Abstract
A critical juncture in early development is the partitioning of cells that will adopt different fates into three germ layers: the ectoderm, the mesoderm, and the endoderm. This step is achieved through the internalization of specified cells from the outermost surface layer, through a process called gastrulation. In Drosophila, gastrulation is achieved through cell shape changes (i.e., apical constriction) that change tissue curvature and lead to the folding of a surface epithelium. Folding of embryonic tissue results in mesoderm and endoderm invagination, not as individual cells, but as collective tissue units. The tractability of Drosophila as a model system is best exemplified by how much we know about Drosophila gastrulation, from the signals that pattern the embryo to the molecular components that generate force, and how these components are organized to promote cell and tissue shape changes. For mesoderm invagination, graded signaling by the morphogen, Spätzle, sets up a gradient in transcriptional activity that leads to the expression of a secreted ligand (Folded gastrulation) and a transmembrane protein (T48). Together with the GPCR Mist, which is expressed in the mesoderm, and the GPCR Smog, which is expressed uniformly, these signals activate heterotrimeric G-protein and small Rho-family G-protein signaling to promote apical contractility and changes in cell and tissue shape. A notable feature of this signaling pathway is its intricate organization in both space and time. At the cellular level, signaling components and the cytoskeleton exhibit striking polarity, not only along the apical–basal cell axis, but also within the apical domain. Furthermore, gene expression controls a highly choreographed chain of events, the dynamics of which are critical for primordium invagination; it does not simply throw the cytoskeletal “on” switch. Finally, studies of Drosophila gastrulation have provided insight into how global tissue mechanics and movements are intertwined as multiple tissues simultaneously change shape. Overall, these studies have contributed to the view that cells respond to forces that propagate over great distances, demonstrating that cellular decisions, and, ultimately, tissue shape changes, proceed by integrating cues across an entire embryo.
Keywords: adherens junction, apical constriction, cytoskeleton, FlyBook, GPCR, morphogen, morphogenesis, myosin, Rho
EPITHELIA are abundant tissue types in metazoan organisms whose structure is established early in embryonic development (Honda 2017). Two defining properties of epithelia are that their constituent cells are (1) physically linked through adhesions to form a sheet, or layer of cells, with important barrier and compartmentalization functions; and (2) polarized across the sheet such that the protein composition on the outer/lumenal side (i.e., apical) of the sheet differs from that on the inner side (i.e., basal). Because epithelial structure is established early in embryonic development, epithelia have to undergo extensive shape changes in order to give rise to the final shape of organs and organisms (Kasza and Zallen 2011; Lecuit et al. 2011; Heisenberg and Bellaiche 2013; Heer and Martin 2017).
The formation of epithelial shape is a process termed epithelial morphogenesis. In Drosophila, and in other organisms, epithelial morphogenesis follows a stereotypical regulatory structure (Figure 1A). First, morphogens create a pattern of gene expression across the embryo. Second, this gene expression leads to the expression of signals, often extracellular, that promote cellular force generation and morphogenesis. This review will focus on actomyosin contractility as the mode of force generation, as opposed to actin polymerization-based protrusion, because of a preponderance of evidence suggesting that contractility is a major driver of epithelial sculpting (Quintin et al. 2008). Contractility can promote cell and tissue shape changes by driving the contraction or shrinkage of a cellular domain.
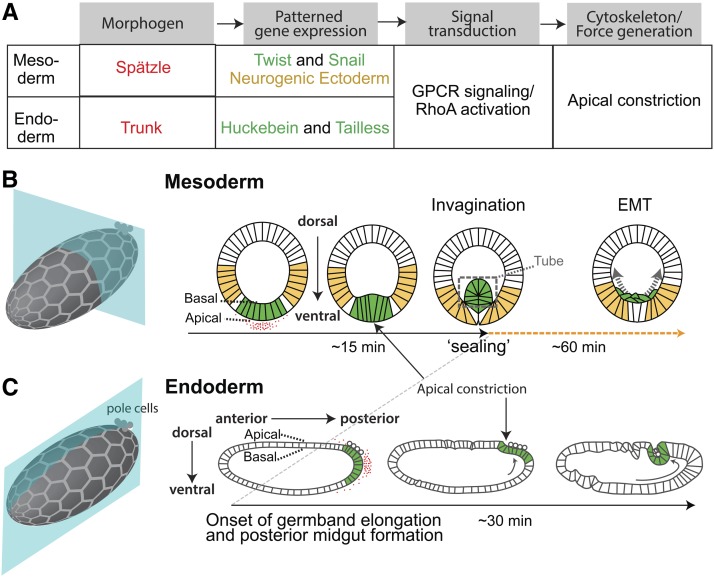
Figure 1.
Drosophila gastrulation overview. (A) Flow chart showing the regulation of cell shape changes that accompany Drosophila gastrulation. Colored text matches the colors in (B and C). (B) Cartoon showing mesoderm invagination in Drosophila. Hexagonal mesh illustrates plane of epithelium, but cell size is not to scale. Red shows pattern of active Spätzle. Green illustrates presumptive mesoderm cells, which express Twist and Snail in response to high levels of Dorsal. Yellow cells show region of ectoderm, which is specified by low levels of Dorsal. (C) Cartoon showing posterior endoderm invagination. Hexagonal mesh illustrates plane of epithelium, but cell size is not to scale. Red shows distribution of the signaling ligand Trunk. Green illustrates presumptive endoderm cells expressing Huckebein and Tailless. Arrow shows the direction of germband elongation.
Cellular contractility is most commonly driven by two proteins, actin and nonmuscle myosin 2 (myosin 2), which are regulated through their assembly into filaments (Murrell et al. 2015). Individual globular actin (G-actin) subunits assemble into filamentous actin (F-actin); actin polymerization is regulated at the level of actin nucleation (by formins and the Arp2/3 complex) and elongation (promoted by Formins and Ena/VASP proteins, and antagonized by Capping protein) (Goode and Eck 2007; Campellone and Welch 2010; Edwards et al. 2014). Myosin 2 forms polymers called bipolar filaments, and is regulated by phosphorylation of the regulatory light chain of the molecule (Heissler and Sellers 2016). Myosin 2 bipolar filaments have the motor heads facing opposite directions, which allows motors on opposing sides of the bipolar filament to bind and walk along F-actin arrays, thereby sliding them past each other (Murrell et al. 2015).
Gastrulation in the Drosophila embryo has served as a major model system for understanding the connection between gene expression and epithelial morphogenesis (Figure 1A) (Leptin 2005). Gastrulation is the process by which a single-layered embryo is converted to multiple “germ” layers. Like many animals, Drosophila establishes domains of cells that invaginate to form either mesoderm or endoderm structures (Figure 1, B and C). Drosophila mesoderm formation involves the inward folding of an epithelial sheet, which results in cell invagination from the outer layer and subsequent formation of an inner layer (Leptin and Grunewald 1990; Sweeton et al. 1991) (Figure 1B). Drosophila gastrulation has played an important role in advancing our understanding of the mechanisms through which actomyosin contractility can shape an embryo (Martin and Goldstein 2014). While some of the details of embryo structure are specific to Drosophila, many of the molecular and cellular mechanisms regulating Drosophila gastrulation are conserved in different contexts. For example, at the cell level, invagination is promoted by a widely utilized cell shape change called apical constriction (Sawyer et al. 2010) (Figure 1, B and C). Apical constriction involves cells contracting on one side of the epithelial layer (i.e., outer or apical), which changes cell shape from columnar to wedge-shaped, thus, promoting inward tissue curvature (Heer et al. 2017). At the molecular level, having a secreted ligand to stimulate apical actomyosin contractility through G-protein coupled receptor signaling is a theme shared by multiple cell types, such as endothelial cells (Shen et al. 2009). At the tissue level, assembly of multicellular actomyosin networks, which propagate force across the hundreds of cells, have been shown to be critical for its proper sculpting (Martin et al. 2010; Yevick et al. 2019). Similarly organized actomyosin networks and means of force propagation have also been shown to operate during gastrulation and neural tube closure in vertebrates (Pfister et al. 2016; Galea et al. 2017), thus rendering Drosophila an increasingly relevant model organism for uncovering the principles that underlie collective cell behavior and morphogenesis.
Gene Regulation and Cell Specification in Drosophila Gastrulation
Morphogen signaling and dorsal activation
During Drosophila gastrulation, presumptive mesoderm and endoderm cells invaginate sequentially (Figure 1, B and C). This chapter will focus on mesoderm invagination, for which the connection between gene expression patterns and morphogenesis is best understood. However, the logic that underlies mesoderm invagination is also true for posterior midgut invagination, and these connections and differences will be discussed.
Drosophila dorsal–ventral polarity is established in the mother’s ovary, where the reciprocal interaction between oocyte and surrounding follicle cells establishes the major body axes prior to fertilization (Roth 2003; Stein and Stevens 2014). Ultimately, signaling via the Toll pathway leads to the graded distribution of nuclear Dorsal in the embryo, which peaks at the ventral midline and decreases in the direction of the dorsal side of the embryo (Figure 2A) (Steward et al. 1988; Roth et al. 1989; Rushlow et al. 1989; Steward 1989). The morphogen that specifies dorsal–ventral polarity and forms a ventral–dorsal gradient is called Spätzle, which binds to the Toll receptor and promotes nuclear translocation of Dorsal (NF-κB) (Figure 2A). The Spätzle protein is synthesized in an inactive form that is proteolytically activated (Figure 2A). Proteolytic activation of Spätzle occurs preferentially in a region of the embryo that was in contact with ovarian follicle cells expressing the pipe gene (Moussian and Roth 2005; Stein and Stevens 2014). The pipe gene encodes a heparan sulfate 2-O-sulfotransferase that modifies the vitelline membrane or some other extracellular matrix component (Sen et al. 1998). It is within this domain of pipe expression that the penultimate protease in a protease cascade, Easter, is activated, which culminates in Spätzle activation by cleavage (Cho et al. 2012; Rahimi et al. 2019). Restriction of Easter-mediated Spätzle activation to a domain of a given width is ensured through negative regulation by a serine protease inhibitor, Serpin27A (Spn27A) (Chang and Morisato 2002; Hashimoto et al. 2003; Ligoxygakis et al. 2003).
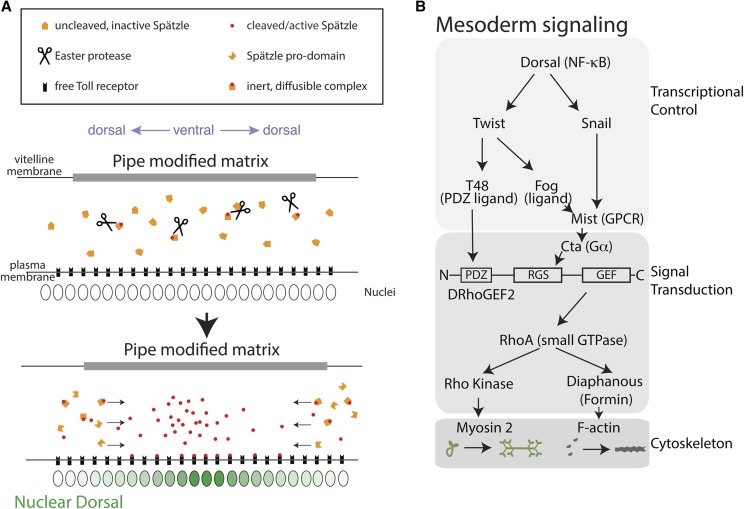
Figure 2.
