ABSTRACT
Global water shortage seriously threatens rice growth especially in irrigated production areas. Association of plants with beneficial soil microbes is one strategy for plant adaption to environmental stresses. In this study, rice (Oryza sativa L.) plants were colonized by the beneficial root-colonizing endophytic fungus Piriformospora indica (P. indica). We demonstrate that grain yield were higher in P. indica-colonized rice plants compared to the uncolonized plants grown in soil. Moreover, P. indica effect on improving water stress tolerance in rice and its physiological mechanism were investigated in a hydroponic culture system. Polyethylene glycol (PEG) was applied to the culture solution to conduct the water stress condition. Water stress-induced leaf wilting and impairments in photosynthetic efficiency were diminished in P. indica-colonized plants. Furthermore, P. indica colonization promotes stomata closure and increases the leaf surface temperature under water stress. The malondialdehyde level (as an indicator for oxidative stress) was lower and the reduced to oxidized glutathione ratio was higher in P. indica-colonized and PEG-exposed rice plants compared to the uncolonized plants. Furthermore, the activities of the antioxidant enzymes catalase and glutathione reductase were up-regulated in inoculated rice seedlings under water stress. In conclusion, P. indica promotes rice performance under water stress by stomata closure and lower oxidative stress.
KEYWORDS: Rice (Oryza sativa L.), Piriformospora indica, water stress, stomata, oxidative stress
Introduction
Sufficient water supply is important to maintain agricultural productivity. Rice belongs to the most dehydration sensitive agricultural plants worldwide. Plants have developed numerous mechanisms to adapt to drought stress, such as increased root lengths promote water uptake and reduced leaf areas diminish transpiration rates. Faster completion of the life cycle is another strategy to respond to drought stress.1,2 Furthermore, water deficiency induces stomatal closure, which reduces CO2 availability and photosynthetic efficiency.3 Limitation of CO2 fixation under high light conditions accelerates photoinhibition and generates reactive oxygen species (ROS), such as H2O2 in chloroplast,4 which cause oxidative damage of proteins, DNA and lipids.5 This results in the activation of ROS-scavenging systems including the antioxidative enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), as well as the antioxidants ascorbate and glutathione which protect plants from oxidative damage under drought stress.6-9
In natural ecosystems, plants continuously interact with a range of soil microorganisms. Mutualistic symbiosis is beneficial for both microbes and plants in plant-microbe interactions.10-12 Endophytic fungi can improve nutrient (such as nitrogen and phosphorus) uptake into the host plants, while the plants provide carbon sources to fungi.13 Piriformospora indica (P. indica) is a well-studied root-colonizing endophytic fungi, and its beneficial effect on plant growth and performance has been described for many plant species including agriculturally important crop plants such as maize, barley and Chinese cabbage.14-16 Beneficial effects of P. indica on plant growth included promotion of root development, seed production, early flowering and secondary metabolite biosynthesis.17 P. indica-colonized barley alleviates the infection of root pathogens.15 In barley, rice, Medicago, Arabidopsis, Chinese cabbage and maize, it was shown that the P. indica-colonized plants performed better under salt or drought stresses.15,18-22 The improved salt tolerance of P. indica-colonized plants was associated with increased antioxidative enzyme activities and proline content.15,18,19 Higher chlorophyll content and stimulation of the expression of drought-responsive genes were observed in P. indica-colonized Arabidopsis and Chinese cabbage under drought stress.20,21 In leaf tissues of P. indica-colonized barley, expressions of several genes involved in oxidative stress defense, photosynthesis and energy metabolism were upregulated.23
Drought causes severe environmental stresses on rice production worldwide. The semiaquatic-environmental and farming system for rice growth and production are quite different from those of other cereal crops. This raises the question whether P. indica has similar beneficial effects on this crop plant in soil and also in hydroponic culture systems, and if so which physiological responses are activated by the fungus in the plants to improve drought tolerance.
Materials and methods
P. indica culture
P. indica was cultured on liquid or agar-containing Kaefer medium.24 For culturing on agar medium, a mycelium clump (1 cm diameter) was transferred from a 14-d old P. indica culture on agar medium to a fresh agar medium. For liquid culture, a mycelium clump was transferred into 50 ml liquid Kaefer medium. The fungus on both agar medium and in liquid culture was incubated at 30°C in the dark.
Plant materials and root colonization
Rice (Oryza sativa L. cv. Tainung 67) seeds were sterilized with sodium hypochlorite, and then germinated at 30°C in dark for 3 d.25 Germinated seeds (30 seedlings) were moved to 500 ml of modified Kimura B nutrient solution (pH 5.5),26 and P. indica (3 g) collected from 12-d liquid culture was mixed into the seedling´s culture solution for inoculation. Rice seedlings were grown at 30/25°C in 12 h light/12 h dark photoperiod. Growth stage of seedlings and the leaf order were counted according to leaf development. The 1st leaf which emerged after the coleoptiles was labeled as 1st leaf.27 For rice experiments in soil, seedlings were transferred into pots (16 cm in diameter and 15 cm in height) after co-cultured with P. indica for 18 d. Three rice plants were planted in one pot and grown in a Phytotron at 30/25°C (day/night) under natural daylight. P. indica spores in colonized rice roots were detected by methyl blue-staining for confirming symbiosis.
Water stress treatment
Experiment 1: The method for the evaluation of dehydration tolerance was modified from Lu et al. (2009).28 P. indica-colonized (and uncolonized control) seedlings were removed from the culture solution and exposed to an airflow for 9.5 hr. Then, the seedlings were rehydrated for 7 d before the survival rate was calculated.
