ABSTRACT
Exercise-induced gastrointestinal syndrome (EIGS) is a common characteristic of exercise. The causes appear to be multifactorial in origin, but stem primarily from splanchnic hypoperfusion and increased sympathetic drive. These primary causes can lead to secondary outcomes that include increased intestinal epithelial injury and gastrointestinal hyperpermeability, systemic endotoxemia, and responsive cytokinemia, and impaired gastrointestinal function (i.e. transit, digestion, and absorption). Impaired gastrointestinal integrity and functional responses may predispose individuals, engaged in strenuous exercise, to gastrointestinal symptoms (GIS), and health complications of clinical significance, both of which may have exercise performance implications. There is a growing body of evidence indicating heat exposure during exercise (i.e. exertional-heat stress) can substantially exacerbate these gastrointestinal perturbations, proportionally to the magnitude of exertional-heat stress, which is of major concern for athletes preparing for and competing in the upcoming 2020 Tokyo Olympic Games. To date, various hydration and nutritional strategies have been explored to prevent or ameliorate exertional-heat stress associated gastrointestinal perturbations. The aims of the current review are to comprehensively explore the impact of exertional-heat stress on markers of EIGS, examine the evidence for the prevention and (or) management of EIGS in relation to exertional-heat stress, and establish best-practice nutritional recommendations for counteracting EIGS and associated GIS in athletes preparing for and competing in Tokyo 2020.
KEYWORDS: Endurance, thermoregulation, epithelial, endotoxin, cytokine, malabsorption, gastric emptying, peristalsis
Introduction
During exercise, perturbations to the gastrointestinal tract, including compromised epithelial integrity and impaired function, with associated upper- and (or) lower-gastrointestinal symptoms (GIS), can result in complications ranging from minor inconvenience to those requiring medical intervention [1–3]. The term “exercise-induced gastrointestinal syndrome” (EIGS) has been reported to describe both the etiology and pathophysiology of exercise-associated gastrointestinal perturbations, as well as the outcomes (i.e. performance and health) that may occur in response to exercise [4]. The onset of EIGS, and associated GIS, varies across different forms of exercise; from reported GIS incidence of ≤10% in strength and power sports, team sports, and endurance exercise <4 h in duration [5–7], to ≥60% in ultraendurance running and triathlon events [1,8–11]. Moreover, GIS incidence and severity appear to be enhanced by exercise activities presenting a running element [6,8], and may also account for a substantial severity warranting exercise performance drawback, cessation, or withdrawal [1,8,10,11], which is a major concern for athletes preparing for and competing in the upcoming 2020 Tokyo Olympic Games.
It is predicted that the Games of the XXXII Olympiad will present ambient temperatures (Tamb) >30°C, with high relative humidity (RH) [12]. From a performance and medical perspective, competitive athletes frequently report greater incidence and severity of EIGS, and associated GIS, when undertaking exercise in hot ambient conditions, compared to temperate or cold ambient conditions [1–3,5]. These also appear to be more pronounced with endurance exercise [4], which include the marathon, 50 km race walk, triathlon, and cycling road race within the Olympic Games competitive program. This is supported by laboratory-controlled studies, where gastrointestinal perturbation (e.g. epithelial integrity and systemic responses) and GIS were increased to a greater extent when endurance exercise was conducted in Tamb 30°C and Tamb 35°C, compared with Tamb 22°C, irrespective of relative humidity (RH) (e.g. field and laboratory study range: 23–82% RH) [2,3,13,14]. High profile examples of EIGS outcomes in recent Olympic Games include Paula Radcliffe’s inability to finish the marathon at the 2004 Athens Olympics, in a reported Tamb 35°C and 31% RH, after which she described abdominal cramping, bloating and urge to defecate, and a history of NSAID use in the days preceding the event (www.theguardian.com/sport/london-2012-olympics-blog/2012/mar/21/50-stunning-moments-paula-radcliffe). More recently, in Tamb 25–28°C and 76% RH, race walker Yohann Diniz was leading the 50 km race walk for the first 2 h of the 2016 Rio de Janeiro Olympics, before suffering abdominal pains and defecation, which developed into fecal blood loss, and episodes of collapse (www.dailymail.co.uk/news/article-3752875).
Given that Olympic athletes are frequently exposed to hot ambient conditions during training and competition, and may be more susceptible to gastrointestinal disturbances, which are common under these conditions, the aims of the current review are to: (1) comprehensively explore the impact of exertional-heat stress on markers of EIGS; (2) examine the evidence for the prevention and (or) management of EIGS in response to exertional-heat stress; and (3) establish best-practice nutritional recommendations to counteract EIGS and associated GIS.
Exercise-induced gastrointestinal syndrome
Primary causal pathways
At the onset of exercise, two primary physiological changes occur that compromise the integrity and function of the gastrointestinal tract (Figure 1). Firstly, redistribution of blood flow to working muscles and peripheral circulation results in reduced total splanchnic perfusion and subsequent gastrointestinal ischemia [15–17]: “the circulatory-gastrointestinal pathway” [4]. Secondly, an increase in sympathetic activation reduces overall gastrointestinal functional capacity through suppressing myenteric and submucosal plexus activity [18–21]: “the neuroendocrine-gastrointestinal pathway” [4]. A comprehensive overview of the impact of exertional stress on markers of EIGS (i.e. gastrointestinal integrity and function, and endotoxin profile) can be viewed in Costa et al. [4]; while a comprehensive review of the cytokine response to exercise can be viewed in Peake et al. [22].
Figure 1.
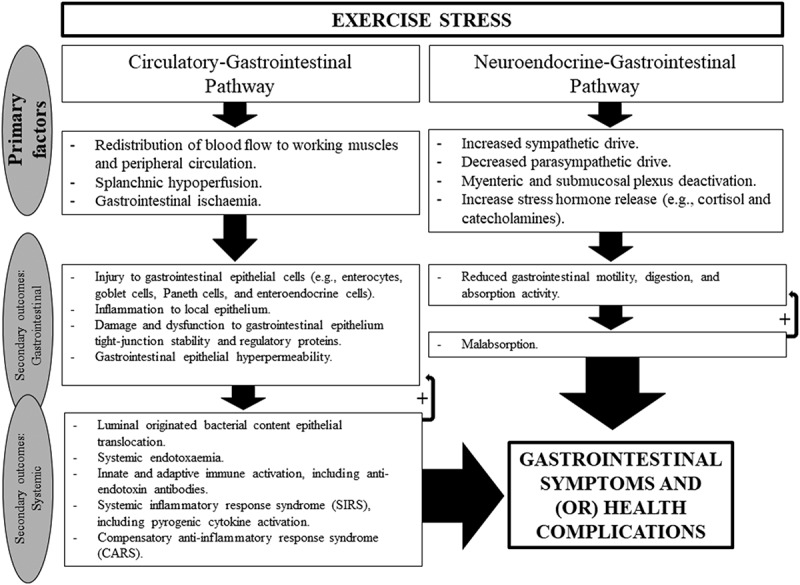
Schematic description of “exercise-induced gastrointestinal syndrome” (EIGS) including the “circulatory-gastrointestinal” and “neuroendocrine-gastrointestinal” pathways. Adopted from Costa et al. [4], with permission.
Secondary outcomes
The secondary outcomes of splanchnic hypoperfusion, and subsequent gastrointestinal ischemia, include injury to cells of the intestinal epithelium (e.g. enterocytes, goblet cells, Paneth cells, and (or) enteroendocrine cells), potentially reducing their functional capabilities, including nutrient digestion and absorption, luminal mucus secretions, production and release of antimicrobial agents as part of immune surveillance and protection, gastrointestinal motility and appetite regulation [15–17,23]. In addition, injury to the gastrointestinal epithelium is associated with increased barrier permeability (i.e. hyperpermeability), by either physical breaks in the intestinal epithelium, damage to tight-junction proteins, and (or) dysfunction to tight-junction regulation [24,25]; thus enhancing the potential translocation of pathogenic content from the lumen into circulation [2–4]. Moreover, such localized intestinal epithelial cell injury promotes acute local inflammatory responses via NFκB gene expression within cells, further promoting epithelial injury, dysregulation, and hyperpermeability [26,27], through a positive feedback loop.
The secondary outcomes of increased sympathetic drive and altered gastrointestinal functional capabilities include reduced gastric emptying and delayed orocecal transit [18–21]. There is also accumulating evidence that exercise stress leads to nutrient malabsorption during and after a bout of strenuous exercise (i.e. resistance, endurance cycling and running) [23,28–30]. It is, however, still unclear if the mechanism(s) underlying impaired nutrient absorption are due to intestinal epithelial enterocyte injury and subsequent transporter damage and (or) malfunction, downregulated intestinal transporter activity, or a combination of both. In either situation, the resultant increase in delivery of nutrients to the distal ileum and colon has the potential to increase the degree of bacterial fermentation. This in turn leads to luminal distension from gas production (e.g. H2, CH4, CO2, and H2S) and osmotically driven increases in intestinal water content. Such a scenario may prompt lower-GIS, which is dependent on the individual’s visceral sensitivity, and may further compromise gastric and duodeno-jejunal motility through the ileal-brake feedback mechanism, albeit predominantly identified and explored in animal models, using either neural or enteroendocrine mediators [23,29,31–35].
Exacerbation factors and implications
Extrinsic factors, such as exercise intensity, duration, modalities, the environmental conditions of the exercise bout, and nonsteroidal anti-inflammatory drug (e.g. aspirin and ibuprofen) use, play a crucial role in determining the magnitude of gastrointestinal disturbance in response to exercise stress [4,18,36–39]. Moreover, intrinsic factors, such as, individual feeding tolerance during exercise, a predisposition or active gastrointestinal disease(s)/disorder(s), and possibly the relative bacterial abundance and diversity of the gastrointestinal microbiota may influence the incidence and magnitude of EIGS [4,23,29]. Nevertheless, if perturbations to gastrointestinal integrity or function, and (or) systemic responses are substantial enough, these can lead to acute or chronic health complications of sub-clinical or clinical significance (Figure 2), including development of chronic gastrointestinal inflammatory disease; or in extreme cases, fatality due to heatstroke, with gastrointestinal and immune originated pathophysiology (e.g. luminal endotoxin-associated sepsis and systemic inflammatory response syndrome (SIRS)) [40–47]. Fatality is rare within the sports and exercise context, or may be underreported. However, it is more frequently reported in occupational health settings (e.g. mining, military, agriculture, and field resourcing) due to these activities showing increased and (or) continued activity, in hot Tamb, after thermoregulatory threshold of clinical significance has progressed beyond the point of self-preservation [48–54]. From an exercise perspective, such gastrointestinal perturbations, and associated GIS, are more commonly reported to present performance implications, including, but not limited to, reduced work output, cessation or withdraw from activity, and reduced feeding ability during and after exercise, potentially impacting both competition (e.g. fueling and hydration) and recovery nutrition (e.g. refueling, rehydration, immunocompetency, optimal muscle synthesis, and training adaptations) [1,8,10,11,23,28–30,55–57].
Figure 2.
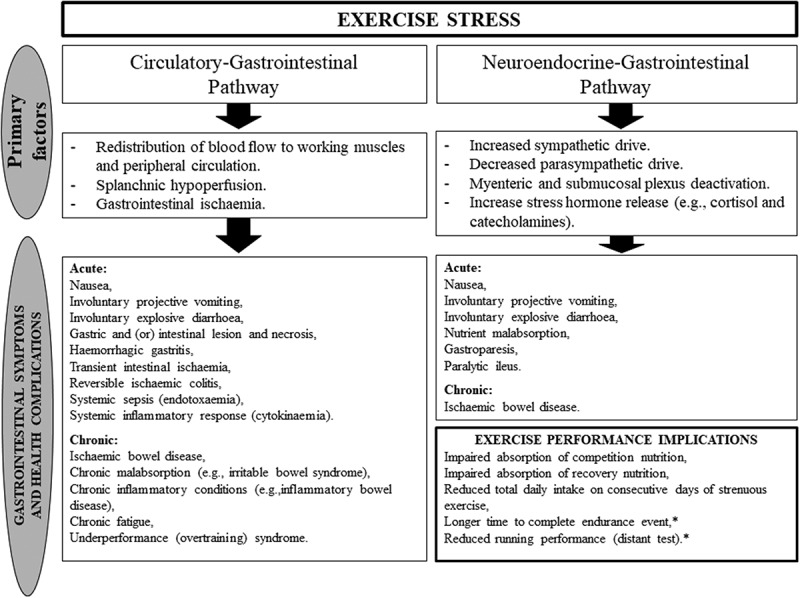
Potential acute and chronic health complications and performance implications arising from the physiological alterations of EIGS. * Symptoms and nutritional status induced. Adopted from Costa et al. [4], with permission.
Impact of exertional-heat stress on exercise-induced gastrointestinal syndrome
The effect of exertional stress, with the additive effect of heat stress, has been studied in relation to both the mechanisms and outcome markers of EIGS. A summary of controlled laboratory and field studies that have comprehensively investigated the impact of exertional-heat stress on gastrointestinal status (i.e. a combination of gastrointestinal integrity and functional markers, systemic endotoxin and immune responses, and using a validated and reliability-tested GIS assessment tool), are presented in Table 1. The outcomes of these studies are hereafter discussed, alongside exertional-heat stress experimental designs that have only assessed one or a limited range of gastrointestinal markers, with or without GIS.
Table 1.
Research studies that have comprehensively assessed the impact of exertional stress, with and without heat stress, on combined markers of gastrointestinal integrity, function, symptoms, and associated systemic responses* (i.e. endotoxin and cytokine profile).
| Population | Exertional and heat stress | Thermoregulatory and cardiovascular stain | BML | post-exercise POsmol | Drinking regime | |
|---|---|---|---|---|---|---|
| Controlled laboratory studies | ||||||
| Gaskell et al. [unpublished observations] | Trained male and female endurance athletes (n = 18), V̇O2max: 60 ml/kg/min. |
2 h running, 60% V̇O2max (10.7 km/h), Tamb 35.7°C and 23% RH. Dietary control: 24 h low FODMAP provisions. |
Peak Tre: 38.9°C Peak TCR: 9 Cardiac drifta: 14 bpm Peak RPE: 13 |
2.0% | 297 mOsmol/kg | Total: 1248 ml water, ad libitum. |
| Costa et al. [59] | Trained male endurance runners (n = 11), V̇O2max: 59 ml/kg/min. |
2 h running, 70% V̇O2max (10.9 km/h), Tamb 24.7°C and 46% RH. Dietary control: 24 h monitoring and breakfast provisions. |
Peak Tre: Not measured Peak TCR: Not measured Cardiac drifta: 11 bpm Peak RPE: 14 |
0.6% | 291 mOsmol/kg | Total: 1689 ml water, programmed. |
| Snipe & Costa [60] | Trained male and female endurance athletes (n = 12), V̇O2max: 56 ml/kg/min. |
2 h running, 60% V̇O2max (9.4 km/h), Tamb 35.1°C and 25% RH. Dietary control: 24 h low FODMAP provisions. |
Peak Tre: 39.6°C Peak TCR: 11 Cardiac drifta: 22 bpm Peak RPE: 16 |
1.1% | 290 mOsmol/kg | Total: 1821 ml water, programmed. |
| Snipe et al. [13] | Trained male and female endurance athletes (n = 10), V̇O2max: 56 ml/kg/min. |
2 h running, 60% V̇O2max (10.4 km/h), Tamb 22.2°C and 44% RH vs. Tamb 35.4°C and 23% RH. Dietary control: 24 h low FODMAP provisions. |
Peak Tre: 38.5°C vs. 39.6°C Peak TCR: 8 vs. 11 Cardiac drifta: 11 vs. 22 bpm Peak RPE: 12 vs. 15 |
1.6% vs. 1.6% | 295 vs. 295 mOsmol/kg | Total: 848 ml vs. 1630 ml water, ad libitum. |
| Snipe et al. [14] | Trained male and female endurance athletes (n = 10), V̇O2max: 56 ml/kg/min. |
2 h running, 60% V̇O2max (10.4 km/h), Tamb 22.2°C and 44% RH vs. Tamb 30.2°C and 35% RH. Dietary control: 24 h low FODMAP provisions. |
Peak Tre: 38.5°C vs. 38.9°C Peak TCR: 8 vs. 10 Cardiac drifta: 11 vs. 16 bpm Peak RPE: 12 vs. 14 |
1.6% vs. 1.6% | 295 vs. 294 mOsmol/kg | Total: 848 ml vs. 1330 ml water, ad libitum. |
| Gill et al. [81]* | Trained male endurance runners (n = 8). V̇O2max: 59 ml/kg/min. |
2 h running, 60% V̇O2max (9.9 km/h), Tamb 34.0°C and 32% RH. Dietary control: 24 h monitoring and breakfast provisions. |
Peak Tre: 39.1°C Peak TCR: 11 Cardiac drifta: 26 bpm Peak RPE: 15 |
1.3% | 297 mOsmol/kg | Total: 1869 ml water, ad libitum. |
| Field Studies | ||||||
| Gill et al. [2]* | Male and female ultraendurance runners (n = 19) | Five-stage 230 km multi-stage ultramarathon (8.1 km/h), maximum daily Tmax range 30°C – 40°C and 31% – 40% RH. Dietary control: ad libitum with real-time monitoring [1,61,62]. |
post-exercise Ttymp: 37.0°C | Stage range: 1.0% – 2.5% | Stage range: 286–297 mOsmol/kg |
758 ml/h, ad libitum |
| Gill et al. [3]* | Male and female ultraendurance runners (n = 25) | 24 h continuous ultramarathon (122–208 km total distance range), Tamb range 0°C – 20°C and 54% – 82% RH. Dietary control: ad libitum with real-time monitoring [1,63]. |
post-exercise Ttymp: 37.5°C | 1.7% | 286 mOsmol/kg | 378 ml/h, ad libitum |
Numerical values reported are indicative of the mean values. a Cardiac drift: ∆ heart rate from baseline steady state (10 min) to exercise cessation (120 min). BML: exercise-induced body mass loss, POsmol: plasma osmolality, FODMAP: fermentable oligo- di – mono-saccharides and polyols, Tamb: ambient temperature, RH: relative humidity, Tre: rectal temperature, TCR: thermal comfort rating, RPE: rating of perceived exertion, Ttymp: tympanic temperature, Tmax: maximal daily temperature'.
