The pathogen Listeria monocytogenes causes the foodborne disease listeriosis, which can be fatal in immunocompromised individuals. L. monocytogenes tolerates several environmental stressors and can persist in food-processing environments and grow in foodstuffs despite traditional control measures such as high salt content. Nonetheless, L. monocytogenes strains differ in their ability to withstand stressors. Elucidating the intraspecies strain variability of L. monocytogenes stress tolerance is crucial for the identification of particularly tolerant strains. To enhance reliable identification of variability in bacterial stress tolerance phenotypes, we compiled a large-scale protocol for the entire data assembly and analysis of microbial growth experiments, providing a systematic approach and checklist for experiments on strain-specific growth ability. Our study illustrated the diversity and strain-specific variation of L. monocytogenes stress tolerance with an unprecedented scope and discovered biologically relevant serovar- and lineage-dependent phenotypes of NaCl tolerance.
KEYWORDS: Listeria, Bioscreen, osmotic, salt, stress tolerance, growth model, model-free spline, Gompertz, modified-Gompertz, logistic, Richards, kinetic parameter, osmotic stress, stress response
ABSTRACT
Listeria monocytogenes causes the severe foodborne illness listeriosis and survives in food-associated environments due to its high stress tolerance. A data assembly and analysis protocol for microbial growth experiments was compiled to elucidate the strain variability of L. monocytogenes stress tolerance. The protocol includes measurement of growth ability under stress (step 1), selection of a suitable method for growth parameter calculation (step 2), comparison of growth patterns between strains (step 3), and biological interpretation of the discovered differences (step 4). In step 1, L. monocytogenes strains (n = 388) of various serovars and origins grown on media with 9.0% NaCl were measured using a Bioscreen C microbiology reader. Technical variability of the growth measurements was assessed and eliminated. In step 2, the growth parameters determined by Gompertz, modified-Gompertz, logistic, and Richards models and model-free splines were compared, illustrating differences in the suitability of these methods to describe the experimental data. In step 3, hierarchical clustering was used to describe the NaCl tolerance of L. monocytogenes measured by strain-specific variation in growth ability; tolerant strains had higher growth rates and maximum optical densities and shorter lag phases than susceptible strains. The spline parameter area under the curve best classified “poor,” “average,” and “good” growers. In step 4, the tested L. monocytogenes lineage I strains (serovars 4b and 1/2b) proved to be significantly more tolerant toward 9.0% NaCl than lineage II strains (serovars 1/2a, 1/2c, and 3a). Our protocol provides systematic tools to gain comparable data for investigating strain-specific variation of bacterial growth under stress.
IMPORTANCE The pathogen Listeria monocytogenes causes the foodborne disease listeriosis, which can be fatal in immunocompromised individuals. L. monocytogenes tolerates several environmental stressors and can persist in food-processing environments and grow in foodstuffs despite traditional control measures such as high salt content. Nonetheless, L. monocytogenes strains differ in their ability to withstand stressors. Elucidating the intraspecies strain variability of L. monocytogenes stress tolerance is crucial for the identification of particularly tolerant strains. To enhance reliable identification of variability in bacterial stress tolerance phenotypes, we compiled a large-scale protocol for the entire data assembly and analysis of microbial growth experiments, providing a systematic approach and checklist for experiments on strain-specific growth ability. Our study illustrated the diversity and strain-specific variation of L. monocytogenes stress tolerance with an unprecedented scope and discovered biologically relevant serovar- and lineage-dependent phenotypes of NaCl tolerance.
INTRODUCTION
The foodborne pathogen Listeria monocytogenes causes listeriosis, a severe illness to which the elderly, infants, and immunocompromised or pregnant individuals are particularly susceptible (1, 2). Preventing the occurrence of L. monocytogenes is difficult due to its flexible lifestyle, which is supported by its ability to survive and adapt to stressors (3–5) in various environments, including foods, soil, water, sewage, and mammalian hosts (6, 7). L. monocytogenes is a problematic contaminant of the food chain, as it has the ability to persist in processing facilities, contaminate foodstuffs, and cause infections predominantly via ready-to-eat foods (7–13). Stress tolerance contributes to the survival of L. monocytogenes within the food chain (14, 15) and facilitates its transition from a saprophyte to a pathogen (16–21). The genetic lineages and sublineages of L. monocytogenes have been described to be unevenly represented among isolates from different environments and hosts (22–26) and to contain differing stress resistance genes (27). L. monocytogenes serovars 1/2a, 1/2c, and 3a belong to the traditionally food-associated lineage II, whereas serovars 1/2b and 4b are assigned to lineage I (28). L. monocytogenes strains possess variable stress tolerance qualities (29, 30), and the overall intraspecies diversity of L. monocytogenes stress tolerance, including lineage-associated traits, remains to be elucidated.
Phenotypic strain variability, if not considered, may cause bias in microbiological investigations (31). To capture overall intraspecies diversity, the investigation of the variability of L. monocytogenes stress tolerance should employ large strain collections of different origins and genotypes. However, large experiments are labor-intensive, necessitating methodologies to perform numerous experiments in bulk. Turbidity via microplate absorbance (optical density [OD]) measurement technologies has been widely used for decades in the study of microbial growth patterns and stress tolerance (32–37). Researchers have considered quantitative approaches, such as comparison with traditional viable counts and calibration of OD measurements to estimate cell counts and kinetic parameters (36–42). Kinetic parameters calculated from OD measurements systematically deviate from parameters obtained using viable counts (36, 37, 39, 40), and since the deviation is systematic, OD is deemed suitable for the relative comparison of growth patterns between strains (41). However, execution of the entire data collection and analysis protocol in a reliable manner, including a stepwise compilation of considerations and tools that yield comparable, qualitative high-throughput data on bacterial stress tolerance phenotypes, has received less attention.
Microbial susceptibility and tolerance toward stressors can be quantified by growth parameters derived from mathematical models (43–47). Commonly used kinetic parameters include lag time (lag phase), maximum specific growth rate, asymptotic (maximum) growth level (44), and area under the curve (48, 49). The importance of selecting a suitable growth model for parameter estimation has been emphasized, as some models assume relationships between parameters or are unsuited for certain types of data (41, 46, 47, 50, 51). Although numerous models with differing underlying principles have been developed and used, one generally has no inherent superiority over another (44). Therefore, determining a parameter estimation approach that suits the purposes of the experiment is relevant for each study design.
The aims of our study were to establish a high-throughput data assembly and analysis protocol for OD measurements of microbial growth and to explore the reliability of the protocol for investigating the intraspecies diversity of L. monocytogenes stress tolerance. Osmotic (salt) stress was chosen as an example due to its relevance at several phases of the bacterial ecology (52–58).
RESULTS
Our data assembly and analysis protocol incorporates the following steps that are essential for the identification of variability in growth ability, i.e., stress tolerance, among bacterial strains: measurement of growth ability under stress (step 1), selection of a suitable method for growth parameter calculation (step 2), comparison of the growth patterns between strains (step 3), and biological interpretation of the discovered differences (step 4) (Table 1).
TABLE 1.
Summary of the data assembly and analysis protocol for strain variability of growth ability under stress conditions
| Protocol step | Specific consideration(s) | Method(s) or example(s)a |
|---|---|---|
| 1. Measurement of growth ability under stress | Optimize stress condition by piloting strain variability. | Fig. S1 |
| Randomize pilot strains and the testing order of strains. | Functions to generate random numbers without repeats, e.g., in R or Excel | |
| Minimize potential technical variation and batch effects: | ||
| Use the same growth medium throughout the entire experiment. | Prepare the required amount all at once | |
| Have one individual perform all experiments, if possible. | Fig. S4, S5 | |
| Utilize within-experiment technical and biological replicates. | Fig. S11 | |
| Utilize between-experiment control strains. | Fig. S11 | |
| When using Bioscreen, leave the outermost rim of the honeycomb plate blank to avoid test broth evaporation. | Fig. S3, S11 | |
| Detect potential contamination and empty wells; retest, if necessary. | Visualize growth curves during or after experiment; culture honeycomb plate wells with unusual growth and no growth | |
| Inspect and deal with outliers. | Fig. S2, Text S10: volume 1 | |
| Inspect and normalize potential technical variation and batch effects. | Fig. S6–9, Text S10: volumes 2 and 3 | |
| 2. Selection of a suitable method for growth parameter calculation | Calculate growth parameters using several methods. | Text S10: volume 4 |
| Compare the fit and values of the parameter calculation methods. | Text S10: volume 5 | |
| 3. Comparison of growth patterns between strains | Visualize growth parameters to see overall parameter variation. | Text S10: volume 6 |
| Visualize growth curves to see entire growth patterns. | Text S10: volumes 3 and 7 | |
| Combine statistical methods and intuitive reasoning to determine a suitable way to quantify strain variability. | Fig. S12–15 | |
| 4. Biological interpretation of the discovered differences | Classify strain variability and interpret it via growth parameters. | Text S10: volumes 6 and 7 |
| Investigate strain variability with biological background variables and draw conclusions. | Data exploration and statistical tests (see Materials and Methods for examples) |
Methods or examples described in the supplemental material published with this article are indicated by their number. Text S10 includes R codes for the data analyses and is divided into volumes 1 to 7 according to their content.
Measurement of growth ability under stress (step 1).
(i) 9.0% NaCl distinguished strain variability of L. monocytogenes growth ability under salt stress. A pilot study using a Bioscreen C microbiology reader determined the sufficient NaCl concentration for identifying differences of stress tolerance between L. monocytogenes strains. At 6.5% NaCl, most pilot strains (Table 2) reached the stationary phase within 7 h and grew to relatively high ODs, displaying little variation in growth patterns between strains (see Fig. S1 in the supplemental material). At 7.5% NaCl, the pilot strains reached the stationary phase within approximately 10 h, and a slight decrease of maximum OD and an increased variability of growth patterns appeared (Fig. S1). Variation between the growth patterns of the pilot strains was apparent at 8.5% NaCl, but the overall decrease in their maximum optical density remained moderate (Fig. S1). To ensure that the selected NaCl concentration would distinguish strain variability, the final test concentration of brain-heart infusion (BHI) broth was set at 9.0% NaCl.
TABLE 2.