Experiment 2: Seedlings at the 4-leaf-stage were transferred to a 29% (w/v) polyethylene glycol 6000 (PEG)-containing Kimura B solution (equivalent to the water potential of −1 MPa). The water potential was measured by the PSYPRO Water Potential System (RS-232, Wescor Inc., USA). After PEG treatment for 4 d, tissue samples were collected for biomass and various further analyzes (cf. below).
Stomata apertures and thermal imaging
For the observation of stomata apertures, the nail polish imprints method was followed as described by Voleníková and Tichá (2001).29 The status of the stomata on the abaxial epidermis of the 4th-leaf of rice seedlings was observed under a light microscope (Axio Imager. M1, Zeiss, Germany). Thirty stomata per leaf located at the middle of a leaf (1 cm of length) were observed in each leaf sample. Thermal images of the leaves were obtained using the InfraRed Camera R300SR (NIPPON AVIONICS CO., LTD, Japan), and the leaf temperature was analyzed by the TAS Version 21.1B software (Ching Hsing Computer-Tech LTD., Taiwan).
Chlorophyll fluorescence measurement
Chlorophyll fluorescence was measured using the MAXI-Imaging-PAM equipment (WALZ, Germany). Maximum quantum yield (Fv/Fm) of photosystem II was determined after dark acclimation for 30 min.
Malondialdehyde (MDA) content analysis
Leaf tissues (0.1 g) were homogenized with 4 ml of 5% (w/v) trichloroacetic acid (TCA) before centrifugation at 10,000 x g for 5 min at 20°C. The supernatant was mixed with 20% (w/v) TCA and 0.5% (w/v) thiobarbituric acid before incubation at 95°C for 30 min. Following, the mixture was centrifuged for 10 min at 3,000 x g, and then the absorbance of the supernatant was detected at 532 nm.30
Determination of reduced ascorbate (AsA) and dehydroascorbate (DHA)
Leaf samples (0.1 g) were ground with 1 ml of 5% (w/v) TCA before centrifugation at 15,000 x g. The supernatant was used to measure AsA and DHA according the procedure described by Law et al. (1983).31
Assay for reduced (GSH) and oxidized glutathione (GSSG)
Leaf samples (50 mg) were ground with 1 ml sulfosaliylic acid (5%, w/v), and the supernatant of the crude extract was collected after centrifugation at 15,000 x g for 10 min. The GSH and GSSG contents were determined by the method of Smith et al. (1985).32
Antioxidant enzyme activity assays
For catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) enzyme activity analyses, leaf samples (0.1 g) were homogenized with 50 mM sodium phosphate buffer (pH 6.8). The supernatant of the crude extract was collected and used for the determination of the enzyme activities. CAT activity was measured by the method of Kato and Shimizu (1987).33 One unit of CAT was defined as the amount of enzyme which degrades 1 μmole H2O2 per min. APX activity was measured by the method of Nakano and Asada (1981).34 One unit of APX activity was defined as the amount of enzyme that degrades 1 μmol of AsA per min, the decrease of AsA was detected at 290 nm. GR was determined according to Foster and Hess (1980),35 and one unit of GR was estimated as the amount of enzyme that degrades 1 μmol of NADPH per min. The decrease of the NADPH concentration was recorded at 340 nm. For SOD activity, leaf samples were extracted by potassium phosphate buffer (50 mM, pH 7.8) containing 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride and 1% (w/v) polyvinylpyrrolidone. After centrifugation, the supernatant was used to determine SOD activity according to the method of Beauchamp and Fridovich (1971).36 The reaction mixture (1.5 ml) contained 25 µl of sample solution, 875 µl of 50 mM potassium phosphate buffer (pH 7.8), 150 µl of methionine (130 mM), 150 µl of nitro-blue tetrazolium chloride (NBT) (75 µM), 150 µl of EDTA (100 µM) and 150 µl of riboflavin (2 µM). The reaction was performed for 10 min before the absorbance was detected at 560 nm. The definition of a unit of SOD activity was the amount of protein that inhibits 50% NBT reduction per min. For all samples, the protein concentration was determined according to Bradford (1976).37
Statistical analysis
The influence of P. indica colonization on the heading time and grain yield of rice plants were investigated in three independent experiments. Results presented here were one of three independently experiments with identical results, and the data are based on 21 biological repeats. All other results are the means of three independent experiments. Significant differences between samples were analyzed by Student’s t-test.
Results
Effects of P. indica colonization on rice grain yield
Figure 1a demonstrates that the roots of rice seedlings are colonized by P. indica under our co-cultivation conditions. We first checked whether P. indica colonization has an influence on the grain production of the rice plants grown in pots in a Phytotron under natural light conditions. P. indica-colonized rice plants had a higher number of panicles per plant (Table 1). All grains were collected from panicles. Before counting the filled grain number, filled and unfilled grains were separated by a seed blower. The results showed the number of filled grain per P. indica-colonized plant was 30% higher than that in uncolonized plants (Table 1). Moreover, the total grain weight of P. indica-colonized plant was 1.3-fold higher than that of uncolonized plants (Table 1). Following, ten filled grains from a plant were grouped and the weight were measured, however, the 10-grains weight was not significant different between the colonized and uncolonized plants (Table 1). It was indicated that the average weight of a grain from P. indica-colonized plant was similar to that from uncolonized plant. In addition, the heading time of P. indica-colonized rice plants were about 4 d earlier than those of the non-inoculated plants (Table 1). Heading time was calculated from the first day that plant was transplanted into soil pot until the panicle began to emerge from the leaf sheath of flag leaf. These results demonstrate that the fungus promotes rice grain yield under non-stress conditions.
