ABSTRACT
Solanaceous plants produce sesquiterpenoid phytoalexins to defend themselves against a variety of pathogens. These toxic compounds are not only harmful to the pathogen but also to the plant, and thus need to be detoxified by the plant after the threat has been eliminated. We report that the detoxification of rishitin, the major phytoalexin in potato tubers and tomato fruits, is mediated by a cytochrome P450 CYP76 family enzyme via the hydroxylation of the isopropenyl group resulting in the formation of 13-hydroxyrishitin, also known as rishitin-M1. We further observed hydroxylation of the potato phytoalexins solavetivone, lubimin and oxylubimin by the same enzyme. Constitutive expression of CYP76 in Nicotiana benthamiana also led to a reduction of the non-potato phytoalexins capsidiol and its derivative capsidiol 3-acetate. We therefore annotated this enzyme as sesquiterpenoid phytoalexins hydroxylase, SPH. This broad range of substrates indicates that SPH functions as a general phytoalexin detoxification enzyme in Solanaceae, and is therefore relevant for a better understanding of plant-pathogen interaction in solanaceous plants, which comprise many economically important crops, such as potato, tomato, eggplant and pepper.
KEYWORDS: Capsidiol, Cytochrome P450, detoxification, phytoalexins, potato, rishitin, Solanaceae plants
Introduction
Production of toxins as a means of protection is a recurring defense pattern in nature and widely spread among all clades. In plants, toxins that primarily target pathogens, are referred to as phytoalexins.1,2 The term phytoalexin is a generic name and encompasses a large number of compounds, which fall into different chemical classes, including flavonoids, indoles and terpenoids. Phytoalexins are but one component in a larger defense arsenal, which includes programmed cell-death, production of ROS (reactive oxygen species) and anti-microbial proteins in PAMP (pathogen-associated molecular patterns) triggered immunity, effector-triggered immunity and systemic acquired resistance.3 Though the way in which the various phytoalexins exert their toxic effect may differ, the general concept appears to be to either eliminate a given pathogen or at least slow its growth down, in order for other defense mechanisms to gain more time to mount an effective defense. This notion is supported by the finding that phytoalexins often accumulate in tissue, which has undergone programmed cell-death,4 thereby hampering a pathogen’s ability to gain a foothold. As a consequence, inhibition of phytoalexin synthesis is commonly observed with a higher disease susceptibility.5–7 This defense strategy is a double-edged sword however, since many of these compounds were shown to also cause damage to the plant cells as well.5,8,9 For this reason, phytoalexin production needs to be contained to the area of infection, surge rapidly, and be detoxified as soon as the infectious threat has been eliminated.
The major phytoalexins present in solanaceous plants are sesquiterpenoids and therefore consist of a 15-carbon backbone which derives from farnesyl diphosphate (FPP) (Figure 1). A multitude of similar phytoalexins exists in different solanaceous species. Probably the most studied representative of these is capsidiol, which is primarily produced in Capsicum and Nicotiana species (Figure 1), where it is found both in leaf and fruit tissue upon elicitation.10,11 Capsidiol has, to our knowledge, never been detected in the closely related Solanum plants. However, Solanum plants appear to produce an extensive range of phytoalexins, such as the accordingly named solavetivone but also rishitin, lubimin, oxylubimin and others.12 The composition of these compounds varies not only between species, but even between different cultivars.13 Some of the phytoalexins present in Solanum can also be found in Nicotiana and Capsicum, though at lower amounts than capsidiol.14,15
Figure 1.
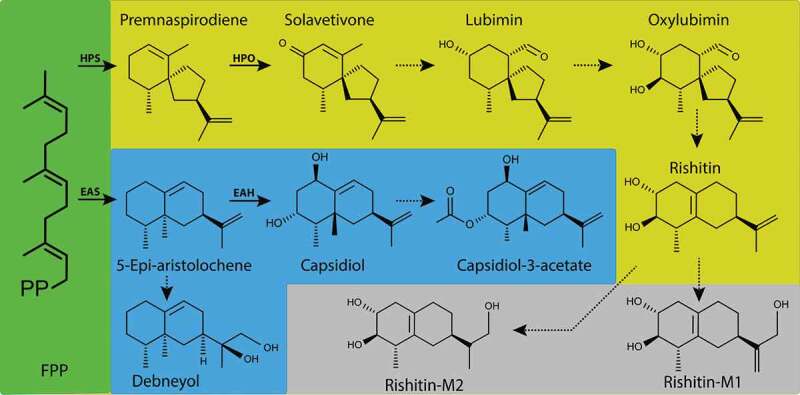
Sesquiterpenoid phytoalexins from solanaceous plants. Molecular structures and proposed pathways for synthesis of sesquiterpenoid phytoalexins in solanaceous plants. Farnesyl diphosphate (FPP) is the common precursor for all compounds, which leads to two separate pathways. The yellow pathway generates solavetivone-type phytoalexins and is present in potato and tomato. The blue pathway leads to the formation of capsidiol and debneyol and is found in Nicotiana benthamiana. In potato tubers, detoxification of rishitin was found to result in rishitin-M1 and rishitin-M2 as depicted in the gray pathway. Solid arrows: Enzymes have been characterized. Dotted arrows: enzyme unknown and intermediates may exist. EAS, 5-epi-aristolochene synthase; EAH (5-epi-aristolochene dihydroxylase; HPS, Hyoscyamus muticus premnaspirodiene synthase; HPO H. muticus premnaspirodiene oxygenase.
