ABSTRACT
Local modulation of the actin cytoskeleton is essential for the initiation and maintenance of strong homotypic adhesive interfaces between neighboring cells. The epithelial adherens junction (AJ) fulfils a central role in this process by mediating E-cadherin interactions and functioning as a signaling scaffold to control the activity of the small GTPase RhoA and subsequent actomyosin contractility. Interestingly, a number of regulatory proteins that modulate RhoA activity at the AJ also control RhoA during cytokinesis, an actomyosin-dependent process that divides the cytoplasm to generate two daughter cells at the final stages of mitosis. Recent insights have revealed that the central player in AJ stability, p120-catenin (p120), interacts with and modulates essential regulators of actomyosin contraction during cytokinesis. In cancer, loss of this modulation is a common event during tumor progression that can induce chromosomal instability and tumor progression.
In this review, we will highlight the functional differences and similarities of the different RhoA-associated factors that have been linked to both the regulation of cell-cell adhesion and cytokinesis.
KEYWORDS: cell adhesion, cytokinesis, Rho activity, p120-catenin, actomyosin contractility
Introduction
Epithelial tissues are polarized as single or multilayer sheets of cells that line the cavities and cover the surfaces of most organs within the body. Epithelial cells protect organ systems against fluctuations in the external milieu by providing a physical barrier while simultaneously allowing for (non)-selective uptake and/or secretion of a broad spectrum of compounds including nutrients and signaling factors. Adhesion complexes control the physical connection between neighboring cells (cell-cell adhesion) and between cells and the extracellular matrix (ECM) (cell-ECM adhesion) rendering them essential for epithelial homeostasis. Cell-cell adhesion in epithelial cells is maintained through distinct multi-protein complexes including desmosomes, tight junctions (TJs), gap junctions and adherens junctions (AJs). AJs are highly dynamic complexes located at the basolateral membrane that are composed of E-cadherin and cadherin-associated catenins including α-catenin, β-catenin and p120-catenin (p120). AJs provide the linkage between homotypic cadherin interactions and the actin and microtubule cytoskeleton. E-cadherin stability at the membrane is controlled through binding of p120 to the juxta-membrane domain (JMD) of E-cadherin, which prevents endocytosis of the AJ.1 The AJ also functions as a cortical signaling platform for different members of the Rho GTPase family, of which RhoA, Rac1, and Cdc42 represent the best studies members.2
Interestingly, the regulatory factors that control RhoA function and actomyosin contractility at sites of cell-cell adhesion also regulate RhoA activity at the equatorial cortex during cell division. Thereby they govern formation of the contractile cytokinetic ring that drives progression of the cleavage furrow, a process that is essential for correct cell division. In this review, we will focus on the shared molecular mechanisms that control RhoA activity and actomyosin contractility during cell-cell adhesion and cell division. First, we will briefly discuss the roles of the different Rho GTPase family members during adhesion and cell division. Next, we will focus on the different factors that regulate RhoA, and highlight the similarities and differences between their function in cell-cell adhesion and cell division.
Rho GTPase activity is spatiotemporally regulated during adhesion and cytokinesis
Rho GTPases are guanine nucleotide binding proteins and members of the Ras superfamily of GTPases.3 Functioning of Rho GTPases is dependent on the intrinsic GTPase cycle: GTP loading induces activation through a conformational change that allows binding of downstream effectors, whereas the intrinsic GTPase activity leads to hydrolysis of GTP to GDP and inactivation of the GTPase.4 Three classes of proteins can regulate the GTPase cycle5: (i) guanine exchange factors (GEFs) that act as activators of GTPases by catalyzing the loading of GTP; (ii) GTPase-activating protein (GAPs) that catalyze the intrinsic GTPase activity and thereby the conversion to the inactive GDP-bound state, and (iii) guanine dissociation inhibitors (GDIs) that function as inhibitors of GTPase activity through binding of the inactive GDP bound form and dissociation from membranes.6
