Abstract
Pirfenidone (PFD) is an anti-fibrotic agent that is clinically used in the treatment of idiopathic pulmonary fibrosis. PFD has been shown to exert protective effects against damage to orbital fibroblasts, endothelial cells, liver cells and renal proximal tubular cells; however, its effect on myocardial cell apoptosis remains unclear. The present study aimed to characterize the effects of PFD on homocysteine (Hcy)-induced cardiomyocyte apoptosis and investigated the underlying mechanisms. H9C2 rat cardiomyocytes were pre-treated with PFD for 30 min followed by Hcy exposure for 24 h. The effects of PFD on cell cytotoxicity were evaluated by CCK-8 assay. The apoptosis rate of each group was determined by flow cytometry. The protein and mRNA levels of connexin 43 (Cx43), Bax, B-cell lymphoma-2 (Bcl-2) and caspase-3 were measured by western blot analysis and reverse transcription-quantitative PCR, respectively. The present results demonstrated that the apoptotic rate increased following Hcy exposure, whereas the apoptotic rate significantly decreased following PFD pre-treatment. Furthermore, the ratio of Bax/Bcl2 was upregulated following Hcy exposure, and Hcy upregulated the expression levels of cleaved caspase-3 and Cx43. Notably, these effects were prevented by PFD. Additionally, the effects of PFD were inhibited by the Cx43 agonist, AAP10. In summary, the findings of the present study demonstrate that PFD protects H9C2 rat cardiomyocytes against Hcy-induced apoptosis by modulating the Cx43 signaling pathway.
Keywords: H9C2 cells, pirfenidone, homocysteine, apoptosis, connexin 43
Introduction
Cardiovascular diseases remain a leading cause of mortality worldwide due to their high morbidity and mortality rates. It has been reported that numerous risk factors may lead to cardiomyocyte dysfunction via the induction of apoptosis, which eventually contributes to the development of human cardiovascular diseases (1). Therefore, understanding the mechanisms responsible for this apoptosis may aid in the enhanced understanding of the pathology of these disorders.
Hypertension, diabetes, sex differences, obesity, smoking and high cholesterol are the most important risk factors for cardiovascular diseases (2,3). Recent studies have demonstrated that the abnormal accumulation of plasma homocysteine (Hcy) is a risk factor for cardiovascular diseases, which may lead to myocardial cell dysfunction by inducing apoptosis (4-7). Therefore, the early identification and timely management of risk factors is critical for reducing morbidity and mortality in patients with cardiovascular diseases.
Several studies have reported that connexin 43 (Cx43) can regulate cell survival and death. Cx43 can initiate apoptotic programs that are involved in arrhythmia, ischemia-reperfusion and heart failure (6,8). Existing data on connexin expression confirm that Cx43 is the principal cardiac connexin, and it forms communication channels for electric and metabolic coupling between cardiomyocytes (9). A previous study demonstrated that Hcy can increase the expression of Cx43 in myocardial cells (10).
Pirfenidone [5-methyl-1-phenyl-2(1H)-pyridone; PFD] is a pyridine compound that exerts anti-fibrotic effects in numerous fibrotic diseases (11,12). However, the exact underlying mechanisms of action of PFD are not yet fully understood. A previous study identified that PFD inhibited the apoptosis of renal tubular cells by maintaining mitochondrial membrane stability, thereby inhibiting the mitochondrial apoptotic signaling pathway (13). Notably, another study demonstrated that PFD significantly reduced the electrical stimulation-induced protein expression levels of Cx43 and α-actin 2 in bone marrow-derived mesenchymal stem cells (14). In a hepatic ischemia/reperfusion injury model, a study identified that PFD reduced SRY-related high mobility group-Box gene 9 overexpression-induced inflammation and apoptosis (15). However, the question of whether PFD is involved in the regulation of the cardiomyocyte apoptosis signaling pathway requires further investigation.
The aim of the present study was to investigate the cardio-protective effects of PFD on the Hcy-induced apoptosis of H9C2 cells. It was hypothesized that PFD protects H9C2 rat cardiomyocytes against Hcy-induced apoptosis by modulating the Cx43 signaling pathway.
Materials and methods
Cell culture and drug administration
H9C2 rat cardio-myocytes were purchased from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The H9C2 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) and supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified 5% CO2 incubator (4). Upon reaching 80% confluency, the cells were subjected to the experimental procedures. H9C2 cells cultured in DMEM/FBS without any treatment served as the control group. H9C2 cells were exposed to Hcy (0.5, 1, 2 or 3 mmol/l; Sigma-Aldrich; Merck KGaA) for 24 h to determine the Hcy treatment concentrations (4). The cells were pre-treated with PFD (Sigma-Aldrich; Merck KGaA) for 30 min followed by exposure to Hcy (3 mmol/l) for 24 h. In addition, the H9C2 cells were treated with the Cx43 agonist, AAP10 (Chinese Peptide Co., 50 nmol/l, 1 h), or the Cx43 inhibitor, Gap26 (cat. no. A1044, APExBIO Technology LLC, 0.5 µmol/l, 30 min), prior to treatment with Hcy. The concentration with maximal protective effects was determined.
