ABSTRACT
Rho GTPases play significant roles in cellular function and their activity is regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), providing activation and inactivation of these GTPases, respectively. Active GTP-bound form of RhoA activates its effector proteins while the inactive GDP-bound form of RhoA exists in a RhoA-RhoGDI (guanine nucleotide dissociation inhibitor) complex in the cytosol. In particular, IκB kinase γ IKKγ/NF-κB essential modulator (NEMO) plays a role as a GDI displacement factor (GDF) for RhoA activation through binding to RhoA-RhoGDI complex. Meanwhile, prion protein inactivates RhoA despite RhoA/RhoGDI association. Novel target proteins for Rho-associated kinase (ROCK) such as glycogen synthase kinase (GSK)-3β and IKKβ are recently discovered. Here, we elaborate on a post-translationally modified version of RhoA, phosphorylated at Tyr42 and oxidized at Cys16/20. This form of RhoA dissociates from RhoA-RhoGDI complex and activates IKKβ on IKKγ/NEMO, thus providing possibly a critical role for tumourigenesis.
KEYWORDS: cancer, GDF, post-translational modification, prion, RhoA, RhoGDI, ROCK
Introduction
Rho GTPases belong to the family of Ras-related small GTP binding proteins that includes RhoA, Cdc42 and Rac1/2 and play several important roles in cellular function including cytoskeletal rearrangement, reactive oxygen species (ROS) production along with regulation of cell morphology, cell movement, and transcription. A dysregulation of Rho GTPases is also linked a variety of diseases including various cancer types. Rho GTPases are activated by association with GTP, catalysed by guanine nucleotide exchange factors (GEFs). The reverse of this reaction involves GTP hydrolysis, a step catalysed by GTPase activating proteins (GAPs), leading to inactivation of Rho GTPases.1-3 For various cellular processes, specific GEFs and GAPs are regulated by a set of stimulants in order to elicit defined states for Rho activity. Rho GTPases are also covalently modified with a lipid moiety that include a prenyl group attached to a defined cysteine residue in their terminal region as part of a CAAX motif (C, cysteine; A, any aliphatic amino acid; X, any amino acid). Inactive GDP-bound Rho exists in the cytosol in a complex with RhoGDI (guanine nucleotide dissociation inhibitor) while GTP-bound Rho is associated with the cell membrane through its prenyl group. For Rho GTPases to be activated, they need to be dissociated from RhoGDI, made possible by GDI displacement factor (GDF), as GEF cannot directly act on the Rho GTPase-RhoGDI complex.
Activated Rho GTPases bind to effector proteins that transmit the Rho-mediated signal to downstream target proteins. These typically include two Rho-associated coiled coil kinases (ROCKs) 1/2 that are activated by RhoA and six p21-activated protein kinases (PAKs) 1–6 that are activated by Cdc42 and Rac1. For RhoA, ROCK then phosphorylates myosin phosphatase, myosin light chain kinase and LIM kinase2 (LIMK2), leading to formation of actin filaments and increased actin-myosin interactions.4 Meanwhile, LIMK1 is activated by PAK1.5 In this mini-review, we focus on the description of novel targets for RhoA and ROCK. We also introduce the prion protein as an additional regulatory protein of RhoA and discuss the post-translational modifications of RhoA involving Cys16/20 oxidation and Ty42 phosphorylation that are likely to be critical for cell tumourigenesis.
Novel target proteins of ROCK identified
ROCK phosphorylates various target proteins in addition to regulatory proteins that control actin filament dynamics.6,7 For example, nuclear ROCK2 phosphorylates p300 acetyltransferase, leading to an increase in overall acetyltransferase activity.8 Also, during tumour cell migration, FilGAP, a Rac GTPase activating protein, is phosphorylated by ROCK, leading to increased tumour invasion.9 For ROCK1, it directly interacts with and phosphorylates c-Myc at Thr58 and/or Ser62, resulting in stabilization of c-Myc and activation of its transcriptional activity.10 In leukemic cells, activated RhoA/ROCK1 binds to Erk1/2, leading to suppression of Erk1/2 activity.11 Also in the context of the insulin binding to its receptor at the cell surface, ROCK subsequently phosphorylates insulin receptor substrate (IRS)-1 Ser632/635, leading to increased glucose uptake by the cell.12 By using GST-ROCK fusion protein as a bait, several additional candidate proteins have been identified to interact with ROCK; these include amyloid precursor protein (APP), receptor-type tyrosine protein phosphatase delta (PTPRD), AP180 and doublecortin (DCX).13 ROCK also binds to and phosphorylates 3-phosphoinositide-dependent kinase 1 (PDK1).14
We also identified ROCK phosphorylating glycogen synthase kinase (GSK)-3β in response to Wnt3A and active ROCK1 was shown to directly phosphorylate GSK-3β at Ser9 in vitro. Although p-Ser9 GSK-3β has been reported not to be related to β-catenin stabilization,15 RhoA/ROCK stabilizes β-catenin through an unknown mechanism(s), leading to increased expression of c-Myc and cyclin D1 and stimulating cell proliferation upon Wnt3A administration.16 RhoA/ROCK-mediated β-catenin activation in response to Wnt3A also induces expression of a chemokine, MIP-1α, leading to an increase in cell migration.16 We also revealed that ROCK1 phosphorylates IKKβ at Ser 177/181 in response to cell treatment with TGF-β1 and in the in vitro setting, active ROCK leads to nuclear factor-κB (NF-κB) activation (Fig. 1).17 As ROCK phosphorylates its various targets, this may also determine the destination of ROCK, localizing in the cell7 (Fig. 1 and Table 1).