Proper cell-cell adhesion is dependent on local modulation of and the interplay between the different members of the Rho GTPase family. For instance, expression of either dominant negative or constrictively active forms of RhoA or Rac1 can disrupt cadherin-dependent adhesion.7,8 During initial cell-cell contact, the coordinated activation of Rac1 and RhoA downstream of E-cadherin regulates the expansion of the adhesive interface and provides lateral actomyosin contractility parallel to the plasma membrane, which is essential for strong adhesion.9
While the interplay between the different Rho GTPases is essential for the initiation, maintenance and functioning of proper cell-cell adhesion, the activity of these GTPases becomes more compartmentalized during mitosis and cytokinesis (Figure 1).10 Although the levels of GTP-bound Rac1 do not change during mitosis, GTP-Cdc42 levels peak during metaphase.11 Here, local Cdc42 activity is thought to mediate bi-oriented attachment of spindle microtubules to kinetochores.12 While Cdc42 represents the primary Rho GTPase during the early stages of mitosis, RhoA activity is crucial during anaphase and telophase where localized GTPase cycling at the equatorial cortex provides the signaling leading to the formation of the cytokinetic contractile ring and ingression of the cleavage furrow.13 Local accumulation and activation of RhoA at the equatorial cortex drive actin-myosin contractility through its downstream effectors ROCK (Rho-associated kinase) and Citron kinase.14 Although overall GTP-Rac1 levels do not fluctuate during mitosis and cytokinesis, its local inhibition seems to be crucial during these processes. Inactivation of Rac1 has been observed at the equatorial cortex during cytokinesis whereas GTP-Rac1 levels are increased at the polar regions after cytokinesis completion.15 Overexpression of dominant active Rac1 impairs cytokinesis and leads to binucleation.16 In contrast, elevated levels of GTP-Rac1 at the spindle poles are thought to mediate cell spreading and adhesion through the induction of actin branching during late telophase.10 In summary, while activity of different members of the Rho GTPase family are crucial to ensure strong cell-cell adhesion, the localization of RhoA, Rac1 and Cdc42 becomes heavily compartmentalized during mitosis and cytokinesis.
Figure 1.
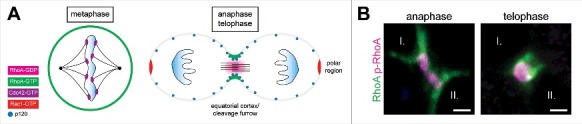
Activity of Rho GTPase family members is compartementalized during cell division. (A) During mitosis and cytokinesis, the activity and localization of RhoA, Rac1 and Cdc42 is compartimentalized. Cdc42 activity peaks during metaphase where it is involved in the attachement of microtubules to the kinetochores. While the overall levels of active Rac1 do not change during cell division, its inactivation at the equatorial cortex is necessary for cytokinesis completions. Here, RhoA activity peaks during anaphase and telophase and drives the ingression of the cleavage furrow. Rac1 activity at the polar regions is thought to contribute to cell spreading and adhesion during and/or after cytokinesis. Both positive (including the RhoGEF Ect2) and negative regulators (including p120 and its interaction partner the RhoGAP MP-GAP; see text for more details) of RhoA localize to the equatorial cortex during cleavage furrow ingression to allow for rapid GTPase cycling to restrict and focus activity of RhoA. (B) Our recent data suggest that an inactive RhoA pool (marked by phosphorylation of Ser188) exists directly adjacent to the active membrane-associated RhoA pool during furrow ingression (anaphase to telophase)38 . The presence of this substantial pool of inactive RhoA might enable rapid GTPase cycling of RhoA to maintain heavily restricted RhoA activity at the eqeatorial cortex to drive the ingression of the cleavage furrow. The molecular mechanisms underlying phosphorylation of RhoA during cytokineses remain undetermined. The two future daughter cells are indicated with I. and II. Error bar = 2 μm. Immunofluorescence images depicted here are reproduced from38.
Shared regulators control RhoA activity during adhesion and cell division
Regulation of Rho GTPase activity and the downstream components that control actin contractility is critical in both cell-cell adhesion and cytokinesis. In the next sections, we will discuss the function of the predominant factors involved in the regulation of RhoA activity and actomyosin contractility during cell adhesion and cytokinesis (Figure 2 for an overview).
Figure 2.
