Abstract
Background
In many countries women are given their own case notes to carry during pregnancy to increase their sense of control over, and satisfaction with, their care.
Objectives
To evaluate the effects of giving women their own case notes to carry during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2015) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials of women given their own case notes to carry during pregnancy.
Data collection and analysis
Two review authors independently applied the inclusion criteria and assessed study quality. One review author extracted data from the included studies using a standard form (checked by second review author). We assessed estimates of effect using risk ratio (RR) with 95% confidence intervals (CI). The quality of the evidence was assessed using the GRADE approach.
Main results
Four trials were included (n = 1176 women). Overall, the quality of the evidence was graded as low to moderate mainly due to the nature of the intervention not allowing blinding. The updated search identified one cluster‐randomised trial, which was included.
Women carrying their own notes were more likely to feel in control (two trials, RR 1.56, 95% CI 1.18 to 2.06; 450 women; moderate quality evidence), although there is no evidence of difference in women's satisfaction (two trials, average RR 1.09, 95% CI 0.92 to 1.29); 698 women; low quality evidence). More women in the case notes group wanted to carry their own notes in a subsequent pregnancy (three trials, RR 1.79, 95% CI 1.57 to 2.03; 552 women; low quality evidence). Overall, the pooled estimate of the two trials (n = 347) that reported on the risk of notes lost or left at home was not significant (average RR 0.38, 95% CI 0.04 to 3.84). There was no evidence of difference for health‐related behaviours (cigarette smoking and breastfeeding (moderate quality evidence)), analgesia needs during labour (low quality evidence), maternal depression, miscarriage, stillbirth and neonatal deaths (moderate quality evidence). More women in the case notes group had operative deliveries (one trial, RR 1.83, 95% CI 1.08 to 3.12; 212 women), and caesarean sections (one trial, average RR 1.51, 95% CI 1.10 to 2.08; 501 women; moderate quality evidence).
Authors' conclusions
The four trials are small, and not all of them reported on all outcomes. The results suggest that there are both potential benefits (increased maternal control and increased availability of antenatal records during hospital attendance) and harms (more operative deliveries). Importantly, all of the trials report that more women in the case notes group would prefer to carry their antenatal records in another pregnancy. There is insufficient evidence on health‐related behaviours (smoking and breastfeeding), women's satisfaction, and clinical outcomes. It is important to emphasise that this review shows a lack of evidence of benefit rather than evidence of no benefit.
Plain language summary
Giving women their own case notes to carry during pregnancy
Overall, the quality of the evidence was graded as low to moderate. The updated search identified one cluster‐randomised trial, which was included.
Women carrying their own case notes improves their sense of control and the availability of antenatal records, but insufficient evidence of additional effects.
In some healthcare systems women are given their own case notes to look after and bring to each antenatal visit. This review of four trials, involving 1176 women, suggests that there are both potential benefits (increased availability of antenatal records during hospital attendance, and increased maternal control) and harms (more operative deliveries). All the trials reported that more women in the case notes group would prefer to hold their antenatal records in another pregnancy, but there was not enough evidence to determine the effect of women carrying their own case notes on health behaviours such as smoking and breastfeeding, women's satisfaction, and clinical outcomes.
Summary of findings
Summary of findings for the main comparison. Case notes versus control.
| Case notes versus control | ||||||
| Population: pregnant women from the time of their first antenatal visit to the end of the puerperium Settings: UK, Australia, Mongol Intervention: any intervention that involved giving women their own case notes to carry during their pregnancy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Case notes versus control | |||||
| Women who felt in control | Study population | RR 1.56 (1.18 to 2.06) | 450 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 235 per 1000 | 367 per 1000 (278 to 485) | |||||
| Moderate | ||||||
| 226 per 1000 | 353 per 1000 (267 to 466) | |||||
| Women's satisfaction with antenatal care | Study population | RR 1.09 (0.92 to 1.29) | 698 (2 studies) | ⊕⊕⊝⊝ low1,2 | Inverse variance. | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Women who wanted to carry case notes in subsequent pregnancy | Study population | RR 1.79 (1.57 to 2.03) | 552 (3 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 494 per 1000 | 885 per 1000 (776 to 1000) | |||||
| Moderate | ||||||
| 516 per 1000 | 924 per 1000 (810 to 1000) | |||||
| Breastfeeding | Study population | RR 1.00 (0.99 to 1.02) | 704 (2 studies) | ⊕⊕⊕⊝ moderate1 | Inverse variance. | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Epidural analgesia used in labour | Study population | RR 1.43 (0.96 to 2.13) | 212 (1 study) | ⊕⊕⊝⊝ low2,4 | ||
| 269 per 1000 | 384 per 1000 (258 to 572) | |||||
| Moderate | ||||||
| 269 per 1000 | 385 per 1000 (258 to 573) | |||||
| Caesarean section | Study population | RR 1.51 (1.1 to 2.08) | 501 (1 study) | ⊕⊕⊕⊝ moderate5 | Inverse variance. | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Stillbirth or neonatal death | Study population | RR 1 (0.99 to 1.01) | 713 (2 studies) | ⊕⊕⊕⊝ moderate1 | Inverse variance. | |
| See comment | See comment | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Most studies contributing data had design limitations. 2 Wide confidence interval crossing the line of no effect. 3 Statistical Heterogeneity (I² > 60%). 4 One study with design limitations. 5 Estimate based on small sample size.
Background
Description of the condition
Over the last few years, there has been much interest regarding the best way in which to deliver antenatal care to women (Munjanja 1996; Dowswell 2015). Challenges to the traditional methods of antenatal care and its effectiveness are being made (Backe 1993), and research from the UK has suggested that women want more control over their care during pregnancy and labour (DoH 1993). One aspect of the change in provision of care is giving women their own case notes to carry throughout their pregnancy. This will empower women by facilitating greater participation in their medical care (Homer 1999), improve the availability of records when needed and reduce the cost. An important benefit of giving women their own case notes is that all healthcare providers will write in one record, which will reduce clinical error and aid carer to carer communication. In addition, it has been hypothesised that women who take responsibility for their own case notes will also exhibit other improved obstetric behaviours such as reduced smoking, reduced need for analgesia in labour and improved breastfeeding (Elbourne 1987).
Description of the intervention
In many low‐income countries record keeping is problematic as hospitals struggle to maintain their infrastructure with limited funds, often resulting in 'lost' records or no access to records. This was part of the motivation to develop the 'Road to Health' card; a parent‐carried card detailing a child's growth, medical history and immunisation record. This was shown to be of value in a low‐income country (Donald 1984). More recent work showed that parents and staff liked the concept of the 'Road to Health' card, but wanted it to be more detailed, including illustrations, charts and more health information, more personalised to each child and in the parents' home language (Harrison 1998).
