ABSTRACT
Passage cells are frequently found in the exodermis and the endodermis of the roots. Because passage cells lack an apoplastic diffusion barrier, they are thought to provide pathways for the transport of nutrients and the entrance of endomycorrhizal fungi. Exodermal passage cells possess Casparian strips but not suberin lamellae. So far, exodermal passage cells have not been associated with a particular internal structure. In some wetland plants, the outer part of the root (i.e., epidermis, exodermis, and sclerenchyma) of emerging lateral root primordia has an oxygen leaky zone called a window. The exodermis at the window site also lacks suberin lamellae, but it remains unclear whether the exodermis at the window site also lacks Casparian strips. Here, we report that several of the exodermal cells in the window of Echinochloa crus-galli grown under aerated or deoxygenated stagnant agar nutrient solution also lack lignin, which is a major constituent of Casparian strips. The sclerenchyma cells that form part of the window also lacked lignin deposits. Sites at which lateral root primordia developed were highly permeable to an apoplastic tracer (periodic acid). These observations indicate that windows consist of a novel type of passage cell at the exodermis that lacks lignin as well as suberin lamellae.
KEYWORDS: Barrier to radial oxygen loss (ROL), Casparian strips, exodermis, passage cell, wetland plants, window
Root growth into hypoxic or anoxic waterlogged soil relies on internal aeration in plants.1 The roots of wetland species have a large volume of aerenchyma, which provides a low-resistance pathway for the diffusion of oxygen from the shoot to the root apex. Oxygen transported via aerenchyma supports the respiration of roots under hypoxic conditions. The loss of oxygen from the aerenchyma to the soil is called radial oxygen loss (ROL). ROL can markedly reduce the availability of oxygen at the root apex for respiration in hypoxic or anoxic waterlogged soil.1,2 The roots of wetland species, including the wild grasses Phragmites australis and Echinochloa crus-galli, possess a barrier to ROL along the basal zones of the roots (for a review of ROL barriers, see Yamauchi et al.3). Thus, oxygen is predominately lost from the apical few centimeters of adventitious roots. A barrier to ROL can conserve oxygen transported to the root apex via the aerenchyma, and thus allow root growth into waterlogged soils.
Suberin and lignin deposits in the apoplast (the outer cellular space) prevent movement of water, ions, and mycorrhizal fungi through the apoplast and thus act as an apoplastic barrier.4 Suberin is a hydrophobic macromolecule built from long-chain fatty acids, glycerol, and aromatic compounds.4,5 Lignin is a complex of polyphenolic polymers.6 Casparian strips, which are present in radial and transverse cell walls in the early developmental stage (State I), are comprised of lignin and suberin.7,8 Suberin lamellae, which are deposited on the inner surface of cell walls and surround the symplast in the subsequent developmental stage (State II), are comprised of suberin.7 Because suberin accumulates at the exodermis and lignin accumulates at the sclerenchyma and exodermis of plants that form a ROL barrier, the barrier is thought to be formed by deposits of suberin and/or lignin in the outer part of the root (OPR).3,9 The ROL barrier is also thought to act as an apoplastic barrier, not only to impede oxygen loss but also to block the entry of phytotoxins (e.g., reduced metal ions) from waterlogged soil.1,3 In wetland species that have a ROL barrier, suberin has been suggested to be a major component of the barrier.10–14
Phragmites australis and Oryza sativa (rice, another wetland species), form not only a ROL barrier but also a unique oxygen leaky zone, called a window, along adventitious roots, which is the site of lateral root emergence in the OPRs.15,16 Interestingly, windows occur in the exodermis at the site of an emerging lateral root, although the tip of the lateral root does not touch the exodermis. Recently, we reported that some of the window cells of Echinochloa crus-galli var. crus-galli and E. crus-galli var. praticola lacked suberin lamellae.14 However, whether the root cells formed Casparian strips and the number of exodermal cells lacking suberin lamellae and/or lignified exodermis were not determined. Here, we examined whether Casparian strips composed of lignin deposits were observed at the exodermis or not and quantified the amounts of suberin and lignin at the window in several varieties of E. crus-galli using fluorescence imaging.
