ABSTRACT
Silencing of SlSUT2 expression in tomato plants leads to a dwarfed phenotype, reduced pollen vitality and reduces pollen germination rate. Male sterility of flowers, together with a dwarfed growth behavior is reminiscent to brassinosteroid defective mutant plants. Therefore we aimed to rescue the SlSUT2 silencing phenotype by local brassinosteroid application. The phenotypical effects of SlSUT2 down-regulation could partially be rescued by epi-brassinolide treatment suggesting that SlSUT2 interconnects sucrose partitioning with brassinosteroid signaling. We previously showed that SlSUT2 silenced plants show increased mycorrhization and, this effect was explained by a putative sucrose retrieval function of SlSUT2 at the periarbuscular membrane. More recently, we reported that the symbiotic interaction between Solanaceous hosts and AM fungi is directly affected by watering the roots with epi-brassinolide. Here we show that the SlSUT2 effects on mycorrhiza are not only based on the putative sucrose retrieval function of SlSUT2 at the periarbuscular membrane. Our analyses argue that brassinosteroids as well as SlSUT2 per se can impact the arbuscular morphology/architecture and thereby affect the efficiency of nutrient exchange between both symbionts and the mycorrhizal growth benefit for the plant.
KEYWORDS: Mycorrhization, sucrose transporter SlSUT2, Brassinosteroids, arbuscular anatomy, male sterility
Introduction
A link between brassinosteroid and primary metabolism was postulated for some time, but the molecular mechanism is still ambiguous. One possible link between BR signaling and carbon metabolism is via the two transcription factors BAM7 and BAM8 containing a BRASSINAZOLE RESISTANT1 (BZR1)-type DNA binding domain on the one side and beta-amylase-like domain on the other. These two transcription factors, BAM7 and BAM8, are able to bind to a BR-responsive cis-regulatory promoter element thereby regulating gene expression. The beta-amylase domain is 1000 times less active in starch breakdown than chloroplastic beta-amylases but it still contains an intact glucan binding site. These transcription factors assumedly affect growth and development by transmitting metabolic signals to BR signaling.1,2
We suggest an additional interaction between BR signaling and carbon partitioning at the level of a sucrose transporter. This is based on the fact that the sucrose transporter SlSUT2 of tomato is in direct contact with the BR co-receptor BAK1, the BR signaling inhibitor MSBP1 and with the sterol reductase DIM1 involved in BR biosynthesis.3,4 We showed recently, that brassinosteroids positively affect the symbiotic interaction between AM fungi and Solanaceous host plants. Overexpression of the MSBP1 inhibitor causes similar morphological modifications on arbuscular anatomy as observed in brassinazole treated plants, an inhibitor of brassinosteroid biosynthesis.5 Here we provide an additional link between brassinosteroids and sucrose transport by analyzing arbuscular anatomy as well as sugar accumulation in tomato plants with strongly reduced SlSUT2 expression. Interestingly, the phenotypic modification of arbuscular architecture previously described for plants with reduced BR response is also seen in SlSUT2 silenced plants, together with reduced pollen viability and germination rate and a dwarf-like growth behavior.
Results
Down-regulation of the SlSUT2 expression has led to reduced pollen tube growth, impeded pollen development and reduced tomato fruit yield.6 Mycorrhization efficiency’s increases, when SlSUT2 expression is decreased3 and we aimed at elucidation of the molecular mechanisms underlying the phenomenon.
We generated SlSUT2 RNAi plants with more severe down-regulation of SlSUT2 expression than the antisense plants published in 2006.6 The phenotypical changes observed in SlSUT2 antisense plants could be reproduced and the degree of inhibition was even stronger and phenotypical changes even more severe. Whereas SlSUT2 antisense plants showed normal growth,6 the RNAi plants were dwarfed with significantly reduced plant height (Figure 1).
Figure 1.
