Bacterial soft rot diseases caused by Pectobacterium spp. and Dickeya spp. are among the most important plant diseases caused by bacteria. Among other crops, they inflict large-scale damage to potatoes. As of today, farmers have few options to control them. The bacteria Bdellovibrio and like organisms (BALOs) are obligate predators of bacteria. We tested their potential to prey on Pectobacterium spp. and Dickeya spp. and to protect potato. We show that different BALOs can prey on soft rot-causing bacteria and prevent their growth in situ, precluding tissue maceration. Dead predators and the supernatant of BALO cultures did not significantly prevent maceration, showing that the effect is due to predation. Soft rot control by the predators was concentration dependent and was higher when the predator was inoculated ahead of the prey. As residual prey remained, we investigated what determines their level and found that initial prey and predator population parameters affect prey response to predation.
KEYWORDS: Predators-prey-potato, Bdellovibrio, rot bacteria, predation resistance, Pectobacterium, biocontrol, potato, predation, soft rot
ABSTRACT
Bacterial soft rot diseases caused by Pectobacterium spp. and Dickeya spp. affect a wide range of crops, including potatoes, a major food crop. As of today, farmers mostly rely on sanitary practices, water management, and plant nutrition for control. We tested the bacterial predators Bdellovibrio and like organisms (BALOs) to control potato soft rot. BALOs are small, motile predatory bacteria found in terrestrial and aquatic environments. They prey on a wide range of Gram-negative bacteria, including animal and plant pathogens. To this end, BALO strains HD100, 109J, and a ΔmerRNA derivative of HD100 were shown to efficiently prey on various rot-causing strains of Pectobacterium and Dickeya solani. BALO control of maceration caused by a highly virulent strain of Pectobacterium carotovorum subsp. brasilense was then tested in situ using a potato slice assay. All BALO strains were highly effective at reducing disease, up to complete prevention. Effectivity was concentration dependent, and BALOs applied before P. carotovorum subsp. brasilense inoculation performed significantly better than those applied after the disease-causing agent, maybe due to in situ consumption of glucose by the prey, as glucose metabolism by live prey bacteria was shown to prevent predation. Dead predators and the supernatant of BALO cultures did not significantly prevent maceration, indicating that predation was the major mechanism for the prevention of the disease. Finally, plastic resistance to predation was affected by prey and predator population parameters, suggesting that population dynamics affect prey response to predation.
IMPORTANCE Bacterial soft rot diseases caused by Pectobacterium spp. and Dickeya spp. are among the most important plant diseases caused by bacteria. Among other crops, they inflict large-scale damage to potatoes. As of today, farmers have few options to control them. The bacteria Bdellovibrio and like organisms (BALOs) are obligate predators of bacteria. We tested their potential to prey on Pectobacterium spp. and Dickeya spp. and to protect potato. We show that different BALOs can prey on soft rot-causing bacteria and prevent their growth in situ, precluding tissue maceration. Dead predators and the supernatant of BALO cultures did not significantly prevent maceration, showing that the effect is due to predation. Soft rot control by the predators was concentration dependent and was higher when the predator was inoculated ahead of the prey. As residual prey remained, we investigated what determines their level and found that initial prey and predator population parameters affect prey response to predation.
INTRODUCTION
Potato (Solanum tuberosum) is a major food crop and the fifth most economically important crop, with a global production of around 380 million tons in 2016 (1). It is estimated that up to 30% of potato yields can be lost in the field and during storage (2). Main contributors to spoilage are rot-causing pectinolytic bacteria belonging to the Enterobacteriaceae family, like Pectobacterium spp. and Dickeya spp. (3). More specifically, these pathogens induce bacterial black leg and soft rot diseases, which spoil, in addition to potatoes, a wide range of fruits, vegetables, and ornamentals, including tomato, onion, pepper, and cabbage (4), in a variety of climates. Pectobacterium carotovorum has the widest host range of all the soft rot bacteria (4–6). The diseases can be particularly destructive, causing total losses at any stage along the agricultural production line (e.g., during planting, the growing season, transport, and storage) (3). They also have increased in prevalence in European countries and in Israel (7, 8), as well as in previously unaffected areas (9). Climate change and global trade are thought to enhance the conditions favorable for their proliferation and global dispersal (10).
As of today, there is no treatment that can stop disease progression once soft rot and blackleg disease develop in a plant. Accordingly, control is largely reliant on prevention, which is spread over various layers. For example, by certifying seed potatoes based on seed grade, a protocol that cannot detect latent infection of progeny tubers from symptomless plants; by the dedication of crops either for seed to be used as planting material or for human consumption (11); and by the application of hygienic measures, such as washing and disinfecting machines used when planting, spraying, haulm flailing, harvesting, and grading in store (5, 12). Also, good agricultural practices, such as draining fields well (5) and the provision of proper plant nutrition (13), positively affect the control of rot diseases and reduce the risk of tuber decay. Several physical treatments to control contamination of potato tubers by soft rot bacteria are also practiced, including the use of hot water, steam-dried hot air, UV radiation, and solar radiation. While the hot water treatment is the most friendly from an environmental perspective, this method is unable to kill plant-pathogenic bacteria that reside deep inside the tubers without also negatively affecting plant growth (11). At the other end of the production chain, good storage procedures are an important factor in reducing disease (11).
Biological control offers an alternative to both physical and chemical controls and may enable disease prevention in contaminated seeds. It can rely on various strategies, including antagonists that directly affect pathogen populations, antibiosis, competition for nutrients, production of secondary metabolites, interference with quorum sensing, and/or the induction of plant-systematic resistance (14–16). Bdellovibrio and like organisms (BALOs) are a group of small, highly motile Gram-negative bacteria that predate exclusively upon other Gram-negative bacteria and are found to be ubiquitous in many terrestrial and aquatic environments (17). Most BALOs belong to the Oligoflexia (18) and, like Bdellovibrio bacteriovorus, the most studied of the BALOs, are periplasmic predators. This lifestyle is characterized by a typically small, highly motile cell, the so-called attack phase, which searches for prey and, upon finding it, penetrates and establishes itself in the prey cell’s periplasm. The predator then engages in filamentous growth at the expense of the prey’s content, finally dividing and producing motile progeny (5 to 7 progeny cells in 3 to 4 h with an Escherichia coli-sized prey cell) that are released from the dead prey cell (19). Noteworthy, different BALO strains differ in prey range, and prey strains of the same species can be differentially preyed upon by the same BALO (20–22).
Although the exploration of the therapeutic potential of BALOs for medicine has dramatically increased in the past years, showing that BALOs can curb infections in vivo (23–26), few studies have explored their potential against phytopathogens (27–29). The aim of this study was to assess the efficiency of B. bacteriovorus against potato rot bacteria. We show that various strains of the predators can prey upon the disease-causing agents. We further show that B. bacteriovorus can completely prevent rot development in vivo. The effect is concentration dependent and is largest when the predator is applied ahead of the pathogen. We then tested a combination of B. bacteriovorus with the Gram-positive species Paenibacillus dendritiformis, a bacterium previously shown to reduce soft rot disease in potato (30). Finally, we explored the effect of predator:prey ratios and initial concentrations on the level of residual prey.
RESULTS
Soft rot disease bacteria are sensitive to BALO predation.
Visual inspection of the turbidity of cultures of the soft rot disease-causing bacteria into which a BALO was inoculated showed that the tested predators preyed on all proposed prey, with the exception of Micavibrio aeruginosavorus ARL-13, which was unable to consume Dickeya solani (Table S1).
