Kinesin-13 and Kinesin-8 regulate microtubule dynamics during mitotic spindle formation and straight tip cell growth in the moss Physcomitrella patens.
Abstract
Kinesin-13 and Kinesin-8 are well-known microtubule (MT) depolymerases that regulate MT length and chromosome movement in animal mitosis. While much is unknown about plant Kinesin-8, Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa) Kinesin-13 have been shown to depolymerize MTs in vitro. However, the mitotic function of both kinesins has yet to be determined in plants. Here, we generated complete null mutants of Kinesin-13 and Kinesin-8 in moss (Physcomitrella patens). Both kinesins were found to be nonessential for viability, but the Kinesin-13 knockout (KO) line had increased mitotic duration and reduced spindle length, whereas the Kinesin-8 KO line did not display obvious mitotic defects. Surprisingly, spindle MT poleward flux, which is mediated by Kinesin-13 in animals, was retained in the absence of Kinesin-13. MT depolymerase activity was not detectable for either kinesin in vitro, while MT catastrophe-inducing activity (Kinesin-13) or MT gliding activity (Kinesin-8) was observed. Interestingly, both KO lines showed waviness in their protonema filaments, which correlated with positional instability of the MT foci in their tip cells. Taken together, the results suggest that plant Kinesin-13 and Kinesin-8 have diverged in both mitotic function and molecular activity, acquiring roles in regulating MT foci positioning for directed tip growth.
INTRODUCTION
Microtubule (MT)-based motor proteins, kinesins, form a large superfamily in animal and plant species (61 genes in Arabidopsis [Arabidopsis thaliana], 78 in Physcomitrella patens, 45 in Homo sapiens, and 25 in Drosophila melanogaster; Reddy and Day, 2001; Miki et al., 2005; Shen et al., 2012). Kinesins show various activities in association with MTs and play pivotal roles in eukaryotic cells, such as cargo transport, MT organization, MT dynamics regulation, and force generation (Walczak and Heald, 2008; Hirokawa et al., 2009). Comprehensive functional analyses in several animal model systems, such as fly and human cell lines, frog egg extracts, and mouse, together with biochemical characterization of each kinesin motor have provided insights into how MT-based intracellular processes are driven and regulated during cell proliferation, differentiation, and animal development. By contrast, the cellular and developmental functions of plant kinesins are less clear, partly due to the difficulty in making and characterizing the phenotypes of complete knockout (KO) lines of paralogous kinesins that likely function redundantly. The use of high-resolution live microscopy, which was particularly critical for assessing kinesin functions during mitosis in animals, has also been limited in plants.
Within the kinesin superfamily, Kinesin-13 and Kinesin-8 commonly show a unique activity in vitro: MT depolymerization (Desai et al., 1999; Howard and Hyman, 2007; Walczak et al., 2013). In vivo, Kinesin-13 is able to depolymerize relatively stable MTs from both ends, while MT catastrophe-inducing activity is limited to the plus end (Rogers et al., 2004; Mennella et al., 2005). The best-studied Kinesin-13, human KIF2C/MCAK, accumulates at MT ends either by diffusion or through recruitment by other MT-associated proteins (Lee et al., 2008). At the ends, it binds to and stabilizes protofilament bends, which promotes strain on the association between protofilaments within the MT lattice (Moores et al., 2002; Ovechkina et al., 2002; Ogawa et al., 2017). In the mitotic spindle, KIF2C/MCAK is localized to the kinetochore and likely triggers depolymerization of MT plus ends that are erroneously attached to the kinetochore (Kline-Smith et al., 2004; Walczak et al., 2013). Another Kinesin-13 (human KIF2A, fly KLP10A) localizes at the pole region and depolymerizes MTs, including relatively stable kinetochore-bound MTs, from the minus end, driving poleward movement of MTs (called spindle MT poleward flux) and chromosome segregation at anaphase (Rogers et al., 2004; Ganem et al., 2005; Walczak et al., 2013). Depletion of Kinesin-13 causes various mitotic errors, such as spindle elongation, spindle monopolarization, erroneous kinetochore–MT attachment, and chromosome lagging at anaphase (Walczak et al., 2013).
While Kinesin-13 does not show motility on MTs, Kinesin-8 possesses both MT-depolymerizing activity and processive plus end-directed motility and thus preferentially destabilizes MT plus ends (Howard and Hyman, 2007; Walczak et al., 2013). During mitosis, Kinesin-8 concentrates at the outer kinetochore region and prevents excessive elongation of kinetochore MTs and stabilizes this kinetochore–MT attachment to promote chromosome alignment to the spindle equator (Mayr et al., 2007; Stumpff et al., 2008, 2012; Edzuka and Goshima, 2019). As a whole, Kinesin-13 and Kinesin-8 MT depolymerization activity is generally required for proper MT length regulation and correct chromosome movement during mitosis of various animal cell types (Walczak et al., 2013). This mitotic activity extends to cytokinesis, where Kinesin-13 and Kinesin-8 also control anaphase spindle length and bundling, respectively (Gatt et al., 2005; Uehara et al., 2013). Kinesin-13 and Kinesin-8 are also repurposed for interphase, where mouse KIF2A (Kinesin-13) suppresses excessive axonal outgrowths (Homma et al., 2003) and KIF24 (Kinesin-13) and KIF19 (Kinesin-8) have roles in regulating primary cilia formation and cilia length, respectively (Kobayashi et al., 2011; Niwa et al., 2012).
Despite protein conservation, the function and activity of Kinesin-13 and Kinesin-8 are not fully understood in plants. Neither mutant phenotypes nor biochemical activity has been reported for Kinesin-8. By contrast, rice (Oryza sativa) and Arabidopsis Kinesin-13s have been shown to preserve some degree of MT depolymerization activity in vitro and in vivo (Oda and Fukuda, 2013; Deng et al., 2015). In Arabidopsis xylem vessel elements, Kinesin-13A is essential to create MT-deficient areas in the cortical MT network that is utilized as a scaffold for cellulose synthase movement. Cellulose is deposited only at areas with patterned MTs, thus creating cellulose-lacking regions in MT-deficient areas, called pits, allowing for lateral transport of solutes and liquids in the plant. Knockdown of Kinesin-13A by RNA interference results in loss of MT patterning and smaller secondary cell wall pit formation (Oda and Fukuda, 2013). Rice Kinesin-13A was shown to be important in regulating MT dynamicity and organization of the cortical MT network in a variety of cell types (Deng et al., 2015). However, the potency of the depolymerization activity is uncertain, since plant Kinesin-13 lacks a domain required for the robust activity of animal Kinesin-13 (Figure 1A; Ovechkina et al., 2002; Lu et al., 2005) and because overexpression of Kinesin-13A in nonxylem cells did not depolymerize MTs unless coexpressed with an additional binding partner, MIDD1 (Oda and Fukuda, 2013). Conversely, Kinesin-13’s function during mitosis is unknown, as the Kinesin-13A mutants in Arabidopsis and rice did not show mitotic defects. Moreover, Arabidopsis Kinesin-13s have been suggested to be functionally redundant as complete null mutants were embryonic lethal (Fujikura et al., 2014).
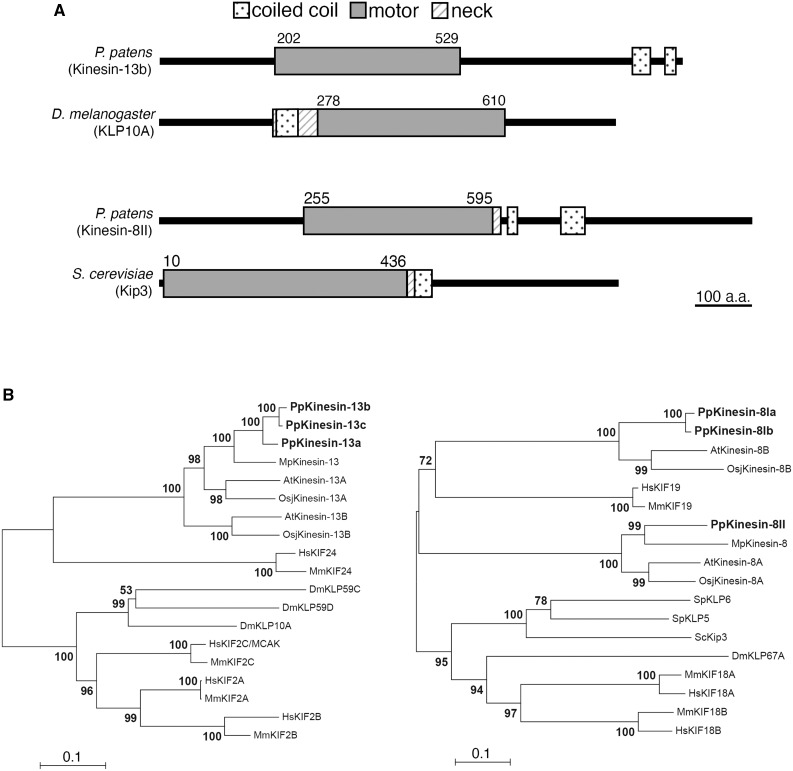
Figure 1.
Conservation of Kinesin-13 and Kinesin-8 in the Moss P. patens.
(A) Protein domains of Kinesin-13 (represented with Kinesin-13b) and Kinesin-8 (represented with Kinesin-8II) of moss, compared with D. melanogaster KLP10A/Kinesin-13 and S. cerevisiae Kip3/Kinesin-8. Domains of Drosophila and budding yeast proteins were obtained from UniProt, whereas moss protein domains were predicted using InterPro.
(B) Kinesin-13 and Kinesin-8 phylogeny across the moss P. patens, the Brassica Arabidopsis, the liverwort Marchantia polymorpha, the rice O. sativa subsp japonica, the fruitfly D. melanogaster, mammalians Mus muculus and H. sapiens, and also for Kinesin-8 in the budding yeast S. cerevisiae and fission yeast Schizosaccharomyces pombe. After amino acid sequences were aligned with MAFFT, gapped regions were removed manually using MacClade. The phylogenetic tree was constructed using neighbor-joining methods using MEGA software. Reliability was assessed with 1000 bootstrapping trials. Protein sequences are presented in Supplemental Data Set 1. Horizontal branch length is proportional to the estimated evolutionary distance. Bar = 0.1 amino acid substitution per site.
In this study, moss (Physcomitrella patens), a model basal plant system, was used to investigate Kinesin-13 and Kinesin-8 function in general cellular processes, such as cell division. Using homologous recombination and clustered regularly interspaced short palindromic repeats (CRISPR) gene editing techniques, all three paralogues of Kinesin-13 and Kinesin-8 were knocked out, generating viable complete null mutants for each of the kinesin subfamilies. We demonstrated that Kinesin-13 has a mitotic role in plants, with the Kinesin-13 triple KO line having a longer prometaphase duration. However, spindle MT flux was still observed and shorter metaphase spindles than the control were formed in the KO lines. By contrast, the Kinesin-8 triple KO line did not display mitotic phenotypes. Unexpectedly, neither kinesin was shown to actively depolymerize MTs in vitro; the Kinesin-13 motor domain was able to induce MT catastrophe, while the gliding activity of the Kinesin-8 motor domain was confirmed. Notably, both KO lines had wavy protonema filaments, which associated with the MT foci abnormally fluctuating at the cell tip. Taken together, functional analyses of Kinesin-13 and Kinesin-8 KO in moss revealed a divergence in mitotic function and molecular activity, while revealing a novel role in regulating MT positioning for directed tip growth.
RESULTS
Kinesin-13 Affects Protonema Growth, but Not Gametophore Morphology
To investigate Kinesin-13’s role in P. patens, all three paralogous Kinesin-13 genes (Kinesin-13a, Kinesin-13b, and Kinesin-13c; Figure 1B; Supplemental Data Set 1) were sequentially deleted by homologous recombination-mediated gene replacement in the moss lines expressing GFP-tubulin and histoneH2B-monomeric red fluorescent protein (mRFP; Supplemental Figures 1A and 1B). Kinesin-13 single and double KO moss colonies did not have observable developmental defects. Moreover, Kinesin-13 triple KO lines (hereafter Kinesin-13 KO) were successfully generated, indicating that Kinesin-13 genes are not essential in moss. There was an overall reduction in colony size in the Kinesin-13 KO compared to the control (Figures 2A and 2B). However, the overall morphology of the protonema colonies, gametophore (leafy shoots encasing gametangia), and rhizoids (root-like filamentous cells differentiated from gametophore basal cells; Cove, 2005; Menand et al., 2007; Kofuji and Hasebe, 2014) were indistinguishable from the control (Figures 2A to 2C), which differs from the case of the rice Kinesin-13A mutant, which has small and round grains with shortened panicles and internodes of the whole rice plant (Kitagawa et al., 2010).
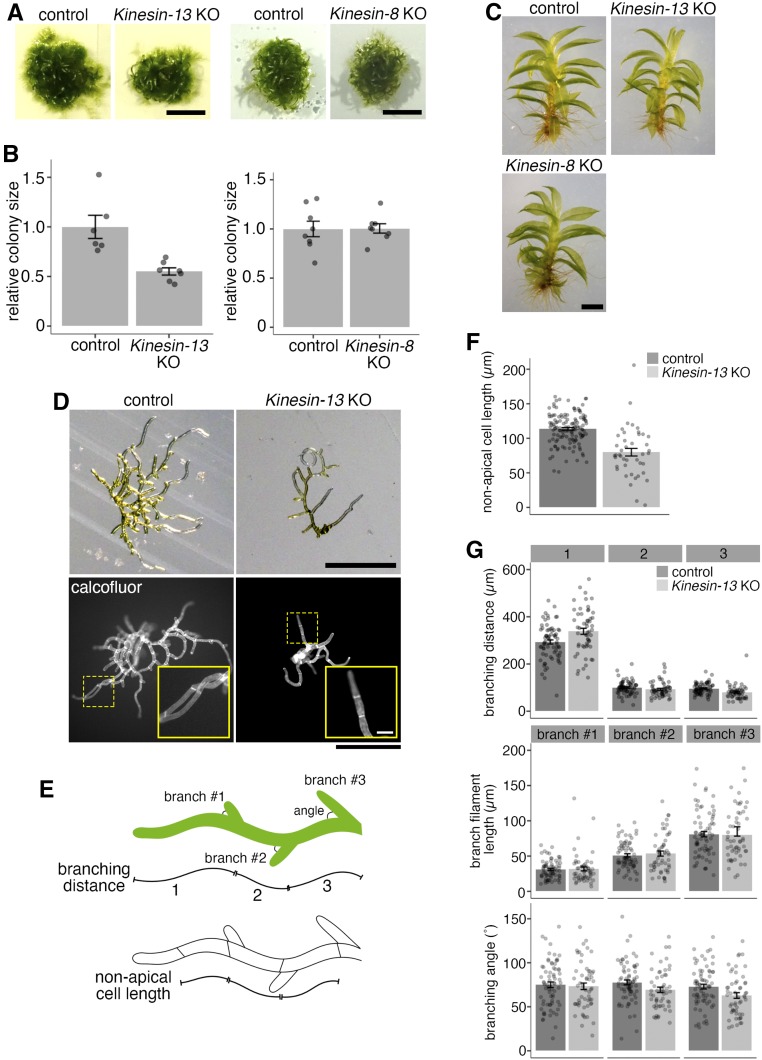
Figure 2.