Figure 1.
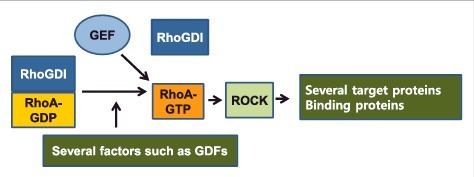
Factors for RhoA-RhoGDI dissociation and ROCK target proteins. RhoA-GDP/RhoGDI complex existing in cytosol needs to be dissociated by several factors such as GDFs (GDI displacement factors) before RhoA can be activated by GEFs. Activated RhoA then binds to ROCK, which then phosphorylates a variety of target proteins or binds to certain proteins, leading to regulation of downstream proteins. Detailed factors and proteins are described in Table 1 and Table 2.
Table 1.
Target proteins for ROCK.
| Target proteins | Function or feature | Reference |
|---|---|---|
| P300 acetyltransferase | Increases of acetyltransferase activity | 8 |
| FilGAP | Rac GAP, Tumour invasion | 9 |
| C-Myc | Stabilization of C-Myc, Increase of transcriptional activity | 10 |
| ERK1/2 | Binding to ERK1/2, Suppression of ERK1/2 activity | 11 |
| IRS-1 | Glucose uptake | 12 |
| IKKβ | Activation | 17 |
| GSK-3β | Inactivation by Ser9 phosphorylation | 16 |
| APP | Binding | 13 |
| PTPRD | Binding | 13 |
| AP180 | Binding | 13 |
| Doublecortin (DCX) | Binding | 13 |
| PDK1 | Binding | 14 |
Abbreviations: APP, amyloid precursor protein; IRS, insulin receptor substrate; PTPRD, Receptor-type tyrosine protein phosphatase dela; DCX, Doublecortin; PDK, 3-phosphinositide-dependent kinase.
Factors affecting the dissociation of Rho GTPases-RhoGDI complex
Agents acting as GDFs and dissociating the Rho-RhoGDI complex: Ezrin/radixin/moesin (ERM) directly interacts with RhoGDI, reducing the activity of RhoGDI and initiating Rho activation. It is then proposed that ERM acts as a GDF.18 Also, the neurotrophin receptor p75NTR directly interacts with RhoGDI and initiates RhoA activation by releasing RhoA from RhoGDI as RhoA inactivation is closely related to neurite outgrowth. Nerve growth factor (NGF), which induces neurite outgrowth, interferes with binding between p75NTR and RhoGDI, whereas Nogo and MAG (myelin-associated glycoprotein), which inhibit neurite outgrowth, strengthen p75NTR-RhoGDI complex formation. Thus, p75NTR acts as a GDF that dissociates the RhoA-RhoGDI complex to activate RhoA (Fig. 1).19
Modifications of RhoGDI regulating its affinity to Rho GTPases: Serine/threonine-protein kinase Pak1 binds to and phosphorylates RhoGDI at Ser101 and Ser174, which causes dissociation of Rac1-RhoGDI, but not RhoA-RhoGDI complexes.20 Also, tyrosine kinase Src phosphorylates RhoGDI at Tyr156 residue, which causes a dramatic decrease in the ability of RhoGDI to form a complex with RhoA, Rac1 and Cdc42.21 Recently, it was reported that RhoGDIα is acetylated at Lys127 and Lys141, which decreases the RhoGDI affinity toward RhoA, leading to facilitation of RhoA activation.22 Furthermore, Lys acetylation of RhoGDI increases GEF-catalysed GTP binding to RhoA. Hence, it is proposed that acetylation of RhoGDI functions as a GDF event.23
Very recently, we found that factors related to the reactive oxygen species (ROS) generation may also affect RhoGDI activity. It is shown that oxidation of RhoGDI by hydrogen peroxide abolishes its complex formation with RhoA.24 As such, RhoGDI oxidation is likely to be a prerequisite for RhoA activation. RhoGDI has only one cysteine residue (Cys79) and yet in the presence of hydrogen peroxide, the RhoGDI Cys79Ala mutant, which is supposed to be an oxidation-resistant form, also does not bind to RhoA in vitro (unpublished data) (Fig. 1). This result suggests that RhoGDI is oxidized at residue(s) other than Cys79. Candidates may include unidentified methionine residue(s) oxidized by hydrogen peroxide for RhoGDI, as sulphur-containing amino acids such as methionine as well as cysteines are particularly vulnerable to oxidation25 (Fig. 1 and Table 2).
