Abstract
Introduction.
Cognitive impairment (CI) increases chemotherapy toxicity risk with need to understand this association utilizing publicly available short screening tools. We evaluated this utilizing a lower threshold on a short screening tool in older adults with cancer.
Materials and Methods.
We analyzed data from the Cancer and Aging Research Group (CARG) Chemotherapy Toxicity Risk tool (CARG score) development and validation cohorts (n=703), which recruited adults age ≥65 with cancer from academic centers. Cognition was evaluated with the Blessed Orientation-Memory-Concentration test (BOMC). Patients with BOMC score ≥11 were excluded. Utilizing cut-points for older adults, we considered moderate BOMC scores (5–10) as potential CI. Logistic regression was used for analysis.
Results.
Patient baseline characteristics included: mean age 73; 85% white; 63% college or higher education; 250 (36%) potential CI; 385 (55%) severe toxicity. Patients with potential CI were more likely non-white (p ≤0.01), to have high school or lower education (p ≤0.01) and high CARG score (p = 0.04). Potential CI was associated with increased severe toxicity risk (OR=1.54, p ≤0.01). After adjusting for CARG score, this association became nonsignificant (OR=1.35; p=0.08). Among patients with lower education (n=258; 36.7%), potential CI remained associated with severe toxicity, even after adjusting for CARG score (OR=1.87, p=0.03).
Conclusions.
Our findings suggest potential cognitive impairment, defined by BOMC score 5–10, in older adults with cancer and lower education is associated with increased severe toxicity risk. Future studies are needed to validate these findings. Healthcare providers should consider cognitive testing before treatment for these vulnerable patients.
Introduction
Both the risk of cognitive impairment (CI) and cancer increase with age.(1, 2) CI is prevalent in older adults with cancer, with 15–48% screening positive.(3–6) Older adults represent the majority of patients diagnosed with cancer, with over 60% of cancer diagnoses occurring in patients aged ≥65.(7) With the aging of our population, older adults will represent an increasing percentage of patients with cancer in the coming two decades.(8) For these patients, it is imperative to improve our understanding about the interplay between cognition and cancer-related outcomes, such as chemotherapy toxicity.
In older adults with cancer, studies have demonstrated that cognitive function plays an important role in the risk for chemotherapy toxicity(9, 10) and overall survival.(4, 11, 12) These studies evaluating the association between cognitive function and chemotherapy toxicity utilized longer cognitive screening tools. National guidelines regarding the management of older adults with cancer recommend screening for CI with common tools such as Blessed Orientation- Memory-Concentration test (BOMC), Mini-Mental Status Exam (MMSE), Montreal Cognitive Assessment (MoCA), and MiniCog.(13, 14) Despite these recommendations, this practice has not been routinely implemented into oncology clinical practice, which in part may be due to concerns with time constraints.(15, 16) The BOMC is a short cognitive screening tool, available in the public domain, which can be completed in less than 5 minutes.(17)
During oncology visits, patients are often given complex instructions regarding their cancer therapy. This assumes that the patient has the ability to follow these complex instructions, identify signs of toxicity, notify the healthcare team in a timely fashion, and take medications as directed. Yet, CI may limit patients’ abilities to follow instructions, thus potentially influencing their risk for adverse events. However, low level of CI can be subtle and easily overlooked. Over a third of patients with CI are not recognized by physicians without screening tools.(18)
The current analysis builds upon prior work by Dr. Arti Hurria and colleagues who have demonstrated that severe chemotherapy-related toxicity is common in older adults with cancer.(9, 19–21) They developed the Cancer and Aging Research Group (CARG) Chemotherapy Toxicity Risk tool which predicts the risk of severe chemotherapy-related toxicity in older adults with cancer. This tool can be utilized when discussing risks and benefits of chemotherapy with older adults. The tool includes patient, tumor, and treatment characteristics, laboratory values as well as geriatric assessment (GA) questions. With the cut-point for severe cognitive impairment, ≥11, the BOMC was not found to be predictive of severe chemotherapy-related toxicity during this prior analysis.
Consistent with Dr. Hurria’s mission to improve the evidence-base for incorporating geriatric-specific endpoints into oncology clinical trials for older adults and understanding how these variables may influence cancer outcomes, our goal is to improve our understanding of how geriatric-related issues (i.e. cognition) influence cancer-related outcomes.(22, 23) The specific objective of this analysis is to evaluate the association between CI identified by a lower threshold on a short cognitive screening tool, the BOMC, and the risk of severe chemotherapy toxicity.