In potatoes and tomatoes, phytoalexin production does not occur in leaves and is best observed in potato tubers,16 as well as the fruits and stems of tomato.17,18 While the discovery of most of these sesquiterpenoids occurred decades ago, little progress has been made in resolving the biosynthetic pathways. Most insights were gained from isotope labeled feeding experiments, but the enzymes and genes required for the biosynthesis of these phytoalexins remain largely unknown. The only exceptions are capsidiol and solavetivone, which merely require two enzymes. In the case of capsidiol, the two enzymes EAS (5-epi-aristolochene synthase) and EAH (5-epi-aristolochene dihydroxylase) are sufficient to produce capsidiol from FPP.19,20 Production of solavetivone requires HPS (Hyoscyamus muticus premnaspirodiene synthase) and HPO (H. muticus premnaspirodiene oxygenase) respectively (Figure 1).21,22
While capsidiol and solavetivone are functional phytoalexins, in many cases they are further converted to other phytoalexins. In Nicotiana benthamiana, production of capsidiol coincides with the formation of capsidiol 3-acetate, though how the esterification occurs is yet unknown. There is an ample amount of research, which establishes a pathway from solavetivone to rishitin, the major phytoalexin in potato and tomato. This pathway suggests that solavetivone is a precursor of lubimin, which subsequently is converted to oxylubimin, and finally to rishitin (Figure 1).23,24
Due to their toxicity not only to the pathogen but also to the host plant, phytoalexins such as rishitin need to be degraded by the host after a certain period of time. The compounds rishitin-M1 (13-hydroxyrishitin) and rishitin-M2 are derivatives of rishitin, which have lost their toxicity to potato tissue, and at the same time, demonstrate a greatly reduced efficiency against Phytophthora infestans (Figure 1).25 The detoxification of rishitin into rishitin-M1 was also found to occur in the fungal pathogen Gibberella pulicaris, which causes dry rot in potato tubers.26 Interestingly rishitin-M1 and rishitin-M2 are created via two distinct mechanisms.27 While rishitin-M1 could be shown to be caused via an oxygenation of the C-13 carbon from molecular oxygen,28 rishitin-M2 requires the intramolecular migration of a hydrogen atom at C-12 (Figure 1).
Solanaceous plants, such as potato, tomato, eggplant and pepper, are an integral part of the diet of many cultures throughout the world. Even though sesquiterpenoid phytoalexins are known to be an important component of pathogen defense in solanaceous plants, research on phytoalexin metabolism has made little progress over the last decades. Gaining insights into the phytoalexin pathway is a requirement for improving our understanding of plant-pathogen interaction in Solanaceae and may help breeding more resistant cultivars in the future.
Results and discussion
In order to gain more insight into the enzymes governing the production of phytoalexins in potato tubers, we’ve investigated a group of cytochrome P450 enzymes, which we suspected to be involved in the phytoalexin pathways of potato. Of those, a gene annotated as cytochrome P450 76A2-like (CYP76A2L), was identified in the genomes of potato, tomato and tobacco, but not in N. benthamiana. This pattern matches the production profile of solavetivone, since out of these plants, N. benthamiana is the only that has never been found to produce solavetivone or phytoalexins derived thereof. Due to this circumstance, N. benthamiana leaves were used as a platform to examine whether CYP76A2L is involved in the pathway of solavetivone derived phytoalexins.
CYP76A2L expression leads to a reduction of all major potato phytoalexins
CYP76A2L was transiently expressed under the control of 35S promoter for two days in N. benthamiana leaves using agroinfiltration, and these leaves were subsequently infiltrated with pure stocks of the potato phytoalexins solavetivone, lubimin, oxylubimin and rishitin. The leaves were harvested and the amounts of residual phytoalexins measured with GC-MS. We detected a reduction of these infiltrated phytoalexins in CYP76A2L expressing leaves after an incubation of 5 h compared to leaves which were only expressing GFP (Figure 2). Since phytoalexins are cytotoxic, we also tested the phytoalexin camalexin from Arabidopsis thaliana as a control for possible effects caused by cellular damage. The infiltrated sesquiterpenoid phytoalexins from Solanum species, solavetivone, lubimin, oxylubimin and rishitin, are all reduced, whereas camalexin, an indole alkaloid phytoalexin, remained unaffected by CYP76A2L expression, indicating that the reduction of the sesquiterpenoids is not an indirect effect but rather caused by the activity of CYP76A2L. These results show that expression of CYP76A2L causes a significant reduction of every tested sesquiterpenoid phytoalexins in the rishitin pathway.
Figure 2.
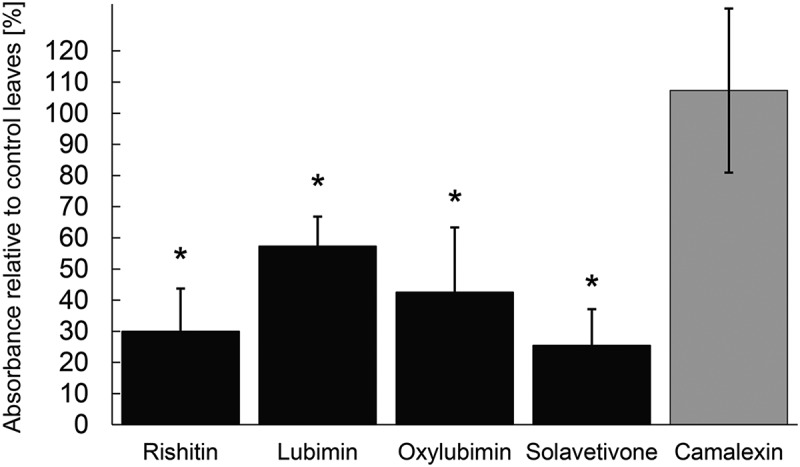
Reduction of Solanaceae phytoalexins in N. benthamiana leaves expressing CYP76A2L. N. benthamiana leaves were inoculated with Agrobacterium strains for expression of GFP or CYP76A2L, and the leaves were infiltrated with 1 mg/ml rishitin, lubimin, oxylubimin, solavetivone or 0.2 mg/ml camalexin (control) 2 d after inoculation. Infiltrated phytoalexins were re-extracted after 5 h and measured using GC-MS. Scores indicate the amount of phytoalexins extracted from leaves expressing CYP76A2L relative to that from GFP expressing control leaves. Data are means ± SD (n = 4). Asterisks indicate a significant difference from camalexin control as assessed by Student’s two-tailed t-test at *P < .05.