Listeria monocytogenes strains used in this study
| Strain | Immediate sourcea | Isolation source | Isolation yrb | Serovar | Pilot (P) or control (C)c |
|---|---|---|---|---|---|
| LE57E | Finland | Processing environment | 2000 | 1/2a | – |
| LU12/2 | Finland | Pig, feces | 2003 | 1/2a | – |
| AE30E | Finland | Processing environment | 1999 | 1/2a | – |
| LK126 | Finland | Fish | 2000 | 1/2a | – |
| LM86 | Finland | Fish | NA | 1/2a | – |
| MJL41 | Finland | Meat | 2009 | 1/2a | – |
| MJL47 | Finland | Meat | 2009 | 1/2a | – |
| TT82E | Finland | Fish | 1997 | 1/2a | – |
| PE4E/1 | Finland | Processing environment | NA | 1/2a | – |
| HT45E | Finland | Poultry | 1998 | 1/2a | – |
| LK132 | Finland | Fish | 2000 | 1/2a | – |
| TT107E | Finland | Fish | 1998 | 1/2a | – |
| LM210 | Finland | Fish | NA | 1/2a | – |
| MJL43 | Finland | Meat | 2009 | 1/2a | – |
| LMML90 | Finland | Meat | 2004 | 1/2a | – |
| LMML100 | Finland | Meat | 2004 | 1/2a | – |
| L34-s | Finland | Meat | 1999–2001 | 1/2a | – |
| LU26/1 | Finland | Pig, feces | 2003 | 1/2a | – |
| LU103/1 | Finland | Meat | 2003 | 1/2a | – |
| MJL14 | Finland | Processing environment | 2009 | 1/2a | – |
| HL34E/1 | Finland | Processing environment | 1999 | 1/2a | – |
| HT65E/1 | Finland | Meat | 1998 | 1/2a | – |
| LK133 | Finland | Fish | 2000 | 1/2a | – |
| RL22E/1 | Finland | Processing environment | 2000 | 1/2a | – |
| LL16/2 | Finland | Wild bird, feces | 1998 | 1/2a | – |
| L12s | Finland | Poultry | 1999–2001 | 1/2a | – |
| LK36 | Finland | Fish | 1993 | 1/2a | – |
| LK129 | Finland | Fish | 2000 | 1/2a | – |
| TR47E | Finland | Fish | NA | 1/2a | – |
| L47s | Finland | Poultry | 1999–2001 | 1/2a | – |
| LM70 | Finland | Dairy product | 1988 | 1/2a | – |
| LL82/1 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LT3/1 | Finland | Meat | 1999 | 1/2a | – |
| IR17V | Finland | Milk | 1998 | 1/2a | – |
| LT26E | Finland | Vegetable | 2000 | 1/2a | – |
| LM69 | Finland | Dairy product | 1988 | 1/2a | – |
| LT14/1 | Finland | Meat | 1999 | 1/2a | – |
| LT4/1 | Finland | Meat | 1999 | 1/2a | – |
| LA40 | Finland | Bird | 1989 | 1/2a | – |
| LT16/1 | Finland | Meat | 1999 | 1/2a | – |
| LK134 | Finland | Fish | 2000 | 1/2a | – |
| LE27E | Finland | Processing environment | 1999 | 1/2a | – |
| JHM70 | Finland | Milk | 1987 | 1/2a | – |
| HE1E | Finland | Processing environment | 1997 | 1/2a | – |
| LK60/1 | Finland | Fish | 1999 | 1/2a | – |
| LE55E | Finland | Processing environment | 2000 | 1/2a | P |
| LU74/1 | Finland | Meat | 2003 | 1/2a | – |
| RE46E | Finland | Processing environment | 1997 | 1/2a | – |
| JHM339 | Finland | Silage | 1988 | 1/2a | – |
| JHM71 | Finland | Cow, feces | 1987 | 1/2a | – |
| LU49/1 | Finland | Meat | 2003 | 1/2a | – |
| LMML10 | Finland | Meat | 2003 | 1/2a | – |
| LA35 | Finland | Sheep | 1998 | 1/2a | – |
| MJL34 | Finland | Meat | 2009 | 1/2a | – |
| LL51/2 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| L10s | Finland | Meat | 2000 | 1/2a | – |
| LL18/3 | Finland | Wild bird, feces | 1998 | 1/2a | – |
| LU102/1 | Finland | Meat | 2003 | 1/2a | – |
| LT9/1 | Finland | Meat | 1999 | 1/2a | – |
| LU118/1 | Finland | Meat | 2003 | 1/2a | – |
| LT13/1 | Finland | Meat | 1999 | 1/2a | – |
| JHM3 | Finland | Cow, feces | 1987 | 1/2a | – |
| JHM110 | Finland | Cow, feces | 1987 | 1/2a | – |
| LM104/1 | Finland | Vegetable | 1999 | 1/2a | – |
| JHM35 | Finland | Cow, feces | 1987 | 1/2a | – |
| LT33/1 | Finland | Meat | 2000 | 1/2a | – |
| LML46 | Finland | NA | NA | 1/2a | – |
| JHM349 | Finland | Silage | 1988 | 1/2a | – |
| 10B | Finland | Fish | 1999–2001 | 1/2a | – |
| JHM387 | Finland | Silage | 1988 | 1/2a | – |
| LL24/2 | Finland | Wild bird, feces | 1998 | 1/2a | – |
| IR18V | Finland | Milk | NA | 1/2a | – |
| LM110 | Finland | Vegetable | 1999 | 1/2a | – |
| LA68 | Finland | Animal | 1999 | 1/2a | – |
| TE9/1 | Finland | Meat | NA | 1/2a | – |
| MJL2 | Finland | Meat | 2009 | 1/2a | – |
| LT12/1 | Finland | Meat | 1999 | 1/2a | – |
| LK130 | Finland | Fish | 2000 | 1/2a | – |
| LL65/1 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LA61 | Finland | Meat | 1993 | 1/2a | – |
| LU123/1 | Finland | Meat | 2003 | 1/2a | – |
| LK121/1 | Finland | Fish | 2001 | 1/2a | – |
| LK51 | Finland | Fish | 1999 | 1/2a | – |
| LMK1 | Finland | Fish | 1996 | 1/2a | – |
| LU56/3 | Finland | Meat | 2003 | 1/2a | – |
| JHM229 | Finland | Cow | 1975 | 1/2a | P |
| TE27/1 | Finland | Meat | NA | 1/2a | – |
| LA42 | Finland | Cow | 1989 | 1/2a | – |
| MJL1 | Finland | Meat | 2009 | 1/2a | – |
| IR16V | Finland | Milk | 1998 | 1/2a | – |
| LA48 | Finland | Sheep | 1990 | 1/2a | – |
| HR5E | Finland | Meat | 1998 | 1/2a | – |
| LK54/1 | Finland | Fish | 1999 | 1/2a | – |
| LU120/1 | Finland | Meat | 2003 | 1/2a | – |
| LT5/1 | Finland | Meat | 1999 | 1/2a | – |
| LL85/1 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LM298 | Finland | Salad | 2002 | 1/2a | – |
| TL1E | Finland | Processing environment | NA | 1/2a | – |
| LT32/1 | Finland | Meat | 2000 | 1/2a | – |
| TE12/1 | Finland | Animal | NA | 1/2a | – |
| LM111 | Finland | Processing environment | NA | 1/2a | – |
| LL22/8 | Finland | Wild bird, feces | 1998 | 1/2a | – |
| LL52/2 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LMML101 | Finland | Meat | 2004 | 1/2a | – |
| 13M | Finland | Poultry | 2000 | 1/2a | – |
| MJL21 | Finland | Meat | 2009 | 1/2a | – |
| TE4/1 | Finland | Meat | NA | 1/2a | – |
| RE1E | Finland | Processing environment | 1997 | 1/2a | – |
| HR19E/1 | Finland | Meat | 2000 | 1/2a | – |
| 18B | Finland | Fish | 1999–2001 | 1/2a | – |
| L96s | Finland | Poultry | 1999–2001 | 1/2a | – |
| LK89/1 | Finland | Fish | 2001 | 1/2a | – |
| LM136 | Finland | Fish | NA | 1/2a | – |
| LA64 | Finland | Cow | 1994 | 1/2a | – |
| LMML117 | Finland | Meat | 2004 | 1/2a | – |
| LT2/1 | Finland | Meat | 1999 | 1/2a | – |
| LK131 | Finland | Fish | 2000 | 1/2a | – |
| LL83/1 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LMK28 | Finland | Fish | 1996 | 1/2a | – |
| LM84 | Finland | Ready-to-eat food | 1999 | 1/2a | – |
| MJL45 | Finland | Meat | 2009 | 1/2a | – |
| L83s | Finland | Meat | 2001 | 1/2a | – |
| LL66/3 | Finland | Wild bird, feces | 2001 | 1/2a | – |
| LU44/1 | Finland | Meat | 2003 | 1/2a | – |
| LML48 | Finland | Meat | 1996 | 1/2a | – |
| LT22/1 | Finland | Meat | 1999 | 1/2a | – |
| LT23/1 | Finland | Meat | 2000 | 1/2a | – |
| LE52E | Finland | Processing environment | 2000 | 1/2a | – |
| JHM15 | Finland | Milk | 1987 | 1/2a | – |
| LA33 | Finland | Cow | 1998 | 1/2a | – |
| HT101E/1 | Finland | Vegetable | 2000 | 1/2a | – |
| HL90E/1 | Finland | Processing environment | 2000 | 1/2b | – |
| RE74E | Finland | Processing environment | 1997 | 1/2b | – |
| L94s | Finland | Poultry | 1999–2001 | 1/2b | – |
| LML44 | Finland | Meat | 1996 | 1/2b | P |
| LM115 | Finland | Vegetable | 1995 | 1/2b | – |
| LSO885/1 | Finland | Processing environment | 1996 | 1/2b | – |
| LML36 | Finland | Processing environment | NA | 1/2b | – |
| LL31/1 | Finland | Wild bird, feces | 1998 | 1/2b | C, P |
| LM103/1 | Finland | Vegetable | NA | 1/2b | – |
| LA46 | Finland | Sheep | 1990 | 1/2b | – |
| LM116 | Finland | Vegetable | 1999 | 1/2b | – |
| L25s | Finland | Meat | 1999–2001 | 1/2b | – |
| 2904 | Finland | Processing environment | 1990 | 1/2b | – |
| 2919 | Finland | Processing environment | 1996 | 1/2b | – |
| 3129 | Finland | Processing environment | 1994 | 1/2b | P |
| 2920 | Finland | Processing environment | 1996 | 1/2b | – |
| LM105 | Finland | Vegetable | NA | 1/2b | – |
| HT69E | Finland | Meat | 1999 | 1/2c | P |
| L125s | Finland | Meat | NA | 1/2a | – |
| MJL40 | Finland | Meat | 2009 | 1/2c | P |
| HE161E/1 | Finland | Processing environment | 1999 | 1/2c | – |
| RE70E | Finland | Processing environment | 1997 | 1/2c | – |
| AR5E | Finland | Poultry | 1999 | 1/2c | – |
| LM89 | Finland | Processing environment | NA | 1/2c | – |
| HL6E | Finland | Processing environment | 1998 | 1/2c | – |
| HE152E | Finland | Processing environment | 1999 | 1/2c | – |
| AT3E | Finland | Meat | 1995 | 1/2c | – |
| HT93E/1 | Finland | Ready-to-eat food | 2000 | 1/2c | – |
| HT100E/1 | Finland | Poultry | 2000 | 1/2c | – |
| HE28E | Finland | Processing environment | 1997 | 1/2c | – |
| L51s | Finland | Meat | 1999–2001 | 1/2c | – |
| LL40/2 | Finland | Wild bird, feces | 1998 | 1/2c | – |
| LMML65 | Finland | Meat | 2003 | 1/2a | – |
| LMML67 | Finland | Meat | 2003 | 1/2c | – |
| KE1E | Finland | Processing environment | 1998 | 1/2c | – |
| E7 | Finland | Dairy product | 1999 | 3a | – |
| LK42/1 | Finland | Fish | 1998 | 3a | P |
| LK55/1 | Finland | Fish | 1999 | 3a | – |
| LM128 | Finland | Vegetable | 1999 | 3a | – |
| LK127 | Finland | Fish | 2000 | 4b | – |
| JHM230 | Finland | Cow | 1984 | 4b | – |
| LL17/3 | Finland | Wild bird, feces | 1998 | 4b | – |
| JHM331 | Finland | Silage | 1988 | 4b | – |
| LT25E | Finland | Vegetable | 2000 | 4b | – |
| LL91/1 | Finland | Wild bird, feces | 2001 | 4b | – |
| LL78/1 | Finland | Wild bird, feces | 2001 | 4b | – |
| LK43/3 | Finland | Fish | 1998 | 4b | – |
| LL67/1 | Finland | Wild bird, feces | 2001 | 4b | – |
| LA22 | Finland | Sheep | 1986 | 4b | – |
| LA30 | Finland | Sheep | 1987 | 4b | – |
| 44M | Finland | Meat | 1999–2001 | 4b | – |
| LL72/1 | Finland | Wild bird, feces | 2001 | 4b | – |
| LE21E | Finland | Processing environment | 1999 | 4b | P |
| LA56 | Finland | Poultry | 1992 | 4b | – |
| LL49/2 | Finland | Wild bird, feces | 2001 | 4b | – |
| AE18E | Finland | Processing environment | 1998 | 4b | – |
| JHM270 | Finland | Cow, feces | 1987 | 4b | – |
| LL87/3 | Finland | Wild bird, feces | 2001 | 4b | – |
| LL4/2 | Finland | Wild bird, feces | 1998 | 4b | P |
| LL1/3 | Finland | Wild bird, feces | 1998 | 4b | – |
| LU97/4 | Finland | Meat | 2003 | 4b | – |
| LL71/1 | Finland | Wild bird, feces | 2001 | 4b | – |
| Lm 217 | Switzerland | Meat | 2000 | 1/2a | P |
| Lm 57 | Switzerland | Meat | 1999 | 1/2b | – |
| Lm 60 | Switzerland | Human | 2006 | 1/2a | – |
| Lm 69 | Switzerland | Human | 2006 | 1/2a | – |
| LmE240 | Switzerland | Carcass | 2011 | 1/2a | P |
| LmE61 | Switzerland | Carcass | 2011 | 1/2a | – |
| LmS1 | Switzerland | Environment | 2011 | 1/2a | – |