Figure 1.
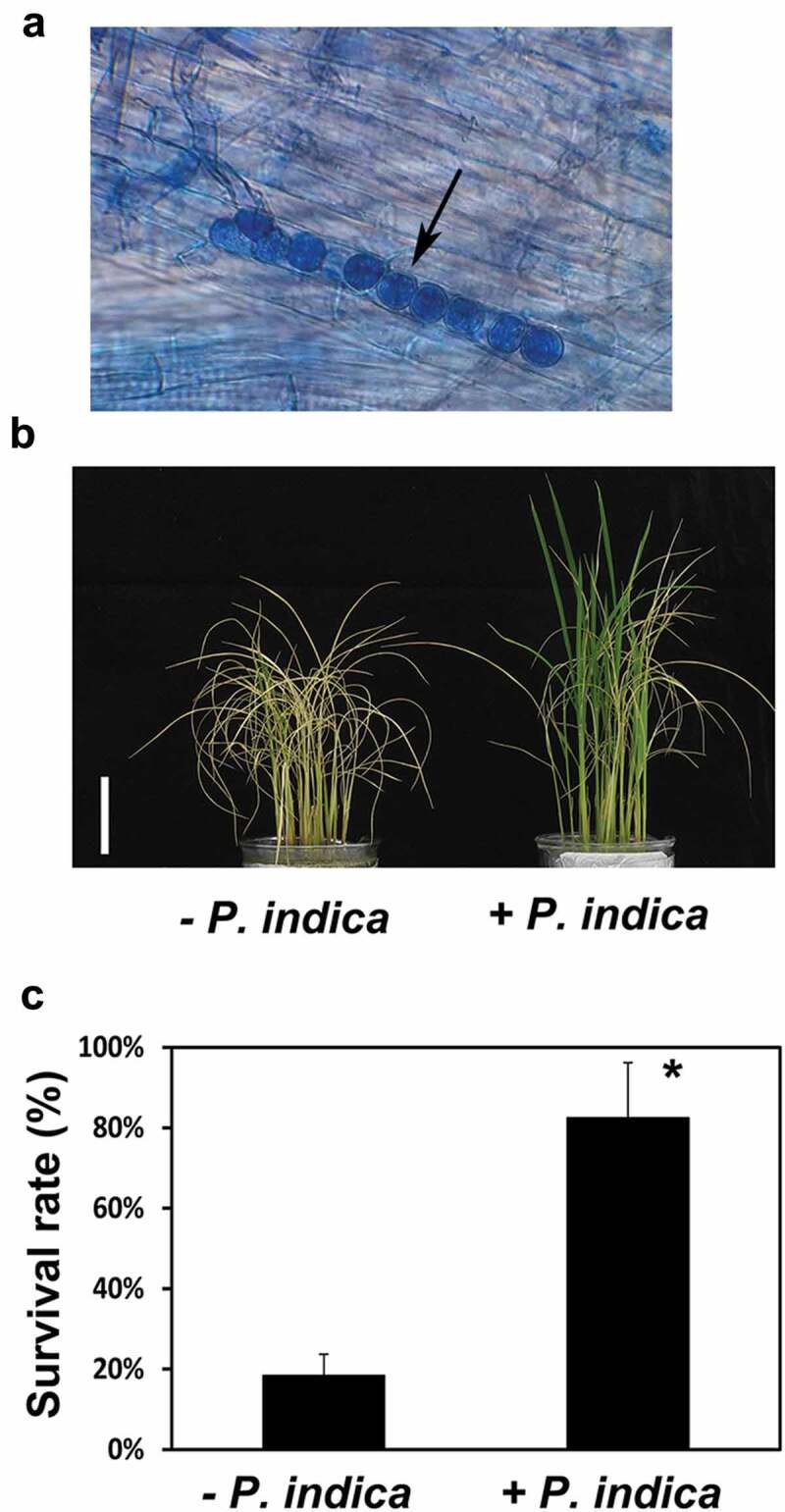
Effects of P. indica colonization on rice growth in dehydration conditions. (a) Efficient root colonization of rice seedlings. Fungal spores can be detected in root tissues of rice plants which were inoculated with P. indica for 12 d. Arrow indicated P. indica spores. Phenotype (b) and survival rate (c) of P. indica-colonized and non-colonized rice plants after 9.5 h drought treatment and 7 d rehydration. Scale bar: 5 cm. * indicates significant difference (* p <0.05).
Table 1.
Heading time and grain yield of rice plants with and without P. indica inoculation.
| Heading time (d) | No. of panicle per plant | No. of filled grains per plant | Grain weight (g) per plant | Ten-grain weight (g) | |
|---|---|---|---|---|---|
| Control | 97.38 ± 0.57 | 1.76 ± 0.10 | 73.90 ± 5.11 | 1.99 ± 0.13 | 0.27 ± 0.00 |
| P. indica inoculation | 92.57 ± 1.25*** | 2.05 ± 0.05** | 96.10 ± 6.18** | 2.54 ± 0.17** | 0.27 ± 0.00 |
Data shown here were from one of three independently repeated experiments and presented as mean ± SE (n = 21). * indicates significant difference (** p < 0.01, *** P< 0.001).
Impact of P. indica colonization on rice drought tolerance
To evaluate the effect of P. indica colonization on drought tolerance of rice plants, we performed first dehydration experiments as described in the Methods and Materials (Experiment 1). Rice seedlings from culture solutions were used for the dehydration treatment, and then seedling´s survival rate was determined after re-watering. Most of the leaves of the non-inoculated plants were wilting or many of them were already dead, while the plants pre-inoculated with P. indica were still green and performed visibly better (Figure 1b). The survival rate of P. indica-colonized rice seedlings was about 82.5% after they were transferred back to culture solutions, while only 18.4% of the non-colonized seedlings survived (Figure 1c).