Kinesin-13 and Kinesin-8 KO Mosses Are Morphologically Normal, but Kinesin-13 KO Moss Shows Retarded Growth and Reduced Cell Length.
(A) and (B) Colony size comparison between control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30, left) or Kinesin-8 KO (GPH0433#9, right) moss. Colonies were cultured from single protoplasts for 27 to 28 d on BCDAT. At least two independent experiments each with at least two plates of colonies were performed. The average colony area for each line on each plate was obtained. Actual areas were then divided by the average area of the control sample to get relative colony size. In the Kinesin-13 KO experiment, KO moss had a relative size of 0.55 ± 0.04 (mean ± se; n = 7), whereas control moss had a relative size of 1.00 ± 0.12 (mean ± se; n = 6). In the Kinesin-8 KO experiment, KO moss had a relative size of 1.01 ± 0.05 (mean ± se; n = 8), whereas control moss had a relative size of 1.00 ± 0.08 (mean ± se; n = 8). Points represent individual colonies; results are from one of at least two independent experiments. Bar = 5 mm.
(C) Gametophore and rhizoids of control moss (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#6) or Kinesin-8 KO (GPH0433#7) moss. Gametophores were isolated from colonies from small colony subcultures cultured on BCDAT for 27 to 28 d. Bar = 1 mm.
(D) Day-8 moss colonies cultured from protoplast of control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30) under bright-field light (top) and with calcofluor staining (bottom). Yellow dashed boxes, inset region. Bars = 500 µm; inset bar = 50 µm.
(E) Cartoon depicting the measurements taken for nonapical cell length in (F) and branching phenotype analysis in (G).
(F) Nonapical cell lengths of caulonema filaments were measured using calcofluor-stained colonies as in (D, bottom) for control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30). Nonapical cell length was reduced in Kinesin-13 KO moss to 79.9 ± 5.5 µm (mean ± se; n = 43), compared with control moss of 113.7 ± 1.9 µm (mean ± se; n = 132). Points represent individual cells; results are pooled from two independent experiments where two independent lines were analyzed.
(G) Branching phenotype analysis of control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30). In particular, branching distance of the first branch site to cell tip (top graph, leftmost bars) was increased in Kinesin-13 KO moss to 338.4 ± 12.9 µm (mean ± se; n = 55), compared with control moss of 293.1 ± 8.8 µm (mean ± se; n = 71). Points represent individual filaments; results are pooled from two independent experiments where two independent lines were analyzed.
To further investigate the colony growth phenotype in Kinesin-13 KO moss, early-stage moss colonies regenerated from single protoplasts cultured for 8 d were analyzed for nonapical cell length and protonema filament branching pattern (Figures 2D to 2G). Nonapical cells, which undergo little cell expansion after cell division, were found to be shorter in the Kinesin-13 KO moss caulonema cells (Figures 2E and 2F), consistent with reduced cell length in rice Kinesin-13A mutants (Deng et al., 2015). The branching pattern was analyzed by measuring the parameters of branching distance (distances from the tip of the protonema filament to the first three branching sites), branch filament length, and branch angle (Figure 2E). While the first branching distance (distance from the tip of the protonema filament to the nearest branching site) increased in the Kinesin-13 KO line, other branching pattern parameters were not observably different from that of the control (Figure 2G).
Kinesin-13 Facilitates Spindle MT Organization and Chromosome Alignment, but Does Not Drive Spindle MT Flux
The protonema tissue propagates by concerted asymmetric cell division and tip growth in the apical stem cells (Rounds and Bezanilla, 2013). Therefore, a reduction in colony size in the Kinesin-13 KO moss could be attributed to a defect in either or both events. To study mitosis in the Kinesin-13 KO moss, localization of moss Kinesin-13s to the mitotic spindle was first confirmed. As previously reported (Miki et al., 2014), moss Kinesin-13s showed spindle localization, most enriched at the spindle equator, with the level of expression varying among the three paralogues; they did not show punctate signals at the spindle pole or kinetochore like animal Kinesin-13 (Supplemental Figure 2A). Next, time-lapse imaging of moss protonema cells revealed that MT-dependent nuclear movement in prophase was abnormal in the Kinesin-13 KO line. In the control, nuclear movement is minimal or mildly apically directed as cells undergo nuclear envelope breakdown (NEBD). By contrast, in the KO line, the nucleus displayed severe retrograde movement leading up into NEBD and often continued moving basally even during spindle establishment (Figures 3A and 3B). This retrograde nuclear movement was also observed in the Kinesin-13ac double KO lines to a lesser degree, but not in the single or Kinesin-13ab double KO lines (Figure 3B). Additionally, overexpression of Kinesin-13b(full-length)-Cerulean under the EF1α promoter complemented the retrograde nuclear movement (Figures 3C and 3D). However, mutant Kinesin-13b constructs in which motor activity (Kinesin-13bRIG-Cerulean; Dawson et al., 2007), conserved MT depolymerization motifs (Kinesin-13bKVD/KEC-Cerulean; Shipley et al., 2004), and a conserved MT binding domain (Kinesin-13bLoop12-Cerulean; Soppina and Verhey, 2014) were compromised could not restore the retrograde nuclear movement (Figure 3D). Overall, these results suggest that Kinesin-13s contribute to nuclear movement redundantly in a motor-dependent manner.
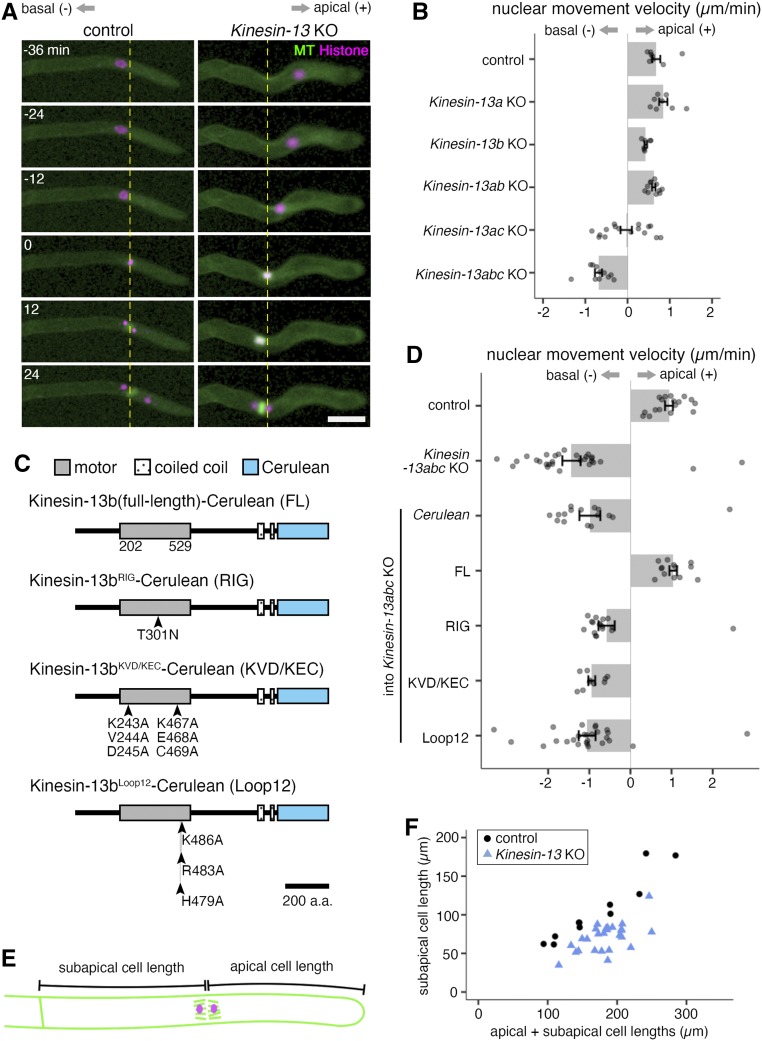
Figure 3.
Kinesin-13abc Triple KO Shows Retrograde Nuclear Movement, While Kinesin-13ac Double KO Shows Both Retrograde and Anterograde Nuclear Movement as Cells Enter Mitosis.
(A) Snapshots of control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30) moss during prophase nuclear/spindle movement. Kinesin-13 KO moss shows retrograde nuclear/spindle movement. Apical side, right, positive value for analysis in (B) and (D); basal side, left, negative values for analysis in (B) and (D); yellow dotted line, nucleus position at NEBD. NEBD, 0 min. Bar = 50 µm.
(B) Nuclear movement velocity during prophase of control (GFP-tubulin/histoneH2B-mRFP; 0.68 ± 0.10 µm/min, mean ± se; n = 8), Kinesin-13a single KO (GPH0411#43; 0.85 ± 0.10 µm/min, mean ± se; n = 8), Kinesin-13b single KO (GPH0412#11; 0.43 ± 0.03 µm/min, mean ± se; n = 7), Kinesin-13ab double KO (GPH0419#33; 0.62 ± 0.04 µm/min, mean ± se; n = 11), Kinesin-13ac double KO (GPH0420#125; –0.03 ± 0.13 µm/min, mean ± se; n = 16), and Kinesin-13abc triple KO (GPH0438#30; –0.69 ± 0.08 µm/min, mean ± se; n = 11). Kinesin-13abc triple KO shows a clear retrograde nuclear movement, whereas Kinesin-13ac double KO shows intermediate retrograde nuclear movement. Apically directed/anterograde movement, positive values; basally directed/retrograde movement, negative values. Points represent individual mitotic events. Results are from one of three independent experiments where two independent lines were analyzed.
(C) Protein domains of Kinesin-13b and mutant proteins for rescue experiments. Point mutations on Kinesin-13b-Cerulean, which was introduced under the EF1α promoter at the PTA1 site in the moss used for rescue experiments are illustrated. a.a., amino acids.
(D) Nuclear movement velocity during prophase of control (GFP-tubulin/histoneH2B-mRFP; 0.94 ± 0.10 µm/min, mean ± se; n = 17), Kinesin-13abc triple KO (GPH0438#30; –1.43 ± 0.22 µm/min, mean ± se; n = 29), Cerulean/Kinesin-13abc triple KO (GPH0903#1; –0.99 ± 0.25 µm/min, mean ± se; n = 16), Kinesin-13b(FL)-Cerulean/Kinesin-13abc triple KO (GPH0899#10; 1.04 ± 0.09 µm/min, mean ± se; n = 13), Kinesin-13bRIG-Cerulean/Kinesin-13abc triple KO (GPH0902#2; –0.58 ± 0.20 µm/min, mean ± se; n = 17), Kinesin-13bKVD/KEC-Cerulean/Kinesin-13abc triple KO (GPH0900#4; –0.94 ± 0.08 µm/min, mean ± se; n = 10), and Kinesin-13bLoop2-Cerulean/Kinesin-13abc triple KO (GPH0901#1; –1.05 ± 0.20 µm/min, mean ± se; n = 27). Apically directed movement, positive values; basally directed movement, negative values. Points represent individual mitotic events. Results are from one of two independent experiments where at least two independent lines were analyzed.
(E) Cartoon depicting how subapical and apical cell lengths were measured for (F).
(F) Subapical cell length was reduced in the Kinesin-13 KO line (GPH0438#30; 70.9 ± 3.6 µm [mean ± se; n = 26; P-value < 0.05, Welch’s two-sample t test]) compared with the control (GFP-tubulin/histoneH2B-mRFP; 105.2 ± 12.4 µm [mean ± se; n = 11]). Each point represents individual mitotic events. Results shown are from one of two independent experiments where two independent lines were analyzed.
The severe retrograde nuclear/spindle movement during prophase likely resulted in cross cell wall–positioning defects in the Kinesin-13 KO moss. Indeed, analysis of subapical and apical cell length at anaphase onset showed that subapical cell length was reduced in the Kinesin-13 KO moss (Figures 3E and 3F). This is associated with a reduction in nonapical cell length of early-stage moss colonies and suggests that moss Kinesin-13 has a role in cell length maintenance.
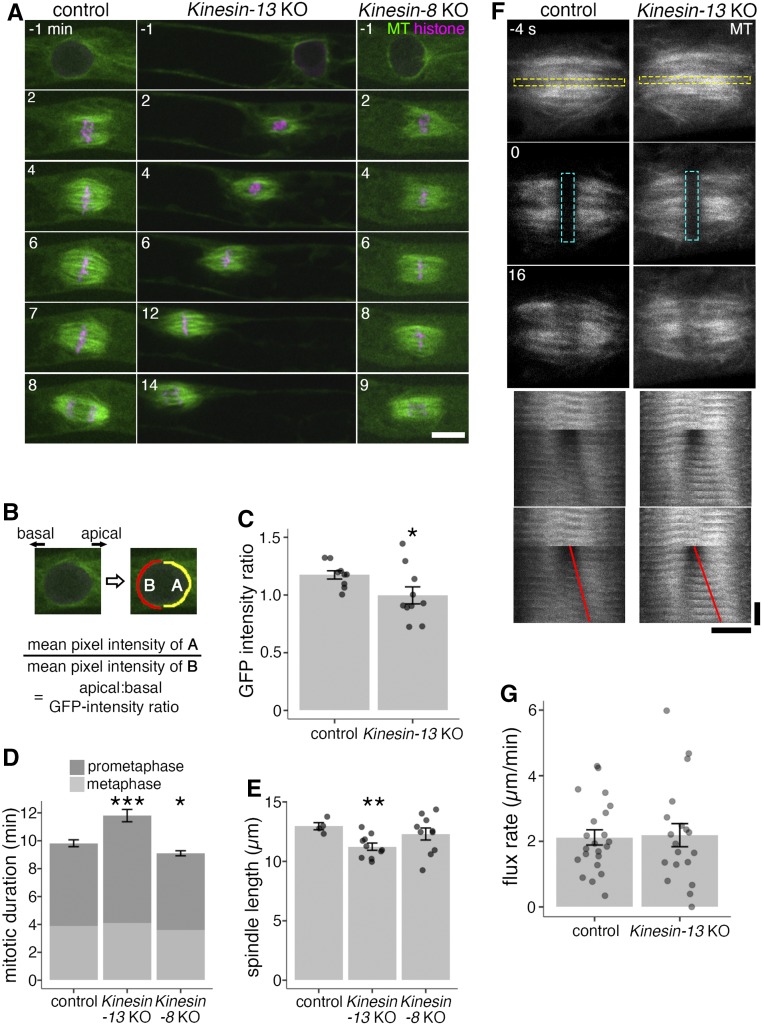
Consistent with the retrograde nuclear/spindle movement, high-resolution time-lapse imaging showed that Kinesin-13 KO moss also has a disparity of the nucleus-surrounding MT array during prophase (Figure 4A; Supplemental Movie 1; Supplemental Movie Legends). In the control, shortly before NEBD, MTs associated asymmetrically to the nucleus, with more MTs gathering on the apical side (Doonan et al., 1985; Nakaoka et al., 2012). By contrast, this apically directed MT asymmetry was altered in the KO line, with the GFP-tubulin intensity ratio of apical-to-basal hemispheres of the nucleus decreasing from ∼1.2 in the control to ∼1.0 (Figures 4B and 4C), suggesting that Kinesin-13s are important for MT organization during prophase.