| LmS9 | Switzerland | Environment | 2011 | 1/2a | – |
| N11-1218 | Switzerland | Poultry | 2011 | 1/2a | – |
| N11-1255 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1285 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1346 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1415 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1515 | Switzerland | Milk | 2011 | 1/2a | – |
| N11-1546 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1617 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-1649 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-1653 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-1696 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-1734 | Switzerland | Ham | 2011 | 1/2a | – |
| N11-1845 | Switzerland | Dairy | 2011 | 1/2a | – |
| N11-1905 | Switzerland | Poultry | 2011 | 1/2a | – |
| N11-2183 | Switzerland | Salad | 2011 | 1/2a | – |
| N11-2215 | Switzerland | Dairy | 2011 | 1/2a | – |
| N11-2272 | Switzerland | Maize | 2011 | 1/2a | – |
| N11-2345 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-2509 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-2538 | Switzerland | Milk | 2011 | 1/2a | – |
| N11-2542 | Switzerland | Cheese | 2011 | 1/2a | – |
| N11-2543 | Switzerland | Environment | 2011 | 1/2a | – |
| N11-2554 | Switzerland | Meat | 2011 | 1/2a | – |
| N11-2662 | Switzerland | Meat | 2011 | 1/2a | – |
| N12-0088 | Switzerland | Fish | 2012 | 1/2a | – |
| N12-0275 | Switzerland | Milk | 2012 | 1/2a | – |
| N12-0299 | Switzerland | Quorn | 2012 | 1/2a | – |
| N12-0303 | Switzerland | Quorn | 2012 | 1/2a | – |
| N12-0373 | Switzerland | Seafood | 2012 | 1/2a | – |
| N12-0402 | Switzerland | Seafood | 2012 | 1/2a | – |
| N12-0435 | Switzerland | Seafood | 2012 | 1/2a | – |
| N12-0459 | Switzerland | Seafood | 2012 | 1/2a | – |
| N12-0494 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-0561 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-0571 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-0677 | Switzerland | Sausage | 2012 | 1/2a | – |
| N12-0762 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-0796 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-0823 | Switzerland | Vegetable | 2012 | 1/2a | – |
| N12-0906 | Switzerland | Milk | 2012 | 1/2a | – |
| N12-0999 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-1002 | Switzerland | Milk | 2012 | 1/2a | – |
| N12-1024 | Switzerland | Meat | 2012 | 1/2a | – |
| N13-0796 | Switzerland | Human | 2013 | 1/2a | – |
| N12-1436 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-2549 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1273 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1641 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-1667 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-1731 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-2118 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-2188 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-2229 | Switzerland | Food | 2012 | 1/2a | – |
| N12-2236 | Switzerland | Meat | 2012 | 1/2a | – |
| N12-2313 | Switzerland | Environment | 2012 | 1/2a | – |
| N12-2320 | Switzerland | Food | 2012 | 1/2a | – |
| N12-2329 | Switzerland | Food | 2012 | 1/2a | – |
| N12-2389 | Switzerland | Milk | 2012 | 1/2a | – |
| N13-0225 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0228 | Switzerland | Milk | 2013 | 4b | P |
| N13-0245 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0254 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0288 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0369 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0406 | Switzerland | Goat | 2013 | 1/2a | – |
| N11-2039 | Switzerland | Human | 2011 | 1/2a | – |
| N13-0474 | Switzerland | Meat | 2013 | 1/2a | – |
| N13-0714 | Switzerland | Human | 2013 | 1/2a | – |
| N11-1547 | Switzerland | Human | 2011 | 1/2a | – |
| N11-1837 | Switzerland | Human | 2011 | 1/2c | – |
| N11-2036 | Switzerland | Human | 2011 | 1/2a | – |
| N11-2134 | Switzerland | Human | 2011 | 1/2a | – |
| N11-2474 | Switzerland | Human | 2011 | 1/2a | – |
| N11-2553 | Switzerland | Human | 2011 | 1/2a | – |
| N12-0123 | Switzerland | Human | 2012 | 1/2a | – |
| N12-0258 | Switzerland | Human | 2012 | 1/2a | – |
| N12-0560 | Switzerland | Human | 2012 | 1/2a | – |
| N12-0588 | Switzerland | Human | 2012 | 1/2a | – |
| N12-0922 | Switzerland | Human | 2012 | 1/2a | – |
| N12-0935 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1107 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1872 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1873 | Switzerland | Human | 2012 | 1/2a | – |
| N12-1917 | Switzerland | Human | 2012 | 1/2a | – |
| N12-2031 | Switzerland | Human | 2012 | 1/2a | – |
| N12-2082 | Switzerland | Human | 2012 | 1/2a | – |
| N12-2169 | Switzerland | Human | 2012 | 1/2a | – |
| N13-0048 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0094 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0119 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0281 | Switzerland | Human | 2013 | 1/2a | – |
| N11-1584 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0698 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0287 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0733 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0739 | Switzerland | Human | 2013 | 1/2a | – |
| N12-0367 | Switzerland | Human | 2012 | 1/2a | – |
| Lm 19 | Switzerland | Human | 2005 | 1/2b | – |
| Lm 45 | Switzerland | Human | 2005 | 1/2b | – |
| Lm 5 | Switzerland | Meat | 1999 | 1/2b | – |
| Lm 9 | Switzerland | Meat | 1999 | 1/2b | – |
| LmE188 | Switzerland | Carcass | 2011 | 1/2b | – |
| LmE212 | Switzerland | Carcass | 2011 | 1/2b | – |
| LmS2 | Switzerland | Environment | 2011 | 1/2b | – |
| LmSB1 | Switzerland | Seafood | 2011 | 1/2b | – |
| N11-1251 | Switzerland | Sausage | 2011 | 1/2b | – |
| N11-1252 | Switzerland | Salad | 2011 | 1/2b | – |
| N11-2675 | Switzerland | Human | 2011 | 1/2a | – |
| N12-1307 | Switzerland | Meat | 2012 | 1/2b | P |
| N12-1608 | Switzerland | Human | 2012 | 1/2b | – |
| N12-1609 | Switzerland | Human | 2012 | 1/2b | – |
| N12-1914 | Switzerland | Milk | 2012 | 1/2b | – |
| N12-2025 | Switzerland | Food | 2012 | 1/2b | – |
| N12-2387 | Switzerland | Meat | 2012 | 1/2b | – |
| N12-2441 | Switzerland | Milk | 2012 | 1/2a | – |
| N12-2449 | Switzerland | Milk | 2012 | 1/2b | – |
| N12-2532 | Switzerland | Human | 2012 | 1/2a | – |
| N12-2533 | Switzerland | Human | 2012 | 1/2a | – |
| N13-0402 | Switzerland | Fish | 2013 | 1/2b | – |
| N13-0581 | Switzerland | Human | 2013 | 1/2b | – |
| N13-0762 | Switzerland | Human | 2013 | 1/2a | – |
| Lm 22/3A | Switzerland | Human | 2004 | 1/2c | – |
| Lm 25/9 | Switzerland | Meat | 2002 | 1/2c | – |
| Lm 28 | Switzerland | Human | 2005 | 1/2c | – |
| Lm 760 | Switzerland | Meat | 2001 | 1/2c | – |
| N11-1514 | Switzerland | Meat | 2011 | 1/2c | – |
| N12-0486 | Switzerland | Human | 2012 | 1/2c | – |
| N12-0563 | Switzerland | Meat | 2012 | 1/2c | P |
| N12-0644 | Switzerland | Meat | 2012 | 1/2c | – |
| N12-0710 | Switzerland | Meat | 2012 | 1/2c | – |
| N12-0822 | Switzerland | Meat | 2012 | 1/2c | – |
| N12-1773 | Switzerland | Meat | 2012 | 1/2c | – |
| N12-1921 | Switzerland | Rice | 2012 | 1/2c | P |
| N12-2151 | Switzerland | Poultry | 2012 | 1/2c | – |
| N12-2271 | Switzerland | Meat | 2012 | 1/2c | – |
| N12-2386 | Switzerland | Environment | 2012 | 1/2c | – |
| N13-0001 | Switzerland | Human | 2013 | 1/2c | – |
| N12-0318 | Switzerland | Quorn | 2012 | 3a | P |
| Lm 49 | Switzerland | Meat | 1999 | 4b | – |
| Lm 58 | Switzerland | Human | 2006 | 4b | – |
| Lm 72 | Switzerland | Human | 2006 | 4b | – |
| Lm 8 | Switzerland | Meat | 1999 | 4b | – |
| Lm LL195 | Switzerland | Human | 1983–87 | 4b | C, P |
| Lm LL201 | Switzerland | Human | 1983–87 | 4b | – |
| LmE131 | Switzerland | Carcass | 2011 | 4b | – |
| LmE153 | Switzerland | Carcass | 2011 | 4b | – |
| LmE154 | Switzerland | Carcass | 2011 | 4b | – |
| LmE162 | Switzerland | Carcass | 2011 | 4b | – |
| N11-1846 | Switzerland | Meat | 2011 | 4b | – |
| N11-1850 | Switzerland | Dairy product | 2011 | 4b | – |
| N11-2292 | Switzerland | Human | 2011 | 4b | – |
| N11-2618 | Switzerland | Human | 2011 | 4b | – |
| N11-2747 | Switzerland | Human | 2011 | 4b | – |
| N11-2801 | Switzerland | Human | 2011 | 4b | – |
| N12-0160 | Switzerland | Meat | 2012 | 4b | – |
| N12-0320 | Switzerland | Human | 2012 | 4b | – |
| N12-0341 | Switzerland | Human | 2012 | 4b | – |
| N12-0432 | Switzerland | Meat | 2012 | 4b | – |
| N12-0466 | Switzerland | Meat | 2012 | 4b | – |
| N12-0529 | Switzerland | Meat | 2012 | 4b | P |
| N12-0551 | Switzerland | Human | 2012 | 4b | – |
| N12-0570 | Switzerland | Human | 2012 | 4b | – |
| N12-0575 | Switzerland | Human | 2012 | 4b | – |
| N12-0605 | Switzerland | Sausage | 2012 | 4b | – |
| N12-0794 | Switzerland | Human | 2012 | 4b | – |
| N12-0869 | Switzerland | Human | 2012 | 4b | – |
| N12-0973 | Switzerland | Meat | 2012 | 4b | – |
| N12-1338 | Switzerland | Human | 2012 | 4b | – |
| N12-1339 | Switzerland | Meat | 2012 | 4b | – |
| N12-1387 | Switzerland | Human | 2012 | 4b | – |
| N12-1655 | Switzerland | Human | 2012 | 4b | – |
| N12-1665 | Switzerland | Environment | 2012 | 4b | – |
| N12-1730 | Switzerland | Human | 2012 | 4b | – |
| N12-1772 | Switzerland | Milk | 2012 | 4b | – |
| N12-1796 | Switzerland | Human | 2012 | 4b | – |
| N12-1859 | Switzerland | Food | 2012 | 4b | – |
| N12-1916 | Switzerland | Environment | 2012 | 4b | – |
| N12-1996 | Switzerland | Environment | 2012 | 4b | – |
| N12-2185 | Switzerland | Environment | 2012 | 4b | – |
| N12-2378 | Switzerland | Human | 2012 | 4b | – |
| N12-2447 | Switzerland | Meat | 2012 | 4b | – |
| N13-0047 | Switzerland | Milk | 2013 | 4b | – |
| N13-0177 | Switzerland | Human | 2013 | 4b | – |
| N13-0677 | Switzerland | Human | 2013 | 4b | – |
| N13-0771 | Switzerland | Human | 2013 | 1/2a | – |
| N13-0772 | Switzerland | Human | 2013 | 4b | P |
| N12-0036 | Switzerland | Human | 2012 | 1/2a | – |
Finland: University of Helsinki, Department of Food Hygiene and Environmental Health strain collection; Switzerland: University of Zürich, Institute for Food Safety and Hygiene strain collection.