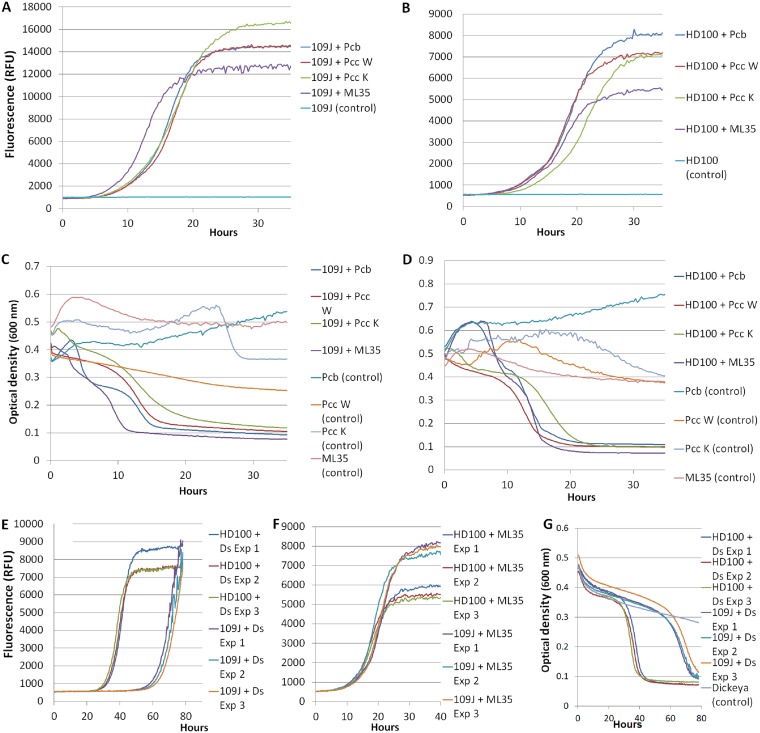
Predation dynamics were explored by using fluorescence to track the growth of B. bacteriovorus strains HD100, 109J, and a ΔmerRNA derivative of HD100, all expressing Td-tomato, and by the concomitant decrease of the prey population by following turbidity (optical density [OD]). B. bacteriovorus 109J grew more slowly on the three tested P. carotovorum strains than on E. coli ML35 (a “standard” laboratory prey) (Fig. 1A and C). B. bacteriovorus HD100-Td-tomato grew as fast on P. carotovorum subsp. brasilense and P. carotovorum subsp. carotovorum WPP14 as on E. coli ML35 but exhibited a slight delay when preying on P. carotovorum subsp. carotovorum KC24 (Fig. 1B and D). B. bacteriovorus HD100-Td-tomato preyed much faster than B. bacteriovorus 109J-Td-tomato on D. solani, reaching maximal population size within 40 h, while at 80 h postinoculation, maximal growth had not yet been reached by B. bacteriovorus 109J-Td-tomato (Fig. 1E and G). E. coli ML35 was preyed upon more rapidly by both predators than was D. solani (Fig. 1E and F). B. bacteriovorus ΔmerRNA-Td-tomato was found to prey as effectively as B. bacteriovorus HD100-Td-tomato on P. carotovorum subsp. brasilense (Fig. S1). The final yields of the predators, as measured by relative fluorescence units (RFU), were higher than 108 cells · ml−1, with some variation (up to ±0.3 × 108 cells · ml−1) in cell number (for technical details, see reference 31). M. aeruginosavorus ARL-13 predation on P. carotovorum subsp. brasilense and P. carotovorum subsp. carotovorum was slow, taking about 40 h, and it was unable to prey on D. solani (Table S2).
FIG 1.
Growth of B. bacteriovorus 109J-Td-tomato and HD100-Td-tomato and decay of Pectobacterium carotovorum subsp. brasiliense (Pcb) and P. carotovorum subsp. carotovorum WPP14 and KC24 (Pcc W and Pcc K, respectively) (A to D), E. coli ML35 (A to D and F), and Dickeya solani (Ds) (E, G) prey populations. The predatory populations were tracked by relative fluorescence units (RFU) (A, B, E, F), and the prey populations were tracked by optical density (OD) (C, D, G).
P. carotovorum subsp. brasilense is more virulent than D. solani in potato slice assays.
P. carotovorum subsp. brasilense, a highly virulent pathogen (32), and D. solani, a disease-causing agent with increasing spread (33), were compared for virulence in a potato slice assay. P. carotovorum subsp. brasilense consistently induced more lesions than D. solani at each of the tested concentrations (Table S3) and was selected for further work.
B. bacteriovorus protects potato slices from soft rot damage.
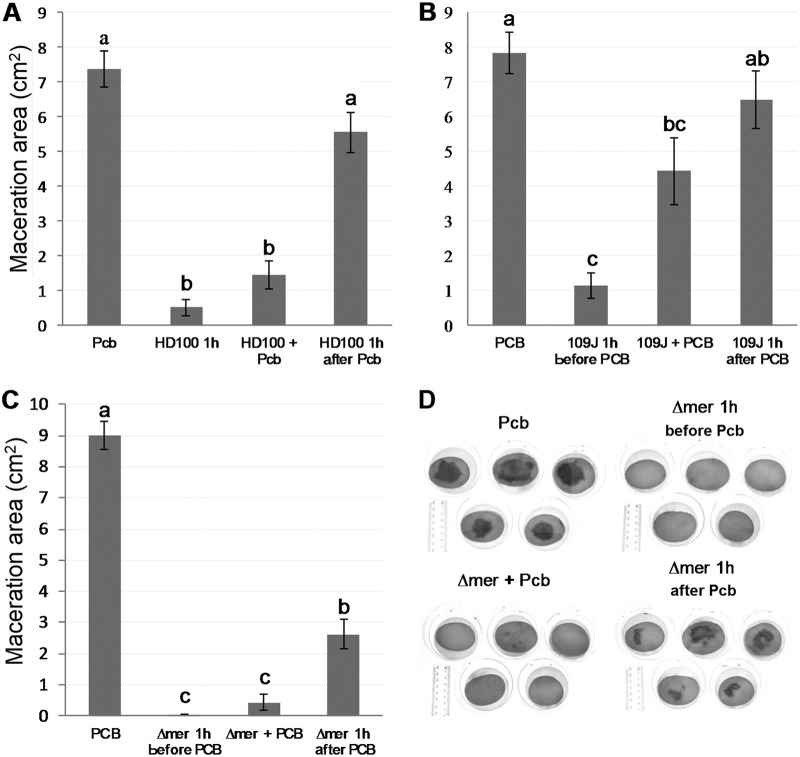
Potato slice assays were used to test the ability of B. bacteriovorus strains HD100, 109J, and the ΔmerRNA mutant to prevent maceration in situ. In all of the experiments, the addition of 109 PFU · ml−1 of any of the B. bacteriovorus strains at 60 min before inoculation with P. carotovorum subsp. brasilense or dual inoculation at the same time significantly reduced (P < 0.02) the maceration area. Yet, differences between strains were observed. HD100 and ΔmerRNA strains were similarly efficient at both inoculation times, but the efficiency of strain 109J was reduced when it was applied together with P. carotovorum subsp. brasilense (Fig. 2). When the predators were applied 60 min after P. carotovorum subsp. brasilense, the ΔmerRNA strain was the only strain able to significantly reduce (P < 0.01) the macerated area (Fig. 2).