Cell viability assay
The viability of the H9C2 rat cardiomyocytes was evaluated using a CCK-8 assay kit (MultiSciences Lianke), according to the manufacturer's protocol. H9C2 cells (1×104/well) seeded in 96-well plates were exposed to Hcy (0.5, 1, 2 or 3 mmol/l) for 24 h. For protective treatment, the cells were pre-treated with PFD (0.1, 0.5, 1 or 1.5 mg/ml) for 30 min and incubated with Hcy for 24 h at 37°C. Subsequently, 10 µl CCK-8 solution were added to each well, the cells were incubated at 37°C for an additional 2 h, and cell viability was detected using a microplate spectrophotometer (BioTek Instruments, Inc.).
Flow cytometric analysis
The Annexin V-FITC Apoptosis Detection kit (MultiSciences Lianke) was used to detect apoptotic cells, according to the manufacturer's protocol. Briefly, the cells were washed with PBS and harvested by trypsinization. The cells (1-2×106) were then incubated with 500 µl 1X binding buffer, 5 µl Annexin V-FITC and 10 µl PI solution. The cells were then gently shaken and incubated at 4°C for 30 min in the dark. The quantitative analysis of apoptosis was conducted using a flow cytometer (Bio-Rad Laboratories, Inc.).
Western blot analysis
The treated H9C2 cardiomyocytes were removed from the CO2 incubator and the medium was discarded. The cells were uniformly plated in a 6-well plate, treated, lysed and the protein concentration was estimated by BCA protein assay. The proteins (50 µg/lane) were separated by 10 or 12% SDS-PAGE, then the proteins were transferred to a 0.45-µm PVDF membrane (EMD Millipore) and immersed in blocking solution for 2 h at room temperature. Subsequently, the membranes were incubated overnight at 4°C with the following primary antibodies: Rabbit anti-Cx43 (cat. no. ab11370; 1:1,000; Abcam), rabbit anti-Bax (cat. no. ab199677; 1:1,000; Abcam), mouse anti-GAPDH (cat. no. ab8245; 1:1,000; Abcam), rabbit anti-Bcl-2 (cat. no. ab196495; 1:1,000; Abcam) and rabbit anti-caspase-3 (cat. no. ab13847; 1:1,000; Abcam). The blots were then washed with TBST and incubated with the secondary antibody (cat. no. ZB-2306; cat. no. ZB-2305; 1:10,000; Beijing Zhongshan Jinqiao Biotechnology Co.) at room temperature for 2 h. Following 3 washes with TBST for 5 min each, the blots were developed using enhanced chemiluminescence (ECL) reagent (GE Healthcare Life Sciences) in a dark room. After images were acquired, analysis was performed using Quantity One software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR (RT-qPCR)
Each group of cells was collected and washed twice with PBS, and total RNA was extracted using the TRIzol kit (Thermo Fisher Scientific, Inc.). The concentration of RNA was detected using an ultraviolet spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Inc.), and complementary DNA was reverse transcribed using a capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Inc.) for qPCR. The gene primers used were as follows: Bax forward, 5′-CCA GGA CGC ATC CAC CAA GAA G-3′ and reverse, 5′-GCT GCC ACA CGG AAG AAG ACC-3′; Bcl-2 forward, 5′-GGT GTG CAG ATG CCG GTT CAG-3′ and reverse, 5′-ACG GTG GTG GAG GAA CTC TTC AG-3′; caspase-3 forward, 5′-GTA CAG AGC TGG ACT GCG GTA TTG-3′ and reverse, 5′-AGT CGG CCT CCA CTG GTA TCT TC-3′; Cx43 forward, 5′-GGA AGC ACC ATC TCC AAC TC-3′ and reverse, 5′-GTA CAG AGC TGG ACT GCG GTA TTG-3′; and β-actin forward, 5′-CAT GTA CGT TGC TAT CCA GGC-3′ and reverse, 5′-CTC CTT AAT GTC ACG CAC GAT-3′. The thermocycling conditions for qPCR were as follows: UDG enzyme activation at 50°C for 2 min, pre-denaturation at 95°C for 2 min, denaturation at 95°C for 15 sec, annealing/extension at 60°C for 1 min, for a total of 40 cycles. The results were analyzed using the 2−ΔΔCq method (16).
Immunofluorescence assay
H9C2 rat cardiomyocytes were uniformly plated at a density of 3×105/ml in a 6-well plate with sterile coverslips to prepare cell slides. After 24 h, the medium was discarded following treatment, and the cells were washed gently with PBS and fixed with paraformaldehyde (40 g/l) for 15 min. The H9C2 rat cardiomyocytes were rewashed with PBS and permeabilized using Triton X-100 (2 g/l) for 3 min. Subsequently, the cells were rewashed with PBS and incubated with BSA (Sigma-Aldrich; Merck KGaA; 50 g/l) at room temperature for 30 min. The primary antibodies [rabbit anti-Cx43 (cat. no. ab11370; 1:100; Abcam), rabbit anti-Bax (cat. no. ab199677; 1:100; Abcam), rabbit anti-Bcl-2 (cat. no. ab196495; 1:100; Abcam) and rabbit anti-caspase-3 (cat. no. ab13847; 1:100; Abcam)] was added, and the negative control group was incubated with PBS. The sample was placed in a wet box overnight at 4°C. The sample was then incubated for 30 min at 37°C, and the primary antibody was discarded. The sample was washed with PBS on a bleaching shaker, and then incubated with a goat anti-rabbit secondary antibody (cat. no. 12-448; 1:50; Sigma-Aldrich) for 1 h at 37°C. Subsequently, the secondary antibody was discarded, and the sample was washed with PBS 3 times for 5 min each time. The cells were then washed with PBS, and the nuclei were stained with DAPI (Solarbio, Inc.) for 15 min at 37°C. Confocal microscopy (Zeiss LSM 510 META, Carl Zeiss AG) was performed to analyze the results (AimImage Examiner; Zeiss LSM Image Examiner version 4.2.0.121).
