to the absorption and utilization of VFA to cope with weaning stress. These results were similar to the research of Boudry et al. (2004), who found that 2 d after weaning dramatically increased the secretion in jejunum and colon and absorption in the jejunum. The concentrations of cecal acetic, propanoic, butyric, isobutyric, pentanoic, and isopentanoic acid declined significantly during the first 24 h post-weaning, which was coincided with the reduction of ADFI, crypt depth, width, and the ratio of Ki67 cells to total epithelial cells in this study. This suggests that weaning may have a dramatic negative impact on the intestinal morphology (Pluske et al., 1997). Previous study also showed that crypt depths and crypt cell production rates were reduced in small intestinal at 3 d after weaning (Hall and Byrne, 1989). In addition, weaning decreased the crypt density in the cecum of piglets (Castillo et al., 2007). The reason for that may be due to the decrease of energy and protein intake during the first 24 h post-weaning, which leads to the decrease of crypt cell proliferation and VFA concentrations, and further influence the morphological development of large intestine. The concentrations of acetic, propionic, and butyric acid were higher than isobutyric, pentanoic, and isopentanoic acid at each time-point post-weaning. This indicates that acetic, propionic, and butyric acid are the predominant forms among the VFA (Guilloteau et al., 2010). In the present study, ADFI was greater with the increase in postweaning time. Moreover, days 3, 7, and 14 had significantly greater ADG, and acetic, propanoic and butyric acid concentrations than day 1, while the crypt depth, width, and the number of total epithelial cells were higher on days 7 and 14 than on days 1 and 3. The improvement of these indexes in the later period of weaning (day 7 to 14) may be the result of intestinal maturation. Boudry et al. (2004) indicated that weaning induces transient dramatic changes in intestinal physiology but is also a period of maturation of the intestine. The change in cecal morphology was approximately also consistent with the change in the concentration of VFA. The reason for this may be that VFA represents the energy-providing substrate for cell growth (den Besten et al., 2013). Yang et al (2016) found that weaning influences energy metabolism, cellular macromolecule organization and localization, and protein metabolism, thereby affecting the proliferation of intestinal epithelial cells in weaned piglets.
mTOR pathway is an important cellular signaling pathway to regulating cell growth and proliferation (Wong, 2013). The ratio of Ki67 cells to the total epithelial cells was higher on day 0 than on all other days and the abundance of p-mTOR protein decreased gradually post-weaning. This indicates a decrease in cecal proliferation after weaning. One of the reasons for this may be related to the decrease of nutrients in the gut. Epithelial cell proliferation is influenced by the presence of food in the gastrointestinal tract (Diamond and Karasov, 1983). Besides, TLR4 activation could lead to reduced proliferation of the intestinal crypts (Neal et al., 2012), while the abundance of TLR4 mRNA expression was higher on days 1, 3, 7, and 14 compared with day 0 in the present study. After weaning, although the cecal proliferation reduced, the proliferation rate may be similar to the shedding rate of cecal epithelial cells, and therefore, the number of total epithelial cells did not change significantly between days 0, 1, and 3. However, the crypt depth and width were lower on days 1 and 3 compared to day 0. One of the reasons could be that weaning leads to a decrease in intercellular space. Crypt depth may depend on the proliferation and shedding rate of epithelial cells at the same time (Hampson, 1986; Williams et al., 2013). The shedding rate of epithelial cells may decrease during late weaning, so even if crypt proliferation does not increase, the number of total epithelial cells also increased on days 7 and 14, ultimately increasing the depth and width of the crypt, and further experiments are needed to confirm this.
As anNa+/H+ exchanger, SLC9A3 is expressed in the stomach, small intestine, cecum, and colon, which plays a significant role in the transepithelial absorption of Na+ in the gut (Fuster and Alexander, 2014). In the current study, the mRNA expression of SLC9A3 decreased significantly during the first 24 h after weaning, then enhanced from day 1 to 3. This indicates that weaning affects the sodium absorption in the cecum. This could be caused by a change in the concentration of VFA. According to previous research, VFA was considered a stimulus affecting sodium absorption (Roediger, 1989).