Gastrointestinal injury and permeability
It is well established that splanchnic hypoperfusion, and subsequent gastrointestinal ischemia, is a key promoting factor for intestinal injury and hyperpermeability [15–17], which may include: mucosal erosion, physical breaks in the epithelium, damage to the multiprotein complex of the epithelial tight-junction (zonulae occludentes) and/or dysfunction to tight-junction regulatory proteins (i.e. claudin, occludin, and (or) junction adhesion molecules). Portal blood flow has been reported to decrease by 20% within 10 min, and by 80% after 1 h, of running at 70% of V̇O2max [58]. Whereas, 1 h of cycling at 70% of maximal power (wattage) output (Wmax) resulted in a 1.7 kPa increase in gastric arterialized PCO2, indicative of splanchnic ischemia, within 10 min of exercise commencement [17].
The effect of exertional-heat stress on intestinal injury is most commonly assessed via the measurement of intestinal fatty acid binding protein (I-FABP), a protein exclusive to the epithelial mucosa of the intestinal wall [15]. Release of I-FABP into circulation indicates enterocyte damage, often due to ischemia of the gastrointestinal tract [17]. Several studies across a range of temperate, warm, and (or) hot ambient conditions have assessed the change in I-FABP pre- to post-exercise, using similar exercise and dietary protocols (Figure 3). Collectively, these studies show that when treadmill running at 60–70% V̇O2max for 2 h, consuming water only to maintain euhydration, the increase in I-FABP is substantially greater once Tamb is ≥35°C, with changes in I-FABP pre- to post-exercise of >1000 pg/ml consistently observed under these hot conditions; compared to increases of ≤600 pg/ml in warm conditions (e.g. Tamb ~30°C), and ≤400 pg/ml in temperate conditions (e.g. Tamb 22–25°C) [13,14,60]. Peak values appear immediately to 1 h after exercise, returning to similar baseline values within 2 h on cessation of exercise, in all trials, possibly linked to systemic clearance. Two of these studies provide a direct comparison, with the same participants exercising in temperate (Tamb 22°C), and either warm (Tamb 30°C) or hot (Tamb 35°C) conditions in randomized crossover trials [13,14]. After nonheat acclimatized endurance athletes performed treadmill running at 60% V̇O2max for 2 h, consuming water ad libitum with no other nutrient intake, the increase in I-FABP from baseline was significantly greater following exercise in Tamb 30°C compared to Tamb 22°C (∆ pre- to post-exercise: 184% vs. 127%, respectively), and substantially greater after running in Tamb 35°C (∆ pre- to post-exercise: 427% vs. 127%, respectively). Together, these studies demonstrate a proportional increase in intestinal injury with increasing exertional-heat stress exposure, and associated proportional increases in exertional and thermoregulatory strain (i.e. body temperature, heart rate, thermal comfort rating, and rating of perceived exertion).
Figure 3.
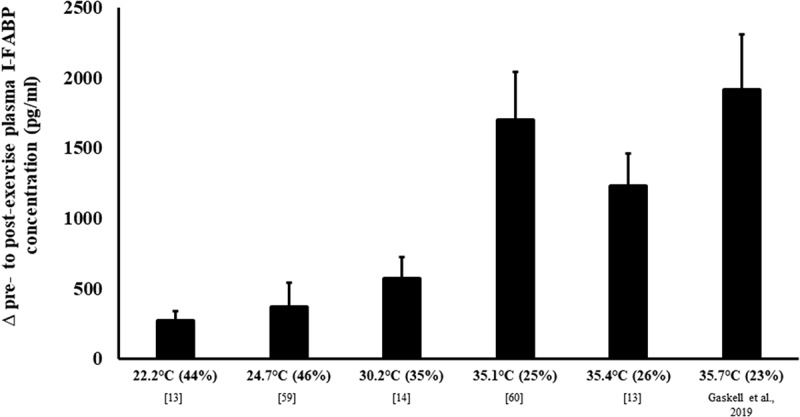
The magnitude of intestinal epithelial injury in response to exertional vs. exertional-heat stress. Mean ± SEM. Tamb (RH). Gaskell et al., 2019 from unpublished observations.
It should be noted that the effect of exertional-heat stress on intestinal integrity appears to also be related to exercise duration. Studies employing exertional-heat stress for ≤2 h, which does not provide sufficient time to induce adequate thermoregulatory strain, and (or) achieving core body temperature measurements (e.g. rectal temperature (Tre)) of <39.0°C, have typically observed a far smaller rise in I-FABP, even when exercise is performed at higher intensities and in hot ambient conditions [64–67]. Considering the precision mechanics of the human body’s thermoregulatory system (i.e. vasodilation adjustments for circulatory redistribution, increased peripheral circulation, body water hemodynamics, sweating responses, upregulated and (or) opportunistic heat loss mechanisms- radiation, evaporation, convection, and conduction), upregulated cooling efficiency allows a trained athlete to cope with a substantial amount of exertional-heat stress before internal heat production through exercise, and external heat exposure through hot ambient conditions, overwhelms these mechanisms and substantial thermoregulatory strain of clinical and performance significance is observed (e.g. mild heat stress at Tamb 30°C resulting in Tre of <39.0°C vs. heat stress at Tamb ≥35°C resulting in Tre of ≥ 39.0°C) [13,14,59,64,65]. In a recent study, 12 male participants undertook 45 min of cycling exercise at 70% of V̇O2max in temperate (Tamb 20°C and 40% RH) and warm (Tamb 30°C and 40% RH) ambient conditions, with an additional passive heat exposure trial [67]. The I-FABP response to exertional stress in temperate and warm trials, which resulted in peak Tre of 38.2°C (95% CI: 38.0°C–38.3°C) and 38.3°C (38.0°C–38.6°C), was a modest 281 pg/ml and 369 pg/ml (i.e. ∆ pre- to post-exercise), respectively. No change in rectal temperature and intestinal injury was observed with passive heating. Clearly, the failure to expose participants to any substantial exertional-heat stress, above the body’s natural ability to thermoregulate, resulted in an insignificant increase in thermoregulatory strain and subsequently intestinal injury, in which values are in accordance with Snipe et al. [14]. It therefore appears, that a prolonged period of exertional-heat stress, that exceeds the thermoregulatory threshold, is a prerequisite for the occurrence of substantial intestinal injury to warrant any clinical or performance significance [4,68,69].
Exercise-associated gastrointestinal hyperpermeability is believed to arise from intestinal barrier dysfunction, induced by splanchnic hypoperfusion, prolonged mechanical trauma during sports such as running (i.e. repetitive jarring of the gastrointestinal tract), oxidative and nitrosative stress, and (or) thermal effects of hyperthermia [70,71]. Gastrointestinal permeability is commonly assessed by urinary excretion of ingested nonmetabolizable sugar probes such as lactulose and rhamnose for the small intestine, sucralose and erythritol for the large intestine, and sucrose for the stomach [70–72]. In terms of assessing small intestinal permeability, the large sugar probe (i.e. lactulose) crosses the epithelium via the paracellular pathway, while the small sugar probe (i.e. rhamnose or mannitol) crosses the epithelium via a transcellular route. Therefore, expressing the excretion rate of these two sugars as a ratio (e.g. lactulose to L-rhamnose ratio (L/R)) controls for factors affecting their urinary excretion (e.g. gastrointestinal transit, intestinal translocation, renal function, and body fluid distribution after absorption) [70–72].
A pre- to post-exercise increase in intestinal permeability has consistently been reported. For example, an increase in L/R (0.040) in excreted urine, indicating a greater degree of small intestinal permeability, was observed in response to 60 min running exercise at 70% V̇O2max in Tamb 30°C (12–20% RH), resulting in a reported core body temperature of 39.5°C [73]. There was however no temperate Tamb comparison. Using the same exercise protocol of 60 min running exercise at 70% V̇O2max, but in Tamb 22°C (62% RH) and Tamb 33°C (50% RH), resulting in a core body temperature of 38.4°C and 39.1°C, respectively, a greater increase in claudin-3, a proposed indirect marker of intestinal permeability, was observed on Tamb 33°C (∆ pre- to post-exercise: 1.6 ng/ml) compared with Tamb 22°C (0.9 ng/ml) [74]. More recent studies compared small intestine permeability (i.e. dual sugar mixture containing 5 g lactulose and 1 g L-rhamnose ingestion 30 min prior to the cessation of exercise), in response to 2 h of running at 60% V̇O2max in Tamb 22°C, 30°C, and 35°C [13,14] (Figure 4). Despite the L/R progressively increasing from Tamb 22°C to Tamb 35°C, the magnitude of difference was not significant. The time course for exercise-associated hyperpermeability returning to baseline levels is currently unknown. This is primarily due to difficulties in sugar test application along a short timeframe, researchers focusing on the magnitude of hyperpermeability, and research employing differing measurement points (e.g. pre-, during-, and post-exercise). It is, however, speculated that recovery would be similar to intestinal injury, and follow a similar recovery trajectory to the primary causal mechanisms-splanchnic hypoperfusion and gastrointestinal ischemia.
Figure 4.
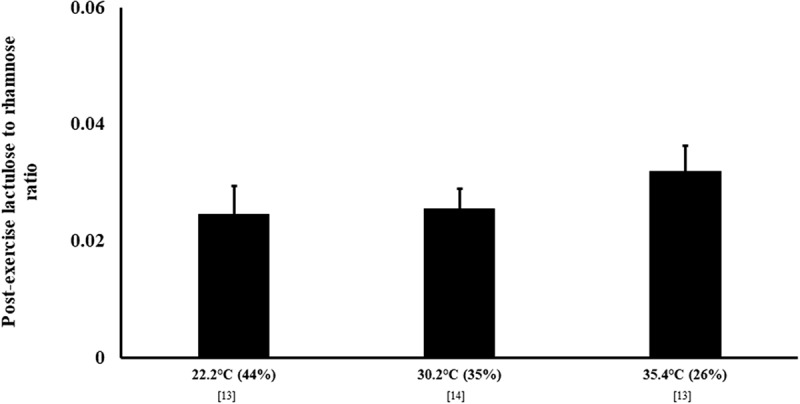
Small intestine permeability in response to exertional vs. exertional-heat stress. Mean ± SEM. Tamb (RH).
A recent systemic literature review confirmed the progressive heightened intestinal hyperpermeability in proportion to core body temperature [75]. Correlation analysis included pre- and post-exercise small intestinal permeability data (r = 0.605, p = 0.013), which may have overemphasized the Tcore to intestinal permeability relationship. A follow-up publication, however, clearly showed an increase in intestinal permeability in proportion to indices of core body temperature using only post-exertional and exertional-heat stress data (r = 0.908, p = 0.033) [76]. This indicates that if a minimal threshold of thermoregulatory strain is not attained (e.g. Tcore ≥39.0°C), the gastrointestinal perturbations are negligible and of no clinical significance or consequence [4]. Moreover, to date, many studies have targeted gastrointestinal permeability as the key variable, and in some studies the only variable, within exercise gastroenterology research [72,74,77,78]. Since intestinal permeability exists on a continuum, is on its own generally asymptomatic, and is not itself a final outcome of EIGS (rather a step within the pathophysiology of EIGS) [4], such variable data on its own, within the context of exercise experimental models, may be considered redundant, and risks data misinterpretations, erroneous conclusions, and erroneous translation into practice. Instead, it is the potential implications of increased gastrointestinal permeability that must be considered, specifically, the translocation of pathogenic bacterial endotoxins resulting in endotoxemia and cytokinemia [2,3,26].
Systemic endotoxemia and cytokinemia
Assessing exercise-associated systemic endotoxemia is usually performed with an assay for gram negative bacterial endotoxin (e.g. limulus amebocyte lysate chromogenic endpoint assay), with or without endotoxin lipopolysaccharide binding protein (LBP) and bacteria detecting soluble CD14 (sCD14) [4,71]. It has been noted in a recent systematic review [4] that exercise of ≥2 h duration, as well as, shorter higher intensity exercise (e.g. ≥70% V̇O2max) in hot ambient conditions, can both result in a concentration of circulating pathogenic bacterial endotoxins (e.g. lipopolysaccharide (LPS)) ≥5 pg/ml above baseline, which is the reference cut-off indicative of systemic endotoxemia [2,3,64,74,79–83]. In addition to the circulating endotoxin concentration, anti-endotoxin antibody (e.g., IgG and IgM) should also be assessed to determine the immune response to endotoxemia, and to interpret the endotoxin concentration in the context of the body’s clearance capacity [80,84–86]. In direct comparison, circulating endotoxin concentration has not been found to be significantly greater following 2 h running at Tamb 30°C or Tamb 35°C, compared to Tamb 22°C [13,14]. However, anti-endotoxin antibodies were substantially reduced after running in Tamb 35°C, compared to an increase after the same exercise bout in Tamb 22°C and Tamb 30°C (Figure 5), indicating that a greater immune response was elicited when running in hot ambient conditions to maintain a similar level of circulating endotoxins. To avoid the potential difficulties in interpreting endotoxin profiles resulting from exertional-heat stress, endotoxin binding factors (e.g. proteins and immune components) can be measured to indirectly detect the response magnitude. For example, plasma sCD14 concentration has been observed to increase more substantially (380 ng/ml) after exertional-heat stress (i.e. 2 h running at 60% V̇O2max in Tamb 35.7°C), compared with exertional stress (i.e. 2 h running at 70% V̇O2max in Tamb 24.7°C; 53 ng/ml) [59, Gaskell et al., unpublished observations]. Moreover, the same exertional-heat stress promoted a significant pre- to post-exercise increase in plasma LBP concentration (1447 ng/ml) [Gaskell et al., unpublished observations]; however, no Tamb comparisons are available to date for plasma LBP concentration. Regardless of environmental conditions, the duration of exercise appears to be critical in the development of endotoxemia. A recent field study, following the first 37 km stage of a 230 km multi-stage ultramarathon event, conducted in hot ambient conditions (Tmax 31–40°C), found an increase in circulating endotoxin from baseline of 40 pg/ml [3], peaking to 80 pg/ml on cessation of Stage 4. Additionally, despite being run in cold to temperate conditions (Tamb range 0–20°C), a 24-h continuous ultramarathon resulted in an increase of 122 pg/ml [2]. The recovery time course of exercise-associated endotoxemia appears dependent on the magnitude of response. Laboratory controlled exertional-heat stress studies demonstrate peak responses immediately post- to 1 h post-exercise [13,14,81], reducing thereafter, and reaching similar baseline concentration within 4 h. In contrast, field-based studies have shown a longer recovery time. In a mountain ultra-distance triathlon, with heat exposure, a modest rise in systemic LPS was observed, returning to baseline levels within 24 h [8]. During multi-stage ultramarathon in the heat (daily running distance range: 37–63 km), a sustained resting and exercise-associated systemic endotoxemia was observed throughout [3].
Figure 5.