How the intervention might work
Many of the same motivations for woman‐carried records apply for the antenatal period. Woman‐carried records are also important when women move from one facility to another during pregnancy, which is common in both high‐ and low‐income countries. In many countries, pregnant women have been issued with a summary of their notes and pregnancy history and progress in a woman‐carried card, which they bring with them for each visit. The objective is to have easy access to each pregnant woman's medical record and also to reduce the administrative costs involved in keeping and retrieving traditional hospital records. The other advantage of woman‐carried records which incorporate clinical, and where available ultrasound fetal growth charts, is that even if the woman moves between health facilities, fetal growth can be monitored and a constant and more accurate assessment of gestational age maintained (Hofmeyr 1994). An indirect benefit of woman‐carried case notes is that the quality of care will improve as communication between the woman and the caregiver is more likely, and the woman is better able to participate in the information exchange.
The content of women‐held maternity care notes is important; they should contain all the information that is pertinent to her pregnancy and childbirth care, particularly if the woman is issued with an abbreviated form of the main case notes. Merely replacing traditional hospital kept records with incomplete or inadequate woman‐carried case notes will not be of benefit.
Why it is important to do this review
A foreseeable problem with woman‐carried case notes is if they are lost, or if the woman does not bring her case notes with her when she attends antenatal care or arrives in labour.
Many countries may be encouraged to implement a practice of women carrying their own case notes before there is clear evidence of benefit or harm. This review aims to evaluate the impact of giving women their own case notes to carry during pregnancy on both the quality of care and clinical outcomes, thus providing evidence as to the benefits or harms of this practice that can be used in health policy decision‐making.
Objectives
To evaluate the effects of giving women their own case notes to carry during pregnancy on administrative outcomes, maternal satisfaction and control, health‐related behaviours and clinical outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, including cluster‐randomised studies.
Types of participants
Pregnant women from the time of their first antenatal visit to the end of the postpartum period.
Types of interventions
Any intervention that involved giving women their own case notes to carry during their pregnancy from the time of their first antenatal visit through the time of hospital admission for the birth of the baby and into the postpartum period.
Types of outcome measures
Primary outcomes
Maternal satisfaction and control
Number of women who felt in control and involved in decision‐making during their pregnancy.
Number of women who reported that they were satisfied with their antenatal care.
Number of women who wanted to carry their own notes in a subsequent pregnancy.
Administrative
Availability of complete antenatal records at time of delivery.
Number of notes lost or left at home when attending hospital.
Secondary outcomes
-
Partner involvement in the pregnancy, during labour and after the birth:
number of partners attending antenatal clinic;
number of partners present during labour;
number of partners actively involved in the care of the baby.
Health‐related behaviours
Number of women who stopped or reduced cigarette smoking.
-
Breastfeeding practices:
number of women choosing to breastfeed;
duration of breastfeeding;
number of women using breast milk supplements.
Clinical
Number of women needing analgesia during labour.
Number of women who had a caesarean section.
Number of women who had an assisted vaginal delivery.
-
Perinatal outcomes:
short‐term morbidity (low five‐minute Apgar scores, transient tachypnoea of the newborn);
mortality;
admissions to special care units.
-
Maternal outcomes:
short‐term morbidity (haemorrhage, infection, blood transfusion, pregnancy loss, intensive care unit admission, depression);
mortality.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeBrown 2004.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons:
number of women who felt in control and involved in decision‐making during their pregnancy;
number of women who reported that they were satisfied with their antenatal care;
number of women who wanted to carry their own notes in a subsequent pregnancy;
number of women choosing to breastfeed;
number of women needing analgesia during labour;
number of women who had a caesarean section;
mortality of neonates (including stillbirth or neonatal death).
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings' table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
No continuous data were analysed in this review update. In future updates, if relevant, we will use the mean difference (MD) if outcomes are measured in the same way between trials. We will use the standardised mean difference (SMD) to combine trials that measured the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We included one cluster‐randomised trial in the analyses along with individually‐randomised trials (Mori 2015). The trial statistician provided cluster‐adjusted RRs for this review upon request. If in future updates we include more cluster trials, we will use appropriate methods to ensure the data are adjusted for design. We may adjust their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial, from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC.
We combined the results from both cluster‐ and individually‐randomised trials using the inverse variance method and random‐effects analysis. There was little heterogeneity between the study designs and no evidence of differences between subgroups according to randomisation unit.
Cross‐over trials
We have not included cross‐over trials in this review as this is not an eligible study design.
Other unit of analysis issues
We considered for each outcome whether the appropriate denominator is the number of babies or the number of women for multiple pregnancy. For all infant outcomes, the number of babies was the appropriate denominator, and for maternal outcomes, the number of women was used.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. We used the inverse variance method and random‐effects analyses for all outcomes where the cluster‐randomised trial and individually‐randomised trials were combined.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and planned to carry out sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Individually‐randomised versus cluster‐randomised trials.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
There were too few studies included to carry out sensitivity analyses. In future updates, we will conduct sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
A updated search in 2015 search identified one report of a trial for possible inclusion. This trial (Mori 2015) was included.
Included studies
Eligibility
Four studies met the inclusion criteria (n = 1176).
Location
Three included studies were conducted within the public health sector of well‐resourced countries, two in the United Kingdom (UK) and one in Australia. One study in the UK was conducted in a teaching hospital antenatal clinic serving a predominantly low‐income population with a large proportion of lone parents, high unemployment and racial groups disproportionately affected by social deprivation (Lovell 1987). The other UK study was conducted in a rural setting at a consultant‐led peripheral antenatal clinic, and there is no comment on the socioeconomic profile of these women (Elbourne 1987). The Australian study was carried out at the antenatal clinic in a teaching hospital in an urban area; no socioeconomic profile of the participants is provided other than that they were all English speaking (Homer 1999). A fourth included study took place in Bulgan, rural Mongolia (Mori 2015).
Participants
All four studies recruited women for the trial at their first antenatal 'booking' visit.
Interventions
In three trials the intervention groups were given their complete antenatal records to carry and the control groups were given a card referred to as a co‐operation or 'co‐op' card, which is a card carried by the pregnant woman but with much abbreviated information and no clinical follow‐up or clinical progress information. In the fourth trial (Mori 2015), women in the intervention group carried a handbook to log maternal health, pregnancy and delivery information as well as child health measures, such as immunisation and growth charts; women in the control arm of this trial received standard antenatal care, and the intervention was rolled out in control clusters after nine months.
