ABSTRACT
Although exclusive breastfeeding has been linked to lower rates of postnatal HIV transmission compared to nonexclusive breastfeeding, mechanisms underlying this are unclear. Across a longitudinally sampled cohort of South African infants, we showed that exclusively breastfed (EBF) infants had altered gut bacterial communities when compared to nonexclusively breastfed (NEBF) infants, as well as reduced peripheral CD4 + T cell activation and lowered chemokine and chemokine receptor expression in the oral mucosa. We further demonstrated that the relative abundance of key taxa was correlated with peripheral CD4 + T cell activation. Here, we supplement those findings by using compositional data analyses to identify shifts in the abundance of several Bifidobacteria strains relative to select strains of Escherichia, Bacteroides, and others that are associated with the transition to NEBF. We illustrate that the abundance ratio of these taxa is tightly correlated with feeding modality and is a strong predictor of peripheral T cell activation. More broadly, we discuss our study in the context of novel developments and explore future directions for the field.
KEYWORDS: Bacterial microbiota, T cell activation, feeding, South Africa, compositional transform, mucosal gene expression
Introduction
More so than by any other mechanism, gut bacterial communities are shaped by dietary practices.1,2 In infants, early life feeding practices are integral in shaping bacterial primary succession patterns in the gut and associated long-term health outcomes.3–5 For example, exclusive breastfeeding (EBF) during the first six months of life has been associated with protection against diarrhea, diabetes, and other morbidities, as compared to nonexclusive breastfeeding (NEBF), where infants are fed breastmilk with a combination of other liquids and solids, or formula feeding (FF).6–8 Recently, studies have begun to elucidate the link between infant feeding practices, microbial community composition, and infant health, 9–11 revealing alterations in gut bacterial community composition and mucosal gene expression between feeding practices.5,10–13
Despite the benefits conferred by exclusive breastfeeding, it is not always a viable option due to maternal disease, dietary restrictions, or availability. In our recent study, we demonstrated that nonexclusive breastfeeding alters the community composition of gut bacteria, increases T cell activation and, possibly, mucosal recruitment of HIV target cells during at least the first 3 months of life. Here, we explore the results of our study in the current context of the field, apply novel compositional data analytical approaches to reveal models between bacterial abundance and measures of immune activation, and discuss next steps and remaining questions.
Feeding practices and HIV transmission
Despite the associated benefits, breastfeeding represents a conduit for HIV transmission in the absence of antiretroviral prophylaxis.14 Though the administration of antiretroviral therapy (ART) has greatly reduced the incidence of mother-to-child transmission, hundreds of thousands of infants still acquire HIV via this mechanism.15 However, previous studies have found that EBF infants have a lower risk of HIV acquisition compared to NEBF infants, 16–18 putatively due to reduced cell-free viral loads in the breastmilk of mothers that exclusively breastfeed.19 In our study conducted in Cape Town, South Africa, we showed that the abundance of specific bacterial taxa was correlated with decreased peripheral CD4 + T cell activation in EBF infants. Further, we demonstrated that gene expression of chemokines and associated receptors involved in HIV target cell recruitment was reduced in the oral mucosa of EBF infants relative to NEBF infants.