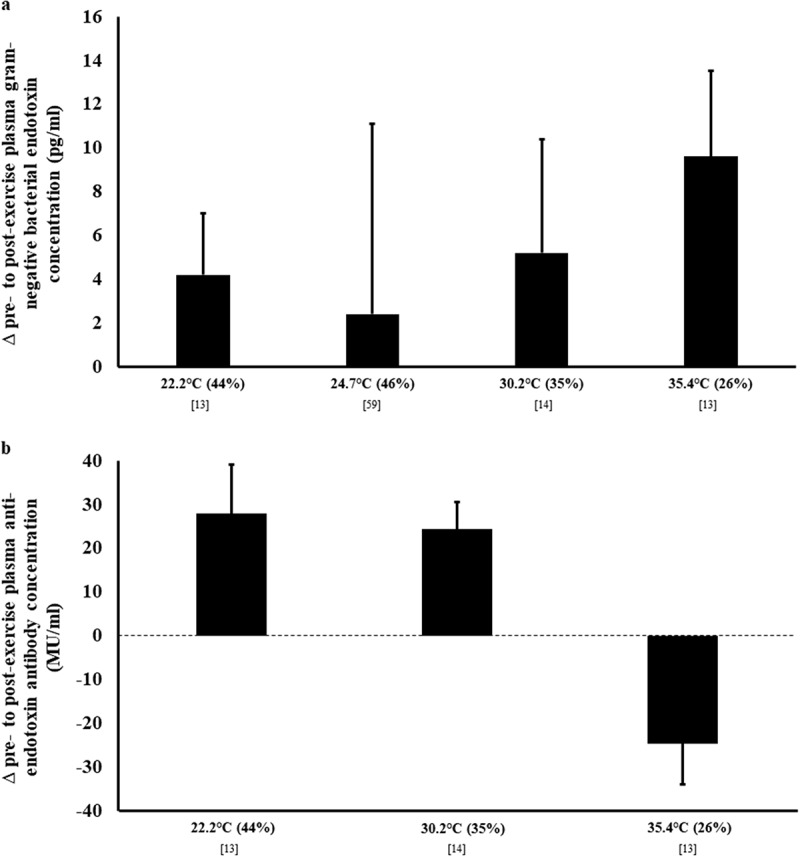
Endotoxin profile in response to exertional and exertional-heat stress. (a) plasma gram negative bacterial endotoxin concentration by limulus amebocyte lysate chromogenic endpoint assay, and (b) plasma IgM anti-endotoxin antibody concentration by Endocab ELISA assay. Mean ± SEM. Tamb (RH).
The effect of endotoxemia, occurring either from intestinal injury creating barrier rupture, epithelial hyperpermeability, or a combination of both, can also be observed from the resulting inflammatory cytokine profile (Figure 6). Indeed, evidence has been presented to show a pronounced systemic cytokinemia, similar to that of clinical sepsis, in the presence of a substantial exercise-associated endotoxemia, compared to exertional stress per se, where the magnitude was not sufficient to induce perturbations to systemic endotoxin profile (e.g. <2 h of exertional or exertional-heat stress) [2–4,22,87]. In general, strenuous exercise leads to an increase in proinflammatory and inflammatory response cytokines, including interleukin (IL)-1B, tumor necrosis factor (TNF)-a, IL-6, and IL-8; albeit findings have been inconsistent and do not appear to be related to Tamb [13,14,60]. However, clear differences, due to heat stress, are observed in anti-inflammatory cytokine responses (peaking 1 to 2 h post-exercise), with substantially greater increases in IL-10 and IL-1 receptor antagonist (ra) observed during recovery from 2 h running at 60% V̇O2max in Tamb 35.1–35.4°C, compared to either Tamb 22.2°C or Tamb 30.2°C [13,14,60]. It appears that the competency of the anti-inflammatory response will dictate the magnitude of proinflammatory cytokine concentration to exertional-heat stress. For example, an absent proinflammatory response after exertional-heat stress exposure was accompanied by the highest anti-inflammatory responses [60]; whilst modest increases in proinflammatory cytokines after exertional stress in temperate and warm conditions were adjunct with more modest anti-inflammatory responses [3,13,14]. Thus, it has previously been suggested that in well trained and (or) heat-adapted athletes, for whom exertional-heat stress is well-tolerated, anti-inflammatory responses predominate, potentially blunting proinflammatory cytokine responses and off-setting any clinically significant episodes associated with pronounced proinflammatory cytokinemia (e.g. SIRS) [2,3,42,52,54,87]. This may also have performance implications, through the proposed role of cytokines (e.g. IL-6) as “fatigogens” [45,88]. On a concerning note, for untrained individuals and under- or deconditioned athletes that are not “fit for task,” athletes presenting immune-dysfunction or are immunocompromised, and (or) athletes that have a predisposition to inflammatory disease(s)/disorder(s), may be high risk individuals for developing either acute or chronic health implications linked to exercise-associated gastrointestinal tract originated and triggered systemic cytokinemia.
Figure 6.
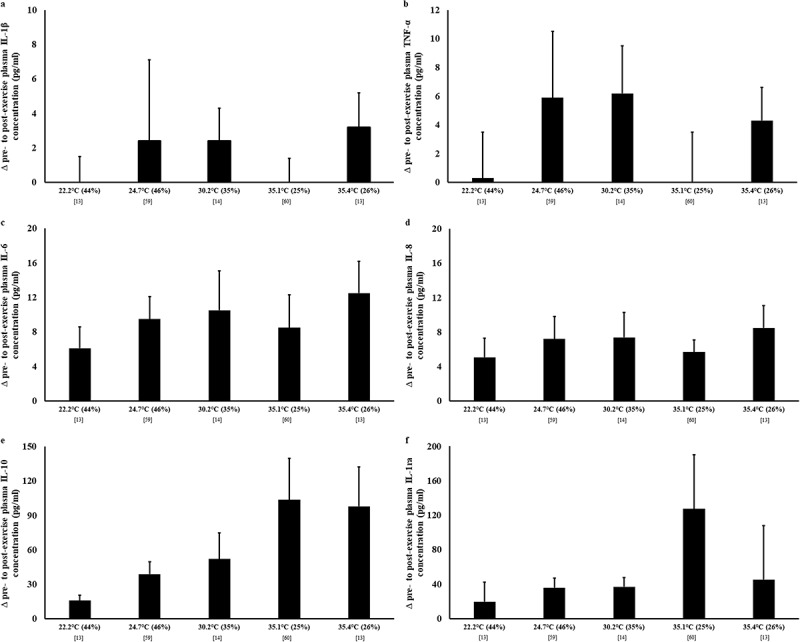
Systemic cytokine profile in response to exertional and exertional-heat stress. Plasma (a) IL-1β, (b) TNF-α, (c) IL-6, (d) IL-8, (e) IL-10, and (f) IL-1ra concentrations by human cytokine/chemokine multiplex assay. Mean ± SEM. Tamb (RH). IL-1β, TNF-α, IL-6, and IL-8 peaking immediately post-exercise; while IL-10 and IL-1ra peaking 1–2 h post-exercise.
Gastrointestinal motility
Gastrointestinal motility can be assessed across different sections of the gastrointestinal tract. The most common measurement techniques for assessing gastrointestinal motility in exercise experimental models are gastric emptying using bolus and aspiration, radio-isotope scanning, radiopaque markers with fluoroscopy, or 13C-acetate solution administration with breath sampling; orocecal transit time (OCTT) using lactulose administration with breath hydrogen (H2) responses; and electrogastrography for gastrointestinal smooth muscle neural activity [4]. Literature relating to gastric emptying and OCTT in response to exercise have typically focused on the effect of exercise intensity, and diverse drink and (or) meal types during and after exercise, generally in short duration exercise, and in temperate ambient conditions [18]. As such, there is currently limited available data to remark on the differences of gastric emptying and (or) OCTT between exertional and exertional-heat stress. However, one study has reported a negative correlation between gastric emptying volume and Tre in response to 3 x 15 min bouts of running at 50% V̇O2max in Tamb 18°C, 35°C, and 49°C [89]. These findings suggest that increased core body temperature during exertional-heat stress may impair gastric emptying, and potentially contribute to the development of upper-GIS arising from increased gastric distension.
Assessment of OCTT, on which gastric emptying will exert some influence, has been made by providing a lactulose solution 30 min before the cessation of exercise, then observing the time course of the breath H2 response post-exercise [4]. This method is used because breath samples taken during exercise do not reflect the expected H2 concentration, due to a potential dilution effect with increased ventilation rate [23,90]. Unpublished data collected from breath sample analysis, as part of Snipe et al. [13,14], showed a rapid rise in breath H2 on cessation from 2 h of running in Tamb 22°C (baseline: 2 ppm to post-exercise: 21 ppm), but a delay of at least 30 min in breath H2 response after 2 h of running in Tamb 30°C (2 ppm to 19 ppm) and Tamb 35°C (2 ppm to 18 ppm). Taken together, these data may indicate an increased OCTT, and therefore reduced gastrointestinal motility with exercise in hot ambient conditions. However, as breath samples were only collected every 30 min in the post-exercise period, it is not possible to ascertain the precise time of breath H2 turning-point and peak, and whether any differences in OCTT occur between Tamb 30°C and Tamb 35°C.
Carbohydrate malabsorption
Breath H2 responses after consuming carbohydrate provide an indicator of both the presence and magnitude of carbohydrate malabsorption before, during, and after exercise [90,91]. Breath samples have been collected and analyzed for H2 during exercise experimental models in temperate conditions, where substantial carbohydrate has been consumed during exercise, and clearly show exertional stress induced carbohydrate malabsorption (i.e. ≥10 ppm from baseline) [91] of a pre-exercise meal, during exercise feeding, and recovery beverage [23,29,59, Gaskell et al., unpublished observations]. However, there are currently no studies that allow comparison of the same diet and exercise intervention in temperate, warm, and (or) hot ambient conditions; whilst consuming foods and (or) fluids that provide potentially fermentable carbohydrates, and avoiding other substances that produce a quantifiable breath H2 response (e.g. lactulose used for the determination of intestinal permeability). Therefore, an inference about the specific effect of exertional-heat stress on carbohydrate malabsorption cannot be made at this time.
Gastrointestinal symptoms
GIS are a common feature of exercise stress, especially endurance exercise [4]. Athletes frequently report an increase in GIS when exercising with the added effect of heat stress [1,13,14]. Until recently, however, no studies had previously assessed GIS in a laboratory-controlled setting to any comprehensive degree, with varying ambient conditions. Table 2 summarizes those studies now published in this area, assessing GIS using a validated and reliability tested modified visual analog scale [92]. The incidence of total-GIS when completing the same exercise protocol (i.e. 2 h running at 60% V̇O2max), and consuming water only to maintain euhydration, was ≤70% in temperate conditions [13,59], increasing to 80% in warm conditions [14], and ≥88% in hot ambient conditions [13,60, Gaskell et al., unpublished observations]. The nature of this relationship appears consistent across gut discomfort, total-, upper-, and lower-GIS. For example, upper-GIS incidence was <65% in temperate conditions, rising to ≥70% in warm and hot conditions [13,14,59,60, Gaskell et al., unpublished observations]. Likewise, lower GIS incidence was ≤30% in temperate conditions, but ≥58% in warm and hot ambient conditions. In addition to increased incidence, the severity of GIS appears greater when heat stress is added to exercise proportionally. Total severity scores for gut discomfort, total-, upper-, and lower-GIS were greater when exercising in warm and hot ambient conditions, compared to temperate conditions (Table 2). It is interesting to note that GIS reported during exertional-heat stress (2 h running at 60% V̇O2max in Tamb 35°C), led into GIS (58%) during the 4 h recovery monitoring period, and into the evening (~12 h post-exercise) [Gaskell et al., unpublished observations]. This suggests that exercise-associated GIS can be long lived, despite gastrointestinal physiological perturbations (i.e. integrity, function, and systemic responses) returning to baseline levels shortly after exercise cessation, and may interfere with recovery nutrition and daily nutritional intake [1]. The underlying causes for such a high incidence of GIS are multifaceted (Figure 1); but may be singular, coupled, or multicausal within and between individual athletes, as previously discussed, and may vary or be similar with each exertional-heat stress exposure. Based on the available laboratory-controlled and field exploratory or observational evidence, the following summary is proposed to depict the relationship between EIGS pathophysiology and GIS [93].
| Pathophysiological pathway: | Factor: | Time course of onset: | Time course of incidence: | |
|---|---|---|---|---|
| Circulatory-Gastrointestinal | Primary | Rapid | Transient | Asymptomatic |
| Circulatory-Gastrointestinal | Secondary | Delayed | Prolonged | Asymptomatic or symptomatic |
| Neuroendocrine-Gastrointestinal | Primary | Rapid | Transient | Symptomatic |
| Neuroendocrine-Gastrointestinal | Secondary | Delayed | Transient or prolonged | Symptomatic |
Table 2.
Research studies that have comprehensively assessed the impact of exertional stress, with and without heat stress, on exercise-associated gastrointestinal symptoms (GIS), in real-time, using a validated and reliability tested modified visual analog scale [92].
| Gut discomfort | Total-GIS1 | Upper-GIS2 | Lower-GIS2 | Nausea | Predominant symptoms | ||
|---|---|---|---|---|---|---|---|
| Snipe et al. [13] Tamb 22.2°C and 44% RH |
Incidence: Severity*: |
NA 6 (7) |
70% 6 (8) |
40% 2 (4) |
30% 3 (8) |
10% 0 (0) |
Belching, bloating, and urge to defecate. |
| Costa et al. [59] Tamb 24.7°C and 46% RH |
Incidence: Severity*: |
NA 16 (11) |
64% 24 (18) |
64% 14 (13) |
27% 4 (4) |
36% 2 (3) |
Belching, upper abdominal pain, bloating, flatulence, nausea, and dizziness. |
| Snipe et al. [14] Tamb 30.2°C and 35% RH |
Incidence: Severity*: |
NA 18 (15) |
80% 38 (37) |
70% 17 (19) |
70% 6 (5) |
40% 8 (13) |
Belching, bloating, upper abdominal pain, urge to defecate, and lower abdominal pain. |
| Snipe & Costa [60] Tamb 35.1°C and 25% RH |
Incidence: Severity*: |
NA 23 (16) |
92% 33 (27) |
83% 20 (20) |
58% 8 (14) |
25% 3 (6) |
Belching, upper abdominal pain, urge to regurgitate, flatulence, urge to defecate, nausea, and dizziness. |
| Snipe et al. [13] Tamb 35.4°C and 26% RH |
Incidence: Severity*: |
NA 25 (21) |
90% 72 (90) |
90% 34 (48) |
70% 9 (13) |
40% 13 (22) |
Belching, bloating, urge to regurgitate, urge to defecate, nausea, dizziness, and acute transient abdominal pain. |
| Gaskell et al. [unpublished observations] Tamb 35.7°C and 23% RH |
Incidence: Severity*: |
NA 9 (13) |
88% 17 (82) |
71% 8 (46) |
59% 7 (22) |
12% 1 (10) |
Belching, upper abdominal bloating, flatulence, lower abdominal bloating, lower abdominal pain, and urge to defecate. |
* Mean (SD) of summative accumulation of rating scale point score of measured time periods (corrected for number of measured time periods). GIS include: upper- (gastroesophageal and gastro-duodenal originated: regurgitation, urge to regurgitate, gastric bloating, belching, stomach pain, and heartburn/gastric acidosis) and lower- (intestinal originated: flatulence including lower-abdominal bloating, urge to defecate, abdominal pain, abnormal defecation including loose water stools, diarrhea, and blood in stools) GIS, and/or other related symptoms (e.g. nausea, dizziness, and acute transient abdominal pain). 1Summative accumulation of upper-, lower-, and other-GIS; 2summative accumulation of upper- or lower-GIS. Tamb: ambient temperature, RH: relative humidity, NA: not applicable.
Prevention and management strategies
Considering the multifactorial etiology and pathophysiology of EIGS, and associated GIS, it is imperative that athletes undergo a comprehensive individual gastrointestinal assessment, [4,23,93]mimicking the ambient temperatures experienced during training and (or) competition, in which GIS occur. Such an assessment aims to identify which primary causal and (or) exacerbation factor(s) (i.e. extrinsic and (or) intrinsic) may be contributing to GIS; and should be performed prior to any programmed intervention, since there is no “one-size-fits-all” approach to preventing or managing EIGS, and associated GIS. Assessment should also include habitual fueling and hydration regimes to ascertain gastrointestinal tolerance in response to the tested ambient temperature. It has consistently been observed within clinical practice and athlete support interventions that the management of these exacerbating factors appears to be the first line action in reducing gastrointestinal perturbations and symptoms associated with exertional-heat stress, as these attempt to attenuate the circulatory-gastrointestinal and neuroendocrine-gastrointestinal pathways’ primary factors. Certain dietary and nutritional strategies before and (or) during exercise may be either beneficial, favorable, neutral, or damaging in supporting gastrointestinal health in athletes exposed to exertional-heat stress, depending on whether or not these strategies address the prime causal and exacerbating factor(s) specific to the athlete. The current evidence for these dietary and nutritional strategies, as part of a targeted approach to address an identified causal and (or) exacerbating factor(s), will now be presented.