Outcomes
For all four trials (Elbourne 1987; Homer 1999; Lovell 1987; Mori 2015), data on the primary and secondary outcomes were collected using self‐administered questionnaires. None of the trials reported whether questionnaires were validated. Mori 2015 also collected data from pregnant women by questionnaire at one month post‐birth; additionally, data collectors visited clinics and hospitals for records of antenatal visits and delivery outcomes.
All four trials reported on the number of women who reported being satisfied with care, but availability of antenatal records at time of delivery was not addressed in any of the trials. Three trials reported the number of notes lost or left at home and the number of women who wanted to carry their own notes (Elbourne 1987; Homer 1999; Lovell 1987); two trials reported on number of women who felt in control (Elbourne 1987; Homer 1999). However, the outcomes were reported differently in each trial; Elbourne 1987 used rate ratios, while Lovell 1987 and Homer 1999 reported percentages.
None of the secondary outcomes were reported in all four trials. Partner involvement was addressed indirectly in two trials (Elbourne 1987; Homer 1999), and the number of women who stopped smoking was reported in two trials (Elbourne 1987; Lovell 1987). Lovell 1987 addressed each of the following outcomes: the number of women needing analgesia; number who had an assisted delivery (caesarean section and forceps); miscarriage; stillbirth and neonatal death; and the number of women breastfeeding after delivery. Mori 2015 reported several secondary outcomes, including breastfeeding; assisted vaginal delivery; caesarean section; stillbirth or neonatal death; admission to neonatal intensive care unit, maternal intensive care unit admission and maternal depression.
Excluded studies
Three studies were excluded because they were not randomised controlled trials (Draper 1986; Phipps 2001; Webster 1996). One study did not involve giving women their own records to carry but instead considered computer stored cards versus hard copy cards (Jenkinson 1989). One study, registered as a planned study in 1997 (Aarts 1997), has been excluded because we have been unable to locate the author to confirm whether the trial was conducted, and if the results are available.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of all "Risk of bias" assessments.
1.
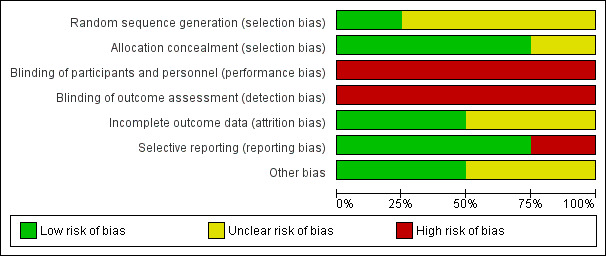
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
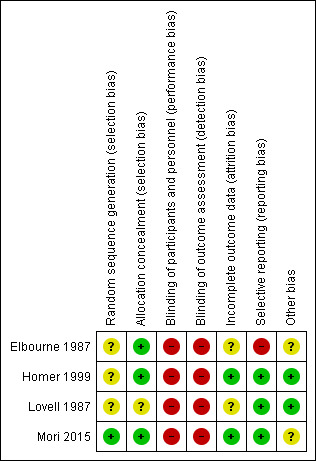
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was not described in three studies (Elbourne 1987; Homer 1999; Lovell 1987) and in one study (Mori 2015) sequence was generated according to the shuffling of sealed envelopes. In three studies concealment of allocation was adequate, all of which used consecutively numbered, sealed, opaque envelopes (Elbourne 1987; Homer 1999; Mori 2015); one trial (Lovell 1987) mentioned random allocation, but did not specify the method of concealment.
Blinding
Due to the nature of the intervention, blinding was not employed for any of the studies included (Elbourne 1987; Homer 1999; Lovell 1987; Mori 2015).
Incomplete outcome data
In the trial by Lovell 1987, 246 women were considered eligible for the study, 11 refused, leaving 235 who were randomised and on whom basic data were available. Seven women miscarried or were lost to follow‐up, leaving 228 (93%) who completed the first questionnaire. In the trial by Homer 1999, 150 women were considered to be eligible for the study, 22 (13%) refused and were not randomised. One‐hundred and twenty‐six women completed the first questionnaire, but not all women answered each question. In Elbourne 1987, 161 women in the case note group and 156 women in the co‐op card group were allocated, and 27 women found ineligible after randomisation and before the initial survey. There were 19 dropouts out of 147 participants in the control group and 22 dropouts out of 143 in the intervention group. In the cluster‐randomised trial (Mori 2015), three areas were excluded before randomisation; one was the subject of a pilot study, and two areas were included in another health study. Nine clusters each received the intervention or the control. Missing outcome data for individual women are reported and minimal.
Selective reporting
Miscarriage, abortion and neonatal deaths are mentioned in the text but not reported according to treatment arm in Elbourne 1987. In all the other included trials, reported outcomes were pre‐specified.
Other potential sources of bias
Elbourne 1987 reported both contamination and Hawthorn effects may have reduced the differences between treatment groups. Mori 2015 reported baseline imbalances in several variables, and adjusted risk ratios (RR) for these variables. Cluster designs were used for meta‐analyses.
Effects of interventions
See: Table 1
Comparison: Case notes versus control
We included four trials involving 1176 women.
Primary outcomes
Women's satisfaction and sense of control
1. Number of women who felt in control and involved in decision‐making during their pregnancy
Two trials contributed data to outcome (n = 450) (Elbourne 1987; Lovell 1987). The pooled estimate shows that women carrying their own case notes were significantly more likely to feel in control (RR 1.56, 95% confidence interval (CI) 1.18 to 2.06; women = 450; studies = two; Analysis 1.1).
1.1. Analysis.
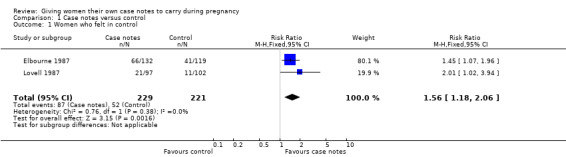
Comparison 1 Case notes versus control, Outcome 1 Women who felt in control.
Homer 1999 used open‐ended questions to ask women about their allocated method of record keeping; positive comments (89%) included a sense of control during the pregnancy (Homer 1999).
2. Number of women who reported that they were satisfied with their antenatal care
Women holding case notes reported a similar level of satisfaction with their antenatal care as women with standard care or data record cards (average RR 1.09, 95% CI 0.92 to 1.29; participants = 698; studies = two; Analysis 1.2). There was moderate heterogeneity for this outcome (Heterogeneity: Tau² = 0.01; I² = 53%), but there was no evidence of a meaningful difference between subgroups based on the randomisation unit (Test for subgroup differences: Chi² = 2.14, df = 1 (P = 0.14), I² = 53.4%). It is possible that the population of pregnant women, trial location (London UK and rural Mongolia), or structure of care may each contribute to heterogeneity.
