ABSTRACT
The plant hormone ethylene is a key regulator of growth, development and stress adaptation at all stages of the plant life cycle. Signal perception and response to the plant hormone are mediated by a family of receptor kinases localized at the ER-Golgi network which gain their high affinity and specificity for the chemically simple ethylene molecule by an essential copper cofactor bound at their transmembrane domain. Transfer of this cofactor from the plant plasma membrane to the ER-localized receptors requires secured cellular transport of the reactive transition metal. In a recent study, we disclosed the transport proteins involved in the copper transfer to the receptors and identified that cytoplasmic chaperones of the ATX1-family and a membrane-bound P-type ATPase are involved in copper routing. Strictly speaking, our data show that receptors can acquire their copper load by different routes and adopt the metal ion from the plasma membrane either by sequential transfer from soluble chaperones of the ATX1-family via the ER-bound copper-transporting ATPase RAN1 or by direct transfer from the soluble chaperones. Here, we have studied the properties of the soluble plant copper chaperone isoforms, ATX1 and CCH, in more detail. Our data support different cellular functions of these isoforms on copper mobilization.
KEYWORDS: Plant copper homeostasis, ethylene receptor, copper chaperone, ATX1, CCH, RAN1
Main
Ethylene signaling is involved in widespread cellular functions of climacteric plants and regulates fundamental plant processes such as fruit ripening, stress and pathogen response, growth and development.1,2 Five ethylene receptors (ETR1, ETR2, ERS1, ERS2 and EIN4) have been identified in A. thaliana. All receptors share a similar architecture harboring the ethylene-binding site at the transmembrane N-terminal region.3 Involvement of a transition metal in ethylene binding has been hypothesized soon after the discovery of ethylene as a plant hormone.4 Nevertheless, only discovery of a mutant allele for copper-transporting ATPase RAN1, showing altered ethylene response, gave the first experimental evidence for copper being the transition metal involved in ethylene signaling.5 However, copper ions are redox-active species and may trigger the formation of reactive oxygen species (ROS). Therefore, cellular transport of free metal is not an option. Instead, specialized proteins, so-called copper chaperones, mediate the transport of the detrimental metal.6 Recent studies in our lab indicate that soluble copper chaperones of the ATX1-family, ATX1 and CCH, participate in copper routing to the ER-localized proteins of the ethylene receptor family.7 Furthermore, our data suggest two distinct routes for copper transfer from the plasma membrane onto the receptors. Binding studies on purified recombinant proteins support the idea that the sequential transfer of the metal ion from soluble cytoplasmic chaperones to the ER-localized transporter RAN1 is the predominant route. In this route, the metal transporter then transfers the copper directly to the transmembrane sensor domain of the receptors presumably via its two N-terminal metal-binding domains (MBDs). Additional copper chaperone interaction studies disclosed an alternative route of a direct transfer from the cytoplasmic chaperones onto the receptors. The 10-fold lower binding constants determined for this direct routing indicate that this route may be less favored. Further domain analysis revealed that RAN1 interacts with both, the transmembrane region and the extra-membranous part of the receptors, while the cytoplasmic chaperones exclusively interact with the transmembrane component. Noteworthy, the interaction of RAN1 with the transmembrane copper target site at the receptors occurs via the N-terminal MBDs of RAN1 which show high similarity to proteins of the ATX1-family.8 Together these proteins share a similar structure consisting of a ferredoxin-like fold and MxCxxC copper-binding motifs. This structural similarity also includes CCS, a copper chaperone targeting superoxide dismutase, which is ubiquitously expressed in all types of cells.9,10 Nonetheless, interaction of CCS with ethylene receptors has not been considered yet. Based on the results of our binding studies of the receptors with different copper chaperones and the structural similarity of the MBD in different types of copper chaperones we hypothesize that copper supply to the ethylene receptor family is not achieved by a distinct single protein, but rather by a range of copper-transport proteins having a certain structural domain (MBD) in common (illustration in Figure 1). Although such an idea is in agreement with the lack of a specific copper chaperone targeting ethylene receptors only, further in vivo analysis is required to validate such multi-protein copper transfer to the receptors.
Figure 1.