Table 2.
Factors to regulate the formation of RhoA-RhoGDI complex.
| Factor | Function or feature | Reference |
|---|---|---|
| Ezrin/radixin/moesin | GDF | 18 |
| p75NTR | GDF | 19 |
| Phosphorylation of RhoGDI by Pak1 | Decrease of RhoGDI activity to bind to Rac1 | 20 |
| Phosphorylation of RhoGDI by Src | Decrease of RhoGDI activity to bind to RhoA, Rac1 and Cdc42 | 21 |
| Acetylation of RhoGDI | Decrease of RhoGDI activity to bind to RhoA | 22 |
| IKKγ/NEMO | GDF | 17 |
| Oxidation of RhoGDI | GDF | 24 |
| Oxidation of RhoA | GDF | 24 |
| Ser188 phosphorylation of RhoA by PKA, PKG, AMPK, ERK | Increase of RhoA binding to RhoGDI, Inactivation of RhoA | 31, 34-36 |
| Tyr42 phosphorylation of RhoA | GDF | 37 |
| Prion | Inactivation of RhoA | 27 |
Abbreviations: GDF, GDI displacement factor; NTR, neurtrophin receptor; Pak, p21-activated kinase; IKKγ, IκB kinase γ; GDI, guanine nucleotide dissociaition inhibitor; NEMO, NF-κB essential modulator; PKA, protein kinase A; PKG, protein kinase G, AMPK, AMP-dependent kinase.
Prion protein: The cellular prion protein (PrPC), a cell-surface glycosylphosphatidylinositol (GPI)-anchored glycoprotein, has also been implicated in neuritogenesis and neuronal differentiation by modulating the RhoA-ROCK-LIMK-cofilin signalling pathway.26,27 Neurite outgrowth by NGF, bFGF, and cAMP in PC12 cells requires the inactivation of RhoA and the mechanism involved has been described.28-30 Contrary to a general notion that proteins binding to RhoGDI activate RhoA through the dissociation of RhoA-RhoGDI complex, the prion protein inactivates RhoA although prion protein binds to both RhoA and RhoGDI (data not shown). Although the function of RhoGDI binding to prion protein remains elusive, we speculate that prion protein provides RhoGDI to the inactivated RhoA. Prion protein induces the phosphorylation of RhoA Ser188 by protein kinase A (PKA), leading to complex formation with RhoGDI.31 P190RhoA, Rap-dependent RhoGAP (ARAP3) and RhoA phosphorylated at Ser188 by PKA attribute to RhoA inactivation in response to NGF, bFGF and cAMP, leading to enhanced complex formation with RhoGDI and neurite outgrowth from PC12 cells.28-30 Notably, PrPC recruits and links RhoA and p190 RhoGAP, resulting in RhoA inactivation in response to NGF stimulation in PC12 cells with PrPC playing a role as a signalling and/or enhancer molecule of neurite outgrowth.27 Interestingly, pathogenic prions (PrPSc) impair both RhoA inactivation and neurite outgrowth in NGF-stimulated PC12 cells (Fig. 2).
Figure 2.
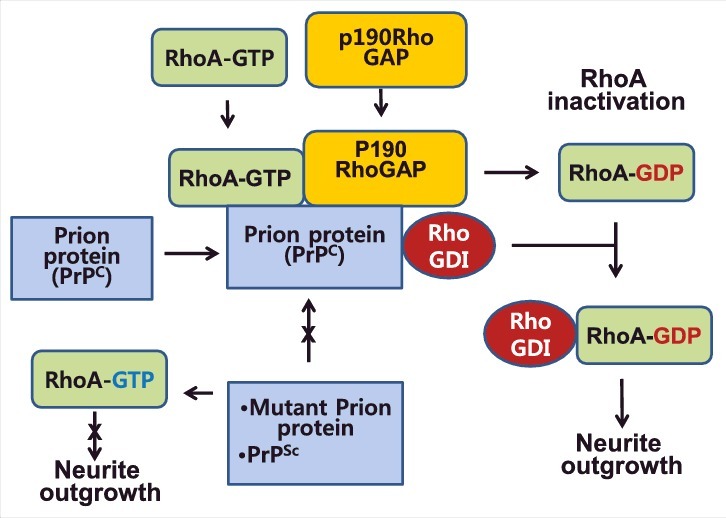
Prion protein as a linker of RhoA and p190RhoGAP, thereby facilitating RhoA inactivation. Prion protein recruits and links RhoA and p190RhoA together, thereby accelerating RhoA inactivation by p190RhoGAP. Subsequently RhoGDI binding with prion protein is likely to be provided to inactivated RhoA. Thus prion protein contributes to neurite outgrowth through RhoA inactivation, whereas mutant prion proteins and pathogenic PrPSc cannot facilitate RhoA inactivation, resulting in high RhoA activity and suppression of neurite outgrowth.