Figure 4.
Kinesin-13 KO Moss Shows Defects in Nuclear-Proximal MT Array, Mitotic Duration, and Spindle Length, but Shows No Difference in Spindle Flux Rate.
(A) Mitosis of control (GFP-tubulin/histoneH2B-mRFP), Kinesin-13 KO (GPH0438#6), and Kinesin-8 KO (GPH0433#9) moss. Kinesin-13 KO showed reduced metaphase spindle length, retrograde nuclear movement during prophase, increased mitotic duration, and loss of apical bias of nuclear MTs. Kinesin-8 KO did not show mitotic defects. NEBD, 0 min; left, basal side; right, apical side. Bar = 10 μm.
(B) and (C) Apical:basal GFP-intensity ratio of GFP-tubulin around the nucleus 1 min before NEBD was measured as the ratio of GFP-tubulin intensities between apical and basal hemispheric circumference. In (C), control (GFP-tubulin/histoneH2B-mRFP), 1.17 ± 0.04 (mean ± se; n = 9; P-value < 0.05, Welch’s two sample t test); Kinesin-13 KO (GPH0438#6, 30), 1.00 ± 0.07 (mean ± se; n = 10). Points represent individual mitotic events.
(D) Mitotic duration of control (GFP-tubulin/histoneH2B-mRFP), Kinesin-13 KO (GPH0438#6, 30), and Kinesin-8 KO (GPH0433#7, 9) moss as measured from NEBD to anaphase onset. Control, 9.8 ± 0.3 min (mean ± se; n = 11); Kinesin-13 KO, 11.8 ± 0.4 min (mean ± se, n = 15; P-value < 0.001, Welch’s two sample t test); Kinesin-8 KO, 9.1 ± 0.2 min (mean ± se; n = 10; P-value < 0.05, Welch’s two sample t test). The duration of prometaphase (from NEBD to chromosome alignment) and metaphase (chromosome alignment to anaphase onset) was also measured and shown. Data shown were pooled from two independent experiments.
(E) Spindle length was measured at metaphase (defined as 1 min before anaphase onset) by obtaining the distance between midpoints of apical and basal spindle widths. Control (GFP-tubulin/histoneH2B-mRFP), 13.0 ± 0.3 µm (mean ± se; n = 4); Kinesin-13 KO (GPH0438#30), 11.2 ± 0.3 µm (mean ± se; n = 10; P-value < 0.01, Welch’s two-sample t test); Kinesin-8 KO (GPH0433#9), 12.3 ± 0.5 µm (mean ± se; n = 10). Points represent individual mitotic events.
(F) Spindle poleward flux of control (GFP-tubulin/histoneH2B-mRFP) and Kinesin-13 KO (GPH0438#30) moss was examined in photobleaching experiments where GFP-tubulin signals on a strip along the metaphase plate was bleached. The bleached regions separating toward the poles are indicative of spindle poleward flux function. Yellow dashed rectangle in the top panel indicates region used to make time series (bottom panel); cyan dashed rectangle represents bleached region; red lines indicate the segmented lines drawn on the kymograph to obtain the flux rate in (G). Horizontal bar = 5 μm; vertical bar = 12 s.
(G) Quantification of spindle poleward flux experiment as shown in (F). Control, 2.1 ± 0.2 µm/min (mean ± se; n = 22); Kinesin-13 KO, 2.2 ± 0.4 µm/min (mean ± se; n = 19). Points represent individual mitotic events, shown are results from four independent experiments.
Upon NEBD, MTs assemble into a bipolar spindle. However, spindle assembly required more time than control cells as anaphase onset was delayed, with the majority of the delay due to slow spindle MT organization as prometaphase was delayed but metaphase was unaffected (Figure 4D). Despite the drastic nuclear movements and mitotic delay, MTs reorganized into the phragmoplast, which is the MT-based machinery required for cell plate formation, and cytokinesis was completed in 15 of 15 cells, indicating that Kinesin-13s are dispensable in the later stages of cell division.
Unexpected from previous studies in animals and the predicted MT depolymerization activity of Kinesin-13 (Walczak et al., 2013), the metaphase spindle was shorter, rather than longer, in the KO cells (Figure 4E). In animal cells, MT depolymerization at the spindle pole by Kinesin-13 is important for poleward flux of spindle MTs, where MTs flow from the spindle equator to the pole regions through the continuous addition and removal of tubulin heterodimers at the plus ends and minus ends, respectively (Rogers et al., 2005). To investigate whether Kinesin-13 depletion affects poleward MT flux in moss, GFP-tubulin at the equator of the mitotic spindle was bleached and the movement of the photobleached strip was monitored. Surprisingly, the strip migrated toward the poles as in control cells, indicating that MT poleward flux took place in spite of complete Kinesin-13s depletion (Figures 4F and 4G; Supplemental Movie 2; Supplemental Movie Legends). Thus, Kinesin-13 contributes to mitosis in an unconventional manner in moss.
Kinesin-13 Regulates Straight Growth of the Protonema Filament by Controlling the Position of MT Focal Points
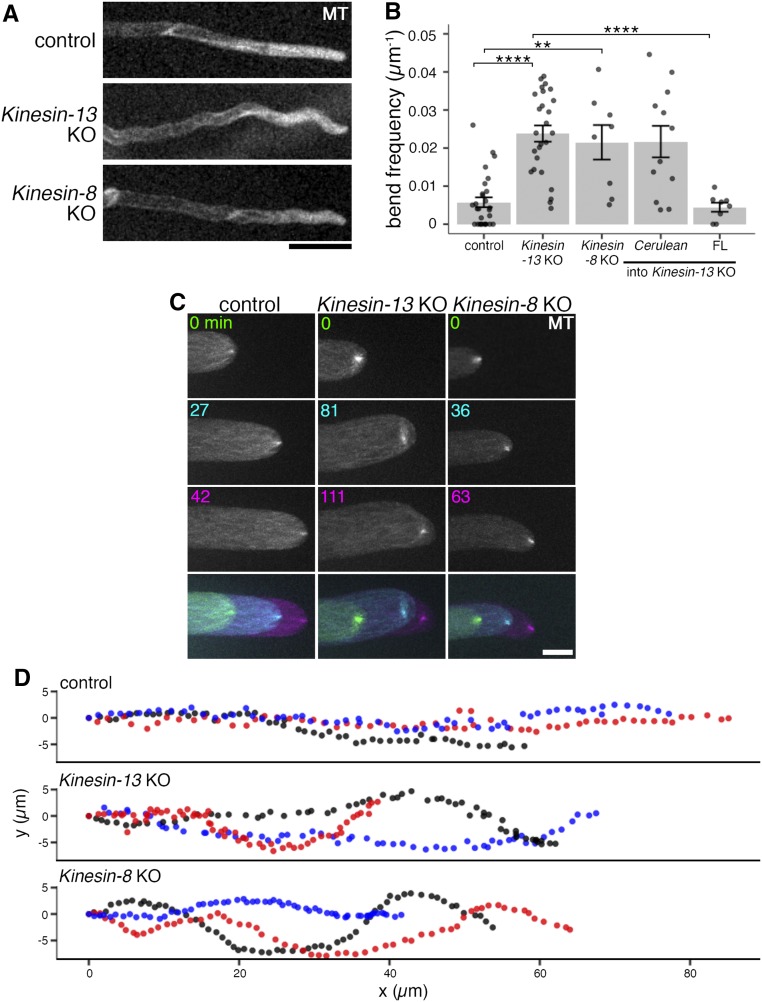
To study the colony growth defect of the Kinesin-13 KO line in detail, long-term time-lapse imaging of protonema filament growth was performed. Protonema filaments were wavy with the protonema cell tip periodically changing growth direction in the Kinesin-13 KO line (Figure 5A; Supplemental Movie 3; Supplemental Movie Legends). The Kinesin-13 KO line was wavier, with a bend frequency of 0.024 ± 0.002 µm−1 (mean ± se; n = 26) compared to the control (0.006 ± 0.001 µm−1, mean ± se; n = 28; Figure 5B). Interestingly, the Kinesin-13ac double KO line showed a milder wavy phenotype, while the single and Kinesin-13ab double KO lines did not (Supplemental Figure 3A). Additionally, ectopic expression of full-length Kinesin-13b rescued the waviness phenotype (Figure 5B; Supplemental Figure 3B). Thus, Kinesin-13s are required for straight tip growth.
Figure 5.
Kinesin-13 and Kinesin-8 KO Moss Have Wavy Protonema Filaments Associated with Unstable MT Foci Positioning.
(A) Protonema filaments of control (GFP-tubulin/histoneH2B-mRFP), Kinesin-13 KO (GPH0438#30), and Kinesin-8 KO (GPH0433#9) moss. Bar = 50 µm.
(B) Waviness of protonema filaments measured as frequency of wavy bend (18° < angle < 90°) of protonema filaments over measured lengths. Control (GFP-tubulin/histoneH2B-mRFP), 0.006 ± 0.001 μm−1 (mean ± se; n = 28 filaments); Kinesin-13 KO (GPH0438#30), 0.024 ± 0.002 µm−1 (mean ± se; n = 26 filaments; P-value < 0.0001); Kinesin-8 KO (GPH0433#7), 0.022 ± 0.005 µm−1 (mean ± se; n = 8 filaments; P-value < 0.01); Cerulean/Kinesin-13 KO (GPH0903#1), 0.022 ± 0.004 µm−1 (mean ± se; n = 12); Kinesin-13b(full-length)-Cerulean/Kinesin-13 KO (GPH0899#10), 0.004 ± 0.001 µm−1 (mean ± se; n = 8; P-value < 0.0001). Points represent individual protonema filaments, and results shown are from one experiment of at least four independent experiments. Tukey’s multiple comparison test was used for statistics (Supplemental Data Set 3).
(C) MT foci at the tip of the caulonema cell of the control (GFP-tubulin/histoneH2B-mRFP), Kinesin-13 KO (GPH0438#30), and Kinesin-8 KO (GPH0433#9) moss. Images were acquired with z-sections at 0.3-µm intervals over a 20-µm range, and maximum z-projections are displayed. Bottom panels show overlaid time series. Colors in time series indicate different time points as labeled in top panels. Bar = 10 μm.
(D) MT foci positions were tracked using the FIJI MOSAIC plug-in 2D/3D particle tracker (Sbalzarini and Koumoutsakos, 2005) in time-lapse imaging data as in (C). (x, y) trajectories of three representative MT foci (shown in different colors) for each line are displayed. Each point represents subsequent positions at each time point, at 3-min intervals for 3 h. Same lines as in (B) are represented.
Directionality of protonema tip growth in moss has been stipulated to be dependent on MTs (Doonan et al., 1988). At the apex of the protonema tip cell, plus ends of MTs converge into a focus known as MT foci (Hiwatashi et al., 2014). This occupies about the same place as the focal point of the actin filament cloud in a mutually dependent manner (Wu and Bezanilla, 2018; Yamada and Goshima, 2018). Tip growth defects including abnormal tip branching, retarded growth, and isotropic growth are the phenotypes observed among transgenic mutants for regulators of cytoskeletal dynamics where its organization at the tip is impaired (actin-related proteins, myo8, KINID kinesin, KCH kinesin; Rounds and Bezanilla, 2013; Hiwatashi et al., 2014; Wu and Bezanilla, 2018; Yamada and Goshima, 2018). As such, it is possible that Kinesin-13 depletion may result in defective MT organization at the cell tip, causing abnormal wavy protonema growth. MT foci behavior in the Kinesin-13 KO line was investigated with spinning disc confocal microscopy where MT foci of the Kinesin-13 KO moss were unstable and fluctuated frequently (Figures 5C and 5D; Supplemental Movie 4; Supplemental Movie Legends). Interestingly, in 19 of 20 bending events observed, the displacement of MT foci occurred prior to cell bending, indicating that MT foci dictated protonema growth direction (Supplemental Figure 4). These results suggest that Kinesin-13s regulate anisotropic growth of protonema filaments by positional maintenance of MT foci at the cell tip.