CYP76A2L can metabolize endogenous sesquiterpenes from N. benthamiana via the isopropenyl tail
When comparing the structures of the phytoalexins tested in the previous section, it becomes apparent that these compounds demonstrate some striking differences, particularly rishitin, which seems to resemble capsidiol, rather than its precursors solavetivone, lubimin and oxylubimin (Figure 1). We thus examined whether CYP76A2L is able to metabolize capsidiol, which does not naturally occur in potato, as well. Elicitation of N. benthamiana with the elicitor INF1, a secretory protein of potato blight pathogen Phytophthora infestans,29 leads to the formation of capsidiol, capsidiol 3-acetate and debneyol.30 Debneyol and capsidiol both derive from 5-epi-aristolochene (Figure 1). Similar to the previous experiment, CYP76A2L was transiently expressed in N. benthamiana leaves for 2 days, followed by elicitation with INF1 for 1 day. In contrast to the infiltrated potato phytoalexins, measuring the impact of CYP76A2L on the endogenous phytoalexins capsidiol and capsidiol 3-acetate is much more difficult. This is because a reduction of capsidiol will necessarily result in a reduced accumulation of capsidiol 3-acetate as well. The opposite also holds true, if the formation of capsidiol 3-acetate is a reversible reaction, which is currently unknown. For this reason, measurements of endogenous capsidiol and capsidiol 3-acetate concentrations do not allow any conclusion over which compound is used as a substrate by CYP76A2L. It is however possible to examine the impact of CYP76A2L on the total accumulation of both capsidiol and its derivative capsidiol 3-acetate, to gain insight into whether CYP76A2L affects the capsidiol pathway. CYP76A2L expression led to a reduction of capsidiol and capsidiol 3-acetate in the N. benthamiana leaves, whereas a reduction of debneyol was not observed (Figure 3). The finding that debneyol levels did not change, indicates that 5-epi-aristolochene is not affected by CYP76A2L, which demonstrates that CYP76A2L directly acts on capsidiol and/or capsidiol 3-acetate. Comparison of the phytoalexins that were metabolized by CYP76A2L, points to the conserved isopropenyl group, which is the same in all these compounds, and differs only for debneyol, where the tail is hydroxylated (Figure 1). Though the tested phytoalexins are similar in size, the two hydroxyl groups in oxylubimin make it a substantially more polar compound than solavetivone, indicating that CYP76A2L does not seem to be very selective in its substrates. It is possible that other compounds containing a similar isopropenyl group may be substrates as well.
Figure 3.
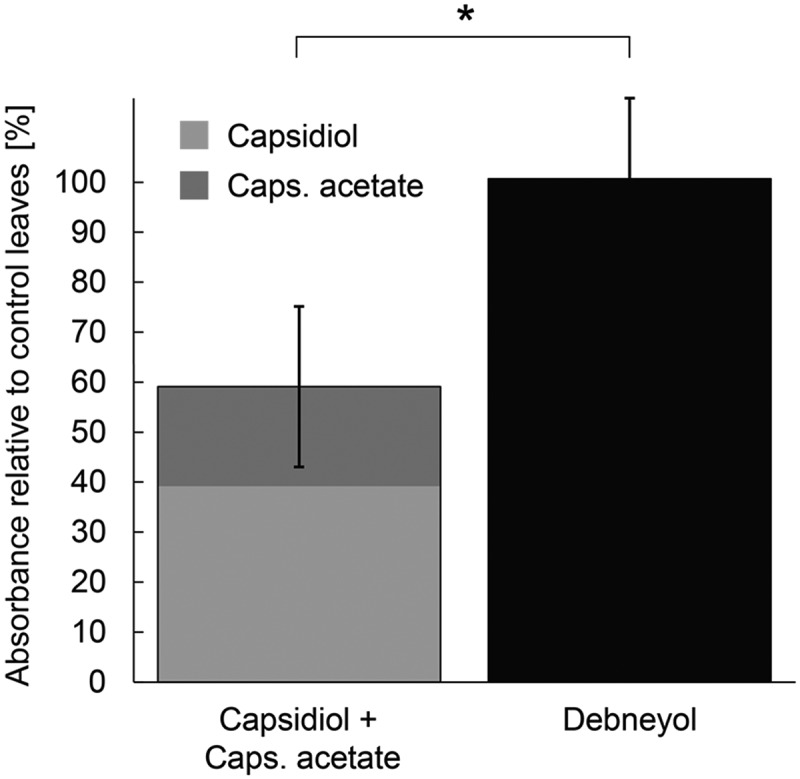
Reduction of capsidiol and capsidiol 3-acetate in N. benthamiana leaves expressing CYP76A2L. N. benthamiana leaves were inoculated with Agrobacterium strains for expression of GFP (control) or CYP76A2L, and the leaves were treated with INF1 elicitor 2 d after inoculation. Phytoalexins were extracted and measured using GC-MS. Scores indicate the amount of phytoalexins extracted from leaves expressing CYP76A2L relative to that from GFP expressing control leaves 1 d after elicitor treatment. Data are means ± SD (n = 6). Data marked with an asterisk are significantly different as assessed by two-tailed Student’s t-tests: *P < .05.
CYP76A2L is a monooxygenase that incorporates a hydroxyl group into solavetivone, lubimin, oxylubimin and rishitin
The reduction of the infiltrated phytoalexins caused by CYP76A2L coincided with the appearance of novel peaks, all of which demonstrated a delayed retention time (Figure 4(a–c)). GFP expressing leaves also differed from CYP76A2L expressing leaves by an additional peak, which corresponded to endogenous capsidiol 3-acetate. The capsidiol 3-acetate peak, which appears to have been produced in response to the infection of A. tumefaciens, had disappeared in the CYP76A2L expressing leaves (Figure 4(a-c)).
Figure 4.
