Abstract
T-cell replete HLA-haploidentical hematopoietic cell transplantation (haplo HCT) with post-transplant cyclophosphamide was originally described using a reduced-intensity conditioning (RIC) regimen. Given that myeloablative conditioning (MAC) is more effective at preventing disease relapse, we compared outcomes of patients receiving MAC and RIC regimens. We evaluated overall survival (OS), disease free survival (DFS), relapse, non-relapse mortality (NRM), and graft versus host disease (GvHD) of 148 patients that underwent haplo HCT with either MAC (n = 61) or RIC (n = 87). Propensity score adjustment (PSA) was used to balance baseline characteristics between groups and more effectively compare outcomes based on conditioning intensity. After the PSA analysis, relapse was significantly decreased with MAC (HR 0.47, 95% CI 0.31–0.70), but was associated with higher NRM (HR 1.74, 1.13–2.67). OS and DFS were not significantly different between groups (HRs for MAC vs. RIC were 0.87, 95% CI 0.64–1.18 and 0.90, 95% CI 0.68–1.18, for OS and DFS, respectively). Rates of acute and chronic GvHD were not significantly different between groups. This analysis suggests that both MAC and RIC regimens are effective in haplo HCT and that MAC regimens may result in less relapse in selected patients. These results need to be verified in a larger registry study.
Keywords: Conditioning intensity, myeloablative, reduced intensity, haploidentical transplantation, post-transplant cyclophosphamide
Introduction
The use of T-cell replete grafts from HLA-haploidentical donors with post-transplant cyclophosphamide (PTCy) as graft versus host disease (GvHD) prophylaxis has been shown to be efficacious in patients who do not have a matched sibling donor.(1, 2) The Hopkins’ group first pioneered this platform of haploidentical hematopoietic cell transplantation (haplo HCT) using PTCy, reduced intensity conditioning (RIC), and bone marrow grafts.(3) Traditionally HLA-mismatch, myeloablative conditioning (with total body irradiation), and peripheral blood-derived grafts are all factors that confer increased risk of acute GvHD (aGvHD), so a RIC regimen of cyclophosphamide, fludarabine, and a single dose of 200 cGy total body irradiation (TBI) on Day −1 was chosen.(4) This Hopkins regimen resulted in low rates of GvHD, non-relapse mortality (NRM), and promising overall survival (OS). Given the initial concern for higher relapse rates, other groups have studied the effect of using the same PTCy platform with busulfan- or TBI-based myeloablative conditioning (MAC) regimens, which have also resulted in acceptable rates of aGvHD.(5–7)
More recently, larger prospective and retrospective studies have evaluated RIC regimens in the haplo HCT setting, but none have directly compared their outcomes with MAC regimens.(8, 9) In other transplant settings, MAC regimens are often preferred for younger, fit patients, as they are associated with lower relapse rates, albeit with high rates of NRM.(10)
Choosing conditioning intensity is a complex decision taking into account age, comorbidities, disease characteristics, and other factors. Since the use of RIC or MAC is not random, pre-transplant patient and disease characteristics are not matched in most studies comparing RIC and MAC, making retrospective comparisons between conditioning intensities difficult. Propensity score adjustment (PSA) is a common strategy used to estimate treatment effect by accounting for covariates that are unequally distributed in treatment groups.
In the absence of prospective, randomized studies, our aim is to compare MAC and RIC regimens for haplo HCT at a single institution and use PSA to mitigate the selection bias inherent in this approach. We hypothesized that using MAC regimens would results in similar OS and disease free survival (DFS) to traditional RIC regimens and reduce post-transplant relapse at the expense of higher NRM.
Methods
Patients and Treatment
We retrospectively evaluated all adult (≥ 18 years of age) patients who underwent haplo HCT using PTCy at Washington University Medical Center from July 2009 through September 2016. This study was approved by the Institutional Review Board of Washington University School of Medicine in Saint Louis. Patient, donor, and transplant characteristics and outcomes were collected by review of the medical record. The primary objective of this study was to evaluate the difference in OS between patients treated with RIC vs. MAC regimens. Secondary objectives were to evaluate relapse, DFS, NRM, and rates of GvHD.