Statistical analysis
SPSS 20.0 (IBM Corp.) was used for all data analyses. All values are expressed as the means ± standard error. Analysis among multiple groups was performed by one-way analysis of variance followed by Tukey's post hoc tests, as appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
PFD prevents Hcy-induced cardiomyocyte cytotoxicity
To investigate the effect of PFD on Hcy-induced H9C2 cell cytotoxicity, the H9C2 cells were cultured with various concentrations (0, 0.1, 0.5, 1, 2 and 3 mmol/l) of Hcy and various concentrations of PFD (0, 0.1, 0.5, 1 and 1.5 mg/ml) for 24 h. The results demonstrated that exposure to 1-3 mmol/l Hcy significantly decreased cell viability compared with the control group (Fig. 1A); treatment with PFD at 0.1-1 mg/ml demonstrated no obvious cytotoxicity towards the H9C2 cells; however, PFD decreased cell viability at the concentration of 1.5 mg/ml (Fig. 1B). The present study then pre-treated the cells with various concentrations of PFD for 30 min, followed by incubation with Hcy for 24 h. Compared with the Hcy group, the cell viability of the co-treatment group increased in a concentration-dependent manner (Fig. 1C). Hcy at 3 mmol/l and PFD at 1 mg/ml were selected for further study. Finally, the cell morphology in each treatment group was observed by microscopy. Cardiomyocytes exposed to 3.0 mmol/l Hcy for 24 h exhibited a disorder of cell alignment and extensive nuclear pyknosis; however, the cell state of the co-treatment group was significantly improved (Fig. 1D). These results demonstrated that PFD alleviated Hcy-induced cytotoxicity in H9C2 cells.
Figure 1.
PFD prevents Hcy-induced cardiomyocyte cytotoxicity. (A) Dose-dependent cytotoxicity of Hcy towards H9c2 cells. H9c2 cells were exposed to 0-3 mmol/l Hcy for 24 h. (B) Cytotoxicity of PFD towards H9c2 cells. Cells were treated with PFD (0-1.5 mg/ml) for 24 h. (C) PFD alleviated Hcy-induced cytotoxicity in H9c2 cells. Cells were pre-treated with or without (0-1.5 mg/ml) PFD for 30 min and co-treated with 3 mM Hcy for 24 h. (D) The cellular morphology of H9C2 cardiomyocytes was examined under an inverted phase contrast microscope (magnification, ×40). Cell viability following treatment was detected by CCK-8 assay. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01, ###P<0.001 vs. Hcy exposure. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone.
Effects of PFD on the Hcy-induced apoptosis, and increased Bax/Bcl2 and cleaved caspase-3 expression levels in H9C2 cells
To investigate the protective effects of PFD on Hcy-induced cardiomyocyte apoptosis, H9C2 rat cardiomyocytes were pre-treated with PFD for 30 min followed by Hcy incubation for 24 h. Annexin V/PI analysis was then performed. As presented in Fig. 2A and B, exposure to Hcy significantly increased the apoptotic rate of the cardiomyocytes, while the effect was significantly inhibited following PFD pre-treatment. Furthermore, the expression levels of related proteins were significantly altered. Hcy upregulated the Bax/Bcl-2 ratio and increased the protein levels of cleaved caspase-3 compared with the control group (Fig. 2C-F); these effects were suppressed by PFD. PFD pre-treatment reduced the level of cleaved caspase-3, suggesting that PFD can inhibit the apoptotic signaling pathway induced by Hcy. The expression and distribution of Bax, Bcl-2 and caspase-3 in the H9C2 cardiomyocytes was detected by immunofluorescence staining. The results demonstrated that Bax and caspase-3 were predominantly distributed in the cytoplasm (Fig. 3A and D), and Bcl-2 was mainly distributed in the nuclear membrane (Fig. 3B). The changes in the expression of Bax, Bcl-2 and caspase-3 in each group were consistent with the results of western blot analysis (Fig. 3C and E). At the same time, RT-qPCR revealed an increase in the ratio of Bax/Bcl-2 and caspase-3 mRNA following exposure to Hcy in the H9C2 cells, whereas these effects were prevented by pre-treatment with PFD (Fig. 3F and G).
Figure 2.
Effects of PFD on the Hcy-induced apoptosis, increased Bax/Bcl2 and cleaved caspase-3 expression levels in H9C2 cells. (A) PFD inhibited H9C2 cell apoptosis following Hcy-induced injury. PFD was used at 1 mg/ml. H9C2 cell apoptosis was determined using Annexin V/PI staining and flow cytometry analysis. (B) Statistical analysis of the apoptotic rate in each group. (C and E) The Hcy-induced increase in Bax/Bcl-2 and cleaved caspase-3 protein level was prevented by PFD. (D and F) Quantification of Bax/Bcl-2 and cleaved caspase-3. *P<0.05, **P<0.01, ***P<0.001 vs. control; #P<0.05, ##P<0.01 vs. Hcy treatment. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone.
Figure 3.
(A, B and D) Fluorescence localization of the apoptotic proteins, Bax, Bcl-2 and caspase-3. Magnification, ×200. (C and E) Half of the relative fluorescence intensity of Bax/Bcl-2 and caspase-3 quantitative analysis. (F and G) Hcy-induced Bax/Bcl-2 and cleaved caspase-3 mRNA upregulation was prevented by PFD. *P<0.05 vs. control; #P<0.05, ##P<0.01 vs. Hcy treatment; &P<0.05 vs. Hcy treatment. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone.