We previously showed that all three varieties of E. crus-galli formed a ROL barrier under aerated or stagnant conditions and that the basal pars of their adventitious roots were clearly surrounded by a well-suberized exodermis.14 We also reported that the sites of lateral root emergence in E. crus-galli var. crus-galli and E. crus-galli var. praticola grown under aerated conditions lacked suberin lamellae at the exodermis.14 In the present study, we confirmed that the site in the exodermis where lateral roots emerged lacked the yellow-green fluorescence of suberin lamellae in E. crus-galli var. praticola grown under stagnant and aerated conditions (Figure 1a, 1b). The intensities of Fluorol Yellow 088 fluorescence at the window site were drastically lower than those at other sites in the exodermal cell wall (Figure 1c, 1d). Similarly, in the other varieties of annual E. crus-galli species (var. praticola, var. crus-galli and var. formosensis) grown under aerated or stagnant conditions, Fluorol Yellow 088 fluorescence was not observed in the exodermis at the site of emerging lateral roots (Supplemental Figure 1a-1f). The number of non-suberized exodermal cells at the window in E. crus-galli accessions ranged from two to six (Figure 1e, 1f; Supplemental Figure 1m, 1n). No obvious differences were observed between aerated and stagnant conditions. Interestingly, in a larger window consisting of six non-suberized exodermis cells (denoted by asterisks in Supplemental Figure 1a), a lateral root primordium (white arrowhead) was larger than the lateral root primordia at the other window sites with fewer non-suberized exodermal cells (cf. Supplemental Figure. 1b, 1c). This raises the possibility that the size of lateral root primordia is related to the number of non-suberized exodermal cells, although we did not observe enough of the larger primordia to confirm this.
Figure 1.
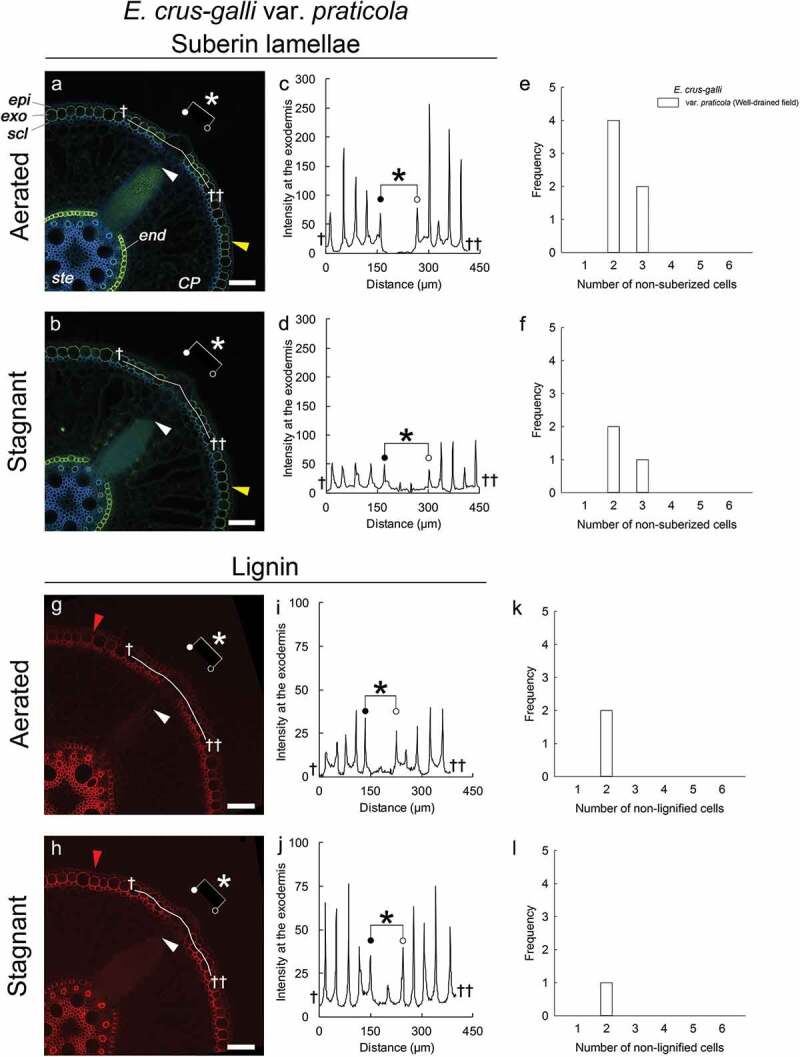
Windows in Echinochloa crus-galli var. praticola lack suberin lamellae and lignin deposits. Plants were grown in aerated nutrient solution for 10 days, and then transferred to deoxygenated stagnant 0.1% agar solution or left in aerated nutrient solution for another 14 days. Basal parts (15–25 mm below root-shoot junction) of 100- to 120-mm-long adventitious roots of length were stained as described in Supplemental methods. (a, b) Suberin lamellae at the exodermis and endodermis under aerated (a) and stagnant (b) conditions. Suberin lamellae are indicated by yellow-green fluorescence with Fluorol Yellow 088 (Yellow arrowhead). (c, d) Line plots of fluorescence intensity of Fluorol Yellow 088 along the exodermis under aerated (c) and stagnant (d) conditions. (e, f) Number of non-suberized exodermal cells at window sites under aerated (e) and stagnant (f) conditions. (g, h) Lignin at the sclerenchyma, exodermis, and endodermis under aerated (g) and stagnant (h) conditions. Basic Fuchsin fluorescence is shown by red color (Red arrowhead). (i, j) Line plots of fluorescence intensity of Basic Fuchsin along exodermis under aerated (i) and stagnat (j) conditions. (k, l) Number of non-lignified exodermal cells at window sites under aerated (k) and stagnant (l) conditions. Asterisks indicate areas of passage cells (windows) that lack both suberin lamellae and lignin deposits. White arrowheads indicate the apex of lateral root primordia. Abbreviations: CP, cortical parenchyma; end, endodermis; epi, epidermis; exo, exodermis; scl, sclerenchyma; ste, stele. Scale bars, 100 µm.