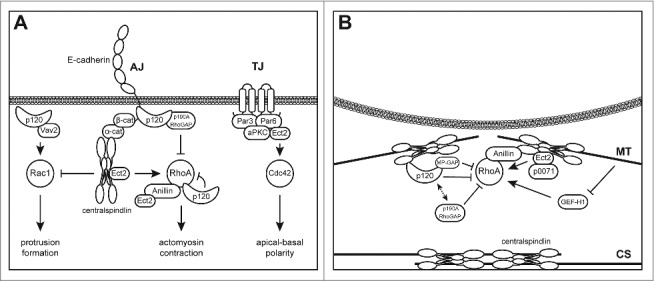
Shared machinery controls Rho GTPases during adhesion and cytokinesis. (A) Regulation of Rho GTPases in cell-cell adhesion complexes. The adherens junction (AJ) complex serves as an adhesion and signaling scaffold that regulates the Rho GTPases. Centralspindlin binds α-catenin and delivers RhoGEF activity via Ect2 to the AJ. In contrast, p120 negatively regulates RhoA activity at the AJ either through recruitment of p190B RhoGAP or through direct binding of inactive GDP-bound RhoA. The presence of centralspindlin at the AJ in turn inhibits the recruitment of p190B RhoGAP to the junction and provides GAP activity towards Rac1 through MgcRacGAP. In turn, the RacGEF Vav2 is recruited to p120 and positively regulates Rac1 near the junction. Besides its function at the AJ, Ect2 has also been associated with regulation of Cdc42 activity at the tight junction (TJ). (B) At the equatorial cortex, local RhoA activity drives ingression of the cleavage furrow. Centralspindlin localizes to the spindle midzone (also known as central spindle; CS) and equatorial cortex through association with both overlapping parallel and astral microtubules (MT). Ect2 is then recruited to centralspindlin and acts as a key RhoGEF during cytokinesis. In addition, the RhoGEF activity of GEF-H1 is necessary during later stages of cytokinesis. The interaction between RhoA and Ect2 is stabilized by the catenin p0071. Another catenin, p120, regulates RhoA activity through complex formation with MP-GAP and GDP-bound RhoA, which might facilitate its N-terminal association with centralspindlin through MKLP1. The presence of positive and negative regulators of RhoA activity near the equatorial cortex provides rapid GTPase cycling and controls the spatial distribution of the active RhoA zone needed for cytokinesis.
Centralspindlin and Ect2
Centralspindlin is a protein complex comprised of MgcRacGAP (male germ cell Rac GTPase activating protein) and the plus-end motor protein MKLP1 (mitotic kinesin-like protein 1). This complex is best known for its essential function during cytokinesis, where it localizes to the spindle midzone and regulates the spatiotemporal activity of RhoA by recruiting Ect2 (epithelial cell transforming 2), the predominant RhoGEF during cytokinesis.17 Remarkably, centralspindlin localizes to the zonula adherens (ZA) and controls junctional Rho GTPase activity in interphase cells.18 Disruption of cortical dynamic microtubules by low concentrations of nocodazole reduced the presence of junctional RhoA and phosphorylated MLC2 (myosin light chain 2), which indicates that the cortical RhoA zone is dependent on a functional microtubule network.18 This microtubule-dependent RhoA activation at the junction is regulated by the centralspindlin subunit motor protein MKLP1 (KIF23) and the recruitment of the Ect2.18 The recruitment of the centralspindlin/Ect2 complex to the ZA depends on binding to α-catenin which connects the actin cytoskeleton to the AJ.18 The presence of the centralspindlin complex near the AJ induces activation of RhoA signaling through two mechanisms: (i) delivering the RhoGEF activity via recruitment of Ect2 and through (ii) inhibition of Rac1 activity which subsequently decreases the junctional recruitment of the RhoA inactivator p190B RhoGAP.18 This junctional inhibition of Rac1 is thought to be mediated by the intrinsic RacGAP activity of the other centralspindlin subunit MgcRacGAP.19 In Xenopus, mutational inactivation of the GAP domain of MgcRacGAP induces increased junctional Rac1 activity and ectopic RhoA activity leading to destabilization of cortical AJ components.20 In addition, MgcRacGAP can also localize to TJs during junction assembly where it associates with the RhoA regulators Cingulin and Paracingulin.21 Interestingly, depletion of Cingulin and Paracingulin effectively depleted MgcRacGAP from the TJ but did not affect the association of Ect2 to the TJ, indicating that Ect2 localization to the TJ does not dependent on the presence of centralspindlin.21 However, depletion of MgcRacGAP had no effect on TJ stability indicating that centralspindlin might not be essential once the TJ has been fully assembled.20
Ect2 was originally identified as a proto-oncogene.22 At the onset of cytokinesis, Ect2 is recruited to the centralspindlin complex upon phosphorylation of MgcRacGAP by Polo-like kinase 1 (Plk1).23,24 As briefly mentioned in the previous section, Ect2 also functions in cell-cell adhesion and cell polarity. Ect2 localizes to cell-cell junctions and co-localizes with the TJ marker ZO-1.25 At the TJ, Ect2 interacts with the Par3/Par6/aPKC polarity complex to regulate local Cdc42 and Rac1 activity.25,26 More evidence for a function of Ect2 in cell polarity comes from 3D culture experiments of MDCK cells.27 Here, expression of either dominant-negative or constitutively-active Ect2 mutants inhibited lumen formation and led to disruption of apical-basal polarity in these cells.27 Given that aPKC activity is crucial for TJ formation and polarity, this disruption may be due to the inability of these Ect2 mutants to recruit the Par3/Par6/aPKC complex to the TJ. As mentioned, centralspindlin-associated Ect2 has been implicated in AJ function.18 Cells depleted for Ect2 displayed severely reduced junctional localization and activity of RhoA and reduced AJ integrity as a consequence.18 Interestingly, the oncogenic function of Ect2 seems to occur independent of its function during cytokinesis. For instance, amplification of Ect2 in non-small cell lung cancer drives transformation through a mechanism dependent on Par6-mediated activation of Rac1.26 Here, expression of dominant-active Rac1 was sufficient to rescue Ect2 depletion, which suggests that Ect2 confers its oncogenic function through Rac1 activation rather than RhoA. In addition, recent insights have revealed that Ect2 also functions in the nucleus where it supports KRAS-driven lung cancer progression by inducing expression of ribosomal RNAs through activation of Rac1.28 However, the molecular mechanisms responsible for the specificity of Ect2 for RhoA (during cytokinesis) and Rac1 (in the cytosol and nucleus) remain elusive.