1.2. Analysis.
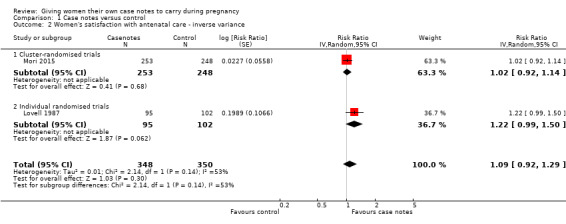
Comparison 1 Case notes versus control, Outcome 2 Women's satisfaction with antenatal care ‐ inverse variance.
Further evidence could not be included in our meta‐analysis. Homer 1999 evaluated maternal satisfaction with overall care using 17 indirect parameters (evaluated using a five‐point Likert scale) in a questionnaire with women; none of these showed statistically significant differences between the two groups (no data given). Elbourne 1987 reported no statistically significant differences in satisfaction with care between women carrying their own notes and women holding only co‐operation cards; no data were provided in the published paper.
3. Number of women who wanted to carry their own notes in a subsequent pregnancy
Three trials reported this outcome (n = 552); each trial found that more women in the case notes group would prefer to hold their antenatal records in another pregnancy. The I² test showed significant heterogeneity (I² = 66.1%), however the resultant random‐effects model was very close to the fixed‐effect model. Whilst there is significant heterogeneity, all of the trials follow the same direction of effect. Overall, the combined result indicates that the number of women wanting to carry their own notes in a subsequent pregnancy was significantly higher in the case notes group (RR 1.79, 95% CI 1.57 to 2.03). This effect is more marked in one trial (Lovell 1987), (RR 2.26, 95% CI 1.75 to 2.93), however, we could not identify any difference between the trials to explain this. Neither trial is cluster‐randomised. See Analysis 1.3.
1.3. Analysis.
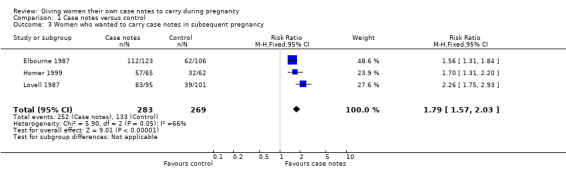
Comparison 1 Case notes versus control, Outcome 3 Women who wanted to carry case notes in subsequent pregnancy.
Administrative
1. Availability of complete antenatal records at time of delivery
None of the included trials reported this outcome.
2. Number of notes lost or left at home when attending hospital
One trial (n = 197) reported no lost or forgotten notes in the women‐held case notes group, but more than 25% of the control group reported that their hospital‐based notes had been lost or mislaid by the hospital at least once during their antenatal care; this difference was statistically significant (P < 0.02; Fishers exact test (Lovell 1987). The same trial (Lovell 1987) reported more mothers in the control group (10/102) had forgotten to bring their notes to an appointment than the case notes group (1/95); RR 0.11, 95% CI 0.01 to 0.82. Another trial (n = 150) reported no difference between the two groups for this outcome (RR 1.03, 95% CI 0.31 to 3.40); on five occasions women in the women‐held notes group failed to bring records to their antenatal appointment, and on five occasions the hospital misplaced records of women in the control group (Homer 1999). The I² test showed significant heterogeneity (74.2%) so a random‐effects analysis was performed. Overall, the pooled estimate of the two trials (n = 347) was not significant (average RR 0.38, 95% CI 0.04 to 3.84; Analysis 1.4).
1.4. Analysis.
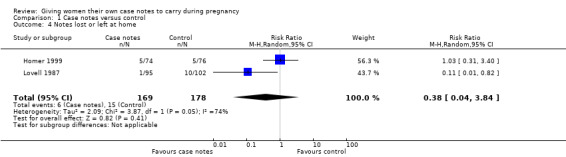
Comparison 1 Case notes versus control, Outcome 4 Notes lost or left at home.
Additionally, Elbourne reported no difference between the women‐held and hospital‐held notes groups in terms of availability of notes in antenatal clinics; however, no data were provided (Elbourne 1987).
Secondary outcomes
1. Partner involvement in the pregnancy, during labour and after the birth
a) number of partners attending antenatal clinic; b) number of partners present during labour; c) number of partners actively involved in the care of the baby.
Two trials commented briefly on partner involvement but did not directly address the outcomes outlined above. In one trial, findings from open‐ended questions suggested that women carrying their case notes felt it gave them an opportunity to share information with their partners, especially important if the partners could not attend antenatal appointments with them (Homer 1999). The other trial (Elbourne 1987), reported no statistically significant differences between the two groups in terms of involvement of the baby's father (no data given).
Health‐related behaviours
1. Number of women who stopped or reduced cigarette smoking
This outcome was addressed in two trials (Elbourne 1987; Lovell 1987). Both reported no significant differences between the women‐held notes and control groups. Data were not provided at all for one trial (Elbourne 1987) that additionally reported no difference in terms of 'within‐person' changes over the study period in the number of cigarettes smoked. Another trial (Lovell 1987), reported no change in smoking behaviour in non smokers, smokers of one to 10 cigarettes per day and smokers of more than 11 per day at eight to 16 weeks or 32 to 34 weeks' gestation. Percentages of women smoking within these groups were provided at the different gestations. Denominators were not provided by the authors, and because of the varying numbers of women completing a given question, they could not be calculated. A RevMan analysis graph could therefore not be generated for this outcome.
Alternatively, Mori 2015 found reduced smoking among other members in the household in intervention clusters compared with control cluster households (RR 0.84, 95% CI 0.71 to 0.99; one trial, 499 women).
2. Breastfeeding practices
a) number of women choosing to breastfeed; b) duration of breastfeeding; c) number of women using breast milk supplements.
Two trials reported this outcome. Lovell 1987 reported the number of women who breastfed after delivery, and Mori 2015 reported women who breastfed at any time between delivery and discharge. There were no group differences in rates of breastfeeding (average RR 1.00, 95% CI 0.99 to 1.02; participants = 704; studies = two; Analysis 1.5).
1.5. Analysis.
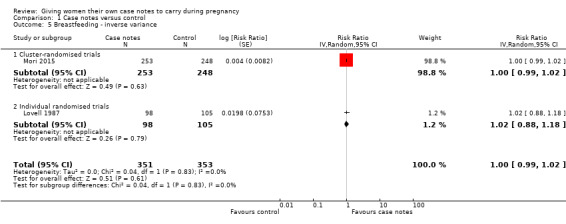
Comparison 1 Case notes versus control, Outcome 5 Breastfeeding ‐ inverse variance.