Passage cells are frequently found in herbaceous and woody species. Passage cells in the endodermis are thought to provide pathways for the transport of nutrients (i.e. calcium and magnesium) because passage cells lack suberin lamellae which inhibit transcellular transport.17,18 A passage cell is defined as a type of endodermal or exodermal cell that possesses Casparian strips, but unlike other dermal cells, does not contain suberin lamellae.18 In Arabidopsis, Casparian strips are composed of lignin but not suberin.8,19 Thus, we also stained for lignin at the window site. Unexpectedly, the sites of emerging lateral roots in the exodermis in all accessions of E. crus-galli lacked Basic Fuchsin fluorescence (Figure 1g, 1h; Supplemental Figure 1g-1l), which is a characteristic of lignin deposits. In E. crus-galli var. praticola under aerated or stagnant conditions, the intensities of Basic Fuchsin fluorescence at the window site were significantly lower than those of other exodermal cell walls (Figure 1i, 1j). In all varieties of E. crus-galli, lignin fluorescence was also suppressed at the sites of emerging lateral roots in the sclerenchyma (Figure 1g, 1h; Supplemental Figure 1g-1l). When Phloroglucinol-HCl was used for lignin staining in E. crus-galli var. praticola under stagnant conditions, a red lignin color was also suppressed in the window site rather than in the OPRs (Supplemental Figure 2b). Here, we should note that the obvious color difference between the window site and the OPRs was not found under aerated conditions (Supplemental Figure 2a), even though the intensity of Basic Fuchsin fluorescence was obviously reduced at the window site (Figure 1g, 1i). Previously, weaker lignification was observed at the window in P. australis using phloroglucinol-HCl.15 Phloroglucinol-HCl has been used as a qualitatively assaying for lignin, and its target site in lignin is 4-O-linked hydroxy cinnamyl aldehyde structures.20 The red color of lignin is usually observed with a light microscope. However, it is difficult to quantify the red stained color due to the thickness of the cross-sections (e.g. 100 µm in the present study). Therefore, to observe Basic Fuchsin fluorescence, we used a confocal microscope, which can focus on a single layer in a thick section. The confocal microscope sensitively detected the fluorescence under aerated conditions (Figure 1g, 1i). To evaluate the permeability of the apoplast at the window site, we observed the penetration of an apoplastic tracer (periodic acid) in E. crus-galli var. praticola under aerated or stagnant conditions. After one hour incubation, the purple color of periodic acid was much stronger near the site of emerging lateral root primordia than at other parts of the roots in the OPRs (Figure 2a, 2b). Such permeable sites were previously shown to be sites where lateral root primordia emerge.21 Similarly, in E. crus-galli var. praticola under aerated or stagnant conditions, the site where lateral root primordia develop was highly permeable to periodic acid (Figure 2).