Methods
This is a secondary data analysis from two multi-center prospective studies, led by Dr. Hurria, whose primary aims were to develop(19) and validate(20) a predictive model of chemotherapy toxicity, CARG Toxicity Risk tool, in older adults with cancer using a GA. The GA includes an evaluation of cognition as well as an evaluation of functional status, comorbidities, polypharmacy, nutritional status, psychological status, and social support.
Patients with cancer were recruited from ten academic centers across the United States and were eligible for enrollment if they were ≥65, scheduled to undergo a new chemotherapy regimen, fluent in English, and able to provide informed consent. Patients were followed from the beginning to the end of their chemotherapy treatment. Each clinical encounter was reviewed by two investigators for chemotherapy-related toxicities and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0. Grade three to five chemotherapy toxicities were considered “severe” (grade 3 - severe; grade 4 - life-threatening; grade 5 - death). All participating site institutional review boards approved these studies. Cognition was evaluated at baseline with the BOMC screening test. The BOMC is a 6-item scale developed from a longer 26-item test, with scores ranging from zero to 28 where higher scores are consistent with severe cognitive impairment.(24) Orientation is evaluated with patient report of the current year, month, and time of day. Concentration is evaluated by having the patient count backward from twenty to one and say the months in reverse order. Memory is evaluated through delayed recall of a brief phrase: “John Brown, 42 Market Street, Chicago.” The BOMC has strong test-retest reliability,(25–27) and is highly correlated (r= 0.81 to 0.92) with the MMSE.(17, 25, 26, 28, 29) The sensitivity and specificity of BOMC score ≥5 for an MMSE score ≤23 were 95% (95% CI 88–98%) and 65% (95% CI 61–67%), respectively.(29)
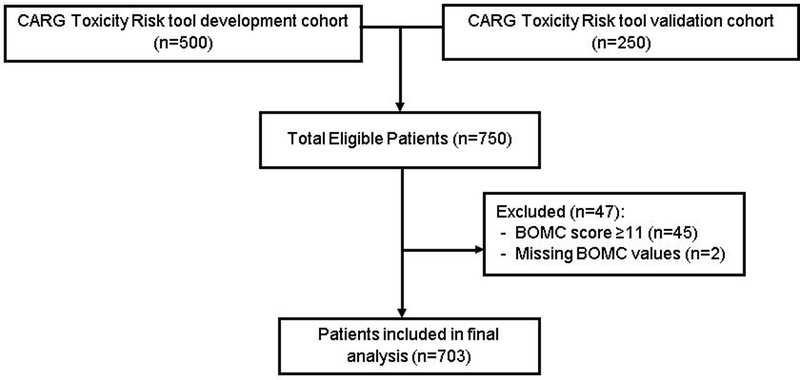
A score of ≥11 on the BOMC is consistent with severe cognitive impairment, suggestive of likely dementia, and therefore providers were informed of this finding.(28–30) Given this, patients with a score of ≥11 (n=45; Figure 1) were excluded from the current analysis as their chemotherapy regimen, choice of dose-intensity, and supportive care plan may have been adjusted by providers to avoid toxicities. In addition, two patients with missing values on the BOMC were also excluded. The BOMC thresholds for less severe cognitive impairment ranges in the literature from four to six.(28, 30, 31) During validation of the 6-item BOMC in four populations of older adults, Katzman et al found that those with normal cognition scored ≤6.(30) During development of a brief clinical and neuropsychological assessment for Alzheimer’s disease by Morris et al, the majority of normal controls scored ≤5 on the BOMC.(28) Evaluating the ability of various cognitive screening tools to identify cognitive dysfunction -- defined by a score of ≤23 on the MMSE -- Carpenter et al found a score of ≥5 on the BOMC to be consistent with cognitive impairment.(31) Based upon this evidence from the general older adult population, we defined a moderate BOMC score of 5–10 as potential cognitive impairment.
Fig. 1.
CONSORT flow diagram for inclusion of patients in analysis.