Clinical
1. Number of women needing analgesia during labour
One trial (n = 212) (Lovell 1987) reported a trend towards more use of epidural analgesia in the case notes group (40/104) compared to the control group (29/108); the difference was not statistically significant (RR 1.43, 95% CI 0.96 to 2.13; Analysis 1.6).
1.6. Analysis.
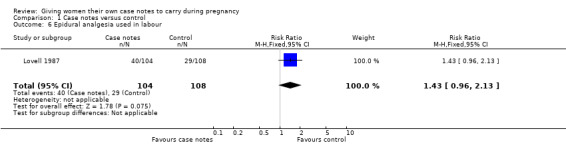
Comparison 1 Case notes versus control, Outcome 6 Epidural analgesia used in labour.
2. Number of women who had a caesarean section/3. an assisted vaginal delivery
Lovell 1987 (n = 212) reported 'assisted delivery' as caesarean and forceps deliveries combined and found that more women in the case notes group (30/104) were delivered by caesarean section or forceps compared to the control group (17/108); (RR 1.83, 95% CI 1.08 to 3.12; Analysis 1.7). The authors do not mention whether ventouse deliveries were included.
1.7. Analysis.
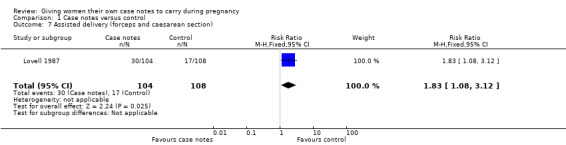
Comparison 1 Case notes versus control, Outcome 7 Assisted delivery (forceps and caesarean section).
Women in the intervention and control groups had similar ranges of assisted vaginal delivery (average RR 0.49, 95% CI 0.04 to 5.55; participants = 501; studies = one; Analysis 1.8), but more women with case notes underwent caesarean section (average RR 1.51, 95% CI 1.10 to 2.08; participants = 501; studies = one; Analysis 1.9).
1.8. Analysis.
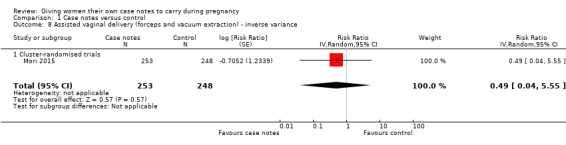
Comparison 1 Case notes versus control, Outcome 8 Assisted vaginal delivery (forceps and vacuum extraction) ‐ inverse variance.
1.9. Analysis.
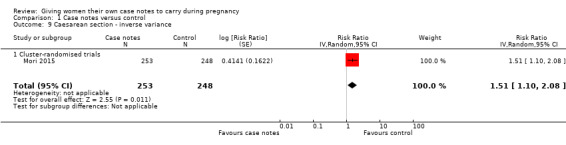
Comparison 1 Case notes versus control, Outcome 9 Caesarean section ‐ inverse variance.
4. Perinatal outcomes
There is no evidence that the case notes intervention benefits new babies. Carrying case notes had no impact on the rate of stillbirth or neonatal death (average RR 1.00, 95% CI 0.99 to 1.01; participants = 713; studies = two; Analysis 1.10). A similar number of neonates was admitted to intensive care (average RR 1.18, 95% CI 0.36 to 3.83; participants = 501; studies = one; Analysis 1.11).
1.10. Analysis.
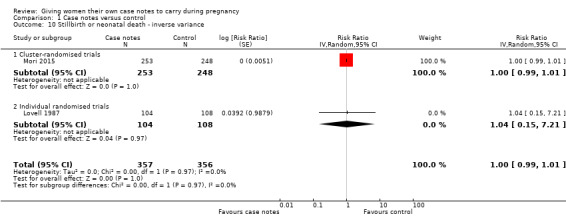
Comparison 1 Case notes versus control, Outcome 10 Stillbirth or neonatal death ‐ inverse variance.
1.11. Analysis.
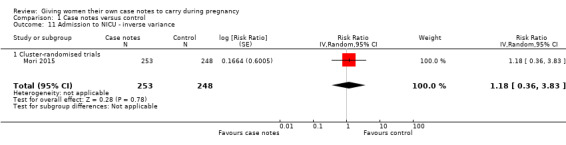
Comparison 1 Case notes versus control, Outcome 11 Admission to NICU ‐ inverse variance.
5. Maternal outcomes
With respect to morbidity, case notes provided no clear advantage to women miscarriage either. There was no evidence of group differences for miscarriage (RR 1.19, 95% CI 0.45 to 3.16; participants = 212; studies = one; Analysis 1.12); maternal admission to intensive care unit during pregnancy (average RR 0.32, 95% CI 0.03 to 3.10; participants = 494; studies = one; Analysis 1.13); or maternal depression (Edinburgh Postnatal Depression Scale cut off 12 points)(average RR 0.99, 95% CI 0.94 to 1.04; participants = 495; studies = one; Analysis 1.14).
1.12. Analysis.
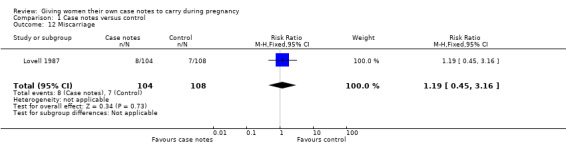
Comparison 1 Case notes versus control, Outcome 12 Miscarriage.
1.13. Analysis.
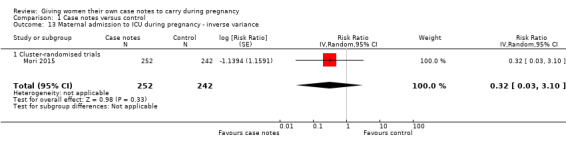
Comparison 1 Case notes versus control, Outcome 13 Maternal admission to ICU during pregnancy ‐ inverse variance.
1.14. Analysis.
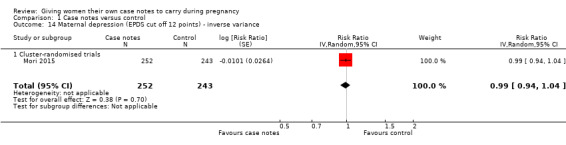
Comparison 1 Case notes versus control, Outcome 14 Maternal depression (EPDS cut off 12 points) ‐ inverse variance.