NA, not available.
Used also as pilot (P) or control (C) strain. –, not applicable.
(ii) Outliers and batch effects were assessed to remove technical variability. During the Bioscreen experiments at 9.0% NaCl, a slight oscillation of OD600 values appeared due to the measurement technology (Fig. S2), and therefore OD600 values at 1-h intervals were used in the analyses. The replicates of the test strains (Table 2) placed in the outermost wells of the Bioscreen honeycomb plate exhibited reduced growth, which increased the variation in the data set; mean coefficient of variance (CV) was higher for replicates from all wells (4.4%) than for those from inner wells (3.2%). Similarly, reduced growth was observed for the replicates of the two control strains (Table 2) placed in the outermost wells throughout all experimental runs (Fig. S3). In addition, variation between the replicates of the control strains was larger in the outermost wells than in the inner wells for Bioscreen runs performed by both laboratory technician A (CV 3.9% versus 3.6%, respectively) and laboratory technician B (CV 6.0% versus 3.4%, respectively), indicating that conditions in the outermost and inner wells differed. The outermost well data were therefore removed as outliers, after which an average of four replicate growth measurements remained per strain to calculate mean OD600 values. Furthermore, both control strains grew systematically more poorly in the experiments performed by laboratory technician A than by laboratory technician B, who performed the experiments for 90 and 298 of the test strains, respectively (Fig. S4 and S5). Although both laboratory technicians used the same growth media and calibrated pipettes, differences could have arisen from different working techniques, speed, and the “signature” of each laboratory technician, even between these two highly experienced individuals. Removal of this batch effect was achieved through a normalization of the test strain mean OD600 values at the measured time points (Fig. S6 to S10). To avoid excessive technical variation, we propose (i) pipetting blank test broth into the outermost wells of a Bioscreen honeycomb plate (see the example of a loading map in Fig. S11) and (ii) assigning one individual to perform all Bioscreen experiments of a particular study.
Selection of a suitable method for growth parameter calculation (step 2).
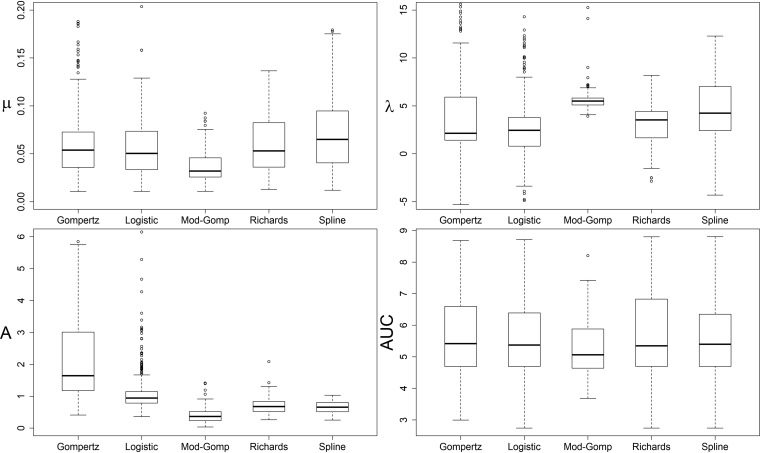
(i) Kinetic growth parameters for the experimental data set varied significantly between different calculation methods. The proportion of strains fitted by each parameter calculation method and the ranges of their parameter values varied notably between the methods used (Table 3 and Fig. 1). Gompertz and logistic models provided negative and excessively large parameter values and included a larger amount of negative values for λ than the other methods (Table 3). All parameter calculation methods differed significantly from one another in pairwise comparison of their respective parameter values for at least two of the parameters λ, μ, MaxOD, and AUC (Friedman’s two-way analysis of variance by ranks for the strains fitted by all methods, n = 128, P ≤ 0.001).
TABLE 3.
Summary of growth parameter estimation for Listeria monocytogenes strains at 9.0% NaCl
| Method used for strain fitting | % of strains (n = 388) fitted | Best model relative to others used for % of strains (n = 388)b | % of λc < 0 of fitted strains | Range for fitted strainsd
|
|||
|---|---|---|---|---|---|---|---|
| μ | λ | MaxOD | AUC | ||||
| Splinea | 100 | NA | 3.1 | 0.012–0.18 | –4.3–12 | 0.25–1.0 | 2.7–8.8 |
| Gompertz | 88 | 0.52 | 13 | 0.011–0.19 | –8.0–57 | 0.41–265 | 3.0–8.7 |
| Modified Gompertz | 41 | 39 | 0 | –0.08–0.09 | 3.9–15 | 0.034–1.4 | 3.7–8.2 |
| Logistic | 97 | 19 | 15 | 0.011–0.65 | –7.7–33 | 0.36–19 | 2.7–8.7 |
| Richards | 77 | 42 | 8.3 | 0.013–0.13 | –2.9–8.2 | 0.26–2.1 | 2.7–8.8 |
Model-free splines according to Kahm et al. (49).
The relative quality of models Gompertz, modified Gompertz, logistic, and Richards to fit each of the strains was assessed by using the Akaike information criterion. NA, not applicable.
λ, lag phase.
μ, maximum growth rate; MaxOD, maximum OD600; AUC, area under the curve.
FIG 1.
Distribution of growth parameter values (μ = maximum growth rate, λ = lag phase, MaxOD = maximum OD600, AUC = area under curve) among the fitted L. monocytogenes strains by Gompertz, logistic, modified Gompertz, and Richards models and model-free spline. Whiskers depict the highest and lowest quartiles, and the box depicts the middle quartiles divided by median line. y axes have been cut to focus on quartiles and remove the most extreme individual outliers.
(ii) Model-free splines provided the most suitable growth parameters for this data set. When assessing the quality of each growth model relative to the other models using the Akaike information criterion (AIC), Richards and modified Gompertz provided the best fit for the largest number of strains, but neither could fit all of the strain growth curves (Table 3). With 77% of the curves fitted, Richards appeared to be a more suitable model for most strains than modified Gompertz. Conversely, the model-free spline provided growth parameters for all tested strains, included relatively few negative λ values, and matched the experimental data points well upon visual inspection. Each spline parameter also correlated significantly with its equivalent Richards model parameter (Spearman’s rho 0.933, 0.722, 0.927, and 1.0 for μ, λ, MaxOD, and AUC, respectively; P < 0.001). Spline parameters were therefore deemed adequate to describe the biological variation of our data set and were chosen for the comparison of strain growth patterns, for the purpose of which the negative spline λ values were changed to 0 after the calculation of all parameters.
Comparison of growth patterns between strains (step 3).
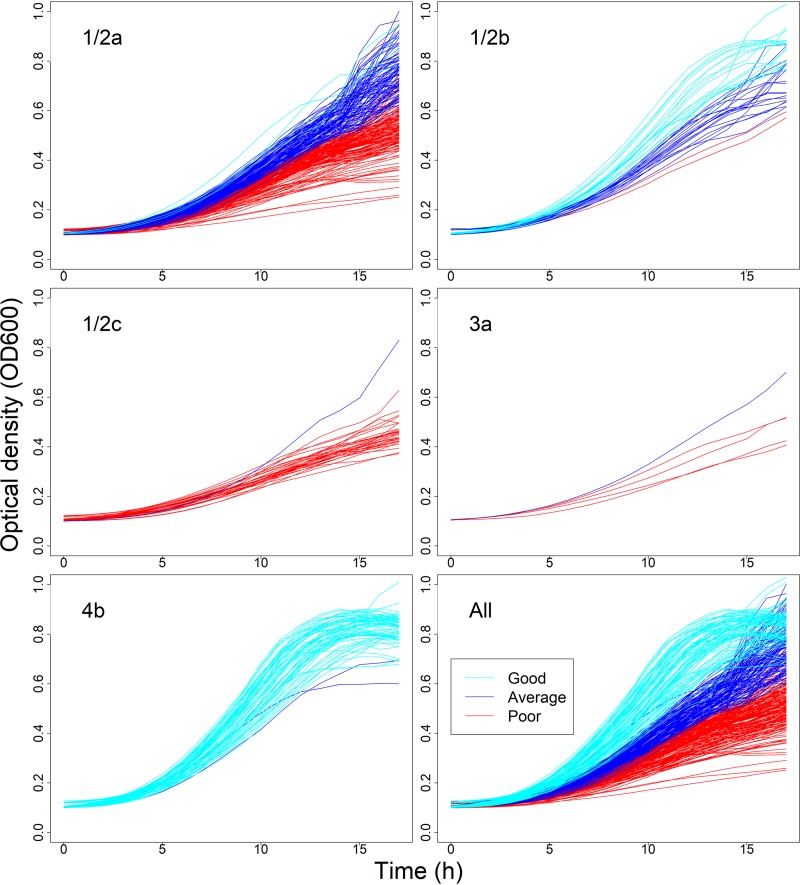
(i) Growth curve and parameter visualization facilitated the identification of growth patterns. Upon visual inspection of strain growth curves, 9.0% NaCl resulted in evident variation of growth patterns between L. monocytogenes strains: tolerant strains displayed higher growth rates and maximum optical densities and shorter lag phases than susceptible strains (Fig. S9). This intraspecies variability indicated that NaCl tolerance of L. monocytogenes could be characterized as a continuous phenotype, measured by gradually increasing growth rate and maximum optical density and decreasing lag phase duration between strains (Fig. S9). Thereby, a classification of the strains into poor, average, and good growers was a priori deemed appropriate to summarize this ordinal variability in the strain-specific growth patterns. The variability between the strain spline parameters was smallest for μ and MaxOD, and highest for λ and AUC (Table 3 and Fig. 1), and all four parameters correlated with each other (Spearman’s rho > 0.8; P < 0.001). As the integral of OD600 measurements, AUC consolidates the other three parameters and was thus hypothesized to be a suitable parameter for strain comparison.