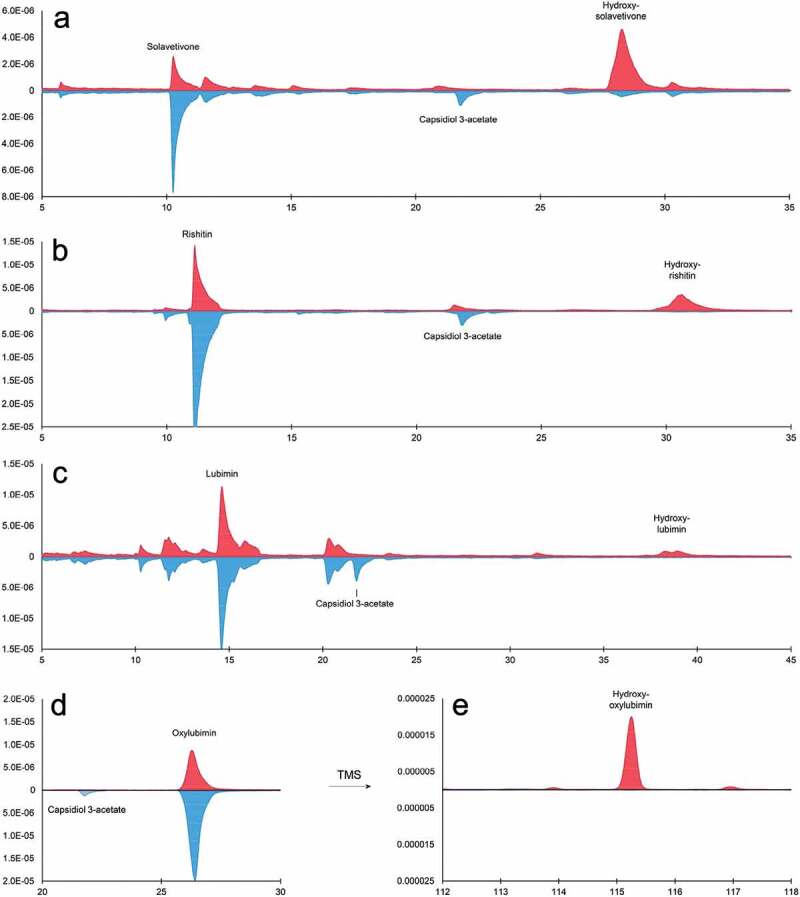
Gas chromatograms of N. benthamiana leaf extracts, expressing CYP76A2L (red) or GFP (blue) and infiltrated with different potato phytoalexins. (a-d) N. benthamiana leaves were inoculated with Agrobacterium strains for expression of GFP (blue, lower side) or CYP76A2L (red, upper side), and the leaves were infiltrated with 1 mg/ml solavetivone (a), rishitin (b), lubimin (c), or oxylubimin (d), 2 d after inoculation. Infiltrated phytoalexins were re-extracted and measured using GC-MS. (e) Chromatogram showing the same extract from D, which has been silylated with TMS. X-axis: time in min; Y-axis: ion abundance relative to total ion signal measured in each extract.
The mass spectra of the CYP76A2L induced peaks were recognized as being sesquiterpenes, due to their resemblance to the mass spectra of the corresponding infiltrated phytoalexins (data not shown). Neither the mass spectra nor retention times of the novel peaks matched any other phytoalexin in the pathway, indicating that the function of CYP76A2L is not the synthesis of lubimin, oxylubimin or rishitin. The delayed retention time, together with the similar mass spectra, hints that these new peaks consist of the infiltrated phytoalexins, which contain at least one additional polar group. We were unable to safely determine the molecular weight from these spectra, since the molecular ion signals were weak, and could not be distinguished from the background. In the case of lubimin, two distinct peaks were observed, which demonstrated similar retention times, and could not be distinguished by their mass spectra (Figure 4(c)). The two peaks could therefore be enantiomers. These enantiomers could either be products of CP76A2L or are the result of two lubimin enantiomers present in the infiltrated lubimin stock. In the case of oxylubimin, which has a relatively low volatility due to its highly oxygenated structure (Figure 4(d)), observation of the CYP76A2L caused peak required even higher vaporization temperatures, which resulted in thermal degradation of the novel peak. To account for the low volatility of the oxylubimin derivative, and to gain more insight into the structure of all novel compounds, we performed trimethylsilyl (TMS) derivatization of the plant extracts with N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) (Figure 4(e)). The MSTFA treatment led to the addition of TMS groups to hydroxyl groups, thus allowing to infer whether the phytoalexins were hydroxylated. The silylated plant extracts showed that CYP76A2L introduced a single hydroxyl group to solavetivone, lubimin, oxylubimin and rishitin, which could be determined from the molecular ion of the silylated peaks (Supplementary Figure 1).
CYP76A2L causes the detoxification of rishitin to rishitin-M1
The finding that rishitin, which is the final compound of the pathway, is hydroxylated by CYP76A2L, indicates that CYP76A2L may not be involved in the biosynthesis of phytoalexins. This is further emphasized by the finding that the hydroxylation of lubimin did not lead to the formation of oxylubimin but rather to a different compound. We therefore examined whether CYP76A2L is involved in the detoxification of phytoalexins instead. Since it is currently unknown how solavetivone, lubimin and oxylubimin are detoxified in plants, we focused on rishitin, where it is reported that it is detoxified into two compounds, rishitin-M1 and rishitin-M2, which accumulate in tubers at a later stage of infection.25 Since the molecular weights of rishitin-M2 and the hydroxylated rishitin do not match, it was concluded that the hydroxylated rishitin could not be rishitin-M2. The molecular weights of rishitin-M1 and the hydroxylated rishitin correspond however, indicating that CYP76A2L may be involved in the detoxification of rishitin to form rishitin-M1.
Therefore, potato tubers were treated with hyphal wall components (HWC) elicitor prepared from Phytophthora infestans, which induces defense responses, including the formation of phytoalexins.4 Phytoalexins were extracted from the tubers 4 d after treatment and compared to the extracts from the CYP76A2L-expressing N. benthamiana leaves which were infiltrated with rishitin. GC-MS measurement of the tuber extracts revealed a peak whose MS and retention time matched that of the hydroxylated rishitin produced by CYP76A2L (Figure 5(a)). The MS also matched the published spectrum for 13-hydroxyrishitin,26 also known as rishitin-M1. This strongly supports that CYP76A2L is the enzyme responsible for the detoxification of rishitin to rishitin-M1 in potato tubers (Figure 5(b)).
Figure 5.