Signaling that promotes mesoderm invagination. (A) Pathway for Spätzle activation and Dorsal nuclear translocation. Spätzle is produced in an inactive proform and is cleaved by a serine protease (Easter) that is activated within the Pipe domain. Binding of cleaved Spätzle to Toll releases the inhibiting prodomain. Active Spätzle bound to its prodomain is free to diffuse. Prodomain is generated preferentially outside the Pipe domain leading to diffusive flux of Spätzle toward the ventral midline. (B) Genetic pathway for cytoskeletal activation in the Drosophila mesoderm. Domain organization of DRhoGEF2 protein is shown, see text for description.
Although pipe expression defines the limits of Spätzle activation, a more refined pattern of active Spätzle is established by a shuttling mechanism that involves active Spätzle rebinding its prodomain, forming a diffusible, but inert, complex (Haskel-Ittah et al. 2012). Because the free prodomain displays a higher concentration at the lateral regions of the embryo, prodomain cleavage and release of free active Spätzle will have different consequences at different regions. In the lateral region, where the concentration of the free prodomain is high, the released ligand will rebind a prodomain molecule and diffuse. However, when active Spätzle is released in the ventral region where the concentration of the prodomain is low, it has a higher chance of binding the Toll receptor. More free Spätzle molecules will be released in the ventral region, forming a dynamic and robust morphogen gradient (Shilo et al. 2013). Binding to Toll functions as a “sink,” and, hence, the diffusive flux of active Spätzle-prodomain complexes toward the ventral midline results in an active Spätzle distribution that narrows over time (Rahimi et al. 2019), resulting in the graded nuclear translocation of the transcription factor, Dorsal, within the pipe domain.
Dorsal’s various target genes have different thresholds for activation: high concentrations of nuclear Dorsal lead to the expression of mesoderm-specific genes (i.e., twist and snail, which have highest threshold for expression), whereas lower nuclear Dorsal concentrations lead to the expression of different genes in the neurogenic ectoderm (Chopra and Levine 2009) (Figure 1B, yellow). In this manner, a single morphogen gradient establishes gene expression domains at different positions along the dorsal–ventral axis, including mesoderm specification in the most ventral domain. High nuclear Dorsal promotes the expression of two transcription factors, Twist and Snail, in the ventral-most cells of the presumptive mesoderm (Figure 2B). The combination of Dorsal, Twist, and Snail can, in turn, induce or repress the expression of other genes that promote contractility and cell shape changes, which result in mesoderm invagination (Leptin 1991).
Gene expression: twist
Twist is a transcriptional activator that induces the expression of hundreds of genes (Furlong et al. 2001). After loss of Twist activity, limited cell shape changes still occur, but the large-scale movement of the mesoderm tissue is lost or unsustained during later stages of development (Leptin and Grunewald 1990; Seher et al. 2007). Analysis of myosin 2 dynamics in twist mutants demonstrates that apical contractions are transient and reversible, resulting in a failure to sustain cell shape changes (i.e., apical constriction) (Martin et al. 2009). Thus, Twist promotes sustained apical contractility, which is required for myosin 2 to form a supracellular meshwork across the apical surface (Martin et al. 2010).
Two to three of the genes induced by Twist act in concert to promote apical contractility and mesoderm invagination during gastrulation (Seher et al. 2007). First, Twist promotes the activation of a G protein-coupled receptor (GPCR) pathway by activating the expression of folded gastrulation (fog), the signaling ligand for this pathway (Costa et al. 1994) (Figure 2B). Most of the downstream components of this GPCR pathway are maternally supplied, including heterotrimeric G protein subunits, the small GTPase RhoA and its regulators, and cytoskeletal proteins. For example, the gene encoding the heterotrimeric Gα12/13 protein associated with this pathway, concertina (cta), has a maternal effect phenotype similar to zygotic fog mutants (Schupbach and Wieschaus 1989; Parks and Wieschaus 1991). Mutations in most of the components of this GPCR pathway do not prevent mesoderm invagination, but result in uncoordinated apical constriction, where some cells constrict while others exhibit delayed or abnormal constriction (Sweeton et al. 1991; Manning et al. 2013; Xie et al. 2016).
Several GPCRs have been identified as Fog receptors. One GPCR, called Mesoderm-invagination signal transducer (mist, also called Methuselah-like 1), is specifically expressed in the mesoderm in a manner that requires Snail activity (Manning et al. 2013) (Figure 2B). In contrast, another GPCR, called Smog, is ubiquitously present in the early embryo (Kerridge et al. 2016). Differential GPCR endocytosis between mesoderm and ectoderm cells is another way through which differential contractility is achieved between these cell populations (Jha et al. 2018). The G protein receptor kinase (Gprk2) and a β-arrestin (Kurtz) are maternally supplied, and modulate GPCR signaling in the mesoderm and ectoderm (Fuse et al. 2013; Jha et al. 2018; Chai et al. 2019). Kurtz also modulates Toll signaling (Anjum et al. 2013), suggesting that signal termination plays a critical role in defining the region of the presumptive mesoderm, as well as differences between mesoderm and ectoderm. Although differences in the modulation of GPCR signaling could contribute to contractility differences between mesoderm and ectoderm, the fact that ectopic Fog expression results in relatively uniform apical myosin 2 activity along the dorsal–ventral axis strongly suggests that Fog expression is a main determinant that differentiates mesoderm behavior from that of the ectoderm (Morize et al. 1998; Dawes-Hoang et al. 2005). Importantly, the higher fog-dependent Smog homo-cluster formation and recruitment to plasma membrane invaginations in the mesoderm, indicate that Fog binds Smog in the mesoderm (Jha et al. 2018).
A second functional output of Twist expression is the expression of a transmembrane protein known as T48 (Kolsch et al. 2007). T48 has a cytoplasmic tail with a PSD95/Dlg1/ZO-1 (PDZ) domain interaction motif. Mutation of T48, on its own, has little consequence on mesoderm invagination. However, disruption of both T48 and cta results in embryos that resemble twist mutants, suggesting that T48 and GPCR signaling function in parallel to promote mesoderm invagination (Kolsch et al. 2007) (Figure 2B). In terms of Twist’s function in myosin 2 regulation, it is also the case that codepletion of both Fog and T48 results in a failure to sustain apical myosin 2 levels, which is a phenotype similar to that of Twist depletion (Martin et al. 2010).
Twist has several other targets that are also important for invagination. First, Twist cooperates with Dorsal to enhance snail expression. Twist-mediated snail expression appears to expand the snail domain, and to sustain high uniform snail levels in the presumptive mesoderm (Leptin 1991; Ip et al. 1992). Other Twist targets are the tribbles and frühstart genes, which play a permissive role in invagination by repressing cell divisions that, otherwise, disrupt the invagination process (Grosshans and Wieschaus 2000; Mata et al. 2000; Seher and Leptin 2000; Grosshans et al. 2003). The tumor necrosis factor (TNF) receptor-associated factor 4 (traf4) gene, which is required for the fine-tuning of apical adherens junction assembly, is another Twist target (Mathew et al. 2011).
Gene expression: snail
In Drosophila, Snail can repress or activate gene expression (Rembold et al. 2014). Only a few Snail target genes have been identified to have functional importance during mesoderm invagination, but these targets provide insight into how contractility is patterned across the embryo. One family of genes that is repressed by Snail is the Bearded family of genes, which inhibit Neuralized-mediated endocytosis of the signaling ligand, Delta (Bardin and Schweisguth 2006; De Renzis et al. 2006). The Bearded family of genes encodes a set of proteins that inhibit the E3 ubiquitin ligase Neuralized (Lai et al. 2001; Yeh et al. 2001). The neuralized gene is expressed in the ventral mesoderm by Twist, and promotes apical constriction through an unknown mechanism (Perez-Mockus et al. 2017). The Bearded proteins inhibit Neuralized, but fail to do so in the ventral mesoderm because they are transcriptionally repressed by Snail (De Renzis et al. 2006). The derepression of neuralized in the ectoderm that occurs in Bearded mutants results in elevated contractility and junction remodeling in ectoderm cells, which causes the mesoderm to unfold after having initiated invagination (Chanet and Schweisguth 2012; Perez-Mockus et al. 2017). Inhibiting neuralized either through mutation or ectopic expression of Bearded genes in the mesoderm does not completely recapitulate the phenotype of a snail mutant, which suggests that other Snail targets are critical for mesoderm invagination (Perez-Mockus et al. 2017).
Another gene that is repressed by Snail is wntD. Counterintuitively, wntD expression is induced in the presumptive mesoderm by Twist, but is repressed by Snail, resulting in low wntD expression (Ganguly et al. 2005). The wntD gene functions as a feedback inhibitor of the Toll/Dorsal pathway, with ectopic wntD expression blocking Dorsal activation (Ganguly et al. 2005; Gordon et al. 2005). The presence of feedback inhibition in this genetic network appears to promote robustness in the positional expression of genes downstream of Dorsal (Rahimi et al. 2016, 2019).
In contrast to gene repression, Snail activates the expression of the GPCR mist in the ventral mesoderm (Figure 2B) (Manning et al. 2013). mist expression in the mesoderm depends on Snail (Figure 2B). However, snail mutants result in a more severe reduction of actomyosin contractility than twist mutants, failing to exhibit even pulsatile myosin 2 dynamics (Martin et al. 2009). Furthermore, in contrast to snail mutants, mist mutants still undergo apical myosin 2 activation and mesoderm invagination (Manning et al. 2013; Kerridge et al. 2016), suggesting that mist is not Snail’s only functional target. Whether bearded gene repression and activating mist expression together account for all of Snail’s function in mesoderm invagination has not yet been tested.
Posterior midgut invagination
Like mesoderm invagination, endoderm invagination also involves patterned gene expression leading to the induction of a signaling cascade that promotes cell and tissue shape changes (Figure 1, A and C). In the case of the posterior endoderm or posterior midgut, fog expression is induced by terminal transcription factors huckebein and tailless (Costa et al. 1994). In contrast to mesoderm invagination, where fog mutants do not prevent invagination in spite of uncoordinated apical constriction, fog mutants completely inhibit posterior midgut invagination (Costa et al. 1994). Thus, fog is required for posterior midgut invagination, but not for mesoderm invagination. It is not clear why this is the case, but one possibility is that mechanical coupling either between cells themselves, or between cells and the overlying extracellular matrix, plays a bigger role during endoderm invagination than during mesoderm invagination. For example, posterior midgut invagination involves an intercellular mechanical signaling relay, which could depend on Fog signaling (Pouille et al. 2009; Mitrossilis et al. 2017; Bailles et al. 2019). Another difference is that part of the posterior midgut tissue is attached to the overlying vitelline membrane via integrin-mediated adhesion, which plays a role in shaping this invagination (Bailles et al. 2019; Münster et al. 2019).
RhoA Signaling—the Importance of Dynamics
Downstream of the GPCR signaling pathway induced by snail and twist transcription is DRhoGEF2, a guanine nucleotide exchange factor (GEF) for the small GTPase RhoA (Barrett et al. 1997; Hacker and Perrimon 1998). RhoA signaling activates actomyosin contractility by coordinately activating myosin 2 and F-actin assembly (Figure 2B) (Etienne-Manneville and Hall 2002; Jaffe and Hall 2005). DRhoGEF2 is maternally deposited into the embryo, and the maternal contribution is required for mesoderm invagination (Barrett et al. 1997; Hacker and Perrimon 1998). DRhoGEF2 is a member of a family of RhoGEFs, including Leukemia-associated Rho GEF (LARG) and PDZ-GEF in humans, which function downstream of heterotrimeric G proteins and also contain PDZ domains (Fukuhara et al. 2000). DRhoGEF2 has an N-terminal PDZ domain that could interact with the PDZ-binding motif of T48 (Kolsch et al. 2007). In addition, DRhoGEF2 has a Regulator of G-protein signaling (RGS) domain, which is thought to interact with the Gα, Concertina. Finally, DRhoGEF2 has a C-terminal DH-PH domain that is thought to be the catalytic GEF domain that activates RhoA. DRhoGEF2 responds to parallel inputs from Concertina and T48 to stimulate myosin 2 contractility (Figure 2B) (Kolsch et al. 2007).