According to a recent report,14 over-activation of ROCK by prion infection leads to an impairment of neurite sprouting through ROCK-induced phosphorylation and activation of 3-phosphoinositide-dependent kinase 1 (PDK1). Subsequent PDK1 overstimulation, which precludes α-cleavage of PrPC by TACE α-secretase, induces PrPSc production.14 Thus, PrPC acts as a signalling module by engaging with RhoA and p190 RhoGAP, leading to efficient RhoA inactivation, followed by controlling neuronal polarity.27,32
Modifications of Rho GTPases regulating their mode of action
Phosphorylations of RhoA GTPase: Rho GTPases undergo post-translational modifications that include phosphorylation, ubiquitination and AMPylation, affecting their function.33 For example, phosphorylation of RhoA Ser188 by protein kinase A (PKA) increases its affinity to RhoGDI.31 Cyclic GMP-dependent protein kinase also phosphorylates RhoA at Ser188 in vascular myocytes, leading to inhibition of RhoA.34 Furthermore, AMP kinase α1 (AMPKα1) phosphorylates RhoA at Ser188 in vascular smooth muscle cells in response to estradiol, also leading to RhoA inhibition.35 In addition, upon EGF stimulation of the cell, ERK phosphorylates RhoA at Ser88 and Thr100, which then upregulate the activity of RhoA.36 It is noteworthy that Tyr42 phosphorylation of RhoA is critical for tumourigenesis,37 also discussed in the later section.
Cys16/20 oxidation and Tyr42 phosphorylation of RhoA: It has been well established that NADPH oxidase (NOX) regulates ROS production. Rac1/2 is involved in activation of p67PHOX, a component of NADPH oxidase, leading to superoxide generation.38 In addition, RhoA has also been reported to regulate superoxide production through indirect activation of NADPH oxidase.39,40 Furthermore, Rap1 and RhoA reveal complementary additive functions for superoxide production in response to IgG-oposonized particles.41
There is also a report of ROS inhibiting RhoA by p190RhoGAP activation, as ROS inhibits low-molecular weight protein tyrosine phosphatase (LMW-PTP), leading to tyrosine phosphorylation and activation of p190RhoGAP. Here Rac is involved in superoxide production, indicating that Rac downregulates RhoA through p190RhoGAP activation.42 Also as noted above, a low concentration of hydrogen peroxide activates RhoA via RhoA oxidation and Vav2 activation.24
Recently, there has also been an evidence of Rho GTPases being regulated by the redox-state of the cell and in turn Rho GTPases regulating the redox-state by controlling enzymes that produce ROS and reactive nitrogen species (RNS).43 Of note, Cys16 and Cys20 in RhoA can be oxidized to form a disulphide bond,44 affecting its activity.
Although there are many reports of RhoA being involved in NF-κB activation,45 its mechanism has not been clearly elucidated. Recently, we proposed a detailed mechanism by which RhoA activates NF-κB. It involves RhoA-GDP/RhoGDI complex binding to IKKγ/NEMO (NF-κB essential modulator). RhoA binds to the N-terminal domain (amino acids 1–43) of IKKγ/NEMO and RhoGDI binds to its large C-terminal domain (amino acids 100–419). Active Dbl, a GEF, slightly increases GTP binding to RhoA in the presence of RhoGDI. Furthermore, IKKγ/NEMO significantly enhances GTP binding to RhoA via active Dbl in the presence of RhoGDI. Therein, we have proposed that IKKγ/NEMO plays a role as a GDF to dissociate the RhoA-RhoGDI complex before GEF action in the TGF-β1 signalling pathway (Fig. 3A).17
Figure 3.