Figure 2.
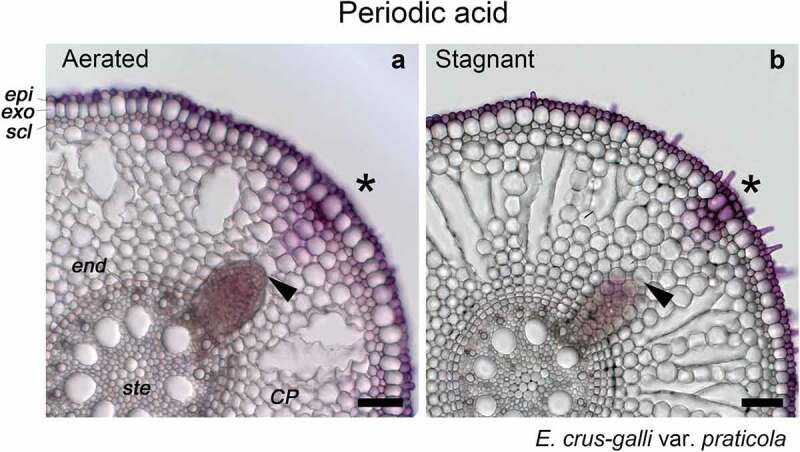
Permeability of the outer part of roots (OPRs) to an apoplastic tracer (periodic acid) in E. crus-galli var. praticola under aerated or stagnant conditions. Permeability was measured as described by Ejiri and Shiono (2019)14 on the basal parts (15–25 mm below root-shoot junction) of 100- to120-mm-long adventitious roots in E. crus-galli var. praticola. Plants were grown in aerated nutrient solution for 10 days, and then transferred to deoxygenated stagnant 0.1% agar solution (b) or left in aerated nutrient solution for another 14 days (a). Purple color indicates where periodic acid penetrated into root tissues. Asterisks indicate areas of passage cells (window). Black arrowheads indicate the apex of lateral root primordia. Abbreviations: CP, cortical parenchyma; end, endodermis; epi, epidermis; exo, exodermis; scl, sclerenchyma; ste, stele. Scale bars, 100 µm.
Window cells can be considered as a novel type of passage cell at the exodermis. Passage cells possess Casparian strips but not suberin lamellae.18 In E. crus-galli varieties, not only suberin lamellae but also lignin were lacking in several exodermal cells from which lateral root primordia emerged (Figure 1, Supplemental Figure 1). The window site was also highly permeable to periodic acid (Figure 2). Because lignin is a major component of Casparian strips and acts as an apoplastic barrier,8,19 our results suggest that window sites lack Casparian strips at the exodermis. However, further studies with Casparian strip-specific histochemical staining (e.g., Berberine-Aniline blue staining) and transmission electron microscopy are needed to confirm this observation.
Plant cells have two types of exodermis, both of which have passage cells. In one type, called uniform exodermis, the cells are similar in shape but their maturation is patchy.18 In the other type, called dimorphic exodermis, the cells have two shapes, short and long. The passage cells in dimorphic epidermis are the short cells. Suberization is delayed or absent in the short cells.18 Exodermal passage cells do not appear to be correlated with internal structure, while endodermal passage cells are consistently positioned near developing xylem cells.18 Windows consist of multi-cellular passage cells that are correlated with lateral root primordia (Figure 1, Supplemental Figure 1). Window cells differ from the patchy passage cells in the uniform exodermis and differ from the short passage cells in the dimorphic exodermis.
What is the purpose of windows? Windows are well correlated with the site of emergence of lateral root primordia.14–16 Sulfide, a toxin, was previously observed to induce strong autofluorescence in rice, implying that the root responded to the sulfide by accumulating suberin and/or lignin in the OPRs.16 Sulfide also prevented lateral roots from penetrating the sclerenchyma and from growing through the cortex of adventitious roots.16 This suggests that well-suberized and/or well-lignified cells in the OPRs block the emergence of lateral roots. Thus, lateral root primordia may in some way induce window formation by degrading well-matured suberin lamellae and lignified cell walls, thereby allowing lateral roots to penetrate the OPRs. The loss of oxygen through the window could be viewed as one of the costs of lateral root emergence.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science [JP16KK0173];Japan Society for the Promotion of Science [JP19K05978];Japan Society for the Promotion of Science [JP17K15211].