Discussion
Summary of main results
Women carrying their own case notes were significantly more likely to feel in control. This is supported by the results from two trials (Elbourne 1987; Lovell 1987) that reported on a sense of control directly. Two trials (Lovell 1987; Mori 2015) reported on women's sense of satisfaction, which showed no evidence of a difference. Three trials suggest that significantly more women in the case notes group would prefer to hold their antenatal records in another pregnancy. In addition to the four trials included in the review, we identified a qualitative study on 21 women who participated in face‐to‐face interviews, which were coded for thematic analysis, to explore the impact on women of carrying their own records during pregnancy. The reaction of the women in the study was overwhelmingly supportive towards carrying their own notes as it improved the level of communication between the women and their healthcare providers and provided a greater sense of sharing and communication (Phipps 2001).The results of three of the four included trials are discordant for one outcome as one trial (Lovell 1987) suggests that women carrying their own notes prevents loss or misplacement of notes and the other two trials (Elbourne 1987; Homer 1999) report no difference.
The evidence for the effect of women‐held notes on health‐related behaviours is inconclusive.
Clinical outcomes were reported in two trials (Lovell 1987; Mori 2015). It is of concern that in this trial women in the case notes group had significantly more assisted deliveries (defined as caesarean section and forceps deliveries), which is not accounted for by the use of epidural analgesia.
Overall completeness and applicability of evidence
The four trials are small (all included fewer than 500 women) and from both middle‐income (rural Mongolia) and high‐income settings (UK and Australia), and not all of them reported on all outcomes; this means we cannot be sure about the effect of giving women their own case notes to carry during pregnancy on administrative outcomes, maternal satisfaction and control, health‐related behaviours, and clinical outcomes.
Quality of the evidence
There is a high risk of Type II error due to the small size of included trials. It is important to emphasise that this review shows a lack of evidence of benefit rather than evidence of no benefit; more research is needed. The nature of the intervention means that there is a possibility of introducing bias due to lack of blinding. We judged the quality of the evidence using GRADE and judged the evidence for giving case notes versus control (Table 1); moderate quality for women who felt in control, breastfeeding, Caesarean section and stillbirth or neonatal death; low quality of evidence for women's satisfaction with antenatal care, women who wanted to carry case notes in a subsequent pregnancy, and epidural analgesia used in labour. We downgraded because most studies had design limitations and wide confidence intervals crossing the line of no effect.
Potential biases in the review process
Three included trials used self‐administered questionnaires to evaluate the effect of women‐held notes; this method is subject to respondent bias and means the outcomes reported in the trials must be interpreted with caution. A patient note audit would provide a more objective measure of quantifiable outcomes. One trial collected and analysed urine samples to obtain a more objective assessment of women's reported smoking behaviour (Elbourne 1987).
Agreements and disagreements with other studies or reviews
There is no similar review and/or other studies.
Authors' conclusions
Implications for practice.
A policy of giving women their own case notes to carry during pregnancy has both potential benefits (increased maternal control and increased availability of antenatal records during hospital attendance) and harms (more operative deliveries). Importantly, all of the trials report that more women in the case notes group would prefer to hold their antenatal records in another pregnancy. There is insufficient evidence of the effect of giving women their own case notes to carry during pregnancy on health‐related behaviours (smoking and breastfeeding), women's satisfaction and clinical outcomes.
Implications for research.
Giving women their own case notes seems to already be in widespread use including in many low‐income countries despite inconclusive evidence and none of the trials having been conducted in a low‐income country. The potential risk of increased caesarean sections and operative vaginal births has significant resource implications in a low‐income setting. For this reason there would be value in a large multicentre trial including low‐income countries that looks specifically at clinical outcomes in women and their babies and administrative issues, and qualitative studies to explore women's satisfaction, empowerment and sense of control.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2015 | New search has been performed | Search updated, one further trial included (Mori 2015). A 'Summary of findings' table incorporated. |
| 31 August 2015 | New citation required but conclusions have not changed | Conclusions remain the same. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 21 March 2011 | New search has been performed | Search updated. No new trials identified. |
| 3 September 2008 | Amended | Converted to new review format. |
| 27 June 2007 | New search has been performed | Search updated. No new trials identified. |
Acknowledgements
Sonja Henderson for ongoing technical support and encouragement with completing this review. We also thank Nancy Medley and Erika Ota for assessing risk of bias and extract data from one of the included trials.
Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Case notes versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women who felt in control | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.18, 2.06] |
| 2 Women's satisfaction with antenatal care ‐ inverse variance | 2 | 698 | Risk Ratio (Random, 95% CI) | 1.09 [0.92, 1.29] |
| 2.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.02 [0.92, 1.14] |
| 2.2 Individual randomised trials | 1 | 197 | Risk Ratio (Random, 95% CI) | 1.22 [0.99, 1.50] |
| 3 Women who wanted to carry case notes in subsequent pregnancy | 3 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.57, 2.03] |
| 4 Notes lost or left at home | 2 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.04, 3.84] |
| 5 Breastfeeding ‐ inverse variance | 2 | 704 | Risk Ratio (Random, 95% CI) | 1.00 [0.99, 1.02] |
| 5.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.00 [0.99, 1.02] |
| 5.2 Individual randomised trials | 1 | 203 | Risk Ratio (Random, 95% CI) | 1.02 [0.88, 1.18] |
| 6 Epidural analgesia used in labour | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.96, 2.13] |
| 7 Assisted delivery (forceps and caesarean section) | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.08, 3.12] |
| 8 Assisted vaginal delivery (forceps and vacuum extraction) ‐ inverse variance | 1 | 501 | Risk Ratio (Random, 95% CI) | 0.49 [0.04, 5.55] |
| 8.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 0.49 [0.04, 5.55] |
| 9 Caesarean section ‐ inverse variance | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.51 [1.10, 2.08] |
| 9.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.51 [1.10, 2.08] |
| 10 Stillbirth or neonatal death ‐ inverse variance | 2 | 713 | Risk Ratio (Random, 95% CI) | 1.00 [0.99, 1.01] |
| 10.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.0 [0.99, 1.01] |
| 10.2 Individual randomised trials | 1 | 212 | Risk Ratio (Random, 95% CI) | 1.04 [0.15, 7.21] |
| 11 Admission to NICU ‐ inverse variance | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.18 [0.36, 3.83] |
| 11.1 Cluster‐randomised trials | 1 | 501 | Risk Ratio (Random, 95% CI) | 1.18 [0.36, 3.83] |
| 12 Miscarriage | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.45, 3.16] |
| 13 Maternal admission to ICU during pregnancy ‐ inverse variance | 1 | 494 | Risk Ratio (Random, 95% CI) | 0.32 [0.03, 3.10] |
| 13.1 Cluster‐randomised trials | 1 | 494 | Risk Ratio (Random, 95% CI) | 0.32 [0.03, 3.10] |
| 14 Maternal depression (EPDS cut off 12 points) ‐ inverse variance | 1 | 495 | Risk Ratio (Random, 95% CI) | 0.99 [0.94, 1.04] |
| 14.1 Cluster‐randomised trials | 1 | 495 | Risk Ratio (Random, 95% CI) | 0.99 [0.94, 1.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Elbourne 1987.