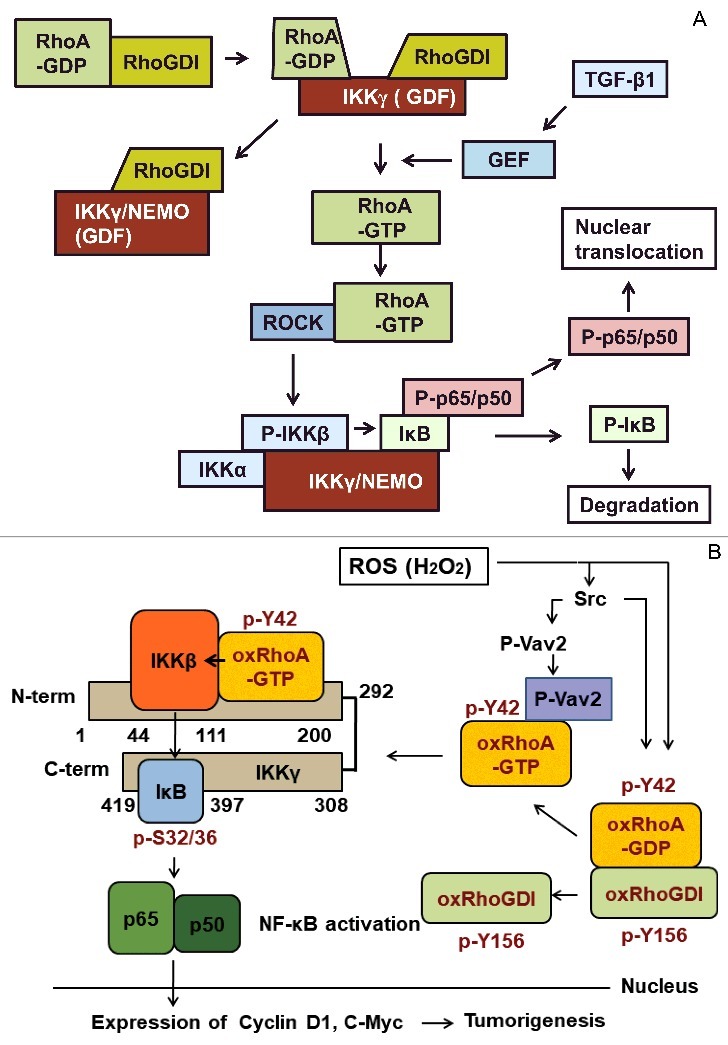
IKKγ/NEMO is required for activation of NF-κB through RhoA GTPase. (A) IKKγ/NEMO facilitates RhoA activation through dissociation of the RhoA-RhoGDI complex. Activated RhoA is first released from IKKγ/NEMO and then activates ROCK, which then phosphorylates IKKβ, leading to IκB phosphorylation and NF-κB activation. (B) Oxidation of Cys16/20 and phosphorylation of Tyr42 of RhoA results in the dissociation of the RhoA-RhoGDI complex. Oxidized/phosphorylated RhoA can be activated by Vav2 GEF and then binds to IKKγ/NEMO, where RhoA directly activates IKKβ juxtaposed to RhoA on IKKγ/NEMO. IKKβ phosphorylates IκB, leading to IκB degradation and NF-κB activation. From this chain of events, ROS induces tumourigenesis through the signalling pathway.
Although RhoA-GTP rarely binds to IKKγ/NEMO,17 RhoA-GTP that is oxidized at Cys16/20 and phosphorylated at Tyr42 readily binds to the region composed of amino acids 100–200 in IKKγ/NEMO and on which, RhoA-GTP stimulates IKKβ, which is juxtaposed with the RhoA. As such, IKKβ directly activated by RhoA oxidized at Cys16/20 and phosphorylated at Tyr42 then phosphorylates IκB, leading to IκB degradation and subsequent NF-κB activation. This in turn leads to expression of C-myc and cyclin D1 and consequent cell proliferation (Fig. 2B).24,37 Also, RhoA oxidized at Cys16/20 and phosphorylated at Tyr42 reduces its affinity to RhoGDI (Fig. 1).24,37 We also speculate that endogenous ROS sources may be in the tumour microenvironment as a co-culture of breast cancer and macrophage cells produced ROS.24,37
Tyr42 phosphorylation of RhoA is brought about by Src tyrosine kinase and ROS contributes to Src activation through hyperoxidization of Src Cys245 and Cys487 residues, leading to Tyr416 phosphorylation of Src.46 P-Tyr42 of RhoA then serves as a binding site for Vav2, which activates RhoA. Thus, RhoA that is oxidized at Cys16/20 and phosphorylated at Tyr42 induces expression of c-Myc and cyclin D1, both essential for cell proliferation. In support of this hypothesis, p-Tyr42 Rho has been detected in patient breast cancer samples, and presence of p-Tyr42 Rho, p-Tyr416 Src and p-Ser527 p65/RelA positively correlate.37 Thereby, we speculate that RhoA Tyr42 phosphorylation and Cys16/20 oxidation are likely to be critical for tumourigenesis in response to ROS (Fig. 3B and Table 2).