Community composition of gut bacterial microbiota is altered between EBF and NEBF infants
Many datasets exist about the effects of EBF vs FF on infant gut microbiota, 20–22 though fewer have surveyed bacterial communities between EBF and NEBF infants. In our 2018 study, we demonstrated that EBF infants possessed altered community composition and diversity of gut bacteria through the first 14 weeks of life, even compared to infants fed breast milk, but along with other solids or liquids (NEBF).11 We reported consistently decreased phylogenetic diversity of EBF bacterial communities at both 6 and 14 weeks of life. Furthermore, we found that overall community profiles significantly differed between EBF and NEBF infants at both time points, using phylogenetically aware (weighted Unifrac) and traditional ecological (Jensen-Shannon) distance metrics. Specifically, we found reduced relative abundance of Streptococcus luteciae in EBF infants at 6 weeks of life compared to NEBF infants. At 14 weeks, we found that EBF infants displayed increased relative abundance of Actinomyces and Atopobium taxa and decreased relative abundance of two members within Bacteroides compared to NEBF infants. These results were recently corroborated by a meta-analysis of the effects of exclusive breastfeeding on gut microbiota across studies and populations, consistently finding reduced α-diversity and decreased relative abundance of Bacteroides in EBF infants.10
Here, we supplement the findings in our original study by applying compositional analyses of bacterial abundance. With the application of compositional data analytical tools, we can further dissect differences in bacterial abundance between EBF and NEBF infants while reducing compositional effects (artifacts associated with relative abundance datasets).23 Microbial community datasets generated from marker gene surveys (e.g., via sequencing 16S/18S rRNA genes) yield abundance measurements that are influenced by the composition of the community, precluding the inference of true shifts in bacterial abundance. Here, we use a special application of the isometric log ratio transformation, 24 a compositional data transformation, to generate ratios of highly informative bacterial taxa that enable more accurate inference of shifts in bacterial abundance. These ratios, termed balances, 25 enable interpretable taxon-level inference of alterations that best discriminate EBF from NEBF infants (Figure 1). To identify the taxa used in Balance 1, we performed a centered log ratio (CLR)26 transformation on our bacterial abundance measurements. Once transformed, we performed a sparse principal component analysis27 to identify taxa positively and negatively associated with each sparse principal component and used these taxa to build a series of orthonormal compositional balances. Penalized (1) logistic regression was then used to identify compositional balances strongly associated with feeding practice. The 1 penalty (lasso) is useful for selecting predictor variables strongly associated with specific responses.28 The ratio identified in Balance 1 yielded the greatest discrimination between feeding modalities.
Figure 1.
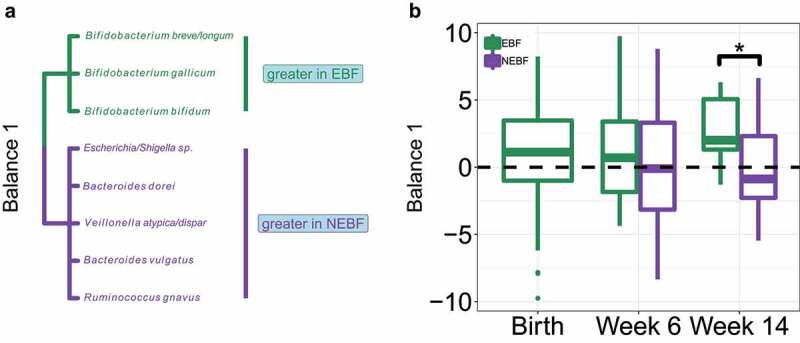
Nonexclusive breastfeeding shifts the abundance of several key bacterial taxa compared to exclusive breastfeeding during the first 14 weeks of life.
A. Bacterial taxa involved in Balance 1. Bacteria with elevated abundance in EBF infants are displayed in green and those with elevated abundance in NEBF infants are displayed in purple. B. Boxplots of Balance 1 values between EBF and NEBF infants across all time points sampled. The dashed black line indicates the value (0) at which bacteria in the numerator (Bifidobacteria) are equal in abundance to bacteria in the denominator (Escherichia et al.). Boxplots display the interquartile range and median value. Taxa in Balance 1 were selected by performing a sparse principal component analysis on centered log ratio transformed bacterial abundance and building isometric log ratio balances from taxa associated with each sparse principal component. Balance 1 was identified as the most strongly correlated with feeding modality using penalized (1) logistic regression. P values: * ≤ 0.05. P values were calculated using unpaired Wilcoxon Rank Sum tests.