Hydration status
Dehydration is a common feature of exercise, especially in hot ambient conditions. There is emerging evidence that dehydration may negatively affect gastrointestinal integrity and function independently of ambient temperature, leading to greater incidence and severity of GIS. Potential mechanisms include: (1) the increase in skin blood flow that occurs during exercise, to aid thermoregulation, is accentuated with the addition of heat stress, potentially exacerbating splanchnic hypoperfusion [94]. (2) The increased body water losses that occur through increased sweating during exercise, are further accentuated with heat stress [95]. If not adequately replaced, body water losses can result in significant reductions in plasma volume Pv [96], which may exacerbate the effects of blood flow redistribution in burdening splanchnic perfusion [97]. And, (3) Dehydration has been independently shown to increase sympathetic drive in response to exercise stress, with the added effects of heat stress [98].
Demonstrating this, pre-exercise dehydration before 90 min of cycling at 70% V̇O2max resulted in impaired gastric emptying, and greater GIS, compared with exercise commenced in a euhydrated state [99]. Additionally, a 1.5% body mass loss in response to 1 h of running at 70% V̇O2max was sufficient to increase gastroduodenal and intestinal permeability above resting levels [100]. Both field and controlled-laboratory studies have shown that dehydration (e.g. >2% exercise-induced body mass loss (BML) and plasma osmolality (POsmol) >300 mOsmol/kg) may exacerbate endotoxemia, and subsequently cytokinemia, in the post-exercise period [3,81]. Laboratory data have confirmed that maintaining euhydration (BML 0.6%, post-exercise POsmol 291 mOsmol/kg, and Δ Pv −1.0%) during 2 h of running at 70% V̇O2max in temperate conditions (Tamb 24.7°C and 46% RH), was more favorable in attenuating markers of EIGS compared with dehydration (BML 3.1%, post-exercise POsmol 306 mOsmol/kg, and Δ Pv −5.8%) [59]. Specifically, the exercise-associated rise in I-FABP was significantly greater in the dehydrated state (∆ pre- to post-exercise: 539 pg/ml vs. 371 pg/ml), as was carbohydrate malabsorption of the pre-exercise meal, observed by a greater increase in breath H2 in the post-exercise period (area under the curve: 1188 ppm/3 h vs. 579 ppm/3 h). However, such modest disturbances to gastrointestinal integrity and function did not result in exacerbation of systemic endotoxemia or cytokinemia with dehydration. Interestingly, whilst the incidence of total-GIS was greater in the dehydrated (82%) compared to euhydrated state (64%), no differences in GIS severity were observed between conditions. This may be because the programmed nature (i.e. forced drinking schedule) of the water consumed during the euhydrated trial (844 ml/h), in itself, contributed to the development of GIS. This likely occurred through gastric intolerance due to overwhelmed intragastric pressure, in comparison to ad libitum intake that would cater for ameliorating these debilitating gastric factors [101]. From a clinical perspective, anecdotally, we have previously observed several endurance athletes with poor gastrointestinal tolerance to fluid intake, despite ingesting a volume that is grossly inadequate to maintain euhydration (i.e. < measured sweat losses). In support of this, endurance athletes commonly report avoiding aggressive fluid replacement strategies, specifically to prevent the onset of GIS during exercise [102].
Regardless of tolerance to fluid intake, over-hydration is also associated with GIS [103]. At its extreme, the onset of exercise-associated hyponatremia (EAH), which can result from an accumulated effect of excessive fluid intake, is associated with multiple GIS [104]. These include nausea with mild EAH, and vomiting resulting from severe EAH and (or) EAH encephalopathy. It is recommended that athletes assess fluid intake and losses in training and competition, using a fluid balance assessment, in order to avoid either significant dehydration or over-hydration during exercise [105]. For endurance exercise, exceeding ≥4 h, where the incidence of EIGS is greatest, body mass changes may not adequately reflect hydration status, and care is needed in interpretation of fluid balance assessments undertaken over this duration [101,106,107]. Drinking fluid ad libitum during such endurance exercise bouts appears to be adequate for most individuals to maintain euhydration [101,103,106]. However, a fluid balance assessment with the additional assessment for gastrointestinal tolerance to fluids may help to identify individuals where ad libitum fluid consumption may not be adequate.
As well as fluid intake, it is a common practice for athletes to supplement with sodium, before and during exercise [108–110]. Anecdotally, one rationale for sodium supplementation is the focus and belief on attenuating dehydration, and subsequently reducing occurrence and severity of GIS. It has been observed that sodium ingestion during submaximal exercise increases thirst and voluntary fluid intake when fluid is consumed ad libitum [111], and this could result in greater Pv over multiple hours of exercise that influence both the circulatory-gastrointestinal and neuroendocrine-gastrointestinal pathways. However, sodium intake does not appear to exert any effect on core body temperature independent of water intake [112]. To date, no research has confirmed whether sodium supplementation, either independent of fluid ingestion or as a means of stimulating increased ad libitum fluid intake, plays a role in preventing or reducing outcomes of EIGS [113,114].
Feeding during exercise
Considering there is a hyperperfusion to the gastrointestinal system with digestion and absorption of nutrients, it is feasible that regular ingestion of nutrients during exercise may play an important role in dampening exercise-associated splanchnic hypoperfusion, and consequently the associated gastrointestinal perturbations [115,116]. Indeed, Qamar & Read [116] originally observed splanchnic blood flow to increase with the ingestion of a liquid test meal during a moderate intensity 15-min treadmill run. Moreover, Rehrer et al. [117] reported a maintained portal vein blood flow to a greater extent with glucose intake pre-exercise and every 20 min, compared with water alone, during 1 h of cycling exercise at 70% V̇O2max in temperate ambient conditions. The absorption of nutrients such as glucose, amino acids, and amino acid precursors (i.e. glutamine, aspartate, glycine, L-arginine, and L-citrulline) provide a stimulus for the production of metabolic vasodilators, including nitric oxide, which may also potentially promote vasodilation of the villi microvasculature, and enhance gastrointestinal perfusion [15,71,115].
In support of the aforementioned, Snipe et al. [118] showed consumption of 15 g of glucose before and every 20 min (45 g/h) during 2 h running at 60% V̇O2max in the heat (Tamb 35°C) completely prevented epithelial injury, reduced small intestinal permeability, supported endotoxin clearance, and reduced markers of physiological strain (Figure 7). Furthermore, GIS incidence and severity did not intensify compared with water. In the same study, equivalent ingestion of hydrolyzed whey protein before and every 20 min during running was investigated. While intestinal injury was similarly prevented and small intestinal permeability substantially reduced compared with water, endotoxin and cytokine profiles were not affected in comparison to glucose supplementation. In addition, protein intake increased GIS incidence and severity compared to both glucose and water, possibly due to tolerance difficulties under exertional-heat stress conditions. Similarly, Lambert et al. [119] observed no additional benefit of glutamine supplementation the night before, immediately prior to, and within a 6% w/v carbohydrate beverage during 60 min of running at 70% V̇O2max in temperate ambient conditions (Tamb 22°C; Tre <39.0°C in all trials) on gastrointestinal permeability, over the 6% w/v carbohydrate beverage alone. It therefore appears that frequent feeding of small amounts of tolerable carbohydrate before and during exertional-heat stress plays an integral part in preventing and (or) attenuating EIGS, while not exacerbating GIS during exertional-heat stress, unlike the ingestion of other nutrients, such as protein.
Figure 7.
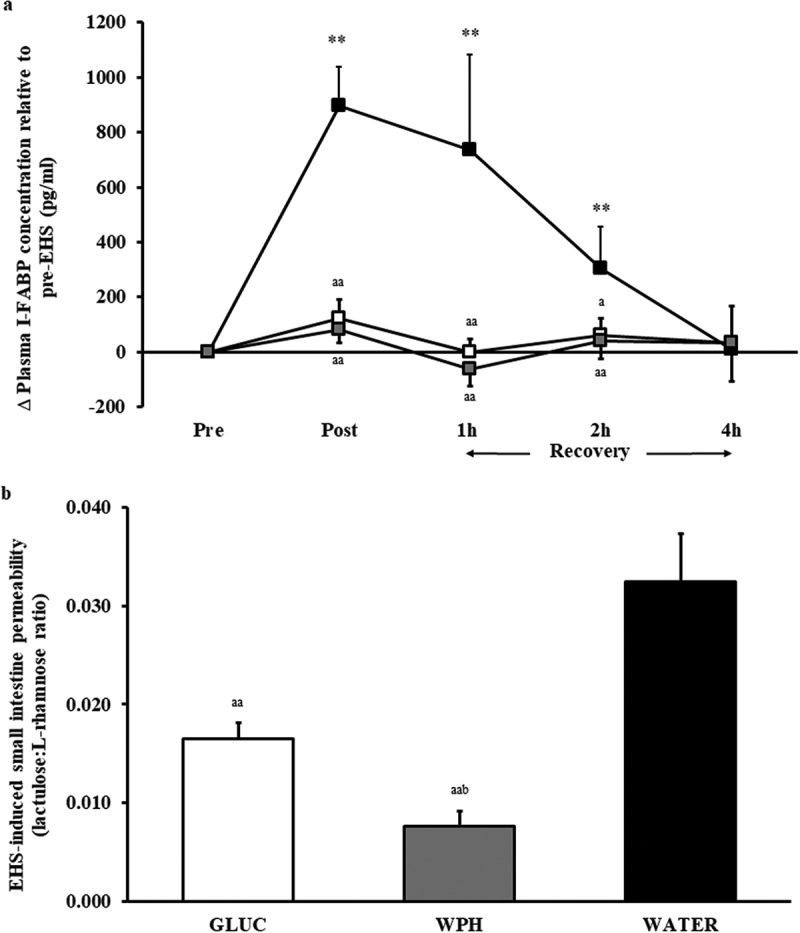
Change in plasma I-FABP concentration (a), and exertional-heat stress (EHS) induced small intestine permeability (b), in response to 2 h of running at 60% V̇O2max in Tamb 35°C on glucose (GLUC: white), hydrolyzed whey protein (WPH: gray), or water (WATER: black). Mean ± SEM (n = 11): **, p < 0.01 vs. pre-EHS; aa p < 0.01 and a p < 0.05 vs. WATER. From Snipe et al. [118], with permission.
Cooling strategies
The rise in core body temperature in response to exertional-heat stress appears to contribute substantially toward the perturbations to gastrointestinal integrity, function, symptoms, and systemic responses. It therefore seems logical that attempting to reduce core body temperature before and (or) during exertional-heat stress would provide a beneficial strategy to avoid such performance debilitating and potentially health issues [120–122]. To date, a small number of studies have investigated the effects of internal cooling during exertional-heat stress (i.e. per-cooling), external cooling before exertional-heat stress (i.e. pre-cooling), and heat acclimation on various aspects of EIGS.
Internal cooling
The ingestion of cold fluid before and during exertional-heat stress, for the purpose of pre- and per-cooling, may help to minimize gastrointestinal perturbations, by attenuating the increase in localized gastrointestinal temperature and peripheral blood flow associated with exercise, and consequently lessening the extent of gastrointestinal hypoperfusion [122,123]. Internal cooling strategies may also reduce overall sympathetic drive, lessening the extent of gastrointestinal dysfunction and (or) paralysis [4]. Core body temperature has been shown to reduce by up to 0.7°C with internal pre- and per-cooling strategies during exertional-heat stress [124–126]. In a counterbalanced randomized control trial, male triathletes (n = 9) ingested 617 g of an ice slurry sports drink (<1°C) during the cycle leg of a simulated Olympic distance triathlon in the heat (Tamb 32–34°C, 20–30% RH) or the same volume of a control solution (i.e. sports drink at the experimental room temperature, ~30°C) [127]. Intragastric temperature was significantly lower with ice slurry ingestion compared to control at 47 min of the cycle leg, after cycling, and at 1.5 km of the run leg; although no significant difference was found for perceptual gastrointestinal discomfort between trials. Using a more comprehensive experimental design and analysis, Snipe and Costa [60] investigated the ingestion of 250 ± 40 ml/15 min of cold (0.4°C) or cool (7.3°C) water during 2 h running at 60% V̇O2max in Tamb 35°C on gastrointestinal integrity, systemic responses, and GIS, compared to the ingestion of temperate water at 22.1°C. In comparison to temperate water ingestion, cold and cool water ingestion during exertional-heat stress ameliorated the rise in Tre (2.0 ± 0.5°C vs. 1.6 ± 0.4°C and 1.7 ± 0.4°C, respectively), resulting in a modestly lower peak Tre (38.9°C vs. 38.6°C and 38.6°C, respectively) and a lower plasma cortisol concentration (507 nMol/L vs. 435 nMol/L and 354 nMol/L, respectively). There was no effect of water temperature on GIS incidence and severity, except for cold and cool water ingestion tending to reduce upper-GIS, predominately urge to regurgitate. There was also no differences for peak post-exercise cytokine profile (plasma IL-6, IL-1β, TNF-α, IL-8, IL-10, and IL-1ra concentration) between cold, cool, and temperate water ingestion. However, a trend was observed for a truncated pre- to post-exercise magnitude in intestinal injury (plasma I-FABP concentration: -460 pg/ml and -428 pg/ml, respectively) with cold and cool water, in comparison to temperate water ingestion. Large individual variations in Tre and post-exercise I-FABP were observed in the cold and cool water trials, suggesting that the ingestion of such fluid temperatures may only benefit those athletes that respond well to internal cooling strategies. Interestingly, outcome measures such as Tre, heart rate, and plasma I-FABP concentration were similar with cold and cool water per-cooling, indicating that the consumption of ~7°C water may be as effective in reducing thermoregulatory strain and intestinal injury as water at 0°C, and more practical due to the difficulties of accessing and ingesting fluids at ~0°C during exertional-heat stress. In summary, cold or cool water ingestion before and during exertional-heat stress appears to have no substantial effect on GIS or systemic responses during prolonged exercise, such as 2 h of running or a simulated Olympic distance triathlon, but may play a role in reducing thermoregulatory and exertional strain, and promoting a small beneficial role on gastrointestinal integrity.
External cooling
From a theoretical perspective, external cooling strategies before and (or) during exertional-heat stress may also reduce exercise-associated splanchnic hypoperfusion and sympathetic drive; and possibly to a greater extent compared to internal cooling strategies. This is due to the larger surface area of the body’s periphery, versus the compacted anatomy of the gastrointestinal tract, allowing for more efficient transfer of heat or cold through temperature transfer mechanisms. To date, no study has comprehensively assessed the impact of external cooling strategies, such as (1) pre-cooling ice bath, cold water emersion, and ice or chill vest systems, and (2) per-cooling cold water shower or wash-down, and ice or chill vest systems, on markers of EIGS. However, attenuating the exercise-induced increase in core body temperature has been shown to ameliorate exercise-associated cytokinemia (i.e. plasma IL-6, IL-12, TNFα, and IL-1ra concentration), in both male and female populations [128,129]. One recent study assessed inflammatory cytokine profile in response to 90 min running at 65% V̇O2max in Tamb 32°C (47% RH), following 1 h of whole-body pre-cooling in a 20°C water bath on one occasion, and 1 h of seated rest in Tamb 20°C (control) on another occasion [130]. Whole-body pre-cooling resulted in an attenuation of eHsp72 responses, and modest attenuation of plasma IL-6 and IL-10 concentrations, but no difference in plasma TNF-α and IL-1ra concentrations were observed. In contrast, pre-cooling with ice-vest and cold towel intervention before 30 min of intermittent sprint exercise in the heat showed no effect on plasma IL-6 concentration compared to no cooling [131].
How these inflammatory cytokine responses relate to exertional-heat stress associated gastrointestinal perturbations is still unknown, and warrants exploration. Prior to further investigation, the following should be considered. Firstly, taking into account the modest mean and peak Tre (38.25°C and 38.88°C, respectively), and modest ∆ pre- to post-exercise cytokine values [130], it is likely the exertional-heat stress was insufficient to promote any substantial degree of gastrointestinal perturbations to effect systemic inflammatory responses [4,13,14]. Secondly, the progressive rise in Tre, from pre- to post-exercise, was more pronounced with whole-body pre-cooling (3.14°C) compared with control (2.19°C), resulting in similar peak Tre (38.85°C vs. 38.88°C) [130]. It is speculated that whole-body pre-cooling would therefore not promote favorable outcome in managing EIGS in response to a substantial degree of exertional-heat stress (e.g. ≥2 h of exercise in Tamb 35°C) [4], and thus additional supportive external cooling strategies would be required. For example, investigation into more aggressive external-cooling strategies, such as pre-cooling in adjunct with per-cooling, tested within a sufficient exertional-heat stress model, is required to determine whether external cooling strategies actually provide some protection against EIGS.