FIG 2.
The effect of B. bacteriovorus strains HD100 (A), 109J (B), and ΔmerRNA (Δmer) (C), on tissue maceration in potato slices induced by the soft rot bacterium P. carotovorum subsp. brasilense (Pcb). B. bacteriovorus (10 μl, 109 PFU · ml−1) inoculated 60 min before, together with, or 60 min after P. carotovorum subsp. brasilense (10 μl, 106 CFU · ml−1). Five photographs of replicates out of 10 per treatment are shown (D). Macerated tissue appears as dark patches. The results shown are the combination of three independent experiments (four for HD100), with 10 replicates per treatment in each experiment. Error bars represent the standard error of 30 replicates (40 replicates for HD100). Treatments not connected by the same letter are significantly different (P < 0.01 for HD100 and ΔmerRNA; P < 0.02 for 109J) according to the Steel-Dwass all-pairs test. The independent experiments are shown in Fig. S2.
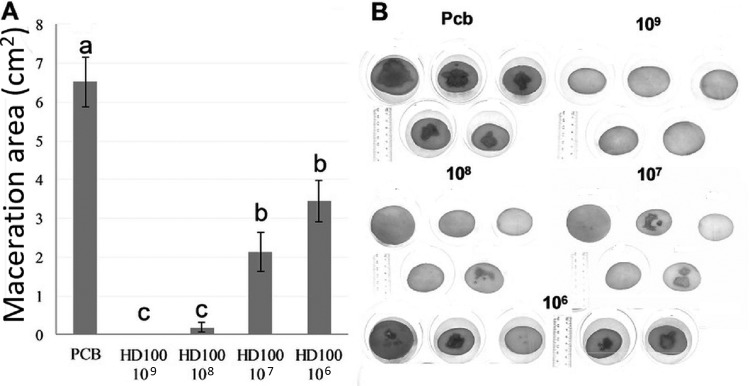
Next, different concentrations (106, 107, 108, and 109 PFU · ml−1) of B. bacteriovorus HD100 were added 60 min before P. carotovorum subsp. brasilense. The maceration area was significantly (P < 0.01) reduced at all concentrations compared to that in the control (P. carotovorum subsp. brasilense only-inoculated treatment). The protective effect induced by the predator was concentration dependent, as the maceration area increased with decreasing predator concentration (Fig. 3). At 109 and 108 PFU · ml−1, maceration was completely prevented, being significantly different (P < 0.01) than that of the unprotected control and from those treated with 107 and 106 PFU · ml−1 of the predator. However, even at these lower B. bacteriovorus concentrations, a significant protective effect was observed (Fig. 3). The results presented here are averages of three independent experiments (see Materials and Methods). Data from each individual experiment are shown in the supplemental material (Fig. S2 and S3).
FIG 3.
The effect of B. bacteriovorus strain HD100 inoculated at concentrations of 106, 107, 108, and 109 PFU · ml−1 (10 μl) on tissue maceration in potato slices induced by the soft rot bacterium P. carotovorum subsp. brasiliense (Pcb) (10 μl, 106 CFU · ml−1) inoculated 60 min after the predator. Five photographs of replicates out of 10 per treatment are shown (B). Macerated tissue appears as dark patches. The results shown are the combination of three independent experiments, with 10 replicates per treatment each. Error bars represent the standard error of 30 replicates. Treatments not connected by the same letter are significantly different (P < 0.01) according to the Steel-Dwass all-pairs test. Results of independent experiments are shown in Fig. S3.
Differential effects of live and dead predators and of culture supernatant.
Next, we ascertained the role of predation in protecting the potato tissues against rot. Explicitly, we aimed at verifying whether the observed effect was not the result of indirect interactions such as elicitation of plant defenses. In that sense, the supernatant of a B. bacteriovorus HD100 lytic culture, heat-deactivated B. bacteriovorus HD100 cells, and live predator cells was inoculated onto potato slides 60 min before P. carotovorum subsp. brasilense, i.e., according to the conditions that yielded the most effective protection. As in the previous experiments, live B. bacteriovorus HD100 significantly reduced (P < 0.01) the maceration area compared to that in the P. carotovorum subsp. brasilense-only control. In contrast, the effects of the lytic culture supernatant and of the heat-killed HD100 cells were not significantly different from that of the control with the pathogen only (Fig. 4), although the average maceration areas were consistently lower in these treatments relative to those in P. carotovorum subsp. brasilense-only controls in the three experiments (Fig. S4).
FIG 4.
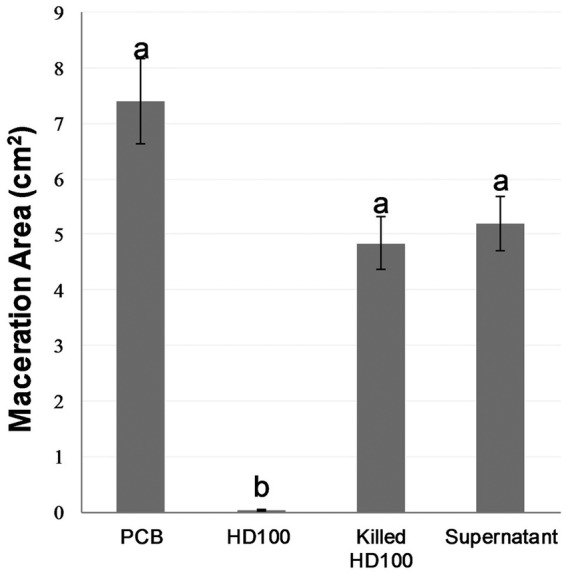
Effect of inoculating B. bacteriovorus HD100 (10 μl, 109 PFU · ml−1), heat-deactivated (killed) HD100, and the supernatant of HD100 60 min before P. carotovorum subsp. brasiliense (Pcb) (10 μl, 106 CFU · ml−1) on soft rot disease development in potato slices. The results shown are the combination of three experiments, with 10 replicates per treatment. Error bars represent standard error of 30 replicates. Treatments not connected by the same letter are significantly different (P < 0.01) according to the Steel-Dwass all-pairs test. Data from each independent experiment are shown in Fig. S4.
Adding P. dendritiformis to B. bacteriovorus does not improve protection.
Paenibacillus dendritiformis was previously shown to protect potato tubers against soft rot (30). Since P. dendritiformis is Gram-positive and is therefore not a BALO prey species, we posited that combining both bacteria may augment protection. It was thus important to ensure that neither bacterium has detrimental effects on the other. The potential of P. dendritiformis to harm BALO prey strains through secretion of metabolites was assessed by streaking LB plates previously inoculated with P. dendritiformis with E. coli ML35, P. carotovorum subsp. brasilense, P. carotovorum subsp. carotovorum WPP14, and P. carotovorum subsp. carotovorum KC24. Overall, no detrimental effects were observed (Fig. S5A). Interactions were further studied by mixing P. dendritiformis and B. bacteriovorus HD100-Td-tomato at ratios of 1:1, 10:1, and 20:1, with E. coli ML35 as prey. Predation dynamics were slightly slower at ratios of 10:1 and 20:1 (Fig. S5B).
Predator populations rapidly decrease in the absence of prey.