Our results demonstrate that the log ratio abundance of three Bifidobacterial taxa (B. breve, B. gallicum, B. bifidum) to Escherichia, Bacteroides dorei, Veillonella dispar, Bacteroides vulgatus, and Ruminococcus gnavus is significantly reduced (P < .027) in NEBF infants, with non-Bifidobacterial taxa surpassing Bifidobacterial abundance by 6 weeks. Specifically, at birth, all infants in our dataset possessed a median value of 1.12 for Balance 1 and this value remained relatively stable for EBF infants during our study (0.7 at 6 weeks; 2.0 at 14 weeks). Conversely, the median value for NEBF infants fell to -0.16 at 6 weeks and −1.14 at 14 weeks, indicating greater abundance of taxa in the denominator (Escherichia et al.) than in the numerator (B. breve, B. gallicum, B. bifidum). Bifidobacteria are canonically beneficial inhabitants of the breastfed infant gut microbiota, 21,29 with select strains possessing the ability to metabolize human milk oligosaccharides.30 Conversely, B. dorei and B. vulgatus have been implicated in negative health outcomes such as the development of autoimmune disease31,32 and necrotizing enterocolitis33 in neonates and infants, and increases in R. gnavus have been associated with intestinal dysbiosis.34
Alterations in the infant immune response associated with feeding modality
In our 2018 study, we assayed peripheral CD4+ T cell activation and buccal mucosal chemokine, chemokine receptor, and keratin gene expression levels longitudinally between EBF and NEBF infants, in order to gain insight into the mechanisms underlying the increased risk of HIV acquisition in NEBF infants. We found that peripheral CD4+ T cell activation was consistently decreased in EBF infants across all time points, with significant reductions in the frequency of CD4+ human leukocyte antigen-DR+ (HLA-DR+) and CD4+ HLA-DR+CD25+ T cells in EBF infants at 6 weeks of life. This relative immune quiescence in EBF infants may confer protection from HIV transmission, in line with previous findings reporting that systemic immune quiescence is associated with protection from sexual HIV transmission.35 Because HIV preferentially infects activated CD4+ T cells, 36 our findings began to provide mechanistic insight into the underlying causes of reduced HIV transmission in EBF infants.
In the primary analysis, we further assayed chemokine, chemokine receptor, and keratin gene expression in the oral mucosa of EBF and NEBF infants, finding reduced levels of several of these in EBF infants at weeks 6 and 14. Specifically, we found significant reductions (measured as log10 fold changes in expression) in CCL5, CCL22, CXCR7, IL7R, and KRT5 in EBF infants, relative to NEBF counterparts. We hypothesized that these decreases in chemokine and chemokine receptor gene expression levels in EBF infants indicated lower immune activation in buccal mucosa, and a consequent decrease in HIV susceptibility. In line with previous work, 37 our results of elevated cytoskeletal gene expression (KRT5) are suggestive of epithelial barrier compromise as a putative mechanism for increased mucosal HIV acquisition risk with NEBF. Together, these results suggest that activation of HIV target cells in conjunction with compromised mucosal barrier integrity may facilitate HIV virion access to target cells, consistent with observations from previous studies of genital mucosa.36–38
The abundance of key bacterial taxa predicts mucosal immune activation and correlates with feeding practices
Our findings led us to propose that the increased bacterial diversity associated with NEBF may induce immune activation, thus explaining the higher incidence of HIV transmission in NEBF infants as compared to EBF infants. Indeed, it has been previously demonstrated that EBF infants have altered gene expression and peripheral T cell activation than formula fed infants.12,39 In our parent study, we identified correlations between ecological distance metrics (Jensen-Shannon and weighted Unifrac) and the expression of several T cell markers, suggesting that bacterial community composition shifted with T cell marker abundance. Using compositional data analytical approaches, we can move beyond correlations between community-wide distance metrics and immune activation markers and into more targeted models involving specific microbial taxa. Here, we use compositional analyses to enable modeling of interactions between bacteria in Balance 1 (Figure 1) and the expression of T cell activation markers (Figure 2). By reducing compositional effects associated with marker gene sequencing, we can more accurately infer interactions between bacterial abundance and external covariates (T cell marker expression).
Figure 2.