Conclusion
Activation of RhoA and ROCK has been known to regulate dynamics of actin filament formation. Novel target proteins and novel functions of RhoA and ROCK have also been discovered. In particular, IKKγ/NEMO functions as a GDF to activate RhoA through binding with RhoA-RhoGDI complex. However, prion protein inactivates RhoA despite RhoA/RhoGDI association; Instead, prion protein plays a role as a platform protein to recruit and link p190RhoA and RhoA, leading to efficient RhoA inactivation and neurite outgrowth. We also focused post-translational modification of RhoA including Tyr42 phosphorylation and Cys16/20 oxidation. The oxidized/phosphorylated RhoA directly activates IKKβ, leading to NF-κB activation and consequently expression of cyclin D1 and C-Myc upon exposure to hydrogen peroxide and as part of events that are closely relevant to tumourigenesis. P-Tyr42 RhoA may also play multiple roles in a variety of unidentified cellular functions.
Abbreviations
- APP
amyloid precursor protein
- DCX
doublecortin
- EGF
epithelial growth factor
- FAK
focal adhesion kinase
- GAP
GTPase activating protein
- GDF
GDI displacement factor
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- GSK
glycogen synthase
- IKK
IκB kinase
- IκB
NF-κB inhibitor
- IRS
insulin receptor substrate
- NEMO
NF-κB essential modulator
- NF-κB
nuclear factor-κB
- NGF
nerve growth factor
- NOX
NADPH oxidase
- NTR
neurotrophin receptor
- PAK
p21-activated kinase
- PDK
phosphatidylinositol-dependent kinase
- PDK1
3-phosphoinositide-dependent kinase 1
- PKA
protein kinase A
- PrP
prion protein
- PTPRD
receptor-type tyrosine proteinphosphatase delta
- ROCK
Rho-associated coiled coil kinase
- ROS
reactive oxygen species
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Sadra AR, Roh SR, and Park SH for the critical reading of the manuscript.
Funding
This research was supported by the Basic Science Research Programme of the National Research Foundation of Korea (NRF) (NRF-2015R1D1A1A01060393) and Hallym University (HRF-S-52).
References
- 1.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13-22. https://doi.org/ 10.1016/S0962-8924(02)00004-1. PMID:12480336 [DOI] [PubMed] [Google Scholar]
- 2.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell. 2007;129:865-77. https://doi.org/ 10.1016/j.cell.2007.05.018. PMID:17540168 [DOI] [PubMed] [Google Scholar]
- 3.Buchsbaum RJ. Rho activation at a glance. J Cell Sci. 2007;120:1149-52. https://doi.org/ 10.1242/jcs.03428. PMID:17376960 [DOI] [PubMed] [Google Scholar]
- 4.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247-69. https://doi.org/ 10.1146/annurev.cellbio.21.020604.150721. PMID:16212495 [DOI] [PubMed] [Google Scholar]
- 5.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253-9. https://doi.org/ 10.1038/12963. PMID:10559936 [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Surma M, Shi S, Lambert-Cheatham N, Shi J. Novel Insights into the Roles of Rho Kinase in Cancer. Arch Immunol Ther Exp (Warsz) 2016;64:259-78. https://doi.org/ 10.1007/s00005-015-0382-6. PMID:26725045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases. 2014;5:e29846. https://doi.org/ 10.4161/sgtp.29846. PMID:25010901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka T, Nishimura D, Wu RC, Amano M, Iso T, Kedes L, Nishida H, Kaibuchi K, Hamamori Y. Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J Biol Chem 2006; 281:15320-9. https://doi.org/ 10.1074/jbc.M510954200. PMID:16574662 [DOI] [PubMed] [Google Scholar]
- 9.Saito K, Ozawa Y, Hibino K, Ohta Y. FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. Mol Biol Cell. 2012;23:4739-50. https://doi.org/ 10.1091/mbc.E12-04-0310. PMID:23097497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Zhang S, Zhang Z, He J, Xu Y, Liu S. ROCK has a crucial role in regulating prostate tumor growth through interaction with c-Myc. Oncogene. 2014;33:5582-91. https://doi.org/ 10.1038/onc.2013.505. PMID:24317511 [DOI] [PubMed] [Google Scholar]
- 11.Li F, Jiang Q, Shi KJ, Luo H, Yang Y, Xu CM. RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 2013;4:e708. https://doi.org/ 10.1038/cddis.2013.243. PMID:23828571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, et al.. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119-29. https://doi.org/ 10.1016/j.cmet.2005.06.011. PMID:16098829 [DOI] [PubMed] [Google Scholar]