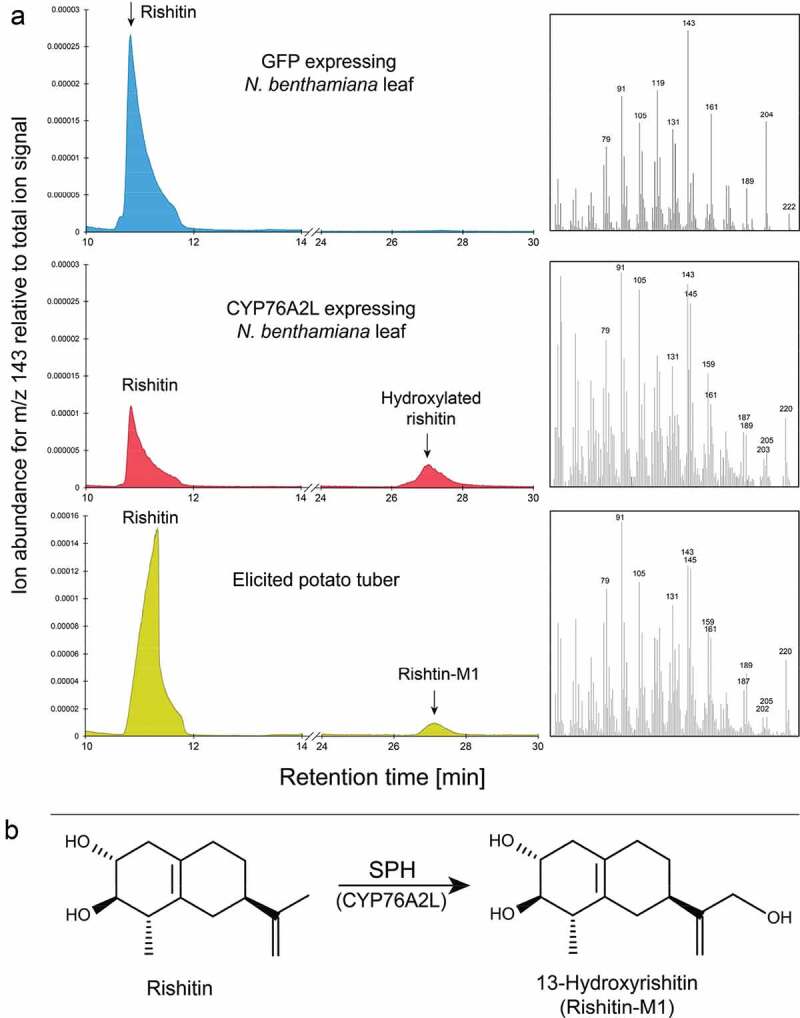
(a): GC-MS measurements of rishitin infiltrated N. benthamiana leaves expressing either GFP or CYP76A2L, and potato tuber treated with HWC elicitor. Left: Ion chromatograms for each sample, right: mass spectra for peaks indicated by black arrows in left chromatograms. (Top and middle) N. benthamiana leaves were inoculated with Agrobacterium strains for expression of GFP (top) or CYP76A2L (middle), and the leaves were infiltrated with 1 mg/ml rishitin 2 d after inoculation. Infiltrated rishitin and hydroxylated rishitin were extracted and measured using GC-MS. (Bottom) Potato tubers were treated with HWC for 4d, rishitin and rishitin-M1 were extracted and measured using GC-MS. (b): Observed reaction of CYP76A2L/SPH when rishitin was used as substrate.
In light of the findings presented, we propose renaming CYP76A2L in a manner that adequately reflects its function. Although the detoxification of rishitin could be used as a basis for naming the enzyme, its broad substrate tolerance, suggests that “sesquiterpenoid phytoalexins hydroxylase”, or short: SPH, describes the enzyme more appropriately. This name also avoids confounding annotations of homolog genes in Solanaceae which do not produce rishitin. CYP76A2L will be referred to as SPH from here on after.
SPH/CYP76A2L is a P450 subfamily specific to Solanaceae species
The results of this study show that the detoxification of rishitin is initiated by SPH via hydroxylation of rishitin to rishitin-M1. SPH was also shown to hydroxylate other potato phytoalexins, as well as affecting the capsidiol pathway, which is not present in potato. A BLAST search of SPH homologs revealed that this cytochrome P450 group is specifically found in Solanaceae species (Supplementary Figure 2). Since close homologs of SPH can also be found in tobacco and pepper (Capsicum annuum), which are known to produce both rishitin and capsidiol,14,15 as well eggplant, which is known to produce neither rishitin nor capsidiol but rather solavetivone and lubimin,31,32 this opens up the notion that SPH may be a general sesquiterpenoid phytoalexin detoxification enzyme. Phylogenetic analysis of SPH and related cytochrome P450 enzymes identified from draft genome sequences of Solanum, Capsicum and Nicotiana species revealed that SPH genes are expanded in potato, tomato and pepper (Supplementary Figure 2). These lineage-specific duplications of the SPH gene suggest the emergence of a specialized pathway during their evolution.33 On the other hand, the finding that N. benthamiana does not contain a close homolog of SPH indicates that capsidiol detoxification occurs in a different way in N. benthamiana.
Concluding remarks
At first glance, a detoxification pathway that targets multiple phytoalexins would indeed be efficient in potato, where several phytoalexins accumulate simultaneously. It would also allow for a certain evolutionary flexibility, since it would allow to quickly respond to pathogen pressure via the generation of novel phytoalexins, without the need for a novel detoxification pathway. The disadvantage of such a strategy is of course, that pathogens could detoxify multiple phytoalexins using the same strategy. However, at least in G. pulicaris, detoxification of rishitin and lubimin is executed by different enzymes in different ways. While rishitin is detoxified via rishitin-M1, lubimin detoxification proceeds via the cyclization of lubimin.26,34,35 Therefore, it may be that lubimin hydroxylated by SPH still exhibits a toxic effect on pathogens. Investigating, whether all SPH hydroxylated phytoalexins lose their efficacy on pathogens, is therefore an important next step for understanding phytoalexin detoxification in solanaceous plants.
Increasing the amount and rate at which phytoalexins are produced in potato tubers upon infection, could help to harden potato tubers against fungal diseases. But even with modern breeding techniques, this is difficult to realize, since the majority of genes responsible for the production of phytoalexins in Solanum are still unknown. A reduction of the SPH-induced rishitin detoxification, on the other hand, could be a relatively simple approach which could lead to a stronger and prolonged defense response. While the identification of SPH is an important first step, further research on SPH will be necessary to determine how SPH is regulated and what role this enzyme plays in other solanaceous plants.
Materials and methods
Biological materials and growth conditions
N. benthamiana (line SNPB-A5)5 was grown in an environmentally controlled growth room at 23°C under a 16 h light/8 h dark per day. Tubers of potato cultivar Rishiri, an interspecific hybrid between Solanum tuberosum and S. demissum carrying the R1 gene for resistance to P. infestans, were stored at 4°C until use. The tubers were washed with water and sterilized with 0.4% sodium hypochlorite. Tissue cylinders (20 mm in diameter) were prepared from parenchymatous tissues with a cork borer and cut into approx. 3-mm-thick discs. The discs were washed with a large volume of cold distilled water and then incubated in a sealed plastic chamber for 20 h at 20°C in the dark before use.