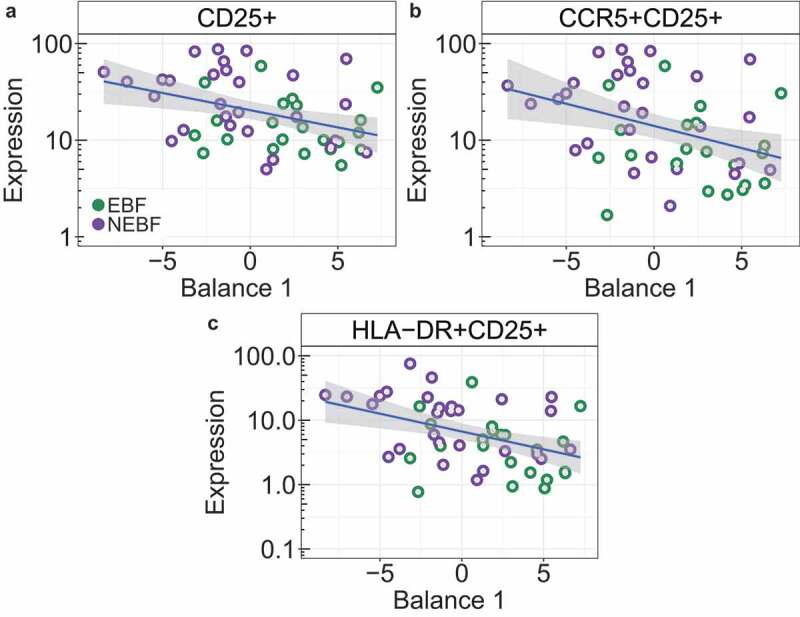
Peripheral CD4 + T cell activation marker and HIV coreceptor expression correlate with shifts in fecal bacterial abundance.
Correlations between Balance 1 and A. CD25+ expression. B. CCR5+ CD25+ expression. C. HLA-DR+CD25+ expression. Linear regression lines are colored blue and the standard error interval is shaded grey. Samples from EBF infants are displayed in green and those from NEBF infants are displayed in purple. Abbreviations: CD25: interleukin-2 receptor alpha chain; CCR5: anti-CC chemokine receptor type 5; HLA-DR: human leukocyte antigen-DR.
Here, we demonstrate that the isometric log ratio of bacteria identified in Balance 1 as associated with feeding practice is a significant negative predictor of immune activation and co-receptor expression (CD25+: P < .0049; CCR5+ CD25+: P < .007; HLA-DR+CD25+: P < .002). Further, this interaction is associated with feeding modality (P < .027) and is correlated with an increase in the ratio of several Bifidobacteria taxa relative to Escherichia and others (Figure 2). Broadly, these findings suggest that, in addition to discriminating feeding practices, Balance 1 also defines a spectrum of immune activation where samples with positive values (greater abundance of taxa in the numerator) display relative immune quiescence and samples with negative values (greater abundance of taxa in the denominator) display relative immune activation, with several plausible mechanisms in play. Concerning immune quiescence, Bifidobacteria have been shown to reduce serum cytokine levels in a diverse range of disease conditions in adults40 and peripheral blood mononuclear cells,41 suggesting beneficial immunomodulatory effects. In adults, supplementation of B. infantis has been shown to reduce cytokine levels in various inflammatory disorders.41 Conversely, several taxa in the denominator of Balance 1 have been associated with elevated immune activation. Increased fecal abundance of R. gnavus has been associated with the development of allergies in infants, and work in murine models has linked R. gnavus to elevated cytokine secretion and airway inflammation.34 Vatanen et al. demonstrated the immunostimulatory nature (upon initial exposure) of E. coli lipopolysaccharide (LPS) compared to that of other gut commensals which are immunoinhibitory.32 Early exposure to immunostimulatory LPS produced by E.coli has been shown to elicit significantly increased cytokine production in human cells in vitro as compared to LPS produced by other gut associates.32 In EBF infants, the reduced activation of these markers may be linked to reduced abundance of E. coli LPS and a reduction in proinflammatory bacteria, and/or elevated abundance of immunomodulatory Bifidobacteria, or a synthesis of both. With respect to HIV acquisition, the infant gut is known to host an abundance of CD4+ CCR5 + T cells which are highly susceptible to HIV-1.42 Our finding of a significant negative correlation between activated, CCR5+ expressing CD4 T cells and Balance 1 suggests that elevated abundance of Bifidobacteria relative to Escherichia, Bacteroides, and other inflammatory taxa may contribute to immune quiescence and reduced HIV target cell abundance, though this should be validated in additional cohorts and/or experimental systems. If these results are found to be reproducible, Bifidobacteria probiotics may represent an attractive intervention to restore the health of NEBF infants and reduce HIV transmission.