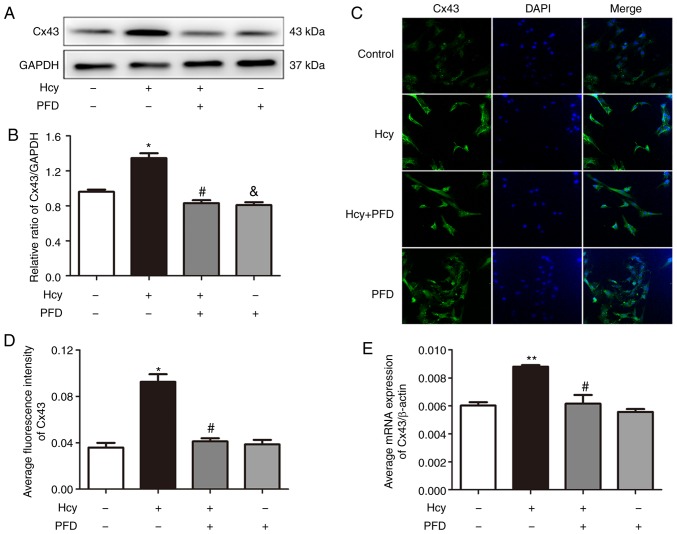
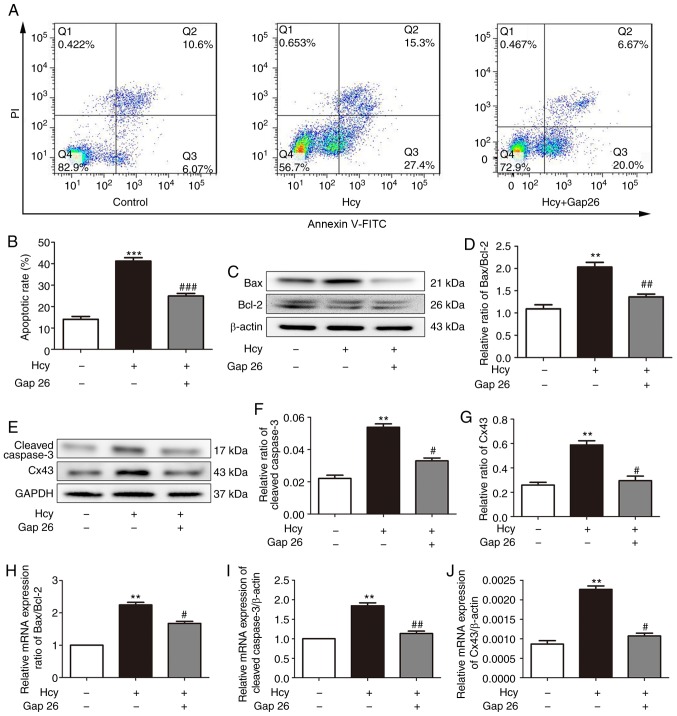
Cx43 is involved in the Hcy-induced apoptosis of H9C2 cardiomyocytes
It has been found that Cx43 is involved in the process of cardiomyocyte apoptosis (8). In investigating the protective mechanisms of PFD in Hcy-induced H9C2 cell injury, it was observed that PFD pre-treatment downregulated the Cx43 protein levels (Fig. 4A and B). The results of immunofluorescence staining of the H9C2 cells demonstrated that Cx43 was predominantly distributed in the cytoplasm and cell membrane (Fig. 4C). The Cx43 fluorescence intensity and mRNA levels of each group were consistent with the results of western blot analysis (Fig. 4D and E). Subsequently, the H9C2 cardiomyocytes were pre-treated with the Cx43 inhibitor, Gap26 (0.5 µmol/l, 30 min), and then exposed to Hcy for 24 h for the following experiments. As shown in Fig. 5A and B, the apoptotic rate was significantly increased following exposure to Hcy, while it was significantly decreased following Gap26 pre-treatment. Moreover, Hcy increased the Bax/Bcl2 ratio and increased the expression levels of cleaved caspase-3 and Cx43. These effects were prevented by Gap26 (Fig. 5C-G). The changes in the mRNA expression levels of Bax, Bcl-2, caspase-3 and Cx43 in each group were consistent with those of western blot analysis (Fig. 5H-J). These results demonstrated that Cx43 is involved in Hcy-induced H9C2 apoptosis.
Figure 4.
Cx43 is involved in the Hcy-induced apoptosis of H9C2 cardiomyocytes. (A) Effects of Hcy and PFD on the expression of Cx43. (B) Statistical analysis of the expression of Cx43. (C) Fluorescence localization of the apoptosis protein Cx43. Magnification, ×200. (D) Half of the relative fluorescence intensity of Cx43 quantitative analysis. (E) The effect of PFD on Cx43 mRNA expression in H9C2 cells was determined by reverse transcription-quantitative PCR. *P<0.05, **P<0.01 vs. control; #P<0.05 vs. Hcy treatment; &P<0.05 vs. Hcy treatment. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone; Cx43, connexin 43.
Figure 5.