The indications for haplo HCT were a lack of a readily available HLA-matched donor in patients otherwise needing allogeneic HCT. Conditioning regimens were classified based on consensus definitions of conditioning regimen intensity.(11) RIC and non-myeloablative conditioning regimens were grouped together in the RIC group. The most common RIC conditioning regimen was the Hopkins regimen of fludarabine 30 mg/m2 day −6 to −2, cyclophosphamide 14.5 mg/m2 day −6 and −5, and TBI 200 cGy TBI on day −1. The most common MAC regimens included intravenous busulfan 110 mg/m2 day −7 to −4, fludarabine 25 mg/m2 day −6 to −2, and cyclophosphamide 14.5 mg/kg day −3 and −2; or fludarabine 30 mg/m2 day −6 to −4 and TBI 150 cGy BID day −3 to 0 (1200 cGy total). All patients received intravenous cyclophosphamide 50 mg/kg on days +3 and +4 after receiving their graft, as previously described.(12)
All grafts were collected from peripheral blood of haploidentical donors using G-CSF 10 mcg/kg daily for 5 consecutive days prior to beginning apheresis. No grafts were subjected to ex vivo T-cell depletion and none of the patients received anti-thymocyte globulin. After a MAC or RIC conditioning regimen, patients were infused with a target of 5.0 × 106 CD34+ cells/kg. For GvHD prophylaxis, in addition to PTCy 50 mg/kg on days +3 and +4, all patients received tacrolimus from day +5 to 180 with a target trough of 5 to 15 ng/mL and mycophenolate mofetil (maximum dose 3g per day) from day +5 to 35 as previously described.(12) Patients were started on daily G-CSF 5 mcg/kg on Day +5 until neutrophil count recovery. Opportunistic infection prophylaxis was administered per institutional protocol with acyclovir, fluconazole, and sulfamethoxazole/trimethoprim. Supportive care including antibiotics for febrile neutropenia and transfusion of leukoreduced, irradiated, CMV-seronegative blood and platelet products were given as clinically necessary. All patients remained hospitalized until neutrophil count recovered to greater than 500/mm2 for three consecutive days.
Definitions
Disease risk for post-transplant relapse and death was defined based on the ASBMT Request for Information (RFI) Disease Classifications (http://www.asbmt.org). Cause of death was determined based on the algorithm proposed by Copelan et al.(13) AML patients were risk stratified by the European LeukemiaNet genetic classification as previously described.(14) The Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) score and Disease Risk Indexes (DRI) were calculated as previously described.(15, 16)
Active disease was defined as lack of a complete remission on the last bone marrow biopsy performed within 4 weeks before starting conditioning. Engraftment of donor cells was determined by PCR assay for short tandem repeats or fluorescence in situ hybridization from bone marrow samples or peripheral blood.(17) Complete donor engraftment was defined as < 5% recipient cells at Day 30 post-transplant. Count recovery was defined as the first of three consecutive days with an absolute neutrophil count greater than 500/mm2 and, for platelets, the first of seven consecutive days with a count greater than 20,000/mm2 without transfusion support in the previous week. Primary graft failure was defined as lack of donor chimerism (<5%) on Day 30 assessment or failure to achieve neutrophil count recovery by Day 28 and either subsequently dying without count recovery or requiring repeat donor cell infusion. Delayed engraftment was defined as failure to achieve neutrophil recovery by day 28 but eventually achieving count recovery of donor origin. Patients underwent bone marrow biopsies at Day 30, Day 100, Day 180, and Day 365 post-transplant, or if peripheral blood counts showed concern for disease relapse.
Disease in remission was defined was defined by disease specific criteria based on the morphologic evaluation of Day 30 post-transplant bone marrow biopsies per International Working Group criteria for leukemia and MDS.(18, 19) Reponses for CML were graded as previously described.(20) Disease status for lymphoma was graded as per International Working Group criteria.(21) Patients with severe aplastic anemia (SAA) were all considered to be in remission prior to transplant and response was based on engraftment. For all other patients who achieved post-transplant remission, relapse was defined by excess blasts in bone marrow or peripheral blood or by biopsy proven extramedullary disease or by disease specific criteria. Acute GvHD was diagnosed clinically based on signs and symptoms rather than the time of onset after transplant. The severity of aGvHD was staged as proposed by International Bone Marrow Transplant Registry (IBMTR).(22) Chronic GvHD (cGvHD) was retrospectively graded in accordance with NIH consensus criteria.(23)
Propensity Score Adjustment
In this observational study, comparison of outcomes such as progression or survival by conditioning regimen is complicated by differences between patients who receive one or the other of these regimens. An analysis that does not adjust for this bias would be unable to attribute any observed differences in outcomes to the conditioning regimen itself rather than to the fact that patients receiving one regimen had stronger risk of poor outcomes (i.e. were older or had higher risk disease) than the patients receiving the other regimen. To adjust for this bias, a logistic regression was used to calculate a propensity score for each patient as the probability of receiving MAC versus RIC, conditional on the patient’s age, diagnosis (leukemia versus other), presence of de novo (versus secondary) disease, DRI, and presence (versus absence) of active disease at the time of transplant.(24) An inverse probability of MAC weight was calculated for each patient and used to adjust analyses of outcomes biased by these patient characteristics. Scores were checked for balance and extreme values.(25)
Statistical Methods
Cox proportional hazards models, propensity score weighted as described above, were used to find 1 year OS and DFS, and Fine-Gray models to find median cumulative incidence of relapse, NRM, aGvHD, cGvHD and times to neutrophil and platelet engraftment. Death was treated as a competing risk for relapse as was relapse for NRM. Fisher’s Exact test was used to compare the proportion of patients with death due to infection. The software packages used for this analysis were Base SAS v9.4 and SAS/STAT 14.2.