Together, these observations indicate a pivotal function for the centralspindlin complex and Ect2 in controlling the activity of Rho GTPases at the cell cortex. Thus, while it is clear that Ect2 mediates proper cell-cell adhesion the signals required to recruit the centralspindlin complex to the ZA remain uncharacterized. Ect2 has also been implicated in establishing and maintaining cell polarity which, at least in part, underlies its function as a tumor suppressor.
Anillin
Anillin is a multi-domain scaffold protein that stabilizes the cytokinetic contractile ring through the crosslinking of F-actin, myosin and septin to their upstream activators RhoA and ROCK1/2.29 In Drosophila, Anillin interacts with the centralspindlin complex through binding of MgcRacGAP, thereby linking the contractile ring to the central spindle.30 Interestingly, Anillin controls actin myosin contractility at the ZA and localizes to sites of cell-cell adhesion in both interphase and mitotic Xenopus embryo cells.31 Depletion of Anillin in Xenopus embryos and human epithelial cell lines disrupted cell-cell adhesion through destabilization of AJ and TJ complexes.31,32 Cells depleted for Anillin displayed reduced cortical F-actin levels accompanied by decreased activity of MLC2.32 Interestingly, in addition to its function downstream of RhoA, Anillin depletion also induced short-lived flares of cortical RhoA activity indicative of junction repair which indicates crosstalk between RhoA and Anillin to some degree.32
During cytokinesis, Anillin controls spatiotemporal RhoA activity through direct interaction with RhoA and MgcRacGAP (either directly or indirectly) and Ect2.29 However, if Anillin regulates actomyosin contractility in complex with centralspindlin and Ect2 at the ZA in complex remains undetermined. Anillin can function, at least partially, independent of centralspindlin during cleavage furrow formation judging from the observation that endogenous Anillin was able to rescue cleavage furrow formation in a fraction of MKLP1-depleted cells.33
In conclusion, the observation that Anillin localizes and functions at sites of cell-cell adhesion further highlights the functional overlap of RhoA and actin-myosin regulators in adhesion and cytokinesis.
Cadherins and catenins
Classical cadherins mediate calcium-dependent cell-cell adhesion through homophilic in cis and in trans interactions and function as signaling scaffolds for Rho GTPases.2 During mitosis and cytokinesis, adhesion interfaces need to be remodeled to sustain apical-basal polarity.34 Polarity during division assures rapid engagement of cell-cell adhesion after cytokinesis to provide and maintain the barrier function of epithelia.34 During furrow ingression, the AJ associates with the actin-myosin contractile ring via β-catenin and α-catenin and induces asymmetric furrow ingression and the subsequent apical formation of the midbody.35,36 In addition, cadherins regulate the new adhesive interface between daughter cells and neighbouring cells following cytokinesis completion.34 Although this mechanism is crucial for polarized midbody positioning and functions in maintaining epithelial architecture during cytokinesis, the function of the AJ is not essential for cytokinesis. Of note, E-cadherin localization during the onset of mitosis has been demonstrated to also provide the spatial cue for the orientation of the mitotic spindle through a conserved interaction with LGN (for its leucine/glycine/asparagine repeats; also known as GPSM2) and NuMa (Nuclear Mitotic Apparatus Protein 1).37 In Drosophila and mammalian cells, cells deficient for E-cadherin are able to undergo normal cytokinesis and symmetric furrow ingression despite the absence of a functional AJ.35,36,38 Interestingly, overexpression of E-cadherin in Drosophila spermatocytes can rescue the cytokinesis defects induced by Anillin depletion which indicates that AJ components may function in stabilization of the contractile ring.39 Conversely, non-junctional E-cadherin clusters were also described to downregulate Rho GTPase activity and inhibit accumulation of non-muscle myosin II at the anterior cortex of C. elegans zygotes. Here, E-cadherin clusters impede the cortical flows to preserve the integrity of the actomyosin cortex.40
An obvious candidate that directly links E-cadherin to actin stability is α-catenin. While cadherin-associated α-catenin provides the physical connection to the underlying actin cytoskeleton under force,41 dimeric α-catenin is also able to interact directly with F-actin independent of cadherin association.42 Interestingly, conditional inactivation of α-catenin in keratinocytes induced the formation of binucleated cells indicating a function for α-catenin during cytokinesis.43 In addition, cadherin-associated α-catenin recruits the centralspindlin complex to sites of cell-cell contact although it is unclear if this also occurs at the equatorial cortex during cytokinesis.18 Together, these data suggest that α-catenin may contribute to furrow ingression by stabilizing the contractile ring and/or anchorage to cadherin complexes.