(A) Pre-treatment with Gap26 inhibited H9C2 cell apoptosis following Hcy-induced injury. Gap26 was used at 0.5 µmol/l. (B) Statistical analysis of the rate of apoptosis in each group. (C and E) Hcy-induced upregulation of Bax/Bcl-2, cleaved caspase-3 and Cx43 protein levels was prevented by Gap26. (D, F and G) Quantification of Bax/Bcl-2, cleaved caspase-3 and Cx43. (H-J) Hcy-induced upregulation of Bax/Bcl-2, cleaved caspase-3 and Cx43 mRNA levels was prevented by Gap26. **P<0.01, ***P<0.001 vs. control; #P<0.05, ##P<0.01 vs. Hcy treatment. Data are shown as the means ± SE (n=3). Hcy, homocys-teine; PFD, pirfenidone; Cx43, connexin 43.
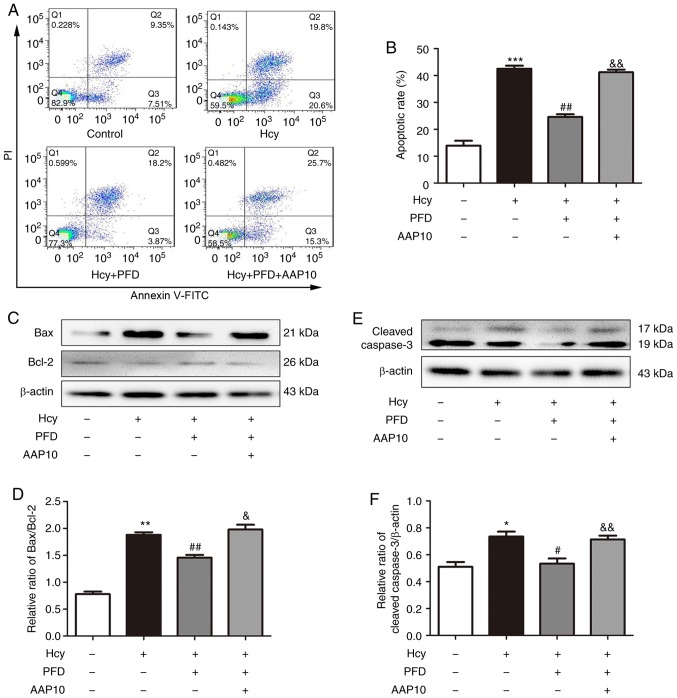
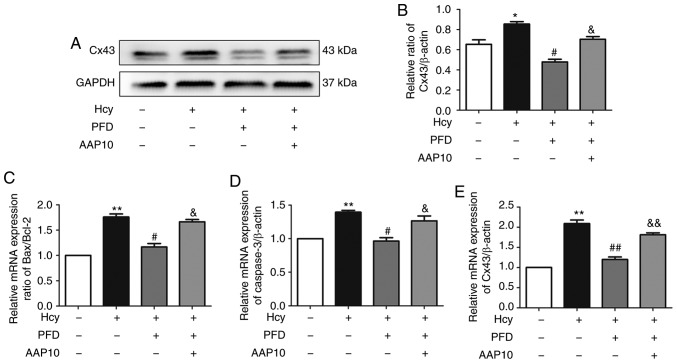
PFD protects the Hcy-induced apoptosis of H9C2 cardiomyocytes via the Cx43 pathway
To investigate whether Cx43 is involved in PFD-regulated protection against Hcy-induced H9C2 cell injury, H9C2 cardiomyocytes were pre-treated with the Cx43 agonist, AAP10 (50 nmol/l, 1 h) and Hcy for the following experiments. Compared with the Hcy group, the rate of apoptosis decreased following pre-treatment with PFD, and this effect was prevented by AAP10 (Fig. 6A and B). Furthermore, Hcy increased the ratio of Bax/Bcl2 and upregulated the expression levels of cleaved caspase-3 and Cx43. These effects were prevented by PFD. Furthermore, the effects of PFD were prevented by AAP10 (Figs. 6C-F, and 7A and B). Compared with PFD pre-treatment, the Cx43 agonist, AAP10, also increased the mRNA ratio of Bax/Bcl-2, and increased the mRNA expression levels of cleaved caspase-3 and Cx43 (Fig. 7C-E). The results demonstrated that PFD protects H9C2 cardiomyocytes against Hcy-induced apoptosis via the Cx43 pathway.
Figure 6.
PFD protects H9C2 cardiomyocytes against Hcy-induced apoptosis via the Cx43 pathway. (A) Hcy-induced apoptosis was attenuated by PFD, and the effects of PFD were reversed by AAP10. AAP10 was used at 50 nmol/l. (B) Statistical analysis of the rate of apoptosis in each group. (C and E) Hcy-induced upregulation of Bax/Bcl-2 and cleaved caspase-3 protein level was prevented by PFD. The effects of PFD were prevented by AAP10. (D and F) Western blot analysis of Bax/Bcl-2 and cleaved caspase-3. *P<0.05, **P<0.01, ***P<0.001 vs. control; #P<0.05, ##P<0.01 vs. Hcy treatment; &P<0.05, &&P<0.01 vs. Hcy + PFD treatment. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone; Cx43, connexin 43.
Figure 7.
(A) The Hcy-induced upregulation of Cx43 protein level was prevented by PFD. The effects of PFD were prevented by AAP10. (B) Western blot analysis of Cx43. (C-E) Hcy-induced upregulation of Bax/Bcl-2, cleaved caspase-3 and Cx43 mRNA levels was prevented by PFD. The effects of PFD were prevented by AAP10. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. Hcy treatment; &P<0.05, &&P<0.01 vs. Hcy + PFD treatment. Data are shown as the means ± SE (n=3). Hcy, homocysteine; PFD, pirfenidone; Cx43, connexin 43.