The effect of P. indica on the tolerance against osmotic stress was also investigated by exposing rice plants to PEG, as described in Experiment 2 (cf. Methods and Materials). In control experiments without PEG, the fresh weights of the shoot and root of P. indica-colonized rice plants were 13.8% and 21.3% higher than those of the uncolonized plants (Figure 2a,b). In PEG-treated conditions, the shoot and root weight of plants colonized with P. indica were increased 25.8% and 25.2% compared to uncolonized plants (Figure 2a,b). Plant growth was strongly repressed by the PEG treatment, and the shoot and root fresh weights of the uncolonized plants were reduced by 60.8% and 47.0%, respectively, after PEG treatment (Figure 2a,b). P. indica-colonized plants exposed to the PEG condition showed a 56.7% decline in shoot weight and 45.2% decline in root weight (Figure 2a,b). Furthermore, non-colonized seedlings showed severe leaf wilting at the 4th-leaf after exposure to PEG for 4 d, while the leaves of P. indica-colonized plants remained green (Figure 2c). Without water stress, the Fv/Fm values of non-colonized and P. indica-colonized plants were about 0.79 and 0.80, respectively. After PEG treatment for 4 d, the Fv/Fm value of the non-colonized seedlings decreased to 0.60, while the value for P. indica-colonized seedlings was 0.76 (Figure 2d). These results indicate that P. indica colonization reduces the negative effects of water stress on the performance of rice plants.
Figure 2.

Changes in biomass and photosynthetic efficiency of P. indica-colonized plants under PEG treated conditions. (a) Shoot fresh weight of non-colonized and P. indica-colonized rice plants after PEG treatment for 4 d. (b) Fresh weight of roots. (c) Leaf damage in non-colonized and P. indica-colonized rice plants after PEG treatment. Arrows indicate the 4th leaf. Scale bar: 5 cm. (d) Fv/Fm values of the 4th-leaf of rice seedlings. * indicates significant difference (* p < 0.05, ** p < 0.01).
Changes in drought-induced stomatal closure in P. indica-colonized plants
Stomata closure was measured on the 4th leaf of the plants 2 h after PEG application. Under non-stress conditions, the percentage of completely closed stomata was not significantly different between P. indica-colonized and uncolonized plants (Figure 3). After PEG treatment, 41.1% of the stomata were completely closed in the uncolonized plants, whereas 50.2% were closed in the colonized plants (Figure 3). Furthermore, the surface temperature on the 4th leaf, detected by an infrared camera (Figure 4a), of the colonized plants was about 0.5°C higher than that of the uncolonized plants (Figure 4b). This indicates that a beneficial effect of P. indica is already detectable in the leaves 2 h after the exposure of the rice plants to drought stress.
Figure 3.

Effects of P. indica colonization on stomatal closure under water stress. * indicates significant difference (* p < 0.05).
Figure 4.

Effects of P. indica colonization on leaf temperature under water stress. (a) Thermal image of P. indica-colonized and non-colonized rice plants after PEG treatment for 2 h. (b) Changes in leaf temperature of the 4th-leaf after plants were treated with PEG for 2 h. * indicates significant difference (* p < 0.05).
Effects of P. indica colonization on antioxidant system in water-stressed plants
The product of lipid peroxidation, MDA, was used as a biomarker to monitor the level of oxidative stress. Figure 5 shows that the MDA level in leaf tissues increased after PEG treatment. The MDA level was lower when the roots were colonized by P. indica, both under normal and water-stress conditions (Figure 5). Next, changes of non-enzymatic and enzymatic ROS scavengers were assayed in the leaf tissues. In both P. indica colonized and uncolonized plants, the ASA levels decreased and the DHA levels slightly increased in PEG-treated plants. Consequently, the ASA/DHA ratio was significantly lower in PEG-exposed plants compared to non-stressed plants (Figure 6). P. indica colonization had no effect on the AsA and DHA levels in the leaves (Figure 6). On the other hand, the GSH level increased and the GSSG level decreased in the leaves of P. indica-colonized and PEG-exposed plants, while no differences were observed without stress (Figure 6). Thus, the higher GSH/GSSG ratio in P. indica-colonized and stress-exposed plants (Figure 6) indicates that the fungus promotes the antioxidant capacity.
Figure 5.

Effects of P. indica colonization on MDA content under water stress. The MDA content were detected in leaf samples harvested after PEG treatment for 4 d. * indicates significant difference (* p < 0.05).
Figure 6.

Effects of P. indica colonization on ascorbate and glutathione levels of leaf tissues after PEG application. Ascorbate and glutathione were analyzed after plants were treated with PEG for 4 d. ASA: ascorbate, DHA: dehydroascorbate, GSH: reduced glutathione, GSSG: oxidized glutathione. * indicates significant difference (* p < 0.05, ** p < 0.01).
To further reveal the effects of P. indica on antioxidant enzymes activities under water stress, the activities of SOD, CAT, APX and GR were analyzed. Figure 7 shows that the fungus stimulated the CAT and GR, but not SOD and APX activities in leaves of stress-exposed seedlings.
Figure 7.

Effects of P. indica colonization on antioxidative enzyme activities under water stress. Enzyme activities of SOD, CAT, APX and GR in leaves of P. indica-colonized and non-colonized rice plants were determined after PEG treatment for 4 d. SOD: superoxide dismutase, CAT: catalase, APX: ascorbate peroxidase, GR: glutathione reductase. * indicates significant difference (* p < 0.05).