At the cell cortex, p120 controls stabilization of cadherin complexes, mediates signaling from cadherin complexes and regulates the local activity of Rho GTPases (reviewed in:44-46). Interestingly, in vitro experiments have determined that p120 negatively regulates RhoA activity by direct binding of inactive GDP-RhoA.38,47 Binding and inhibition of RhoA by p120 occurs via a small lysine-rich domain (residues 622–628) and appears to be mutually exclusive with cadherin binding.47 Functional evidence for a role of p120 in control of cytokinesis comes from conditional mouse models generated o study the tumor suppressive functions of p120 in the skin and the mammary gland.48,49 Conditional inactivation of p120 in keratinocytes resulted in a high frequency of binucleated cells and chromosome segregation defects.50 Recently, our lab demonstrated that mammary-specific inactivation of p120 in combination with p53 loss induced the formation of highly-invasive and metastatic breast carcinomas that are characterized by abundant bi- and multinucleated tumor cells.38,48 These studies showed that p120 controls the spatiotemporal activity of RhoA at the equatorial cortex through concomitant association with RhoA, MP-GAP (ARHGAP11A) and MKLP1.38 Inducible loss of p120 expression induced mislocalized RhoA activity during anaphase and telophase, resulting in cytokinesis defects and subsequent binucleation. Interestingly, bi- and multinucleation did not occur in conditional E-cadherin knockout tumor models.38 In this context, reconstitution of a p120 mutant deficient in cadherin engagement (K401M substitution mutant) fully rescued the cytokinesis defects upon p120 loss, which demonstrated that the regulation of cytokinesis by p120 is independent of its association with classical cadherins.38
In addition, the p120 family member p0071 (or plakophilin-4; PKP4) has also been linked to RhoA activation during furrow formation.51 Depletion or overexpression of p0071 induced cytokinesis defects and the subsequent formation of binucleated cells.51 During metaphase, p0071 localizes to centrosomes and the mitotic spindle and accumulates at the spindle midzone during anaphase.51 Here, p0071 is proposed to function as a positive modulator of RhoA activity by stabilizing the interaction between Ect2 and RhoA. In contrast to p120, p0071 appears to have a higher affinity for GTP-RhoA and mediates efficient GTP-GDP exchange by stabilizing the interaction between RhoA and Ect2.51 Interestingly, localization of p0071 to the midbody is dependent on RhoA activity, while p0071 itself acts as a positive regulator of RhoA. A similar dependency has been observed for p120 during cytokinesis: while p120 functions as inhibitor of RhoA activity, localization to the active Rho zone during cytokinesis depends on its ability to interact with RhoA.38 Deletion of the lysine-rich region required for binding to RhoA was sufficient to prevent p120 localization to the equatorial cortex and induce cytokinesis defects, while binding of MKLP1 to the N-terminal head region of p120 remained unaltered.38
Together, these data indicate that p120 and p0071 function as important scaffold proteins that bridge and stabilize the interactions between RhoA and its upstream regulators such as centralspindlin and Ect2 during cytokinesis. Importantly, while the presence of AJ complexes near the equatorial cortex functions in positioning the contractile ring, they are not essential for the successful completion of cytokinesis.