Kinesin-13 Is an Interphase MT Plus End Tracking Protein
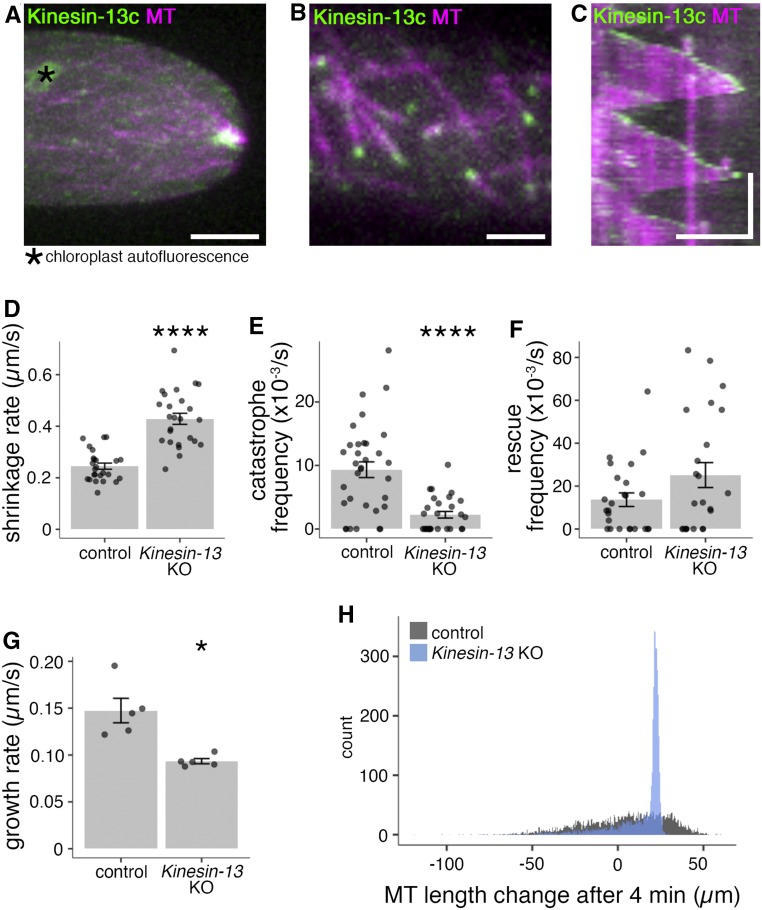
To investigate Kinesin-13’s localization during interphase, endogenously tagged Kinesin-13-Citrine lines (Miki et al., 2014) were observed with spinning disc confocal microscopy. Consistent with the depletion data, Kinesin-13s localized to MT foci (Figure 6A; Supplemental Figure 2B). To determine whether Kinesin-13 also associates with individual MTs in the endoplasm, we used oblique illumination fluorescence microscopy that enables observation of single MTs near the cell cortex with reduced effect of chloroplast autofluorescence (Jonsson et al., 2015; Nakaoka et al., 2015). In the interphase MT array, Kinesin-13s accumulated at the ends of growing MTs and disappeared from the ends when MTs switched to the shrink phase (Figures 6B and 6C; Supplemental Figure 2C; Supplemental Movie 5). Since MT minus ends are stabilized and exhibit little to no dynamicity in this cell type (Leong et al., 2018), we concluded that Kinesin-13 localizes to the plus ends of growing MTs. The plus end tracking behavior is reminiscent of human KIF2C/MCAK and Drosophila KLP10A, which are recruited by End Binding1 (EB1) to growing plus ends (Mennella et al., 2005; Lee et al., 2008).
Figure 6.
Kinesin-13 Localizes to the Interphase MT Network and Depletion of Kinesin-13 Results in Increased Shrinkage Rate, Reduced Catastrophe Frequency, Increased Rescue Frequency, and Reduced Growth Rate.
(A) MT foci of Kinesin-13c-Citrine/mCherry-tubulin (GPH0100#15) moss. Images were acquired at 0.3-µm intervals over a 10-µm range; shown is maximum z-projection. Bar = 5 µm.
(B) Interphase MT network of Kinesin-13c-Citrine/mCherry-tubulin (GPH0100#15) moss. Images were acquired by oblique illumination fluorescence split-view microscopy to avoid chloroplast autofluorescence. Bar = 2 µm.
(C) Kymograph of MT growth taken from imaging as in (B), taken every 3 s. Vertical bar = 2 min; horizontal bar = 5 µm.
(D) Interphase MT plus end shrinkage rate of control (GFP-tubulin/histoneH2B-mRFP, 0.245 ± 0.012 µm/s [mean ± se; n = 25 cells]) and Kinesin-13 KO (GPH0438#30, 0.429 ± 0.021 µm/s [mean ± se; n = 25 cells; P-value < 0.0001, Welch’s two-sample t test]) moss. Points represent individual cells; results shown are from one experiment of two independent experiments.
(E) Interphase MT catastrophe frequency of control (GFP-tubulin/histoneH2B-mRFP, 9.3 ± 1.2 × 10−3/s [mean ± se; n = 33 cells]) and Kinesin-13 KO (GPH0438#30, 2.2 ± 0.5 × 10−3/s [mean ± se; n = 28 cells; P-value < 0.0001, Welch’s two-sample t test]). Points represent individual cells; results shown are from two independent experiments.
(F) Interphase MT rescue frequency of control (GFP-tubulin/histoneH2B-mRFP, 14 ± 3 × 10−3/s [mean ± se; n = 25 cells]) and Kinesin-13 KO (GPH0438#30, 25 ± 6 × 10−3/s [mean ± se; n = 23 cells]). Points represent individual cells; results shown are from two independent experiments.
(G) Interphase MT plus end growth rate of control (EB1-Citrine/mCherry-tubulin, GPH0379#2, 0.147 ± 0.013 µm/s [mean ± se; n = 5 cells, 50 MTs]) and Kinesin-13 KO moss (GPH0577#11, 0.093 ± 0.003 µm/s [mean ± se; n = 5 cells, 50 MTs; P-value < 0.05, Welch’s two-sample t test]). Points represent individual cells.
(H) Simulation of MT growth of 4000 MTs in 4 min based on a probability model established using MT dynamics parameters from in vivo interphase MT dynamics analyses (D) to (G) (see Methods and Table 1). Control MT dynamics parameters yielded approximately normal distributions of MT lengths and tended to have a larger population of MTs with longer lengths, with the longest 25% of MTs ranging between 23.4 to 59.8 µm in length. For MTs under Kinesin-13 KO conditions, the distribution of MT length was narrower, with 50% of all MTs being from 11.5 to 22.6 µm in length, whereas the longest 25% of MTs ranged from 22.6 to 29.4 µm in length. Histogram bin width = 0.5 µm.
MT Shrinkage Rate and Rescue Frequency Increase, While MT Growth Rate and Catastrophe Frequency Reduce, upon Kinesin-13 Depletion
Since Kinesin-13s tracked growing MT plus ends, the effect of Kinesin-13 deletion on MT plus end dynamics during interphase was analyzed using time-lapse oblique illumination imaging of GFP-tubulin. MT shrinkage rate increased upon Kinesin-13 depletion, from 0.25 ± 0.01 µm/s (mean ± se; 5 MTs per cell analyzed, n = 25 cells) in the control to 0.43 ± 0.02 µm/s (mean ± se; 5 MTs per cell analyzed, n = 25 cells) in the KO line (Figure 6D). The catastrophe frequency was reduced from 9.3 ± 1.2 × 10−3/s (mean ± se; n = 33) in the control to 2.2 ± 0.5 × 10−3/s (mean ± se; n = 28) in the KO line (Figure 6E), while the rescue frequency was increased from 14 ± 3 × 10−3/s (mean ± se; n = 25) in the control to 25 ± 6 × 10−3/s (mean ± se; n = 23) in the KO line (Figure 6F). To analyze MT growth rate, Kinesin-13 KO moss expressing EB1-Citrine (Supplemental Figure 1C), a tracker of growing MT plus ends, was imaged with oblique illumination fluorescence microscopy. MT growth rate based on EB1-Citrine comet movement was reduced from 0.147 ± 0.013 µm/s (mean ± se; 10 MTs per cell analyzed, n = 5 cells) in the control to 0.093 ± 0.003 µm/s (mean ± se; 10 MTs per cell analyzed, n = 5 cells) in the KO lines (Figure 6G). These results suggest that Kinesin-13 plays a role in regulating MT dynamics in the interphase MT network.
Altered MT Dynamics Parameters May Underlie MT Length Phenotypes in Kinesin-13 KO
Depletion of MT depolymerases or catastrophe-promoting factors causes cytoplasmic MT lengthening and spindle expansion (Howard and Hyman, 2007; Goshima and Scholey, 2010). For example, in fission yeast cells lacking catastrophe-promoting factors, cytoplasmic MTs are more frequently polymerized beyond the limits of the cell, resulting in MT bending and curling (West et al., 2001). However, shorter metaphase spindle formation (Figure 4E) and the observation that MT foci often are unable to reach the apex of the cell tip in Kinesin-13 KO lines (Supplemental Figure 5; Supplemental Movie 4; Supplemental Movie Legends) appear to contradict this general rule. We reasoned that a decrease in MT growth rate and increase in shrinkage rate might limit overall MT length, despite a significant reduction in catastrophe frequency. To evaluate this possibility, we built a probability model fixed by the parameters of MT growth rate, shrinkage rate, catastrophe frequency, and rescue frequency and ran a simulation in which 4000 MTs exhibit dynamic instability for 4 min (Figure 6H; Table 1). With control parameters, a normal distribution was obtained where 50% of MTs ranged from –12.4 to 23.4 µm in length and the longest 1000 MTs ranged from 23.4 to 59.8 µm in length. By contrast, with MT dynamics parameters of Kinesin-13 KO cells, a narrower normal distribution was obtained, with 50% of MTs having lengths of 11.5 to 22.6 µm and the longest 1000 MTs ranging from 22.6 to 29.4 µm. Thus, the formation of shorter metaphase spindles and apex-displaced MT foci is a theoretically possible outcome, and actually a more likely outcome, associated with depletion of moss Kinesin-13 that affects both MT catastrophe frequency and growth/shrinkage rate.
Table 1. Parameters Used in MT Length Simulations.
| Dynamics Parameter | Control | Kinesin-13 KO |
|---|---|---|
| Growth rate (µm/s) | 0.147 ± 0.029 | 0.093 ± 0.006 |
| Shrinkage rate (µm/s) | 0.245 ± 0.059 | 0.429 ± 0.107 |
| Catastrophe frequency (x 10−3/s) | 9.3 | 2.2 |
| Rescue frequency (x 10−3/s) | 14 | 25 |
Values displayed are mean ± sd.
Kinesin-13 Motor Domain Induces Catastrophe in Vitro
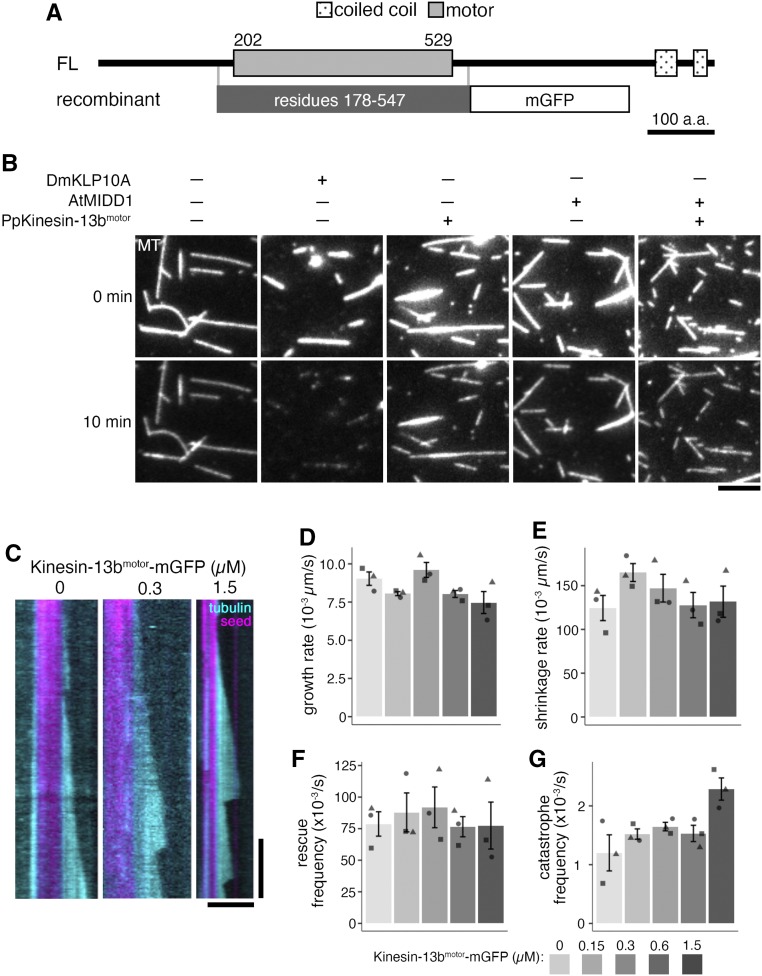
To investigate the direct effect of moss Kinesin-13 on MT dynamics, recombinant Kinesin-13bmotor-momomeric GFP (mGFP) was expressed and purified from a bacterial expression system (Figure 7A; Supplemental Figure 5A; full-length Kinesin-13 could not be obtained in either bacteria or insect culture cell expression system). The purified Kinesin-13bmotor-mGFP was subjected to binding-release experiments to confirm ATP hydrolysis activity: it bound to MTs in the presence of a nonhydrolyzable ATP analog (AMPPNP) and dissociated from MTs upon ATP addition (Supplemental Figure 5B). The ATPase-active protein was added to GMPCPP-stabilized MTs, but it did not show active MT depolymerization like that of animal Kinesin-13 protein (Drosophila KLP10A; Figure 7B; Rogers et al., 2004; Moriwaki and Goshima, 2016). We considered the possibility of moss Kinesin-13 requiring a binding partner like MIDD1 for Arabidopsis Kinesin-13 (Oda and Fukuda, 2013). A BLAST search showed that moss does not have MIDD1 homologues; so, moss Kinesin-13 was tested for MT depolymerization activity in the presence of Arabidopsis MIDD1, but it also did not depolymerize MTs (Figure 7B). Overall, the purified Kinesin-13bmotor-mGFP construct did not exhibit MT depolymerase activity under these experimental conditions.
Figure 7.
Recombinant Kinesin-13 Does Not Depolymerize Stabilized GMPCPP-MT Seeds but Shows MT Catastrophe-Inducing Activity.
(A) Protein domains of the Kinesin-13b (FL) and recombinant Kinesin-13bmotor-mGFP (recombinant) construct. Protein domains were determined using InterPro. His-tag for affinity purification was attached to the C terminus of the recombinant protein. a.a., amino acids.
(B) In vitro MT depolymerization assay using GMPCPP-stabilized MT seeds was performed using purified DmKLP10A, recombinant Kinesin-13bmotor-mGFP construct, AtMIDD1, AtMIDD1 and Kinesin-13bmotor-mGFP construct, and also under buffer only conditions. Only DmKLP10A successfully depolymerized MT seeds. The slight reduction in intensity in the bottom panels is due to photobleaching during imaging. All proteins were used at 200 nM except for AtMIDD1, which was at 100 nM. Bar = 5 µm.
(C) Representative kymographs of in vitro MT dynamics polymerization assays with Kinesin-13bmotor-mGFP construct at 0, 0.3, and 1.5 µM. Time-lapse imaging was performed with TIRF microscopy taken every 3 s. Brightness and contrast were manually adjusted. Vertical bar = 2 min; horizontal bar = 5 µm.