In field settings, the timing of predator and prey interacting on a tuber or in the surrounding soil is impossible to control. We therefore measured the survival of B. bacteriovorus in situ. The inoculum substantially decreased after 24 h by ∼2 orders of magnitude to 107 PFU · ml−1, followed by another drop of 2.5 orders of magnitude in the following 24 h, while a control culture kept in amended HEPES (aHEPES) showed no such decrease (Fig. S6).
Predation persisters are affected by predator:prey ratio.
In BALO-prey interactions, the prey population is not eradicated. The remaining population exhibits reversible, plastic resistance (34) and is thus a potential seed for regrowth of the pathogen onto the potato host. In order to understand if plastic resistance originates in a fixed ratio of cells that are intrinsically less sensitive to predation or results from elicitation by predatory activity, we examined the relationships between resistance, predator and prey population sizes, and their ratios.
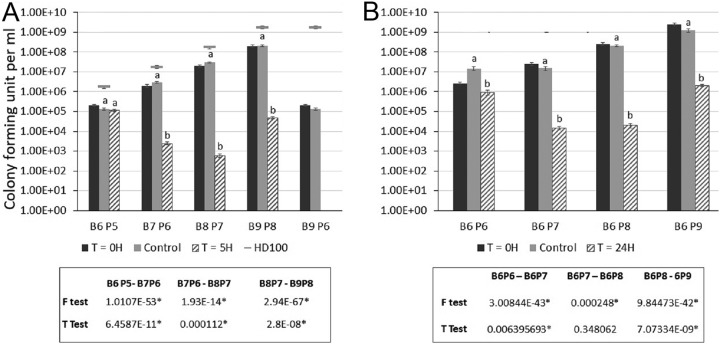
At low concentrations of predator and prey, when the concentration of the former was higher than that of (106 PFU · ml−1 predators:105 CFU · ml−1 prey [B6:P5]) or equal (B6:P6) to that of the latter, the remaining prey population size was similar to that of the inoculum or was slightly reduced. The remaining prey level however, dropped by 3 and 4 orders of magnitude at prey inocula 10 and 100 times higher, respectively, unrelated to predator concentration (B7:P6 and B6:P7, and B8:P7 and at B6:P8, respectively). A further increase in initial prey concentration reduced the ratio between initial and residual population sizes, unrelated to initial predator inoculum levels (B9:P8 and B6:P9). Finally, at the high predator:prey ratio of B9:P6, residual prey numbers were below the detection level (Fig. 5, Fig. S7 and S8).
FIG 5.
Surviving P. carotovorum subsp. brasiliense (CFU · ml−1) after mixing with B. bacteriovorus HD100 in amended HEPES (aHEPES) at initial concentrations of (A) 106, 107, 108, and 109 PFU · ml−1 predators and 105, 106, 107, and 108 CFU · ml−1 prey, 5 h (T = 5 h) after mixing predator and prey. Predator:prey ratio was kept at 10:1. Horizontal bars are the concentration of the predator at the start of the experiment. (B) Predators (106 PFU · ml−1) and prey (106, 107, 108, and 109 CFU · ml−1), 24 h (T = 24 h) after mixing. B, predator; P, prey; the number denotes the exponent of the initial concentration. The results shown are the combination of three (A) and two (B) independent experiments. Error bars indicate standard error. Within each treatment (BiPj), bars not connected by the same letter are significantly different (P < 0.01). The independent experiments are shown in Fig. S7 and S8.
Glucose may inhibit predation in potato tissues.
As the inoculation of B. bacteriovorus after that with P. carotovorum subsp. brasilense proved to be less efficient than the other treatments, and since Dashiff et al. (35) demonstrated that sugars can inhibit predation, we tested the effect of glucose concentrations found in potatoes on predation. Glucose concentrations from the center of the potato, the edge, and blended potatoes were measured and were found to be 3, 22, and 7 mM, respectively. pH measured on control and macerated slices was consistently found to be 6 and 8, respectively (Fig. S9). Predation by B. bacteriovorus HD100 on live P. carotovorum subsp. brasilense and E. coli ML35 at these glucose concentrations was inhibited in a concentration-dependent manner. On heat-deactivated E. coli ML35 cells, however, B. bacteriovorus HD100 grew as well as the control, independent of glucose concentration (Fig. S9).
DISCUSSION
Being obligate predators of bacteria, BALOs have been dubbed “living antibiotics” (36). This potential is being explored, mostly for applications in the medical field (23–26). Here, we show that BALOs are able to prevent bacterial infections in plant tissues. The need for biocontrol approaches is particularly sore for bacterial diseases of plants, as very few treatments exist, and most of the practices to keep crops protected from such diseases, especially soft rots, are based on sanitation, nutrition, and growth management.
The different BALOs used on the potato slices were, to different extents, able to prey upon most of the pectinolytic bacterial strains tested. Predation was shown to stand behind the protective effects against soft rot, as dead predators and a filtered predatory culture supernatant (which also contained prey debris) were unable to effectively protect the potato tissues against maceration. However, these latter treatments consistently reduced the average maceration area in the replicated experiments, suggesting that a mild protective effect may still be induced. Bacterial determinants such as microbe-associated molecular patterns residues, including flagellum, EF-Tu, or exopolysaccharide, may elicit plant immune response (37). Interestingly, the flagellum of Bdellovibrio predators is sheathed (38). Moreover, their lipid A has a unique composition that includes mannose instead of phosphate residues (39). These peculiar structural and chemical features may explain why, in animals, exposition to BALOs generates only minimal immune or inflammatory responses (24, 40, 41). We propose that the low response observed in plants may similarly be due to low levels of defense elicitation.
Differences were observed between predators, with M. aeruginosavorus showing a more restricted prey range than B. bacteriovorus, and slower predation. It should be noted, however, that BALO-prey interactions can be extremely specific, since BALO strains, even predators belonging to the same species, have differential affinities for strains of prey belonging to the same species, ranging from very “palatable” to highly resistant to predation (21, 22). This was confirmed in this study, as the different BALOs, including closely related strains (B. bacteriovorus HD100 and 109J are >99% identical at the genome sequence level [42]), showed differential efficiencies when preying upon closely related strains of pathogens (e.g., P. carotovorum subsp. brasilense, P. carotovorum subsp. carotovorum WPP14 and KC24).
It should be noted that pathogen virulence did not affect BALO efficiency, as the B. bacteriovorus strains were more effective against the most virulent pathogen (P. carotovorum subsp. brasilense) in the potato slice assay than against the less virulent D. solani. Thus, any particular BALO may not be a generic biocontrol agent that is similarly effective against different soft rot agents. While this may be viewed as a limitation in their use and a cause of concern for their application against D. solani, a pathogen with an increasing spread (33), it should be pointed out that novel predatory strains can be rapidly isolated against emerging pathogens—as long as these are Gram-negative—providing a flexible and “customized” answer to specific diseases. This approach has been put into practice in the development of BALO-based control of diseases in aquaculture (43, 44).
All three B. bacteriovorus strains (HD100, 109J, and the ΔmerRNA derivative of HD100) were very effective in preventing maceration of potato slices when applied before the pathogen or along with it, with 109J showing a somewhat reduced performance in the latter setting. The effect was concentration dependent, completely preventing disease spread at high predator concentrations (109 and 108 PFU · ml−1) and at high predator:prey ratios (1,000:1 and 100:1, respectively) but also showing significant efficiency at lower values (107 CFU · ml−1 and 10:1; 106 CFU · ml−1 and 1:1). These results are particularly remarkable because they further support predation as the main mechanism standing behind the observed phenomenon. Indeed, at high predator concentrations and a high predator:prey ratio (tens of predators per prey), prey cells may be targeted by multiple predators at once, causing rapid premature lysis but preventing predator multiplication. However, at low concentrations and ratios (1:1 and 10:1), prey lysis is not a concern (45).