Discussion
P. indica enhanced rice yield
P. indica-induced root growth and alterations in the root architecture were observed in Chinese cabbage, Arabidopsis, maize and rice.16,38-40 We demonstrate that in rice plants, the root biomass was also promoted by P. indica (Figure 2b). Acceleration of root development enhances nutrients and water uptake, which subsequently results in promotion of the aerial parts of the plants. In Arabidopsis and tobacco roots, P. indica stimulated the expression of enzymes involved in nitrate metabolism including nitrate reductase,41 and a positive effect of P. indica on phosphate uptake was shown in maize.42 Achatz et al. (2010) reported that P. indica enhanced grain yield of barley,43 and this was caused by growth promotion at early development stages. In our study, the panicle and filled grain number of P. indica-colonized rice plants were significantly higher compared to non-inoculated control plants, whereas the ten-grain weight was not changed (Table 1). This indicates that the higher grain yield in P. indica-colonized rice plants is caused by promoting panicle formation and grain filling. In barley, P. indica colonization increased the number of ears per plant.43 Filling efficiency of rice spikelet is dependent on photosynthesis of the mature leaves during grain filling and the mobilization of carbohydrate in the leaf sheath and culm of the upper leaves.44,45 Stimulation of the carbohydrate metabolism and expression of sugar transporter genes was described for several plant species associated with mycorrhizal fungi.46,47 The heading time of P. indica-colonized rice plants was 4 d earlier than that of non-colonized plants, which suggests that P. indica promotes the transition from the vegetative to the reproductive stage. All these data suggest that P. indica promotes developmental processes such as panicle formation by stimulating the translocation of resources to the sink tissues. This is also consistent with earlier flowering which was observed for P. indica-colonized Coleus forskohlii and Arabidopsis plants,48,49 and Kim et al. (2017) suggested that P. indica promotion in Arabidopsis is mediated by gibberellin.49
P. indica improved water stress tolerance in rice plants
Under PEG-induced water stress conditions, P. indica-colonized rice plants showed less drought-caused leaf wilting and maintained higher biomass than non-colonized plants (Figure 2). We demonstrate that P. indica increases water stress tolerance of rice plants by modulating stomata closure and leaf temperature after PEG treatment (Figures 3, 4), which are indicators for water deficit.50 Regulation of stomata closure is mediated by abscisic acid (ABA), H2O2, nitric oxide, and involves Ca2+ signaling.3,7,51 The expression of a Ca2+-sensing regulator required for stomata closure was up-regulated in leaves of P. indica-colonized Chinese cabbage.21 In snapdragon, it was observed that arbuscular mycorrhizal fungi colonization can help to maintain higher water potential and relative water content of host plants under water deficient conditions.52
Furthermore, water stress impairs the electron transport in photosynthesis and induces ROS production.53 The chlorophyll fluorescence was used to monitor the maximum quantum efficiency of PSII photochemistry.54 P. indica-colonized rice showed better photosynthetic efficiency than non-colonized plants in PEG-induced water stresses (Figure 2d). Thus, the fungus reduces PSII damage under water stresses. Consistent with these observations, Saddique et al. (2018) observed that P. indica colonization stabilizes grana in chloroplast.55 ROS scavenging is one of the first mechanisms to protect the photosystems from oxidation damage.3 The generation of MDA, a product of lipid peroxidation, is regarded as an indicator of drought-induced oxidative damage.56 Since P. indica-colonized rice plants showed lower MDA accumulation (Figure 5), the fungus reduces the level of ROS-induced lipid peroxidation. Furthermore, abiotic stress tolerance is caused by higher antioxidative capacity of the stress-exposed plants. SOD, which catalyzes the dismutation of O2− to H2O2,57 was no significantly affected by P. indica colonization in leaf tissues (Figure 7). However, activities of CAT, which converts H2O2 to H2O, increased in the leaves of P. indica-colonized and water-stressed seedlings (Figure 7). CAT is located in peroxisomes and indispensable for ROS scavenging especially under stress.58,59 APXs have different affinities for H2O2 than CATs. Since APX activity was not affected by P. indica (Figure 7), CAT appears to be the major target of the fungus for H2O2 detoxification under PEG stress. Furthermore, the GR activity was increased in leaves of P. indica-colonized rice seedling under PEG treatment (Figure 7). GR catalyzes the ascorbate-glutathione cycle, maintains a proper GSH/GSSG redox ratio in cells and is necessary for ascorbate regeneration.57,60,61 In Arabidopsis, stomata aperture is reduced after exogenous application of GSH. This correlated with higher ABA levels and let to the idea that GSH-induced stomata behavior is mediated via ABA signaling.62 Our results showed that the GSH content and the GSH/GSSG ratio were higher in leaves of P. indica-inoculated plants than those in non-inoculated plants (Figure 6). However, how this is related to the more efficient stomatal closure in stress-exposed plant colonized by P. indica, requires further investigations.