Next steps and open questions
Despite the advances in our ability to quantitate and analyze bacterial and immunological dynamics in parallel, many questions remain regarding the effects of feeding practices on infant gut microbiota and immune development. That is, most of our insights into interactions between the microbiome and external variables are correlative in nature. As we progress toward causal studies of the microbiome using germ-free animal models, insights from large observational studies will translate into mechanistic insights. In our 2018 study, we demonstrated altered bacterial community compositions correlated with various measurements of immune activation. Here, we further interrogated those interactions by fitting models between specific bacterial taxa and feeding mode (Figure 1) and immune marker expression (Figure 2). However, whether altered bacterial communities elicited immune activation themselves, or if other variables between feeding practices induced immune activation, which in turn altered the associated bacterial communities, remains unknown. Our original study and further work here reveal that a link exists between these variables, but the directionality of these interactions remains an open question. Further efforts to interrogate these interactions in animal models may enable the fine-scale resolution needed to pinpoint mechanistic interactions and infer directionality between specific bacterial strains and immune activation. Validation of these models in additional cohorts will be imperative to determine the generality of these interactions between gut bacterial associates and immune responses.
As with any bacterial microbiome study, these data represent one piece of an incredibly elaborate network of interactions between bacteria, fungi, viruses, and the immune response. It is likely that these other microbial communities, as well as various environmental factors, act as determinants of gut bacterial succession and community dynamics. Our collective analyses on this cohort have begun to elucidate mechanistic links between the reduction in HIV susceptibility associated with exclusive breastfeeding versus nonexclusive breastfeeding in African infants. With this establishment, future studies will help to mitigate the risks associated with nonexclusive breastfeeding for all infants, regardless of HIV-exposure or geographic location. The bacteria included in models illustrated here (Balance 1) may guide targets for therapeutic interventions to mitigate the negative effects of NEBF. Our results, in conjunction with the growing body of literature analyzing the gut microbiota of EBF and NEBF infants, 10,12,13,43 nominate Bifidobacteria as an attractive target for potential probiotics and therapeutics. Ultimately, insights gained from previous and future studies can be applied toward the improvement of health outcomes for all infants for which exclusive breastfeeding is not possible.
Funding Statement
This work was supported by the Center for AIDS Research, University of Washington [AI027757]; National Institutes of Health [K08 HD069201].
Data availability
The data and R scripts required to reproduce the analyses used in this study are available at https://github.com/itsmisterbrown/GutMicrobes_analyses
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ.. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171(7):647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. 2016;7(1):5–14. doi: 10.1017/S2040174415001233. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth – first 1000 days and beyond. Trends Microbiol. 2019;27(2):131–147. doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, Perez-Bravo F, Memon A, Gimeno SG, Wadsworth EJK, et al. Breast-feeding and childhood-onset type 1 diabetes: a pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35(11):2215–2225. doi: 10.2337/dc12-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2002;8:CD003517. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14(1):1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokulich NA, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, Helman LJ, Kastan MB, Knapp DW, Levin WJ, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, Bender JM, Azad MB, Thompson AL, Weiss ST, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):4169. doi: 10.1038/s41467-018-06473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood LF, Brown BP, Lennard K, Karaoz U, Havyarimana E, Passmore J-AS, Hesseling AC, Edlefsen PT, Kuhn L, Mulder N, et al. Feeding-Related Gut Microbial Composition Associates With Peripheral T-Cell Activation and Mucosal Gene Expression in African Infants. Clin Infect Dis. 2018;67(8):1237–1246. doi: 10.1093/cid/ciy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Praveen P, Jordan F, Priami C, Morine MJ. The role of breast-feeding in infant immune system: a systems perspective on the intestinal microbiome. Microbiome. 2015;3:41. doi: 10.1186/s40168-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, Herman D, Wang M, Donovan SM, Chapkin R. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13(4):r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNICEF . For every child, end AIDS–seventh stocktaking report. New York, NY: UNICEF; 2016. [Google Scholar]