Heat acclimatization and acclimation
Heat acclimatization and acclimation protocols have been extensively investigated, with the aim of creating acute adaptations, such as increased plasma volume, which improve thermoregulatory and cardiovascular strain to exertional-heat stress; and more prolonged adaptations, such as increased sweating responses, renal hormonal adjustments, and enhanced thermal perception, which improve overall tolerance to exertional-heat stress [64,132,133]. Therefore, if thermoregulatory strain and intolerance is reduced by such adaptations, it is speculated and seems logical that the magnitude of EIGS, and associated GIS, would also be reduced. To date, only one study has investigated the impact of consecutive days of exertional-heat stress exposure on markers of gastrointestinal integrity. Over five consecutive days, participants ran at ~11.1 km/h (running speed equivalent to 4mMol/L lactate) in Tamb 40°C (40% RH), either to volitional exhaustion or until an increase of 2°C core body temperature was reached (day range: 24–26 min of exercise), with water provided ad libitum [64]. The magnitude of Tre rise was less on Day 5 (peak: 38.7°C) vs. Day 1 (39.0°C); however, no differences were seen for physiological strain index, running distance, circulating LPS levels, plasma I-FABP concentration, and cytokine profile (except for a modest reduction in plasma TNF-α concentration). It appears the exertional-heat stress, both total daily exposure and total number of repetitive exposures, was insufficient to warrant any substantial adaptation, especially increased plasma volume, which is a prerequisite to support thermoregulation and exertional-heat stress tolerance. Indeed, frequent (24–48 h) exertional-heat stress accumulative to ≥6 h, with each exposure attaining substantial thermoregulatory strain (e.g. Tre >38.5°C) has been reported to result in a substantial rise in Pv, and subsequently improved cardiovascular and thermoregulatory function, to the equivalent exertional-heat stress exposure [2,68,134]. It is therefore proposed that greater daily exertional-heat stress and a greater number of exposures may support the management of EIGS, and warrants substantiation through further exploration.
Dietary FODMAP modification
Often diet in the pre-, during-, and post-exercise period can play a key role in influencing exercise-associated GIS. It has almost become traditional for athletes to alter their diets and (or) be advised to limit their pre- and during-exercise dietary fiber, fat, and protein intake, in order to help prevent exercise-associated GIS. Moreover, it has been reported that athletes without diagnosed coeliac disease are seeking a gluten-free dietary approach, in the belief it may prevent symptoms [135]. However, such a diet has failed to show any gastrointestinal benefits in experimental models [136]. Interestingly, in a cohort of 910 athletes, >50% were found to unknowingly be avoiding foods high in fermentable oligo-di-mono-saccharide and polyols (FODMAPs) with the purpose to prevent exercise-associated GIS [137]. Remarkably, 80% of the cohort self-reported general symptom improvement.
A collection of short-chain rapidly fermentable carbohydrates, FODMAPs are important dietary triggers of irritable bowel syndrome [138,139]; and are found in a range of foods, including fruits such as apples and mangoes, lactose containing products such as milk and yoghurt, and a variety of vegetable and grain sources including legumes, wheat, rye, barley, onion, and garlic. The malabsorption of ingested FODMAPs can increase intestinal luminal content through gas production and osmotic water translocation, owing to bacterial fermentation and the osmotic properties of carbohydrate types. Consequently, lower-GIS such as excessive flatulence, lower abdominal bloating, and urge to defecate may be triggered, and lower abdominal pain in individuals with visceral hypersensitivity due to increased luminal distention [31,138,139]. These same lower-GIS mimic those experienced by athletes undertaking strenuous exercise in hot ambient conditions. From a translational perspective, compromised digestion and absorption that may occur in response to exertional-heat stress, risks malabsorption of FODMAPs ingested before, during, or after exertional-heat stress [4,140]. As a result, lower-GIS may be triggered, and plausibly, rapid onset of upper-GIS via the ileal brake mechanism [23,29,32,34]. Athletes expectedly have a high dietary FODMAP intake, due to the variety and volume of food and nutrient rich fluids consumed to meet energy requirements. Indeed, in a typical diet of a multisport athlete, 80 g/day of dietary FODMAPs [141] was detected, in comparison to the average Western intake of approximately ~25 g/day [142].
Dietary FODMAP modification has shown some potential to reduce GIS in athletes at rest and during exercise; albeit current evidence is primarily derived from case studies [141,143]. There has recently been much excitement and growing anecdotal advocacy for the use of a low FODMAP diet, for the purpose of reducing EIGS and associated GIS during exercise, in athletes presenting recurrent episodes of GIS during exercise [144]. Much of this attention appears related not only to the published case studies [141,143], but also to a recent single blind randomized cross-over study, conducted in temperate ambient conditions [145]. General reductions in GIS were observed at rest following 6 days of low (8.1 ± 3.5 g/day) dietary FODMAP provisions, compared with 6 days of high (41.4 ± 7.9 g/day) dietary FODMAP provisions, in a cohort (n = 11) of recreational competitive runners with symptomatic history. However, the study appears not to have provided sufficient exertional stress (i.e. sessions comprised of 5 × 1000 m at 100% V̇O2max, plus a 7 km threshold run) to prompt GIS incidence and sufficient severity within the exercise protocol, to adequately test the impact of the dietary intervention on GIS outcomes of concern to athletes (i.e. during exercise) [4]. Similarly, another study suggested a low FODMAP diet may support a reduction in exercise-associated GIS, however failed to identify GIS incidence and severity during exercise, with prime focus on the resting period [146]. Moreover, both of these studies discussed the role of EIGS pathophysiology in the incidence and severity of exercise-associated GIS, but neither study comprehensively measured any supportive circulatory-gastrointestinal or neuroendocrine-gastrointestinal pathway markers in response to the exertional stress [145,146]. The outcomes from both of these studies do not present novel findings, since it is already well established that low FODMAP dietary interventions can reduce GIS at rest in both free-living healthy and functional gastrointestinal disorder populations [142,147,148]. To date, only one study has comprehensively investigated the impact of dietary FODMAPs prior to exertional-heat stress on markers of EIGS and associated GIS. Non-heat acclimatized male and female endurance and ultra-endurance runners (n = 17) consumed a 24-h high (47 g/day) FODMAP diet or a 24-h low (2 g/day) FODMAP diet, in a double blind randomized cross-over design, before completing 2 h of running at 60% V̇O2max in hot ambient conditions (Tamb 35.7°C, 23% RH) [Gaskell et al., unpublished observations]. A recovery beverage matching the dietary intervention was consumed immediately after exertional-heat stress. In contrast to the low FODMAP diet, upper- and lower-GIS incidence and severity before and during running, and across the total intervention period (including acute and prolonged recovery periods) were greater following the high FODMAP diet (summative accumulation of rating scale point score of measured time periods and individual reported participant range: 44 (4–157) vs. 22 (2–76)). Breath H2 concentration was substantially greater throughout the high FODMAP trial (area under the curve (mean and 95% CI): high FODMAP diet 2525 (1452–3597) ppm/4h, and low FODMAP diet 1505 (1031–1978) ppm/4 h). Interestingly, the magnitude of intestinal injury was found to be substantially greater on the low FODMAP diet, compared with the high FODMAP diet, and a trend for a greater lipopolysaccharide binding protein response (i.e. endotoxemia magnitude marker) was also found in the low FODMAP trial, possibly due to greater hypoperfusion associated with lesser nutrient trafficking along the intestinal epithelium [23,118]. In summary, findings taken from this study demonstrate a low FODMAP diet reduces the incidence and severity of exercise-associated GIS, possibly through reducing carbohydrate malabsorption and associated compromise to gastrointestinal functional responses, but results in greater perturbations to gastrointestinal integrity. Since low FODMAP carbohydrate (i.e. glucose) ingested prior to and frequently during exertional-heat stress has been found to completely prevent intestinal epithelial injury [118,149]; best practice suggests that dietary advice targeting both GIS and gastrointestinal integrity, comprises a combination of at least a 24-h low FODMAP diet before, and low FODMAP carbohydrate sources ingested frequently during exertional-heat stress.
Nutritional supplementation
Within the public domain, there exists a substantial amount of testimonials and anecdotal comments suggesting a wide array of nutritional supplements to “prevent” exercise-associated GIS. Despite a substantial amount of research being conducted to understand the potential causal mechanisms, very limited research has been done in determining the effectiveness of certain nutritional supplements in the prevention and management of EIGS. To date, the only nutritional supplement interventions that have received research attention, in regards to gastrointestinal integrity, function, and (or) systemic responses, with or without GIS, using exertional-heat stress experimental designs in humans, have been antioxidants, amino acids L-arginine and glutamine, bovine colostrum, curcumin, and probiotics (Table 3). Due to the varying degrees of exertional-heat stress, the limited and inconsistent scope of EIGS markers within and between experimental protocols, the modest magnitude of gastrointestinal perturbation marker amelioration (including no abolishment responses in several studies) that do not reflect values of clinical significance, and variability in supplementation interventions (e.g. form, dosage, frequency, and timescale), substantial discrepancies between study outcomes are evident. Therefore, the translational and practical message from these nutrition supplementation interventions is difficult to ascertain, and the clinical and performance relevance is unclear. Nevertheless, considering nutritional supplement interventions target the secondary outcomes of EIGS, it is unlikely that these play any substantial role in preventing the primary causal mechanisms (i.e. splanchnic hypoperfusion and sympathetic drive), with only amelioration of secondary outcomes are potentially feasible.
Table 3.
Summary of human research studies investigating the potential role of nutritional supplement interventions for the prevention or attenuation of exercise-induced gastrointestinal syndrome (EIGS) markers, and exercise-associated gastrointestinal symptoms (GIS), in response to exertional-heat stress and (or) field competitive events, in comparison to placebo or control.
| Supplement | Supplementation intervention | Exertional-heat stress and (or) field competitive events | Gastrointestinal integrity, function, symptom, and systemic outcomes (pre- to post-exercise magnitude vs. placebo or control) |
|---|---|---|---|
| Antioxidants [77] |
2 weeks Vitamin E, 1000 IU/day. |
Marathon competition, Tamb not reported. | ↔ Intestinal permeability. ↔ Overall incidence and severity of gastrointestinal symptoms. |
| L-Arginine [150] |
14 days 30 g/day. | Marathon competition in Tamb -2°C | ↔ Intestinal permeability. ↔ Incidence and severity of gastrointestinal symptoms. |
| Glutamine [73][151][152] |
7 days 0.9g/kgFFM/day. Pre-exercise 0.9g/kgFFM. Pre-exercise 0.25, 0.5, and 0.9 g/kgFFM. |
1 h running at 70% V̇O2max in Tamb 30°C. 1 h running at 70% V̇O2max in Tamb 30°C. 1 h running at 70% V̇O2max in Tamb 30°C. |
↓ Intestinal permeability. ↑ IκBα expression. ↑ HSF-1, HSP70 and occludin expression in response to heat stress. ↔ Intestinal permeability. ↔ Endotoxemia. ↔ TNF-α. ↔ HSP70 and IκBα expression. ↓ Intestinal permeability, ↔ Epithelial injury, ↔ Gastrointestinal symptoms. |
| Bovine colostrum [65][153] |
7 days 1.7 g/kg/day. 14 days 20 g/day. |
90 min of altered intensity cycling and running exercise in Tamb 30ºC 46 min running at 95% ventilatory threshold in Tamb 40ºC. |
↔ Intestinal permeability ↔ endotoxemia and cytokinemia (IL-1β, TNF-α, IL-6, IL-10 and IL-8). ↔ I-FABP. |
| Probiotics [154][81][155][156][157] |
4 weeks multistrain probiotic capsule. 7 days 1.0 ×1010 L. casei CFU/day beverage24. 12-weeks multi-strain probiotic + prebiotic + antioxidant capsule vs. multi-strain probiotic + prebiotic capsule. 3 months 4.0 × 1010 L. rhamnosus CFU/day beverage. 12-weeks multi-strain probiotic capsule vs. multi-strain probiotic + glutamine powder. |
Running exercise to fatigue at 80% of ventilatory threshold (~35 min) in Tamb 35ºC. 2 h running exercise at 60% V̇O2max in Tamb 34.0ºC. Long distance triathlon competition, Tamb not reported. Marathon competition, Tamb not reported. Multi-stage ultra-marathon (7 days 249 km, Sahara Desert, Tmax 39ºC). |
↔ Intestinal permeability. ↔ Neutrophil function. ↔ LPS and anti-LPS IgM. ↔ Cytokinemia (TNF-α, IL-6, IL-10 and IL-1ra). ↔ Incidence and severity of gastrointestinal symptoms. ↑ Endotoxemia. ↔ Cytokinemia (IL-1β, TNF-α, IFN-γ, IL-6, IL-10 and IL-8). ↔ Incidence of gastrointestinal symptoms. ↔ Intestine permeability.a ↔ Endotoxemia and anti-endotoxin.a Unclear description of gastrointestinal symptoms during and after exercise. ↔ Incidence of Gastrointestinal symptoms. ↓ Duration of Gastrointestinal symptoms incidence. ↔ Incidence of oral and upper respiratory tract symptoms. ↔ eHSP72. |
| Curcumin [158] |
3-days 500 mg/day curcumin. | 1 h running at 65% V̇O2max in Tamb 37ºC. | ↓ I-FABP. ↓ IL-1ra. ↔ IL-6, MCP-1, and TNF-α. |
Significant reduction ↓↓ (p < 0.01) and ↓ (p < 0.05) vs. placebo or control, significant increase ↑ (p < 0.05) vs. placebo or control, ↔ no significant change or unclear statistical consistency and representation vs. placebo or control. BM: Body mass, FFM: fat free mass, Tamb: ambient temperature, Tmax: maximal ambient temperature, CFU: colony forming units. a Measured day prior to event and then at 6-days postevent.
Probiotics
With respect to gastrointestinal health, there is significant interest and belief amongst the general public for the administration of probiotic supplementation, with the aim of optimizing gastrointestinal health and preventing gastrointestinal related issues, despite multiple systematic reviews that do not support such claims [159–161]. Similarly, it is a common habit for athletes to consume probiotics (e.g. pharmaceutical style capsules or powders, and commercially available beverages or yogurts) leading into competition, during periods of intensified training, and (or) episodes of ill health; whether self-administered or recommended by the athlete’s professional support network. Considering probiotic bacteria have demonstrated favorable mechanistic effects in vitro on epithelial integrity, albeit using Lactobacillus plantarum [162,163], and may have favorable clinical outcomes in some populations presenting compromised intestinal integrity (e.g. infection and inflammation) [164], it is suggested that probiotic supplementation may also ameliorate exercise-associated gastrointestinal perturbations. Previous research has established that as the dosage of probiotic supplementation (e.g. Bifidobacterium animalis ssp. lactis × 1010 vs. × 1011 colony-forming unit (CFU)/day) increases, the proportion of bacteria-specific positive identification also increases [165]. Thus, it is generally speculated that providing a higher probiotic dosage over a shorter duration, or lower probiotic dosage over a more prolonged time period, will create a scenario of bacterial abundance of specific strain(s) and a subsequent protective effect. The mechanisms of action by which increasing the relative abundance of certain short chain fatty acid-producing bacteria (e.g. butyrate producing Lactobacillus and Bifidobacterium) may provide a protective effect to the secondary outcomes of EIGS include, but are not limited to: enhancing the mucosal lining, enterocyte stability and functional capabilities, antimicrobial protein and immunoglobulin secretions, immune regulation of luminal pathogenic agents including enhanced regulation of local inflammatory responses, and epithelial tight-junction protein stability and regulatory function [166–169].