(ii) The parameter AUC differentiated L. monocytogenes growth patterns in salt stress. Four alternative hierarchical clustering outcomes using different variables were investigated to select the best clustering method. The alternative clusterings (numbered 1 to 4) included the following sets of variables: OD600 values from 0 to 17 h (alternative 1), spline growth parameters λ, μ, MaxOD, and AUC (alternative 2), spline growth parameters λ, μ, and MaxOD (alternative 3), and spline growth parameter AUC (alternative 4). For each alternative clustering outcome, dendrograms based on Ward linkage suggested a division of the entire data set into two clusters, both of which could be further divided into two clusters. The alternative clustering outcomes 1 to 4 were initially visualized by their strain growth curves in Microsoft Office Excel 2013 (Fig. S12 to S15) and assessed for their ability to recognize the a priori deemed ordinal growth patterns. Only alternative 4, which utilized AUC as the clustering variable, resulted in ordinal division of the growth clusters (Fig. S15) and thus was chosen for the growth pattern classification of L. monocytogenes strains.
Biological interpretation of the discovered differences (step 4).
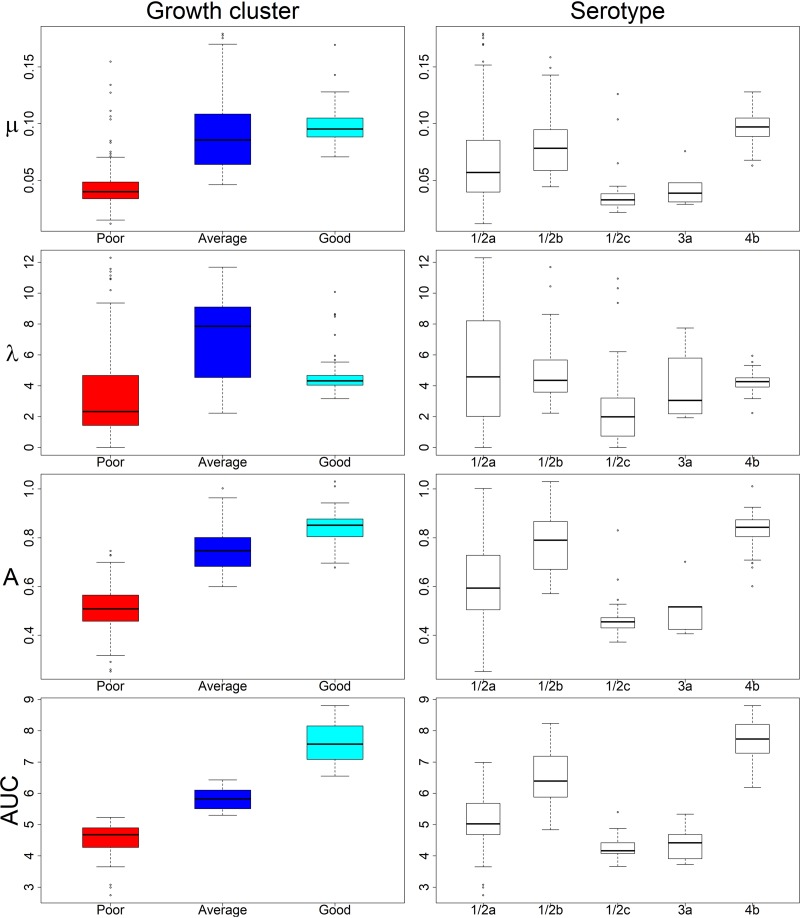
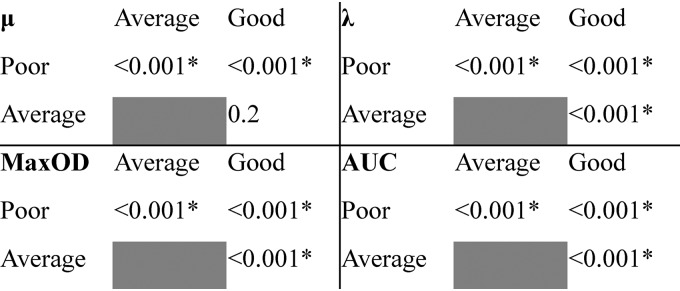
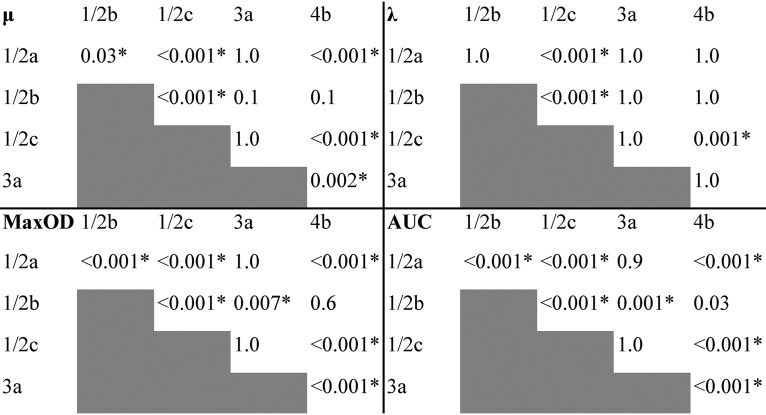
(i) The growth of L. monocytogenes strains varied from poor to good at 9.0% NaCl. Clusters of strains displaying poor, average, and good growth at 9.0% NaCl, including 182, 116, and 90 strains, respectively, were labeled accordingly (Fig. 2). Variation between the strains within each cluster (CVs “good” [52%], “average” [62%], and “poor” [63%]) was higher than variation between the individual replicates of a strain (CVs 0.0 to 36%). The cluster “poor” was characterized by the lowest values of all growth parameters, whereas “good” included strains with the highest MaxOD and AUC values (Fig. 3). The “average” cluster included strains with highly varying μ and λ, but intermediary MaxOD and AUC (Fig. 3). The spline growth parameters differed significantly from one another between the three growth clusters (Kruskal-Wallis test, P < 0.001), except for μ of clusters “average” and “good” (Fig. 4).
FIG 2.
L. monocytogenes strains (n = 388) by serovar and growth cluster in 9.0% NaCl. The panels depict strain growth curves belonging to serovars 1/2a, 1/2b, 1/2c, 3a, 4b, and all serovars. Division to growth clusters is visualized by color, as indicated in legend.
FIG 3.
Variation of spline growth parameters of L. monocytogenes strains (n = 388) in 9.0% NaCl within identified growth clusters and serovars. Whiskers indicate the highest and lowest quartiles, and the box depicts the middle quartiles divided by median line. Dots indicate outliers. Growth parameters are calculated from spline fit: μ corresponds to growth rate (OD600 U/h), λ to lag time (h), MaxOD to maximum growth (OD600 units), and AUC to the area under curve calculated by an integral.
FIG 4.
Kruskal-Wallis test P values for the associations of each spline growth parameter between the growth clusters “poor,” “average,” and “good” determined for L. monocytogenes strains (n = 388) at 9.0% NaCl. Significant associations are indicated with an asterisk. μ corresponds to growth rate (OD600 U/h), λ to lag time (h), MaxOD to maximum growth (OD600 units), and AUC to the area under curve calculated by an integral.
(ii) Strain variability at 9.0% NaCl uncovered serovar- and lineage-dependent salt stress tolerance of L. monocytogenes. The best-growing L. monocytogenes strains belonged to the serovars 1/2b and 4b of lineage I, and the poorest growers to serovars 1/2a, 1/2c, and 3a of lineage II (Fig. 2). Serovar 4b was significantly overrepresented in the cluster “good,” and 1/2b in the “good” and “average” clusters, whereas serovar 1/2a was significantly underrepresented in cluster “good” and 1/2c in clusters “good” and “average” (z-test, P < 0.05; Table 4). In our data set, the serovar 1/2a strains were characterized by a large variation of their growth parameter values (Fig. 3). Serovars 1/2b and 4b exhibited the highest and 1/2c and 3a the lowest growth parameter median values (Fig. 3). Each serovar differed significantly from another serovar in the pairwise comparison of their growth parameter values for at least two of the parameters λ, μ, MaxOD, and AUC (Kruskal-Wallis test, P ≤ 0.03, Fig. 5).
TABLE 4.
Numbers and proportions of Listeria monocytogenes strains of each serovar belonging to three 9.0% NaCl growth clustersa
| Serovar | No. (%) of strains by growth cluster |
||
|---|---|---|---|
| Poor | Average | Good | |
| 1/2a | 144 (59.8)A | 93 (38.6)A | 4 (1.7)B |
| 1/2b | 2 (5.3)A | 19 (50.0)B | 17 (44.7)B |
| 1/2c | 32 (97.0)A | 1 (3.0)B | 0 (0.0)B |
| 3a | 4 (80.0)A | 1 (20.0)A | 0 (0.0)A |
| 4b | 0 (0.0)A | 2 (2.8)A | 69 (97.2)B |
The numbers of L. monocytogenes strains in each serovar (1/2a, n = 241; 1/2b, n = 38; 1/2c, n = 33; 3a, n = 5; 4b, n = 71) belonging to the 9.0% NaCl growth clusters “poor,” “average,” and “good” are presented. The same superscript capital letter indicates proportions that do not differ significantly at the 0.05 level within rows (z-test with Bonferroni correction).
FIG 5.
Kruskal-Wallis test P values for the associations of each spline growth parameter between the tested serovar 1/2a, 1/2b, 1/2c, 3a, and 4b Listeria monocytogenes strains (n = 388) at 9.0% NaCl. Significant associations are indicated with an asterisk. μ corresponds to growth rate (OD600 U/h), λ to lag time (h), MaxOD to maximum growth (OD600 units), and AUC to the area under curve calculated by an integral.