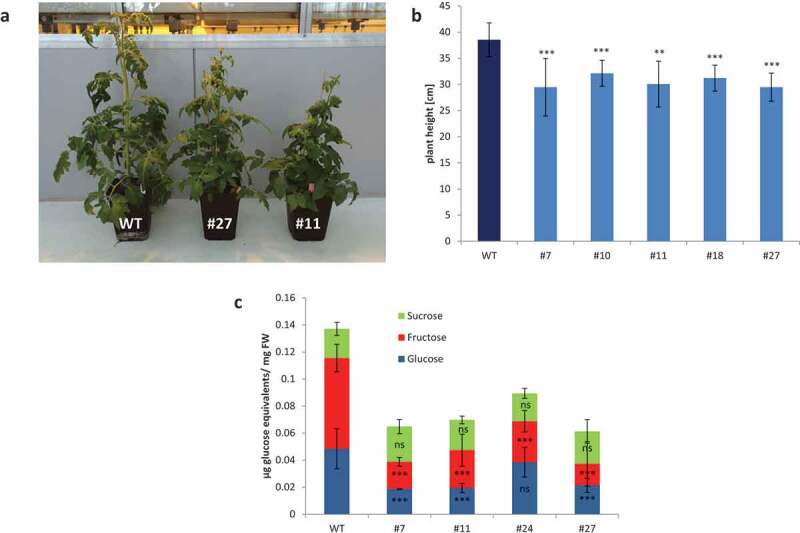
Phenotype of SlSUT2-RNAi plants.
(a) SlSUT2-RNAi tomato plants are smaller than WT plants. (b) Plant size of StSUT2-RNAi plants is reduced compared to tomato WT plants 8 weeks after transfer to soil. ***p < .001; **p < .01. (c) The content of soluble sugars in source leaves of SlSUT2-RNAi plants is reduced compared to WT plants. ***p < .001.
The level of soluble sugars was significantly reduced only in one strongly inhibited SlSUT2 antisense line #48.6 In case of SlSUT2 RNAi tomato plants, the reducetion of soluble sugars, mainly hexoses is more prominent: in two out of four transgenic lines was only 50% of the WT sugar content, which was mainly due to a reduction in glucose and fructose (Figure 1c). It is therefore unlikely that SlSUT2 promotes phloem loading as shown for SlSUT1,6 whose inhibition is leading to high accumulation of soluble sugars in leaves ()
Consistently to earlier observations,6 the RNAi plants revealed reduced pollen germination rate and reduced pollen viability (Figure 2a). In order to test whether reduced germination rate is simply due to reduced pollen vitality, aniline blue staining of pollen was performed (Figure 2b). Only viable pollen show bright blue fluorescence after staining. Slightly reduced viability of pollen was confirmed for all tested RNAi lines (Figure 2c). Flowers, that have been sprayed with 1 µM epi-brassinolide for a period of 2 weeks show a partial rescue of this defect; differences between the pollen vitality of WT and transgenic plants are slightly diminished by the epi-BL treatment, but the SlSUT2 RNAi plants do not reach WT levels (Figure 2d).
Figure 2.
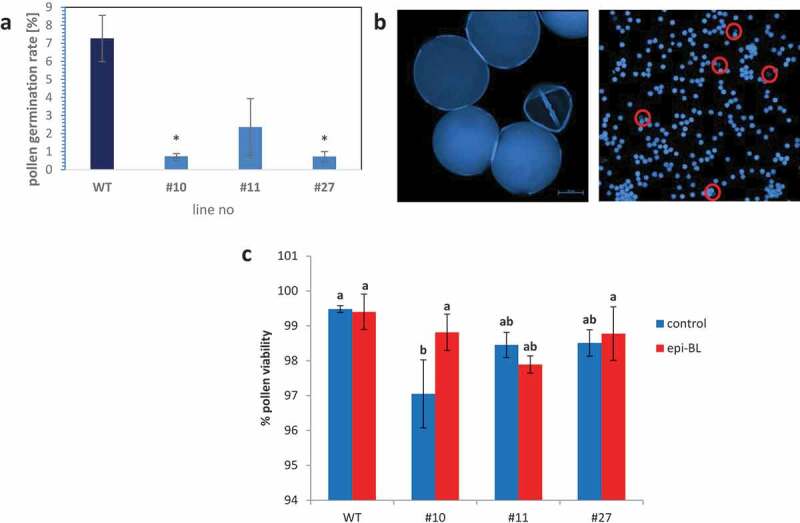
Pollen germination rate and vitality.