The beneficial effect of P. indica on the performance of osmotic stress-exposed rice plants has been repeatedly reported, either by the application of salt,18,63-65 PEG55 or heavy metals.66-68 In all instances, ROS scavenging compounds, as well as mechanisms for the protection of photosynthesis are targets of the endophyte. However, the mechanisms by which P. indica diminishes osmotic stress in the hosts and how this information travels from the colonized roots to the aerial parts is still enigmatic. Recent investigations demonstrate that the plant response to osmotic stress is quite different for different stress treatments. For instance, Darko et al. (2019) showed that various osmolytes activate different metabolic processes even under iso-osmotic conditions and these changes also differ in the leaves and roots.69 Elmaghrabi et al (2019) demonstrated quite different gene expression profiles in response to salt and osmotic stresses in Medicago truncatula.70 Similar results were reported for osmotic and ionic stress conditions by Fatemi et al. (2019).71 Thus, it is important to understand which are the key regulatory targets of the fungus for improving water stress tolerance of rice and which of them are activated in response to a given stimulus. Here, we focused on PEG-induced responses, however, the experimental set-up now allows also the identification of the P. indica targets induced by other stressors, such as salt or heavy metal stress.
Drought stress responses have been investigated in numerous other plant systems colonized by root-colonizing fungi.72 The beneficial effects for plants might dependent on the colonization efficiency which is both plant- and genotype-specific.40,73 Kord et al. (2019) demonstrated that miRNAs participate in P. indica-mediated salt tolerance in rice.65 Putative target mRNAs encoded proteins involved in nutrient uptake, sodium transporters, growth regulators, auxin-responsive proteins, transcription factors involved in the import of potassium ions or the export of sodium ions into the root cells were identified.74-81 The amounts of phytohormones, ROS scavenging enzymes, metabolites, phosphatases, kinases, ion, proton and metabolite transporters, osmolytes and lipids, as well as the genes for their biosyntheses, are targeted by beneficial fungi to promote osmotic stress tolerance responses in various symbiotic interactions including rice.18,40,63,82,83
In summary, P. indica colonization promotes rice growth and grain production. The fungus also protects the plants against drought stress. We observed that the colonized plants exhibited higher ROS scavenging efficiency and faster stomatal closure under PEG-induced osmotic stress conditions. The co-cultivation conditions allow to identify the primary targets of the fungus as well as the signals involved in root-to-shoot signaling.
Funding Statement
This research was supported by grant [MOST104-2311-B-002-010 and 105-2311-B-002-003] from the Ministry of Science and Technology in Taiwan.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ngara R, Ndimba BK.. Understanding the complex nature of salinity and drought-stress response in cereals using proteomics technologies. Proteomics. 2014;14:1–10. doi: 10.1002/pmic.201300351. [DOI] [PubMed] [Google Scholar]
- 2.Kooyers NJ. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015;234:155–162. doi: 10.1016/j.plantsci.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandra Reddy A, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Tripathy BC, Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 7.Osakabe Y, Osakabe K, Shinozaki K, Tran LS. Response of plants to water stress. Front Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kogel KH, Franken P, Huckelhoven R. Endophyte or parasite-what decides? Curr Opin Plant Biol. 2006;9:358–363. doi: 10.1016/j.pbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Johnson J, Oelmüller R. Mutualism or parasitism: life in an unstable continuum. What can we learn from the mutualistic interaction between Piriformospora indica and Arabidopsis thaliana. Endocyt Cell Res. 2009;19:81–111. [Google Scholar]
- 12.Singh LP, Gill SS, Tuteja N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav. 2011;6:175–191. doi: 10.4161/psb.6.2.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 14.Varma A, Verma S, Sahay N, Butehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol. 1999;65:2741–2744. doi: 10.1128/AEM.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YC, Johnson JM, Chien CT, Sun C, Cai D, Lou B, Oelmüller R, Yeh KW. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Mol Plant Microbe Interact. 2011;24:421–431. doi: 10.1094/mpmi-05-10-0110. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J, Alex T, Oelmüller R. Piriformospora indica: the versatile and multifunctional root endophytic fungus for enhanced yield and tolerance to biotic and abiotic stress in crop plants. J Trop Agric. 2014;52:103–122. [Google Scholar]
- 18.Jogawat A, Saha S, Bakshi M, Dayaman V, Kumar M, Dua M, Varma A, Oelmüller R, Tuteja N, Johri AK. Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signal Behav. 2013;8:e26891. doi: 10.4161/psb.26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Li L, Wang X, Zhu P, Wu H, Qi S. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol Biochem. 2017;119:211–223. doi: 10.1016/j.plaphy.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Sherameti I, Tripathi S, Varma A, Oelmuller R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Mol Plant Microbe Interact. 2008;21:799–807. doi: 10.1094/mpmi-21-6-0799. [DOI] [PubMed] [Google Scholar]
- 21.Sun C, Johnson JM, Cai D, Sherameti I, Oelmuller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol. 2010;167:1009–1017. doi: 10.1016/j.jplph.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Wang A, Wang J, Wei Q, Zhang W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017;5:251–258. doi: 10.1016/j.cj.2016.10.002. [DOI] [Google Scholar]