The CARG Toxicity Risk score was calculated for each patient. This score is based on 11 variables including patient age, tumor type, treatment regimen, laboratory values (hemoglobin, creatinine clearance), and GA questions (falls, ability to walk one block, ability to take medications without assistance, decrease in social activities, hearing).(19) The CARG Toxicity Risk score ranges from zero to nineteen which can then be further categorized into low (score 0–5), medium (6–9), and high (10–19) risk groups. This predictive model has acceptable predictive ability (AUC=0.72 for development cohort, and 0.65 for validation cohort) for severe chemotherapy toxicity.(32) Full details of this measure and toxicities can be found elsewhere.(19, 20)
Statistical Analysis
For the current analysis, a moderate BOMC score of 5 to 10 was used to define potential cognitive impairment, as described above. Demographic or clinical characteristic frequency distributions between patients with normal cognition versus potential cognitive impairment were compared using Chi-squared tests. Continuous BOMC score between patients with and without severe chemotherapy toxicity were compared using t- test. Correlation between BOMC score and CARG Toxicity Risk score was assessed using Spearman’s correlation coefficient.
Univariate and multivariate logistic regression models were used to compute the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for the associations between potential cognitive impairment and risk of severe chemotherapy toxicity.(33) Exploratory subgroup analyses were conducted to examine whether the associations between potential cognitive impairment and severe chemotherapy toxicity differed by education and race/ethnicity. Because CARG Toxicity Risk score is the primary predictor for chemotherapy toxicity, multivariable models were adjusted for CARG Toxicity Risk group (low, medium, and high). Thirty-four patients with missing data on the CARG Toxicity Risk score were excluded from multivariable analyses. There were no differences in baseline patient characteristics between these 34 patients and those with available CARG Toxicity Risk score data (Supplemental Table 1). All statistical tests were two-sided and p-values less than 0.05 were considered statistically significant. Data were analyzed using SAS 9.4 (analytic software; SAS Institution, Cary, NC).
Results
Patient Characteristics
The current analysis included 703 patients with a mean age of 73 (range 65–94) and the majority with stage III or IV cancer (81.1%). Among them, 250 (36%) patients had potential cognitive impairment. The frequency distributions of patient demographics overall and by potential cognitive impairment are shown in Table 1. Most patients were female (56.6%), married (60.3%) and lived with a spouse, partner, or child (77.0%). The majority of patients also had at least a college or advanced degree (63.2%) and were white (84.6%). Compared with patients having normal cognition, those with potential cognitive impairment were less likely to be white (p<0.01), and more likely to have a high school or lower education (p<0.01) and be in the high CARG Toxicity Risk group (p = 0.04). No differences were observed for the other patient characteristics (all p>0.05).
Table 1.
Patient Characteristics
| Characteristics | Overall (n=703) | By Cognition |
||
|---|---|---|---|---|
| Normal (n=453, 64.4%) | Potential Impairment (n=250, 35.6%) | p-value* | ||
| Age, years | 0.06 | |||
| 65–69 | 249 | 66.7 | 33.3 | |
| 70–74 | 184 | 68.5 | 31.5 | |
| 75–79 | 155 | 63.9 | 36.1 | |
| ≥80 | 115 | 53.9 | 46.1 | |
| Sex | 0.07 | |||
| Female | 398 | 67.3 | 32.7 | |
| Male | 305 | 60.7 | 39.3 | |
| Race/Ethnicity | 0.009 | |||
| White | 595 | 66.7 | 33.3 | |
| Black | 56 | 55.4 | 44.6 | |
| Other | 52 | 48.1 | 51.9 | |
| Education Level | 0.007 | |||
| Less than high school | 22 | 36.4 | 63.6 | |
| High school graduate | 236 | 61.4 | 38.6 | |
| Associate/bachelor’s degree | 298 | 65.1 | 34.9 | |
| Advanced degree | 146 | 71.9 | 28.1 | |
| Missing† | 1 | |||
| Marital Status | 0.42 | |||
| Married | 424 | 65.3 | 34.7 | |
| Widowed | 160 | 59.4 | 40.6 | |
| Single | 25 | 72.0 | 28.0 | |
| Separated/divorced | 94 | 67.0 | 33.0 | |
| Household | ||||
| Composition Lives lone | 148 | 0.12 | ||
| Lives with spouse, partner, or child | 541 | 70.3 | 29.7 | |
| Missing† | 14 | 63.4 | 36.6 | |
| Cancer Type | 0.39 | |||
| Breast | 109 | 67.9 | 32.1 | |
| GI | 186 | 64.5 | 35.5 | |
| GU | 78 | 62.8 | 37.2 | |
| GYN | 102 | 70.6 | 29.4 | |
| Lung | 195 | 62.1 | 37.9 | |
| Other | 33 | 51.5 | 48.5 | |
| Cancer Stage | 0.63 | |||
| I | 32 | 62.5 | 37.5 | |
| II | 94 | 70.2 | 29.8 | |
| III | 168 | 62.5 | 37.5 | |
| IV | 402 | 63.9 | 36.1 | |
| Missing | 7 | |||
| No. of Comorbidities | 0.97 | |||
| 0–1 | 220 | 64.6 | 35.4 | |
| ≥2 | 483 | 64.4 | 35.6 | |
| CARG Toxicity Risk Group | 0.04 | |||
| Low | 182 | 69.8 | 30.2 | |
| Medium | 339 | 65.5 | 34.5 | |
| High | 148 | 56.8 | 43.2 | |
| Missing† | 34 | |||
Abbreviations: GI = gastrointestinal; GU = genitourinary; GYN = gynecologic
Chi-squared test
Missing categories not included in statistical analysis
The mean BOMC score for all patients was 3.9 (standard deviation [SD]= 3.3). Patients with severe chemotherapy toxicity had modestly higher BOMC scores (mean 4.1, SD= 3.4) compared with those who did not have severe chemotherapy toxicity (mean 3.6, SD= 3.1; p= 0.05). BOMC score was weakly correlated with CARG Toxicity Risk score (Spearman Correlation Coefficient 0.12, p<0.01).