Preparation and treatment of elicitors
INF1 elicitor was prepared from Escherichia coli (DH5α) carrying an expression vector for INF1, pFB53, as previously reported.29,36 N. benthamiana leaves were treated with 150 nM INF1 solution as previously described.36 Hyphal wall components (HWC) elicitor was prepared from mycelia of P. infestans strain PI1234-137 that has been grown in liquid rye medium for approx. 2 weeks at 20°C as described previously38 and aged potato tuber discs for 20 h were treated with 1 mg/ml HWC and incubated in a moist chamber at 20°C in the dark.
Construction of vector and Agrobacterium-mediated transient expression
RNA was extracted from potato tuber treated with 1 mg/ml HWC elicitor for 1 d using the RNeasy Plant Mini Kit (Qiagen, Germany) and cDNA synthesis was conducted using ReverTra Ace-α- (Toyobo, Japan) according to the manufacturer’s instructions. cDNA of SPH (CYP76A2L) was amplified with primers contained a 15 bp 5ʹ overhang for In-Fusion (Takara Bio, Japan) cloning into the vector pNPP4030 digested with BamHI.
Forward primer: TACATCTAGAGGATCATGGAATATGAATGGAGCTATCTGT.
Reverse Primer: CGAGGTTAACGGATCTCAAGCCTTTTTTGGTATTACTTTC.
Transformation of A. tumefaciens strain GV3101 with resulting vector pNPP40-StSPH via electroporation, and transient expression of GFP (pNPP40-GFP)30 or SPH in N. benthamiana leaves was performed as previously described.36
Infiltration of potato phytoalexins into N. benthamiana leaves
Synthesized rishitin, lubimin, oxylubimin and solavetivone39-41 were provided by Prof. Akira Murai (Hokkaido University, Japan) and stored at −30°C. The purity of stored phytoalexins was confirmed by GC-MS analysis. Capsidiol and capsidiol 3-acetate standards were purified as previously reported.30 Camalexin was obtained from Sigma-Aldrich, USA (CA No. SML1016). Phytoalexins standards were dissolved in DMSO and then in water to a final DMSO concentration of 2%. The solution was then infiltrated into the leaf tissue from the abaxial side using 1 ml needleless syringes.
Extraction of phytoalexins from N. benthamiana leaves and potato tubers
The leaf tissue was cut using a scalpel and a maximum of 150 mg leaf tissue was used for extraction. The tissue was frozen in liquid nitrogen, pulverized and extracted for 2 h at room temperature in 1 ml ethyl acetate. Due to the lower polarity of solavetivone, samples infiltrated with solavetivone were extracted in 1:1 (v/v) cyclohexane:ethyl acetate instead. The raw extract was measured using GC-MS. Phytoalexins produced in potato tubers treated with HWC elicitor were extracted by shaking tuber discs in 5 ml (per disc) ethyl acetate overnight. The extract was then dried in a vacuum evaporator and resuspended in 1 ml ethyl acetate.
GC/MS measurements
Gas chromatography was performed on an Agilent Technologies 7890A GC System using a DuraBond Ultra Inert column (length 30 m; diameter 0.25 mm; film 0.25 µm, Agilent Technologies, USA). Injection volume 1 µl, splitless. Default measurement method for leaf extracts: Injection at 270°C; Oven temperature: 3 min 170°C, then increasing 0.2°C/min to 195°C. Camalexin and TMS treated samples were measured at an injection temperature of 250°C; Oven temperature 3 min 100°C, then increasing 1°C/min to 270°C. Potato tuber extract and compared leaf extracts: Injection at 270°C; Oven temperature: 3 min 170°C, then increasing 0.5°C/min to 250°C. Mass spectrometry: EI using Agilent 5975C; scan m/z 33–500. TMS derivatization of plant extracts was performed as follows: 50 µl raw plant extract was dried in a vacuum evaporator and resuspended in 50 µl N-Methyl-N-trimethylsilyl trifluoroacetamide (MSTFA, Fujifilm Wako Pure Chemical, Japan) and incubated at room temperature for 30 min.
DNA sequencing and bioinformatics
The constructed vector was sequenced by the dideoxynucleotide chain termination method using Big-Dye (Version 3) chemistry (Applied BioSystems). Products were separated on an ABI3130 analyzer (Applied BioSystems, USA). Sequence data was analyzed in MacVectror (ver. 15.1, MacVector Inc., USA). The gene sequence for S. tuberosum SPH (CYP76A2L) has been deposited in the DDBJ/EMBL/GenBank databases (Accession Number LC506385). Predicted amino acid sequences of plant SPH and related cytochrome P450 were collected from Solanaceae Genomics Network (https://solgenomics.net)42 for S. tuberosum (ITAG release 1, ST1.0), S. lycopersicum (ITAG release 4), S. melongena (release 2.5.1), C. annuum (cv. CM334 release 1.55), N. attenuata (v2 annot v5), N. benthamiana (v1.0.1), N. sylvestris,43 N. tabacum (Nitab v4.5 Edwards2017) and N. tomentosiformis,43 or from SoyBase (https://www.soybase.org) for G. max (Wm82.a2.v1), or from PlantGDB (http://www.plantgdb.org/AtGDB/) for A. thaliana (TAIR9/10 v171). Protein sequences were aligned using ClustalW44 with default settings and phylogenetic analysis was conducted using the neighbor-joining method45 using MacVectror ver. 15.1 (MacVector Inc.).
Funding Statement
This work was supported by the Japan Society for the Promotion of Science under Grant-in-Aid for Scientific Research (B) (26292024 and 17H03771).