| Methods | Randomised controlled trial. | |
| Participants | 290 pregnant women (147 case note and 143 card) less than 34 weeks' gestation. 161 women were randomised to the case note group and 156 women to the card group. 27 women found ineligible after randomisation. | |
| Interventions | Intervention group: woman to hold own obstetric case notes until 10 days after delivery; control group: women carried abbreviated form of notes (a 'co‐operation card') and full obstetric case notes held by medical records department records. | |
| Outcomes | 5 hypotheses were made about women carrying their own notes: 1) they would feel more satisfied with their maternity care; 2) women would feel better about their pregnancy, better informed, less anxious, more in control, more confident and that the babies father was more involved in the pregnancy, labour and care of the child; 3) women would be less likely to suffer depression and find it easier to communicate with staff; 4) women carrying their own notes would result in increased availability of notes and a saving of clerical resources. | |
| Notes | Peripheral consultant clinic Newbury, West Berkshire, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described; trial described as randomised. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered series of opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible for this intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Randomised: 161 case note and 156 co‐op card. 27 women found ineligible after randomisation and before the initial survey. Eligible for first questionnaire: 147 case note and 143 card. Attrition documented: 2 refusals, 3 miscarriages and 1 abortion did not complete the initial questionnaire. Further questionnaires were not mailed to 13 women, due to spontaneous or induced abortion (9), stillbirth (1), neonatal death (3) or immigration (1). A final 2 women were not sent the third questionnaire due to serious maternal and neonatal morbidity. Women who completed all 3 questionnaires: 128 case note group and 119 in the co‐operation card group. |
| Selective reporting (reporting bias) | High risk | Miscarriage, abortion and neonatal deaths are mentioned in the text but not reported according to treatment arm. We are therefore unable to use these data in meta‐analyses. 2 women were not followed up due to serious maternal or neonatal morbidity. |
| Other bias | Unclear risk | The authors report 13 women whose actual record‐holding differed from their group allocation. Group characteristics similar at baseline. The authors report both contamination and Hawthorn effects may have reduced the differences between treatment groups. |
Homer 1999.
| Methods | Randomised controlled trial. | |
| Participants | 150 English speaking women attending antenatal clinic (74 women in the intervention group and 76 women in the control group). | |
| Interventions | Intervention group: women given entire antenatal record through pregnancy. Control group: women given small, abbreviated version ('co‐op card') to carry and full notes kept at the hospital. Jan ‐ Dec, 1997. Questionnaire at 34‐38 weeks' gestation. |
|
| Outcomes | Women's sense of control, involvement in care and levels of communication, availability of records at antenatal visit. | |
| Notes | Antenatal clinic in a NSW teaching hospital, Australia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Study described as randomised. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible for this intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 150 women were considered to be eligible for the study, 22 (13%) refused and were not randomised.128 women randomised. 126 completed questionnaire at 35 weeks, but not all women answered each question. |
| Selective reporting (reporting bias) | Low risk | Specified outcomes are reported. |
| Other bias | Low risk | Groups comparable at baseline. |
Lovell 1987.
| Methods | Randomised controlled trial. | |
| Participants | 246 women attending antenatal clinic were eligible and data were obtained from 104 women in the intervention group and 108 women in the control group | |
| Interventions | Intervention group: women carried full set of antenatal records up until admission. Control group: women carried 'co‐op card' and maternity notes retained by hospital. Both trial arms had access to notes during appointments. Questionnaires self‐administered at 8‐16 weeks, 32‐42 weeks and postnatally (while still in hospital). Women recruited 20 June to 7 November 1984. |
|
| Outcomes | Women's satisfaction with care, sense of control and self‐confidence, communication with staff and involvement of baby's father. Also health‐related behaviour: attendance at antenatal clinic, breastfeeding, smoking and alcohol consumption. | |
| Notes | Antenatal clinic, St Thomas's Hospital, London, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Study described as randomised. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not described and not feasible for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not described and not feasible for this intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 246 women eligible. 11 refused (unclear whether before or after randomisation). Miscarriage, moved and lost to follow‐up reduced sample to 228. Women who suffered late miscarriage were included in the analyses of clinical outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported. |
| Other bias | Low risk | No significant baseline group differences. |
Mori 2015.
| Methods | Cluster‐randomised controlled trial in Bulgan, Mongolia. | |
| Participants | Pregnant women living in Bulgan, Mongolia. The unit of randomisation was the Soum and bag, small geographic areas in Mongolia. Each Soum has a healthcare facility where women must register their newborn. 18 geographic areas were randomised, after selection for administrative convenience and to avoid contamination. 501 women (253 women in the intervention group and 248 women in the control group) participated in the study. |
|
| Interventions | Distribution of maternal and child health handbooks during pregnancy. The MCH handbook logged maternal health and personal information, pregnancy, delivery and postpartum health and weight, dental health, parenting classes, child developmental milestones from 0‐6 years, immunisation records and height and weight charts for children. | |
| Outcomes | Primary: number of antenatal visits; proportion of women attending 6 or more antenatal visits. (The national standard for antenatal care in Mongolia is 6 visits.) Secondary: maternal outcomes: morbidity during pregnancy, mode of delivery, breastfeeding initiation, maternal depression and health (EPDS and GHQ). Infant outcomes: birthweight, Apgar score, NICU admission, neonatal mortality at discharge. Maternal healthy behaviours. |
|
| Notes | Significant group differences noted for distances travelled to nearest health centre (greater in the intervention group) and for wealth index (the control group was poorer). The authors report that travel time did not function as an effect modifier; however, women from a higher socioeconomic background attended more antenatal care visits. Trial authors provided unpublished outcome data upon request. The trial statistician (HN) calculated RRs and 95% confidence using the GEE method to adjust for cluster design and baseline differences, including wealth index. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence according to the shuffling of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes at time of randomisation. All areas were randomised at the same time. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking was not possible for this intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Masking was not possible for this intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 areas were excluded before randomisation; 1 was the subject of a pilot study, and 2 areas were included in another health study. 9 clusters each received the intervention or the control. Missing outcome data for individual women are reported and minimal. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes have been reported. Addtional analyses were obtained from the authors upon request. The trial data file has been published online with the trial report. |
| Other bias | Unclear risk | The authors reported baseline imbalances between clusters for travel time to health centre and wealth. The authors reported that recall bias may exist due to data collection at 1 month after birth. Analyses were undertaken with methods appropriate for cluster trials; the authors used GEE methods to adjust for the effects of cluster design and baseline variables. A sample size calculation was undertaken and met. Recruitment bias ‐ All of the eligible women were registered with the health centres in their soums, and had been visited by the doctors. There is a possibility for them to go to another health centre in a different soum for their antenatal visit or any other visit. Recruitment was done by home visiting of their doctors, not by women's visiting health centres, as the home visits are mandatory for these doctors. |
EPDS: Edinburgh Postnatal Depression Scale GEE: Generalised estimating equations GHQ: General Health Questionnaire MCH: Maternal and Child Health NICU: neonatal intensive care unit RR: risk ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aarts 1997 | Unable to contact authors to determine whether this trial was completed and published or not. |
| Draper 1986 | Not a randomised clinical trial. |
| Jenkinson 1989 | Randomised clinical trial, but women were not given their own notes; instead, women were given their antenatal record in the form of an optical memory computer card. |
| Phipps 2001 | Qualitative research, not a randomised clinical trial. |
| Webster 1996 | Not a randomised clinical trial. |
Differences between protocol and review
We added maternal intensive care unit admission and maternal depression to the list of outcomes under maternal morbidity.