An important feature in BALO-prey interactions is that prey populations are not eradicated. Shemesh and Jurkevitch (34) discovered that the remaining prey cells in a lytic culture were resistant to predation, but that resistance was plastic and not heritable. It was shown that it was not due to secreted compounds or residues found in the lytic culture. Plastic resistance occurs in planktonic populations, as well as in biofilm-forming prey populations (46, 47), and varies between predator and prey combinations (21, 22). Consequently, resistant cells of the pathogen may remain, constituting a potential for regrowth and infection. We further examined how resistance is generated, showing that a low concentration of predator and prey (B6:P5 or B6:P6) was not conducive to predation and that low prey concentrations slow predation (31, 48). This may explain why, at B6:P5 and B6:P6, the original and final prey populations after incubation with the predator for 5 and 24 h were similar. Treatments in which the prey population was increased led to very large increases in the ratio of initial to remaining prey population sizes, which ranged from about 103 (B7:P6 and B6:P7) to more than 104 (B8:P7 and B6:P8). Since at these ratios and population sizes, premature prey lysis is minimal (45), these findings suggest that the ratio of resistance in the prey population is not a fixed proportion but a variable dependent upon the numbers of predators and prey and, thus, on population dynamics. The absolute number of remaining prey cells also fluctuated, but strikingly more under conditions where predators outnumbered prey and over a short incubation time than under conditions where prey outnumbered predators (B6:P7 and B6:P8), even though incubation was longer in the latter case, further supporting that plastic resistance results from the interaction of the predator with the prey. At the higher ratios of B6:P9, predation may not be completed within the time of the experiment, and as mentioned above, at a ratio of B9:P6, prey cells may experience rapid lysis (45).
The application of B. bacteriovorus HD100 and 109J 1 h after P. carotovorum subsp. brasilense resulted in a nonsignificant decrease of the maceration area by only circa 20%. When the predator was applied alone for the same amount of time on a potato slice, it survived and generated plaques, demonstrating that loss of viability was not the cause of the loss in efficiency. Moreover, B. bacteriovorus ΔmerRNA remained partly efficient under the same conditions. However, longer exposure of the predator to starvation conditions may have detrimental effects for field applications, an issue that should be further evaluated. Taken together, these results suggest that the pathogen alters the microenvironment, leading to the inhibition of predation. The growth of soft rot bacteria on potato is accompanied by a shift from pH 6 to pH 8 in the tissues that results from the activity of quorum-sensing (QS)-dependent, maceration-causing lytic enzymes (5). The small inoculum used in our experiment (10 μl of 106 CFU · ml−1, i.e., 104 cells) spread on the surface of the potato and may therefore not be sufficiently dense to induce QS and thus to initiate pH increase in the microenvironment within that short time frame. Glucose is present in the tissues that may be readily used by P. carotovorum subsp. brasilense, providing the energy and carbon required for population expansion. Glucose metabolism by soft rot-causing bacteria produces acids (49), reducing pH, which is a condition detrimental to predatory activity (35). Accordingly, we posit that during the first phase of pathogen establishment, the microenvironment turns acidic, preventing predation. Supporting this, and as found by Dashiff et al. (35), we observed that predation on dead prey cultures in the presence of glucose was not inhibited.
Finally, the addition of the Gram-positive bacterium P. dendritiformis neither induced protection nor improved the performance of B. bacteriovorus but rather caused slight, concentration-dependent delays in predation, possibly through a decoy effect (50). This shows that the combination of a Gram-positive biocontrol agent with a BALO predator incurs a price. Depending upon the efficiency of the added strain against the deleterious agent, this strategy may or may not be beneficial. Further strategies to improve the efficiency of the BALOs may be explored, including cocktails of predatory strains and their packaging, e.g., by encapsulation, as recently described for the protection of the whiteleg shrimp (Penaeus vannamei) from the pathogen Vibrio vulnificus (51). In conclusion, we showed that BALOs have the potential to prevent rot from spreading in healthy tissue and that protective action is more effective than corrective action.
MATERIALS AND METHODS
Bacterial strains, growth media, and growth conditions.
The bacterial strains used in this study are shown in Table 1. The prey bacteria P. carotovorum spp., Dickeya solani, and Pseudomonas corrugata, as well as the Gram-positive bacterium Paenibacillus dendritiformis, were grown in LB medium at 28°C in shaking flasks at 180 rpm. Escherichia coli was grown at 37°C. All were started from single colonies originating from laboratory stocks kept at −80°C. Overnight cultures were centrifuged at 4,200 × g for 10 min at 4°C, resuspended in HEPES buffer amended with 2 mM CaCl2 and 3 mM MgCl2·7H2O (pH 7.8) (aHEPES). Optical density at 600 nm (OD600) was adjusted to 10 by concentration to provide a stock solution, which was stored at 4°C for up to 2 weeks until use. The bacterial predators Bdellovibrio bacteriovorus HD100, HD100-Td-tomato, 109J, the ΔmerRNA mutant, and Micavibrio aeruginosavorus ARL-13 were from lab stock kept at −80°C. merRNA is a putative standalone cyclic-di-GMP riboswitch that is highly expressed during the attack phase (52); the ΔmerRNA mutant exhibits increased motility (53). A bacteriological loopful from the frozen stock of predators was transferred to a 250-ml Erlenmeyer flask containing 1 ml of a prey suspension (OD600 = 10) diluted in 25 ml of aHEPES. E. coli ML35 and P. corrugata were used as prey for growth of B. bacteriovorus and M. aeruginosavorus, respectively. The flask was shaken at 250 rpm and 28°C. Growth of the predator was monitored by a decrease in turbidity of the suspension (due to lysis of the prey) and visually verified by microscopy. After most of the prey cells were lysed, the suspension was filtered through a 0.45-μm filter (Sartorius) to separate predatory cells from any leftover prey. The filtrate was stored at 4°C for up to 1 day until use. Prey and predator cultures were routinely counted by dilution plating as CFU per ml (CFU · ml−1) and PFU per ml (PFU · ml−1), respectively. The latter plating was performed in double-layered agar (54). P. dendritiformis cultures were counted using the most probable number (MPN) method. Bacterial suspensions were 10× serially diluted in aHEPES, and 10 μl aliquots were dropped in a row (5 drops per row) onto an LB agar plate and incubated at 28°C for up to 2 days.
TABLE 1.
Bacterial strains and plasmids used in this work
| Species, strain, or plasmid | Use | Reference or source |
|---|---|---|
| Escherichia coli | ||
| ML35 | Prey | 55 |
| SM10 | Prey, conjugal transfer | 56 |
| Pseudomonas corrugata | Prey | Gyora Kryztman, ARO |
| Pectobacterium carotovorum | ||
| Pectobacterium carotovorum subsp. brasiliense | Prey | 57 |
| Pectobacterium carotovorum subsp. carotovorum | ||
| KC24 | Prey | 6 |
| WPP14 | Prey | 6 |
| Dickeya solani | Prey | 8 |
| Paenibacillus dendritiformis T | Biocontrol agent | 30 |
| Bdellovibrio bacteriovorus | ||
| HD100 | Predator | 58 |
| HD100-Td-tomato | Predator | 59 |
| 109J | Predator | 20 |
| 109J-Td-tomato | Predator | 59 |
| ΔmerRNA-Td-tomato | Predator | This study |
| Micavibrio aeruginosavorus | ||
| ARL-13 | Predator | 60 |
| Plasmid | ||
| pMQ414 | Constitutive for Td-tomato, Gma resistant | 59 |
Gm, gentamicin.