During apical constriction, RhoA is not simply turned on. Instead, downstream outputs of the RhoA pathway, such as apical Rho-Kinase recruitment and myosin 2 activation are dynamic (Martin et al. 2009; Vasquez et al. 2014), which require dynamic regulation of RhoA (Figure 3A) (Mason et al. 2016). Myosin 2 activity exhibits pulsing behavior with bursts of myosin 2 activation followed by either myosin 2 inactivation or remodeling (Figure 3B) (Martin et al. 2009). Pulsatile dynamics are also observed with a RhoA activity biosensor or by imaging DRhoGEF2 itself (Munjal et al. 2015; Mason et al. 2016).
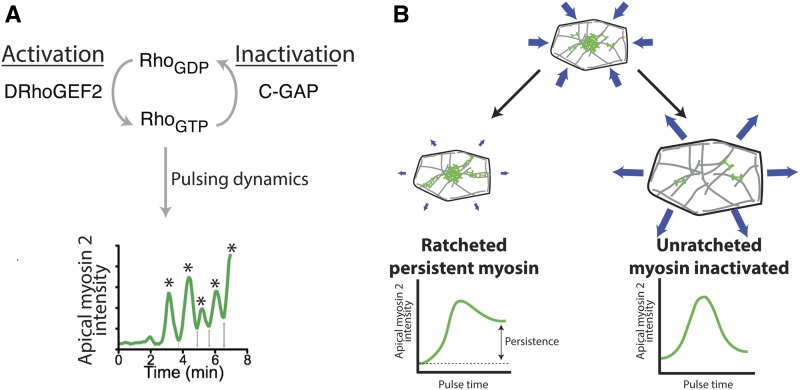
Figure 3.
The dynamics of RhoA regulation in mesoderm cells. (A) Cyclical activation and inactivation of RhoA. DRhoGEF2 promotes formation of active RhoA-GTP. RhoA is inactivated by C-GAP. The combination of DRhoGEF2 and C-GAP is required for pulses of myosin 2 activation (asterisks). (B) RhoA activity levels determine the outcome of contractile pulse. High RhoA activity levels maintain apical myosin 2 after pulse, which stabilizes cell shape and promotes ratcheted constriction. Low RhoA activity levels fail to maintain myosin 2 after the pulse, which results in cell shape relaxation and unratcheted constriction.
Rho GTPase signaling turnover is a key feature of contractile systems, including apical constriction (Denk-Lobnig and Martin 2019). Apical constriction itself is dynamic, occurring as a series of pulses where phases of apex contraction are interrupted by phases of apex relaxation or stabilization (Martin et al. 2009). Contractile pulses are correlated with bursts of myosin 2 assembly in the middle of the apical surface (Figure 3B) (Martin et al. 2009; Blanchard et al. 2010; Rauzi et al. 2010). These contractile pulses are initiated by bursts of DRhoGEF2, which precede apical myosin 2 by ∼10 sec (Mason et al. 2016). Interestingly, a Rab protein, Rab35, precedes myosin 2 localization by ∼45–60 sec, further suggesting that membrane trafficking organizes signaling events upstream of DRhoGEF2 (Miao et al. 2019). In mesoderm cells, a RhoA GTPase activating protein (GAP) called Cumberland-GAP, C-GAP, or RhoGAP71E mediates pulse termination. C-GAP depletion results in a continuous increase in myosin 2 activation without periods of myosin 2 inactivation (Mason et al. 2016). The fact that removing C-GAP disrupts pulsing suggests that pulsing in mesoderm cells involves RhoA signaling dynamics (Figure 3A). Indeed, RhoA signaling has been observed to exhibit hallmarks of excitable dynamics in other systems, such as pulsatile and wave-like behavior (Bement et al. 2015; Bischof et al. 2017; Michaux et al. 2018; Segal et al. 2018).
C-GAP and presumably control of RhoA activity level also determine the outcome of a contractile pulse. One outcome of a myosin 2 pulse is that cell apex constriction is stabilized (Figure 3B, left), such that the cell undergoes stepwise constriction like a ratchet (Figure 4A) (Martin et al. 2009). In twist mutants, apex constrictions resulting from myosin 2 pulses are predominantly reversed, with the apical area relaxing (Figure 3B, right). Whether apex constriction is stabilized or reversed is correlated with the amount of apical myosin 2 that persists following a pulse (Figure 3B) (Xie and Martin 2015). C-GAP overexpression disrupts apical myosin 2 persistence, which leads to cell apex relaxation and ineffective apical constriction (Mason et al. 2016). Interestingly, C-GAP overexpression is the perturbation that most closely resembles Twist depletion, suggesting that signaling downstream of Twist establishes a proper balance between RhoA activation by a GEF and inactivation by a GAP, which is critical for proper morphogenesis.
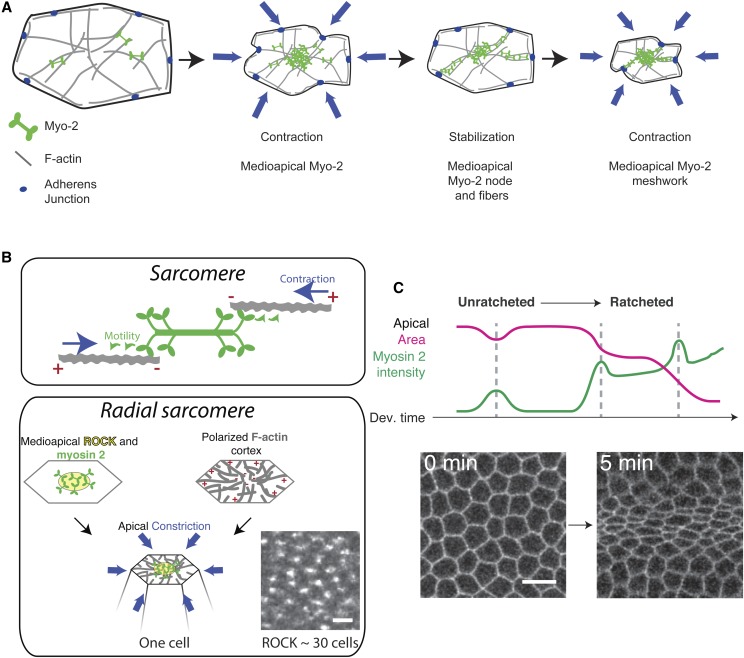
Figure 4.
Spatial and temporal organization of myosin 2 contractility in mesoderm cells. (A) Ratcheted apical constriction of mesoderm cells. Medioapical actomyosin pulls centripetally on adherens junctions in stepwise constriction of the cell apex. (B) Apical cortex has a radial organization. Top, myosin 2 interacting with antiparallel actin filaments with plus ends facing out enables filament sliding and contraction. Bottom, medioapical myosin 2 activation and radial organization of actin filaments with outward facing plus ends enable actin network to be pulled toward center, constricting the apex. Image shows ROCK localization during apical constriction of the mesoderm, which shows a clear periodic pattern across the tissue—each spot represents the center of a contractile unit, which is a single cell. Bar, 3 μm. (C) Temporal progression of myosin 2 pulsing. Initially unratcheted pulses occur and then cells transition to having ratcheted pulses, which leads to sustained changes in apical area. Images show unconstricted and then constricted cell apices in the process of invagination. Images are reproduced from Chanet et al. (2017). Bar, 10 μm.
Cytoskeletal Regulation and Organization During Invagination
RhoA activates myosin 2 through its effector Rho-Kinase (ROCK), which promotes myosin 2 activation by direct phosphorylation of myosin regulatory light chain and also by phosphorylating and inhibiting the myosin phosphatase (Amano et al. 1996; Mizuno et al. 1999; Winter et al. 2001). As with mammalian myosin 2, phosphorylation of the regulatory light chain (spaghetti squash, or sqh, in Drosophila) switches the motor activity from an “off” to an “on” state (Jordan and Karess 1997; Vasquez et al. 2016). During Drosophila gastrulation, ROCK is required for apical myosin 2 accumulation in apically constricting cells (Dawes-Hoang et al. 2005). Furthermore, acute ROCK inhibition results in the rapid (∼10 sec) disappearance of apical myosin 2, suggesting that ROCK is continuously required to balance inactivation by myosin phosphatase to maintain apical myosin 2 (Coravos and Martin 2016). Myosin phosphatase colocalizes with apical myosin 2 and promotes rapid myosin 2 turnover, which is required for myosin 2 pulsing (Vasquez et al. 2014; Munjal et al. 2015).
In addition to regulating myosin 2, RhoA also regulates actin cytoskeleton organization in the Drosophila mesoderm (Figure 2B) (Fox and Peifer 2007). Another RhoA effector, the formin Diaphanous (Goode and Eck 2007), is required for mesoderm invagination (Homem and Peifer 2008; Mason et al. 2013). However, despite RhoA activation in constricting cells, overall levels of cortical F-actin are decreased in mesoderm cells prior to furrow formation (Jodoin et al. 2015). This decline in cortical F-actin levels in mesoderm cells is attributed to higher levels of active Cofilin, an F-actin severing protein (Jodoin et al. 2015). Actin turnover, including formin-mediated F-actin assembly and cofilin-mediated disassembly, is important for maintaining intercellular connections in the tissue, as will be discussed in the next section.
Spatial organization
Myosin 2 activation and F-actin assembly are spatially choreographed across the apical cortex of presumptive mesoderm cells. Active, GTP-bound RhoA and ROCK are enriched in the middle of the apical surface (medioapical), which promotes medioapical myosin 2 accumulation (Mason et al. 2013, 2016; Munjal et al. 2015) (Figure 4B). This polarization requires C-GAP, suggesting that precise regulation of RhoA activity establishes medioapical myosin 2 enrichment (Mason et al. 2016). In contrast, actin subunit incorporation at F-actin plus ends occurs predominantly at intercellular junctions (Coravos and Martin 2016). However, F-actin minus ends are medioapical and colocalize with myosin 2. The enrichment of the different F-actin ends in distinct apical regions suggests that the apical actin cortex is radially polarized (Figure 4B). Given that F-actin plus ends face outward and the minus ends and myosin 2 activation is predominantly medioapical, the apical actin cortex resembles a contractile unit, such as a sarcomere. Indeed, ROCK localization exhibits a polka-dot pattern across the tissue, with each dot representing the medioapical domain of one cell (Figure 4B).
Recent data has suggested that other cells may have a similar contractile organization. For example, the leading edge of the epidermis during Drosophila dorsal closure has a repeated pattern of myosin 2 localization that resembles “bars-on-a-string,” with each bar representing a single leading edge cell (Franke et al. 2005). Recent super-resolution microscopy of leading edge cells has shown that Ena—a protein that promotes F-actin plus end elongation—localizes to the adherens junctions, suggesting that F-actin plus ends are enriched facing outwards from a central myosin 2 bar (Manning et al. 2019). Given that leading edge cells have centrally localized myosin 2 and peripherally enriched F-actin plus ends, this topology again resembles a sarcomere. Adherens junctions exhibit F-actin plus end enrichment in several epithelial cell types (Tang and Brieher 2012; Verma et al. 2012); thus, this apical cortex organization may play a general role in nonmuscle contractility.