Results
Between July 2009 and September 2016, 148 patients underwent haplo HCT with a median follow up of 8.2 months overall and 20.3 months for surviving patients. RIC regimens were used in 87 patients and MAC in 61 patients. Baseline patient, disease, and transplant characteristics are described in Table 1. These groups were relatively well matched, with the exception of patients receiving RIC regimens were less likely to have active disease at time of transplant and had a non-significant trend toward older age and lower DRI. The median age for patients in the MAC and RIC cohorts was 48 years and 55 years (p = 0.08). Fifty nine percent of patients had active disease in the MAC arm compared to only 32% in the RIC arm (p = 0.001). The DRI was “high” or “very high” in 57% of patients who received MAC and in 48% of patients who received RIC (p = 0.09).
Table 1.
Patient, disease, and transplant characteristics
| Variable | Myeloablative conditioning (n = 61) | Reduced Intensity conditioning (n = 87) | p-value |
|---|---|---|---|
| Age, years | 0.077 | ||
| Median (range) | 48 (range 19–70) | 55 (19–73) | |
| <40 | 19 (31%) | 26 (30%) | |
| 40–49 | 13 (21%) | 12 (14%) | |
| 50–59 | 19 (31%) | 18 (21%) | |
| 60–69 | 9 (15%) | 23 (26%) | |
| >70 | 1 (2%) | 8 (9%) | |
| Age ≤ 60 | 51 (84%) | 56 (64%) | 0.015 |
| Age > 60 | 10 (16%) | 31 (36%) | |
| Age ≤ 65 | 55 (90%) | 68 (78%) | 0.074 |
| Age > 65 | 6 (10%) | 19 (22%) | |
| Sex | 0.19 | ||
| Female | 34 (56%) | 39 (45%) | |
| Male | 27 (44%) | 48 (55%) | |
| Donor relationship to patient | 0.53 | ||
| Sibling | 31 (51%) | 41 (47%) | |
| Child | 18 (30%) | 33 (38%) | |
| Parent | 11 (18%) | 12 (14%) | |
| Other | 1 (2%) | 1 (1%) | |
| Donor Sex | 0.51 | ||
| Female | 25 (41%) | 31 (36%) | |
| Male | 36 (59%) | 56 (64%) | |
| Female donor into male recipient | 11 (18%) | 16 (18%) | 0.99 |
| Donor Age, median, years | 45.5 (range 16–68) | 42 (range 15–70) | 0.66 |
| Ethnicity | 0.67 | ||
| Caucasian | 52 (85%) | 67 (77%) | |
| Black | 7 (11%) | 16 (18%) | |
| Hispanic | 1 (2%) | 2 (2%) | |
| Asian | 1 (2%) | 2 (2%) | |
| Disease | 0.090 | ||
| AML | 45 (74%) | 50 (57%) | |
| MDS | 5 (8%) | 12 (14%) | |
| ALL | 7 (11%) | 9 (10%) | |
| CML | 1 (2%) | 2 (2%) | |
| Lymphoma | 1 (2%) | 6 (7%) | |
| SAA | 0 | 7 (8%) | |
| Other | 2 (3%) | 1 (1%) | |
| Previous transplant | 0.36 | ||
| None | 43 (70%) | 61 (70%) | |
| Autologous | 3 (5%) | 5 (6%) | |
| Matched unrelated donor | 10 (16%) | 14 (16%) | |
| Matched sibling donor | 5 (8%) | 7 (8%) | |
| Disease Risk Index | 0.057 | ||
| Very high | 13 (21%) | 10 (11%) | |
| High | 22 (36%) | 32 (37%) | |
| Intermediate | 25 (41%) | 34 (39%) | |
| Low | 1 (2%) | 4 (5%) | |
| N/A | 0 | 7 (8%) | |
| HCT-CI Score | 0.153 | ||
| 0 | 3 (5%) | 5 (6%) | |
| 1 | 8 (13%) | 7 (8%) | |
| 2 | 8 (13%) | 10 (11%) | |
| 3 | 15 (25%) | 21 (24%) | |
| ≥4 | 27 (44%) | 44 (51%) | |
| ELN Risk (for AML) | 0.21 | ||
| Favorable | 6 (10%) | 5 (6%) | |
| Intermediate I | 14 (23%) | 18 (21%) | |
| Intermediate II | 9 (15%) | 6 (7%) | |
| Adverse | 16 (26%) | 21 (24%) | |
| N/A | 16 (26%) | 37 (43%) | |
| Cytogenetic Risk | 0.50 | ||
| Low | 3 (5%) | 6 (7%) | |
| Intermediate | 33 (54%) | 36 (41%) | |
| High | 13 (21%) | 18 (21%) | |
| N/A | 7 (11%) | 16 (18%) | |