Oxidative stress caused by weaning stress affects antioxidative stress capability, which in turn affects piglet health (Yuan et al., 2007; Zhu et al., 2012). Excessive production of reactive-oxygen and reactive-nitrogen species may result in oxidative stress (Valko et al., 2007). MDA is one of the final products of polyunsaturated fatty acids peroxidation in the cells, which is commonly known as a marker of oxidative stress (Gaweł et al., 2004). Xu et al. (2014) indicated that 3 d after weaning, piglets significantly reduced the antioxidant capacities of intestine and increased the levels of MDA and hydroxyl radicals as well as H2O2. Cao et al. (2018) reported that the MDA concentration was significantly increased, and the activities of SOD and GSH-Px, as well as the mRNA expression of antioxidant genes (Cu/Zn-SOD, Mn-SOD, glutathione peroxidise1 and 4 [GPx1 and GPx4]), were declined in intestinal mucosa of piglets during 1 wk after weaning. Yin et al. (2014) demonstrated that the concentrations of MDA and 8-hydroxydeoxyguanosine (DNA oxidative injury) were increased in plasma, and the mRNA expression of Cu/Zn-SOD, Mn-SOD, GPx1, and GPx4 were decreased in small in intestine of piglets after 3 d after weaning. Consistently, our study also confirmed that MDA concentration increased significantly from day 1 to 3 and was highest on day 3. This suggests that the piglets may have been exposed to oxidative stress after weaning. The concentration of MDA was significantly lower on days 7 and 14 than on day 3, possibly due to the presence of the antioxidant defense systems to reduce MDA, which includes enzymatic antioxidant system. When oxidative stress occurs severely, a defense system promotes the regulation and expression of enzymes, including SOD and CAT (MatÉs et al., 1999). SOD can catalyze the conversion of toxic superoxide anion radicals to H2O2 and CAT can detoxify H2O2 into oxygen and water (Gopavajhula et al., 2013; Glorieux and Calderon, 2017; Wang et al., 2017). In contrast to the results of Cao et al. (2018), our T-SOD, Mn-SOD, and CuZn-SOD activities increased during the first 24 h after weaning. It indicates that oxidative stress caused by weaning stimulates the body to produce a large amount of SOD rapidly. Afterwards, SOD catalyzes the conversion of toxic superoxide anion radicals into H2O2. The large amount of H2O2 generated further stimulates the production of CAT, so the activity of CAT increased significantly from day 1 to 3 post-weaning.
LPS is one of the outer components of gram-negative bacteria such as Escherichia coli (E. coli) (Lu et al., 2008), and is present in large quantities in the gut. LPS induces the secretion of a large number of proinflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-4, and IL-12 (Pålsson-McDermott and O’Neill, 2004), which may result in severe intestinal disease. TLR4 recognizes specific pathogen-associated molecular patterns, including LPS and heat-shock proteins (Takeuchi et al., 1999; Pålsson-McDermott and O’Neill, 2004). A previous study on intestinal microbiota of weaned piglets showed that E. coli concentrations increased after weaning (Konstantinov et al., 2006). Therefore, the concentration of LPS was higher on days 1, 3, 7, and 14 compared to day 0, this may cause intestinal mucosal injury and barrier dysfunction (Maes et al., 2008). Then, the stimulation of TLR4 by LPS may have occurred (Gerold et al., 2007), resulting in TLR4 mRNA expression also being higher on days 1, 3, 7, and 14 than on day 0. The stimulation of TLR4 by LPS induces the release of proinflammatory cytokines (Lu et al., 2008; Takeuchi and Akira, 2010). This may be why the mRNA abundance of IFN-γ and IL-1β increased rapidly within 24 h after weaning. However, the mRNA expression of IFN-γ and IL-1β decreased rapidly from day 1 to 3. This downregulation may have taken place due to a rapid adaptation at the time of weaning, at least in terms of expression of proinflammatory cytokines (Pié et al., 2004), because proinflammatory cytokines excessive release could lead to intestinal morphology and function damage (Chen et al., 2019a). In the current study, the concentration of LPS increased from day 3 to 7 after weaning. Since LPS can stimulate TLR4 and then induce the release of cytokines (Kawai et al., 2001; Lu et al., 2008), the mRNA expression of TLR4, IFN-γ, and IL-1β also increased from day 3 to 7. A previous study had reported that the expression of TNF-α remained elevated in the distal small intestine at day 8 post-weaning, which might be the result of an active immune stimulation by microbial products (Pié et al., 2004). In this study, TNF-α mRNA expression did not change significantly between days 0 and 7 until from day 7 to 14. The reason may be that LPS concentration in the early stage is not high enough to stimulate the expression of TNF-α; it was the highest on day 7, which probably reached the level to stimulate TNF-α expression. Therefore, a significant increase in TNF-α mRNA abundance occurred from day 7 to 14. The concentration of LPS decreased significantly from day 7 to 14, probably due to the presence of the body’s immune and antioxidant system, which relieved stress and inhibited harmful bacteria.