(a) The pollen germination rate of SlSUT2-RNAi plants is significantly reduced. Asterisks indicate significant differences between WT and RNAi lines (*p < 0,05). (b) Viability of pollen was analyzed by aniline blue staining. Viable pollen grains show bright blue fluorescence whereas non-viable pollen are not stained (red circles). (c) Pollen viability of all tested SlSUT2-RNAi plants is reduced compared to WT pollen. The viability can be rescued by epi-brassinolide treatment in one out of three transgenic lines. A Tukey HSD test was performed with alpha = 0.05.
In SlSUT2 silenced plants, AM fungi show increased mycorrhization, while the growth promoting effect of AM fungi on tomato WT plants vanishes.3 The newly generated SlSUT2 RNAi tomato plants were inoculated with Rhizoglomus irregularis (Figure 3). At first view, the arbuscules in SlSUT2 RNAi roots (Figure 3b–d) look bushier than in corresponding WT roots (Figure 3a). Increased RNA accumulation of the fungal RiGAPDH and the arbuscule–specific phosphate transporter gene SlPT4 of tomato (Figure 4a) confirmed the increased mycorrhization formerly seen in the roots of the SlSUT2 silenced plants.3 Quantification of the diameter of the finest tubular branches of arbuscules revealed significantly reduced tubule diameters in SlSUT2-RNAi plants line #11 and #27 (Figure 4c). At the same time, a high number of septate hyphae were observed in SlSUT2-RNAi line #27 (Figure 4b).
Figure 3.
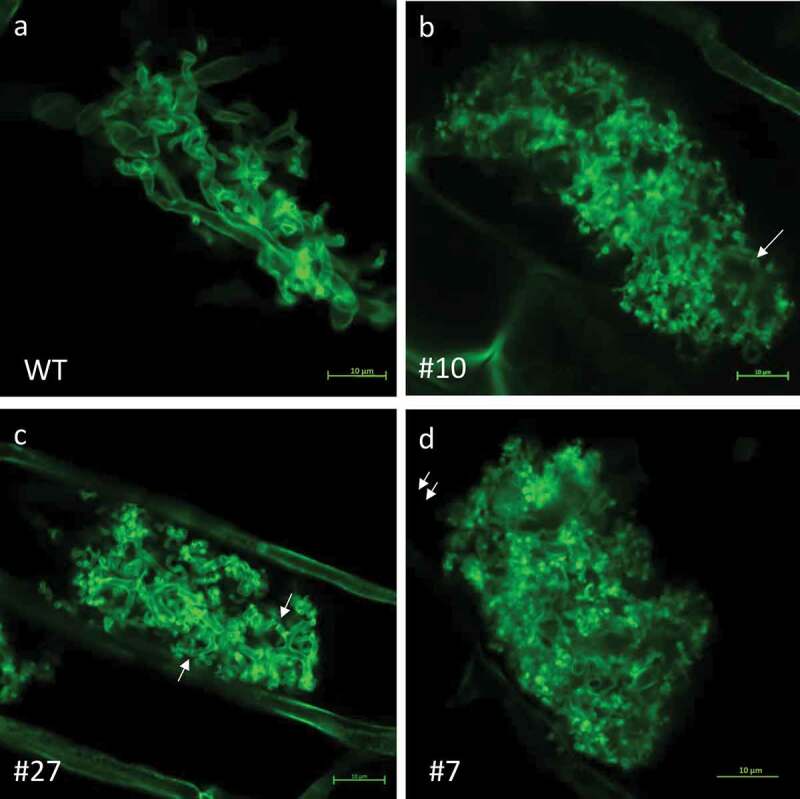
Arbuscular morphology.