(D) to (G) In vitro MT dynamics parameters were analyzed from time-lapse imaging of in vitro MT dynamics polymerization assays with Kinesin-13bmotor-mGFP construct at 0, 0.15, 0.3, 0.6, and 1.5 µM taken using TIRF microscopy at 3-s intervals. In particular, growth rate (D) was observed to reduce slightly, from 9.0 ± 0.4 × 10−3µm/s (mean ± se; n = 3) in buffer only conditions, to 7.5 ± 0.7 × 10−3µm/s (mean ± se; n = 3) in 1.5 µM protein. Shrinkage rate and rescue frequency were shown in (E) and (F), respectively. Catastrophe frequency (G) was observed to reproducibly increase with high concentrations of Kinesin-13bmotor-mGFP, having a catastrophe frequency of 2.3 ± 0.2 × 10−3/s (mean ± se; n = 3) at 1.5 µM protein, compared to 1.2 ± 0.3 × 10−3/s in buffer only conditions. Points represent mean values from independent experiments, differently shaped points represent the different independent experiments.
The purified Kinesin-13bmotor-mGFP was also subjected to an in vitro MT polymerization assay at concentrations of 0, 0.15, 0.3, 0.6, and 1.5 µM. While the growth rate was somewhat reduced with a higher Kinesin-13bmotor concentration (Figure 7D), the shrinkage rate and rescue frequency were not obviously affected by Kinesin-13bmotor-mGFP addition (Figures 7E and 7F). Interestingly, in the presence of Kinesin-13bmotor-mGFP, the catastrophe frequency was reproducibly increased (Figure 7G). This result is consistent with in vivo data in which MT catastrophe frequency was lower in the Kinesin-13 KO line. By contrast, the recombinant protein did not reproduce the plus end accumulation seen in vivo, indicating that the truncated region and/or a separate factor may be required for plus end recruitment.
Chromosome Segregation and Cell Division Proceed Normally in the Absence of Kinesin-8
The mitotic phenotype associated with Kinesin-13 deletion was fairly mild. We reasoned that another MT depolymerase instead might have a major role in MT depolymerization in mitosis of protonema filaments, and we decided to investigate Kinesin-8, which shows strong depolymerization activity during mitosis of yeast (Hildebrandt and Hoyt, 2000; Unsworth et al., 2008). To study Kinesin-8 function in the moss, all three paralogous genes phylogenetically classified into the moss Kinesin-8 subfamily (Kinesin-8Ia, Kinesin-8Ib, and Kinesin-8II; Figure 1B; Supplemental Data Set 1; Shen et al., 2012; Miki et al., 2014) were knocked out (Supplemental Figure 1D). Moss colonies, gametophores, and rhizoids were normal in the Kinesin-8 KO line (Figures 2A to 2C). High-resolution mitosis imaging did not show any defect in prophase MT organization, spindle formation, chromosome alignment, anaphase chromosome segregation, and cytokinesis (9.1 ± 0.2 min from NEBD to anaphase onset; n = 10; Figures 4A, 4D, and 4E). We concluded that Kinesin-8s are dispensable for mitotic cell division in moss protonema filaments.
Kinesin-8 Controls the Positioning of MT Foci for Straight Tip Growth
Interestingly, the Kinesin-8 KO line also had wavy protonema filaments with bends occurring at smaller magnitudes, with a bend frequency of 0.022 ± 0.005 µm−1 (mean ± se; n = 8; Figures 5A and 5B; Supplemental Movie 3; Supplemental Movie Legends). Tracking of the MT foci at tip cells showed that it fluctuates more frequently in the Kinesin-8 KO line than in the control or Kinesin-13 KO line (Figures 5C and 5D; Supplemental Movie 4; Supplemental Movie Legends), consistent with its smaller magnitudes of bends.
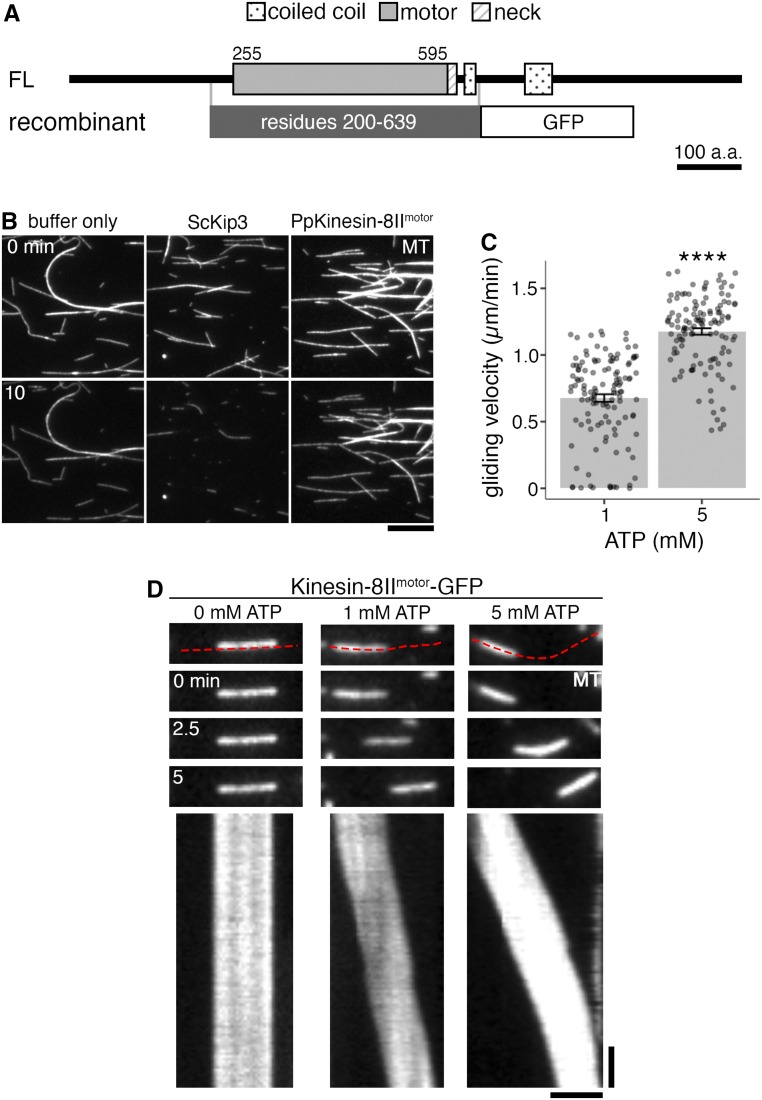
Kinesin-8IImotor Glides MTs, but Does Not Show a MT Depolymerization Activity in Vitro
To analyze the intrinsic activity of moss Kinesin-8, the recombinant Kinesin-8IImotor-GFP protein was expressed and purified from a bacterial expression system (Figure 8A; Supplemental Figure 5C) and was subjected to a MT depolymerization assay. Next, 200 nM Kinesin-8IImotor-GFP was added to GMPCPP-stabilized MTs, but it could not depolymerize them, while 200 nM budding yeast (Saccharomyces cerevisiae) Kinesin-8/Kip3 could depolymerize the MTs (Figure 8B). Kinesin-8IImotor-GFP was then tested for MT gliding activity. Protein immobilized on silanized coverglass showed ability to glide GMPCPP-stabilized MTs in an ATP-dependent manner, but it also could not depolymerize those MTs (Figures 8C and 8D).
Figure 8.
Recombinant Kinesin-8 Motor Does Not Depolymerize MTs but Shows MT Gliding Activity.
(A) Protein domains of Kinesin-8II (FL) and recombinant Kinesin-8IImotor-GFP (recombinant) construct. Protein domains were identified using InterPro. His-tag for affinity purification was attached to the C terminus of the recombinant protein. a.a., amino acids.
(B) In vitro MT depolymerization assay using GMPCPP-stabilized MT seeds was performed using purified ScKip3, recombinant Kinesin-8IImotor-GFP, and also under buffer only conditions. Only ScKip3 showed MT depolymerization activity. The slight reduction in intensity in the bottom panels is due to photobleaching during imaging. All proteins were used at 200 nM. Bar = 10 µm.
(C) ATP-dependent MT gliding velocity of Kinesin-8IImotor-GFP. 1 mM ATP, 0.68 ± 0.03 µm/min (mean ± se; n = 124 MTs); 5 mM ATP, 1.18 ± 0.02 µm/min (mean ± se; n = 121 MTs, P-value < 0.0001, Welch’s two-sample t test).
(D) In vitro MT gliding assay using GMPCPP-stabilized MTs on Kinesin-8IImotor-GFP, which was immobilized on glass, at 0, 1, and 5 mM ATP. Red dotted line in the top panel indicates the segmented line used to draw kymographs (bottom panels). Gliding activity of Kinesin-8IImotor-GFP was verified in three independent experiments. Vertical bar = 45 s; horizontal bar = 2 µm.
DISCUSSION
The KO lines generated in this study showed some characteristic phenotypes unreported in previous plant kinesin mutants, such as wavy cell growth accompanying MT foci positional fluctuation (Kinesin-13 and Kinesin-8) and prophase MT disorganization (Kinesin-13). Intriguingly, several processes driven by these motors in many animal and yeast species were normal in their absence in moss (Physcomitrella patens), such as spindle MT flux and chromosome segregation. Moreover, the hallmark activity of these kinesins, MT depolymerization, was not detected in vitro. Overall, this study provides a comprehensive view on the roles of Kinesin-13 and Kinesin-8 in a single plant species. Furthermore, our results reinforce the emerging view that the kinesin superfamily is well conserved in plants but that members of this superfamily have diverged in their function (Gicking et al., 2018; Nebenführ and Dixit, 2018).
Are Plant Kinesin-13 and Kinesin-8 MT Depolymerases?
Kinesin-13 is a well-known MT depolymerase in animals. Arabidopsis and rice Kinesin-13s have also been shown to depolymerize stabilized MTs (Oda and Fukuda, 2013; Deng et al., 2015). However, moss Kinesin-13 only exhibited catastrophe-inducing activity in vitro and could not depolymerize GMPCPP-stabilized MTs. This in vitro result is consistent with the reduced catastrophe frequency seen for interphase MTs in the Kinesin-13 KO moss. Nevertheless, negative results obtained in vitro are not necessarily conclusive: inappropriate expression systems or unsuitable biochemical environments could prevent full activity of the protein. In this study, a motor-only construct was used due to technical constraints. Thus, it is possible that another domain(s) on the Kinesin-13 protein is required for MT depolymerization activity. One such element may be the coiled coil, which in animal Kinesin-13 dimerizes the protein and increases MT depolymerization activity (Hertzer et al., 2006). However, the coiled coil region required for dimerization is located immediately upstream/downstream of the motor domain in animal Kinesin-13 (Maney et al., 2001), but it is located further down the C terminus in moss Kinesin-13 (Figure 1A); it is unclear whether dimerization of moss Kinesin-13 could enhance the activity in a similar manner to animal homologues. Furthermore, animal Kinesin-13 monomers are capable of depolymerizing MTs in vitro (Maney et al., 2001; Hertzer et al., 2006). Moss and also Arabidopsis Kinesin-13s lack the neck domain that is important for strong MT depolymerization activity in animals (Ovechkina et al., 2002); based on this feature, it was indeed originally speculated that plant Kinesin-13 might not have MT-depolymerizing activity (Lu et al., 2005). Thus, although we cannot rule out the possibility that moss Kinesin-13 has MT-depolymerizing activity, possibly with the aid of a specific binding partner, it is enticing to speculate that it has diverged structurally and functionally from animal Kinesin-13.
In cells, there is even less evidence to support Kinesin-13 as a MT depolymerase. Upon Kinesin-13 KO, interphase MTs show reduced MT growth rate and increased shrinkage rate. Such results instead point to Kinesin-13 being a MT growth promoter. However, MT growth-promoting activity was not observed in vitro. This may be due to the use of the motor-only construct with which we could not recapitulate the plus end enrichment of Kinesin-13. Alternatively, considering the decrease and increase in catastrophe and rescue frequency of interphase MTs, it is possible the Kinesin-13 regulates the growth and shrinkage rate indirectly via tubulin cycling: reduced catastrophe would result in reduced availability of tubulin in the free tubulin pool, which might affect MT growth and shrinkage rates, as was proposed in studies of Arabidopsis Armadillo Repeat-containing Kinesin (ARK) proteins (Eng and Wasteneys, 2014) and more recently for the plant-specific MT nucleator MACERATOR4 (MACET4; Schmidt and Smertenko, 2019).
Similar to observations of Kinesin-13, we could not observe MT depolymerization of the Kinesin-8 motor in our assay, which differs from human and yeast Kinesin-8. This might be due to our use of a truncated construct (∼440 amino acids), as we failed to purify the longer fragment (∼640 amino acids). However, we recently found that Drosophila Kinesin-8 (full length) shows plus end-directed motility and induces MT catastrophe at the plus end, but that it is not able to depolymerize stable MTs in vitro (Edzuka and Goshima, 2019). Moss Kinesin-8 might have similar activities.
Kinesin-13 and Kinesin-8 for Mitosis
We could not detect any aberrant phenotypes in Kinesin-8 KO lines during mitotic cell division, such as chromosome misalignment or mitotic delay, which are common phenotypes observed in yeast and animal cells, suggesting that Kinesin-8 has lost mitotic functions in moss. By contrast, some, but not all, known mitotic functions of Kinesin-13 (Walczak et al., 2013) were observed in moss. In animal mitosis, centrosomal MTs (astral MTs) are overly developed during prophase in the absence of Kinesin-13 (Goshima and Vale, 2003; Rogers et al., 2004). Similarly, disorganized MTs were observed around the nucleus, despite the loss of centrosomes in moss (and all other land plants). MTs surrounding the nucleus may act as MTOCs equivalent to animal centrosomes. During prometaphase, the kinetochore–MT attachment appears to be less efficient, since prometaphase duration was slightly prolonged in the Kinesin-13 KO moss; whether this is due to overall changes in MT dynamics or the lack of error correction, as is the case of KIF2C/MCAK depletion in animal cells, remains elusive. Spindle monopolarization, as reported in centrosome-containing animal cells (Goshima and Vale, 2003), was not detected. At metaphase, Kinesin-13 in animal cells acts as a MT depolymerase at the pole, driving MT poleward flux and halting spindle extension. Surprisingly, we did not obtain data that moss Kinesin-13 plays such a role: Kinesin-13 does not localize to the spindle pole, MT flux was detected in the Kinesin-13 KO line, and the spindle was shorter, rather than longer, in the complete absence of Kinesin-13. MT dynamics is a major contributor to spindle length regulation in animal somatic cells (Goshima and Scholey, 2010); therefore, shortening might be due to the reduced MT growth rate observed in the endoplasm, consistent with Kinesin-13 localizing to the spindle equator where MT plus ends are enriched. Chromosome segregation during anaphase A was normal, further supporting the notion that Kinesin-13 does not act as a MT depolymerase at the pole. These data indicate that the moss mitotic spindle possesses a mechanism that drives spindle MT poleward flux independently of Kinesin-13.