In conclusion, weaning in piglets is associated with a transient dramatic response in cecal morphology and cell proliferation, luminal VFA and LPS concentration, antioxidant enzymes activity, mRNA expression of inflammatory cytokine, and sodium absorption in the cecum. These results indicate that cecal morphology, function, and health are significantly changed during the postweaning period, and dietary supplementation with VFA or modulating antioxidant or inflammatory properties may be able to improve cecal morphology, function, and health in weaned piglets.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Acknowledgments
This work was supported by Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), National Key R & D Program ( 2016YFD0501201), Natural Science Foundation of Hunan Province (2017JJ1020), and Hunan Provincial Innovation Foundation for Postgraduate (CX20190393).
Glossary
Abbreviations
- BCA
bicinchoninic acid
- CAT
catalase
- CuZn-SOD
CuZn-superoxide dismutase
- dd
double-distilled
- FFAR
free fatty acid receptor
- GP
glutathione peroxidise
- IFN-γ
interferon-γ
- KCNMA
potassium calcium-activated channel subfamily M alpha 1
- LPS
lipopolysaccharide
- MCT1
monocarboxylate transporter 1
- MDA
malondialdehyde
- Mn-SOD
Mn-superoxide dismutase
- p-mTOR
phosphorylated mammalian target of rapamycin
- prot
protein
- SLC9A3
solute carrier family 9 member A3
- SMCT
sodium-coupled monocarboxylate transporter
- SOD
superoxide dismutase
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor-α
- T-SOD
total superoxide dismutase
- VFA
volatile fatty acid
Literature Cited
- Bomba L., Minuti A., Moisá S. J., Trevisi E., Eufemi E., Lizier M., Chegdani F., Lucchini F., Rzepus M., Prandini A., . et al. 2014. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genomics 14:657–671. doi: 10.1007/s10142-014-0396-x [DOI] [PubMed] [Google Scholar]
- Boudry G., Péron V., Le Huërou-Luron I., Lallès J. P., and Sève B.. . 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256 [DOI] [PubMed] [Google Scholar]
- Buchet A., Belloc C., Leblanc-Maridor M., and Merlot E.. . 2017. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. Plos One 12:e0178487. doi: 10.1371/journal.pone.0178487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S. T., Wang C. C., Wu H., Zhang , L. F. Jiao Q. H., and Hu C. H.. . 2018. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 96:1073–1083. doi: 10.1093/jas/skx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M., Martín-Orúe S. M., Nofrarías M., Manzanilla E. G., and Gasa J.. . 2007. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet. Microbiol. 124:239–247. doi: 10.1016/j.vetmic.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Chen W. B., Fang R. J., Wu X., Cheng Z. B., and Tian Y. B.. . 2019a. The effects of zinc methionine chelate and ZnSO4 on the growth performance and immune function of the weaned piglets and on IPEC-J2 cell immune function. Kafkas. Univ. Vet. Fak. Derg. 25:185–192. doi: 10.9775/kvfd.2018.20654 [DOI] [Google Scholar]
- Chen C., Wang Z., Li J., Li Y., Huang P., Ding X., Yin J., He S., Yang H., and Yin Y.. . 2019b. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets1. J. Anim. Sci. 97:1212–1221. doi: 10.1093/jas/skz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D. J., and Bakker B. M.. . 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54:2325–2340. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., and Karasov W. H. 1983. Gut physiology: trophic control of the intestinal mucosa. Nature. 304:18. doi: 10.1038/304018a0 [DOI] [PubMed] [Google Scholar]
- Fuster D. G., and Alexander R. T.. . 2014. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 466:61–76. doi: 10.1007/s00424-013-1408-8 [DOI] [PubMed] [Google Scholar]
- Gaweł S., Wardas M., Niedworok E., and Wardas P.. . 2004. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 57:453–455. [PubMed] [Google Scholar]
- Gerold G., Zychlinsky A., and de Diego J. L.. . 2007. What is the role of toll-like receptors in bacterial infections? Semin. Immunol. 19:41–47. doi: 10.1016/j.smim.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Glorieux C., and Calderon P. B.. . 2017. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 398:1095–1108. doi: 10.1515/hsz-2017-0131 [DOI] [PubMed] [Google Scholar]