DISCUSSION
This growth data assembly and analysis protocol for L. monocytogenes salt stress tolerance studies provides a systematic tool and checklist for the investigation of strain variability of stress tolerance (Table 1). Strain variability of growth ability is an important practical factor to consider, for instance, prior to conducting quantitative microbiological risk assessments (QMRAs), as it has implications on the selection of strains for challenge tests estimating shelf-lives of food products (31, 59). Our protocol compiles a systematic approach to estimate the scope of intraspecies stress tolerance variability and identify strains that grow better than others under examined stress conditions, aiding the selection of suitable strains for QMRAs. Phenotypic knowledge gained by our approach can also be used to identify exceptionally tolerant or susceptible strains for further analysis of underlying dynamics and mechanisms of stress tolerance.
Although tools for data generation and handling depend upon the preferences of each researcher, the considerations to ensure the reliability of extensive growth studies are the same. To begin with, our results highlight the importance of incorporating an appropriate level of stress when aiming to examine strain variability of stress tolerance. Although NaCl concentrations of 8% and below have been used in L. monocytogenes stress experiments to illustrate strain variability (33, 35, 60, 61), our results imply that a high NaCl concentration was required to differentiate notable variation in strain growth ability. The ability of our study to affirm, on an unprecedentedly large scale, some previously described serovar- and lineage-dependent osmotic stress phenomena (30, 61, 62, 77) emphasizes the relevance and reliability of our data assembly and analysis protocol for L. monocytogenes stress tolerance investigations.
When assessing the strain variability of growth ability, we must initially make sure to draw conclusions on biological, not technical, variability (63) by the use of technical and biological controls and careful data exploration. Technical variation and data exploration steps should be discussed in growth experiment publications to review the reproducibility and reliability of the results. Measurement oscillation due to the machinery and differences between strain replicates depending on their placement in the Bioscreen honeycomb plate were resolved by removing outliers. The reduced growth of the replicates placed alongside the outermost rim of the plate might have been due to, for instance, a higher rate of evaporation of liquid from the 300 μl of culture dilution placed in the outermost wells than in the inner wells, which would have caused an irregular increase of the NaCl concentration in the outermost wells. Although the overall variation between the control strain replicates was negligible, the systematic well effect indicated a biologically noteworthy phenomenon, which should be considered when designing loading maps for the Bioscreen honeycomb plate (Fig. S11). Due to the extreme precision required in the labor-intensive pipetting of the Bioscreen honeycomb plates, individual differences in, e.g., pipetting techniques may result in systematic variation, even between highly experienced professionals. Following standard statistical procedures, the observed batch effect between the two laboratory technicians was removed by setting one group as a reference and normalizing the other by adding the calculated difference of the mean OD600 values to its OD600 values. The emphasis of our experiments on phenotypic variability was on the comparison of growth curves and kinetic parameters proportionate to one another, not on their absolute values. Thereby, the performed normalization was adequate, as well as imperative, to create comparable distributions and enable the identification of strains with dissimilar growth patterns.
The second step of our data assembly and analysis protocol was to choose a suitable method to quantify growth ability for the comparison of strains, a purpose which did not require substantial assumptions on the underlying kinetics of growth. Our analysis revealed marked differences in the ability of the tested growth models and model-free splines to fit all growth curves, as well as significant variation in the respective parameter estimates by the different methods. Unlike in previous studies (38, 41, 64), none of the tested growth models were adequate to describe our entire data set, but model-free splines (49) fitted all the curves. The spline parameters correlated with those derived from the Richards model which has been indicated to suit absorbance measurement data (38, 64). When utilizing absorbance to evaluate strain differences, it is valuable to note that an apparent increase in cell size without increase in cell numbers may increase the OD at highly stressful conditions (42). Under NaCl stress, L. monocytogenes cells may elongate to form filaments which are divided by septa (65–67) and consist of several normal-sized cells on the verge of division (67). Consequently, a potential increase in OD caused by such filaments would still reflect cell numbers.
The third step of our data assembly and analysis protocol included the comparison of alternative hierarchical clustering outcomes to facilitate the strain comparison and classification of growth patterns. In foodstuffs, the delay at the beginning of growth or the maximum growth level reached may sometimes be more important than growth rate, which can be similar for curves with differing λ and MaxOD (44). While μ may be an often-used practically convenient metric, the variation of both λ and MaxOD within our data set of 388 L. monocytogenes strains advocated for the consideration of the entire growth pattern in strain comparison. AUC was deemed an appropriate variable to differentiate between L. monocytogenes strain growth patterns under salt stress, as it suits to summarize and compare entire growth patterns in cases where λ increases while μ and MaxOD decrease in an ordinal fashion between the different strains. However, growth curves with a high μ and MaxOD could reach a similar AUC to curves with a low μ and MaxOD, if the λ were long for the former and short for the latter. Consequently, no single all-purpose solution exists, and both statistical tests and intuitive reasoning should be used to classify the overall growth patterns present in a given data set, keeping in mind the suitability of the chosen approach with respect to the data and purpose of the study. The identified ordinal growth clusters “poor,” “average,” and “good” displayed, on average, significantly different growth parameters, but overlapped slightly. Thereby, a strict distinction into groups of differing growth patterns was not possible, which indicates that L. monocytogenes growth under salt stress should be approached as a continuous rather than a categorized phenotype.
The final step of our data assembly and analysis protocol was to draw biological interpretations on stress tolerance based on the strain differences observed in the growth experiments. The clusters of L. monocytogenes strains displaying poor, average, and good growth appeared to provide a meaningful representation of the differing growth patterns, as they also captured biological differences of salt stress tolerance among the studied L. monocytogenes serovars. In our experiments, L. monocytogenes lineage I strains (serovars 1/2b and 4b) grew significantly better than lineage II strains (serovars 1/2a, 1/2c, and 3a) at 9.0% NaCl, which indicates that the ability to grow under NaCl osmotic stress is connected to the phylogenetic composition of the species. Serovar 4b is frequently associated with human listeriosis, while occasional human outbreaks by serovar 1/2b have been reported (23, 68), and serovar 1/2a is common in human cases in the Nordic countries (69, 70). The apparent overrepresentation of 1/2a and 1/2c among isolates from food-related sources and their underrepresentation in human cases in some countries have been hypothesized to be related to attenuated virulence of many lineage II strains (22, 27, 71) or their endurance of environmental conditions in food-associated environments (28, 61). Our results are particularly congruent with the former hypothesis, since L. monocytogenes virulence has been linked to its osmotolerance (17, 52, 53), but do not conflict with the latter hypothesis either, as 9.0% NaCl is a condition rarely encountered in the food chain, except for brining solutions.
In our experiments, many L. monocytogenes strains were able to grow relatively well in saline conditions that are among the most extreme found in the food chain. However, food matrices and establishments form complex systems that involve multiple varying stress conditions. Although L. monocytogenes is highly tolerant toward several conditions used for controlling bacterial growth during food production and storage (14, 15), the overall stress exposure and response depend on all environmental conditions, such as temperature, pH, water activity, nutrients, and sanitizing agents (3, 4, 30, 35). The growth patterns described here for NaCl osmotic stress may therefore be altered by other factors present in food systems. Nevertheless, the notable strain variability during salt stress uncovered by the present study implies that L. monocytogenes has a large reservoir of growth ability. This may help the population adapt to prevailing environmental conditions and hence conquer various niches, a strategy referred to as “bet-hedging” (72). Based on our observations, high salinity environments could provide a growth niche particularly for L. monocytogenes strains of lineage I.
MATERIALS AND METHODS
Strain collection.
L. monocytogenes wild-type strains (n = 388, Table 2) isolated from animals, raw materials, food, processing environment, and patients were used. Stock cultures were stored at –70°C in bead tubes. Serotyping was performed using the Listeria Antisera Set with O- and H-factor antisera (Denka Seiken, Tokyo, Japan) according to the manufacturer’s instructions. Serovars of 14 strains yielding uncertain results were confirmed using multiplex-PCR as described elsewhere (73). Strains represented serovars 1/2a (n = 241), 1/2b (n = 38), 1/2c (n = 33), 3a (n = 5), and 4b (n = 71).
Optimization of the stress condition.
To select an appropriate osmotic stress condition, separate pilot study broths were prepared by adding NaCl to final concentrations of 6.5, 7.5, and 8.5% into BHI broth (Oxoid, Cheshire, England). In the pilot, 20 L. monocytogenes strains (Table 2) were chosen by generating random numbers for each strain in R and selecting 10 strains with the highest random number values from both lineage I and II while ensuring even distribution of serovars. A single colony of each pilot strain was grown in duplicate (technical replicates) in each NaCl concentration using a Bioscreen C microbiology reader (Growth Curves, Helsinki, Finland) following the protocol described for the growth experiments at salt stress below. Growth curves were visualized in Microsoft Office Excel 2013 to determine which condition displayed increased variation among strain growth ability, i.e., sufficient stress to distinguish differences between strains (Fig. S1).
Growth experiments of NaCl tolerance.
Stock cultures plated on blood agar were incubated at 37°C for 24 h. Three separate colonies (biological replicates) were individually inoculated into 5 ml of BHI broth and incubated at 37°C with shaking (250 rpm) for 18 h to reach an average cell count of 109 CFU ml−1. Overnight cultures were diluted (1:100) in 1.5 ml of fresh BHI broth including 9% NaCl (test broth), and 300 μl of each diluted culture was loaded into separate wells of a 100-well honeycomb plate in duplicate (technical replicates). Four wells were left with blank test broth as controls for no growth. The three biological replicates of the same strain were loaded at scattered positions on the plate. A cover was used on the plate to prevent evaporation.
Growth experiments were performed in the Bioscreen C microbiology reader at 37°C for 17 h, and the optical densities at 600 nm (OD600) were automatically measured at 15-min intervals. Two control strains were used in each Bioscreen run throughout the study; three individual isolates of the two controls were grown in the same positions of the honeycomb plate to exclude systematic technical variation between separate Bioscreen runs. The order in which the 388 strains were tested throughout the experiments was determined via random numbers generated in R version 3.4.3 (74). During each experiment the growth curves were routinely visualized at the computer connected to the Bioscreen machine. At the end of the experiment, the wells showing no growth or exceptional shape of growth curve were sampled and cultured on blood agar (37°C for 24 h) to detect lack of sample or potential contaminants. If this culture displayed no growth (empty well) or mixed growth (contaminated well) based on visual inspection, the data from such wells were disregarded and the strains concerned retested.
Raw data exploration.
Data exploration was performed in Microsoft Office Excel 2013 and R version 3.4.3. To mitigate the slight oscillation of the measured OD600 values, which appeared due to the measurement technology (Fig. S2), the OD600 values at 1-h intervals were used in the analyses. The replicate growth curves of the two control strains were visually inspected to evaluate potential systematic differences within the experiments. As differences were observed between the replicates grown in the inner and outermost wells of the Bioscreen honeycomb plate (Fig. S3 to S5), the outermost-well replicates were excluded as outliers. Initially, three biological and two technical replicate sets of OD600 values (six in total) were obtained for every strain. The technical and biological replicate curves of each strain were visualized, and their similarity was evaluated. The strain was retested if individual replicates displayed deviations that could not be explained by contamination or their position in an outermost well.
Identification and normalization of batch effects.