In mesoderm cells, the polarity of ROCK and myosin 2 is important for apical surface contraction, consistent with a sarcomere-like contraction (Mason et al. 2013; Coravos and Martin 2016). Furthermore, the rate of apical constriction is proportional to the ATPase activity of the myosin 2 motor (Vasquez et al. 2016), which is what is expected from a sarcomere-like model of contraction (Barany 1967). However, there are several important differences between the observed organization in mesoderm cells and that of a sarcomere. First, in mesoderm cells, the apical actin network is arranged radially around a signaling center, rather than being linear. Second, medioapical actomyosin networks are extremely dynamic and undergo self-organizing behaviors. One way in which actomyosin networks can undergo self-organization is through contraction-driven advection of plasma membrane associated proteins (Munro et al. 2004; Munjal et al. 2015), some of which (i.e., Rho/ROCK advection) could feed back to regulate myosin 2 activity. Furthermore, as apical myosin 2 accumulates, medioapical actomyosin changes from medioapical spots to a fibrous organization that aligns relative to the mechanics of the surrounding tissue (Chanet et al. 2017). Thus, these apical actomyosin networks can exhibit self-organizing properties that enable them to change over time.
In addition to the radial organization of the apical F-actin cortex, the microtubule cytoskeleton also exhibits a radial polarity across the apex of constricting cells. During gastrulation, apical actomyosin contraction drives the formation of an medioapical microtubule organizing center, which promotes microtubule growth from the apical center out toward the cell junctions (Ko et al. 2019). Although the microtubule-associated protein EB1 binds DRhoGEF2 (Rogers et al. 2004), microtubule organization is not required for myosin 2 activation or initiating apical constriction, but promotes rapid actin dynamics in the apical cortex (Ko et al. 2019).
Temporal organization
Cytoskeletal behavior during gastrulation is also organized in time. In the mesoderm, myosin 2 assembly occurs in pulses with phases of assembly followed by disassembly or remodeling (Martin et al. 2009). The outcome of a constriction is correlated with myosin 2 behavior after the pulse (Xie and Martin 2015). If medioapical myosin 2 is disassembled, then the apical cortex relaxes, and the cell fails to undergo net constriction (unratcheted pulse, Figure 3B). In contrast, if medioapical myosin 2 persists, then the constricted apical shape can be stabilized—an event that is often associated with formation of F-actin and myosin 2-containing fibers or cables that span the apical surface (ratcheted pulse, Figure 3B and Figure 4A). During mesoderm invagination, there is a temporal progression in cell behavior: initial pulses fail to exhibit myosin 2 persistence, and cells relax (Figure 4C, unratcheted); subsequent pulses lead to persistent myosin 2 accumulation, resulting in ratcheted contraction, which ultimately promotes collective tissue contraction (Figure 4C) (Xie and Martin 2015). Membrane trafficking through the Rab35 GTPase and its GEF, Sbf, is also important for ratcheted apical constriction (Miao et al. 2019). One effect of Rab35- and Sbf-depletion in embryos is that cells have heterogeneous myosin 2 levels, with some cells lacking and others having an abundance of myosin 2 (Miao et al. 2019). Because this phenotype is reminiscient of mutants in the Fog pathway (Parks and Wieschaus 1991; Sweeton et al. 1991; Costa et al. 1994; Xie et al. 2016), one hypothesis is that Sbf/Rab35 regulates GPCR signaling at plasma membrane invaginations (Jha et al. 2018). Alternatively, the removal of apical plasma membrane could be a process that acts in concert with contractility to change cell shape, as was proposed for Xenopus gastrulation (Lee and Harland 2010).
Work in Caenorhabditis elegans suggested that apical constriction is initiated by the onset of a molecular clutch that engages between the apical actomyosin cortex and the intercellular junctions (Roh-Johnson et al. 2012). It was suggested that a clutch mechanism was also responsible for the onset of mesoderm invagination in Drosophila, but several factors make this unclear. First, in the Drosophila mesoderm, pulsed actomyosin contractions are initially weak, and then strengthen over developmental time, making it difficult to know whether the initial contractions are not attached to intercellular junctions, or are simply too weak to change cell shape (Xie and Martin 2015). Second, adherens junctions are not initially present at the apical surface, but both adherens junctions and polarity proteins are pulled apically by actomyosin contractility (Weng and Wieschaus 2016, 2017). Therefore, initial contractions might not change cell shape, but still be connected to adherens junctions and be involved in pulling them apically. Finally, actomyosin contractility itself promotes the apical accumulation of adherens junction proteins during both Drosophila and C. elegans gastrulation (Marston et al. 2016; Weng and Wieschaus 2016). Therefore, greater coupling between the cell surface and the plasma membrane could result from an increase in the number of adhesion molecules recruited to adherens junction structures rather than a regulated link between the two. In summary, while a clutch-like mechanism was convincingly shown to trigger C. elegans gastrulation (Roh-Johnson et al. 2012), the trigger for Drosophila gastrulation appears to be more complicated, and to involve the coordination and change in both contractility and adherens junctions.
The F-actin cortex is also highly dynamic during apical constriction, and its dynamics are critical for force transmission across the tissue. During contractility the apical F-actin network continuously fragments (Jodoin et al. 2015). F-actin network fragmentation is especially prevalent next to adherens junctions, which separates the adherens junction from medioapical actomyosin (Figure 5A). Apical F-actin network turnover is responsible for repairing network fragmentation, thus, re-establishing the connection between the medioapical actomyosin and the adherens junctions (Figure 5A) (Jodoin et al. 2015). Importantly, the proper microtubule cytoskeleton organization is required to rapidly reestablish these lost connections, suggesting that cooperation between actin and microtubule cytoskeletal systems is critical for propagating force between cells (Ko et al. 2019).
Figure 5.
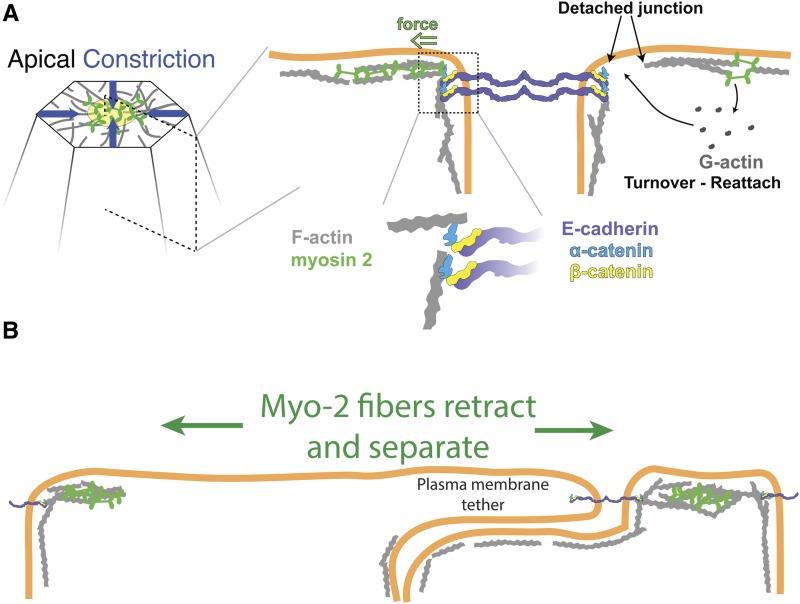
Integrating forces between cells during gastrulation. (A) Cartoon showing apical–basal cross-section through a wild-type cell, highlighting the adherens junction and its connection to the underlying F-actin cortex. The cell on the right illustrates a detachment event where actin turnover is required to reconnect the junction and medioapical actomyosin cortex. (B) Cartoon illustrating the outcome of depleting adherens junction components in the cell (e.g., armadillo mutant). Note that medioapical actomyosin networks contract away from each other, with actomyosin fibers retracting. Also, note the plasma membrane tether that is pulled from the left-hand cell toward the cell on the right.
While apical constriction in the endoderm cells of the posterior midgut is similar to that of the mesoderm (e.g., apical constriction is pulsatile (Chanet et al. 2017), and myosin 2 is enriched medioapically), the distribution of medioapical myosin 2 is different. In contrast to mesoderm cells, where myosin 2 is organized into a medioapical node with fibers that span the apical surface (Figure 4A), endoderm cells contain myosin 2 organized into medioapical rings (Chanet et al. 2017). The cause of this different distribution will be discussed in the next section.
Force Integration Across the Tissue and Resulting Cell Shape
For cells to promote a tissue-wide change, cellular forces have to be integrated across the tissue. During mesoderm invagination, adherens junctions are required to mechanically couple cells (Dawes-Hoang et al. 2005; Sawyer et al. 2009; Martin et al. 2010). Adherens junctions contain the adhesion receptor E-cadherin, which forms clusters that physically link cells on the extracellular side of the plasma membrane (Figure 5A) (Yap et al. 2015). On the cytoplasmic side of the adherens junctions, adaptor proteins such as α- and β- catenin and Canoe/Afadin link E-cadherin receptors to the underlying actomyosin cytoskeleton (Lecuit and Yap 2015; Vasquez and Martin 2016). All of these proteins are required to integrate forces across the invaginating mesoderm.
In Drosophila, adherens junctions initially form plaque-like structures known as spot adherens junctions before forming a continuous zonula adherens (Tepass and Hartenstein 1994). In mesoderm cells, fibrous actomyosin structures spanning the apical surface connect to spot adherens junctions in an end-on manner, which pulls adherens junctions centripetally, toward the center of the cell apex (Martin et al. 2009) (Figure 4A and Figure 5A). The weakest point of this transmission appears to be the connection between the actomyosin cytoskeleton and the cytoplasmic interface of the adherens junction. For example, live imaging of the F-actin cortex in wild-type embryos has revealed repeating fragmentation events that separate medioapical actomyosin from the adherens junctions (Figure 5A) (Jodoin et al. 2015). Furthermore, lowering adherens junction protein levels results in actomyosin networks tearing away from one side of an intercellular junction, which results in a plasma membrane tube or “tether” being pulled from the unattached cell toward the cell whose junctional attachment remains (Figure 5B) (Sawyer et al. 2009; Martin et al. 2010).
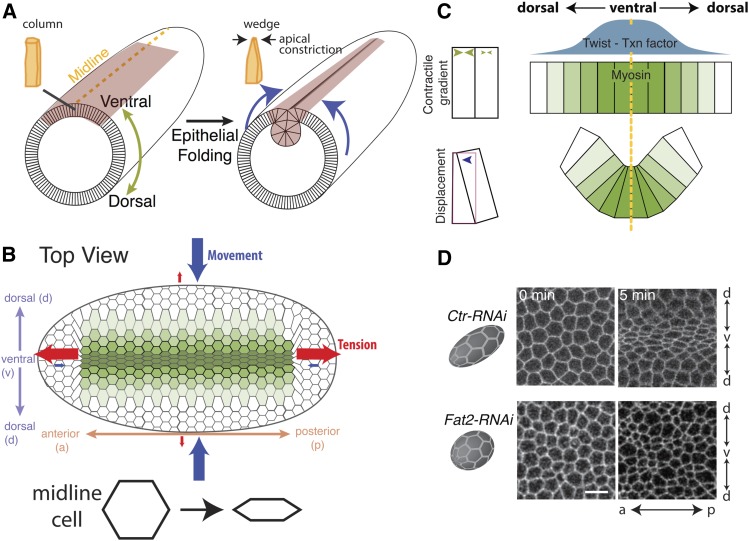
The result of integrating force across the tissue is the generation of global tissue movement and mechanical tension. These two outcomes tend to be anticorrelated: tension is highest when movement is restrained, and movement is often associated with lower tension. Mesoderm invagination is associated with anisotropic tension, with the highest tension oriented along the anterior-posterior axis, along which cells fail to constrict efficiently (Figure 6, A and B) (Martin et al. 2010). This anisotropic tension results from the shapes of the contractile domain and the embryo, which are longer along the anterior–posterior axis than the dorsal–ventral axis (Figure 6, A and B) (Spahn and Reuter 2013; Chanet et al. 2017; Guglielmi and De Renzis 2017). In addition, contractility is graded along the dorsal–ventral axis, which means that there is a force imbalance between cells tugging along that direction (Figure 6, B and C) (Spahn and Reuter 2013; Heer et al. 2017; Lim et al. 2017). The force balance along the anterior–posterior axis means that there is greater resistance to apical constriction along this axis, which results in higher tension and less cell shape change. Thus, the anisotropic tension in the mesoderm causes anisotropic apical constriction, with ventral midline cells remaining more elongated along the anterior–posterior axis, and more apically constricted and wedge-shaped along the dorsal–ventral axis (Figure 6, B and D, control) (Sweeton et al. 1991; Martin et al. 2010). This anisotropy in tension and cell shape depends on ellipsoidal embryo shape: changing the embryo to a more spherical shape disrupts this anisotropy, thereby illustrating the interdependence between cell and embryo form (Figure 6D) (Chanet et al. 2017). Thus, embryo and tissue shape feeds back on cell behavior, resulting in wedge-shaped rather than cone-shaped cells and a dorsal–ventral axis of curvature in the mesoderm (Figure 6, A and B).