Heat-killing treatments and culture supernatants.
When needed, prey and predator cultures were killed by heating to 60°C for 30 and 50 min, respectively, in a dry bath block (Major Science Elite, USA). Supernatant from B. bacteriovorus cultures grown as described above were obtained by 5× concentrating the culture and filtering three times through a 0.2-μm filter (Sartorius, Germany). Aliquots were plated to confirm the absence of propagules.
Predation dynamics in the presence of P. dendritiformis.
B. bacteriovorus HD100-Td-tomato, E. coli ML35, and P. dendritiformis were grown as above. The P. dendritiformis culture was either concentrated or serially diluted to obtain various E. coli:P. dendritiformis ratios upon mixing. Twenty μl of the predator, 100 μl of the prey, 100 μl of P. dendritiformis, and 780 μl amended HEPES (25 mM HEPES, 2 mM CaCl2·2H2O, 3 mM MgCl2·6H2O) buffer were mixed. Growth of the predator was tracked using fluorescence in a plate reader (31). Three experiments with three replicates were conducted.
Construction of predators and prey strains expressing Td-tomato.
A B. bacteriovorus 109J overnight culture (100 μl) was mixed with 200 μl of a 10-fold concentrated E. coli SM10 donor culture carrying pMQ414::Td-tomato grown in LB with 10 μg · ml−1 gentamicin (Gm) to an OD600 of 0.4, and then spread over a sterile membrane (0.2 μm) placed upon an NB medium diluted 10× (DNB) agar plate. Following overnight incubation at 28°C, the mixture was diluted in aHEPES and plated in double-layer agar plates containing 10 μg · ml−1 Gm using E. coli SM10 as the prey. Lytic plaques indicating growth of the predator were screened for the presence of the Td-tomato gene by PCR and for Td-tomato expression by fluorescence microscopy (Eclipse Ti; Nikon).
Sensitivity of the rot pathogens to predation.
Five hundred microliters of prey culture (OD600 = 10) and 100 μl of a BALO lytic culture were diluted 50 times while mixed in aHEPES buffer in 50-ml Falcon tubes, resulting in final concentrations of approximately 108 CFU · ml−1 and 106 PFU · ml−1, respectively. The cocultures were incubated at 28°C for up to 72 h with constant shaking at 250 rpm. Predation was visually assessed by clarification of the suspension, and confirmed by phase-contrast microscopy, for the presence of a high density of predators (small, rapidly swimming cells) and a low density of prey (larger cells). Furthermore, predation by B. bacteriovorus HD100, 109J, and the ΔmerRNA mutant expressing Td-tomato was tracked by fluorescence (predator growth) and OD (prey decay) in a plate reader (Tecan Spark 10M; Switzerland) (31). Three experiments with three replicates were conducted for each combination of predator and prey in both settings.
Potato slice assay.
The red-skinned Desiree potato cultivar was sourced from local supermarkets and used in all experiments. Potatoes were surface sterilized by dipping for 1 min in a 1% sodium hypochlorite solution, washed twice with sterile distilled water, and left to dry in a laminar flow hood. After potato surfaces were dried, potatoes were washed with 70% ethanol and again dried on sterile petri dishes. Slices of about 0.5-cm width were cut using a sterilized knife, and were transferred using sterile tweezers to petri dishes containing sterile 70-mm-diameter filter paper (Whatman 1) dampened with 330 μl of sterile distilled water. BALO cultures were prepared as described in “Bacterial strains, growth media, and growth conditions,” 5× concentrated to reach ∼109 PFU · ml−1, and diluted as required for inoculation at lower concentrations. P. carotovorum subsp. brasilense was grown as above, subcultured (∼100 μl) in fresh LB to an OD600 of ∼0.2, and washed twice in aHEPES buffer; the OD600 was adjusted to 0.2, then diluted to yield ∼106 CFU · ml−1. P. dendritiformis was grown overnight on an LB plate, then resuspended in aHEPES buffer to an OD600 adjusted to 0.2. Potato slices were inoculated with 10-μl drops of the tested cultures applied to the center of the slice. BALOs were applied either 60 min ahead of, together with, or 60 min after P. carotovorum subsp. brasilense. In the appropriate experiments, P. dendritiformis was applied along with the predator. The potato slices were incubated at 28°C for up to 48 h. All experiments were repeated three times, with 10 replicates per treatment (unless otherwise specified). After incubation, each slice was photographed with a Canon PowerShot S120 camera, using a ruler for scale. The maceration area was calculated using the ImageJ image processing program.
Bdellovibrio bacteriovorus survival on potato slices.
Potato slices 0.5 cm thick and 1.7 cm wide were prepared using a sterile knife and a sterile cork borer. Bacterial suspensions (109 PFU · ml−1) were inoculated into 0.5-cm thick slices for 0, 1, 24, and 48 h. The surviving predators were retrieved by vigorously vortexing the potato plugs for ∼7 min in 10 ml of HEPES buffer. Predators were quantified by plating, as described above.
Predation persisters.
The remaining P. carotovorum subsp. brasilense population levels after predation by B. bacteriovorus HD100 was measured by dilution plating. Predator and prey were mixed at ratios ranging from 1:1,000 to 1,000:1 and at concentrations of 106 to 109 PFU · ml−1 predators and 105 to 108 CFU · ml−1 prey, denoted B5 to B9 and P6 to P9, respectively. Prey:predator ratios (1:10) of various inoculum concentrations were incubated for 5 h; predator:prey ratios of 1:1, 1:10, 1:100, 1:1,000 at a predator inoculum concentration of 106 PFU · ml−1 were incubated for 24 h.
Effect of glucose on predator growth.
The concentration of glucose was measured at several sites in the potato slices using the Infinity glucose oxidase liquid stable reagent kit. aHEPES solutions that included glucose at the measured concentrations were prepared and used to measure the effect of glucose on predation, measured as above.
pH measurements in potato slices.
The pH was measured from the center, the edge, and in blended potatoes, using MColorpHast nonbleeding pH indicator strips (Germany) with a pH range of 5 to 10 with 0.5 increments. The strips were compared to measurements obtained with a Mettler Toledo SevenCompact S220 benchtop pH meter (USA).
Statistical analysis.