- Gopavajhula V. R., Chaitanya K. V., Akbar Ali Khan P., Shaik J. P., Reddy P. N., and Alanazi M.. . 2013. Modeling and analysis of soybean (Glycine max. L) Cu/Zn, Mn and Fe superoxide dismutases. Genet. Mol. Biol. 36:225–236. doi: 10.1590/S1415-47572013005000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., and Van Immerseel F.. . 2010. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23:366–384. doi: 10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- Hall G. A., and Byrne T. F.. . 1989. Effects of age and diet on small intestinal structure and function in gnotobiotic piglets. Res. Vet. Sci. 47:387–392. doi: 10.1016/S0034-5288(18)31267-0 [DOI] [PubMed] [Google Scholar]
- Hampson D. J. 1986. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 40:32–40. doi: 10.1016/S0034-5288(18)30482-X [DOI] [PubMed] [Google Scholar]
- Heo J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., and Nyachoti C. M.. . 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Kawai T., Takeuchi O., Fujita T., Inoue J., Mühlradt P. F., Sato S., Hoshino K., and Akira S.. . 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887–5894. doi: 10.4049/jimmunol.167.10.5887 [DOI] [PubMed] [Google Scholar]
- Kirat D., and Kato S.. . 2006. Monocarboxylate transporter 1 (MCT1) mediates transport of short-chain fatty acids in bovine caecum. Exp. Physiol. 91:835–844. doi: 10.1113/expphysiol.2006.033837 [DOI] [PubMed] [Google Scholar]
- Kolver E. S., and de Veth M. J.. . 2002. Prediction of ruminal pH from pasture-based diets. J. Dairy Sci. 85:1255–1266. doi: 10.3168/jds.S0022-0302(02)74190-8 [DOI] [PubMed] [Google Scholar]
- Konstantinov S. R., Awati A. A., Williams B. A., Miller B. G., Jones P., Stokes C. R., Akkermans A. D., Smidt H., and de Vos W. M.. . 2006. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 8:1191–1199. doi: 10.1111/j.1462-2920.2006.01009.x [DOI] [PubMed] [Google Scholar]
- Lu Y. C., Yeh W. C., and Ohashi P. S.. . 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. doi: 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Maes M., Kubera M., and Leunis J. C.. . 2008. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro. Endocrinol. Lett. 29:117–124. doi: 10.1038/ncpendmet0726 [DOI] [PubMed] [Google Scholar]
- MatÉs J. M., Pérez-Gómez C., and De Castro I. N.. . 1999. Antioxidant enzymes and human diseases. Clin. Biochem. 32:595–603. doi: 10.1016/S0009-9120(99)00075-2 [DOI] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Le Huërou-Luron I., Lallès J. P., and Sève B.. . 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi: 10.1017/S000711450720580X [DOI] [PubMed] [Google Scholar]
- Mortensen P. B., and Clausen M. R.. . 1996. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. Suppl. 216:132–148. doi: 10.3109/00365529609094568 [DOI] [PubMed] [Google Scholar]
- Nabuurs M. J. A. 1998. Weaning piglets as a model for studying pathophysiology of diarrhea. Vet. Quart. 20:42–45. doi: 10.1080/01652176.1998.9694967 [DOI] [PubMed] [Google Scholar]
- Neal M. D., Sodhi C. P., Jia H., Dyer M., Egan C. E., Yazji I., Good M., Afrazi A., Marino R., Slagle D., . et al. 2012. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J. Biol. Chem. 287:37296–37308. doi: 10.1074/jbc.M112.375881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neolaka G. M. G., Yustisia I., Sadikin M., and Wanandi S. I.. . 2017. The effect of extracellular alkalinization on lactate metabolism of breast cancer stem cells: overview of LDH-A, LDH-B, MCT1 and MCT4 gene expression. J. Phys.: Conf. Ser. 884:012033. doi: 10.1088/1742-6596/884/1/012033 [DOI] [Google Scholar]
- Pålsson-McDermott E. M., and O’Neill L. A.. . 2004. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology 113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J. P., Blazy F., Laffitte J., Sève B., and Oswald I. P.. . 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134:641–647. doi: 10.1093/jn/134.3.641 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/s0301-6226(97)00057-2 [DOI] [Google Scholar]