GEF-H1
GEF-H1 was originally identified as a novel GEF that possesses activity towards RhoA and Rac1.52 Interestingly, GEF-H1 shows a striking association with microtubules in interphase and mitotic cells.52 Furthermore, association of GEF-H1 with microtubules inhibits its GEF activity leading to reduced RhoA activity.53 GEF-H1 localizes to sites of cell-cell contact where it associates with the TJ component paracingulin and regulates RhoA and Rac1 activity during TJ formation and maintenance.54,55 Besides cell-cell adhesion, GEF-H1 has also been shown to function in focal adhesions where it mediates stiffening of the actin cytoskeleton in response to mechanical force.56 During mitosis, GEF-H1 localizes to the mitotic spindle and accumulates at the contractile ring during cytokinesis.57 Depletion of GEF-H1 led to ectopic cleavage furrow formation and membrane deformations during anaphase. Ultimately, these cytokinesis defects induced the formation of binucleated cells as expected from a RhoA-associated regulatory factor in cytokinesis. GEF-H1 is phosphorylated by the mitotic kinase Aurora A early in mitosis and dephosphorylation during telophase is crucial to induce GTP-loading of RhoA.57 Interestingly, GEF-H1 seems to function during the final stages of cytokinesis, whereas Ect2 is responsible for RhoA activation during the early stages of cytokinesis.57 Although the functions of GEF-H1 have not been as well described as Ect2, it is evident from these data that GEF-H1 functions in the regulation of RhoA during cytokinesis and adhesion.
p190A RhoGAP
The presence of both positive (GEFs) as negative regulators (GAPs and GDIs) of RhoA at the ingression furrow is crucial to maintain a highly focused active Rho zone by restricting lateral diffusion of GTP-RhoA during cytokinesis.58 In fibroblasts, p190A RhoGAP (ARHGAP35; not be mistaken for the aforementioned p190B RhoGAP) is recruited to N-cadherin-associated p120 in response to Rac1 activation.59 Depletion of p190A RhoGAP in this setting destabilized the AJ complex and reduced cell-cell adhesion.59 During cytokinesis, p190A RhoGAP localizes to the cleavage furrow where it regulates the activity of RhoA through its GAP function.60,61 The levels of p190A RhoGAP are controlled through ubiquitin-mediated degradation and are reduced during mitosis and cytokinesis.60 Overexpression of p190A RhoGAP induced cytokinesis defects and binucleation which indicates that its GAP activity needs to be restrained for successful furrowing.62 Interestingly, P190A RhoGAP binds Anillin and Ect2 at the equatorial complex suggesting the presence of a signaling module that couples GEF and GAP activity to the contractile ring during furrow ingression.62,63 Together, the presence of negative RhoA regulators such as p190A RhoGAP and p120 at the active RhoA zone during cytokineses further underlines the need for rapid GTPase cycling to spatially restrict RhoA signaling and elicit a strong and heavily localized downstream response.
Concluding remarks
Several core factors that control Rho GTPase activity and actomyosin contractility have been identified to function both in cell-cell adhesion and cytokinesis. These observations raise the interesting question whether these components may have evolved this dual function to orchestrate coordinated regulation of cell-cell adhesion and cell division, which would allow epithelial tissue to maintain its barrier function during cell divisions. Loss of cell-cell adhesion, for instance through loss of the tumor suppressor p120, induces cytokinesis defects and the formation of chromosomally instable binucleated cancer cells.38 Chromosomal instability may contribute to tumor progression by providing the tumor cells with a highly plastic genome that allows for rapid adaptation. However, besides p120 no other components have been identified to possess a similar dual function in cell-cell adhesion and cytokinesis and, in addition, are found to be frequently lost or mutated in human cancers.
One remaining outstanding question is how the dual function of RhoA regulatory proteins is regulated. While the spatiotemporal regulation of RhoA activation at the equatorial cortex during cytokinesis by mitotic kinases is relatively well understood, the signals that control the association between p120 and MKLP1 or the recruitment of centralspindlin to the AJ remain undetermined and deserve to be the focus of future research.