| Missing | 5 (8%) | 11 (13%) | |
| Disease status at transplant | 0.001 | ||
| Active disease | 36 (59%) | 28 (32%) | |
| Remission | 25 (41%) | 52 (60%) | |
| BM failure | 0 | 7 (8%) | |
| Karnofsky performance status | 0.54 | ||
| 100 | 1 (2%) | 3 (3%) | |
| 90 | 27 (44%) | 38 (44%) | |
| 80 | 24 (39%) | 30 (34%) | |
| 70 | 8 (13%) | 8 (9%) | |
| ≤60 | 1 (2%) | 8 (9%) | |
| Conditioning Regimens | Flu-TBI(fx) 44 (72%) | Flu-Cy-TBI(sd) 74 (85%) | |
| Flu-Bu4-Cy 13 (21%) | Flu-Mel 6 (7%) | ||
| Others 4 (7%) | Others 7 (8%) | ||
| Donor/Recipient CMV Status | 0.5 | ||
| Negative/negative | 20 (33%) | 25 (29%) | |
| Negative/positive | 16 (26%) | 23 (26%) | |
| Positive/negative | 10 (16%) | 9 (10%) | |
| Positive/positive | 15 (25%) | 30 (34%) | |
| Graft Composition (median, range) | |||
| T-cell dose (CD3 × 107/kg) | 17.4 (0.2–68.5) | 17.9 (0.1–59.4) | 0.457 |
| CD34 cell dose (× 106/kg) | 5.5 (3.1–11.2) | 5.5 (1.6–14.2) | 0.744 |
In the whole patient population, overall survival appeared to be adversely impacted by low Karnofsky Performance Score (KPS) (p = 0.01), HCT-CI score (p = 0.02), age (p = 0.04), and DRI (p = 0.13). Low KPS (p = 0.04) and DRI (p = 0.09) were associated with worse DFS. Cumulative incidence of relapse was associated with diagnosis (p < 0.01) and DRI (p = 0.08). Cumulative incidence of NRM was associated with diagnosis (p < 0.01), age (p = 0.03), KPS (p = 0.03), and HCT-CI score (p = 0.02). Univariate analysis was performed for the subpopulation of patients with AML and MDS (n = 112), which showed similar results with worse OS associated with DRI (p = 0.02), age (p = 0.05), and HCT-CI score (p = 0.06). Worse DFS was associated with DRI (p = 0.02) and relapse was associated with high DRI (p = 0.02) and presence of secondary vs de novo disease (p = 0.04). Increased NRM was associated with low KPS (p = 0.07) and HCT-CI score (p = 0.10).
On our analysis of the total population, approximately 20% of the choice of conditioning intensity was related to patient age, diagnosis, having de novo disease (versus secondary), DRI, and active disease at transplant. Other baseline patient characteristics such as HCT-CI score, performance status, cytogenetic risk, previous allogeneic transplant, ABO mismatch, and CMV status were not statistically associated with the choice of RIC versus MAC.
Based on this data, a propensity score was developed to adjust for differences in age at transplant, diagnosis, de novo disease, DRI, and active disease at transplant. After PSA, the hazard ratio for OS based on receiving a MAC regimen was 0.87 (95% CI 0.64–1.18). The 1year OS for the MAC and RIC groups was not statistically different at 50.8% (43.3–59.8%) and 43.0% (36.1–51.3%), respectively (Figure 1B). After similar propensity score weighting, the hazard ratio for DFS based on receiving a MAC regimen was 0.90 (068–1.18). The 1-year DFS for the MAC and RIC groups was not statistically different at 40.5% (33.4–49.1%) and 36.6% (29.9–44.8%), respectively (Figure 1A). There was no difference in cause of death by conditioning intensity (p = 0.36). Disease recurrence was the most common cause of death in both groups, accounting for 33% of deaths in the MAC group and 58% in the RIC group (p = 0.45).
Figure 1.