- 13.Amano M, Tsumura Y, Taki K, Harada H, Mori K, Nishioka T, Kato K, Suzuki T, Nishioka Y, Iwamatsu A, et al.. A proteomic approach for comprehensively screening substrates of protein kinases such as Rho-kinase. PLoS One. 2010;5:e8704. https://doi.org/ 10.1371/journal.pone.0008704. PMID:20090853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alleaume-Butaux A, Nicot S, Pietri M, Baudry A, Dakowski C, Tixador P, Ardila-Osorio H, Haeberlé AM, Bailly Y3, Peyrin JM, et al.. Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation. PLoS Pathog. 2015;11:e1005073. https://doi.org/ 10.1371/journal.ppat.1005073. PMID:26241960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571-83. https://doi.org/ 10.1038/sj.emboj.7600633. PMID:15791206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JG, Kim MJ, Choi WJ, Moon MY, Kim HJ, Lee JY, et al.. Wnt3A Induces GSK-3beta Phosphorylation and beta-Catenin Accumulation Through RhoA/ROCK. J Cell Physiol. 2017;232:1104-13. https://doi.org/ 10.1002/jcp.25572. PMID:27575935 [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim JG, Moon MY, Park SH, Park JB. IkappaB kinase gamma/nuclear factor-kappaB-essential modulator (IKKgamma/NEMO) facilitates RhoA GTPase activation, which, in turn, activates Rho-associated KINASE (ROCK) to phosphorylate IKKbeta in response to transforming growth factor (TGF)-beta1. J Biol Chem. 2014;289:1429-40. https://doi.org/ 10.1074/jbc.M113.520130. PMID:24240172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371-5. https://doi.org/ 10.1074/jbc.272.37.23371. PMID:9287351 [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461-7. PMID:12692556 [DOI] [PubMed] [Google Scholar]
- 20.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117-27. https://doi.org/ 10.1016/j.molcel.2004.05.019. PMID:15225553 [DOI] [PubMed] [Google Scholar]
- 21.DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17:4760-8. https://doi.org/ 10.1091/mbc.E06-06-0533. PMID:16943322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhlmann N, Wroblowski S, Scislowski L, Lammers M. RhoGDIalpha Acetylation at K127 and K141 Affects Binding toward Nonprenylated RhoA. Biochemistry. 2016;55:304-12. https://doi.org/ 10.1021/acs.biochem.5b01242. PMID:26695096 [DOI] [PubMed] [Google Scholar]
- 23.Kuhlmann N, Wroblowski S, Knyphausen P, de Boor S, Brenig J, Zienert AY, Meyer-Teschendorf K, Praefcke GJ, Nolte H, Krüger M, et al.. Structural and Mechanistic Insights into the Regulation of the Fundamental Rho Regulator RhoGDIalpha by Lysine Acetylation. J Biol Chem. 2016;291:5484-99. https://doi.org/ 10.1074/jbc.M115.707091. PMID:26719334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JG, Kwon HJ, Wu G, Park Y, Lee JY, Kim J, Kim SC, Choe M, Kang SG, Seo GY, et al.. RhoA GTPase oxidation stimulates cell proliferation via nuclear factor-kappaB activation. Free Radic Biol Med. 2017;103:57-68. https://doi.org/ 10.1016/j.freeradbiomed.2016.12.013. PMID:27974245 [DOI] [PubMed] [Google Scholar]
- 25.Drazic A, Winter J. The physiological role of reversible methionine oxidation. Biochim Biophys Acta. 2014;1844:1367-82. https://doi.org/ 10.1016/j.bbapap.2014.01.001. PMID:24418392 [DOI] [PubMed] [Google Scholar]
- 26.Pietri M, Dakowski C, Hannaoui S, Alleaume-Butaux A, Hernandez-Rapp J, Ragagnin A, Mouillet-Richard S, Haik S, Bailly Y, Peyrin JM, et al.. PDK1 decreases TACE-mediated alpha-secretase activity and promotes disease progression in prion and Alzheimer's diseases. Nat Med. 2013;19:1124-31. https://doi.org/ 10.1038/nm.3302. PMID:23955714 [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Choi HS, Park JH, Kim MJ, Lee HG, Petersen RB, Kim YS, Park JB, Choi EK. Regulation of RhoA activity by the cellular prion protein. Cell Death Dis. 2017;8:e2668. https://doi.org/ 10.1038/cddis.2017.37. PMID:28300846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon CY, Kim HJ, Lee JY, Kim JB, Kim SC, Park JB. p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells. Exp Mol Med. 2010;42:335-44. https://doi.org/ 10.3858/emm.2010.42.5.035. PMID:20200473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon CY, Kim HJ, Morii H, Mori N, Settleman J, Lee JY, Kim J, Kim SC, Park JB. Neurite outgrowth from PC12 cells by basic fibroblast growth factor (bFGF) is mediated by RhoA inactivation through p190RhoGAP and ARAP3. J Cell Physiol. 2010;224:786-94. https://doi.org/ 10.1002/jcp.22184. PMID:20578246 [DOI] [PubMed] [Google Scholar]