For the potato slice assay experiments, three or more repeated experiments with 10 replicates each were combined and analyzed in JMP 14 statistical software. Not all groups within the aggregates followed a normal distribution, and at least one group did not have equal variance; therefore, nonparametric comparisons were used, specifically the Steel-Dwass all-pairs test. Individual experiments were analyzed using the F test and Student’s t test (see Supplemental Material). Resistance levels were analyzed using the F test or Student’s t test.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by The Israel Ministry of Agriculture and Rural Development as part of the “Root of the Matter—the Root Zone Knowledge Center for Leveraging Modern Agriculture” (Core Knowledge Center grant 391/15).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.FAO Statistics. 2018. World food and agriculture statistical pocketbook 2018. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 2.Gustafsson J, Cederberg C, Sonesson U, Emanuelsson A. 2013. The methodology of the FAO study: “Global food losses and food waste—extent, causes and prevention” —FAO, 2011. SIK Institutet för livsmedel och bioteknik, Gothenburg, Sweden. [Google Scholar]
- 3.Charkowski AO. 2018. The changing face of bacterial soft-rot diseases. Annu Rev Phytopathol 56:269–288. doi: 10.1146/annurev-phyto-080417-045906. [DOI] [PubMed] [Google Scholar]
- 4.Ma B, Hibbing ME, Kim H-S, Reedy RM, Yedidia I, Breuer J, Breuer J, Glasner JD, Perna NT, Kelman A, Charkowski AO. 2007. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology 97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- 5.Pérombelon M. 2002. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol 51:1–12. [Google Scholar]
- 6.Toth IK, Bell KS, Holeva MC, Birch PRJ. 2003. Soft rot erwiniae: from genes to genomes. Mol Plant Pathol 4:17–30. doi: 10.1046/j.1364-3703.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 7.Lumb VM, Pérombelon MCM, Zutra D. 1986. Studies of a wilt disease of the potato plant in Israel caused by Erwinia chrysanthemi. Plant Pathol 35:196–202. doi: 10.1111/j.1365-3059.1986.tb02004.x. [DOI] [Google Scholar]
- 8.Tsror L, Erlich O, Lebiush S, Hazanovsky M, Zig U, Slawiak M, Grabe G, van der Wolf JM, van de Haar JJ. 2009. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. Eur J Plant Pathol 123:311–320. doi: 10.1007/s10658-008-9368-0. [DOI] [Google Scholar]
- 9.Palacio-Bielsa A, Cambra MA, Lopez MM. 2006. Characterisation of potato isolates of Dickeya chrysanthemi in Spain by a microtitre system for biovar determination. Ann Appl Biol 148:157–164. doi: 10.1111/j.1744-7348.2006.00045.x. [DOI] [Google Scholar]
- 10.Coakley SM, Scherm H, Chakraborty S. 1999. Climate change and plant disease management. Annu Rev Phytopathol 37:399–426. doi: 10.1146/annurev.phyto.37.1.399. [DOI] [PubMed] [Google Scholar]
- 11.Czajkowski R, Pérombelon MCM, Van Veen JA, Van der Wolf JM. 2011. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathology 60:999–1013. doi: 10.1111/j.1365-3059.2011.02470.x. [DOI] [Google Scholar]
- 12.Perombelon MCM, Kelman A. 1980. Ecology of the soft rot erwinias. Annu Rev Phytopathol 18:361–387. doi: 10.1146/annurev.py.18.090180.002045. [DOI] [Google Scholar]
- 13.Platero M, Tejerina G. 1976. Calcium nutrition in Phaseolus vulgaris in relation to its resistance to Erwinia carotovora. J Phytopathol 85:314–319. doi: 10.1111/j.1439-0434.1976.tb01675.x. [DOI] [Google Scholar]
- 14.Compant S, Duffy B, Nowak J, Clément C, Barka EA. 2005. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin D, Moënne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O’Gara F. 2006. Ecological interaction of a biocontrol Pseudomonas fluorescens strain producing 2,4-diacetylphloroglucinol with the soft rot potato pathogen Erwinia carotovora subsp. atroseptica. FEMS Microbiol Ecol 23:95–106. doi: 10.1111/j.1574-6941.1997.tb00394.x. [DOI] [Google Scholar]
- 16.Jafra S, Przysowa J, Czajkowski R, Michta A, Garbeva P, Van der Wolf JM. 2006. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can J Microbiol 52:1006–1015. doi: 10.1139/w06-062. [DOI] [PubMed] [Google Scholar]
- 17.Rotem O, Pasternak Z, Jurkevitch E. 2014. Bdellovibrio and like organisms, p 3–17. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes: deltaproteobacteria and epsilonproteobacteria. Springer Berlin Heidelberg, Berlin, Germany. [Google Scholar]
- 18.Hahn MW, Schmidt J, Koll U, Rohde M, Verbarg S, Pitt A, Nakai R, Naganuma T, Lang E. 2017. Silvanigrella aquatica gen. nov., sp. nov., isolated from a freshwater lake, description of Silvanigrellaceae fam. nov. and Silvanigrellales ord. nov., reclassification of the order Bdellovibrionales in the class Oligoflexia, reclassification of the families Bacteriovoracaceae and Halobacteriovoraceae in the new order Bacteriovoracales ord. nov., and reclassification of the family Pseudobacteriovoracaceae in the order Oligoflexales. Int J Syst Evol Microbiol 67:2555–2568. doi: 10.1099/ijsem.0.001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton AK, Kanna M, Woods RD, Aizawa SI, Sockett RE. 2010. Shadowing the actions of a predator: backlit fluorescent microscopy reveals synchronous nonbinary septation of predatory Bdellovibrio inside prey and exit through discrete bdelloplast pores. J Bacteriol 192:6329–6335. doi: 10.1128/JB.00914-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurkevitch E, Minz D, Ramati B, G B. 2000. Prey range characterization, ribotyping, and diversity of soil and rhizosphere Bdellovibrio spp. isolated on phytopathogenic bacteria. Appl Environ Microbiol 66:2365–2371. doi: 10.1128/aem.66.6.2365-2371.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dashiff A, Junka R, Libera M, Kadouri D. 2011. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol 110 doi: 10.1111/j.1365-2672.2010.04900.x. [DOI] [PubMed] [Google Scholar]
- 22.Dashiff A, Kadouri DE. 2011. Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol Oral Microbiol 26:19–34. doi: 10.1111/j.2041-1014.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 23.Romanowski EG, Stella NA, Brothers KM, Yates KA, Funderburgh ML, Funderburgh JL, Gupta S, Dharani S, Kadouri DE, Shanks R. 2016. Predatory bacteria are nontoxic to the rabbit ocular surface. Sci Rep 6:30987. doi: 10.1038/srep30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shatzkes K, Singleton E, Tang C, Zuena M, Shukla S, Gupta S, Dharani S, Onyile O, Rinaggio J, Connell ND, Kadouri DE. 2016. Predatory bacteria attenuate Klebsiella pneumoniae burden in rat lungs. mBio 7:e01847-16. doi: 10.1128/mBio.01847-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis AR, Moore C, Mazon-Moya M, Krokowski S, Lambert C, Till R, Mostowy S, Sockett RE. 2016. Injections of predatory bacteria work alongside host immune cells to treat Shigella infection in zebrafish larvae. Curr Biol 26:3343–3351. doi: 10.1016/j.cub.2016.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo R, Chae R, Mukherjee S, Singleton E, Occi J, Kadouri D, Connell N. 2015. Susceptibility of select agents to predation by predatory bacteria. Microorganisms 3:903–912####912. doi: 10.3390/microorganisms3040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherff RH. 1973. Control of bacterial blight of soybean by Bdellovibrio bacteriovorus. Phytopathology 63:400–402. doi: 10.1094/Phyto-63-400. [DOI] [Google Scholar]