Colonized roots of tomato WT (a) and SlSUT2-RNAi plants (b–d) were stained with WGA-Alexa488. Arbuscules of SlSUT2-RNAi plants appear bushier and the diameter of finest arbuscular branches is significantly reduced in line #11 and #27 (see results of quantification in Figure 4c).
Figure 4.
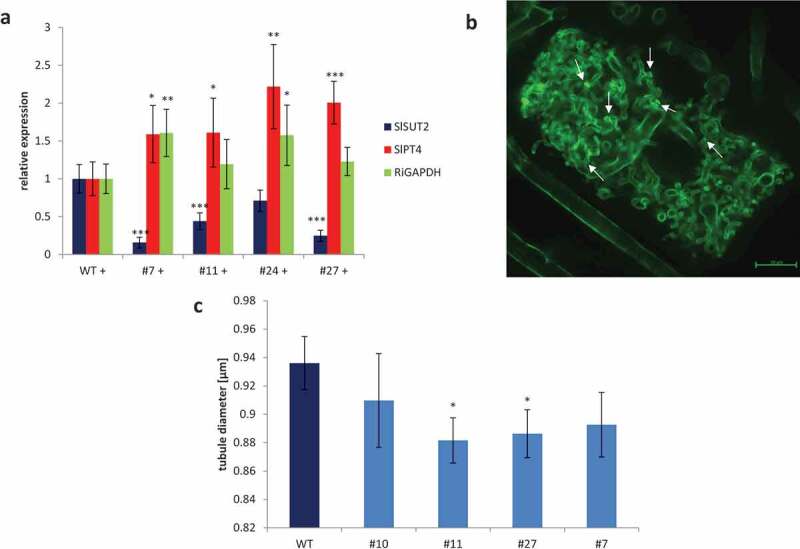
Mycorrhization.
(a) Real time PCR confirmed increased mycorrhizal colonization and function. Reduced SlSUT2 expression (blue) is accompanied by increased gene expression of the fungal RiGAPDH (green) or the mycorrhization-specific phosphate transporter gene SlPT4 (red). ***p < .001; **p < .01; *p < .05. (b) Arbuscules of SlSUT2-RNAi #27 show a high number of septa (arrows). (c) Tubule diameter of arbuscule branches is reduced in SlSUT2-RNAi plant. The diameter of the finest arbuscular branches was analyzed using the confocal Zeiss software ZEN 2.0 blue edition. In case of WT plants, 15 tubules from 4 different arbuscules in 8 different plants were measured (n = 480), in case of transgenic SlSUT2-RNAi plants, 15 tubules from 4 different arbuscules in 3 different plants per transgenic line were measured (n = 180). The tubule diameter of finest arbuscular branches was significantly reduced in two out of four SlSUT2-RNAi plants (*p < .05).
In contrast to SlSUT2 antisense plants, the fruit yield of SlSUT2-RNAi plants was not reduced and fruits are not sterile. The number of fruits per plant is slightly increased whereas the total fruit yield was slightly lower (data not shown) indicating smaller fruits.