Two blinded cross-over randomized controlled laboratory studies and three field-based intervention studies have reported on the impact of probiotic supplementation on markers of gastrointestinal integrity, with or without GIS, in response to exertional-heat stress and (or) competition (Table 3). Four weeks of capsule form Lactobacillus, Bifidobacterium, and Streptococcus (4.5 × 109 CFU) supplementation (n = 10 male runners) did not substantially alter exercise-associated perturbations to gastrointestinal integrity, endotoxemia and cytokinemia, compared with placebo, in response to running exercise to fatigue (~35 min) at 80% of ventilatory threshold in Tamb 35ºC [154]. A more recent study observed that oral ingestion of a commercially available probiotic beverage containing L. casei (volume equivalent for x1011 CFU/day) (n = 8 male runners) for seven consecutive days, before 2 h running at 60% V̇O2max in Tamb 34°C, resulted in a substantially greater endotoxemia (i.e. ∆ pre- to post-exercise: 15 pg/ml and 1 h post-exercise: 46 pg/ml) in all participants, compared with matched placebo [81]. Despite a higher cytokinemia in the probiotic group, the magnitude of difference to the placebo group was not substantial enough to warrant significance. It was speculated that these outcomes are potentially due to the increased dosage of fermentable carbohydrates (i.e. fructose and lactose) contained within the probiotic beverage formulation (vs. placebo). This in turn may have altered the gastrointestinal microbiota composition (e.g. increase in total bacterial abundance per se), promoting greater predisposition to exercise-associated endotoxin translocation, although this was not of clinical significance (i.e. no cytokinemia indicative of sepsis was observed). Indeed, a high FODMAP diet (95% CI: 16.9–30.6 g/day) has been shown to result in a significantly higher absolute bacteria abundance in the gastrointestinal tract, compared with a low FODMAP diet (95% CI: 1.9– 4.2 g/day) [170]. Moreover, acute consumption of fructose and lactose based FODMAP has been linked to small intestinal bacterial overgrowth, albeit in clinical populations [171,172]. On an additional note, recent reviews [173,174] have referred to the study by Lamprecht et al. [175], suggesting a multispecies probiotic supplementation (1010 CFU/day) for 14 weeks reduced intestinal hyperpermeability in response to strenuous exercise. Firstly, the biomarker used to establish intestinal permeability (i.e. fecal zonulin) is not an established marker to detect hyperpermeability. Secondly, the ELISA kit used to detect this none-established marker has recently been confirmed to contain analysis issues, warranting caution with results interpretation [176].
In regards to field based interventions, 12-week multistrain probiotic and prebiotic (fructooligosaccharide) capsule supplementation, with or without an antioxidant formulation (lipoic-acid and N-acetyl-carnitine hydrochloride), prior to an Ironman distance triathlon (n = 30), did not result in any substantial pre- to post-exercise differences in intestinal permeability and endotoxin profile [155]. It should be noted that researchers collected blood samples at baseline, 1 day before and 6 days after competition, and therefore the time periods of these resting blood samples failed to assess exercise-induced changes. Additionally, the modest, but clinically irrelevant, change reported in intestinal permeability (e.g. lactulose-mannitol ratio on placebo: baseline to pre-competition +0.03, and pre- to post-competition samples +0.02) and gram-negative bacterial endotoxin concentration (∆ range: 1–4 pg/ml) from baseline resting samples, are consistent with normal daily fluctuations [2]. Three months supplementation of 4.0 × 1010 L. rhamnosus CFU/day beverage (n = 141) did not reduce the incidence of GIS to a marathon competition [156]. In addition, 12-week multistrain probiotic supplementation (n = 32), with fructooligosaccharide or glutamine, did not differ eHSP72 responses to a multi-stage ultramarathon competition conducted in hot ambient conditions, compared with placebo [157].
On the contrary to common belief within the public domain, and unclear or erroneous reporting within scientific reviews on the role of probiotics on markers of gastrointestinal status (i.e. integrity, function, symptoms, and systemic responses) in response to exercise in human populations, which have not provided evidenced substantiation [173,174,177,178], it appears that probiotic supplementation (i.e. capsule or beverage) does not result in any clear and substantial beneficial outcomes on markers of gastrointestinal status in response to exertional-heat stress, which is in accordance with a recently published systematic literature review [179]. This is not surprising, considering the mechanisms of action proposed for beneficial effects are targeted toward enhancing secondary outcomes (i.e. integrity of the epithelial barrier), and not on prevention or amelioration of primary causal pathways (i.e. hypoperfusion and sympathetic drive). Therefore, at present, there is no justification for advising probiotic supplementation, and safety investigation is warranted, especially in response to exertional-heat stress, due to potentially unknown and/or underlying hidden outcomes.
Glutamine
Glutamine is proposed to play a role in supporting gastrointestinal health during exertional-heat stress by enhancing the expression of heat shock proteins (e.g. hsp70), which can prevent injury to the intestinal epithelium, and subsequently reduce intestinal permeability and localized and systemic inflammatory responses [24,25]. Additionally, protein intermediates, such as glutamine, may promote a scenario for improved villi microvascular hyperemia, through the production of localized metabolic vasodilators, principally nitric oxide that improves intestinal microvillus perfusion during exercise [24,73,115]. It has been demonstrated that glutamine supplementation (e.g. up to 0.9 g/FFM/day) for 7 days and pre-exercise reduced intestinal permeability, and increased expression of protective heat-shock factors (i.e. cellular protection) in response to 1 h of running at 70% V̇O2max in Tamb 30°C. However, no substantial improvements in other markers of EIGS were observed (i.e. intestinal injury, GIS, endotoxin, and cytokine profile) [73,151,152]. Moreover, the addition of glutamine before (2 × 1300 mg) and during exercise (0.6% w/v) in adjunct to a 6% w/v carbohydrate beverage did not provide any further benefits in attenuating intestinal injury in response to 1 h of running at 70% V̇O2max, albeit in temperate ambient conditions [119].
Bovine colostrum
Similarly, bovine colostrum is proposed to play a role in supporting gastrointestinal health in response to exertional-heat stress, by enhancing the expression of cellular protection factors, which may protect intestinal epithelial enterocytes, epithelial tight-junction stability proteins, and regulators, and thus maintain intestinal epithelial integrity [65,78,180–183]. To date, only one research group has found consistent attenuation in intestinal permeability, with one study showing an attenuation in intestinal injury with 14-day bovine colostrum supplementation (20 g/day), vs. placebo, prior to 20 min of running exercise at 80% V̇O2max, without heat stress [78,180,181,183]. The clinical and performance relevance of the reduced intestinal permeability is unclear, considering this marker of gastrointestinal integrity has limited meaning without other integrity and systemic response markers. The transferability of these findings is also limited due to the exertional stress used within the experimental design, without heat exposure. Overall, such exertional stress is not associated with gastrointestinal perturbations of clinical significance or pronounced GIS [4]; subsequently outcomes observed were modest changes to intestinal permeability and injury [78,180,181,183]. Interesting, other research studies have found no positive effect with varying doses of bovine colostrum supplementation, compared with placebo, using more pronounced exertional stress models, with or without heat stress, and assessing a wider variety of EIGS markers[65,153,182]. For example, 7 days of bovine colostrum supplementation (1.7 g/kg/day) did not alter (vs. placebo) intestinal permeability, endotoxemia, and cytokine profile in response to 90 min of alternating cycling and running exercise in Tamb 30°C [65], whilst 14 days of bovine colostrum supplementation (20 g/day) did not alter (vs. placebo) intestinal injury in response to 46 min of running exercise at 95% ventilatory threshold in Tamb 40°C [153].
Curcumin
The anti-inflammatory properties of curcumin have been suggested to ameliorate intestinal epithelial inflammatory mediators, subsequently reducing the local inflammation that promotes intestinal epithelial injury and hyperpermeability, and potentially reducing systemic endotoxin concentration and cytokine responses [158]. Only one study to date has investigated the effects of curcumin supplementation prior to exertional-heat stress on markers of EIGS. Curcumin supplementation for 3 days (500 mg/day) resulted in an attenuated increase (vs. placebo) in intestinal injury and inflammatory markers in response to 60 min of running exercise at 65% V̇O2max in Tamb 37°C [158]. It is, however, important to note that the magnitude of intestinal epithelial injury was modest in nature (∆ pre- to post-exercise plasma I-FABP concentration: curcumin 480 pg/ml vs. placebo 768 pg/ml) compared to more pronounced exertional-heat stress models [13,60]. The attenuation on curcumin vs. placebo was also negligible (plasma I-FABP concentration: 288 pg/ml). Such outcomes appear to be less effective than other strategies that are likely to have broader performance and (or) health benefits for athletes (e.g. cooling strategies, short-term low FODMAP diet, maintaining euhydration, and (or) carbohydrate ingestion before and during exertional-heat stress) [59,60,118,149, Gaskell et al. unpublished observations].
Summary and practical recommendations
Considering the anticipated hot and humid ambient conditions of the 2020 Tokyo Olympic Games, it is likely that a high incidence of reported or unreported EIGS may occur. It is now well established that exertional-heat stress plays a potent role in exacerbating the primary causal mechanisms of EIGS, and clearly the severity of exertional-heat stress has a direct impact on the magnitude of secondary outcomes. EIGS has the potential to impair nutrient delivery, exercise performance, and increases the risk of exercise-associated GIS and health implications. Thus, prevention and management strategies will be a key feature for any athlete attempting to optimize their performance in such environmental extremes. Emerging evidence indicates that attenuating the rise in thermoregulatory strain in response to exertional-heat stress may ameliorate the magnitude of secondary outcomes; however, individual tolerance will dictate the success of any particular intervention. As such, prior to any individualized intervention, a tailored gastrointestinal-assessment is recommended as the essential first line action, to ascertain the singular or multifaceted causal mechanisms of an athlete’s gastrointestinal issues in response to exertional-heat stress. Thereafter, recent findings indicate management of exacerbation factors, maintenance of euhydration using cool fluids, consuming tolerable amounts of carbohydrate frequently (e.g., every ~20 min) during exercise, and short-term dietary FODMAP restriction, as promising strategies to moderate alterations in gastrointestinal integrity and function, and associated symptoms, in response to exertional-heat stress. Other aforementioned strategies are more ambiguous in nature, and their true clinical relevance in preventing or managing aspect of EIGS are not certain. Table 4 provides an overview of practical recommendations for the management of EIGS, and associated GIS, as a result of exertional-heat stress.
Table 4.
Practical recommendations for the management of exercise-induced gastrointestinal syndrome, and associated gastrointestinal symptoms, as a result of exertional-heat stress.
|
Essential: - Undertake a gastrointestinal assessment (e.g. gut challenge protocols) during exertional-heat stress, for identification of primary causal mechanism and secondary gastrointestinal integrity and functional outcomes [4,23,93]. - Undertake a gastrointestinal assessment during exertional-heat stress in a training or competition mirrored simulation, to establish individual tolerance to fuel and fluid types, forms, and concentrations [23,29,93]. - Identify and manage extrinsic and intrinsic exacerbating factors [4]. |
|
Strategies to consider: - Begin exercising in hot ambient conditions euhydrated, and maintain euhydration throughout (within individual tolerance) using ad libitum fluid intake behaviors [59,106]. - Avoid overconsuming fluids, and scenarios of exercise-associated hyponatremia [29,103,104,106]. - Carbohydrate before and frequently throughout exertional-heat stress [118]. - Undertake a gastrointestinal assessment during exertional-heat stress to establish exercise-associated nutrient malabsorption, especially of high FODMAP sources (e.g., fructose, lactose, sorbitol, mannitol, and lupin flour) that are common ingredients found in sports nutrition products, and adapt pre- and during-exercise nutrition accordingly [23,29,143]. - Trial and practice competition nutrition in training sessions, including specific food and fluid quality and quantity [23]. - Avoid NSAID medication in the day leading up to, immediately before, and during exertional-heat stress [39]. - Gut training protocols [23,29]. - Short-term low FODMAP diet, with or without low residue, after assessment to establish tolerance [4,93,143]. - Internal and external cooling strategies to limit thermoregulatory strain; such as pre- and per-cooling using cool fluids, and cool water bathing, showers, or vests [60]. - Undertake appropriate heat acclimation/acclimitization protocols prior to competition in hot ambient conditions. Consider specific exercise mode, intensity, duration, and Tamb per exercise bout; and also, frequency of exertional-heat exposures and timescale between exposures [69]. |
|
Unlikely to be of benefit (unclear outcomes or requiring further substantiation): - Gluten free dietary interventions in athletes without diagnosed coeliac disease [136]. - Nutritional supplementation interventions (e.g. antioxidants, specific amino-acids, bovine colostrum, and curcumin) (Table 3). - Probiotic supplementation (Table 3). - Exercise or dietary regimes to alter gastrointestinal microbiota bacterial relative abundance, diversity, and (or) functional properties [4]. |
Acknowledgments
AM and RC contributed to the introduction. RC and RS contributed to the description of EIGS. AM, RC, and RS contributed to the impact of exertional-heat stress on EIGS. RC, AM, SG, and RS contributed to various parts of the prevention and management strategies. RC, AM, and SG contributed to various parts of the practical recommendations. RC was responsible to compiling the manuscript sections. All authors reviewed the full manuscript and approved the final version. The material within is the result of work supported with resources and the use of facilities at BASE Facility, Monash University, Department of Nutrition Dietetics & Food. Funding support from BASE Facility, Monash University, Department of Nutrition Dietetics & Food; Monash University, Faculty of Medicine Nursing & Health Sciences, Strategic Grant Scheme; and Sports Medicine Australia Research Foundation Grant.