Kinesin-13 and Kinesin-8 for Tip Growth
The most prominent phenotype observed both in the Kinesin-13 and Kinesin-8 KO lines was the tip growth defect. Recent studies suggest the importance of MT-converging centers, the MT foci, in protonema tip growth in moss, where F-actin, which is absolutely essential for tip growth, is concentrated near the MT foci. In several mutants of MT-associated motors in which the tip grows more slowly, the MT foci are not persistently formed (Hiwatashi et al., 2014; Wu and Bezanilla, 2018; Yamada and Goshima, 2018). The transient MT foci produced bursts of MT concentration at random locations along the tip region of the apical cell, causing the bending of the protonema filament at abrupt angles (Hiwatashi et al., 2014). Such phenotypes are similar to the tip growth defects seen when moss is treated with MT-disruptive drugs (Doonan et al., 1988). In contrast to those mutants, we persistently observed single MT foci in the Kinesin-13 or Kinesin-8 KO. However, their positions were unstable, exhibiting the waviness of rather regular amplitude and frequencies. This suggests that Kinesin-13 and Kinesin-8 play a role in MT foci positional guidance, rather than MT foci formation/maintenance, which ensures straight growth of the protonema filament. While straight tip growth with limited MT foci fluctuations would allow the fastest propagation of moss, wavy growth would be an advantageous mechanism to facilitate innovative exploration of the environment. Our study highlights the regulation of MT plus end dynamics by MT-associated proteins as a possible intracellular mechanism to modulate cell growth in response to environmental cues, reminiscent of axon guidance in neurons (Sabry et al., 1991; Tanaka et al., 1995; Menon and Gupton, 2016).
METHODS
Molecular Cloning and Gene Targeting Experiments
All transgenic moss lines, plasmids, and primers used in this study are listed in Supplemental Data Set 2; all lines originated from the Physcomitrella patens Gransden 2004 strain. Moss culture, transformation, and transgenic line selection were performed as described previously (Yamada et al., 2016). In brief, moss cells were cultured on BCDAT or BCD media under 24 h of light illumination (FL40SD [NEC], 2500 luces), and transformation was performed by the standard polyethylene glycol–mediated method.
KO Moss Generation
Kinesin-13 KO (GPH0438) and Kinesin-8 KO (GPH0433) were generated in the moss strain expressing GFP-tubulin and histoneH2B-mRFP (Nakaoka et al., 2012) sequentially replacing the targeted genes with antibiotic resistance using homologous recombination (HR). To do this, 1 kb of genomic DNA sequences upstream/downstream of start/stop codons of the target genes were cloned around an antibiotic resistance cassette and then transformed into the moss. To knock out Kinesin-13 genes in the moss expressing EB1-Citrine and mCherry-tubulin (Kosetsu et al., 2013), Kinesin-13b was first removed by antibiotic resistance–mediated HR as described before. For Kinesin-13a and Kinesin-13c, CRISPR-mediated gene removal was utilized. Next, 20-bp guide RNAs (gRNAs) targeting the start and end of the gene were designed using CRISPOR (http://crispor.tefor.net/; Supplemental Figure 1A). gRNAs were then integrated into a BsaI site of a vector carrying the U6 promoter and RNA scaffold (pCasGuide/pUC18; Lopez-Obando et al., 2016; Collonnier et al., 2017), and then the CRISPR cassettes were cloned into a vector carrying nourseothricin resistance (pSY034) with InFusion (Takara) to assemble multiple gRNA cassettes into a plasmid (pSY062) following methods described previously (Leong et al., 2018). Equal amounts of this circular multicassette plasmid and Streptococcus pyogenes Cas9 expression vector (pGenius; Collonnier et al., 2017) were transformed into the Kinesin-13b KO/EB1-Citrine/mCherry-tubulin background. Confirmation of Kinesin-13 and Kinesin-8 KO lines (Supplemental Figures 1B to 1D) were performed by PCR as described previously by Yamada et al. (2016) and Leong et al. (2018).
Endogenous C-Terminal Citrine Tagging
C-Terminal endogenous Kinesin-13 and Kinesin-8 Citrine tagging lines from Miki et al. (2014) were used. In these lines, Kinesin-13 and Kinesin-8 were tagged endogenously with Citrine at the C terminus via HR where C-terminal sequence and downstream sequence of the stop codon of the target gene (both 1-kb lengths) flanking an antibiotic resistance cassette was used as the HR template. Confirmation of this line was by PCR using primers designed to target the C-terminal region and outside the 3′ untranslated region of the target gene. To make rescue plasmids, the Kinesin-13 sequence was cloned from a cDNA library and ligated into the pENTR/D-TOPO vector. The Kinesin-13 mutant plasmids (Kinesin-13bRIG-Cerulean, Kinesin-13bKVD/KEC-Cerulean, and Kinesin-13bLoop12-Cerulean) were generated by mutagenesis with the full-length Kinesin-13 plasmid, and primers are listed in Supplemental Data Set 2. The cloned Kinesin-13 sequences were ligated into the pMN603 vector by a Gateway LR reaction, which contains the EF1α promoter, Cerulean gene, nourseothricin-resistance cassette, and PTA1 sequences designated for HR-mediated integration (Miki et al., 2016; Yoshida et al., 2019).
Imaging Sample Preparation
Agar Pad Sample
Moss samples used in time-lapse imaging were prepared either in six-well glass-bottomed plates or 35-mm glass-bottomed dishes as described previously by Yamada et al. (2016). Briefly, protonema cells were plated onto glasses coated with BCD agar medium and cultured for 4 to 6 d.
Microdevice Sample
Samples used for oblique illumination fluorescence microscopy were prepared in BCD liquid medium in 15-µm-height polydimethylsiloxane microfluidic chambers mostly following methods previously described by Leong et al. (2018) and Kozgunova and Goshima (2019). Briefly, protonema cells were homogenized in BCD liquid media, filtered through a sheet of 50-µm nylon mesh, and injected into microfluidic chambers attached to 24 m × 32 mm micro coverglasses (No. 1-S, Matsunami) and cultured for 4 to 6 d.
Calcofluor-Stained Sample
Eight-day-old moss colonies regenerated from single protoplasts were mounted on 35-mm glass-bottomed dishes with BCD agar medium. The moss colonies were stained with 100 μL of 0.1 mg/mL calcofluor solution diluted with water and covered with a coverglass. The samples were then imaged with an Eclipse TE2000-E inverted microscope (Nikon).
Moss Colony Assay
A protoplast regeneration assay was performed following the method described by Vidali et al. (2007) and Yamada et al. (2016), with some modifications. In brief, sonicated moss on cellophane-lined BCDAT plate was digested by 8% (w/v) mannitol solution supplemented with 2% (w/v) driselase. The generated protoplasts were washed three times with 8% (w/v) mannitol solution, and 1.5 × 105 mL−1 of cells was resuspended with 7 mL of protoplast regeneration liquid. After overnight incubation in darkness, the protoplasts were centrifuged at 510g for 2 min at 25°C and resuspended in 4 mL of Protoplast Regeneration Media (PRM) solution in which CaCl2 was added after autoclave. Next, 0.5 to 1 mL of 4 mL of protoplast solution was spread on a cellophane-lined PRM plate. The protoplasts were cultured for 4 d and transferred to a BCDAT plate, followed by 4 d of culture. The 8-d-old moss colonies were observed with a stereomicroscope.
Live in Vivo Imaging
Spinning disc confocal microscopy using a 100× 1.45-NA lens and ImagEM camera (Hamamatsu Photonics) was performed with a Nikon Ti inverted microscope as described previously by Yamada et al. (2016). The z-series were taken using a Nano-Z Series nanopositioner combined with a Nano-Drive controller (Mad City Labs), where z-stack imaging was performed at 0.3 µm. Oblique illumination fluorescence microscopy was performed with a Ti microscope (Nikon) with a total internal reflection fluorescence (TIRF) unit, a 100× 1.49-NA lens, GEMINI split view (Hamamatsu Photonics), and electron multiplication charge-coupled device camera (Evolve, Roper). The microscopes were controlled by a Micromanager or NIS-Elements (Nikon). Low-magnification epifluorescence imaging was performed using an Eclipse TE2000-E inverted microscope (Nikon) with 10×/0.3 LN1C Plain Fluo lens and Zyla 5.5 sCMOS camera (Andor), controlled with IQ3 (Andor). Photobleaching experiments were performed using an LSM780-DUO-NLO confocal microscopy system (Zeiss) using a Plan-Apochromat 63×/1.40 oil differential interference contrast lens controlled using Zen (Zeiss) with a 489-nm diode laser (time-lapse imaging taken at 2 mW and bleaching at 100 mW). Moss expressing GFP-tubulin (GPH0438#30 for Kinesin-13 KO and GFP-tubulin/histoneH2B-mRFP for control) were used for photobleaching experiments where images were acquired every 3 s. All imaging was performed at room temperature in the dark. Moss colonies and gametophores were imaged with SMZ800N (Nikon) and ILCE-QX1 (SONY) cameras. The stereomicroscope system was controlled with PlayMemories software (SONY).
Computer Simulations
Simulations were built in R (version 3.6.0; https://github.com/TomoyaEdzuka/MT_dyanamics_simulation). Parameters used in this simulation for each condition (control or Kinesin-13 KO) are listed in Table 1. In this simulation, catastrophe and rescue frequency parameters were used to determine the probability of individual steps (1 s) undergoing a transition change or to continue a growth/shrink phase. At each phase transition (i.e., catastrophe/rescue event) new growth/shrinkage rates were assigned following a log normal distribution of the growth and shrink parameters. MT lengths were then simulated for 4000 trials (i.e., 4000 MTs) for 240 steps (i.e., 4 min).
Protein Purification
The motor domain Kinesin-13b, which is the most highly expressed protein of the three paralogous Kinesin-13 genes, was cloned from moss cDNA and transgenically linked to mGFP and 6×His at the C terminus (plasmid pGG885; Supplemental Data Set 2). Kinesin-13bmotor-mGFP expression was induced in SOLBL21 Escherichia coli with 0.1 mM isopropyl β-d-thiogalactoside (IPTG) for 20 h at 18°C. Harvested cells were lysed using the Advanced Digital Sonifier D450 (Branson) in lysis buffer (50 mM Tris-HCl, pH 8.0, 100 mM KCl, 2 mM MgCl2, 20 mM imidazole, and 0.1 mM ATP) supplemented with 10 mM β-mercaptoethanol and protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF] and peptide inhibitor cocktail: 5 mg/mL aprotinin, 5 mg/mL chymostatin, 5 mg/mL leupeptin, and 5 mg/mL pepstatin A). After rotation with nickel-nitrilotriacetic acid (Ni-NTA)–coated beads for 2 h at 4°C, the proteins were eluted using 500 μL of elution buffer (50 mM Tris-HCl, pH 8.0, 100 mM KCl, 2 mM MgCl2, 300 mM imidazole, and 0.1 mM ATP). Elution was repeated five to seven times. The eluates were then further purified through an AMPPNP-ATP binding-release experiment. Eluates were first bound with 1 mM AMPPNP to 76.5 µM of 1 mM GMPCPP-stabilized MTs and then sedimented through an 60% glycerol cushion. Finally, the proteins were released from the pellet with 10 mM ATP. The motor domain and nearby coiled-coil domain (residues 200 to 639) of Kinesin-8II were cloned from moss cDNA and transgenically joined to GFP and 6×His tag at the C terminus (pTM266; Supplemental Data Set 2) and introduced into a pET-23 E. coli expression vector. The recombinant protein was purified from SOLBL21 E. coli induced with 0.2 mM IPTG for 20 h at 18°C. Harvested cells were lysed using the Advanced Digital Sonifier D450 (Branson) in lysis buffer (25 mM MOPS, pH 7.0, 250 mM KCl, 2 mM MgCl2, 5% [w/v] Suc, 30 mM imidazole, and 0.1 mM ATP) supplemented with 5 mM β-mercaptoethanol and protease inhibitors (0.5 mM PMSF and peptide inhibitor cocktails). After rotation with Ni-NTA–coated beads (0.5-mL bed volume) for 2 h at 4°C, proteins were eluted with 500 μL of elution buffer (25 mM MOPS, pH 7.0, 250 mM KCl, 2 mM MgCl2, 400 mM imidazole, 5% [w/v] Suc, 1 mM ATP, and 5 mM β-mercaptoethanol). Elution was repeated five to seven times. Eluates were used immediately. For the in vitro MT depolymerization assay, the buffer for the elution was exchanged to 1× standard assay buffer (SAB: 25 mM MOPS, pH 7.0, 75 mM KCl, 2 mM MgCl2, and 1 mM EGTA) supplemented with 1 mM ATP to remove imidazole using a PD-25 column (GE Healthcare). Drosophila KLP10A (plasmid pGG885) was purified referencing (Moriwaki and Goshima, 2016) and was purified with the same protocol as Kinesin-13bmotor-mGFP. Instead of the binding-release experiment, elutions of KLP10A were subjected to buffer exchange to 1× MRB80 with 75 mM KCl and 0.1 mM ATP using a PD-25 column (GE Healthcare). AtMIDD1 was purified following Oda and Fukuda (2013), with some modifications. Briefly, GST-AtMIDD1 expression was induced in SOLBL21 E. coli using 0.2 mM IPTG and cultured for 20 h at 18°C. Harvested cells were lysed using the Advanced Digital Sonifier D450 (Branson) in lysis buffer (10 mM HEPES, pH 7.4, 1 mM EGTA, 1 mM MgCl2, and 150 mM KCl) supplemented with 1 mM DTT and protease inhibitors (0.5 mM PMSF and peptide inhibitor cocktails). After rotation with glutathione Sepharose 4B beads (GE Healthcare; 0.5-mL bed volume) for 2 h at 4°C, proteins were eluted with 500 μL of elution buffer (100 mM HEPES, pH 7.4, 100 mM KCl, and 30 mM reduced glutathione). Elution was repeated five to seven times. Buffer was then exchanged using a PD-10 column (GE Healthcare). Budding yeast Kip3 was purified following Kip3 purification (Gupta et al., 2006) and Drosophila KLP67A purification (Edzuka and Goshima, 2019). In brief, ScKip3-sfGFP-6xHis (pED273) was expressed in Sf21 moth cells at 27°C for 72 h. Cells were lysed with 1% (v/v) Triton X-100 in lysis buffer (50 mM MOPS, pH 7.0, 250 mM NaCl, 2 mM MgCl2, 1 mM EGTA, 20 mM imidazole, and 0.1 mM ATP) supplemented with 2 mM β-mercaptoethanol and protease inhibitors (0.5 mM PMSF and peptide inhibitor cocktails) for 30 min at 25°C. After rotation with Ni-NTA–coated beads (0.5-mL bed volume) for 2 h at 4°C, proteins were eluted with 500 μL of elution buffer (25 mM MOPS, pH 7.0, 250 mM KCl, 2 mM MgCl2, 400 mM imidazole, 5% [w/v] Suc, 1 mM ATP, and 5 mM β-mercaptoethanol). Buffer was then exchanged to 1× SAB supplemented with 1 mM ATP to remove imidazole using a PD-25 column (GE Healthcare).