- 23.Ghabooli M, Khatabi B, Ahmadi FS, Sepehri M, Mirzaei M, Amirkhani A, Jorrin-Novo JV, Salekdeh GH. Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J Proteomics. 2013;94:289–301. doi: 10.1016/j.jprot.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Hill T, Käfer E. Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet Newsl. 2001;48:20–21. doi: 10.4148/1941-4765.1173. [DOI] [Google Scholar]
- 25.Chen HJ, Chen JY, Wang SJ. Molecular regulation of starch accumulation in rice seedling leaves in response to salt stress. Acta Physiol Plant. 2008;30:135–142. doi: 10.1007/s11738-007-0101-y. [DOI] [Google Scholar]
- 26.Chu C, Lee T. The relationship between ethylene biosynthesis and chilling tolerance in seedlings of rice (Oryza sativa L.). Bot Bull Acad Sin. 1989;30:263–273. [Google Scholar]
- 27.Chang YA, Dai NC, Chen HJ, Tseng CH, Huang ST, Wang SJ. Regulation of rice sucrose transporter 4 gene expression in response to insect herbivore chewing. J Plant Inteact. 2019;14:525–532. doi: 10.1080/17429145.2019.1662099. [DOI] [Google Scholar]
- 28.Lu G, Gao C, Zheng X, Han B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta. 2009;229:605–615. doi: 10.1007/s00425-008-0857-3. [DOI] [PubMed] [Google Scholar]
- 29.Voleníková M, Tichá I. Insertion profiles in stomatal density and sizes in Nicotiana tabacum L. plantlets. Biol Plant. 2001;44:161–165. doi: 10.1023/a:1017982619635. [DOI] [Google Scholar]
- 30.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 31.Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith IK, Kendall AC, Keys AJ, Turner JC, Lea PJ. The regulation of the biosynthesis of glutathione in leaves of barley (Hordeum vulgare L.). Plant Sci. 1985;41:11–17. doi: 10.1016/0168-9452(85)90059-7. [DOI] [Google Scholar]
- 33.Kato M, Shimizu S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot. 1987;65:729–735. doi: 10.1139/b87-097. [DOI] [Google Scholar]
- 34.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 35.Foster JG, Hess JL. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980;66:482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 38.Sirrenberg A, Gobel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Santos P, Feussner I, Pawlowski K. Piriformospora indica affects plant growth by auxin production. Physiol Plant. 2007;131:581–589. doi: 10.1111/j.1399-3054.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 39.Kumar M, Yadav V, Tuteja N, Johri AK. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology. 2009;155:780–790. doi: 10.1099/mic.0.019869-0. [DOI] [PubMed] [Google Scholar]
- 40.Bertolazi AA, de Souza SB, Ruas KF, Campostrini E, de Rezende CE, Cruz C, Melo J, Colodete CM, Varma A, Ramos AC. Inoculation with Piriformospora indica is more efficient in wild-type rice than in transgenic rice over-expressing the vacuolar H+-PPase. Front Microbiol. 2019;10:1087. doi: 10.3389/fmicb.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmuller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem. 2005;280:26241–26247. doi: 10.1074/jbc.M500447200. [DOI] [PubMed] [Google Scholar]
- 42.Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathi T, Tuteja N, Saxena AK, Johri AK. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem. 2010;285:26532–26544. doi: 10.1074/jbc.M110.111021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Achatz B, Kogel KH, Franken P, Waller F. Piriformospora indica mycorrhization increases grain yield by accelerating early development of barley plants. Plant Signal Behav. 2010;5:1685–1687. doi: 10.4161/psb.5.12.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa T, Akita S, Li Q. Relationship between contents of non-structural carbohydrates before panicle initiation stage and grain yield in rice (Oryza sativa L.). Jpn J Crop Sci. 1993;62:130–131. doi: 10.1626/jcs.62.130. [DOI] [Google Scholar]
- 45.Samonte SOP, Wilson LT, McClung AM, Tarpley L. Seasonal dynamics of nonstructural carbohydrate partitioning in 15 diverse rice genotypes. Crop Sci. 2001;41:902–909. doi: 10.2135/cropsci2001.413902x. [DOI] [Google Scholar]
- 46.Doidy J, van Tuinen D, Lamotte O, Corneillat M, Alcaraz G, Wipf D. The Medicago truncatula sucrose transporter family: characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Mol Plant. 2012;5:1346–1358. doi: 10.1093/mp/sss079. [DOI] [PubMed] [Google Scholar]
- 47.Wu HH, Zou YN, Rahman MM, Ni QD, Wu QS. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci Rep. 2017;7:42389. doi: 10.1038/srep42389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, Tuteja N, Johri AK, Varma A. The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal Behav. 2012;7:103–112. doi: 10.4161/psb.7.1.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D, Abdelaziz ME, Ntui VO, Guo X, Al-Babili S. Colonization by the endophyte Piriformospora indica leads to early flowering in Arabidopsis thaliana likely by triggering gibberellin biosynthesis. Biochem Biophys Res Commun. 2017;490:1162–1167. doi: 10.1016/j.bbrc.2017.06.169. [DOI] [PubMed] [Google Scholar]
- 50.Grant OM, Chaves MM, Jones HG. Optimizing thermal imaging as a technique for detecting stomatal closure induced by drought stress under greenhouse conditions. Physiol Plant. 2006;127:507–518. doi: 10.1111/j.1399-3054.2006.00686.x. [DOI] [Google Scholar]
- 51.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 52.Asrar AA, Abdel-Fattah GM, Elhindi KM. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica. 2012;50:305–316. doi: 10.1007/s11099-012-0024-8. [DOI] [Google Scholar]
- 53.Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2011;16:53–60. doi: 10.1016/j.tplants.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- 55.Saddique MAB, Ali Z, Khan AS, Rana IA, Shamsi IH. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice (NY). 2018;11:34. doi: 10.1186/s12284-018-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Noctor G, Foyer CH. Ascorbate and Glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 58.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 59.Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K, Mohamed AA, Thangavel P, Devi GD, Vasudhevan P, et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res Int. 2016;23:19002–19029. doi: 10.1007/s11356-016-7309-6. [DOI] [PubMed] [Google Scholar]