The presence of potentially confounding batch effects, i.e., systematic nonbiological differences between sample groups, was estimated using the control strain replicates included in each Bioscreen experiment. Laboratory technicians A and B conducted the experiments for 90 and 298 strains, respectively, and an evident batch effect was observed between them (Fig. S3 to S6). A nonparametric approach was employed in the batch effect normalization to avoid assuming any specific parametric shape of the growth data over time, as the observed batch effect itself was nonlinear (Fig. S7 to S9). At each time point t, the mean ODs— and —of control samples belonging to each group were calculated. When LA and LB are the two groups of control samples defined by the batch (laboratory technician), the difference——s the estimated batch effect at time point t. The normalization was performed by adding Δt to all samples belonging to batch LA. The custom R scripts for normalization are provided as supplemental material (Text S10 in the supplemental material).
Growth parameters and data visualization.
Growth modeling and growth curve visualization were performed with R version 3.4.3 (74). Using the normalized mean OD600 values, kinetic growth parameters were determined for each strain with the R package grofit (49) (archived from the CRAN repository on 17 June 2018) using default settings. The parameters were calculated with logistic, Gompertz, modified-Gompertz, and Richards models, as well as model-free splines based on cubic spline interpolation, for which the smoothing parameter, smooth.gc, was set to default (NULL), allowing the program to select an optimal value via cross-validation techniques (49). The resulting five sets of kinetic growth parameters included λ (lag phase duration), μ (growth rate), MaxOD (maximum OD600), and AUC (area under the curve). In addition, the program assesses and selects the best-fitting growth model for each individual strain by the comparison of nonlinear least-squares regression of each model via Akaike information criterion (AIC) (75). Since the same parameter calculation method had to be used for all strains to enable their comparison, we selected the best-fitting parameter calculation method based on the following criteria: (i) the ability to calculate parameters for all growth curves, (ii) a low proportion of negative values of λ (76), and (iii) a good fit on visual inspection of the modeled curve and data points. After the selection of the best method, the negative λ values were transformed to 0, because negative measurements of time duration are not meaningful. The custom R scripts for growth curve and parameter visualization are provided as supplemental material (Text S10).
Statistical analyses.
Statistical analyses were performed with R version 3.4.3 and IBM SPSS Statistics for Windows version 25.0 (IBM, Armonk, NY). The variability between the replicates of each strain and the strains within each growth cluster was estimated using the coefficient of variation (CV, i.e., ratio of standard deviation to mean) calculated from their respective OD600 values. Nonnormal distribution of the growth parameter values was confirmed by Kolmogorov-Smirnov test and visualization of normal Q-Q plots. Friedman’s two-way analysis of variance by ranks and Spearman’s rho were used to compare the growth parameters of the different models and model-free splines, while removing strains with missing values from the calculation of these statistics. Hierarchical clustering with Ward’s method by squared Euclidian distance was utilized to classify the strain growth patterns. Four alternative clustering outcomes were formed using either the strain mean-OD600 values from 0 to 17 h at 1-h intervals (alternative 1) or a selection of the best-fitting growth parameters (alternatives 2 to 4 described in Results). Utilizing the visualization of the cluster growth curves in Microsoft Office Excel 2013, we chose the clustering alternative that best captured the growth patterns emerging from the data set. A Kruskal-Wallis test was used to investigate the associations between serovar, growth cluster, and growth parameters.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Finnish Ministry of Agriculture and Forestry (Makera grant 1796/312/2013), the Doctoral Program in Food Chain and Health of the University of Helsinki, and the Finnish Foundation of Veterinary Research. The funders were not involved in study design, data analysis, or writing of the article.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. 2001. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farber JM, Losos JZ. 1988. Listeria monocytogenes: a foodborne pathogen. CMAJ 138:413–418. [PMC free article] [PubMed] [Google Scholar]
- 3.Fonnesbech Vogel B, Hansen LT, Mordhorst H, Gram L. 2010. The survival of Listeria monocytogenes during long-term desiccation is facilitated by sodium chloride and organic material. Int J Food Microbiol 140:192–200. doi: 10.1016/j.ijfoodmicro.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira A, O’Byrne CP, Boor KJ. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl Environ Microbiol 67:4454–4457. doi: 10.1128/aem.67.10.4454-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker LA, Cetin MS, Hutkins RW, Benson AK. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol 180:4547–4554. doi: 10.1128/JB.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray ML, Killinger AH. 1966. Listeria monocytogenes and listeric infections. Bacteriol Rev 30:309–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenlon DR, Wilson J, Donachie W. 1996. The incidence and level of Listeria monocytogenes contamination of food sources at primary production and initial processing. J Appl Bacteriol 81:641–650. doi: 10.1111/j.1365-2672.1996.tb03559.x. [DOI] [PubMed] [Google Scholar]
- 8.Blatter S, Giezendanner N, Stephan R, Zweifel C. 2010. Phenotypic and molecular typing of Listeria monocytogenes isolated from the processing environment and products of a sandwich-producing plant. Food Control 21:1519–1523. doi: 10.1016/j.foodcont.2010.04.025. [DOI] [Google Scholar]
- 9.Lundén JM, Autio TJ, Sjöberg AM, Korkeala HJ. 2003. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J Food Prot 66:2062–2069. doi: 10.4315/0362-028x-66.11.2062. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen MK, Bjorkroth J, Korkeala HJ. 1999. Characterization of Listeria monocytogenes from an ice cream plant by serotyping and pulsed-field gel electrophoresis. Int J Food Microbiol 46:187–192. doi: 10.1016/S0168-1605(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen MK, Siitonen A, Heiskanen P, Haajanen H, Bjorkroth KJ, Korkeala HJ. 1999. Molecular epidemiology of an outbreak of febrile gastroenteritis caused by Listeria monocytogenes in cold-smoked rainbow trout. J Clin Microbiol 37:2358–2360. doi: 10.1128/JCM.37.7.2358-2360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephan R, Althaus D, Kiefer S, Lehner A, Hatz C, Schmutz C, Jost M, Gerber N, Baumgartner A, Hächler H, Mäusezahl-Feuz M. 2015. Foodborne transmission of Listeria monocytogenes via ready-to-eat salad: a nationwide outbreak in Switzerland, 2013–2014. Food Control 57:14–17. doi: 10.1016/j.foodcont.2015.03.034. [DOI] [Google Scholar]
- 13.Holch A, Webb K, Lukjancenko O, Ussery D, Rosenthal BM, Gram L. 2013. Genome sequencing identifies two nearly unchanged strains of persistent Listeria monocytogenes isolated at two different fish processing plants sampled 6 years apart. Appl Environ Microbiol 79:2944–2951. doi: 10.1128/AEM.03715-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucur FI, Grigore-Gurgu L, Crauwels P, Riedel CU, Nicolau AI. 2018. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front Microbiol 9:2700. doi: 10.3389/fmicb.2018.02700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NicAogáin K, O’Byrne CP. 2016. The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front Microbiol 7:1865. doi: 10.3389/fmicb.2016.01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gahan CGM, Hill C. 2005. Gastrointestinal phase of Listeria monocytogenes infection. J Appl Microbiol 98:1345–1353. doi: 10.1111/j.1365-2672.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. 2004. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol 186:3355–3362. doi: 10.1128/JB.186.11.3355-3362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallipolitis BH, Ingmer H. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol Lett 204:111–115. doi: 10.1111/j.1574-6968.2001.tb10872.x. [DOI] [PubMed] [Google Scholar]
- 19.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J Bacteriol 185:5722–5734. doi: 10.1128/jb.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter PD, Emerson N, Gahan CGM, Hill C. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J Bacteriol 181:6840–6843. doi: 10.1128/JB.181.21.6840-6843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray MJ, Freitag NE, Boor KJ. 2006. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect Immun 74:2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton DM, Scarlett JM, Horton K, Sue D, Thimothe J, Boor KJ, Wiedmann M. 2001. Characterization and pathogenic potential of Listeria monocytogenes isolates from the smoked fish industry. Appl Environ Microbiol 67:646–653. doi: 10.1128/AEM.67.2.646-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trott DJ, Robertson ID, Hampson DJ. 1993. Genetic characterization of isolates of Listeria monocytogenes from man, animals and food. J Med Microbiol 38:122–128. doi: 10.1099/00222615-38-2-122. [DOI] [PubMed] [Google Scholar]
- 24.Haase JK, L. monocytogenes MLST Study Group, Didelot X, Lecuit M, Korkeala H, Achtman M. 2014. The ubiquitous nature of Listeria monocytogenes clones: a large-scale multilocus sequence typing study. Environ Microbiol 16:405–416. doi: 10.1111/1462-2920.12342. [DOI] [PubMed] [Google Scholar]
- 25.Jeffers GT, Bruce JL, McDonough PL, Scarlett J, Boor KJ, Wiedmann M. 2001. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology 147:1095–1104. doi: 10.1099/00221287-147-5-1095. [DOI] [PubMed] [Google Scholar]
- 26.Hong E, Doumith M, Duperrier S, Giovannacci I, Morvan A, Glaser P, Buchrieser C, Jacquet C, Martin P. 2007. Genetic diversity of Listeria monocytogenes recovered from infected persons and pork, seafood and dairy products on retail sale in France during 2000 and 2001. Int J Food Microbiol 114:187–194. doi: 10.1016/j.ijfoodmicro.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Moura A, Criscuolo A, Pouseele H, Maury MM, Leclercq A, Tarr C, Björkman JT, Dallman T, Reimer A, Enouf V, Larsonneur E, Carleton H, Bracq-Dieye H, Katz LS, Jones L, Touchon M, Tourdjman M, Walker M, Stroika S, Cantinelli T, Chenal-Francisque V, Kucerova Z, Rocha EPC, Nadon C, Grant K, Nielsen EM, Pot B, Gerner-Smidt P, Lecuit M, Brisse S. 2016. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat Microbiol 2:16185. doi: 10.1038/nmicrobiol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsi RH, den Bakker HC, Wiedmann M. 2011. Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol 301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Lianou A, Stopforth JD, Yoon Y, Wiedmann M, Sofos JN. 2006. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J Food Prot 69:2640–2647. doi: 10.4315/0362-028x-69.11.2640. [DOI] [PubMed] [Google Scholar]