Discussion
The present study observed that PFD attenuates Hcy-induced cardiomyocyte apoptosis by modulating the Cx43 signaling pathway. A recent study reported that PFD can reduce tubular cell apoptosis by inhibiting mitochondrial apoptotic signaling pathways (13). However, the mechanism of the protective effect of PFD on myocardial apoptosis has not yet been determined.
In recent years, apoptosis has been found to be widely involved in the pathological processes of various cardiovascular diseases, such as atherosclerosis, heart failure and arrhythmias. Elevated levels of Hcy in plasma are a risk factor for cardiovascular disease and can cause a range of pathological processes. Several studies have demonstrated that Hcy can activate the apoptotic signaling pathway in cardiomyocytes (4-6). In the present study, first, cytotoxicity was measured to obtain understanding into the Hcy-induced toxic effects on cardiomyocytes. The results demonstrated that exposure to 3 mmol/l Hcy can significantly reduce cell viability. Cardiomyocyte apoptosis can be regulated by Bcl-2 family proteins (17,18). Numerous studies have reported that Hcy can enhance apoptosis in a variety of cell types, including endothelial cells, mesangial cells and human umbilical vein endothelial cells (19-21). The present study found that Hcy increased the ratio of Bax/Bcl-2, promoted caspase-3 activation and induced cardiomyocyte apoptosis.
PFD has been shown to reduce the fibrosis of different organs, including the lungs, kidney and heart (22,23). A recent study demonstrated that PFD exerts cardiovascular protective effects in a variety of noxious stimulation models (24). In a cardiac hypertrophic mouse model induced by Ang II infusion, echocardiography exhibited a significant increase in left ventricular hypertrophy in the vehicle group compared with the control group. Notably, PFD significantly inhibited this effect (24). These results demonstrated that PFD exerts a certain protective effect on cardiovascular disease; however, the specific mechanisms have not yet been determined. In this study, it was found that treatment with PFD at 0.1-1 mg/ml had no significant effect on H9c2 cell viability. At the same time, compared with Hcy treatment alone, the cell viability of the PFD pre-treatment group increased in a concentration-dependent manner. Chen et al found that PFD significantly inhibited HK2 cell apoptosis in a dose-dependent manner (13). The results of the present study are consistent with those of this previous study. Of note, it was found that the PFD (1.5 mg/ml) and Hcy co-treatment group exhibited an enhanced viability even they exhibited cytotoxicity. This study found similar findings to those of previous studies. Tsuchiya et al found that PFD increased the survival rate of LPS-treated rats at 24 h dose dependently; however, PFD did not lead to a further increase in the survival rate at 650 mg/kg (25). Gefitinib and cisplatin are commonly used anticancer drugs that inhibit the growth of tumor cells. Tsai et al found that the simultaneous administration of gefitinib and cisplatin in a panel of previously untreated non-small cell lung cancer cell lines caused overall antagonism, which was directly associated with gefitinib sensitivity (26). Thus, it was hypothesized that co-treatment with PFD and Hcy may counteract the toxic effects; however, the specific mechanisms warrant further investigation. Therefore, PFD can alleviate Hcy-induced cytotoxicity in H9C2 cells.
In a nephrotoxicity animal model, researchers observed that PFD was capable of partly reversing the pro-apoptotic properties of chronic cyclosporine A (CsA) by decreasing the expression of p53 and Fas-L and increasing that of the survival gene Bcl-xL (27). Another study found that PFD treatment inhibited caspase-9 and caspase-3 cleavage in renal proximal tubular cells (13). The results of the present study revealed that PFD pre-treatment reduced the ratio of Bax/Bcl-2 in the Hcy-induced cardiomyocyte apoptosis model. At the same time, the activation of caspase-3 was suppressed by PFD. These results demonstrate that PFD can protect against Hcy-induced H9C2 myocardial cell apoptosis by reducing the expression of pro-apoptotic factors; however, the specific regulatory mechanisms have not yet been elucidated.
Cell communication refers to the transmission of information from one cell to another through a medium, which produces a corresponding response. The maintenance of homeostasis is controlled by utilizing the interaction between extracellular, intracellular and intercellular signaling pathways (28). Gap junctions are a special membrane structure that connects adjacent cells. These junctions have a wide distribution range and exist in the majority of animal cells. Connexin is the basic building block of the gap junction channel (17). Cx43 is widely involved in the development of diseases, such as the regulation of cell growth, proliferation, apoptosis and homeo-stasis (17,29). Connexin mimetic peptides, such as Gap26, are known to be inhibitors of gap junction channels (30). It has been indicated that the Cx43 agonist resists-arrhythmias peptide, AAP10, improves gap-junctional intercellular coupling in human cardiomyocytes and prevents separation caused by acidification (31). A recent study demonstrated that Cx43 is a key regulator of cardiomyocyte apoptosis under pathological and physiological conditions (8). To investigate the protective mechanisms of PFD in Hcy-induced H9C2 cell injury, the present study found a significant decrease in the Cx43 protein level following PFD pre-treatment. Subsequently, the H9C2 cardiomyocytes were pre-treated with the Cx43 inhibitor, Gap26, and incubated with Hcy. Hcy increased the ratio of Bax/Bcl2 and upregulated the protein expression levels of cleaved caspase-3 and Cx43. These effects were prevented by Gap26. In a model of intestinal damage, a previous study found that intestinal damage can be significantly relieved following administration of Cx43 inhibitors (32). Another study demonstrated that stretch-induced apoptosis in human trabecular meshwork cells is accompanied by upregulation of Cx43 (33). The present findings indicated that Cx43 is involved in the apoptosis of Hcy-induced H9C2 cardiomyocytes. The beneficial effect of PFD was then abolished using the connexin agonist AAP10. The present study found that Hcy induces apoptosis of H9C2 cardiomyocytes through Cx43, and this phenomenon can be prevented by PFD, which can aggravate cardiomyocyte apoptosis following administration of the connexin agonist AAP10.