Figure 6.
The mechanics of Drosophila mesoderm invagination. (A) Cartoon showing cell and tissue shape changes during mesoderm invagination. Orange cells illustrate cell shape. Blue arrows show movement of ectoderm tissue during mesoderm invagination (maroon). Note that ventral side is shown facing up to highlight the mesoderm. (B) Birds-eye view of presumptive mesoderm. Green illustrates the level of cell contractility. Red arrows denote tension and blue arrows denote movement. (C) Cross-section view of mesoderm cells during invagination. A gradient in twist expression (blue curve) results in a gradient in myosin 2 contractility (green), which promotes efficient apical constriction in the ventral–dorsal direction. (D) Apical constriction anisotropy depends on embryo shape. Images are subapical views of cells showing cell outlines. In the control, ellipsoidal embryos (Ctr-RNAi), cells constrict mostly in the ventral–dorsal direction. In round embryos (Fat2-RNAi), cell constrict isotropically. Images are reproduced from Chanet et al. (2017). Bar, 10 μm.
Mechanosensing during Drosophila gastrulation
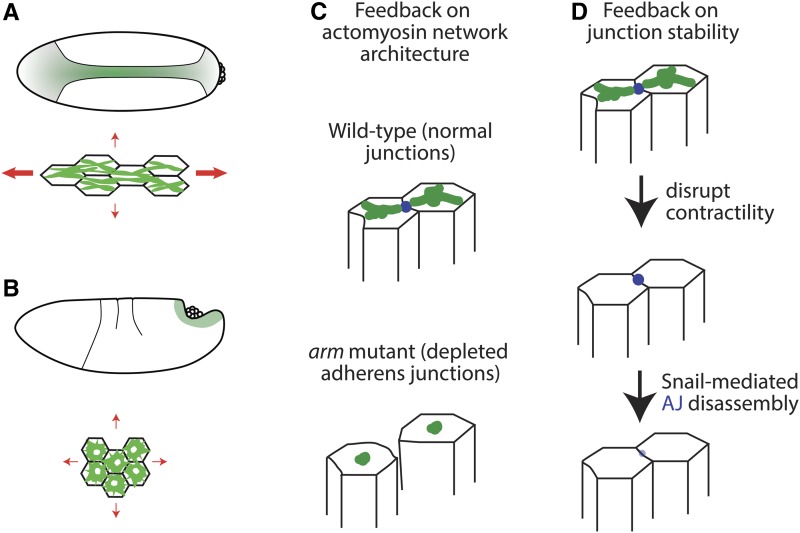
In addition to generating force, the cytoskeleton and adherens junctions also respond to force through mechanosensing or mechanotransduction mechanisms (Hannezo and Heisenberg 2019). During gastrulation, the surrounding mechanical constraints to invagination regulate the geometrical properties of cells and their internal cytoarchitecture, suggesting long-range mechanical coupling and feedback (Chanet et al. 2017). As described above, the higher tension along the long axis of the embryo prevents cells from constricting along this axis, resulting in an elongated cell shape (Figure 7A) (Martin et al. 2010). In contrast, endoderm invagination is associated with low isotropic tension, leading to isotropic apical constriction (Figure 7B) (Chanet et al. 2017). In the mesoderm, myosin 2-containing fibers that connect between cells align and straighten with the axis of tension in the embryo (Figure 7A) (Chanet et al. 2017; Yevick et al. 2019). In contrast, perturbations that result in isotropic resistance to constriction or the natural process of endoderm invagination, result in the formation of myosin 2-containing rings (Figure 7B) (Chanet et al. 2017). Mechanical signals between cells have also been suggested to be responsible for inducing apical myosin 2 accumulation by inhibiting Fog/receptor endocytosis (Pouille et al. 2009; Mitrossilis et al. 2017). However, in the mesoderm, Fog activity is not required for invagination or myosin 2 stabilization, suggesting that mechanical feedback is not the primary mechanism of myosin 2 induction (Costa et al. 1994; Kolsch et al. 2007; Xie et al. 2016). Adherens junctions and the mechanical integration between cells are similarly not required for mesodermal apical myosin 2 accumulation, but do strongly impact actomyosin network geometry (Dawes-Hoang et al. 2005; Sawyer et al. 2009; Martin et al. 2010). In the absence of counterbalancing forces mediated by adherens junctions, myosin 2 fibers contract into medioapical spots (Figure 5B and Figure 7C). These data suggest that, in mesoderm cells, myosin 2 activation is cell autonomous, but that apical actomyosin network morphology and alignment depends strongly on mechanical context through force balance (Figure 7, A and B).
Figure 7.
Mechanical feedback mechanisms in Drosophila gastrulation. (A) Cartoon illustrating the apical myosin 2 meshwork during mesoderm invagination. Note myosin 2 fibers oriented along the anterior–posterior axis are formed. Red arrows denote tension and, thus, resistance to contraction. (B) Cartoon illustrating the apical myosin 2 meshwork during posterior endoderm invagination. Note tension is isotropic (red arrows) and myosin 2 rings are formed. (C) Integration of mechanical force is necessary for apical myosin 2 meshwork structure. Top image illustrates a wild-type meshwork while bottom image illustrates the change in meshwork morphology in response to disrupting adherens junctions (i.e., armadillo mutants). (D) Mechanical force is required for adherens junction stability. Cartoon illustrates the effect of disrupting contractility: adherens junctions disassemble in the absence of contractility.
Mechanical feedback can also operate at the level of intercellular attachments. Recent work has shown that adherens junctions function as mechanical integrators of contractility in a tissue (Lecuit and Yap 2015). During mesoderm invagination, myosin 2 contractility stabilizes adherens junctions in the face of Snail-mediated disassembly (Figure 7D) (Weng and Wieschaus 2016). Indeed, acute pharmacological inhibition of ROCK decreases E-cadherin levels in spot junctions within 1 min of inhibition, demonstrating the dependence of adherens junction stability on myosin 2 contractility and a continuous pulling force (Coravos and Martin 2016).
Tissue-Wide Mechanical Coupling of Morphogenetic Movements During Gastrulation
Like most other events in development, gastrulation does not occur in isolation. Presumptive mesoderm and endoderm tissues are connected to ectoderm tissue, and movements of mesoderm and endoderm depend on the deformability and other responses of the neighboring tissues. This coupling is illustrated by the fact that gastrulation is accompanied by global morphogenetic flows that occur across the entire embryo (Lye et al. 2015; Rauzi et al. 2015; Streichan et al. 2018). The global morphogenetic flows that occur in the embryo before and after mesoderm invagination are predicted with >>90% accuracy by the spatial distribution of myosin 2 across the embryo and its anisotropy (i.e., orientation), suggesting that unbalanced myosin 2 activity promotes global tissue movement (Streichan et al. 2018).
One consequence of the invagination of mesoderm and endoderm tissues is that the remaining ectoderm must fill the significant surface void left by the internalization of these cells. Mesoderm invagination induces a dorsal–ventral tensile stretch on lateral germband cells (Lye et al. 2015). However, it appears that the cells that compensate most for mesoderm invagination are the dorsal-most cells, which stretch considerably in response to mesoderm invagination (Rauzi et al. 2015).
Posterior midgut invagination results in anterior–posterior tensile stress in the ectoderm (Collinet et al. 2015; Lye et al. 2015). Associated with the anterior–posterior elongation of ectoderm cells is the intercalation of these cells to elongate the germband, which allows the endoderm to internalize fully. Germband extension involves the planar polarized distribution of myosin 2, which causes junctions with one type of orientation to contract, while junctions with an orthogonal orientation elongate, resulting in directional cell intercalation and tissue elongation along the anterior–posterior axis (Irvine and Wieschaus 1994; Bertet et al. 2004; Zallen and Wieschaus 2004). Drosophila germband extension also involves planar polarized protrusions typical of cell crawling behavior, which could contribute to, or even lead, cell intercalation movements (Sun et al. 2017). Given that anterior–posterior cell stretching is intensified when cell intercalation is blocked, it is possible that germband elongation relieves stress in the ectoderm to allow the posterior midgut to efficiently internalize (Butler et al. 2009; Lye et al. 2015). Importantly, it has been shown that elongation of anterior–posterior oriented junctions, rather than shrinkage of myosin 2-containing dorsal–ventral oriented junctions, requires external pulling force from posterior midgut formation, suggesting that tissue elongation requires the combination of cell-scale forces that shrink dorsal–ventral oriented junctions and tissue-scale forces that promote elongation of anterior–posterior oriented junctions (Collinet et al. 2015).
Consequences of Apical Constriction: Invagination vs. Ingression
Apical constriction of cells in an epithelial monolayer is associated with cell and tissue shape changes (i.e., folding), as well as cell elimination or extrusion from the monolayer (Heer and Martin 2017; Fadul and Rosenblatt 2018). In contrast to the coordinated folding and invagination of the Drosophila mesoderm, the inward movement of individual cells or cell ingression is a feature of gastrulation in other animals (Lee et al. 2006; Wu and McClay 2007; Roh-Johnson et al. 2012; Williams et al. 2012). What determines whether cells completely constrict and are eliminated from the epithelium, or undergo coordinated, but incomplete, constriction and maintain tissue integrity? While the factors that determine the extent of apical constriction in different contexts are still unclear, several experiments have shed light on critical processes that promote tissue invagination.
Apical constriction initially leads to an apical–basal elongation of mesoderm cells (Sweeton et al. 1991; Gelbart et al. 2012). It has been suggested that constriction of the cell apex could be balanced by passive forces in the cell, such as hydrodynamic forces in the cytoplasm or by basal or lateral cortex tension. Consistent with the possibility that cytoplasmic pressure resists deformation and enables apical forces to be propagated basally, the volume of mesoderm cells remains more-or-less constant during apical constriction (Gelbart et al. 2012). Force from apical constriction induces hydrodynamic flows that lead to nuclear movement and apical–basal elongation (Gelbart et al. 2012; He et al. 2014). As mesoderm cells achieve a more wedge-shaped morphology they undergo basal expansion and apical–basal shortening, which is correlated with basal myosin 2 depletion (Polyakov et al. 2014). Indeed, ectopic myosin 2 activation after cell lengthening inhibits apical–basal cell shortening and tissue invagination, demonstrating a similarly important role for basal relaxation in cells achieving a final wedge shape and tissue invagination (Krueger et al. 2018).
Several mechanical models have been put forth to describe the process of mesoderm invagination, and these has been reviewed in detail by Rauzi and colleagues (Rauzi et al. 2013). A key feature of many of these models is that apical constriction shrinks the outer, apical surface relative to the inner, basal surface, which generates an inward curvature when the two surfaces are connected. In the simplest case, this principle can create curvature without accounting for other cell shape changes (Heer et al. 2017), although these other shape changes (e.g., apical–basal forces and forces from ectoderm cells) are likely to affect the speed or the extent of invagination (Conte et al. 2012; Perez-Mockus et al. 2017; Gracia et al. 2019). Consistent with the proposed key role for apical constriction, the patterned optogenetic activation of RhoA in the early embryo can induce ectopic invaginations (Izquierdo et al. 2018). While these ectopic invaginations do not reproduce the exact shape of the invaginated mesoderm, these experiments nevertheless illustrate that apical myosin 2 activation plays a key role in initiating cell invagination.