We carried out the following subgroup analyses in the 2015 update:
Individually‐randomised versus cluster‐randomised trials.
A 'Summary of findings' table has been added for the 2015 update.
Contributions of authors
The original version of the protocol was written and revised by Heather Brown. Trials were identified by a single review author (Heather Brown) and checked by the co‐author (Helen Smith) in the original version of the review. Inclusion criteria were applied, quality assessed and data extracted independently by both authors. The review was written and revised by both authors.
For the 2015 update, Rintaro Mori updated the text based upon the inclusion of the new trial and prepared the draft. Erika Ota and a second Researcher assessed risk of bias and extracted data from the new trial. Hisashi Noma conducted statistical analyses for the cluster trial. All the other authors critically reviewed the draft and agree with the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Rintaro Mori is the lead author of one of the included studies (Mori 2015) and this was assessed by Erika Ota and Nancy Medley. No other conflicts of interest noted.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Elbourne 1987 {published data only}
- Elbourne D, Richardson M, Chalmers I, Waterhouse I, Holt E. The Newbury Maternity Care Study: a randomized controlled trial to assess a policy of women holding their own obstetric records. British Journal of Obstetrics and Gynaecology 1987;94:612‐9. [DOI] [PubMed] [Google Scholar]
Homer 1999 {published data only}
- Homer CSE, Davis GK, Everitt LS. The introduction of a woman‐held record into a hospital antenatal clinic: the bring your own records study. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(1):54‐7. [DOI] [PubMed] [Google Scholar]
Lovell 1987 {published data only}
- Lovell A, Zander LI, James CE, Foot S, Swan AV, Reynolds A. St. Thomas' Maternity Case Notes Study: Why not give mothers their own case notes?. London: Cicely Northcote Trust, 1986. [Google Scholar]
- Lovell A, Zander LI, James CE, Foot S, Swan AV, Reynolds A. The St. Thomas's Hospital maternity case notes study: a randomised controlled trial to assess the effects of giving expectant mothers their own maternity case notes. Paediatric and Perinatal Epidemiology 1987;1:57‐66. [DOI] [PubMed] [Google Scholar]
Mori 2015 {published data only}
- Mori R, Yonemoto N, Noma H, Ochirbat T, Barber E, Soyolgerel G, et al. The Maternal and Child Health (MCH) Handbook in Mongolia: a cluster‐randomized, controlled trial. Plos One 2015;10(4):e0119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aarts 1997 {published data only (unpublished sought but not used)}
- Aarts FV. Trial to determine the value of introducing a "state of pregnancy" passport on Curacao. Personal communication 1997.
Draper 1986 {published data only}
- Draper J, Field S, Thomas H, Hare M. Should women carry their antenatal records?. BMJ 1986;292(6520):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkinson 1989 {published data only}
- Jenkinson SD, House MJ. The entire computerised antenatal record collected prospectively and stored on patient held optical memory. Proceedings of Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 July 4‐7; London, UK. 1989.
Phipps 2001 {published data only}
- Phipps H. Carrying their own medical records: the perspective of pregnant women. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(4):398‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Webster 1996 {published data only}
- Webster J, Foster S, Thomas I, Griffin A, Timms H. Sharing antenatal care: client satisfaction and use of 'patient held' record. Australian and New Zealand Journal of Obstetrics and Gynaecology 1996;36(1):11‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Backe 1993
- Backe B, Nakling J. Effectiveness of antenatal care: a population based study. British Journal of Obstetrics and Gynaecology 1993;100:727‐32. [DOI] [PubMed] [Google Scholar]
DoH 1993
- Expert Maternity Group, Department of Health. Changing Childbirth. London: HMSO, 1993. [Google Scholar]
Donald 1984
- Donald PR, Hesseling PB. The 'Road to Health' card ‐ a cornerstone of preventative and promotive paediatrics. South African Medical Journal 1984;65:423‐5. [PubMed] [Google Scholar]
Dowswell 2015
- Dowswell T, Carroli G, Duley L, Gates S, Gülmezoglu AM, Khan‐Neelofur D, Piaggio G. Alternative versus standard packages of antenatal care for low‐risk pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD000934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 1998
- Harrison D, V Heese H, Harker H, Mann MD. An assessment of the 'Road to Health' card based on perceptions of clinic staff and mothers. South African Medical Journal 1998;88:1424‐7. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 1994
- Hofmeyr GJ, Pattinson RC, Nikodem VC, Gulmezoglu AM. Charting fetal growth. Journal of Comprehensive Health 1994;5:62‐7. [Google Scholar]
Munjanja 1996
- Munjanja SP, Lindmark G, Nystrom L. Randomised controlled trial of a reduced visits programme of antenatal care in Harare, Zimbabwe. Lancet 1996;348:364‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Brown 2004
- Brown HC, Smith HJ. Giving women their own case notes to carry during pregnancy. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002856.pub2] [DOI] [PubMed] [Google Scholar]
Hodnett 1995
- Hodnett ED. Women carrying their own case‐notes during pregnancy. [revised 12 May 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.