- 30.Jeon CY, Moon MY, Kim JH, Kim HJ, Kim JG, Li Y, Jin JK, Kim PH, Kim HC, Meier KE, et al.. Control of neurite outgrowth by RhoA inactivation. J Neurochem. 2012;120:684-98. https://doi.org/ 10.1111/j.1471-4159.2011.07564.x. PMID:22035369 [DOI] [PubMed] [Google Scholar]
- 31.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510-9. PMID:8599934 [PMC free article] [PubMed] [Google Scholar]
- 32.Linden R. The Biological Function of the Prion Protein: A Cell Surface Scaffold of Signaling Modules. Front Mol Neurosci. 2017;10:77. https://doi.org/ 10.3389/fnmol.2017.00077. PMID:28373833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson MF. Rho GTPases their post-translational modifications, disease-associated mutations and pharmacological inhibitors. Small GTPases. 2016;12:1-13. https://doi.org/ 10.1080/21541248.2016.1218407. PMID:27548350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722-9. https://doi.org/ 10.1074/jbc.M000753200. PMID:10783386 [DOI] [PubMed] [Google Scholar]
- 35.Gayard M, Guilluy C, Rousselle A, Viollet B, Henrion D, Pacaud P, Loirand G, Rolli-Derkinderen M. AMPK alpha 1-induced RhoA phosphorylation mediates vasoprotective effect of estradiol. Arterioscler Thromb Vasc Biol. 2011;31:2634-42. https://doi.org/ 10.1161/ATVBAHA.111.228304. PMID:21852563 [DOI] [PubMed] [Google Scholar]
- 36.Tong J, Li L, Ballermann B, Wang Z. Phosphorylation and Activation of RhoA by ERK in Response to Epidermal Growth Factor Stimulation. PLoS One. 2016;11:e0147103. https://doi.org/ 10.1371/journal.pone.0147103. PMID:26816343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JG, Choi KC, Hong CW, Park HS, Choi EK, Kim YS, Park JB. Tyr42 phosphorylation of RhoA GTPase promotes tumorigenesis through nuclear factor (NF)-kappaB. Free Radic Biol Med. 2017;112. https://doi.org/ 10.1016/j.freeradbiomed.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 38.Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533-48. https://doi.org/ 10.1089/ars.2006.8.1533. PMID:16987009 [DOI] [PubMed] [Google Scholar]
- 39.Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB. Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem 2004; 279:21589-97. https://doi.org/ 10.1074/jbc.M308386200. PMID:14970220 [DOI] [PubMed] [Google Scholar]
- 40.Moon MY, Kim HJ, Li Y, Kim JG, Jeon YJ, Won HY, Kim JS, Kwon HY, Choi IG, Ro E, et al.. Involvement of small GTPase RhoA in the regulation of superoxide production in BV2 cells in response to fibrillar Abeta peptides. Cell Signal. 2013;25:1861-9. https://doi.org/ 10.1016/j.cellsig.2013.05.023. PMID:23707391 [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Kim JG, Kim HJ, Moon MY, Lee JY, Kim J, Kim SC, Song DK, Kim YS, Park JB, et al.. Small GTPases Rap1 and RhoA regulate superoxide formation by Rac1 GTPases activation during the phagocytosis of IgG-opsonized zymosans in macrophages. Free Radic Biol Med. 2012;52:1796-805. https://doi.org/ 10.1016/j.freeradbiomed.2012.02.004. PMID:22330068 [DOI] [PubMed] [Google Scholar]
- 42.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236-41.https://doi.org/ 10.1038/ncb938. PMID:12598902 [DOI] [PubMed] [Google Scholar]
- 43.Hobbs GA, Zhou B, Cox AD, Campbell SL. Rho GTPases, oxidation, and cell redox control. Small GTPases. 2014;5:e28579. https://doi.org/ 10.4161/sgtp.28579. PMID:24809833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heo J, Raines KW, Mocanu V, Campbell SL. Redox regulation of RhoA. Biochemistry. 2006;45:14481-9. https://doi.org/ 10.1021/bi0610101. PMID:17128987 [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Kim JG, Moon MY, Jeon CY, Won HY, Kim HJ, Jeon YJ, Seo JY, Kim JI, Kim J, et al.. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood. 2006;108:1821-9. https://doi.org/ 10.1182/blood-2005-10-009191. PMID:16705092 [DOI] [PubMed] [Google Scholar]
- 46.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarufgi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391-403. https://doi.org/ 10.1128/MCB.25.15.6391-6403.2005. PMID:16024778 [DOI] [PMC free article] [PubMed] [Google Scholar]