Acknowledgments
We thank Dr. Akio Murai (former Professor of Hokkaido University, Japan) for providing synthetic sesquiterpenoid phytoalexins, Dr. David A. Jones (Australian National University, Australia) for providing N. benthamiana seeds, Prof. Sophien Kamoun (The Sainsbury Laboratory, UK) for the pFB53 vector. We also thank Dr. Kenji Asano and Mr. Seiji Tamiya (National Agricultural Research Center for Hokkaido Region, Japan) and Mr. Yasuki Tahara and Ms. Mayu Hioki (Nagoya University, Japan) for providing tubers of potato, and Prof. Kazuhito Kawakita, Drs. Sotaro Chiba and Ikuo Sato (Nagoya University, Japan) for valuable discussions. This work was supported by a Grant-in-Aid for Scientific Research (B) (Nos. 26292024 and 17H03771).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Müller KO, Borger H.. Experimentelle Untersuchungen über die Phytophthora-resistenz der Kartoffel. Arb Biol Reichsanst Land Forstwirtsch. 1940;23:1–9. [Google Scholar]
- 2.Hammerschmidt R. Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol. 1999;37::285–306. doi: 10.1146/annurev.phyto.37.1.285. [DOI] [PubMed] [Google Scholar]
- 3.Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Sakai S, Doke N, Tomiyama K. Relation between necrosis and rishitin accumulation in potato tuber slices treated with hyphal wall components of Phytophthora infestans. Ann Phytopath Soc Japan. 1982;48:238–240. doi: 10.3186/jjphytopath.48.238. [DOI] [Google Scholar]
- 5.Shibata Y, Ojika M, Sugiyama A, Yazaki K, Jones DA, Kawakita K, Takemoto D. The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre- and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell. 2016;28:1163–1181. doi: 10.1105/tpc.15.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takemoto D, Shibata Y, Ojika M, Mizuno Y, Imano S, Ohtsu M, Sato I, Chiba S, Kawakita K, Rin S, et al. Resistance to Phytophthora infestans: exploring genes required for disease resistance in Solanaceae plants. J Gen Plant Pathol. 2018;84:312–320. doi: 10.1007/s10327-018-0801-8. [DOI] [Google Scholar]
- 7.Yoshioka M, Adachi A, Sato Y, Doke N, Kondo T, Yoshioka H. RNAi of the sesquiterpene cyclase gene for phytoalexin production impairs pre- and post-invasive resistance to potato blight pathogens. Mol Plant Pathol. 2019;20:907–922. doi: 10.1111/mpp.2019.20.issue-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraishi T, Oku H, Isono M, Ouchi S. The injurious effect of pisatin on the plasma membrane of pea. Plant Cell Physiol. 1975;16:939–942. doi: 10.1093/oxfordjournals.pcp.a075218. [DOI] [Google Scholar]
- 9.Lyon GD. Evidence that the toxic effect of rishitin may be due to membrane damage. J Exp Bot. 1980;37:957–966. doi: 10.1093/jxb/31.4.957. [DOI] [Google Scholar]
- 10.Bailey JA, Burden RS, Vincent GG. Capsidiol: an antifungal compound produced in Nicotiana tabacum and Nicotiana clevelandii following infection with tobacco necrosis virus. Phytochemistry. 1975;14:597. doi: 10.1016/0031-9422(75)85148-X. [DOI] [Google Scholar]
- 11.Baker FC, Brooks CJW. Biosynthesis of the sesquiterpenoid, capsidiol, in sweet pepper fruits inoculated with fungal spores. Phytochemistry. 1976;15:689–694. doi: 10.1016/S0031-9422(00)94422-4. [DOI] [Google Scholar]
- 12.Jadhav SJ, Mazza G, Salunkhe DK. Terpenoid phytoalexins in potatoes: a review. Food Chem. 1991;41:195–217. doi: 10.1016/0308-8146(91)90043-N. [DOI] [Google Scholar]
- 13.Desjardins AE, McCormick SP, Corsini DL. Diversity of sesquiterpenes in 46 potato cultivars and breeding selections. J Agric Food Chem. 1995;43:2267–2272. doi: 10.1021/jf00056a056. [DOI] [Google Scholar]
- 14.Fuchs A, Slobbe W, Mol MC, Posthumus MA. GC/MS analysis of fungitoxic terpenoids from tobacco. Phytochemistry. 1983;22:1197–1199. doi: 10.1016/0031-9422(83)80221-0. [DOI] [Google Scholar]
- 15.Hoshino T, Chida M, Yamaura T, Yoshizawa Y, Mizutani J. Phytoalexin induction in green pepper cell cultures treated with arachidonic acid. Phytochemistry. 1994;36:1417–1419. doi: 10.1016/S0031-9422(00)89733-2. [DOI] [Google Scholar]
- 16.Katsui N, Murai A, Takasugi M, Imaizumi K, Masamune T, Tomiyama K. The structure of rishitin, a new antifungal compound from diseased potato tubers. Chem Commun. 1968;1:43–44. doi: 10.1039/c19680000043. [DOI] [Google Scholar]
- 17.McCance DJ, Drysdale RB. Production of tomatine and rishitin in tomato plants inoculated with Fusarium oxysporum f. sp. lycopersici. Physiol Plant Pathol. 1975;7:221–230. doi: 10.1016/0048-4059(75)90027-2. [DOI] [Google Scholar]
- 18.Glazener JA, Wouters CH. Detection of rishitin in tomato fruits after infection with Botrytis cinerea. Physiol Plant Pathol. 1981;19:243–248. doi: 10.1016/S0048-4059(81)80027-6. [DOI] [Google Scholar]
- 19.Facchini PJ, Chappell J. Gene family for an elicitor-induced sesquiterpene cyclase in tobacco. Proc Natl Acad Sci USA. 1992;89:11088–11092. doi: 10.1073/pnas.89.22.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralston L, Kwon ST, Schoenbeck M, Ralston J, Schenk DJ, Coates RM, Chappell J. Cloning, heterologous expression, and functional characterization of 5-epi-aristolochene- 1,3-dihydroxylase from tobacco (Nicotiana tabacum). Arch Biochem Biophys. 2001;393:222–235. doi: 10.1006/abbi.2001.2483. [DOI] [PubMed] [Google Scholar]