Discussion
During the last years, evidences accumulate that brassinosteroid (BR) signaling is linked to carbon partitioning and sugar availability. Tomato mutant plants defective in BR biosynthesis (dx) show reduced levels of sugars and starch in leaves and reduced dry matter content in fruits.7 BR application to leaves partially rescued this phenotype.7 In sugar cane, down-regulation of the LRR-receptor kinase and brassinosteroid co-receptor BAK1 affects carbon accumulation. ScBAK1 is mainly expressed in the bundle sheath cells of leaves and its expression is correlated with high levels of sugar.8 The photomorphogenic mutant bsl1 (brassinosteroid, light and sugar1) from Arabidopsis is disturbed not only in its BR response but also in sugar sensitivity, causing a dwarfed phenotype in the light and delayed bolting. The bsl1 mutant is hypersensitive to metabolizable sugars. The short root and short hypocotyl phenotype together with the sugar hypersensitivity could be rescued by BR application.9 It is assumed that bls1 integrates light, hormone and sugar signaling. The BR regulated gene EXORDIUM-LIKE 1 is also induced by sugar starvation, prolonged darkness and anoxia. The carbon status in leaves also affects EXL1 protein levels suggesting a role in the carbon starvation response in order to maintain biomass production and survival even under carbon or energy limited conditions.10,11
Based on these results, a hierarchical model of sugar and brassinosteroid signaling was postulated also regarding hypocotyl graviresponse of etiolated Arabidopsis seedlings.1 BR deficiency induced by brassinazole treatment also revealed a link between BR-promoted plant growth and the availability of carbohydrates and energy in Arabidopsis.10 A transcriptomic study suggests that BRs can stimulate both anabolic and catabolic pathways.10 In some of the BR defective mutants exogenous sugar application antagonizes BR inhibition.7,9,11–17
Analysis of BR deficient mutant plants that are devoid of constitutive photomorphogenesis and display a dwarfed phenotype like cpd or show reduced expression of the sterol reductase DIMINUTO1 (DIM1) revealed altered carbohydrate accumulation and reduced starch levels.18 Interestingly, these BR defective mutant plants also show impaired carbohydrate uptake and altered enzyme activities and expression patterns of cell wall invertases, sucrose synthases and beta-amylases. This suggests that BR biosynthesis and function are required for carbon uptake and carbohydrate metabolism. The reduced growth of BR defective mutants might be explained by reduced carbon availability and/or photosynthetic capacity.18
SlSUT2 directly interacts with components of the BR signaling pathway, namely, the BR co-receptor BAK1 and the BR signaling inhibitor MSBP1.3 Whereas SlSUT2 expression is increased in response to biotic interactions, the expression of MSBP1 is reduced according to the TomExpress database.5 Interestingly, the overexpression of MSBP1 and roots treated with the BR biosynthesis inhibitor Brassinazole reveal the same effect on arbuscular morphology; both reduced the diameter of arbuscular branches and arbuscules appeared bushier. In parallel, the exchange capacity of nutrients between host plants and mycorrhiza seems to decline;5 mycorrhizal plants do not show an increase in biomass, nor an increase in mineral nutrients such as Mn, K, Ca, Fe, Zn and P in response to mycorrhization.5 Unexpectedly higher branching of arbuscules is paralleled by diminished nutrient exchange and membrane transport between plants and fungi.
SlSUT2 RNAi plants confirmed previous observations with SlSUT2 antisense plants and it became obvious that SlSUT2 silencing is accompanied with a dwarfed phenotype and reduced pollen germination and viability leading to male sterility of flowers. This characteristic phenotype could partially be rescued by BR treatment (Figure 2d).
Arbuscular architecture responded similarly to silencing of SlSUT2 and MSBP1 overexpression or Brassinazole treatment5 (Figures 3 and 4). Here again, the decrease of the diameter of arbuscular branches is accompanied by the loss of the plant’s growth benefit by root colonization.3 This suggests contrary functions of SlSUT2 and MSBP1 on mycorrhizal colonization and development within the plant root. Thus, the SlSUT2-silencing effects on mycorrhization efficiency cannot exclusively be explained by its putative retrieval function at the periarbuscular membrane. It cannot be excluded that SlSUT2-mediated effects on mycorrhization are also due to de-regulation of BR signaling. Further experiments are needed to analyze whether brassinosteroids affect carbon partitioning between the host plant and the fungus.