- Roediger W. E. 1989. Short chain fatty acids as metabolic regulators of ion absorption in the colon. Acta Vet. Scand. Suppl. 86:116–125. [PubMed] [Google Scholar]
- Takeuchi O., and Akira S.. . 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., and Akira S.. . 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451. doi: 10.1016/s1074-7613(00)80119-3 [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., and Telser J.. . 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39:44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- van Beers-Schreurs H. M. G., Nabuurs M. J. A., Vellenga L., Kalsbeek-van der Valk H. J., Wensing T., and Breukink H. J.. . 1998. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J. Nutr. 128:947–953. doi: 10.1093/jn/128.6.947 [DOI] [PubMed] [Google Scholar]
- van Beers-Schreurs H. M., Vellenga L., Wensing T., and Breukink H. J.. . 1992. The pathogenesis of the post-weaning syndrome in weaned piglets: a review. Vet. Q. 14:29–34. doi: 10.1080/01652176.1992.9694322 [DOI] [PubMed] [Google Scholar]
- Wang L., Yan S., Li J., Li Y., Ding X., Yin J., Xiong X., Yin Y., and Yang H.. . 2019. Rapid communication: the relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 97:353–358. doi: 10.1093/jas/sky388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang X., Deng F., Yuan R., and Shen F.. . 2017. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypiumhirsutum. BMC. Genomics. 18:376. doi: 10.1186/s12864-017-3768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Duckworth C. A., Watson A. J., Frey M. R., Miguel J. C., Burkitt M. D., Sutton R., Hughes K. R., Hall L. J., Caamaño J. H., . et al. 2013. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis. Model. Mech. 6:1388–1399. doi: 10.1242/dmm.013284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. A., Verstegen M. W., and Tamminga S.. . 2001. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 14:207–228. doi: 10.1079/NRR200127 [DOI] [PubMed] [Google Scholar]
- Wong M. 2013. Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomed. J. 36:40–50. doi: 10.4103/2319-4170.110365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., and Wang T.. . 2014. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 30:584–589. doi: 10.1016/j.nut.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Yang H. S., Fu D. Z., Kong X. F., Wang W. C., Yang X. J., Nyachoti C. M., and Yin Y. L.. . 2013. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 91:2740–2748. doi: 10.2527/jas.2012-5795 [DOI] [PubMed] [Google Scholar]
- Yang H., Xiong X., Wang X., Li T., and Yin Y.. . 2016. Effects of weaning on intestinal crypt epithelial cells in piglets. Sci. Rep. 6:36939. doi: 10.1038/srep36939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wu M. M., Xiao H., Ren W. K., Duan J. L., Yang G., Li T. J., and Yin Y. L. 2014. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92:612-619. doi: 10.2527/jas.2013-6986 [DOI] [PubMed] [Google Scholar]
- Yuan S., Chen D., Zhang K., and Yu B. 2007. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian. Austral. J. Anim. 20:1600–1605. doi: 10.5713/ajas.2007.1600 [DOI] [Google Scholar]
- Zhou Z., Zhang J., Zhang X., Mo S., Tan X., Wang L., Li J., Li Y., Ding X., Liu X., . et al. 2019. The production of short chain fatty acid and colonic development in weaning piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 103:1530–1537. doi: 10.1111/jpn.13164 [DOI] [PubMed] [Google Scholar]
- Zhu L. H., Zhao K. L., Chen X. L., and Xu J. X.. . 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90:2581–2589. doi: 10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]
- Zong E., Huang P., Zhang W., Li J., Li Y., Ding X., Xiong X., Yin Y., and Yang H.. . 2018. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets. J. Anim. Sci. 96:1130–1139. doi: 10.1093/jas/skx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn T. M., Zúñiga M., Madrid E., Tostes R., Fortes Z., Giachini F., and San Martín S.. . 2011. Maternal diabetes affects cell proliferation in developing rat placenta. Histol. Histopathol. 26:1049–1056. doi: 10.14670/HH-26.1049 [DOI] [PubMed] [Google Scholar]