- 60.Meloni DA, Oliva MA, Martinez CA, Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot. 2003;49:69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- 61.Pyngrope S, Bhoomika K, Dubey RS. Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma. 2013;250:585–600. doi: 10.1007/s00709-012-0444-0. [DOI] [PubMed] [Google Scholar]
- 62.Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012;158:340–351. doi: 10.1104/pp.111.181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jogawat A, Vadassery J, Verma N, Oelmüller R, Dua M, Nevo E, Johri AK. PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Sci Rep. 2016;6:36765. doi: 10.1038/srep36765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheteiwy MS, Shao H, Qi W, Hamoud YA, Shaghaleh H, Khan NU, Yang R, Tang B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int J Mol Sci. 2019;20:5709. doi: 10.3390/ijms20225709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kord H, Fakheri B, Ghabooli M, Solouki M, Emamjomeh A, Khatabi B, Sepehri M, Salekdeh GH, Ghaffari MR. Salinity-associated microRNAs and their potential roles in mediating salt tolerance in rice colonized by the endophytic root fungus Piriformospora indica. Funct Integr Genomics. 2019;19:659–672. doi: 10.1007/s10142-019-00671-6. [DOI] [PubMed] [Google Scholar]
- 66.Mohd S, Shukla J, Kushwaha AS, Mandrah K, Shankar J, Arjaria N, Saxena PN, Narayan R, Roy SK, Kumar M. Endophytic fungi Piriformospora indica mediated protection of host from arsenic toxicity. Front Microbiol. 2017;8:754. doi: 10.3389/fmicb.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohd S, Kushwaha AS, Shukla J, Mandrah K, Shankar J, Arjaria N, Saxena PN, Khare P, Narayan R, Dixit S, et al. Fungal mediated biotransformation reduces toxicity of arsenic to soil dwelling microorganism and plant. Ecotoxicol Environ Saf. 2019;176:108–118. doi: 10.1016/j.ecoenv.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 68.Dabral S, Yashaswee, Varma A, Choudhary DK, Bahuguna RN, Nath M. Biopriming with Piriformospora indica ameliorates cadmium stress in rice by lowering oxidative stress and cell death in root cells. Ecotoxicol Environ Saf. 2019;186:109741. doi: 10.1016/j.ecoenv.2019.109741. [DOI] [PubMed] [Google Scholar]
- 69.Darko E, Végh B, Khalil R, Marček T, Szalai G, Pál M, Janda T. Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions. PLoS One. 2019;14:e0226151. doi: 10.1371/journal.pone.0226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elmaghrabi AM, Rogers HJ, Francis D, Ochatt S. Toward unravelling the genetic determinism of the acquisition of salt and osmotic stress tolerance through in vitro selection in Medicago truncatula. Methods Mol Biol. 2018;1822:291–314. doi: 10.1007/978-1-4939-8633-0_19. [DOI] [PubMed] [Google Scholar]
- 71.Fatemi F, Hashemi-Petroudi SH, Nematzadeh G, Askari H, Abdollahi MR. Exploiting differential gene expression to discover ionic and osmotic-associated transcripts in the halophyte grass Aeluropus littoralis. Biol Proced Online. 2019;21:14. doi: 10.1186/s12575-019-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osmolovskaya N, Shumilina J, Kim A, Didio A, Grishina T, Bilova T, Keltsieva OA, Zhukov V, Tikhonovich I, Tarakhovskaya E, et al. Methodology of drought stress research: experimental setup and physiological characterization. Int J Mol Sci. 2018;19:4089. doi: 10.3390/ijms19124089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, Senthilkumar R, Ma G, Zou Q, Zhu K, Shen X, Tian D, Hua MS, Oelmüller R, Yeh KW. Piriformospora indica-induced phytohormone changes and root colonization strategies are highly host-specific. Plant Signal Behav. 2019;14:1632688. doi: 10.1080/15592324.2019.1632688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 75.Hadiarto T, Tran LS. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011;30:297–310. doi: 10.1007/s00299-010-0956-z. [DOI] [PubMed] [Google Scholar]
- 76.Jogaiah S, Govind SR, Tran LS. Systems biology-based approaches toward understanding drought tolerance in food crops. Crit Rev Biotechnol. 2013;33:23–39. doi: 10.3109/07388551.2012.659174. [DOI] [PubMed] [Google Scholar]
- 77.Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci. 2015;6:84. doi: 10.3389/fpls.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lou D, Wang H, Yu D. The sucrose non-fermenting-1-related protein kinases SAPK1 and SAPK2 function collaboratively as positive regulators of salt stress tolerance in rice. BMC Plant Biol. 2018;18:203. doi: 10.1186/s12870-018-1408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buti M, Baldoni E, Formentin E, Milc J, Frugis G, Lo Schiavo F, Genga A, Francia E. A meta-analysis of comparative transcriptomic data reveals a set of key genes involved in the tolerance to abiotic stresses in rice. Int J Mol Sci. 2019;20:5662. doi: 10.3390/ijms20225662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhan X, Shen Q, Chen J, Yang P, Wang X, Hong Y. Rice sulfoquinovosyltransferase SQD2.1 mediates flavonoid glycosylation and enhances tolerance to osmotic stress. Plant Cell Environ. 2019;42:2215–2230. doi: 10.1111/pce.13554. [DOI] [PubMed] [Google Scholar]
- 81.Chen YS, Ho TD, Liu L, Lee DH, Lee CH, Chen YR, Lin SY, Lu CA, Yu SM. Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proc Natl Acad Sci U S A. 2019;116:21925–21935. doi: 10.1073/pnas.1904818116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das J, Ramesh KV, Maithri U, Mutangana D, Suresh CK. Response of aerobic rice to Piriformospora indica. Indian J Exp Biol. 2014;52:237–251. [PubMed] [Google Scholar]
- 83.Lata R, Chowdhury S, Gond SK, White JF Jr.. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol. 2018;66:268–276. doi: 10.1111/lam.12855. [DOI] [PubMed] [Google Scholar]