In Vitro MT Depolymerization
The in vitro MT depolymerization assay in Moriwaki and Goshima (2016) and Gell et al. (2010) was followed, with some modifications. DmKLP10A, Kinesin-13bmotor-mGFP, and AtMIDD1 were mixed with 30% Alexa Fluor 568–labeled GMPCPP-MT seeds immobilized on silanized coverglass in assay buffer (1× MRB80, 1 mM ATP, 50 mM Glc, 0.5 µg/μL κ-casein, and 0.1% [w/v] methylcellulose) supplemented with an oxygen scavenger system. Similarly, Kinesin-8IImotor-GFP and ScKip3 were also introduced to immobilized GMPCPP-MT seeds, but in a different assay buffer (1× SAB, 0.1% [w/v] methylcellulose, 50 mM Glc, 0.5 µg/μL κ-casein, 1 mM ATP, and 75 mM KCl, supplemented with oxygen scavenger system). Proteins were used at 200 nM concentrations, except AtMIDD1, which was used at 100 nM following Oda and Fukuda (2013). TIRF imaging was taken every 3 s for 10 min at 25°C.
In Vitro MT Polymerization Assay
We largely followed methods described previously (Li et al., 2012; Moriwaki and Goshima, 2016; Leong et al., 2018) for the MT polymerization assay. MT growth was initiated by flowing 20 µM porcine brain tubulin (containing 10% Alexa Fluor 647–labeled tubulin) and 0, 0.15, 0.3, 0.6, and 1.5 µM purified Kinesin-13bmotor-mGFP in assay buffer (1× MRB80, 75 mM KCl, 1 mM ATP, 50 nM Glc, 1 mM GTP, 0.8 µg/μL κ-casein, and 0.1% [w/v] methylcellulose) supplemented with an oxygen scavenger system. TIRF imaging of Alexa Fluor 568–labeled GMPCPP-MT seeds and Alexa Fluor 647–labeled free tubulin was taken every 3 s for 10 min at 25°C. Kymographs were drawn for 25 trackable dynamic MTs and were analyzed for catastrophe events and rescue events. Catastrophe frequency was then defined by the number of catastrophe events per growth time (minutes), whereas rescue frequency was defined by the number of rescue events per shrink time (minutes). Growth and shrinkage rates were taken from the corresponding slopes from the same kymographs. Three independent experiments, each with a different batch of purified proteins were performed.
In Vitro MT Gliding Assay
For the gliding assay with purified Kinesin-8IImotor-GFP, methods described in Miki et al. (2015) were referenced. Briefly, 10 μL of the freshly purified recombinant protein was introduced into the flow chamber and incubated at room temperature for 2 min in the dark and then washed with 20 μL of 1×SAB (25 mM MOPS, pH 7.0, 75 mM KCl, 2 mM MgCl2, and 1 mM EGTA). Next, 10 μL of reaction mix (1×SAB, 0.1% [w/v] methylcellulose, 50 mM Glc, 0.5 µg/μL κ-casein, 50 mM GMPCPP-MT seeds, and 75 mM KCl, supplemented with oxygen scavenger system and varying concentrations of ATP) was introduced into the flow chamber, and it was sealed with candle wax. TIRF imaging of Alexa Fluor 647–labeled GMPCPP-MT seeds in in vitro MT gliding assays with Kinesin-8IImotor-GFP supplemented with various concentrations of ATP was taken every 3 s for 10 min at 23 to 25°C. Kymographs were drawn for ∼30 to 50 trackable dynamic MTs per sample, and the slopes of the kymographs were taken as gliding velocity.
Data Analysis
Phylogenetic Analysis
Protein sequences used for phylogenetic analysis are presented in Supplemental Data Set 1. After amino acid sequences were aligned with MAFFT, gapped regions were removed manually using MacClade. The phylogenetic tree was constructed using neighbor-joining methods using the MEGA software. Reliability was assessed with 1000 bootstrapping trials.
Moss Colony Growth Rate
GPH0438#30 (Kinesin-13 KO) and GPH0002#5 (control) protoplasts were made following Yamada et al. (2016), with some modifications. In brief, moss was incubated with 2% (w/v) driselase solution (in 8% [w/v] mannitol), washed thrice with 8% (w/v) mannitol, incubated overnight in protoplast liquid medium, and then plated in PRM/T medium on cellophane-lined PRM plates, cultured at 25°C under continuous light. On day 2, moss-lined cellophane was transferred to BCDAT plates. Approximately day 7 when individual colonies were larger, they were picked and inoculated on BCDAT plates and then cultured at 25°C under continuous light. On day 27 to 28, images of plates with grown colonies were taken with the in-built camera of Xperia X Performance (23 MP Type 1/2.3′ Exmor RS sensor, 24-mm equivalent lens with f/2.0 aperture). Images were converted to 8-bit, automatically thresholded, binarized, and then analyzed with image processing software FIJI. Colonies were automatically outlined with the wand tool. Data in Figure 2B are pooled from three independent experiments.
Nonapical Cell Length
Low-magnification calcofluor-stained images of moss colonies were used to measure nonapical cell length (Figure 2E), where only caulonema cells were measured. To distinguish between caulonema and chloronema cells, protonema filaments were first judged by eye in bright-field images of the same colonies, in which chloroplast density was used as an indicator of cell state (caulonema, chloroplast sparse; chloronema, or chloroplast dense).
Branching Distance, Branch Filament Length, and Branching Angle
Low-magnification images of 8-d-old moss colonies regenerated from single protoplasts were used to analyze branching pattern parameters. Branching parameters were manually measured as shown in the cartoon in Figure 2E.
Nuclear Movement Velocity
Samples from epifluorescence imaging of moss protonema filaments undergoing mitosis were analyzed. Kymographs were drawn on the filaments, and nuclear movement velocity was obtained from the slopes of nuclear movement in these kymographs where positive and negative values were assigned to apical and basal directions, respectively.
Subapical Cell Length
Samples from epifluorescence imaging of moss protonema filaments undergoing mitosis were analyzed. Kymographs were drawn on the filaments, and the length from the middle of the spindle at anaphase to the cell tip and to the previous cell plate was measured as apical and subapical cell length, respectively (Figure 3E, cartoon).
GFP-Tubulin Intensity around the Nucleus
Spinning disc confocal fluorescence time-lapse imaging of GFP-tubulin and histoneH2B-mRFP moss taken every 1 min was used for analysis. Segmented line built-in tool in FIJI was used to mark the hemispheric circumference around the nucleus on the apical and basal side at 1 min before NEBD (Figure 4B, cartoon), and the mean pixel intensity was measured. The apical:basal GFP-intensity ratio was defined as the ratio of the mean pixel intensity of the apical hemisphere over that of the basal hemisphere.
Mitotic Duration
Mitosis images taken every 1 min with a spinning disc confocal microscope (Nikon Ti; EM charge-coupled device camera ImagEM [Hamamatsu Photonics]; CSU-X1 [Yokogawa]) were used for analysis. Mitotic duration was defined as time from NEBD to anaphase onset, whereas prometaphase is the time from NEBD to chromosome alignment, and metaphase duration is the time from chromosome alignment to anaphase onset.
Spindle Length
Mitosis images taken every 1 min with a spinning disc confocal microscope (Nikon Ti; electron multiplication charge-coupled device camera ImagEM [Hamamatsu Photonics ]; CSU-X1 [Yokogawa]) were used for analysis. The metaphase spindle (defined as 1 min before anaphase onset) was used to analyze spindle area. Four points (the two limits of basal and apical pole widths) were marked and their (x, y) positions were obtained, where spindle length was obtained from the distance between the midpoints of the basal and apical pole widths.
Spindle MT Flux Rate
Mitosis images were taken every 3 s with a LSM780-DUO-NLO confocal microscope. Kinesin-13 KO (GPH0438#30) and control (GFP-tubulin/histoneH2B-mRFP) mitosis were used for analysis. Cells were bleached along the equator of the mitotic spindle shortly after NEBD once the spindle shape was formed, before anaphase entry. Next, 38-pixel width slices covering the length of the spindle were cut out and arranged using the montage tool in FIJI for easy viewing of the movement of the bleached region. Their slopes were then taken as the MT flux rate.
Protonema Filament Bend Frequency
Epifluorescence images of moss protonema filaments cultured on BCDAT media were used for analysis in FIJI. Contrast was adjusted to make edges of protonema filaments clearer. A blind test was performed to ascertain the waviness threshold; acute angles of above 18° were determined to be wavy. Randomly chosen protonema filaments were analyzed for all the angles of bends along a length of protonema filament using the FIJI in-built angle tool. The number of bends that were wavy (18° < angle < 90°) were counted and taken over the length measured as bend frequency.
MT Foci Trajectories
The MT foci of Kinesin-13 KO, Kinesin-8 KO, and control moss expressing GFP-tubulin were imaged at ×60 magnification with a z-series every 0.3 µm for a range of 20 µm every 3 min for 3 h. The maximum z-projection of the files was analyzed using a FIJI MOSAIC plug-in (Sbalzarini and Koumoutsakos, 2005) particle tracker 2D/3D (radius, 20; cutoff, 0.001; per/abs, 0.005; link range, 5; displacement, 20; dynamics, Brownian) to automatically generate the MT foci trajectories. The linear regression of the trajectories was rotated to horizontal and normalized to start at (x, y) = (0, 0) to plot the graphs in Figure 5D.
Interphase Endoplasmic MT Plus End Dynamics
For plus end shrinkage rate, catastrophe frequency, and rescue frequency, oblique illumination time-lapse images taken every 3 s of the interphase endoplasmic MT network of Kinesin-13 KO (GPH0438#30) and control (GFP-tubulin/histoneH2B-mRFP) were analyzed. For MT shrinkage rate, kymographs were drawn on five randomly chosen MTs per cell (cell n = 25; total MT n = 125), and the slope was taken as shrinkage rate. To analyze catastrophe and rescue frequencies, an ∼5 × 6-µm area of a cell was randomly selected whereupon every traceable MT plus end was traced for a kymograph to count the number of catastrophe or rescue events over the observed growth or shrink time, respectively. For ease of tracking, areas with fewer MTs, but that were not near the cell plate, were preferred. Two independent experiments were performed and consistent results were obtained. To determine the MT growth rate, Kinesin-13 KO moss expressing EB1-Citrine was used, where Citrine marks growing MT plus ends. Spinning disc time-lapse images taken every 3 s of the interphase endoplasmic MT array in Kinesin-13 KO (GPH0577 no. 2, 11) and control (EB1-Citrine/mCherry-tubulin, GPH0379 no. 2) moss were analyzed. Kymographs were taken along the edge of the apical side of the tip cell of protonema filaments. Slopes of EB1-Citrine comets in kymographs were measured and taken as the growth rate.
Statistics
Welch’s two-sample t test (two sided) was used when samples were approximately normally distributed. Tukey multiple comparison test was used to analyze the data presented in Figure 5B. Results from statistic tests are in Supplemental Data Set 3. Significance with the following P-values are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001.
Accession Numbers
Sequence data from this article can be found in the Joint Genome Institute, U.S. Department of Energy)Phytozome data library under the following accession numbers: Kinesin-13a (Pp3c1_27370); Kinesin-13b (Pp3c14_9830); Kinesin-13c (Pp3c10_106980); Kinesin-8Ia (Pp3c1_32120); Kinesin-8Ib (Pp3c2_3070); Kinesin-8II (Pp3c21_9290).
Supplemental Data
Supplemental Figure 1. PCR confirmation of Kinesin-13 and Kinesin-8 KO lines.
Supplemental Figure 2. Localization of Kinesin-13 paralogues during mitosis and interphase.
Supplemental Figure 3. Protonema filaments of Kinesin-13 single, double, and triple KO moss, Kinesin-13b full-length rescue moss, and Kinesin-13b mutant moss.
Supplemental Figure 4. MT foci and cell tip directional changes during bending of protonema filament.
Supplemental Figure 5. Coomassie blue staining of purified proteins used in in vitro assays.
Supplemental Data Set 1. Alignments used to generate the phylogenies presented in Figure 1B.
Supplemental Data Set 2. Materials used in this study.
Supplemental Data Set 3. Welch’s two-sample t test and Tukey multiple comparison test results.
Supplemental Movie 1. Mitosis of control, Kinesin-13, and Kinesin-8 KO moss.
Supplemental Movie 2. Spindle poleward flux of control and Kinesin-13 KO moss.
Supplemental Movie 3. Protonema filament growth of control, Kinesin-13 and -8 KO moss.
Supplemental Movie 4. MT foci in protonema growth of control, Kinesin-13 and -8 KO moss.
Supplemental Movie 5. Localization of Kinesin-13 during interphase.
Supplemental Movie Legends. Legends for Supplemental Movies 1 to 5.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Yoshihisa Oda, Momoko Nishina, and Tomohiro Miki for moss lines and plasmids; Yoshikatsu Sato and Advanced Bioimaging Support (ABiS) platform (Japan Society for the Promotion of Science Grant JP16H06280) for help with the photobleaching experiments; Elena Kozgunova and Rie Inaba for technical assistance; Mitsuyasu Hasebe for reagents; and Shogo Takatani for discussions. This work was funded by Japan Society for the Promotion of Science KAKENHI (17H06471 to G.G. and 19K23723 to M.Y.) and by Japan Society for the Promotion of Science and DFG under the Joint Research Projects-LEAD with UKRI (to G.G.).