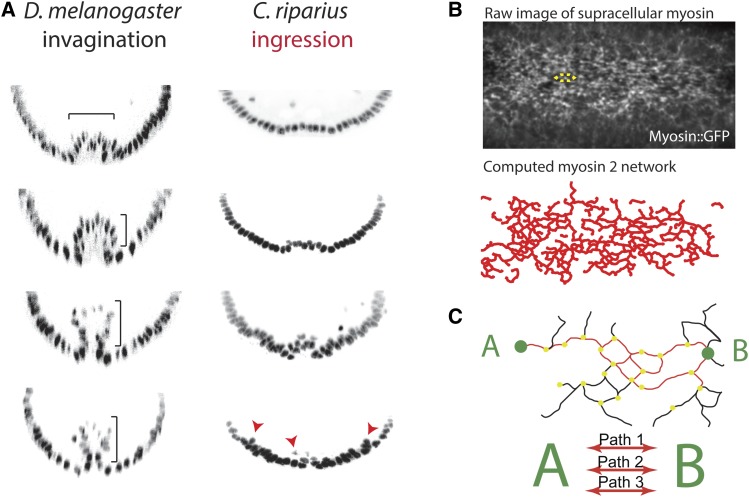
Insight into why apical constriction leads to invagination, rather than ingression, has also come from comparing gastrulation in Drosophila to that in other insects such as the midge, Chironomus riparius. C. riparius and D. melanogaster last shared a common ancestor ∼250 MYA and exhibit different modes of mesoderm internalization (Urbansky et al. 2016). D. melanogaster exhibits epithelial folding and invagination as discussed in this chapter (Leptin and Grunewald 1990; Sweeton et al. 1991). In contrast, C. riparius exhibits cell ingression during its gastrulation (Figure 8A). The presumptive mesoderm of both C. riparius and D. melanogaster express twist and snail, and these transcription factors are also required for mesoderm internalization of C. riparius (Urbansky et al. 2016). The main difference in mesoderm gene expression between these two species is that C. riparius fails to express fog and T48, which together activate sustained actomyosin contractility in Drosophila mesoderm cells (Figure 2B) (Urbansky et al. 2016). Interestingly, ectopic expression (i.e., not restricted to mesoderm) of either fog or T48 in C. riparius changes mesoderm internalization from ingression to invagination mode (Urbansky et al. 2016).
Figure 8.
Mechanisms that promote collective cell invagination. (A) Cross-section images of embryos with labeled nuclei comparing D. melanogaster and C. riparius mesoderm internalization. Bracket shows collective tissue invagination of Drosophila and arrows show individual cell ingression in C. riparius. Images are reproduced from Urbansky et al. (2016). (B) Image is a Z-projection of sqh::GFP showing the medioapical supracellular myosin 2 meshwork. The approximate size of an individual cell is highlighted by the yellow dotted line. A computed trace of the myosin 2 network is below the raw image. Images are from one of the data sets used in Yevick et al. (2019). (C) Cartoon illustrating structural redundancy of the supracellular myosin 2 network. Reproduced part of Graphical abstract from Yevick et al. (2019).
In contrast to C. riparius, Fog signaling plays a role in mesoderm internalization for the flour beetle, Tribolium castaneum. In T. castaneum, the homologs of twi, cta, fog, and mist are expressed in the mesoderm, similar to Drosophila (Handel et al. 2005; Benton et al. 2019). Furthermore, fog depletion in T. castaneum delays mesoderm internalization and T. castaneum mesoderm cells undergo pulsatile contractions, similar to Drosophila (Benton et al. 2019). Interestingly, Fog signaling in T. castaneum and several other insect species plays a role in cellularization, suggesting that this might be the ancestral function of the Fog pathway (Benton et al. 2019).
Is there an advantage for mesoderm invagination occurring collectively vs. stochastically? Urbansky and colleagues noted that Drosophila mesoderm invagination occurs faster than C. riparius mesoderm ingression, and suggested that invagination might provide a more robust mechanism for internalization (Urbansky et al. 2016). Indeed, recent work suggests that the network of actomyosin connections that forms across the Drosophila presumptive mesoderm tissue promotes the robustness of the invagination process in several ways (Figure 8B) (Yevick et al. 2019). First, redundancy in mechanical connections between cells ensures overall network connectivity and tissue function in the face of local or cell damage (Figure 8C). Second, directional connectivity and stiffening of the network along the anterior–posterior axis enables furrow formation at lower contractility levels and ensures proper furrow/fold orientation.
In addition to the expression of fog and T48, other mechanisms exist to prevent cell extrusion during invagination. During normal Drosophila mesoderm invagination, tissue integrity is maintained during folding, and is only lost after internalization when cells undergo epithelial-to-mesenchymal transition (EMT) (Figure 1B) (McMahon et al. 2008, 2010; Clark et al. 2011). Depletion of the Abelson nonreceptor tyrosine kinase (Abl) results in abnormal extrusion of mesoderm cells as they invaginate (Jodoin and Martin 2016). Abl regulates numerous cellular processes and components, including the cytoskeleton, where Abl negatively regulates an actin assembly factor, Enabled (Ena) (Gertler et al. 1990, 1995). In Drosophila blastoderm cells, Abl loss results in excessive apical microvilli at the expense of other actin structures (Grevengoed et al. 2003). Furthermore, Abl loss in mesoderm cells results in ectopic later F-actin (Fox and Peifer 2007). Cell extrusion and other mesoderm defects result from abnormal Ena activity because these phenotypes are suppressed by codepletion of Ena (Fox and Peifer 2007; Jodoin and Martin 2016). Cell extrusion that results from Abl depletion is accompanied by the disruption of apical–basal cell polarity, suggesting that cells undergo a premature EMT-like process (Jodoin and Martin 2016). Therefore, in Drosophila, it appears that mechanisms are in place to properly time EMT, and, thus, to maintain tissue integrity during mesoderm invagination.
Acknowledgments
The author thanks members of the Martin laboratory past and present for their contributions to the field and for their comments on the manuscript. The author thanks Ben-Zion Shilo and Matthew Benton for helpful discussions about their work. In addition, the author thanks the anonymous reviewers and the editors for their suggestions to improve the manuscript.
Footnotes
Communicating editor: T. Schüpbach
Literature Cited
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K. et al. , 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271: 20246–20249. 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- Anjum S. G., Xu W., Nikkholgh N., Basu S., Nie Y. et al. , 2013. Regulation of Toll signaling and inflammation by beta-arrestin and the SUMO protease Ulp1. Genetics 195: 1307–1317. 10.1534/genetics.113.157859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailles A., Collinet C., Philippe J. M., Lenne P. F., Munro E. et al. , 2019. Genetic induction and mechanochemical propagation of a morphogenetic wave. Nature 572: 467–473. 10.1038/s41586-019-1492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany M., 1967. ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50: 197–218. 10.1085/jgp.50.6.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A. J., and Schweisguth F., 2006. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell 10: 245–255. 10.1016/j.devcel.2005.12.017 [DOI] [PubMed] [Google Scholar]
- Barrett K., Leptin M., and Settleman J., 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91: 905–915. 10.1016/S0092-8674(00)80482-1 [DOI] [PubMed] [Google Scholar]
- Bement W. M., Leda M., Moe A. M., Kita A. M., Larson M. E. et al. , 2015. Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17: 1471–1483. 10.1038/ncb3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M. A., Frey N., Nunes da Fonseca R., von Levetzow C., Stappert D. et al. , 2019. Fog signaling has diverse roles in epithelial morphogenesis in insects. eLife 8:pii: e47346. 10.7554/eLife.47346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C., Sulak L., and Lecuit T., 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–671. 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]
- Bischof J., Brand C. A., Somogyi K., Majer I., Thome S. et al. , 2017. A cdk1 gradient guides surface contraction waves in oocytes. Nat. Commun. 8: 849 [corrigenda: Nat. Commun. 9: 200 (2018)]. 10.1038/s41467-017-00979-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard G. B., Murugesu S., Adams R. J., Martinez-Arias A., and Gorfinkiel N., 2010. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development 137: 2743–2752. 10.1242/dev.045872 [DOI] [PubMed] [Google Scholar]
- Butler L. C., Blanchard G. B., Kabla A. J., Lawrence N. J., Welchman D. P. et al. , 2009. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 11: 859–864. 10.1038/ncb1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G., and Welch M. D., 2010. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11: 237–251. 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai F., Xu W., Musoke T., Tarabelsi G., Assaad S. et al. , 2019. Structure-function analysis of beta-arrestin Kurtz reveals a critical role of receptor interactions in downregulation of GPCR signaling in vivo. Dev. Biol. 455: 409–419. 10.1016/j.ydbio.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet S., and Schweisguth F., 2012. Regulation of epithelial polarity by the E3 ubiquitin ligase Neuralized and the Bearded inhibitors in Drosophila. Nat. Cell Biol. 14: 467–476. 10.1038/ncb2481 [DOI] [PubMed] [Google Scholar]
- Chanet S., Miller C. J., Vaishnav E. D., Ermentrout B., Davidson L. A. et al. , 2017. Actomyosin meshwork mechanosensing enables tissue shape to orient cell force. Nat. Commun. 8: 15014 10.1038/ncomms15014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. J., and Morisato D., 2002. Regulation of Easter activity is required for shaping the Dorsal gradient in the Drosophila embryo. Development 129: 5635–5645. 10.1242/dev.00161 [DOI] [PubMed] [Google Scholar]
- Cho Y. S., Stevens L. M., Sieverman K. J., Nguyen J., and Stein D., 2012. A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity. Curr. Biol. 22: 1013–1018. 10.1016/j.cub.2012.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V. S., and Levine M., 2009. Combinatorial patterning mechanisms in the Drosophila embryo. Brief. Funct. Genomics Proteomics 8: 243–249. 10.1093/bfgp/elp026 [DOI] [PubMed] [Google Scholar]
- Clark I. B., Muha V., Klingseisen A., Leptin M., and Muller H. A., 2011. Fibroblast growth factor signalling controls successive cell behaviours during mesoderm layer formation in Drosophila. Development 138: 2705–2715. 10.1242/dev.060277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C., Rauzi M., Lenne P. F., and Lecuit T., 2015. Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 17: 1247–1258. 10.1038/ncb3226 [DOI] [PubMed] [Google Scholar]
- Conte V., Ulrich F., Baum B., Munoz J., Veldhuis J. et al. , 2012. A biomechanical analysis of ventral furrow formation in the Drosophila melanogaster embryo. PLoS One 7: e34473 10.1371/journal.pone.0034473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coravos J. S., and Martin A. C., 2016. Apical sarcomere-like actomyosin contracts nonmuscle Drosophila epithelial cells. Dev. Cell 39: 346–358. 10.1016/j.devcel.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Wilson E. T., and Wieschaus E., 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76: 1075–1089. 10.1016/0092-8674(94)90384-0 [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang R. E., Parmar K. M., Christiansen A. E., Phelps C. B., Brand A. H. et al. , 2005. Folded gastrulation, cell shape change and the control of myosin localization. Development 132: 4165–4178. 10.1242/dev.01938 [DOI] [PubMed] [Google Scholar]
- Denk-Lobnig M., and Martin A. C., 2019. Modular regulation of Rho family GTPases in development. Small GTPases 10: 122–129. 10.1080/21541248.2017.1294234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzis S., Yu J., Zinzen R., and Wieschaus E., 2006. Dorsal-ventral pattern of Delta trafficking is established by a Snail-Tom-Neuralized pathway. Dev. Cell 10: 257–264. 10.1016/j.devcel.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Edwards M., Zwolak A., Schafer D. A., Sept D., Dominguez R. et al. , 2014. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 15: 677–689 (erratum: Nat. Rev. Mol. Cell Biol 15: 677–689). 10.1038/nrm3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., and Hall A., 2002. Rho GTPases in cell biology. Nature 420: 629–635. 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- Fadul J., and Rosenblatt J., 2018. The forces and fates of extruding cells. Curr. Opin. Cell Biol. 54: 66–71. 10.1016/j.ceb.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. T., and Peifer M., 2007. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 134: 567–578. 10.1242/dev.02748 [DOI] [PubMed] [Google Scholar]
- Franke J. D., Montague R. A., and Kiehart D. P., 2005. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr. Biol. 15: 2208–2221. 10.1016/j.cub.2005.11.064 [DOI] [PubMed] [Google Scholar]
- Fukuhara S., Chikumi H., and Gutkind J. S., 2000. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 485: 183–188. 10.1016/S0014-5793(00)02224-9 [DOI] [PubMed] [Google Scholar]