- 15.Shapiro RL, Kitch D, Ogwu A, Hughes MD, Lockman S, Powis K, Souda S, Moffat C, Moyo S, McIntosh K, et al. HIV transmission and 24-month survival in a randomized trial of HAART to prevent MTCT during pregnancy and breastfeeding in Botswana (The Mma Bana Study). AIDS. 2013;27(12):1911. doi: 10.1097/QAD.0b013e32836158b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell M-L. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369(9567):1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 17.Kourtis AP, Ibegbu CC, Wiener J, King CC, Tegha G, Kamwendo D, Kumwenda J, Kaur SP, Flax V, Ellington S, et al. Role of intestinal mucosal integrity in HIV transmission to infants through breast-feeding: the BAN study. J Infect Dis. 2013;208(4):653–661. doi: 10.1093/infdis/jit221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunney KM, Iliff P, Mutasa K, Ntozini R, Magder LS, Moulton LH, Humphrey JH. Associations between breast milk viral load, mastitis, exclusive breast-feeding, and postnatal transmission of HIV. Clin Infect Dis. 2010;50(5):762–769. doi: 10.1086/650535. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn L, Kim H-Y, Walter J, Thea DM, Sinkala M, Mwiya M, Kankasa C, Decker D, Aldrovandi GM. HIV-1 concentrations in human breast milk before and after weaning. Sci Transl Med. 2013;5(181):181ra51–181ra51. doi: 10.1126/scitranslmed.3005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–67. [DOI] [PubMed] [Google Scholar]
- 22.Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, Sogin ML, Li H, Moore JH, Karagas MR. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016;170(3):212–219. doi: 10.1001/jamapediatrics.2015.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, et al. Isometric logratio transformations for compositional data analysis. Math Geol. 2003;35(3):279–300. doi: 10.1023/A:1023818214614. [DOI] [Google Scholar]
- 25.Egozcue JJ, Pawlowsky-Glahn V. Groups of parts and their balances in compositional data analysis. Math Geol. 2005;37(7):795–828. doi: 10.1007/s11004-005-7381-9. [DOI] [Google Scholar]
- 26.Aitchison J. The statistical analysis of compositional data. J R Stat Soc. 1982;44:139–177. doi: 10.1111/rssb.1982.44.issue-2. [DOI] [Google Scholar]
- 27.Witten DM, Tibshirani R, Hastie T. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics. 2009;10(3):515–534. doi: 10.1093/biostatistics/kxp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc. 1996;58:267–288. doi: 10.1111/rssb.1996.58.issue-1. [DOI] [Google Scholar]
- 29.Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105(48):18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis-Richardson AG, Yang C-L, Ge M-Y, Ibrahim M, Li B, Zhao W-J, Chen G-Y, Zhu B, Xie G-L. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol. 2014;5:678. doi: 10.3389/fmicb.2014.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen A-M, et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165(4):842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heida FH, van Zoonen AGJF, Hulscher JBF, Te Kiefte BJC, Wessels R, Kooi EMW, Bos AF, Harmsen HJM, de Goffau MC. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin Infect Dis. 2016;62(7):863–870. doi: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 34.Chua HH, Chou H-C, Tung Y-L, Chiang B-L, Liao -C-C, Liu -H-H, Ni Y-H. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology. 2018;154(1):154–167. doi: 10.1053/j.gastro.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Begaud E, Chartier L, Marechal V, Ipero J, Léal J, Versmisse P, Breton G, Fontanet A, Capoulade-Metay C, Fleury H, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286(5443):1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 37.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, Abou M, Westmacott GR, McCorrister S, Kwatampora J, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016;9(1):194–205. doi: 10.1038/mi.2015.51. [DOI] [PubMed] [Google Scholar]
- 38.Masson L, Passmore J-AS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, Williamson C, Little F, Mansoor LE, Naranbhai V, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61(2):260–269. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardeshir A, Narayan NR, Méndez-Lagares G, Lu D, Rauch M, Huang Y, Van Rompay KKA, Lynch SV, Hartigan-O’Connor DJ. Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci Transl Med. 2014;6(252):252ra120. doi: 10.1126/scitranslmed.3008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145. doi: 10.1136/bmj.j5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EMM. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4(4):325–339. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, Wilde JCH, de Vries N, van Lier RAW, Kootstra N, Pals ST, et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120(22):4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 43.Turroni F, Milani C, Duranti S, Ferrario C, Lugli GA, Mancabelli L, van Sinderen D, Ventura M. Bifidobacteria and the infant gut: an example of co-evolution and natural selection. Cell Mol Life Sci. 2018;75(1):103–118. doi: 10.1007/s00018-017-2672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and R scripts required to reproduce the analyses used in this study are available at https://github.com/itsmisterbrown/GutMicrobes_analyses