AUTHOR CONTRIBUTIONS
M.Y. and G.G. conceived project; S.Y.L. and M.Y. designed and performed experiments; S.Y.L., T.E., and M.Y. analyzed experimental data; T.E. performed computer simulation; S.Y.L., G.G., and M.Y. wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Collonnier C., Epert A., Mara K., Maclot F., Guyon-Debast A., Charlot F., White C., Schaefer D.G., Nogué F. (2017). CRISPR-Cas9-mediated efficient directed mutagenesis and RAD51-dependent and RAD51-independent gene targeting in the moss Physcomitrella patens. Plant Biotechnol. J. 15: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. (2005). The moss Physcomitrella patens. Annu. Rev. Genet. 39: 339–358. [DOI] [PubMed] [Google Scholar]
- Dawson S.C., Sagolla M.S., Mancuso J.J., Woessner D.J., House S.A., Fritz-Laylin L., Cande W.Z. (2007). Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryotic Cell 6: 2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.Y., Liu L.T., Li T., Yan S., Kuang B.J., Huang S.J., Yan C.J., Wang T. (2015). OsKinesin-13A is an active microtubule depolymerase involved in glume length regulation via affecting cell elongation. Sci. Rep. 5: 9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Verma S., Mitchison T.J., Walczak C.E. (1999). Kin I kinesins are microtubule-destabilizing enzymes. Cell 96: 69–78. [DOI] [PubMed] [Google Scholar]
- Doonan J.H., Cove D.J., Lloyd C.W. (1985). Immunofluorescence microscopy of microtubules in intact cell lineages of the moss, Physcomitrella patens. I. Normal and CIPC-treated tip cells. J. Cell Sci. 75: 131–147. [DOI] [PubMed] [Google Scholar]
- Doonan J.H., Cove D.J., Lloyd C.W. (1988). Microtubules and microfilaments in tip growth: Evidence that microtubules impose polarity on protonemal growth in Physcomitrella patens. J. Cell Sci. 89: 533–540. [Google Scholar]
- Edzuka T., Goshima G. (2019). Drosophila kinesin-8 stabilizes the kinetochore-microtubule interaction. J. Cell Biol. 218: 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R.C., Wasteneys G.O. (2014). The microtubule plus-end tracking protein ARMADILLO-REPEAT KINESIN1 promotes microtubule catastrophe in Arabidopsis. Plant Cell 26: 3372–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura U., Elsaesser L., Breuninger H., Sánchez-Rodríguez C., Ivakov A., Laux T., Findlay K., Persson S., Lenhard M. (2014). Atkinesin-13A modulates cell-wall synthesis and cell expansion in Arabidopsis thaliana via the THESEUS1 pathway. PLoS Genet. 10: e1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Upton K., Compton D.A. (2005). Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 15: 1827–1832. [DOI] [PubMed] [Google Scholar]
- Gatt M.K., Savoian M.S., Riparbelli M.G., Massarelli C., Callaini G., Glover D.M. (2005). Klp67A destabilises pre-anaphase microtubules but subsequently is required to stabilise the central spindle. J. Cell Sci. 118: 2671–2682. [DOI] [PubMed] [Google Scholar]
- Gell C., et al. (2010). Microtubule dynamics reconstituted in vitro and imaged by single-molecule fluorescence microscopy. Methods Cell Biol. 95: 221–245. [DOI] [PubMed] [Google Scholar]
- Gicking A.M., Swentowsky K.W., Dawe R.K., Qiu W. (2018). Functional diversification of the kinesin-14 family in land plants. FEBS Lett. 592: 1918–1928. [DOI] [PubMed] [Google Scholar]
- Goshima G., Scholey J.M. (2010). Control of mitotic spindle length. Annu. Rev. Cell Dev. Biol. 26: 21–57. [DOI] [PubMed] [Google Scholar]
- Goshima G., Vale R.D. (2003). The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell Biol. 162: 1003–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.L. Jr., Carvalho P., Roof D.M., Pellman D. (2006). Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat. Cell Biol. 8: 913–923. [DOI] [PubMed] [Google Scholar]
- Hertzer K.M., Ems-McClung S.C., Kline-Smith S.L., Lipkin T.G., Gilbert S.P., Walczak C.E. (2006). Full-length dimeric MCAK is a more efficient microtubule depolymerase than minimal domain monomeric MCAK. Mol. Biol. Cell 17: 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt E.R., Hoyt M.A. (2000). Mitotic motors in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1496: 99–116. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Tanaka Y., Niwa S. (2009). Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10: 682–696. [DOI] [PubMed] [Google Scholar]
- Hiwatashi Y., Sato Y., Doonan J.H. (2014). Kinesins have a dual function in organizing microtubules during both tip growth and cytokinesis in Physcomitrella patens. Plant Cell 26: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma N., Takei Y., Tanaka Y., Nakata T., Terada S., Kikkawa M., Noda Y., Hirokawa N. (2003). Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114: 229–239. [DOI] [PubMed] [Google Scholar]
- Howard J., Hyman A.A. (2007). Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 19: 31–35. [DOI] [PubMed] [Google Scholar]
- Jonsson E., Yamada M., Vale R.D., Goshima G. (2015). Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants. Nat. Plants 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K., Kurinami S., Oki K., Abe Y., Ando T., Kono I., Yano M., Kitano H., Iwasaki Y. (2010). A novel kinesin 13 protein regulating rice seed length. Plant Cell Physiol. 51: 1315–1329. [DOI] [PubMed] [Google Scholar]
- Kline-Smith S.L., Khodjakov A., Hergert P., Walczak C.E. (2004). Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell 15: 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Tsang W.Y., Li J., Lane W., Dynlacht B.D. (2011). Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145: 914–925. [DOI] [PubMed] [Google Scholar]
- Kofuji R., Hasebe M. (2014). Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr. Opin. Plant Biol. 17: 13–21. [DOI] [PubMed] [Google Scholar]
- Kosetsu K., de Keijzer J., Janson M.E., Goshima G. (2013). MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in Physcomitrella patens. Plant Cell 25: 4479–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozgunova E., Goshima G. (2019). A versatile microfluidic device for highly inclined thin illumination microscopy in the moss Physcomitrella patens. Sci. Rep. 9: 15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Langford K.J., Askham J.M., Brüning-Richardson A., Morrison E.E. (2008). MCAK associates with EB1. Oncogene 27: 2494–2500. [DOI] [PubMed] [Google Scholar]
- Leong S.Y., Yamada M., Yanagisawa N., Goshima G. (2018). SPIRAL2 stabilises endoplasmic microtubule minus ends in the moss Physcomitrella patens. Cell Struct. Funct. 43: 53–60. [DOI] [PubMed] [Google Scholar]
- Li W., Moriwaki T., Tani T., Watanabe T., Kaibuchi K., Goshima G. (2012). Reconstitution of dynamic microtubules with Drosophila XMAP215, EB1, and Sentin. J. Cell Biol. 199: 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Obando M., Hoffmann B., Géry C., Guyon-Debast A., Téoulé E., Rameau C., Bonhomme S., Nogué F. (2016). Simple and efficient targeting of multiple genes through CRISPR-Cas9 in Physcomitrella patens. G3 (Bethesda) 6: 3647–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Lee Y.R., Pan R., Maloof J.N., Liu B. (2005). An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol. Biol. Cell 16: 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Wagenbach M., Wordeman L. (2001). Molecular dissection of the microtubule depolymerizing activity of mitotic centromere-associated kinesin. J. Biol. Chem. 276: 34753–34758. [DOI] [PubMed] [Google Scholar]
- Mayr M.I., Hümmer S., Bormann J., Grüner T., Adio S., Woehlke G., Mayer T.U. (2007). The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr. Biol. 17: 488–498. [DOI] [PubMed] [Google Scholar]
- Menand B., Calder G., Dolan L. (2007). Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J. Exp. Bot. 58: 1843–1849. [DOI] [PubMed] [Google Scholar]
- Mennella V., Rogers G.C., Rogers S.L., Buster D.W., Vale R.D., Sharp D.J. (2005). Functionally distinct kinesin-13 family members cooperate to regulate microtubule dynamics during interphase. Nat. Cell Biol. 7: 235–245. [DOI] [PubMed] [Google Scholar]
- Menon S., Gupton S.L. (2016). Building blocks of functioning brain: cytoskeletal dynamics in neuronal development. Int. Rev. Cell Mol. Biol. 322: 183–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H., Okada Y., Hirokawa N. (2005). Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 15: 467–476. [DOI] [PubMed] [Google Scholar]
- Miki T., Nakaoka Y., Goshima G. (2016). Live cell microscopy-based RNAi screening in the moss Physcomitrella patens. Methods Mol. Biol. 1470: 225–246. [DOI] [PubMed] [Google Scholar]
- Miki T., Naito H., Nishina M., Goshima G. (2014). Endogenous localizome identifies 43 mitotic kinesins in a plant cell. Proc. Natl. Acad. Sci. USA 111: E1053–E1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Nishina M., Goshima G. (2015). RNAi screening identifies the armadillo repeat-containing kinesins responsible for microtubule-dependent nuclear positioning in Physcomitrella patens. Plant Cell Physiol. 56: 737–749. [DOI] [PubMed] [Google Scholar]
- Moores C.A., Yu M., Guo J., Beraud C., Sakowicz R., Milligan R.A. (2002). A mechanism for microtubule depolymerization by KinI kinesins. Mol. Cell 9: 903–909. [DOI] [PubMed] [Google Scholar]
- Moriwaki T., Goshima G. (2016). Five factors can reconstitute all three phases of microtubule polymerization dynamics. J. Cell Biol. 215: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka Y., Kimura A., Tani T., Goshima G. (2015). Cytoplasmic nucleation and atypical branching nucleation generate endoplasmic microtubules in Physcomitrella patens. Plant Cell 27: 228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka Y., Miki T., Fujioka R., Uehara R., Tomioka A., Obuse C., Kubo M., Hiwatashi Y., Goshima G. (2012). An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell 24: 1478–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A., Dixit R. (2018). Kinesins and myosins: Molecular motors that coordinate cellular functions in plants. Annu. Rev. Plant Biol. 69: 329–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa S., Nakajima K., Miki H., Minato Y., Wang D., Hirokawa N. (2012). KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev. Cell 23: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Oda Y., Fukuda H. (2013). Rho of plant GTPase signaling regulates the behavior of Arabidopsis kinesin-13A to establish secondary cell wall patterns. Plant Cell 25: 4439–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Saijo S., Shimizu N., Jiang X., Hirokawa N. (2017). Mechanism of catalytic microtubule depolymerization via KIF2-tubulin transitional conformation. Cell Reports 20: 2626–2638. [DOI] [PubMed] [Google Scholar]
- Ovechkina Y., Wagenbach M., Wordeman L. (2002). K-loop insertion restores microtubule depolymerizing activity of a “neckless” MCAK mutant. J. Cell Biol. 159: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Day I.S. (2001). Kinesins in the Arabidopsis genome: A comparative analysis among eukaryotes. BMC Genomics 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G.C., Rogers S.L., Schwimmer T.A., Ems-McClung S.C., Walczak C.E., Vale R.D., Scholey J.M., Sharp D.J. (2004). Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427: 364–370. [DOI] [PubMed] [Google Scholar]
- Rogers G.C., Rogers S.L., Sharp D.J. (2005). Spindle microtubules in flux. J. Cell Sci. 118: 1105–1116. [DOI] [PubMed] [Google Scholar]
- Rounds C.M., Bezanilla M. (2013). Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol. 64: 243–265. [DOI] [PubMed] [Google Scholar]
- Sabry J.H., O’Connor T.P., Evans L., Toroian-Raymond A., Kirschner M., Bentley D. (1991). Microtubule behavior during guidance of pioneer neuron growth cones in situ. J. Cell Biol. 115: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbalzarini I.F., Koumoutsakos P. (2005). Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 151: 182–195. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Smertenko A. (2019). Identification and characterization of the land-plant-specific microtubule nucleation factor MACET4. J. Cell Sci. 132: jcs232819. [DOI] [PubMed] [Google Scholar]
- Shen Z., Collatos A.R., Bibeau J.P., Furt F., Vidali L. (2012). Phylogenetic analysis of the Kinesin superfamily from physcomitrella. Front. Plant Sci. 3: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley K., Hekmat-Nejad M., Turner J., Moores C., Anderson R., Milligan R., Sakowicz R., Fletterick R. (2004). Structure of a kinesin microtubule depolymerization machine. EMBO J. 23: 1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V., Verhey K.J. (2014). The family-specific K-loop influences the microtubule on-rate but not the superprocessivity of kinesin-3 motors. Mol. Biol. Cell 25: 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpff J., von Dassow G., Wagenbach M., Asbury C., Wordeman L. (2008). The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev. Cell 14: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpff J., Wagenbach M., Franck A., Asbury C.L., Wordeman L. (2012). Kif18A and chromokinesins confine centromere movements via microtubule growth suppression and spatial control of kinetochore tension. Dev. Cell 22: 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E., Ho T., Kirschner M.W. (1995). The role of microtubule dynamics in growth cone motility and axonal growth. J. Cell Biol. 128: 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R., Tsukada Y., Kamasaki T., Poser I., Yoda K., Gerlich D.W., Goshima G. (2013). Aurora B and Kif2A control microtubule length for assembly of a functional central spindle during anaphase. J. Cell Biol. 202: 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth A., Masuda H., Dhut S., Toda T. (2008). Fission yeast kinesin-8 Klp5 and Klp6 are interdependent for mitotic nuclear retention and required for proper microtubule dynamics. Mol. Biol. Cell 19: 5104–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Augustine R.C., Kleinman K.P., Bezanilla M. (2007). Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 19: 3705–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C.E., Heald R. (2008). Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 265: 111–158. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Gayek S., Ohi R. (2013). Microtubule-depolymerizing kinesins. Annu. Rev. Cell Dev. Biol. 29: 417–441. [DOI] [PubMed] [Google Scholar]
- West R.R., Malmstrom T., Troxell C.L., McIntosh J.R. (2001). Two related kinesins, klp5+ and klp6+, foster microtubule disassembly and are required for meiosis in fission yeast. Mol. Biol. Cell 12: 3919–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.Z., Bezanilla M. (2018). Actin and microtubule cross talk mediates persistent polarized growth. J. Cell Biol. 217: 3531–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Goshima G. (2018). The KCH kinesin drives nuclear transport and cytoskeletal coalescence to promote tip cell growth in Physcomitrella patens. Plant Cell 30: 1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Miki T., Goshima G. (2016). Imaging mitosis in the moss Physcomitrella patens. Methods Mol. Biol. 1413: 263–282. [DOI] [PubMed] [Google Scholar]
- Yoshida M.W., Yamada M., Goshima G. (2019). Moss Kinesin-14 KCBP accelerates chromatid motility in anaphase. Cell Struct. Funct. 44: 95–104. [DOI] [PubMed] [Google Scholar]