Kaplan-Meier plots of (A) Disease free survival; and (B) Overall survival according to conditioning intensity. Propensity score adjusted Cox proportional hazard models of (C) Cumulative incidence of relapse; and (D) Non-relapse mortality.
The most common cause of transplant failure for both groups was disease relapse, which occurred in 19 (31%) patients who received MAC and 37 (43%) patients who received RIC regimens. After PSA, the hazard ratio for cumulative incidence of disease relapse after transplant with MAC was 0.47 (0.31–0.70). The 1 year cumulative incidence of relapse was 21.3% (13.9–32.6%) and 36.6% (27.8–48.0%) in the MAC and RIC groups, respectively (Figure 1C). Relapse occurred at a median of 19 months for patients who received RIC and the median was not reached among patients who received MAC. Seven and 14 patients received donor lymphocyte infusions for disease relapse in the MAC and RIC groups.
After PSA, the hazard ratio for NRM after receiving a MAC regimen was 1.74 (1.132.67). The cumulative incidence of NRM at 1 year was 38.6% (25.4–58.6%) for MAC and 27.4% (20.0–37.6%) for RIC (Figure 1D). Deaths due to infection were not significantly different between conditioning intensities, with 8 deaths (13%) and 10 deaths (11%) in the MAC and RIC groups, respectively. Other causes of death were less frequent and not different between groups. Rates of aGvHD did not significantly differ between conditioning intensities. Cumulative incidence of grades II-IV aGvHD was 29.6% and 35.8% for MAC and RIC, and after propensity score weighting the hazard ratio for grade II-IV aGvHD after MAC was 0.79 (95% CI 0.53–1.18). Cumulative incidence of grades III-IV aGvHD was 17.1% and 7.3% in both groups with a hazard ratio of 1.76 after propensity score adjustment (0.93–3.33). cGvHD was not different between groups, with rates at 1 year of 34% and 30% for the MAC and RIC groups (HR 1.17, 0.75–1.81). Severe cGvHD was uncommon and only developed in 2 patients in each group (Figure 2).
Figure 2.
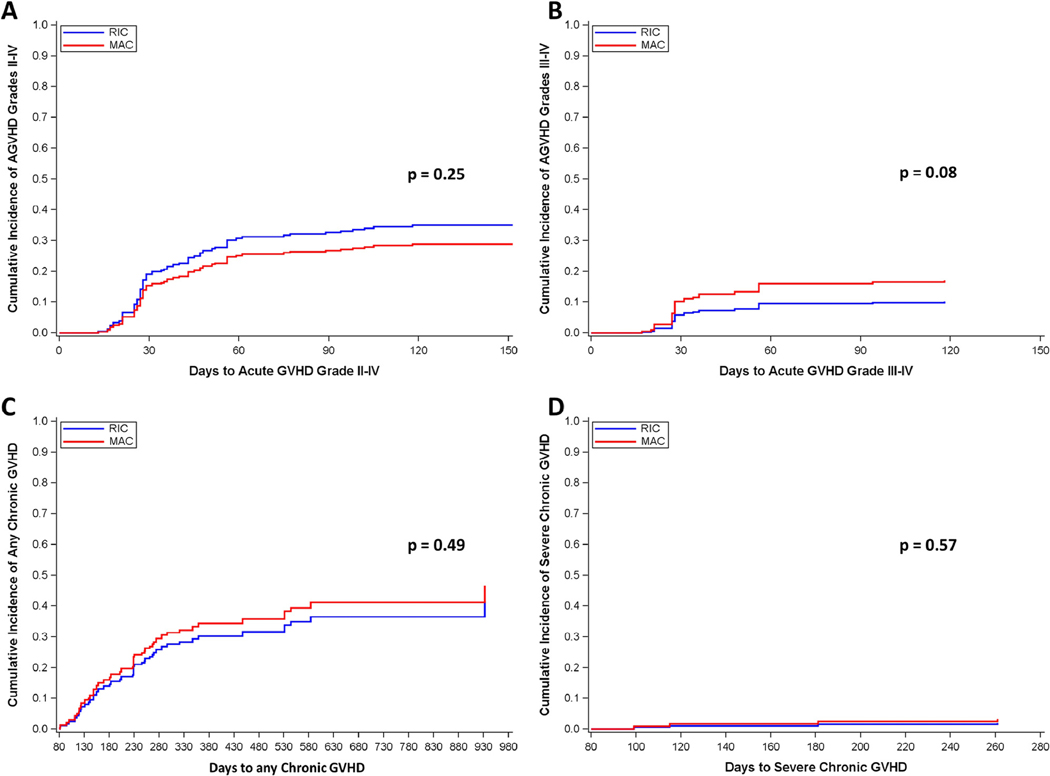
Propensity score adjusted Cox proportional hazard models of cumulative incidence of graft versus host disease (GvHD) for patients based on conditioning intensity. (A) Acute GvHD grades II-IV; (B) Acute GvHD grade III-IV; (C) Chronic GvHD; (D) Severe chronic GvHD.