Acknowledgments
Prof. Toshihito Yoshioka (Fukui Prefectural University) is thanked for providing seeds of Echinochloa crus-galli accessions for the experiments. This work was partly supported by JSPS KAKENHI (JP16KK0173, JP17K15211, and JP19K05978 to KS).
Author Contributions
ME and KS designed the experiments. ME performed most of the experiments and analyses. KS wrote the article and supervised the experiments.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Armstrong W. Aeration in higher plants. Adv Bot Res. 1979;7:1–5. [Google Scholar]
- 2.Armstrong W, Beckett PM.. Internal aeration and the development of stelar anoxia in submerged roots - a multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol. 1987;105:221–245. doi: 10.1111/j.1469-8137.1987.tb00860.x. [DOI] [Google Scholar]
- 3.Yamauchi T, Colmer TD, Pedersen O, Nakazono M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018;176:1118–1130. doi: 10.1104/pp.17.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enstone DE, Peterson CA, Ma F. Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul. 2003;21:335–351. doi: 10.1007/s00344-003-0002-2. [DOI] [Google Scholar]
- 5.Graça J. Suberin: the biopolyester at the frontier of plants. Front Chem. 2015;3:62. doi: 10.3389/fchem.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros J, Serk H, Granlund I, Pesquet E. The cell biology of lignification in higher plants. Ann Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber L, Franke RB. Endodermis and exodermis in roots. eLS. Chichester: Chichester, UK: John Wiley & Sons Ltd; 2011. p. 1–7. [Google Scholar]
- 8.Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA. 2012;109:10101–10106. doi: 10.1073/pnas.1205726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe K, Nishiuchi S, Kulichikhin K, Nakazono M. Does suberin accumulation in plant roots contribute to waterlogging tolerance? Front Plant Sci. 2013;4:178. doi: 10.3389/fpls.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Simone O, Haase K, Müller E, Junk WJ, Hartmann K, Schreiber L, Schmidt W. Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species. Plant Physiol. 2003;132:206–217. doi: 10.1104/pp.102.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulichikhin K, Yamauchi T, Watanabe K, Nakazono M. Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ. 2014;37:2406–2420. doi: 10.1111/pce.12294. [DOI] [PubMed] [Google Scholar]
- 12.Shiono K, Yamauchi T, Yamazaki S, Mohanty B, Malik AI, Nagamura Y, Nishizawa NK, Tsutsumi N, Colmer TD, Nakazono M. Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J Exp Bot. 2014;65:4795–4806. doi: 10.1093/jxb/eru235. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant Cell Environ. 2017;40:304–316. doi: 10.1111/pce.v40.2. [DOI] [PubMed] [Google Scholar]
- 14.Ejiri M, Shiono K. Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Front Plant Sci. 2019;10:254. doi: 10.3389/fpls.2019.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot. 2000;86:687–703. doi: 10.1006/anbo.2000.1236. [DOI] [Google Scholar]
- 16.Armstrong J, Armstrong W. Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann Bot. 2005;96:625–638. doi: 10.1093/aob/mci215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison-Murray RS, Clarkson DT. Relationships between structural development and the absorption of ions by the root system of Cucurbita pepo. Planta. 1973;114:1–16. doi: 10.1007/BF00390280. [DOI] [PubMed] [Google Scholar]
- 18.Peterson CA, Enstone DE. Functions of passage cells in the endodermis and exodermis of roots. Physiol Plantarum. 1996;97:592–598. doi: 10.1111/j.1399-3054.1996.tb00520.x. [DOI] [Google Scholar]
- 19.Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64:531–558. doi: 10.1146/annurev-arplant-050312-120050. [DOI] [PubMed] [Google Scholar]
- 20.Pomar F, Merino F, Barceló AR. O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma. 2002;220:17–28. doi: 10.1007/s00709-002-0030-y. [DOI] [PubMed] [Google Scholar]
- 21.Soukup A, Votrubová O, Čížková H. Development of anatomical structure of roots of Phragmites australis. New Phytol. 2002;153:277–287. doi: 10.1046/j.0028-646X.2001.00317.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