- Furlong E. E., Andersen E. C., Null B., White K. P., and Scott M. P., 2001. Patterns of gene expression during Drosophila mesoderm development. Science 293: 1629–1633. 10.1126/science.1062660 [DOI] [PubMed] [Google Scholar]
- Fuse N., Yu F., and Hirose S., 2013. Gprk2 adjusts Fog signaling to organize cell movements in Drosophila gastrulation. Development 140: 4246–4255. 10.1242/dev.093625 [DOI] [PubMed] [Google Scholar]
- Galea G. L., Cho Y. J., Galea G., Mole M. A., Rolo A. et al. , 2017. Biomechanical coupling facilitates spinal neural tube closure in mouse embryos. Proc. Natl. Acad. Sci. USA 114: E5177–E5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Jiang J., and Ip Y. T., 2005. Drosophila WntD is a target and an inhibitor of the Dorsal/Twist/Snail network in the gastrulating embryo. Development 132: 3419–3429. 10.1242/dev.01903 [DOI] [PubMed] [Google Scholar]
- Gelbart M. A., He B., Martin A. C., Thiberge S. Y., Wieschaus E. F. et al. , 2012. Volume conservation principle involved in cell lengthening and nucleus movement during tissue morphogenesis. Proc. Natl. Acad. Sci. USA 109: 19298–19303. 10.1073/pnas.1205258109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler F. B., Doctor J. S., and Hoffmann F. M., 1990. Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science 248: 857–860. 10.1126/science.2188361 [DOI] [PubMed] [Google Scholar]
- Gertler F. B., Comer A. R., Juang J. L., Ahern S. M., Clark M. J. et al. , 1995. Enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 9: 521–533. 10.1101/gad.9.5.521 [DOI] [PubMed] [Google Scholar]
- Goode B. L., and Eck M. J., 2007. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76: 593–627. 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Dionne M. S., Schneider D. S., and Nusse R., 2005. WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature 437: 746–749. 10.1038/nature04073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia M., Theis S., Proag A., Gay G., Benassayag C. et al. , 2019. Mechanical impact of epithelial-mesenchymal transition on epithelial morphogenesis in Drosophila. Nat. Commun. 10: 2951 10.1038/s41467-019-10720-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed E. E., Fox D. T., Gates J., and Peifer M., 2003. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J. Cell Biol. 163: 1267–1279. 10.1083/jcb.200307026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans J., and Wieschaus E., 2000. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101: 523–531. 10.1016/S0092-8674(00)80862-4 [DOI] [PubMed] [Google Scholar]
- Grosshans J., Müller H. A., and Wieschaus E., 2003. Control of cleavage cycles in Drosophila embryos by fruhstart. Dev. Cell 5: 285–294. 10.1016/S1534-5807(03)00208-9 [DOI] [PubMed] [Google Scholar]
- Guglielmi G., and De Renzis S., 2017. Optogenetic inhibition of apical constriction during Drosophila embryonic development. Methods Cell Biol. 139: 167–186. 10.1016/bs.mcb.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Hacker U., and Perrimon N., 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12: 274–284. 10.1101/gad.12.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel K., Basal A., Fan X., and Roth S., 2005. Tribolium castaneum twist: gastrulation and mesoderm formation in a short-germ beetle. Dev. Genes Evol. 215: 13–31. 10.1007/s00427-004-0446-9 [DOI] [PubMed] [Google Scholar]
- Hannezo E., and Heisenberg C. P., 2019. Mechanochemical feedback loops in development and disease. Cell 178: 12–25. 10.1016/j.cell.2019.05.052 [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Kim D. R., Weiss L. A., Miller J. W., and Morisato D., 2003. Spatial regulation of developmental signaling by a serpin. Dev. Cell 5: 945–950. 10.1016/S1534-5807(03)00338-1 [DOI] [PubMed] [Google Scholar]
- Haskel-Ittah M., Ben-Zvi D., Branski-Arieli M., Schejter E. D., Shilo B. Z. et al. , 2012. Self-organized shuttling: generating sharp dorsoventral polarity in the early Drosophila embryo. Cell 150: 1016–1028. 10.1016/j.cell.2012.06.044 [DOI] [PubMed] [Google Scholar]
- He B., Doubrovinski K., Polyakov O., and Wieschaus E., 2014. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508: 392–396. 10.1038/nature13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heer N. C., and Martin A. C., 2017. Tension, contraction and tissue morphogenesis. Development 144: 4249–4260. 10.1242/dev.151282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heer N. C., Miller P. W., Chanet S., Stoop N., Dunkel J. et al. , 2017. Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 144: 1876–1886. 10.1242/dev.146761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg C. P., and Bellaiche Y., 2013. Forces in tissue morphogenesis and patterning. Cell 153: 948–962. 10.1016/j.cell.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Heissler S. M., and Sellers J. R., 2016. Various themes of myosin regulation. J. Mol. Biol. 428: 1927–1946. 10.1016/j.jmb.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem C. C., and Peifer M., 2008. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development 135: 1005–1018. 10.1242/dev.016337 [DOI] [PubMed] [Google Scholar]
- Honda H., 2017. The world of epithelial sheets. Dev. Growth Differ. 59: 306–316. 10.1111/dgd.12350 [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., and Levine M., 1992. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 6: 1518–1530. 10.1101/gad.6.8.1518 [DOI] [PubMed] [Google Scholar]
- Irvine K. D., and Wieschaus E., 1994. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120: 827–841. [DOI] [PubMed] [Google Scholar]
- Izquierdo E., Quinkler T., and De Renzis S., 2018. Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun. 9: 2366 10.1038/s41467-018-04754-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A. B., and Hall A., 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21: 247–269. 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- Jha A., van Zanten T. S., Philippe J. M., Mayor S., and Lecuit T.. 2018. Quantitative control of GPCR organization and signaling by endocytosis in epithelial morphogenesis. Curr. Biol. 28: 1570–1584.e6. 10.1016/j.cub.2018.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodoin J. N., and Martin A. C., 2016. Abl suppresses cell extrusion and intercalation during epithelium folding. Mol. Biol. Cell 27: 2822–2832. 10.1091/mbc.e16-05-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodoin J. N., Coravos J. S., Chanet S., Vasquez C. G., Tworoger M. et al. , 2015. Stable force balance between epithelial cells arises from F-actin turnover. Dev. Cell 35: 685–697. 10.1016/j.devcel.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P., and Karess R., 1997. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 139: 1805–1819. 10.1083/jcb.139.7.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza K. E., and Zallen J. A., 2011. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr. Opin. Cell Biol. 23: 30–38. 10.1016/j.ceb.2010.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge S., Munjal A., Philippe J. M., Jha A., de las Bayonas A. G. et al. , 2016. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat. Cell Biol. 18: 261–270. 10.1038/ncb3302 [DOI] [PubMed] [Google Scholar]
- Ko C. S., Tserunyan V., and Martin A. C., 2019. Microtubules promote intercellular contractile force transmission during tissue folding. J. Cell Biol. 218: 2726–2742. 10.1083/jcb.201902011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V., Seher T., Fernandez-Ballester G. J., Serrano L., and Leptin M., 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315: 384–386. 10.1126/science.1134833 [DOI] [PubMed] [Google Scholar]
- Krueger D., Tardivo P., Nguyen C., and De Renzis S., 2018. Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J. 37:pii: e100170. 10.15252/embj.2018100170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C., Deblandre G. A., Kintner C., and Rubin G. M., 2001. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1: 783–794. 10.1016/S1534-5807(01)00092-2 [DOI] [PubMed] [Google Scholar]
- Lecuit T., and Yap A. S., 2015. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17: 533–539. 10.1038/ncb3136 [DOI] [PubMed] [Google Scholar]
- Lecuit T., Lenne P. F., and Munro E., 2011. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 27: 157–184. 10.1146/annurev-cellbio-100109-104027 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., and Harland R. M., 2010. Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Curr. Biol. 20: 253–258. 10.1016/j.cub.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Marston D. J., Walston T., Hardin J., Halberstadt A. et al. , 2006. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr. Biol. 16: 1986–1997. 10.1016/j.cub.2006.08.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M., 1991. Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5: 1568–1576. 10.1101/gad.5.9.1568 [DOI] [PubMed] [Google Scholar]
- Leptin M., 2005. Gastrulation movements: the logic and the nuts and bolts. Dev. Cell 8: 305–320. 10.1016/j.devcel.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Leptin M., and Grunewald B., 1990. Cell shape changes during gastrulation in Drosophila. Development 110: 73–84. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P., Roth S., and Reichhart J. M., 2003. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr. Biol. 13: 2097–2102. 10.1016/j.cub.2003.10.062 [DOI] [PubMed] [Google Scholar]
- Lim B., Levine M., and Yamazaki Y., 2017. Transcriptional pre-patterning of Drosophila gastrulation. Curr. Biol. 27: 610 10.1016/j.cub.2017.01.067 [DOI] [PubMed] [Google Scholar]
- Lye C. M., Blanchard G. B., Naylor H. W., Muresan L., Huisken J. et al. , 2015. Mechanical coupling between endoderm invagination and axis extension in Drosophila. PLoS Biol. 13: e1002292 10.1371/journal.pbio.1002292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. J., Peters K. A., Peifer M., and Rogers S. L., 2013. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Sci. Signal. 6: ra98 10.1126/scisignal.2004427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L. A., Perez-Vale K. Z., Schaefer K. N., Sewell M. T., and Peifer M., 2019. The Drosophila Afadin and ZO-1 homologues Canoe and Polychaetoid act in parallel to maintain epithelial integrity when challenged by adherens junction remodeling. Mol. Biol. Cell 30: 1938–1960. 10.1091/mbc.E19-04-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston D. J., Higgins C. D., Peters K. A., Cupp T. D., Dickinson D. J. et al. , 2016. MRCK-1 drives apical constriction in C. elegans by linking developmental patterning to force generation. Curr. Biol. 26: 2079–2089. 10.1016/j.cub.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., and Goldstein B., 2014. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141: 1987–1998. 10.1242/dev.102228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Kaschube M., and Wieschaus E. F., 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457: 495–499. 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M., and Wieschaus E. F., 2010. Integration of contractile forces during tissue invagination. J. Cell Biol. 188: 735–749. 10.1083/jcb.200910099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F. M., Tworoger M., and Martin A. C., 2013. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 15: 926–936. 10.1038/ncb2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F. M., Xie S., Vasquez C. G., Tworoger M., and Martin A. C., 2016. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214: 603–617. 10.1083/jcb.201603077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J., Curado S., Ephrussi A., and Rorth P., 2000. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101: 511–522. 10.1016/S0092-8674(00)80861-2 [DOI] [PubMed] [Google Scholar]