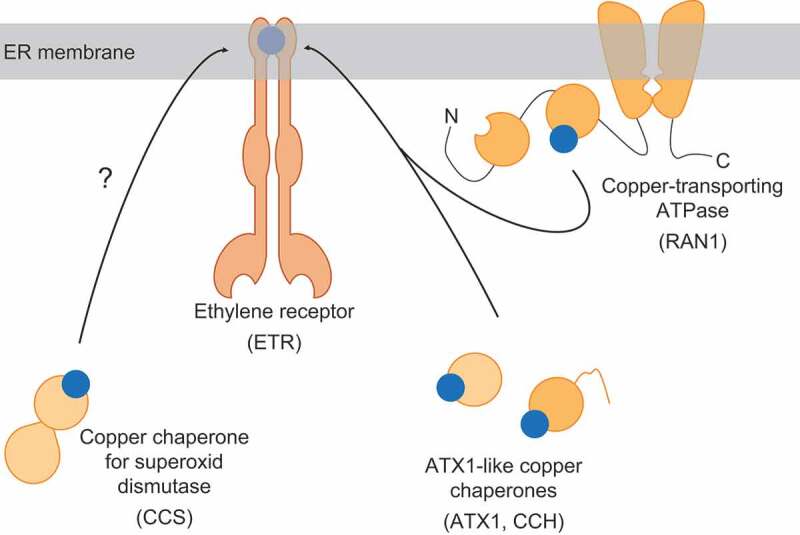
Illustration of copper chaperones involved in copper transport to ER-bound ethylene receptors. Copper-transporting ATPase RAN1 and soluble cytoplasmic copper chaperones ATX1 and CCH deliver copper to the ER-localized receptors. Due to their structural and functional homology, further copper chaperones with ferredoxin-like folds, such as CCS may provide an additional transfer route. Protein-bound copper is shown as blue circles, whereas the different apo-proteins are drawn in orange.
In the biological context, the stepwise transport from cytoplasmic copper chaperones to the receptors via RAN1 makes logical sense. First, due to the involvement of the ER-localized RAN1 metal transporter, cytoplasmic copper chaperones are “recruited” to the endomembrane system bringing copper in close spatial proximity to the receptors without the need for the development of a specific copper chaperone targeting these receptors. Secondly, employing a copper transfer mediator rather than a specific chaperone offers additional paths for regulation in ethylene signaling as receptors lacking the cofactor are known to be inactive even in the absence of the plant hormone.11 In this context, RAN1 is a suitable mediator linking intracellular copper availability and ethylene signaling as indicated by ethylene independent growth defects.12 Shortcutting the sequential transport to the receptors by the direct transfer of the metal ion from the chaperones of the ATX1-family may assure a backup strategy at certain environmental, developmental or physiological conditions, e.g. strong copper deficiency.
Although our data clearly support copper transfer from cytoplasmic chaperones to the receptor the opposite path namely copper uptake from the receptors by the cytoplasmic chaperones upon receptor turnover is also entirely feasible. Such a scenario is further supported by considering the contrary roles of ATX1 and CCH in copper mobilization proposed for higher plants.13-15 While ATX1 is assumed to predominantly mediate intracellular copper transport, CCH is thought to be involved in intercellular copper mobilization, e.g. from senescing to young organs.13,14 To further support such divergent roles of the two plant cytoplasmic chaperones, we addressed the oligomerization state of ATX1 and CCH in the absence and presence of their copper load by microfluidic analysis of their hydrodynamic radius (Figure 2). These experiments clearly show that ATX1 remains monomeric regardless of the copper content. In contrast, dimerization of CCH is observed upon addition of the metal ion. A truncation mutant of CCH, named CCHΔ, lacking the C-terminal extension that is characteristic for this plant-specific chaperone isoform, was used in further studies to pinpoint the dimerization domain in CCH. Noteworthy, CCHΔ shows hydrodynamic characteristics similar to ATX1, but not to CCH. Hence, we conclude that the C-terminal extension of CCH is required for dimerization, which might be a precondition for long-distance copper transport as it provides additional shielding of the reactive metal load.
Figure 2.
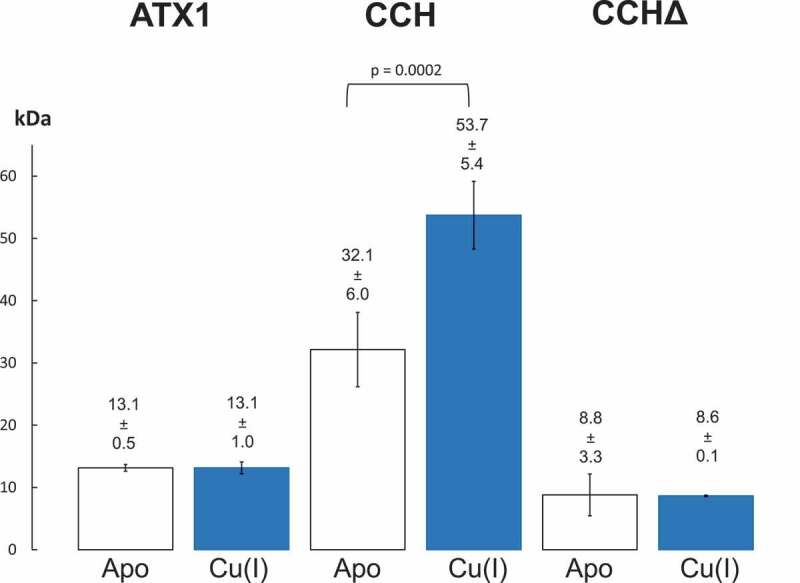
Molecular mass determination of soluble copper chaperones. Protein size data were collected using a Fluidity One instrument (Fluidic Analytics Ltd, Cambridge, UK). Mass in kDa was calculated based on the hydrodynamic radius16 in the absence or presence of copper (I). Alexa488-labeled proteins ATX1, CCG or CCHΔ (1μM) were incubated with 10 µM DTT (Apo) or Cu(I)-BCA2 (Cu(I)), respectively. Data are represented as mean molecular mass in kDa ± s.d. of at least triplicate measurements. Mass increase of CCH in the presence of copper was analyzed statistically using Student’s t-test.