Cx43 phosphorylation/dephosphorylation plays an important role in regulating cell survival and death processes. Evidence has also demonstrated that high levels of phosphorylated Cx43 mainly regulates cell survival. Cx43 dephosphorylation is involved in numerous pathological processes. Yang et al found that Cx43 phosphorylation at S282 mediates cardiomyocyte survival and S282 dephosphorylation induces cardiomyocyte apoptosis under normal conditions (8). The present study had some limitations. It is important to perform an assay for Cx43 phosphorylation in future studies.
In conclusion, the present study elucidated the protective role of PFD in cardiomyocyte apoptosis. The findings demonstrated that PFD modulates the expression of apoptosis-related proteins through the Cx43 signaling pathway to attenuate Hcy-induced apoptosis in H9C2 cells. These results provide new insight into the clinical application of PFD in the treatment of cardiovascular diseases.
Acknowledgments
The authors would like to thank the Key Laboratory of Xinjiang Endemic and Ethnic Diseases, the 3rd Department of Cardiology, the First Affiliated Hospital of the Medical College, Shihezi University and the Departments of Physiology and Pathophysiology of Shihezi University School of Medicine for their assistance.
Funding
The study was supported by the National Natural Science Foundation of China (grant nos. 81860286 and 81660271 to KM), Shihezi University International Science and Technology Cooperation Promotion Project (grant no. GJHZ201603 to KM), Corps Young and Middle-aged Science and Technology Innovation Leadership Program of China (grant no. 2016BC006 to KM) and Hospital Level Key Area Innovation Team Project (The First Affiliated Hospital of Shihezi University Medical College, grant no. TJ2016-001 to LW).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KM and LW conceived and designed the experiments. YO, JS and LL were involved in the study design. KC, LC, YO, LZ and XL performed the experiments. KC and LC analyzed the data. KC, YO and LC wrote and revised the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shekhar A, Heeger P, Reutelingsperger C, Arbustini E, Narula N, Hofstra L, Bax JJ, Narula J. Targeted imaging for cell death in cardiovascular disorders. JACC Cardiovasc Imaging. 2018;11:476–493. doi: 10.1016/j.jcmg.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Madonna R, Balistreri CR, De Rosa S, Muscoli S, Selvaggio S, Selvaggio G, Ferdinandy P, De Caterina R. Impact of sex differences and diabetes on coronary atherosclerosis and ischemic heart disease. J Clin Med. 2019;8 doi: 10.3390/jcm8010098. pii: E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo F, Das A, Chen J, Wu P, Li X, Fang Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc Diabetol. 2019;18:54. doi: 10.1186/s12933-019-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aminzadeh A, Mehrzadi S. Cardioprotective effect of levosimendan against homocysteine-induced mitochondrial stress and apoptotic cell death in H9C2. Biochem Biophys Res Commun. 2018;507:395–399. doi: 10.1016/j.bbrc.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Fan CD, Sun JY, Fu XT, Hou YJ, Li Y, Yang MF, Fu XY, Sun BL. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Front Physiol. 2017;8:1041. doi: 10.3389/fphys.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Zhao L, Zhou Y, Lu X, Wang Z, Wang J, Li W. Taurine ameliorated homocysteine-induced H9C2 cardiomyocyte apoptosis by modulating endoplasmic reticulum stress. Apoptosis. 2017;22:647–661. doi: 10.1007/s10495-017-1351-9. [DOI] [PubMed] [Google Scholar]
- 7.Ostrakhovitch EA, Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res Rev. 2019;49:144–164. doi: 10.1016/j.arr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Yan X, Xue J, Zheng Y, Chen M, Sun Z, Liu T, Wang C, You H, Luo D. Connexin43 dephosphorylation at serine 282 is associated with connexin43-mediated cardiomyocyte apop-tosis. Cell Death Differ. 2019;26:1332–1345. doi: 10.1038/s41418-019-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vozzi C, Dupont E, Coppen SR, Yeh HI, Severs NJ. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol. 1999;31:991–1003. doi: 10.1006/jmcc.1999.0937. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart Circ Physiol. 2002;282:H2124–H2133. doi: 10.1152/ajpheart.01028.2001. [DOI] [PubMed] [Google Scholar]