Abbreviations
- BM
Body mass
- BML
Exercise-induced body mass loss
- CFU
Colony-forming units
- CH4
Methane
- CO2
Carbon dioxide
- CARS
Compensatory anti-inflammatory response syndrome
- EAH
Exercise-associate hyponatremia
- EIGS
Exercise-induced gastrointestinal syndrome
- FFM
Fat free mass
- FODMAP
Fermentable oligo-di-mono-saccharides and polyols
- GIS
Gastrointestinal symptoms
- H2
Hydrogen
- H2S
Hydrogen sulfide
- I-FABP
Intestinal fatty acid binding protein
- IL
Interleukin
- LBP
Lipopolysaccharide binding protein
- LPS
Lipopolysaccharide
- L/R
Lactulose to rhamnose ratio
- NFκβ
Nuclear factor kappa beta
- OCTT
Orocecal transit time
- POsmol
Plasma osmolality
- Pv
Plasma volume
- RH
Relative humidity
- RPE
Rating of perceived exertion
- sCD14
Soluble CD14
- SIRS
Systemic inflammatory response syndrome
- Tamb
Ambient temperature
- Tmax
Maximal temperature
- TNF
Tumor necrosis factor
- Tre
Rectal temperature
- Ttymp
Tympanic temperature
- V̇O2max
Maximal oxygen uptake
- Wmax
Maximal power (wattage) output
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Costa RJS, Snipe R, Camões-Costa V, et al. The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events. Sports Med Open. 2016;2(16):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Gill SK, Hankey J, Wright A, et al. The impact of a 24-hour ultra-marathon on circulatory endotoxin and cytokine profile. Int J Sports Med. 2015;36:688–695. [DOI] [PubMed] [Google Scholar]
- [3].Gill SK, Teixeira A, Rama L, et al. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc Immunol Rev. 2015;21:114–128. [PubMed] [Google Scholar]
- [4].Costa RJS, Snipe R, Kitic C, et al. Systematic review: exercise-induced gastrointestinal syndrome–Implication for health and disease. Aliment Ther Pharmacol. 2017;46(3):246–265. [DOI] [PubMed] [Google Scholar]
- [5].Engebretsen L, Soligard T, Steffen K, et al. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br J Sports Med. 2013;47(7):407–414. [DOI] [PubMed] [Google Scholar]
- [6].Pfeiffer B, Stellingwerff T, Hodgson AB, et al. Nutritional intake and gastrointestinal problems during competitive endurance events. Med Sci Sports Exerc. 2012;44:344–351. [DOI] [PubMed] [Google Scholar]
- [7]. ter Steege RWF, Van Der Palen J, Kolkman JJ. Prevalence of gastrointestinal complaints in runners competing in a long-distance run: an internet-based observational study in 1281 subjects. Scand J Gastroenterol. 2008;43:1477–1482. [DOI] [PubMed] [Google Scholar]
- [8].Jeukendrup AE, Vet-Joop K, Sturk A, et al. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin Sci. 2000;98:47–55. [PubMed] [Google Scholar]
- [9].Stuempfle KJ, Hoffman MD, Hew-Butler T. Association of gastrointestinal distress in ultramarathoners with race diet. Int J Sport Nutr Exerc Metab. 2013;23:103–109. [DOI] [PubMed] [Google Scholar]
- [10].Stuempfle KJ, Hoffman MD. Gastrointestinal distress is common during a 161-km ultramarathon. J Sports Sci. 2015;33(17):1814–1821. [DOI] [PubMed] [Google Scholar]
- [11].Hoffman MD, Fogard K. Factors related to successful completion of a 161-km ultramarathon. Int J Sports Physiol Perform. 2011;6(1):25–37. [DOI] [PubMed] [Google Scholar]
- [12].Kakamu T, Wada K, Smith DR, et al. Preventing heat illness in the anticipated hot climate of the Tokyo 2020 Summer Olympic Games. Environ Health Prev Med. 2017;22(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Snipe R, Khoo A, Kitic C, et al. Heat stress during prolonged running results in exacerbated intestinal epithelial injury and gastrointestinal symptoms. Eur J Appl Physiol. 2018a;118(2):389–400. [DOI] [PubMed] [Google Scholar]
- [14].Snipe R, Khoo A, Kitic C, et al. Mild Heat stress during prolonged running results in exacerbated intestinal epithelial injury and gastrointestinal symptoms. Int J Sports Med. 2018b;39(4):255–263. [DOI] [PubMed] [Google Scholar]
- [15].Grootjans J, Lenaerts K, Buurman WA, et al. Life and death at the mucosal-luminal interface: new perspectives on human intestinal ischemia-reperfusion. World J Gastroenterol. 2016;22(9):2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].ter Steege RW, Kolkman JJ. The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment Pharmacol Ther. 2012;35:516–528. [DOI] [PubMed] [Google Scholar]
- [17].van Wijck K, Lenaerts K, van Loon LJ, et al. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One. 2011;6(7):e22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Horner KM, Schubert MM, Desbrow B, et al. Acute exercise and gastric emptying: a meta-analysis and implications for appetite control. Sports Med. 2015;45(5):659–678. [DOI] [PubMed] [Google Scholar]
- [19].Leiper JB. Fate of ingested fluids: factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. 2015;73(S2):57–72. [DOI] [PubMed] [Google Scholar]
- [20].Strid H, Simrén M, Störsrud S, et al. Effect of heavy exercise on gastrointestinal transit in endurance athletes. Scand J Gastroenterol. 2011;46:673–677. [DOI] [PubMed] [Google Scholar]
- [21].van Nieuwenhoven MA, Brouns F, Brummer R-JM. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol. 2004;91:429–434. [DOI] [PubMed] [Google Scholar]
- [22].Peake JM, Della Gatta P, Suzuki K, et al. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. [PubMed] [Google Scholar]
- [23].Costa RJS, Miall A, Khoo A,et al. Gut training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl Physiol Nutr Metab. 2017;42(5):547–557. [DOI] [PubMed] [Google Scholar]
- [24].Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2016;120(6):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zuhl M, Schneider S, Lanphere K, et al. Exercise regulation of intestinal tight junction proteins. Br J Sports Med. 2014;48(12):980–986. [DOI] [PubMed] [Google Scholar]
- [26].Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. [DOI] [PubMed] [Google Scholar]
- [28].Lang JA, Gisolfi CV, Lambert GP. Effect of exercise intensity on active and passive glucose absorption. Int J Sport Nutr Exerc Metab. 2006;16(5):485–493. [DOI] [PubMed] [Google Scholar]
- [29].Miall A, Khoo A, Rauch C, et al. Two weeks of repetitive gut-challenge reduces exercise associated gastrointestinal symptoms and malabsorption. Scand J Med Sci Sports. 2018;28:630–640. [DOI] [PubMed] [Google Scholar]
- [30].van Wijck K, Pennings B, van Bijnen AA, et al. Dietary protein digestion and absorption are impaired during acute postexercise recovery in young men. Am J Phyiol Regul Integr Comp Physiol. 2013;304(5):R356–R361. [DOI] [PubMed] [Google Scholar]
- [31].Zhu Y, Zheng X, Cong X, et al. Bloating and distention in IBS. Am J Gastro. 2013;108(9):1516–1525. [DOI] [PubMed] [Google Scholar]
- [32].Layer P, Peschel S, Schlesinger T, et al. Human pancreatic secretion and intestinal motility: effects of ileal nutrient perfusion. Am J Physiol Gastrointest Liver Physiol. 1990;258:G196–201. [DOI] [PubMed] [Google Scholar]
- [33].Putkonen L, Yao CK, Gibson PR. Fructose malabsorption syndrome. Curr Opin Clin Nutr Metab Care. 2013;6(4):473–477. [DOI] [PubMed] [Google Scholar]
- [34].Shin HS, Ingram JR, McGill AT, et al. Lipids: can all macronutrients put a ‘brake’ on eating? Physiol Behav. 2013;120:114–123. [DOI] [PubMed] [Google Scholar]
- [35].Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43(2):181–196. [DOI] [PubMed] [Google Scholar]
- [36].Lambert GP, Boylan M, Laventure JP, et al. Effect of aspirin and ibuprofen on GI permeability during exercise. Int J Sports Med. 2007;28(9):722–726. [DOI] [PubMed] [Google Scholar]
- [37].Ryan AJ, Chang RT, Gisolfi CV. Gastrointestinal permeability following aspirin intake and prolonged running. Med Sci Sports Exerc. 1996;28(6):698–705. [DOI] [PubMed] [Google Scholar]
- [38].van Wijck K, Lenaerts K, Van Bijnen AA, et al. Aggravation of exercise-induced intestinal injury by Ibuprofen in athletes. Med Sci Sports Exerc. 2012;44(12):2257–2262. [DOI] [PubMed] [Google Scholar]
- [39].Warden SJ. Prophylactic use of NSAIDs by athletes: a risk/benefit assessment. Phys Sportsmed. 2010;38(1):132–138. [DOI] [PubMed] [Google Scholar]
- [40].Caradonna L, Amati L, Magrone T, et al. Enteric bacteria, lipopolysaccharide and related cytokines in irritable bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- [41].Hodgin KE, Moss M. The epidemiology of sepsis. Curr Pharm Des. 2008;14:1833–1839. [DOI] [PubMed] [Google Scholar]
- [42].Lim CL. Heat sepsis precedes heat toxicity in the pathophysiology of heat stroke- a new paradigm on an ancient disease. Antioxidant. 2018;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morris G, Berk M, Galecki P, et al. The emerging role of autoimmunity in myalgicencephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol Neurobiol. 2014;49:741–756. [DOI] [PubMed] [Google Scholar]
- [44].Opal SM. Endotoxins and other sepsis triggers. Contrib Nephol. 2010;167:14–24. [DOI] [PubMed] [Google Scholar]
- [45].Robson-Ansley P, Costa RJS. Overreaching and unexplained underperformance syndrome: nutritional interventions. In: Maughan R, editor. The encyclopaedia of sports medicine, an IOC medical commission publication- Volume 19: sports Nutrition. NJ, USA: Wiley-Blackwell publishers; 2014. Chapter 33, p. 404–414. [Google Scholar]
- [46].Thomas JL. Helpful or harmful? Potential effects of exercise on select inflammatory conditions. Phys Sportsmed. 2013;41:93–100. [DOI] [PubMed] [Google Scholar]
- [47].Grames C, Berry-Cabán CS. Ischemic colitis in an endurance runner. Case Rep Gastrointest Med. 2012;2012:356895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Donoghue A. Heat illness in the U.S. mining industry. Am J Ind Med. 2004;45:351–356. [DOI] [PubMed] [Google Scholar]
- [49].Fortune M, Mustard C, Etches J. Work-attributed illness arising from excess heat exposure in Ontario, 2004–2010. Can J Public Health. 2013;104(5):E420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hunt A, Parker A, Stewart I. Symptoms of heat illness in surface mine workers. Int Arch Occup Environ Health. 2013;86:519–527. [DOI] [PubMed] [Google Scholar]
- [51].Hyman M, Wu S, Hafizi H. Prevalence of gastrointestinal problems in occupations: a retrospective study. J Occup Environ Med. 2011;53:117. [DOI] [PubMed] [Google Scholar]
- [52].O’Connor FG, Casa DJ, Bergeron MF, et al. American College of Sports Medicine round table on exertional heat stroke. Return to duty/return to play: conference Proceedings. Curr Sports Med Rep. 2010;9:314–321. [DOI] [PubMed] [Google Scholar]
- [53].Nag P, Nag A, Ashtekar S. Thermal limits of men in moderate to heavy work in tropical farming’. Ind Health. 2007;45(1):107–117. [DOI] [PubMed] [Google Scholar]
- [54].Rav-Acha M, Hadad E, Epstein Y, et al. Fatal exertional heat stroke: a case series. Am J Med Sci. 2004;328:84–87. [DOI] [PubMed] [Google Scholar]
- [55].Costa RJS, Oliver SJ, Laing SJ, et al. Influence of timing of postexercise carbohydrate-protein ingestion on selected immune indices. Int J Sport Nutr Exerc Metab. 2009;19(4):284–366. [DOI] [PubMed] [Google Scholar]
- [56].Costa RJS, Walters R, Bilzon JLJ, et al. Effects of immediate post-exercise carbohydrate ingestion with and without protein on neutrophil degranulation. Int J Sport Nutr Exerc Metab. 2011;21(3):205–213. [DOI] [PubMed] [Google Scholar]
- [57].Costa RJS, Richardson K, Fortes MB, et al. The effects of post-exercise feeding on saliva IgA and anti-microbial proteins. Int J Sport Nutr Exerc Metab. 2012;22(3):184–191. [PubMed] [Google Scholar]
- [58].Rehrer NJ, Smets A, Reynaert H, et al. Effect of exercise on portal vein blood flow in man. Med Sci Sports Exerc. 2001;33:1533–1537. [DOI] [PubMed] [Google Scholar]
- [59].Costa RJS, Camões-Costa V, Snipe RMJ, Dixon D, Russo I, Huschtscha Z. The impact of exercise-induced hypohydration on intestinal integrity, function, symptoms, and systemic endotoxin and inflammatory responses. J Appl Physiol. 2019. (In press). [DOI] [PubMed] [Google Scholar]
- [60].Snipe R, Costa RJS. Does the temperature of water ingested during exertional-heat stress influence gastrointestinal injury, symptoms, and systemic inflammatory profile? J Sci Med Sport. 2018;21(8):771–776. [DOI] [PubMed] [Google Scholar]
- [61].Costa RJS, Swancott A, Gill S, et al . Compromised energy and nutritional intake of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment. Int J Sports Sci. 2013;3(2): 51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Costa RJS, Teixiera A, Rama L, et al. Water and sodium intake habits and status of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment: An observational study. Nutri.J. 2013;12(13): 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Costa RJS, Gill SK, Hankey J, et al. Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Br.J.Nutri. 2014;112, 428–437. [DOI] [PubMed] [Google Scholar]
- [64].Barberio MD, Elmer DJ, Laird RH, et al. Systemic LPS and inflammatory response during consecutive days of exercise in heat. Int J Sports Med. 2015;36(3):262–270. [DOI] [PubMed] [Google Scholar]
- [65].Morrison SA, Cheung SS, Cotter JD. Bovine colostrum, training status, and gastrointestinal permeability during exercise in the heat: a placebo-controlled double-blind study. Appl Physiol Nutr Metab. 2014;39(9):1070–1082. [DOI] [PubMed] [Google Scholar]
- [66].Sessions J, Bourbeau K, Rosinski M, et al. Carbohydrate gel ingestion during running in the heat on markers of gastrointestinal distress. Eur J Sport Sci. 2016;16(8):1064–1072. [DOI] [PubMed] [Google Scholar]
- [67].Sheahen BL, Fell JW, Zadow EK, et al. Intestinal damage following short-duration exercise at the same relative intensity is similar in temperate and hot environments. Appl Physiol Nutr Metab. 2018;43(12):1314–1320. [DOI] [PubMed] [Google Scholar]
- [68].Costa RJS, Crockford MJ, Moore JP, et al. Heat acclimation responses of an ultra-endurance running group preparing for hot desert based competition. Eur J Sport Sci. 2014;14(S1):S131–S141. [DOI] [PubMed] [Google Scholar]
- [69].Pryor JL, Johnson EC, Robert WC, et al. Application of evidence-based recommendations for heat accumulation: individual and team sport perspective. Temperature. 2018;6(1):37–49. doi: 10.1080/23328940.2018.1516537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev. 2004;32(4):185–190. [DOI] [PubMed] [Google Scholar]
- [71].van Wijck K, Lenaerts K, Grootjans J, et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and preventions. Am J Physiol. 2012;303:G155–G168. [DOI] [PubMed] [Google Scholar]
- [72].Pals KL, Chang RT, Ryan AJ, et al. Effect of running intensity on intestinal permeability. J Appl Physiol. 1997;82(2):571–576. [DOI] [PubMed] [Google Scholar]
- [73].Zuhl MN, Lanphere KR, Kravitz L, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014;116(1):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yeh Y, Law L, Lim C. Gastrointestinal response and endotoxemia during intense exercise in hot and cool environments. Eur J Appl Physiol. 2013;113(6):1575–1583. [DOI] [PubMed] [Google Scholar]
- [75].Pires W, Veneroso CE, Wanner SP, et al. Association between exercise-induced hyperthermia and intestinal permeability: A systematic review. Sports Med. 2017;47(7):1389–1403. [DOI] [PubMed] [Google Scholar]
- [76].Pires W, Wanner SP, Soares DD, et al. Author’s Reply to Kitic: comment on: “Association between exercise-induced hyperthermia and intestinal permeability: A systematic review”. Sports Med. 2018;48(12):2887–2889. [DOI] [PubMed] [Google Scholar]
- [77].Buchman AL, Killip D, Ou CN, et al. Short-term vitamin E supplementation before marathon running: a placebo-controlled trial. Nutr. 1999;15(4):278–283. [DOI] [PubMed] [Google Scholar]
- [78].Marchbank T, Davison G, Oakes JR, et al. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G477–G484. [DOI] [PubMed] [Google Scholar]
- [79].Ashton T, Young IS, Davison GW, et al. Exercise-induced endotoxemia: the effect of ascorbic acid supplementation. Free Radic Biol Med. 2003;35(3):284–291. [DOI] [PubMed] [Google Scholar]
- [80].Camus G, Nys M, Poortmans JR, et al. Endotoxemia production of tumor necrosis factor alpha and polymorphonuclear neutrophil activation following strenuous exercise in humans. Eur J Appl Physiol. 1998;79:62–68. [DOI] [PubMed] [Google Scholar]
- [81]. Gill SK, Allerton DM, Ansley-Robson P, et al. Does acute high dose probiotic supplementation containing lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia? Int J Sports Nutr Exercise Metab. 2016;26:268–275. [DOI] [PubMed] [Google Scholar]
- [82].Guy JH, Pyne DB, Deakin GB, et al. Acclimation training improves endurance cycling performance in the heat without inducing endotoxemia. Front Physiol. 2016;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lim CL, Pyne D, Horn P, et al. The effects of increased endurance training load on biomarkers of heat intolerance during intense exercise in the heat. Appl Physiol Nutr Metab. 2009;34(4):616–624. [DOI] [PubMed] [Google Scholar]
- [84].Bosenberg AT, Brock-Utne JG, Gaffin SL, et al. Strenuous exercise causes systemic endotoxemia. J Appl Physiol. 1988;65:106–108. [DOI] [PubMed] [Google Scholar]
- [85].Brock-Utne JG, Gaffin SL, Wells MT, et al. Endotoxemia in exhausted runners after a long-distance race. S Afr Med J. 1988;73:533–536. [PubMed] [Google Scholar]
- [86].Camus G, Poortmans J, Nys M, et al. Mild endotoxaemia and the inflammatory response induced by a marathon race. Clin Sci. 1997;92:415–422. [DOI] [PubMed] [Google Scholar]
- [87].Willmott AGB, Hayes M, James CA, et al. Once- and twice-daily heat acclimation confer similar heat adaptations, inflammatory responses and exercise tolerance improvements. Physiol Rep. 2018;6(24):e13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vargas N, Marino F. Heat stress, gastrointestinal permeability and IL-6 signalling- implications for exercise performance and fatigue. Temperature. 2016;3(2):240–251. doi: 10.1080/23328940.2016.1179380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Neufer PD, Young AJ, Sawka MN. . Gastric emptying during exercise: effects of heat stress and hypohydration. Eur J Appl Physiol. 1989;58:433-439. [DOI] [PubMed] [Google Scholar]