Engraftment was similar between the two groups with 2 and 3 patients experiencing primary graft failure in the MAC and RIC groups, respectively. Delayed engraftment occurred in 6 (9.8%) and 10 (11.5%) patients in the MAC and RIC groups. Mixed chimerism was observed at day 30 in 3 (5% of patients alive) and 9 patients (11% of patients alive) in the MAC and RIC groups (p = 0.70). Of the 9 patients receiving RIC with mixed chimerism at day 30, 3 patients achieved full donor chimerism by day 100, 2 patients had persistent disease causing mixed chimerism, 1 patient had stable mixed chimerism, and 3 patients died prior to reassessment of their chimerism. Median times to neutrophil and platelet count recovery were 18 and 34 days in the MAC group and 17 and 26 days in the RIC group. CD34+ selected donor cell infusions were used for patients that were fully engrafted (>95% chimerism) with poor count recovery or secondary graft failure in 6 patients (6.8%) who received MAC and 12 (13.8%) who received RIC.
Discussion
To our knowledge, this is the first study to directly compare outcomes between MAC and RIC regimens for patients undergoing haplo HCT with PTCy. Choosing a conditioning regimen is a complex decision, taking into account many clinical variables that can laden retrospective analyses with bias. We attempted to mitigate some of this bias by identifying the patient and disease characteristics that contributed to conditioning regimen selection and creating a propensity score to standardize study groups. Based on this analysis, MAC resulted in half the rate of disease relapse, but caused significantly more NRM so that OS and DFS were not significantly different between conditioning intensities.
The role of conditioning intensity has not been prospectively evaluated in the haplo HCT setting with PTCy. A non-myeloablative regimen with low dose cyclophosphamide, fludarabine, and low dose TBI was first used by the Hopkins group, which reported good outcomes for engraftment, GvHD, and NRM; but the 1 year relapse rate was high at 51%.(3) Subsequent studies evaluated busulfan-based and TBI-based MAC regimens with haplo HCT and PTCy that showed a 1 year DFS near 50%.(5, 6) A large registry study comparing outcomes of haplo HCT with PTCy to matched unrelated donor (MUD) transplants showed relapse rates at 1 year to be 43% with RIC and 41% with MAC regimens.(26) Another large retrospective study that included patients who received the Hopkins RIC regimen again demonstrated a 1 year DFS of 51% in patients undergoing haplo HCT with PTCy.(27) Other reports of outcomes after haplo HCT with MAC have varied, although all had small sample sizes and most groups used predominantly the standard RIC regimen.(7, 28, 29) An EBMT registry study of Haplo-HCT also showed no difference in OS or DFS between RIC and MAC although only a minority of patients received PTCy.(30)
MAC has been compared to RIC in other transplant settings, but there is little data from prospective, randomized trials to definitively guide practice. The BMT CTN 0901 trial comparing MAC and RIC in AML and MDS was stopped early after it showed the 18 month cumulative incidence of relapse was 13.5% for MAC and 48.3% for RIC (p < 0.001). MAC had a trend toward better 18 month OS, at 77.5% versus 67.7% (p = 0.07).(31) Otherwise, data comparing conditioning intensities is largely limited to retrospective reports. A meta-analysis comparing RIC and MAC in AML and ALL showed equivalent OS between the groups with MAC having higher rates of PFS and NRM.(32)
Other studies have compared RIC and MAC in the setting of different donor types. A comparison in the matched related donor (MRD) setting showed higher NRM and cGvHD rates but lower relapse rates with MAC, with equivalent OS between groups.(33) A study comparing conditioning intensities in MUD transplants for AML showed RIC was associated with a higher rate of relapse and decreased NRM in patients older than 50 years.(34) An EBMT study compared RIC and MAC for patients with AML using mismatched unrelated donors and found that for patients over 50 years old, relapse rates were similar across conditioning intensities with RIC associated with better NRM and OS.(35)
These data in aggregate suggest a preference for MAC regimens as they result is less relapse, but may have more NRM (especially in older patients) and cGvHD, but often without significant change in OS. As such, MAC regimens may be preferred for most disease types if patients have a good performance status.
The aim of this study was to evaluate if using MAC regimens could improve outcomes and reduce the risk of relapse after haplo HCT with PTCy. The results from this analysis of 148 patients show that after PSA to control for differences in baseline characteristics between groups, 1 year DFS was not significantly different at 36.6% for patients receiving RIC (85% of patients received the Hopkin’s regimen) and 40.5% for MAC (p = 0.45). OS was also not significantly different between patients who received MAC or RIC regimens for haplo HCT with PTCy with a hazard ratio of 0.87 (0.64–1.18). Similar to other transplant settings, this comes at the expense of higher NRM (HR 1.74).