- 11.Komiya C, Tanaka M, Tsuchiya K, Shimazu N, Mori K, Furuke S, Miyachi Y, Shiba K, Yamaguchi S, Ikeda K, et al. Antifibrotic effect of pirfenidone in a mouse model of human nonalcoholic steatohepatitis. Sci Rep. 2017;7:44754. doi: 10.1038/srep44754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahnke T, Kowtharapu BS, Stachs O, Schmitz KP, Wurm J, Wree A, Guthoff RF, Hovakimyan M. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS One. 2017;12:e0172592. doi: 10.1371/journal.pone.0172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JF, Liu H, Ni HF, Lv LL, Zhang MH, Zhang AH, Tang RN, Chen PS, Liu BC. Improved mitochondrial function underlies the protective effect of pirfenidone against tubulointerstitial fibrosis in 5/6 nephrectomized rats. PLoS One. 2013;8:e83593. doi: 10.1371/journal.pone.0083593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Li L, Tang M, Zeng Y, Li H, Yu X. Biomimetic electrical stimulation induces rat bone marrow mesenchymal stem cells to differentiate into cardiomyocyte-like cells via TGF-beta 1 in vitro. Prog Biophys Mol Biol. 2019;148:47–53. doi: 10.1016/j.pbiomolbio.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Fan XD, Zheng HB, Fan XS, Lu S. Increase of SOX9 promotes hepatic ischemia/reperfusion (IR) injury by activating TGF-β1. Biochem Biophys Res Commun. 2018;503:215–221. doi: 10.1016/j.bbrc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Decrock E, Vinken M, De Vuyst E, Krysko DV, D'Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: To live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 18.Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Luo M, Xie N, Wang H, Wang J. Bioinformatics-based analysis of the involvement of AC005550.3, RP11-415D17.3, and RP1-140K8.5 in homocysteine-induced vascular endothelial injury. Am J Transl Res. 2018;10:2126–2136. [PMC free article] [PubMed] [Google Scholar]
- 20.Majumder S, Ren L, Pushpakumar S, Sen U. Hydrogen sulphide mitigates homocysteine-induced apoptosis and matrix remodelling in mesangial cells through Akt/FOXO1 signalling cascade. Cell Signal. 2019;61:66–77. doi: 10.1016/j.cellsig.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Hong Y, Zhang C, Shen Y, Pan YS, Chen RZ, Zhang Q, Chen YH. Picroside II attenuates hyperhomocysteinemia-induced endothelial injury by reducing inflammation, oxidative stress and cell apoptosis. J Cell Mol Med. 2019;23:464–475. doi: 10.1111/jcmm.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neri T, Lombardi S, Faìta F, Petrini S, Balìa C, Scalise V, Pedrinelli R, Paggiaro P, Celi A. Pirfenidone inhibits p38-mediated generation of procoagulant microparticles by human alveolar epithelial cells. Pulm Pharmacol Ther. 2016;39:1–6. doi: 10.1016/j.pupt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Liu X, Wang B, Nie Y, Wen J, Wang Q, Gu C. Pirfenidone suppresses MAPK signalling pathway to reverse epithelial-mesenchymal transition and renal fibrosis. Nephrology (Carlton) 2017;22:589–597. doi: 10.1111/nep.12831. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Yamashita N, Izumi Y, Nakamura Y, Shiota M, Hanatani A, Shimada K, Muro T, Iwao H, Yoshiyama M. The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res. 2012;35:34–40. doi: 10.1038/hr.2011.139. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya H, Kaibori M, Yanagida H, Yokoigawa N, Kwon AH, Okumura T, Kamiyama Y. Pirfenidone prevents endo-toxin-induced liver injury after partial hepatectomy in rats. J Hepatology. 2004;40:94–101. doi: 10.1016/j.jhep.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Tsai CM, Chen JT, Stewart DJ, Chiu CH, Lai CL, Hsiao SY, Chen YM, Chang KT. Antagonism between gefitinib and cisplatin in non-small cell lung cancer cells why randomized trials failed? J Thorac Oncol. 2011;6:559–568. doi: 10.1097/JTO.0b013e3182021ff5. [DOI] [PubMed] [Google Scholar]
- 27.Shihab FS, Bennett WM, Yi H, Andoh TF. Effect of pirfeni-done on apoptosis-regulatory genes in chronic cyclosporine nephrotoxicity. Transplantation. 2005;79:419–426. doi: 10.1097/01.TP.0000151721.99418.48. [DOI] [PubMed] [Google Scholar]
- 28.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: Novel roles of 'hemi-channels'. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooreman A, Van Campenhout R, Ballet S, Annaert P, Van Den Bossche B, Colle I, Cogliati B, Vinken M. Connexin and pannexin (hemi)channels: Emerging targets in the treatment of liver disease. Hepatology. 2019;69:1317–1323. doi: 10.1002/hep.30306. [DOI] [PubMed] [Google Scholar]
- 30.Wang N, De Bock M, Antoons G, Gadicherla AK, Bol M, Decrock E, Evans WH, Sipido KR, Bukauskas FF, Leybaert L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagen A, Dietze A, Dhein S. Human cardiac gap-junction coupling: Effects of antiarrhythmic peptide AAP10. Cardiovasc Res. 2009;83:405–415. doi: 10.1093/cvr/cvp028. [DOI] [PubMed] [Google Scholar]
- 32.Zou Z, Liu B, Zeng L, Yang X, Huang R, Wu C, Zhu H, Gao Y, Yuan D, Yu J. Cx43 inhibition attenuates sepsis-induced intestinal injury via downregulating ROS transfer and the activation of the JNK1/Sirt1/FoxO3a signaling pathway. Mediators Inflamm. 2019;2019:7854389. doi: 10.1155/2019/7854389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tellios N, Feng M, Chen N, Liu H, Tellios V, Wang M, Li X, Chang CA, Hutnik C. Mechanical stretch upregulates connexin43 in human trabecular meshwork cells. Clin Exp Ophthalmol. 2019;47:787–794. doi: 10.1111/ceo.13492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.