Acknowledgments
We gratefully appreciate helpful suggestions and Agrobacterial strains provided by Caterina Brancato, University of Tübingen. This work has received funding from the Ministry of Consumer Protection, Food and Agriculture of the Federal Republic of Germany, from the Ministry for Science, Research and Culture of the State of Brandenburg, and from the Thuringian Ministry of Infrastructure and Agriculture.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Gupta A, Singh M, Jones AM, Laxmi A.. Hypocotyl directional growth in Arabidopsis: a complex trait. Plant Physiol. 2012;159:1–5. doi: 10.1104/pp.112.195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinhold H, Soyk S, Simkova K, Hostettler C, Marafino J, Mainiero S, Vaughan CK, Monroe JD, Zeeman SC.. β-amylase–like proteins function as transcription factors in Arabidopsis, controlling shoot growth and development. Plant Cell. 2011;23:1391–1403. doi: 10.1105/tpc.110.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitterlich M, Krügel U, Boldt‐Burisch K, Franken P, Kühn C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. 2014;78:877–889. doi: 10.1111/tpj.2014.78.issue-5. [DOI] [PubMed] [Google Scholar]
- 4.Bitterlich M, Krügel U, Boldt-Burisch K, Franken P, Kühn C. Interaction of brassinosteroid functions and sucrose transporter SlSUT2 regulate the formation of arbuscular mycorrhiza. Plant Signal Behav. 2014;9:e970426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Sivers L, Jaspar H, Johst B, Roese M, Bitterlich M, Franken P, Kühn C. Brassinosteroids affect the symbiosis between the AM fungus rhizoglomus irregularis and Solanaceous host plants. Front Plant Sci. 2019;10:571. doi: 10.3389/fpls.2019.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006;45:180–192. doi: 10.1111/tpj.2006.45.issue-2. [DOI] [PubMed] [Google Scholar]
- 7.Lisso J, Altmann T, Müssig C. Metabolic changes in fruits of the tomato dx mutant. Phytochemistry. 2006;67:2232–2238. doi: 10.1016/j.phytochem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Vicentini R, Felix Jde M, Dornelas MC, Menossi M. Characterization of a sugarcane (Saccharum spp.) gene homolog to the brassinosteroid insensitive1-associated receptor kinase 1 that is associated to sugar content. Plant Cell Rep. 2009;28:481–491. doi: 10.1007/s00299-008-0656-0. [DOI] [PubMed] [Google Scholar]
- 9.Laxmi A, Paul LK, Peters JL, Khurana JP. Arabidopsis constitutive photomorphogenic mutant, bls1, displays altered brassinosteroid response and sugar sensitivity. Plant Mol Biol. 2004;56:185–201. doi: 10.1007/s11103-004-2799-x. [DOI] [PubMed] [Google Scholar]
- 10.Schröder F, Lisso J, Obata T, Erban A, Maximova E, Giavalisco P, Kopka J, Fernie AR, Willmitzer L, Müssig C, et al. Consequences of induced brassinosteroid deficiency in Arabidopsis leaves. BMC Plant Biol. 2014;14:309. doi: 10.1186/s12870-014-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schröder F, Lisso J, Müssig C. EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 2011;156:1620–1630. doi: 10.1104/pp.111.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Singh M, Laxmi A. Interaction between glucose and brassinosteroid during the regulation of lateral root development in Arabidopsis. Plant Physiol. 2015;168:307–320. doi: 10.1104/pp.114.256313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljung K, Nemhauser JL, Perata P. New mechanistic links between sugar and hormone signalling networks. Curr Opin Plant Biol. 2015;25:130–137. doi: 10.1016/j.pbi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Matsoukas IG. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front Genet. 2014;5:218. doi: 10.3389/fgene.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu F, Xi ZM, Zhang H, Zhang CJ, Zhang ZW. Brassinosteroids are involved in controlling sugar unloading in Vitis vinifera ‘Cabernet Sauvignon’ berries during veraison. Plant Physiol Biochem. 2015;94:197–208. doi: 10.1016/j.plaphy.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, He J. Sugar-induced plant growth is dependent on brassinosteroids. Plant Signal Behav. 2015;10:e1082700. doi: 10.1080/15592324.2015.1082700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu Z, Wang J, Chen Y, Bi Y, He J. Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta. 2015;242:881–893. doi: 10.1007/s00425-015-2328-y. [DOI] [PubMed] [Google Scholar]
- 18.Schlüter U, Köpke D, Altmann T, Müssig C. Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ. 2002;25:783–791. doi: 10.1046/j.1365-3040.2002.00860.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