Copper routing to the receptors is still an exciting topic and not yet fully understood. Remarkably, no copper chaperones targeting exclusively the ethylene receptor family have been identified yet. However, recent studies in our lab have identified two distinct routes by which copper can be transferred to or from the receptors. As the proteins involved in these routes share a common structural element (MBD), involvement of additional proteins with this motif for receptor copper homeostasis has to be considered and should be addressed in further studies.
Funding Statement
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 267205415 – SFB 1208 (project B06 to GG).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:1–3. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- 2.Lin Z, Zhong S, Grierson D.. Recent advances in ethylene research. J Exp Bot. 2009;60:3311–3336. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- 3.Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- 4.Burg SP, Burg EA. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967;42:144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR. RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–393. doi: 10.1016/S0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 6.Mira H, Sancenón V, Peñarrubia L. Copper homeostasis in plants. In: Massaro EJ, editor. Handbook of copper pharmacology and toxicology. Totowa, NJ: Humana Press; 2002. [Google Scholar]
- 7.Hoppen C, Muller L, Hänsch S, Uzun B, Milic D, Meyer AJ, Weidtkamp-Peters S, Groth G. Soluble and membrane-bound protein carrier mediate direct copper transport to the ethylene receptor family. Sci Rep. 2019;9:10715. doi: 10.1038/s41598-019-47185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann M, Clarke O, Gulbis JM, Keizer DW, Jarvis RS, Cobbett CS, Hinds MG, Xiao Z, Wedd AG. Metal binding affinities of Arabidopsis zinc and copper transporters: selectivities match the relative, but not the absolute, affinities of their amino-terminal domains. Biochemistry. 2009;48:11640–11654. doi: 10.1021/bi901573b. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Ghany SE, Muller-Moule P, Niyogi KK, Pilon M, Shikanai T. Two P-type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell. 2005;17:1233–1251. doi: 10.1105/tpc.104.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohu CM, Abdel-Ghany SE, Gogolin Reynolds KA, Onofrio AM, Bodecker JR, Kimbrel JA, Niyogi KK, Pilon M. Copper delivery by the copper chaperone for chloroplast and cytosolic copper/zinc-superoxide dismutases: regulation and unexpected phenotypes in an Arabidopsis mutant. Mol Plant. 2009;2:1336–1350. doi: 10.1093/mp/ssp084. [DOI] [PubMed] [Google Scholar]
- 11.Zhao XC, Qu X, Mathews DE, Schaller GE. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol. 2002;130:1983–1991. doi: 10.1104/pp.011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder BM, Rodriguez FI, Bleecker AB. The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J Biol Chem. 2010;285:37263–37270. doi: 10.1074/jbc.M110.170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mira H, Martinez-Garcia F, Penarrubia L. Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 2001;25:521–528. doi: 10.1046/j.1365-313x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 14.Puig S, Mira H, Dorcey E, Sancenon V, Andres-Colas N, Garcia-Molina A, Burkhead JL, Gogolin KA, Abdel-Ghany SE, Thiele DJ, et al. Higher plants possess two different types of ATX1-like copper chaperones. Biochem Biophys Res Commun. 2007;354:385–390. doi: 10.1016/j.bbrc.2006.12.215. [DOI] [PubMed] [Google Scholar]
- 15.Shin LJ, Lo JC, Yeh KC. Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol. 2012;159:1099–1110. doi: 10.1104/pp.112.195974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyn MT, Gusek TW. Prediction of diffusion coefficients of proteins. Biotechnol Bioeng. 1990;35:327–338. doi: 10.1002/(ISSN)1097-0290. [DOI] [PubMed] [Google Scholar]