- [90].Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome consensus conference. 1st Rome H2-breath testing consensus conference working group. Aliment Pharmacol Ther. 2009;29(Suppl1):1–49. [DOI] [PubMed] [Google Scholar]
- [91].Bate JP, Irving PM, Barrett JS, et al. Benefits of breath hydrogen testing after lactulose administration in analysing carbohydrate malabsorption. Eur J Gastroenterol Hepatol. 2010;22(3):318–326. [DOI] [PubMed] [Google Scholar]
- [92].Gaskell SK, Snipe RMJ, Costa RJS. Test re-test reliability of a modified visual analogue scale assessment tool for determining incidence and severity of gastrointestinal symptom in response to exercise stress. Int J Sports Nutr Exercise Metab 2019. In press. [DOI] [PubMed] [Google Scholar]
- [93].Costa RJS, Hoffman MD, Stellingwerff T. Considerations for ultra-endurance activities: part 1 - nutrition. Res Sports Med. 2018. 27(2):166–181. [DOI] [PubMed] [Google Scholar]
- [94].Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78(5):603–612. [DOI] [PubMed] [Google Scholar]
- [95].Shirreffs SM, Sawka MN. Fluid and electrolyte needs for training, competition, and recovery. J Sports Sci. 2011;29(Suppl 1):S39–S46. [DOI] [PubMed] [Google Scholar]
- [96].Costill DL, Fink WJ. Plasma volume changes following exercise and thermal dehydration. J Appl Physiol. 1974;37(4):521–525. [DOI] [PubMed] [Google Scholar]
- [97].Trangmar SJ, González-Alonso J. New insights into the impact of dehydration on blood flow and metabolism during exercise. Exercise Sports Sci Rev. 2017;45(3):146–153. [DOI] [PubMed] [Google Scholar]
- [98].González-Alonso J, Mora-Rodríguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol Heart Circ Physiol. 2000;278:H321–H330. [DOI] [PubMed] [Google Scholar]
- [99].van Nieuwenhoven MA, Vriens BE, Brummer RJ, et al. Effect of dehydration on gastrointestinal function at rest and during exercise in humans. Eur J Appl Physiol. 2000;83(6):578–584. [DOI] [PubMed] [Google Scholar]
- [100].Lambert CP, Lang J, Bull A, et al. Fluid restriction during running increases gl permeability. Int J Sports Med. 2008;29(3):194–198. [DOI] [PubMed] [Google Scholar]
- [101].Hoffman MD, Snipe RMJ, Costa RJS. Ad libitum drinking adequately supports hydration during 2 h of running in different ambient temperatures. Eur J Appl Physiol. 2018;118(12):2687–2697. [DOI] [PubMed] [Google Scholar]
- [102].Winger JM, Dugas JP, Dugas RL. Beliefs about hydration and physiology drive drinking behaviours in runners. Br J Sports Med. 2011;45(8):646–649. [DOI] [PubMed] [Google Scholar]
- [103].Hoffman MD, Pasternak A, Rogers IR, et al. Medical services at ultra-endurance foot races in remote environments: medical issues and consensus guidelines. Sports Med. 2014;44(8):1055–1069. [DOI] [PubMed] [Google Scholar]
- [104].Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the 3rd International exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Br J Sports Med. 2015;49(22):1432–1446. [DOI] [PubMed] [Google Scholar]
- [105].Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–568. [DOI] [PubMed] [Google Scholar]
- [106].Hoffman MD, Stellingwerff T, Costa RJS. Considerations for ultra-endurance activities: part 2 - hydration. Res Sports Med. 2018. 27(2):182–194. [DOI] [PubMed] [Google Scholar]
- [107].Hoffman MD, Goulet EDB, Maughan RJ. Considerations in the use of body mass change to estimate change in hydration status during a 161-kilometer ultramarathon running competition. Sports Med. 2018;48(2):243–250. [DOI] [PubMed] [Google Scholar]
- [108].McCubbin AJ, Costa RJS. Impact of sodium ingestion during exercise on endurance performance: a systematic review. Int J Sports Sci. 2018;8(3):97–107. [Google Scholar]
- [109].McCubbin AJ, Costa RJS. The impact of dietary sodium intake on sweat sodium concentration in response to endurance exercise: a systematic review. Int J Sports Sci. 2018;8(1):25–37. [Google Scholar]
- [110].McCubbin AJ, Cox G, Costa RJS. Sodium intake beliefs, information sources, and intended practices of endurance athletes before and during exercise. Int J Sports Nutr Exercise Metab. 2018. (in press) [DOI] [PubMed] [Google Scholar]
- [111].Del Coso J, González-Millán C, Salinero JJ, et al. Effects of oral salt supplementation on physical performance during a half-ironman: A randomized controlled trial. Scand J Med Sci Sports. 2016;26(2):156–164. [DOI] [PubMed] [Google Scholar]
- [112].Earhart EL, Weiss EP, Rahman R, et al. Effects of oral sodium supplementation on indices of thermoregulation in trained, endurance athletes. J Sports Sci Med. 2014;14(1):172–178. [PMC free article] [PubMed] [Google Scholar]
- [113].Hoffman MD, Stuempfle KJ, Valentino T. Sodium intake during an ultramarathon does not prevent muscle cramping, dehydration, hyponatremia, or nausea. Sports Med Open. 2015;1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hoffman MD, Stuempfle KJ. Is sodium supplementation necessary to avoid dehydration during prolonged exercise in the heat? J Strength Cond Res. 2016;30(3):615–620. [DOI] [PubMed] [Google Scholar]
- [115].Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. [DOI] [PubMed] [Google Scholar]
- [116].Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28:583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rehrer NJ, Goes E, Du Gardeyn C, et al. Effect of carbohydrate on portal vein blood flow during exercise. Int J Sports Med. 2005;26(3):171–176. [DOI] [PubMed] [Google Scholar]
- [118].Snipe RMJ, Khoo A, Kitic C, et al. Carbohydrate and protein intake during exertional-heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl Physiol Nutr Metab. 2017;42(12):1283–1292. [DOI] [PubMed] [Google Scholar]
- [119].Lambert GP, Broussard LJ, Mason BL, et al. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol. 2001;90(6):2075–2080. [DOI] [PubMed] [Google Scholar]
- [120].Racinaiz S, Cocking S, Jd P. Sports and environmental temperature: from warming-up to heating-up. Temperature. 2017;4(3):227–257. doi: 10.1080/23328940.2017.1356427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bongers CC, Hopman MT, Eijsvogels TM. Cooling interventions for athletes: an overview of effectiveness, physiology, mechanisms, and practical considerations. Temperature. 2017;4(1):60–87. doi: 10.1080/23328940.2016.1179380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Morris NB, Jay O. To drink or to pour. How should athletes use water to cool themselves. Temperature. 2016;3(2):191–194. doi: 10.1080/23328940.2016.1185206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Morris NB, Coombs G, Jay O. Ice slurry ingestion leads to a lower net heat loss during exercise in the heat. Med Sci Sports Exerc. 2016;48(1):114–122. [DOI] [PubMed] [Google Scholar]
- [124].Tan PM, Lee JK. The role of fluid temperature and form on endurance performance in the heat. Scand J Med Sci Sports. 2015;25(Suppl 1):39–51. [DOI] [PubMed] [Google Scholar]
- [125].Burdon CA, Hoon MW, Johnson NA, et al. The effect of ice slushy ingestion and mouthwash on thermoregulation and endurance performance in the heat. Int J Sport Nutr Exerc Metab. 2013;23(5):458–469. [DOI] [PubMed] [Google Scholar]
- [126].Yeo ZW, Fan PW, Nio AQ, et al. Ice slurry on outdoor running performance in heat. Int J Sport Med. 2012;33(11):859–866. [DOI] [PubMed] [Google Scholar]
- [127].Stevens CJ, Dascombe B, Boyko A, et al. Ice slurry ingestion during cycling improves Olympic distance triathlon performance in the heat. J Sports Sci. 2013;31(12):1271–1279. [DOI] [PubMed] [Google Scholar]
- [128].Snipe RMJ, Costa RJS. Does biological sex impact intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress? J Sports Sci. 2018;36(24):2827–2835. [DOI] [PubMed] [Google Scholar]
- [129].Rhind SG, Gannon GA, Shephard RJ, et al. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int J Hyperthermia. 2004;20(5):503–516. [DOI] [PubMed] [Google Scholar]
- [130].Lee BJ, Clarke ND, Hankey J, et al. Whole body precooling attenuates the extracellular HSP72, IL-6 and IL-10 responses after an acute bout of running in the heat. J Sports Sci. 2018;36(4):414–421. [DOI] [PubMed] [Google Scholar]
- [131].Duffield R, Steinbacher G, Fairchild TJ. The use of mixed-method, part-body pre-cooling procedures for team-sport athletes training in the heat. J Strength Cond Res. 2009;23(9):2524–2532. [DOI] [PubMed] [Google Scholar]
- [132].Daanen HAM, Racinais S, Périard JD. Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 2018;48(2):409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Heathcote SL, Hassmén P, Zhou S, et al. Passive heating: reviewing practical heat acclimation strategies for endurance athletes. Front Physiol. 2018;9:1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rama L, Minuzzi LG, Carvalho HM, et al. Changes of hematological markers during a multi-stage ultra-marathon competition in the heat. Int J Sports Med. 2016;37(02):104–111. [DOI] [PubMed] [Google Scholar]
- [135].Lis DM, Stellingwerff T, Shing CM, et al. Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int J Sport Nutr Exerc Metab. 2015;25(1):37–45. [DOI] [PubMed] [Google Scholar]
- [136].Lis D, Stellingwerff T, Kitic CM, et al. No effects of a short-term gluten-free diet on performance in Nonceliac Athletes. Med Sci Sports Exerc. 2015;47(12):2563–2570. [DOI] [PubMed] [Google Scholar]
- [137].Lis D, Ahuja KD, Stellingwerff T, et al. Food avoidance in athletes: FODMAP foods on the list. Appl Physiol Nutr Metab. 2016;41(9):1002–1004. [DOI] [PubMed] [Google Scholar]
- [138].Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8):1517–1527. [DOI] [PubMed] [Google Scholar]
- [139].Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31(8):874–882. [DOI] [PubMed] [Google Scholar]
- [140].Costa RJS, Snipe RMJ, Kitic C, et al. Low-FODMAP diet for exercise-induced gastrointestinal syndrome - authors’ reply. Aliment Ther Pharmacol. 2017;46(10):1023–1024. [DOI] [PubMed] [Google Scholar]
- [141].Lis DM, Ahuja KDK, Stellingwerff T, et al. Utilizing a low FODMAP diet to combat exercise-induced gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2016;26:481–487. [DOI] [PubMed] [Google Scholar]
- [142].Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterol. 2014;146(1):67–75. [DOI] [PubMed] [Google Scholar]
- [143].Gaskell SK, Costa RJS. Case Study: applying a low FODMAP dietary intervention to a female ultra-endurance runner with irritable bowel syndrome during a multi-stage ultra-marathon. Int J Sports Nutr Exercise Metab. 2019;29(1):61–67. [DOI] [PubMed] [Google Scholar]
- [144].Lis DM. Exit Gluten-Free and Enter Low FODMAPs: a novel dietary strategy to reduce gastrointestinal symptoms in athletes. Sports Med. 2019;49(suppl 1):87–97. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lis DM, Stellingwerff T, Kitic CM, et al. A preliminary strategy to reduce gastrointestinal distress in athletes. Med Sci Sports Exerc. 2018;50(1):116–123. [DOI] [PubMed] [Google Scholar]
- [146].Wiffin M, Smith L, Antonio J, et al. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet on exercise-related gastrointestinal symptoms. J Int Soc Sports Nutr. 2019;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hill P, Muir JG, Gibson PR. Controversies and recent developments of the low-FODMAP diet. Gastroenterol Hepatol. 2017;13(1):36–45. [PMC free article] [PubMed] [Google Scholar]
- [148].Staudacher, H.M., Irving, P.M. and Lomer, M.C.E. 2014. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol. Hepatol., 11(4): 256-266. doi: 10.1038/nrgastro.2013.259. [DOI] [PubMed] [Google Scholar]
- [149].Alcock R, McCubbin A, Camões-Costa V, et al. Nutritional support for self-sufficient multi-stage ultra-marathon: rationed versus full energy provisions. Wilderness Environ Med. 2018;29(4):508–520. [DOI] [PubMed] [Google Scholar]
- [150].Buchman AL, O’Brien W, Ou CN, et al. The effect of arginine or glycine supplementation on gastrointestinal function, muscle injury, serum amino acid concentrations and performance during a marathon run. Int J Sport Med. 1999;20(5):315–321. [DOI] [PubMed] [Google Scholar]
- [151].Zuhl M, Dokladny K, Mermier C, et al. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chap. 2015;20(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Pugh JN, Sage S, Hutson M, et al. Glutamine supplementation reduces markers of intestinal permeability during running in the heat in a dose-dependent manner. Eur J Appl Physiol. 2017;117(12):2569–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].McKenna Z, Berkemeier Q, Naylor A, et al. Bovine colostrum supplementation does not affect plasma I-FABP concentrations following exercise in a hot and humid environment. Eur J Appl Physiol. 2017;117(12):2561–2567. [DOI] [PubMed] [Google Scholar]
- [154].Shing CM, Peake JM, Lim CL, et al. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur J Appl Physiol. 2014;114:93–103. [DOI] [PubMed] [Google Scholar]
- [155]. Roberts JD, Suckling CA, Peedle GY, et al. An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutr. 2016;17(11):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Kekkonen RA, Vasankari TJ, Vuorimaa T, et al. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17(4):352–363. [DOI] [PubMed] [Google Scholar]
- [157].Marshall H, Chrismas BCR, Suckling CA, et al. Chronic probiotic supplementation with or without glutamine does not influence the eHsp72 response to a multi-day ultra-endurance exercise event. Appl Physiol Nutr Metab. 2017;42(8):876–883. [DOI] [PubMed] [Google Scholar]
- [158].Szymanski MC, Gillum TL, Gould LM, et al. Short term dietary curcumin supplementation reduces gastrointestinal barrier damage and physiological strain responses during exertional heat stress. J Appl Physiol. 2018;124(2):330–340. [DOI] [PubMed] [Google Scholar]
- [159].McKenzie YA, Thompson J, Gulia P, et al. (IBS dietetic guideline review group on behalf of gastroenterology specialist group of the British dietetic association). British dietetic association systematic review of systematic reviews and evidence-based practice guidelines for the use of probiotics in the management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):576–592. [DOI] [PubMed] [Google Scholar]
- [160].Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. [DOI] [PubMed] [Google Scholar]
- [161].Parker EA, Roy T, D’Adamo CR, et al. Probiotics and gastrointestinal conditions: an overview of evidence from the Cochrane collaboration. Nutr. 2018;45:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Karczewski J, Troost FJ, Konings I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G851–G859. [DOI] [PubMed] [Google Scholar]
- [163].Mujagic Z, de Vos P, Boekschoten MV, et al. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep. 2017;7:40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bron PA, Kleerebezem M, Brummer RJ, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Christensen HR, Larsen CN, Kaestel P, et al. Immunomodulating potential of supplementation with probiotics: a dose–response study in healthy young adults. FEMS Immunol Med Microbiol. 2006;47:380–390. [DOI] [PubMed] [Google Scholar]
- [166].Anand S, Mandal S, Patil P, et al. Pathogen-induced secretory diarrhea and its prevention. Eur J Clin Microbiol Infect Dis. 2016;35(11):1721–1739. [DOI] [PubMed] [Google Scholar]
- [167].Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160–174. [DOI] [PubMed] [Google Scholar]
- [168].Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 2009;27(4):450–454. [DOI] [PubMed] [Google Scholar]
- [169].Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001;73(6):1124S–1130S. [DOI] [PubMed] [Google Scholar]
- [170].Halmos EP, Christophersen CT, Bird AR, et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- [171].Enko D, Kriegshäuser G, Kimbacher C, et al. Carbohydrate Malabsorption and putative carbohydrate-specific small intestinal bacterial overgrowth: prevalence and diagnostic overlap observed in an austrian outpatient center. Digestion. 2015;92(1):32–38. [DOI] [PubMed] [Google Scholar]
- [172].Parodi A, Dulbecco P, Savarino E, et al. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43(10):962–966. [DOI] [PubMed] [Google Scholar]
- [173].Armstrong L, Lee EC, Armstrong EM. Interactions of gut microbiota, endotoxemia, immune function, and diet in exertional heatstroke. J Sports Med. 2018;Article ID: 5724575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Leite GSF. Resende master student AS, West NP, Lancha AH Jr. Probiotics and sports: a new magic bullet? Nutrition. 2018;60:152–160. [DOI] [PubMed] [Google Scholar]
- [175].Lamprecht M, Bogner S, Schippinger G, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebocontrolled trial. J Int Soc Sports Nutr. 2012;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 2018;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bermon S, Castell LM, Calder PC, et al. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- [178].Guy JH, Vincent GE. Nutrition and supplementation considerations to limit endotoxemia when exercising in the heat. Sports. 2018;6(1):E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Möller GB, Goulart M, Nicoletto BB, et al. Supplementation of probiotics and its effects on physically active individuals and athletes: systematic review. Int J Sports Nutr Exercise Metab 2019. (In press.) [DOI] [PubMed] [Google Scholar]
- [180].Davison G, Marchbank T, March DS, et al. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. 2016;104(2):526–536. [DOI] [PubMed] [Google Scholar]
- [181].March DS, Marchbank T, Playford RJ, et al. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Eur J Appl Physiol. 2017;117(5):931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Carol A, Witkamp RF, Wichers HJ, et al. Bovine colostrum supplementation’s lack of effect on immune variables during short-term intense exercise in well-trained athletes. Int J Sport Nutr Exerc Metab. 2011;21(2):135–145. [DOI] [PubMed] [Google Scholar]
- [183].March DS, Jones AW, Thatcher R, et al. The effect of bovine colostrum supplementation on intestinal injury and circulating intestinal bacterial DNA following exercise in the heat. Eur J Nutr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