Similar to what has been previously reported with haplo HCT with PTCy, rates of primary graft failure in this study were low at 5%.(3, 36, 37) An interesting finding of this study was that the rates of grade II-IV and III-IV aGvHD were similar at 26% and 11% for MAC and 38% and 11% for RIC. Rates of cGvHD were also similar at 34% and 30% for MAC and RIC. Rates of grade III-IV aGvHD and severe cGvHD were also low, again similar to previously reported outcomes of haplo HCT with PTCy.(27)
All patients in this study received peripheral blood stem cell (PBSC) grafts. The original Hopkins’ regimen used bone marrow as the graft source in an effort to reduce the rate of GvHD. However, many groups have published outcomes using PBSC grafts given the relative ease of collection and possible faster engraftment and reduced risk of relapse.(5, 7, 38, 39) A more recent comparison showed bone marrow (BM) grafts had an increased risk disease relapse relative to PBSC grafts at the cost of more aGvHD and cGvHD in the haplo HCT with PTCy setting.(40) Using PBSCs as a graft source for all patients in this study limits the confounding effect of graft source on relapse.
Using a propensity score to standardize baseline characteristics for the two study groups has some inherent limitations – not all the differences between these groups can be accounted for and propensity score analysis assumes that patients could have been treated with either conditioning intensity. While it is possible some patients receiving RIC may have been deemed unfit to receive a MAC regimen, the baseline characteristics of this study show that there were no absolute criteria restricting patients to a particular conditioning intensity, making propensity score analysis feasible. Other limitations of this study include patient heterogeneity by including all disease types and that a variety of conditioning regimens, although the vast majority of patients in the RIC group received the standard Hopkins regimen and the majority of patients in the MAC group received fludarabine and fractionated TBI conditioning. Univariate analyses of the subpopulation with AML and MDS showed similar results to the entire cohort. Many of the patients had high risk characteristics, including active disease at time of transplant or had relapsed after a prior allogeneic transplant, which likely contributed to worse outcomes that limited the median follow up and possibly the ability to detect meaningful differences between conditioning intensities.
Strengths of this study are that all patients were treated at a single institution with identical supportive care measures. All patients received PBSC grafts with G-CSF until count recovery. This uniform treatment and evaluation of patient outcomes limits heterogeneity between treatment groups that may be present in multi-institutional or registry studies.
In conclusion, this analysis suggests that conditioning intensity does not significantly affect OS or DFS after haplo HCT with PTCy. However, MAC regimens were associated with significantly less risk of disease relapse at the cost of a higher incidence of NRM. These findings are consistent with outcomes of MAC and RIC in the matched related and MUD transplant settings. These results need to be verified prospectively or in the setting of a large registry study but suggest selectively using MAC regimens in fit patients for haplo HCT with PTCy is efficacious and reduces risk of disease relapse, while choosing RIC does not appear to be harmful.
Table 2.
Comparison of outcomes based on conditioning intensity.
| Myeloablative conditioning (n=61) | Reduced Intensity conditioning (n=87) | p-value | |
|---|---|---|---|
| Median follow up, days, (range) | 273 (6–1069) | 242 (5–2006) | |
| Median follow up for survivors, days | 532 (87–1069) | 661 (189–2006) | |
| Primary Graft Failure | 2 | 3 | 0.99 |
| Median time to neutrophil engraftment, days (range) | 18 (12–78) | 17 (10–70) | 0.77 |
| Cumulative incidence of neutrophil engraftment, day 30 | 83.6% (95% CI 71.3– 91.0%) | 80.5% (70.3–87.4%) | |
| Median time to platelet engraftment days (range) | 34 (16–214) | 26 (8–134) | 0.20 |
| Cumulative incidence of platelet engraftment, Day 100 | 95.6% (95% CI 80.6– 99.1%) | 95.4% (85.1–98.8)% | |
| Acute GvHD, 6 months | |||
| Grades II-IV | 29.6% (18.2–48.0%) | 35.8% (26.4–47.9%) | 0.25 |
| Grades III-IV | 17.1% (7.3–40.1%) | 10.1 (5.1–20.0) | 0.083 |
| Chronic GvHD, 1 year | |||
| Any | 34.4% (22.7–52.0%) | 30.3% (20.8–44.2%) | 0.49 |
| Severe | 3.3% (0.81–13.7%) | 2.1% (0.5–9.1%) | 0.57 |
| Non-Relapse mortality – 1year (after PSA) | 38.6% (25.4–58.6%) | 27.4% (20.0–37.6%) | 0.033 |
| Cumulative incidence of relapse - 1 year (after PSA) | 21.3% (13.9–32.6%) | 36.6% (27.4–48.8%) | 0.001 |
| Disease Free Survival – 1 year (after PSA) | 40.5% (33.4–49.1%) | 36.6% (293.9–44.8%) | 0.45 |
| Overall survival – 1 year (after PSA) | 50.8% (43.3–59.8%) | 43.0% (36.1–51.3%) | 0.15 |
| Cause of death | 0.36 | ||
| Disease relapse/persistence | 10 | 30 | |
| Infection | 8 | 10 | |
| GvHD | 5 | 11 | |
| Other | 7 | 7 | |
Highlights.