- 28.Uematsu T. 1980. Ecology of Bdellovibrio parasitic to rice bacterial leaf blight pathogen, Xanthomonas oryzae. Rev Plant Prot Res 13:12–26. [Google Scholar]
- 29.Saxon E, Jackson R, Bhumbra S, Smith T, Sockett R. 2014. Bdellovibrio bacteriovorus HD100 guards against Pseudomonas tolaasii brown-blotch lesions on the surface of post-harvest Agaricus bisporus supermarket mushrooms. BMC Microbiol 14:163. doi: 10.1186/1471-2180-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapidot D, Dror R, Vered E, Mishli O, Levy D, Helman Y. 2015. Disease protection and growth promotion of potatoes (Solanum tuberosum L.) by Paenibacillus dendritiformis. Plant Pathol 64:545–551. doi: 10.1111/ppa.12285. [DOI] [Google Scholar]
- 31.Sathyamoorthy R, Maoz A, Pasternak Z, Im H, Huppert A, Kadouri D, Jurkevitch E. 2019. Bacterial predation under changing viscosities. Environ Microbiol 21:2997–3010. doi: 10.1111/1462-2920.14696. [DOI] [PubMed] [Google Scholar]
- 32.van der Wolf JM, de Haan EG, Kastelein P, Krijger M, de Haas BH, Velvis H, Mendes O, Kooman-Gersmann M, van der Zouwen PS. 2017. Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathol 66:571–583. doi: 10.1111/ppa.12600. [DOI] [Google Scholar]
- 33.Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, Tsror L, Elphinstone JG. 2011. Dickeya species: an emerging problem for potato production in Europe. Plant Pathology 60:385–399. doi: 10.1111/j.1365-3059.2011.02427.x. [DOI] [Google Scholar]
- 34.Shemesh Y, Jurkevitch E. 2004. Plastic phenotypic resistance to predation by Bdellovibrio and like organisms in bacterial prey. Environ Microbiol 6:12–12. doi: 10.1046/j.1462-2920.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 35.Dashiff A, Keeling TG, Kadouri DE. 2011. Inhibition of predation by Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus via host cell metabolic activity in the presence of carbohydrates. Appl Environ Microbiol 77:2224–2231. doi: 10.1128/AEM.02565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sockett RE. 2009. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol 63:523–539. doi: 10.1146/annurev.micro.091208.073346. [DOI] [PubMed] [Google Scholar]
- 37.Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomashow LS, and, Rittenberg SC. 1985. Waveform analysis and structure of flagella and basal complexes from Bdellovibrio bacteriovorus 109J. J Bacteriol 163:1038–1046. doi: 10.1128/JB.163.3.1038-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwudke D, Linscheid M, Strauch E, Appel B, Zähringer U, Moll H, Müller M, Brecker L, Gronow S, Lindner B. 2003. The obligate predatory Bdellovibrio bacteriovorus possesses a neutral lipid A containing α-d-mannoses that replace phosphate residues. J Biol Chem 278:27502–27512. doi: 10.1074/jbc.M303012200. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Tang C, Tran M, Kadouri DE. 2016. Effect of predatory bacteria on human cell lines. PLoS One 11:e0161242. doi: 10.1371/journal.pone.0161242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanks RMQ, Davra VR, Romanowski EG, Brothers KM, Stella NA, Godboley D, Kadouri DE. 2013. An eye to a kill: using predatory bacteria to control Gram-negative pathogens associated with ocular infections. PLoS One 8:e66723. doi: 10.1371/journal.pone.0066723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wurtzel O, Dori-Bachash M, Pietrokovski S, Jurkevitch E, Sorek R. 2010. Mutation detection with next-generation resequencing through a mediator genome. PLoS One 5:e15628. doi: 10.1371/journal.pone.0015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai J, Zhao J, Wang Z, Zou D, Sun I. 2008. Lysis of vibrios by Bdellovibrio-and-like organisms (BALOs) isolated from marine environment. J Food Saf 28:220–235. doi: 10.1111/j.1745-4565.2008.00116.x. [DOI] [Google Scholar]
- 44.Cao H, An J, Zheng W, He S. 2015. Vibrio cholerae pathogen from the freshwater-cultured whiteleg shrimp Penaeus vannamei and control with Bdellovibrio bacteriovorus. J Invertebr Pathol 130:13–20. doi: 10.1016/j.jip.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Im H, Kim D, Ghim C-M, Mitchell R. 2013. Shedding light on microbial predator-prey population dynamics using a quantitative bioluminescence assay. Microb Ecol doi: 10.1007/s00248-013-0323-z. [DOI] [PubMed] [Google Scholar]
- 46.Kadouri D, O'Toole GA. 2005. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol 71:4044–4051. doi: 10.1128/AEM.71.7.4044-4051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadouri D, Venzon NC, O’Toole GA. 2007. Vulnerability of pathogenic biofilms to Micavibrio aeruginosavorus. Appl Environ Microbiol 73:605–614. doi: 10.1128/AEM.01893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varon M, Zeigler B. 1978. Bacterial predator-prey interaction at low prey density. Appl Environ Microbiol 36:11–17. doi: 10.1128/AEM.36.1.11-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham DC, Dowson WJ. 1960. The coliform bacteria associated with potato black-leg and other soft rots. Ann Applied Biology 48:58–64. doi: 10.1111/j.1744-7348.1960.tb03504.x. [DOI] [Google Scholar]
- 50.Hobley L, King JR, Sockett RE. 2006. Bdellovibrio predation in the presence of decoys: three-way bacterial interactions revealed by mathematical and experimental analyses. Appl Environ Microbiol 72:6757–6765. doi: 10.1128/AEM.00844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao H, Wang H, Yu J, An J, Chen J. 2019. Encapsulated Bdellovibrio powder as a potential bio-disinfectant against whiteleg shrimp-pathogenic vibrios. Microorganisms 7:244. doi: 10.3390/microorganisms7080244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karunker I, Rotem O, Dori-Bachash M, Jurkevitch E, Sorek R. 2013. A global transcriptional switch between the attack and growth forms of Bdellovibrio bacteriovorus. PLoS One 8:e61850. doi: 10.1371/journal.pone.0061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rotem O, Pasternak Z, Shimoni E, Belausov E, Porat Z, Pietrokovski S, Jurkevitch E. 2015. Cell-cycle progress in obligate predatory bacteria is dependent upon sequential sensing of prey recognition and prey quality cues. Proc Natl Acad Sci U S A 112:E6028–E6037. doi: 10.1073/pnas.1515749112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jurkevitch E. 2012. Isolation and classification of Bdellovibrio and like organisms Curr Prot Microbiol 26:7B.1.1–7B.1.20. doi: 10.1002/9780471729259.mc07b01s26. [DOI] [PubMed] [Google Scholar]
- 55.Buttin G, Cohen G, Monod J, Rickenberg H. 1956. Galactoside-permease of Escherichia coli. Ann Inst Pasteur (Paris) 91:829–857. (In French.) [PubMed] [Google Scholar]
- 56.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 57.Duarte V, De Boer SH, Ward LJ, de Oliveira A. 2004. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. J Appl Microbiol 96:535–545. doi: 10.1111/j.1365-2672.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 58.Stolp H, Starr MP. 1963. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29:217–248. doi: 10.1007/bf02046064. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee S, Brothers KM, Shanks RMQ, Kadouri DE. 2015. Visualizing Bdellovibrio bacteriovorus by using the tdTomato fluorescent protein. Appl Environ Microbiol 82:1653–1661. doi: 10.1128/AEM.03611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambina VA, Afinogenova AV, Romaĭ Penabad S, Konovalova SM, Pushkareva AP. 1982. Micavibrio admirandus gen. et sp. nov. Mikrobiologiia 51:114–117. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.