- 30.Van Der Veen S, Moezelaar R, Abee T, Wells-Bennik M. 2008. The growth limits of a large number of Listeria monocytogenes strains at combinations of stresses show serotype- and niche-specific traits. J Appl Microbiol 105:1246–1258. doi: 10.1111/j.1365-2672.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 31.Lianou A, Koutsoumanis KP. 2013. Strain variability of the behavior of foodborne bacterial pathogens: a review. Int J Food Microbiol 167:310–321. doi: 10.1016/j.ijfoodmicro.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Faleiro ML, Andrew PW, Power D. 2003. Stress response of Listeria monocytogenes isolated from cheese and other foods. Int J Food Microbiol 84:207–216. doi: 10.1016/s0168-1605(02)00422-1. [DOI] [PubMed] [Google Scholar]
- 33.Hingston P, Chen J, Dhillon BK, Laing C, Bertelli C, Gannon V, Tasara T, Allen K, Brinkman FSL, Hansen LT, Wang S. 2017. Genotypes associated with Listeria monocytogenes isolates displaying impaired or enhanced tolerances to cold, salt, acid, or desiccation stress. Front Microbiol 8:369. doi: 10.3389/fmicb.2017.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keto-Timonen R, Pöntinen A, Aalto-Araneda M, Korkeala H. 2018. Growth of Yersinia pseudotuberculosis strains at different temperatures, pH values, and NaCl and ethanol concentrations. J Food Prot 81:142–149. doi: 10.4315/0362-028X.JFP-17-223. [DOI] [PubMed] [Google Scholar]
- 35.Magalhães R, Ferreira V, Brandão TRS, Palencia RC, Almeida G, Teixeira P. 2016. Persistent and non-persistent strains of Listeria monocytogenes: a focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol 57:103–108. doi: 10.1016/j.fm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Augustin J, Rosso L, Carlier V. 1999. Estimation of temperature dependent growth rate and lag time of Listeria monocytogenes by optical density measurements. J Microbiol Methods 38:137–146. doi: 10.1016/S0167-7012(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 37.McClure PJ, Cole MB, Davies KW, Anderson WA. 1993. The use of automated tubidimetric data for the construction of kinetic models. J Ind Microbiol 12:277–285. doi: 10.1007/BF01584203. [DOI] [Google Scholar]
- 38.Dalgaard P, Koutsoumanis K. 2001. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J Microbiol Methods 43:183–196. doi: 10.1016/S0167-7012(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 39.Dalgaard P, Ross T, Kamperman L, Neumeyer K, McMeekin TA. 1994. Estimation of bacterial growth rates from turbidimetric and viable count data. Int J Food Microbiol 23:391–404. doi: 10.1016/0168-1605(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 40.Francois K, Devlieghere F, Standaert AR, Geeraerd AH, Cools I, Van Impe JF, Debevere J. 2005. Environmental factors influencing the relationship between optical density and cell count for Listeria monocytogenes. J Appl Microbiol 99:1503–1515. doi: 10.1111/j.1365-2672.2005.02727.x. [DOI] [PubMed] [Google Scholar]
- 41.Pla M, Oltra S, Esteban M, Andreu S, Palop A. 2015. Comparison of primary models to predict microbial growth by the plate count and absorbance methods. Biomed Res Int 2015:365025. doi: 10.1155/2015/365025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson K, McVey AF, Clark IBN, Swain PS, Pilizota T. 2016. General calibration of microbial growth in microplate readers. Sci Rep 6:38828. doi: 10.1038/srep38828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esser DS, Leveau JHJ, Meyer KM. 2015. Modeling microbial growth and dynamics. Appl Microbiol Biotechnol 99:8831–8846. doi: 10.1007/s00253-015-6877-6. [DOI] [PubMed] [Google Scholar]
- 44.Peleg M, Corradini MG. 2011. Microbial growth curves: what the models tell us and what they cannot. Crit Rev Food Sci Nutr 51:917–945. doi: 10.1080/10408398.2011.570463. [DOI] [PubMed] [Google Scholar]
- 45.Baranyi J, Roberts TA. 1995. Mathematics of predictive food microbiology. Int J Food Microbiol 26:199–218. doi: 10.1016/0168-1605(94)00121-l. [DOI] [PubMed] [Google Scholar]
- 46.Zwietering MH, Jongenburger I, Rombouts FM, van ‘t Riet K. 1990. Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881. doi: 10.1128/AEM.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchanan RL, Whiting RC, Damert WC. 1997. When is simple good enough: a comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol 14:313–326. doi: 10.1006/fmic.1997.0125. [DOI] [Google Scholar]
- 48.Korkeala H, Alanko T, Tiusanen T. 1992. Effect of sodium nitrite and sodium chloride on growth of lactic acid bacteria. Acta Vet Scand 33:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahm M, Hasenbrink G, Lichtenberg-Fraté H, Ludwig J, Kschischo M. 2010. grofit: fitting biological growth curves with R. J Stat Softw 33:1–21. doi: 10.18637/jss.v033.i07.20808728 [DOI] [Google Scholar]
- 50.Baranyi J, Roberts TA. 1994. A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol 23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 51.Huang L. 2013. Optimization of a new mathematical model for bacterial growth. Food Control 32:283–288. doi: 10.1016/j.foodcont.2012.11.019. [DOI] [Google Scholar]
- 52.Watson D, Sleator RD, Casey PG, Hill C, Gahan C. 2009. Specific osmolyte transporters mediate bile tolerance in Listeria monocytogenes. Infect Immun 77:4895–4904. doi: 10.1128/IAI.00153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Payne A, Schmidt TB, Nanduri B, Pendarvis K, Pittman JR, Thornton JA, Grissett J, Donaldson JR. 2013. Proteomic analysis of the response of Listeria monocytogenes to bile salts under anaerobic conditions. J Med Microbiol 62:25–35. doi: 10.1099/jmm.0.049742-0. [DOI] [PubMed] [Google Scholar]
- 54.Motes ML. 1991. Incidence of Listeria spp. in shrimp, oysters, and estuarine waters. J Food Prot 54:170–173. doi: 10.4315/0362-028X-54.3.170. [DOI] [PubMed] [Google Scholar]
- 55.Colburn KG, Kaysner CA, Abeyta CJ, Wekell MM. 1990. Listeria species in a California coast estuarine environment. Appl Environ Microbiol 56:2007–2011. doi: 10.1128/AEM.56.7.2007-2011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duché O, Trémoulet F, Glaser P, Labadie J. 2002. Salt stress proteins induced in Listeria monocytogenes. Appl Environ Microbiol 68:1491–1498. doi: 10.1128/aem.68.4.1491-1498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang CA, Sheen S, Juneja VK. 2009. Effect of salt, smoke compound, and temperature on the survival of Listeria monocytogenes in salmon during simulated smoking processes. J Food Sci 74:M522–M529. doi: 10.1111/j.1750-3841.2009.01377.x. [DOI] [PubMed] [Google Scholar]
- 58.Cornu M, Beaufort A, Rudelle S, Laloux L, Bergis H, Miconnet N, Serot T, Delignette-Muller ML. 2006. Effect of temperature, water-phase salt and phenolic contents on Listeria monocytogenes growth rates on cold-smoked salmon and evaluation of secondary models. Int J Food Microbiol 106:159–168. doi: 10.1016/j.ijfoodmicro.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 59.Uyttendaele M, Rajkovic A, Benos G, François K, Devlieghere F, Debevere J. 2004. Evaluation of a challenge testing protocol to assess the stability of ready-to-eat cooked meat products against growth of Listeria monocytogenes. Int J Food Microbiol 90:219–236. doi: 10.1016/S0168-1605(03)00305-2. [DOI] [PubMed] [Google Scholar]
- 60.Vialette M, Pinon A, Chasseignaux E, Lange M. 2003. Growths kinetics comparison of clinical and seafood Listeria monocytogenes isolates in acid and osmotic environment. Int J Food Microbiol 82:121–131. doi: 10.1016/s0168-1605(02)00249-0. [DOI] [PubMed] [Google Scholar]
- 61.Bergholz TM, den Bakker HC, Fortes ED, Boor KJ, Wiedmann M. 2010. Salt stress phenotypes in Listeria monocytogenes vary by genetic lineage and temperature. Foodborne Pathog Dis 7:1537–1549. doi: 10.1089/fpd.2010.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro VB, Destro MT. 2014. Listeria monocytogenes serotype 1/2b and 4b isolates from human clinical cases and foods show differences in tolerance to refrigeration and salt stress. J Food Prot 77:1519–1526. doi: 10.4315/0362-028X.JFP-13-548. [DOI] [PubMed] [Google Scholar]
- 63.Aryani DC, den Besten HMW, Hazeleger WC, Zwietering MH. 2015. Quantifying strain variability in modeling growth of Listeria monocytogenes. Int J Food Microbiol 208:19–29. doi: 10.1016/j.ijfoodmicro.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 64.López S, Prieto M, Dijkstra J, Dhanoa MS, France J. 2004. Statistical evaluation of mathematical models for microbial growth. Int J Food Microbiol 96:289–300. doi: 10.1016/j.ijfoodmicro.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 65.Jørgensen F, Stephens PJ, Knøchel S. 1995. The effect of osmotic shock and subsequent adaptation on the thermotolerance and cell morphology of Listeria monocytogenes. J Appl Bacteriol 79:274–281. doi: 10.1111/j.1365-2672.1995.tb03137.x. [DOI] [Google Scholar]
- 66.Zaika LL, Fanelli JS. 2003. Growth kinetics and cell morphology of Listeria monocytogenes Scott A as affected by temperature, NaCl, and EDTA. J Food Prot 66:1208–1215. doi: 10.4315/0362-028X-66.7.1208. [DOI] [PubMed] [Google Scholar]
- 67.Hazeleger WC, Dalvoorde M, Beumer RR. 2006. Fluorescence microscopy of NaCl-stressed, elongated Salmonella and Listeria cells reveals the presence of septa in filaments. Int J Food Microbiol 112:288–290. doi: 10.1016/j.ijfoodmicro.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 68.McLauchlin J. 1990. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur J Clin Microbiol Infect Dis 9:210–213. doi: 10.1007/bf01963840. [DOI] [PubMed] [Google Scholar]
- 69.Lukinmaa S, Miettinen M, Nakari UM, Korkeala H, Siitonen A. 2003. Listeria monocytogenes isolates from invasive infections: variation of sero- and genotypes during an 11-year period in Finland. J Clin Microbiol 41:1694–1700. doi: 10.1128/jcm.41.4.1694-1700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parihar VS, Lopez-Valladares G, Danielsson-Tham M, Peiris I, Helmersson S, Unemo M, Andersson B, Arneborn M, Bannerman E, Barbuddhe S, Bille J, Hajdu L, Jacquet C, Johansson C, Löfdahl M, Möllerberg G, Ringberg H, Rocourt J, Tjernberg I, Ursing J, Henriques-Normark B, Tham W. 2008. Characterization of human invasive isolates of Listeria monocytogenes in Sweden 1986–2007. Foodborne Pathog Dis 5:755–761. doi: 10.1089/fpd.2008.0123. [DOI] [PubMed] [Google Scholar]
- 71.Jacquet C, Doumith M, Gordon JI, Martin PMV, Cossart P, Lecuit M. 2004. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J Infect Dis 189:2094–2100. doi: 10.1086/420853. [DOI] [PubMed] [Google Scholar]
- 72.Veening J, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 73.Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol 42:3819–3822. doi: 10.1128/JCM.42.8.3819-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 75.Akaike H. 1973. Information theory and an extension of the maximum likelihood principle. Proceedings of 2nd International Symposium on Information Theory, p 267–281. International Symposium on Information Theory, Budapest, Hungary. [Google Scholar]
- 76.Solieri L, Dakal TC, Bicciato S. 2014. Quantitative phenotypic analysis of multistress response in Zygosaccharomyces rouxii complex. FEMS Yeast Res 14:586–600. doi: 10.1111/1567-1364.12146. [DOI] [PubMed] [Google Scholar]
- 77.Ringus DL, Ivy RA, Wiedmann M, Boor KJ. 2012. Salt stress-induced transcription of sigma b- and CtsR-regulated genes in persistent and non-persistent Listeria monocytogenes strains from food processing plants. Foodborne Pathog Dis 9:198–206. doi: 10.1089/fpd.2011.1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.