- Mathew S. J., Rembold M., and Leptin M., 2011. Role for Traf4 in polarizing adherens junctions as a prerequisite for efficient cell shape changes. Mol. Cell. Biol. 31: 4978–4993. 10.1128/MCB.05542-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A., Supatto W., Fraser S. E., and Stathopoulos A., 2008. Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science 322: 1546–1550. 10.1126/science.1167094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A., Reeves G. T., Supatto W., and Stathopoulos A., 2010. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development 137: 2167–2175. 10.1242/dev.051573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Vanderleest T. E., Jewett C. E., Loerke D., and Blankenship J. T., 2019. Cell ratcheting through the Sbf RabGEF directs force balancing and stepped apical constriction. J. Cell Biol. 218: 3845–3860. 10.1083/jcb.201905082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux J. B., Robin F. B., McFadden W. M., and Munro E. M., 2018. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J. Cell Biol. 217: 4230–4252. 10.1083/jcb.201806161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrossilis D., Roper J. C., Le Roy D., Driquez B., Michel A. et al. , 2017. Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun. 8: 13883 10.1038/ncomms13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Amano M., Kaibuchi K., and Nishida Y., 1999. Identification and characterization of Drosophila homolog of Rho-kinase. Gene 238: 437–444. 10.1016/S0378-1119(99)00351-0 [DOI] [PubMed] [Google Scholar]
- Morize P., Christiansen A. E., Costa M., Parks S., and Wieschaus E., 1998. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 125: 589–597. [DOI] [PubMed] [Google Scholar]
- Moussian B., and Roth S., 2005. Dorsoventral axis formation in the Drosophila embryo–shaping and transducing a morphogen gradient. Curr. Biol. 15: R887–R899. 10.1016/j.cub.2005.10.026 [DOI] [PubMed] [Google Scholar]
- Munjal A., Philippe J. M., Munro E., and Lecuit T., 2015. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524: 351–355. 10.1038/nature14603 [DOI] [PubMed] [Google Scholar]
- Munro E., Nance J., and Priess J. R., 2004. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7: 413–424. 10.1016/j.devcel.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Münster S., Jain A., Mietke A., Pavlopoulos A., Grill S. W. et al. , 2019. Attachment of the blastoderm to the vitelline envelope affects gastrulation of insects. Nature 568: 395–399 (erratum: Nature 568: E14). 10.1038/s41586-019-1044-3 [DOI] [PubMed] [Google Scholar]
- Murrell M., Oakes P. W., Lenz M., and Gardel M. L., 2015. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16: 486–498. 10.1038/nrm4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S., and Wieschaus E., 1991. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell 64: 447–458. 10.1016/0092-8674(91)90652-F [DOI] [PubMed] [Google Scholar]
- Perez-Mockus G., Mazouni K., Roca V., Corradi G., Conte V. et al. , 2017. Spatial regulation of contractility by Neuralized and Bearded during furrow invagination in Drosophila. Nat. Commun. 8: 1594 10.1038/s41467-017-01482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Shook D. R., Chang C., Keller R., and Skoglund P., 2016. Molecular model for force production and transmission during vertebrate gastrulation. Development 143: 715–727. 10.1242/dev.128090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakov O., He B., Swan M., Shaevitz J. W., Kaschube M. et al. , 2014. Passive mechanical forces control cell-shape change during Drosophila ventral furrow formation. Biophys. J. 107: 998–1010. 10.1016/j.bpj.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille P. A., Ahmadi P., Brunet A. C., and Farge E., 2009. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2: ra16 10.1126/scisignal.2000098 [DOI] [PubMed] [Google Scholar]
- Quintin S., Gally C., and Labouesse M., 2008. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 24: 221–230. 10.1016/j.tig.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Rahimi N., Averbukh I., Haskel-Ittah M., Degani N., Schejter E. D. et al. , 2016. A WntD-dependent integral feedback loop attenuates variability in Drosophila Toll signaling. Dev. Cell 36: 401–414. 10.1016/j.devcel.2016.01.023 [DOI] [PubMed] [Google Scholar]
- Rahimi N., Averbukh I., Carmon S., Schejter E. D., Barkai N. et al. , 2019. Dynamics of Spaetzle morphogen shuttling in the Drosophila embryo shapes gastrulation patterning. Development 146: dev181487. 10.1242/dev.181487 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Lenne P. F., and Lecuit T., 2010. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468: 1110–1114. 10.1038/nature09566 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Hocevar Brezavscek A., Ziherl P., and Leptin M., 2013. Physical models of mesoderm invagination in Drosophila embryo. Biophys. J. 105: 3–10. 10.1016/j.bpj.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M., Krzic U., Saunders T. E., Krajnc M., Ziherl P. et al. , 2015. Embryo-scale tissue mechanics during Drosophila gastrulation movements. Nat. Commun. 6: 8677 10.1038/ncomms9677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold M., Ciglar L., Yanez-Cuna J. O., Zinzen R. P., Girardot C. et al. , 2014. A conserved role for Snail as a potentiator of active transcription. Genes Dev. 28: 167–181. 10.1101/gad.230953.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. L., Wiedemann U., Hacker U., Turck C., and Vale R. D., 2004. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14: 1827–1833. 10.1016/j.cub.2004.09.078 [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M., Shemer G., Higgins C. D., McClellan J. H., Werts A. D. et al. , 2012. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 335: 1232–1235. 10.1126/science.1217869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., 2003. The origin of dorsoventral polarity in Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 1317–1329, discussion 1329. 10.1098/rstb.2003.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., Stein D., and Nusslein-Volhard C., 1989. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59: 1189–1202. 10.1016/0092-8674(89)90774-5 [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Han K., Manley J. L., and Levine M., 1989. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59: 1165–1177. 10.1016/0092-8674(89)90772-1 [DOI] [PubMed] [Google Scholar]
- Sawyer J. K., Harris N. J., Slep K. C., Gaul U., and Peifer M., 2009. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J. Cell Biol. 186: 57–73. 10.1083/jcb.200904001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. M., Harrell J. R., Shemer G., Sullivan-Brown J., Roh-Johnson M. et al. , 2010. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341: 5–19. 10.1016/j.ydbio.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T., and Wieschaus E., 1989. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics 121: 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D., Zaritsky A., Schejter E. D., and Shilo B. Z., 2018. Feedback inhibition of actin on Rho mediates content release from large secretory vesicles. J. Cell Biol. 217: 1815–1826. 10.1083/jcb.201711006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seher T. C., and Leptin M., 2000. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 10: 623–629. 10.1016/S0960-9822(00)00502-9 [DOI] [PubMed] [Google Scholar]
- Seher T. C., Narasimha M., Vogelsang E., and Leptin M., 2007. Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev. 124: 167–179. 10.1016/j.mod.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Sen J., Goltz J. S., Stevens L., and Stein D., 1998. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell 95: 471–481. 10.1016/S0092-8674(00)81615-3 [DOI] [PubMed] [Google Scholar]
- Shen Q., Wu M. H., and Yuan S. Y., 2009. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet. 2009: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Haskel-Ittah M., Ben-Zvi D., Schejter E. D., and Barkai N., 2013. Creating gradients by morphogen shuttling. Trends Genet. 29: 339–347. 10.1016/j.tig.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Spahn P., and Reuter R., 2013. A vertex model of Drosophila ventral furrow formation. PLoS One 8: e75051 10.1371/journal.pone.0075051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. S., and Stevens L. M., 2014. Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip. Rev. Dev. Biol. 3: 301–330. 10.1002/wdev.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R., 1989. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59: 1179–1188. 10.1016/0092-8674(89)90773-3 [DOI] [PubMed] [Google Scholar]
- Steward R., Zusman S. B., Huang L. H., and Schedl P., 1988. The dorsal protein is distributed in a gradient in early Drosophila embryos. Cell 55: 487–495. 10.1016/0092-8674(88)90035-9 [DOI] [PubMed] [Google Scholar]
- Streichan S. J., Lefebvre M. F., Noll N., Wieschaus E. F., and Shraiman B. I., 2018. Global morphogenetic flow is accurately predicted by the spatial distribution of myosin motors. eLife 7: pii: e27454. 10.7554/eLife.27454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Amourda C., Shagirov M., Hara Y., Saunders T. E. et al. , 2017. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol. 19: 375–383 [corrigenda: Nat. Cell Biol. 20: 503 (2018)]. 10.1038/ncb3497 [DOI] [PubMed] [Google Scholar]
- Sweeton D., Parks S., Costa M., and Wieschaus E., 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112: 775–789. [DOI] [PubMed] [Google Scholar]
- Tang V. W., and Brieher W. M., 2012. alpha-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J. Cell Biol. 196: 115–130. 10.1083/jcb.201103116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., and Hartenstein V., 1994. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161: 563–596. 10.1006/dbio.1994.1054 [DOI] [PubMed] [Google Scholar]
- Urbansky S., González Avalos P., Wosch M., and Lemke S., 2016. Folded gastrulation and T48 drive the evolution of coordinated mesoderm internalization in flies. eLife 5: pii: e18318. 10.7554/eLife.18318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C. G., and Martin A. C., 2016. Force transmission in epithelial tissues. Dev. Dyn. 245: 361–371. 10.1002/dvdy.24384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C. G., Tworoger M., and Martin A. C., 2014. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J. Cell Biol. 206: 435–450. 10.1083/jcb.201402004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C. G., Heissler S. M., Billington N., Sellers J. R., and Martin A. C., 2016. Drosophila non-muscle myosin II motor activity determines the rate of tissue folding. eLife 5: pii: e20828. 10.7554/eLife.20828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Han S. P., Michael M., Gomez G. A., Yang Z. et al. , 2012. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol. Biol. Cell 23: 4601–4610. 10.1091/mbc.e12-08-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M., and Wieschaus E., 2016. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J. Cell Biol. 212: 219–229. 10.1083/jcb.201508056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M., and Wieschaus E., 2017. Polarity protein Par3/Bazooka follows myosin-dependent junction repositioning. Dev. Biol. 422: 125–134. 10.1016/j.ydbio.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Burdsal C., Periasamy A., Lewandoski M., and Sutherland A., 2012. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn. 241: 270–283. 10.1002/dvdy.23711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R. et al. , 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. 10.1016/S0092-8674(01)00298-7 [DOI] [PubMed] [Google Scholar]
- Wu S. Y., and McClay D. R., 2007. The Snail repressor is required for PMC ingression in the sea urchin embryo. Development 134: 1061–1070. 10.1242/dev.02805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., and Martin A. C., 2015. Intracellular signalling and intercellular coupling coordinate heterogeneous contractile events to facilitate tissue folding. Nat. Commun. 6: 7161 10.1038/ncomms8161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Mason F. M., and Martin A. C., 2016. Loss of Galpha12/13 exacerbates apical area dependence of actomyosin contractility. Mol. Biol. Cell 27: 3526–3536. 10.1091/mbc.e16-05-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A. S., Gomez G. A., and Parton R. G., 2015. Adherens junctions revisualized: organizing cadherins as nanoassemblies. Dev. Cell 35: 12–20. 10.1016/j.devcel.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Yeh E., Dermer M., Commisso C., Zhou L., McGlade C. J. et al. , 2001. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr. Biol. 11: 1675–1679. 10.1016/S0960-9822(01)00527-9 [DOI] [PubMed] [Google Scholar]
- Yevick H. G., Miller P. W., Dunkel J., and Martin A. C., 2019. Structural redundancy in supracellular actomyosin networks enables robust tissue folding. Dev. Cell 50: 586–598.e3. 10.1016/j.devcel.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A., and Wieschaus E., 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6: 343–355. 10.1016/S1534-5807(04)00060-7 [DOI] [PubMed] [Google Scholar]