Funding Statement
Netherlands Organization for Scientific Research (NWO/ZonMW-VIDI 0616.096.318); Dutch Cancer Society (KWF-UU-2011-5230).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199(2):365-380. https://doi.org/ 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratheesh A, Priya R, Yap AS. Coordinating Rho and Rac. In: The Molecular Biology of Cadherins. Vol 116. Progress in Molecular Biology and Translational Science. Elsevier; 2013:49-68. https://doi.org/ 10.1016/B978-0-12-394311-8.00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Wennerberg K, Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. 2005;118(5):843-846. https://doi.org/ 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe AB, Hall A. RHO GTPASES: Biochemistry and Biology. Annu Rev Cell Dev Biol. 2005;21(1):247-269. https://doi.org/ 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 5.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell. 2007;129(5):865-877. https://doi.org/ 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Mata R, Boulter E, Burridge K. The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493-504. https://doi.org/ 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137(6):1421-1431. /pmc/articles/PMC2132529/?report = abstract. https://doi.org/ 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139(4):1047-1059. https://doi.org/ 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol. 2007;178(3):517-527. https://doi.org/ 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases. 2014;5(2):e29770. https://doi.org/ 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168(2):221-232. https://doi.org/ 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428(6984):767-771. https://doi.org/ 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- 13.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15(12):651-658. https://doi.org/ 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15(7):371-377. https://doi.org/ 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizaki H, Ohba Y, Kurokawa K, et al.. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162(2):223-232. https://doi.org/ 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshizaki H, Ohba Y, Parrini M-C, Dulyaninova NG, Bresnick AR, Mochizuki N, Matsuda M. Cell Type-specific Regulation of RhoA Activity during Cytokinesis. J Biol Chem. 2004;279(43):44756-44762. https://doi.org/ 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 17.White EA, Glotzer M. Centralspindlin: At the heart of cytokinesis. Robinson DN, Bement WM, Balasubramanian MK, Sanger JW, Bement W, eds. Cytoskeleton. 2012;69(11):882-892. https://doi.org/ 10.1002/cm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS.et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14(8):818-828. https://doi.org/ 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touré A, Dorseuil O, Morin L, et al.. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273(11):6019-6023. https://doi.org/ 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 20.Breznau EB, Semack AC, Higashi T, Miller AL. MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell-cell junctions in epithelial cells. Mol Biol Cell. 2015;26(13):2439-2455. https://doi.org/ 10.1091/mbc.E14-11-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillemot L, Guerrera D, Spadaro D, Tapia R, Jond L, Citi S. MgcRacGAP interacts with cingulin and paracingulin to regulate Rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol Biol Cell. 2014;25(13):1995-2005. https://doi.org/ 10.1091/mbc.E13-11-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miki T, Smith CL, Smith CL, et al.. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature. 1993;362(6419):462-465. https://doi.org/ 10.1038/362462a0. [DOI] [PubMed] [Google Scholar]
- 23.Yüce O, Piekny A, Glotzer M. An ECT2–centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170(4):571-582. https://doi.org/ 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petronczki M, Glotzer M, Kraut N, Peters J-M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12(5):713-725. https://doi.org/ 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Liu XF, Ishida H, Raziuddin R, Miki T. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Czeta (PKCzeta) and regulates PKCzeta activity. Mol Cell Biol. 2004;24(15):6665-6675. https://doi.org/ 10.1128/MCB.24.15.6665-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Justilien V, Fields AP. Ect2 links the PKCiota-Par6alpha complex to Rac1 activation and cellular transformation. Oncogene. 2009;28(41):3597-3607. https://doi.org/ 10.1038/onc.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XF, Ohno S, Miki T. Nucleotide exchange factor ECT2 regulates epithelial cell polarity. Cell Signal. 2006;18(10):1604-1615. https://doi.org/ 10.1016/j.cellsig.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Baker MJ, Cooke M, Kazanietz MG. Nuclear PKCι-ECT2-Rac1 and Ribosome Biogenesis: A Novel Axis in Lung Tumorigenesis. Cancer Cell. 2017;31(2):167-169. https://doi.org/ 10.1016/j.ccell.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Piekny AJ, Maddox AS. The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol. 2010;21(9):881-891. https://doi.org/ 10.1016/j.semcdb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell Division Requires a Direct Link between Microtubule-Bound RacGAP and Anillin in the Contractile Ring. Current Biology. 2008;18(1):25-29. https://doi.org/ 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Reyes CC, Jin M, Breznau EB, et al.. Anillin regulates cell-cell junction integrity by organizing junctional accumulation of Rho-GTP and actomyosin. Curr Biol. 2014;24(11):1263-1270. https://doi.org/ 10.1016/j.cub.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Chadha GK, Feygin A, Ivanov AI. F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells. Cell Mol Life Sci. 2015;72(16):3185-3200. https://doi.org/ 10.1007/s00018-015-1890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18(1):30-36. https://doi.org/ 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 34.Le Bras S, Le Bras S, Le Borgne R. Epithelial cell division – multiplying without losing touch. 2014;127(24):5127-5137. doi: 10.1242/jcs.151472. [DOI] [PubMed] [Google Scholar]