- 21.Back K, Chappell J. Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J Biol Chem. 1995;270:7375–73781. doi: 10.1074/jbc.270.13.7375. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S, Yeo YS, Zhao Y, O’Maille PE, Greenhagen BT, Noel JP, Coates RM, Chappell J. Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio- and stereo-specific hydroxylations of diverse sesquiterpene substrates. J Biol Chem. 2007;282:31744–31754. doi: 10.1074/jbc.M703378200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K, Ishiguri Y, Doke N, Tomiyama K, Yagishashi F, Murai A, Katsui N, Masamune T. Biosynthesis of the sesquiterpenoid phytoalexin rishitin from acetate via oxylubimin in potato. Phytochemistry. 1978;17:1901–1902. doi: 10.1016/S0031-9422(00)88729-4. [DOI] [Google Scholar]
- 24.Whitehead IM, Atkinson AL, Threlfall DR. Studies on the biosynthesis and metabolism of the phytoalexin lubimin and related compounds in Datura stramonium L. Planta. 1990;182:81–88. doi: 10.1007/BF00239988. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguri Y, Tomiyama K, Murai A, Katsui N, Masamune T. Toxicity of rishitin, rishitin-M-1 and rishitin-M-2 to Phytophthora infestans and potato tissue. Ann Phytopath Soc Japan. 1978;44:52–56. doi: 10.3186/jjphytopath.44.52. [DOI] [Google Scholar]
- 26.Gardner HW, Desjardins AE, McCormick SP, Weisleder D. Detoxification of the potato phytoalexin rishitin by Gibberella pulicaris. Phytochemistry. 1994;37:1001–1005. doi: 10.1016/S0031-9422(00)89517-5. [DOI] [Google Scholar]
- 27.Murai A, Yoshizawa Y, Ikura M, Katsui N, Masamune T. Metabolism of rishitin. The NIH-like shift in formation of rishitin M-2. J Chem Soc Chem Commun. 1986;2:891–892. doi: 10.1039/C39860000891. [DOI] [Google Scholar]
- 28.Brindle PA, Coolbear T, Kuhn PJ, Threlfall DR. An 18O2 study of the biosynthesis and metabolism of rishitin. Phytochemistry. 1985;24:1219–1222. doi: 10.1016/S0031-9422(00)81105-X. [DOI] [Google Scholar]
- 29.Kamoun S, van West P, de Jong AJ, de Groot KE, Vleeshouwers VGAA, Govers F. A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Mol Plant-Microbe Interact. 1997;10:13–20. doi: 10.1094/MPMI.1997.10.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Matsukawa M, Shibata Y, Ohtsu M, Mizutani A, Mori H, Wang P, Ojika M, Kawakita K, Takemoto D. Nicotiana benthamiana calreticulin 3a is required for the ethylene-mediated production of phytoalexins and disease resistance against oomycete pathogen Phytophthora infestans. Mol Plant-Microbe Interact. 2013;26:880–892. doi: 10.1094/MPMI-12-12-0301-R. [DOI] [PubMed] [Google Scholar]
- 31.Ward EWB, Unwin CH, Hill J, Stoessl A. Sesquiterpenoid phytoalexins from fruits of eggplants. Phytopathology. 1975;65:859–863. doi: 10.1094/Phyto-65-859. [DOI] [Google Scholar]
- 32.Yoshihara T, Hagihara Y, Nagaoka T, Chiba S, Sakamura S. Fungitoxic compounds from the roots of the eggplant stock. Ann Phytopath Soc Japan. 1988;54:453–459. doi: 10.3186/jjphytopath.54.453. [DOI] [Google Scholar]
- 33.Panchy N, Lehti-Shiu M, Shiu SH. Evolution of gene duplication in plants. Plant Physiol. 2016;171:2294–2316. doi: 10.1104/pp.16.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner HW, Desjardins AE, Weisleder D, Plattner RD. Biotransformation of the potato phytoalexin, lubimin, by Gibberella pulicaris. Identification of major products. Biochim Biophys Acta. 1988;966:347–356. doi: 10.1016/0304-4165(88)90084-0. [DOI] [Google Scholar]
- 35.Desjardins AE, Gardner HW, Weltring KM. Detoxification of sesquiterpene phytoalexins by Gibberella pulicaris (Fusarium sambucinum) and its importance for virulence on potato tubers. J Ind Microbiol. 1992;9:201–211. doi: 10.1007/BF01569624. [DOI] [Google Scholar]
- 36.Shibata Y, Kawakita K, Takemoto D. Age-related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene- and salicylic acid-mediated signaling pathways. Mol Plant-Microbe Interact. 2010;23:1130–1142. doi: 10.1094/MPMI-23-9-1130. [DOI] [PubMed] [Google Scholar]
- 37.Shibata Y, Kawakita K, Takemoto D. SGT1 and HSP90 are essential for age-related non-host resistance of Nicotiana benthamiana against the oomycete pathogen Phytophthora infestans. Physiol Mol Plant Pathol. 2011;75:120–128. doi: 10.1016/j.pmpp.2010.10.001. [DOI] [Google Scholar]
- 38.Doke N, Tomiyama K. Effect of hyphal wall components from Phytophthora infestans on protoplasts of potato tuber tissues. Physiol Plant Pathol. 1980;16:169–176. doi: 10.1016/0048-4059(80)90031-4. [DOI] [Google Scholar]
- 39.Murai A, Nishizakura K, Katsui N, Masamune T. The synthesis of rishitin. Tetrahedron Lett. 1975;16:4399–4402. doi: 10.1016/S0040-4039(00)91135-3. [DOI] [Google Scholar]
- 40.Murai A, Sato S, Masamune T. Efficient synthesis of (±)-solavetivone. J Chem Soc Chem Commun. 1981;17:904–905. doi: 10.1039/C39810000904. [DOI] [Google Scholar]
- 41.Murai A, Sato S, Masamune T. Total synthesis of (±)-lubimin and (±)-oxylubimin. J Chem Soc Chem Commun. 1982;9:513–514. doi: 10.1039/C39820000513. [DOI] [Google Scholar]
- 42.Fernandez-Pozo N, Menda N, Edwards JD, Saha S, Tecle IY, Strickler SR, Bombarely A, Fisher-York T, Pujar A, Foerster H, et al. The Sol Genomics Network (SGN) – from genotype to phenotype to breeding. Nucleic Acids Res. 2015;43:D1036–1041. doi: 10.1093/nar/gku1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierro N, Battey JN, Ouadi S, Bovet L, Goepfert S, Bakaher N, Peitsch MC, Ivanov NV. Reference genomes and transcriptomes of Nicotiana sylvestris and Nicotiana tomentosiformis. Genome Biol. 2013;14:R60. doi: 10.1186/gb-2013-14-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JD, Higgins DG, Gibson TJ, CLUSTAL W. improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