MAC regimens for Haplo-HCT with PTCy were associated with reduced relapse but increased NRM
There were no differences in OS or DFS between RIC and MAC Haplo-HCT patients
Rates of acute and chronic GvHD were not different between our RIC and MAC patients
Acknowledgments:
SCC Biostatistics Shared Resource and NCI Cancer Center Support Grant #P30 CA091842, Eberlein, PI; National Center For Advancing Translational Sciences of the National Institutes of Health, Award Number TL1TR002344.
Funding: none
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–62. [DOI] [PubMed] [Google Scholar]
- 2.Rashidi A, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Cashen AF, et al. Comparison of Outcomes after Peripheral Blood Haploidentical versus Matched Unrelated Donor Allogeneic Hematopoietic Cell Transplantation in Patients with Acute Myeloid Leukemia: A Retrospective Single-Center Review. Biol Blood Marrow Transplant. 2016;22(9):1696–701. [DOI] [PubMed] [Google Scholar]
- 3.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859–66. [DOI] [PubMed] [Google Scholar]
- 6.Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–22. [DOI] [PubMed] [Google Scholar]
- 7.Huselton E, Slade M, DiPersio JF, Westervelt P, Vij R, Uy GL, et al. Single institution experience with G-CSF mobilized T-cell replete haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(5):769–71. [DOI] [PubMed] [Google Scholar]
- 8.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolanos-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisdorf DJ. Reduced-intensity versus myeloablative allogeneic transplantation. Hematol Oncol Stem Cell Ther. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–6. [DOI] [PubMed] [Google Scholar]
- 13.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–76. [DOI] [PubMed] [Google Scholar]
- 14.Mrozek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinelli G, Trabetti E, Farabegoli P, Testoni N, Bandini G, Motta MR, et al. Early detection of bone marrow engraftment by amplification of hypervariable DNA regions. Haematologica. 1997;82(2):156–60. [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Annals of oncology : official journal of the European Society for Medical Oncology. 2017;28(7):1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PR RD. Reducing bias in observational studies using subclassification on the propensity score. Journal of the American Statistical Association. 1984;79:516–24. [Google Scholar]
- 25.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCurdy SR, Kasamon YL, Kanakry CG, Bolanos-Meade J, Tsai HL, Showel MM, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with posttransplantation cyclophosphamide. Haematologica. 2017;102(2):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50 Suppl 2:S37–9. [DOI] [PubMed] [Google Scholar]
- 29.Huselton E, Slade M, Rizwan R. Use of Myeloablative or Reduced Intensity Conditioning with Haploidentical Hematopoietic Cell Transplantation for Acute Leukemia and MDS is Associated with Similar Outcomes. Biology of Blood and Marrow Transplantation. 2017;23(3, S279). [Google Scholar]
- 30.Rubio MT, Savani BN, Labopin M, Piemontese S, Polge E, Ciceri F, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul Wahid SF, Ismail NA, Mohd-Idris MR, Jamaluddin FW, Tumian N, Sze-Wei EY, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells Dev. 2014;23(21):2535–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimoni A, Labopin M, Savani B, Volin L, Ehninger G, Kuball J, et al. Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol. 2016;9(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7. [DOI] [PubMed] [Google Scholar]
- 35.Savani BN, Labopin M, Kroger N, Finke J, Ehninger G, Niederwieser D, et al. Expanding transplant options to patients over 50 years. Improved outcome after reduced intensity conditioning mismatched-unrelated donor transplantation for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2016;101(6):773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donnell PV, Eapen M, Horowitz MM, Logan BR, DiGilio A, Brunstein C, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant. 2016;51(12):1599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castagna L, Crocchiolo R, Furst S, Bramanti S, El Cheikh J, Sarina B, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20(5):724–9. [DOI] [PubMed] [Google Scholar]
- 40.Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. 2017;35(26):3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