- 35.Guillot C, Lecuit T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell. 2013;24(3):227-241. https://doi.org/ 10.1016/j.devcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Morais-de-Sá E, Sunkel C. Adherens junctions determine the apical position of the midbody during follicular epithelial cell division. 2013;14(8):696-703. doi: 10.1038/embor.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gloerich M, Bianchini JM, Siemers KA, Cohen DJ, Nelson WJ. Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat Commun. 2017;8:13996. https://doi.org/ 10.1038/ncomms13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Ven RAH, de Groot JS, Park D, et al.. p120-catenin prevents multinucleation through control of MKLP1-dependent RhoA activity during cytokinesis. Nat Commun. 2016;7:13874. https://doi.org/ 10.1038/ncomms13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldbach P, Goldbach P, Wong R, et al.. Stabilization of the Actomyosin Ring Enables Spermatocyte Cytokinesis in Drosophila. 2010;21(9):1482-1493. doi: 10.1091/mbc.E09-08-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padmanabhan A, Ong HT, Zaidel-Bar R. Non-junctional E-Cadherin Clusters Regulate the Actomyosin Cortex in the C. elegans Zygote. Curr Biol. 2017;27(1):103-112. https://doi.org/ 10.1016/j.cub.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Buckley CD, Tan J, Anderson KL, et al.. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346(6209):1254211-1254211. https://doi.org/ 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin Is a Molecular Switch that Binds E-Cadherin-β-Catenin and Regulates Actin-Filament Assembly. Cell. 2005;123(5):903-915. https://doi.org/ 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104(4):605-617. https://doi.org/ 10.1016/S0092-8674(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 44.Schackmann RCJ, Tenhagen M, van de Ven RAH, Derksen PWB. p120-catenin in cancer – mechanisms, models and opportunities for intervention. 2013;126(Pt 16):3515-3525. doi: 10.1242/jcs.134411. [DOI] [PubMed] [Google Scholar]
- 45.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409-432. https://doi.org/ 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kourtidis A, Lu R, Pence LJ, Anastasiadis PZ. A central role for cadherin signaling in cancer. Exp Cell Res. 2017;358(1):78-85. https://doi.org/ 10.1016/j.yexcr.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13(5):604-610. https://doi.org/ 10.1016/S0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 48.Schackmann RCJ, Klarenbeek S, Vlug EJ, Stelloo S, van Amersfoort M, Tenhagen M, Braumuller TM, Vermeulen JF, van der Groep P, Peeters T et al.. Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer Res. 2013;73(15):4937-4949. https://doi.org/ 10.1158/0008-5472.CAN-13-0180. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-Catenin Mediates Inflammatory Responses in the Skin. Cell. 2006;124(3):631-644. https://doi.org/ 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Moreno M, Perez-Moreno M, Song W, Williams SE, Fuchs E. et al.. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proceedings of the National Academy of Sciences. 2008;105(40):15399-15404. https://doi.org/ 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf A, Keil R, Götzl O, Mun A, Schwarze K, Lederer M, Hüttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol. 2006;8(12):1432-1440. https://doi.org/ 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 52.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273(52):34954-34960. https://doi.org/ 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 53.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4(4):294-301. https://doi.org/ 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 54.Benais-Pont G, Punn A, Flores-Maldonado C, Eckert J, Raposo G, Fleming TP, Cereijido M, Balda MS, Matter K. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160(5):729-740. https://doi.org/ 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guillemot L, Guillemot L, Paschoud S, Foglia A, Citi S. Paracingulin Regulates the Activity of Rac1 and RhoA GTPases by Recruiting Tiam1 and GEF-H1 to Epithelial Junctions. 2008;19(10):4442-4453. doi: 10.1091/mbc.E08-06-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13(6):724-729. https://doi.org/ 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12(5):699-712. https://doi.org/ 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bement WM, Miller AL, Dassow von G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28(10):983-993. https://doi.org/ 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127(5):1027-1039. https://doi.org/ 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 60.Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163(3):571-582. https://doi.org/ 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su L, Pertz O, Mikawa M, Hahn K, Parsons SJ. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp Cell Res. 2009;315(8):1347-1359. https://doi.org/ 10.1016/j.yexcr.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manchinelly SAS, Manchinelly SAS, Miller JA, Miyake T, Palmer L, Mikawa M, Parsons SJ. Mitotic Down-regulation of p190RhoGAP Is Required for the Successful Completion of Cytokinesis. J Biol Chem. 2010;285(35):26923-26932. https://doi.org/ 10.1074/jbc.M110.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikawa M, Su L, Parsons SJ. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle. 2008;7(13):2003-2012. /pmc/articles/PMC2791401/?report = abstract. https://doi.org/ 10.4161/cc.7.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]