BOMC Score and Severe Chemotherapy Toxicity
The overall incidence of severe chemotherapy toxicity was 54.8% (n= 385). The incidence was 51.0% (n= 231) in patients with normal cognition and 61.6% (n= 154) in patients with potential cognitive impairment (p <0.01). Compared to patients with normal cognition, those with potential cognitive impairment had increased risk of severe chemotherapy toxicity in univariate analysis (OR= 1.54, p<0.01). However, the association was attenuated and became not statistically significant after adjusting for CARG Toxicity Risk group (OR = 1.35; p = 0.08; Table 2).
Table 2.
Associations between Potential Cognitive Impairment and Severe Chemotherapy Toxicity
| Univariate Analysis | Adjusted Analysis* | |||||||
|---|---|---|---|---|---|---|---|---|
| Severe Chemotherapy Toxicity | OR (95% CI) | p-value | Severe Chemotherapy Toxicity | OR (95% CI) | p-value | |||
| No | Yes | No | Yes | |||||
| Overall | ||||||||
| Cognition | 0.007 | 0.08 | ||||||
| Normal | 222 | 231 | 211 | 222 | ||||
| Potential Impairment | 96 | 154 | 1.54 (1.13–2.11) | 92 | 144 | 1.35 (0.96–1.90) | ||
| By Race/ Ethnicity | ||||||||
| White | ||||||||
| Normal Cognition | 191 | 206 | 183 | 197 | ||||
| Potential Impairment | 80 | 118 | 1.37 (0.97–1.93) | 0.08 | 79 | 111 | 1.24 (0.86–1.80) | 0.25 |
| Non-White | ||||||||
| Normal Cognition | 31 | 25 | 28 | 25 | ||||
| Potential Impairment | 16 | 36 | 2.79 (1.27–6.15) | 0.01 | 13 | 33 | 1.98 (0.76–5.17) | 0.16 |
| By Education Level | ||||||||
| High School Education or Less | ||||||||
| Normal Cognition | 75 | 78 | 72 | 77 | ||||
| Potential Impairment | 36 | 69 | 1.84 (1.10–3.08) | 0.02 | 33 | 64 | 1.87 (1.06–3.29) | 0.03 |
| Greater than High School Education | ||||||||
| Normal Cognition | 146 | 153 | 138 | 145 | ||||
| Potential Impairment | 60 | 85 | 1.35 (0.91–2.02) | 0.14 | 59 | 80 | 1.13 (0.73–1.74) | 0.58 |
Abbreviations: OR = odds ratio; CI = confidence interval
Adjusted for CARG Toxicity Risk group (low, middle, high), 34 patients excluded due to missing CARG Toxicity Risk score data
In order to examine whether the association between potential cognitive impairment and severe chemotherapy toxicity was modified by education and race/ethnicity, subgroup analyses were performed. In the univariate analysis, potential cognitive impairment was significantly associated with higher risk of severe chemotherapy toxicity in non-white patients (n= 108, 15.4%, OR= 2.79, p= 0.01) and those with a high school or lower education (n= 258, 36.7%, OR= 1.84, p= 0.02). After adjustment for CARG Toxicity Risk group, potential cognitive impairment was significantly associated with increased risk of chemotherapy toxicity only among those with a high school or lower education level (OR= 1.87, p= 0.03). No interaction was found between potential cognitive impairment and race/ethnicity (p= 0.59) or potential cognitive impairment and education (p= 0.35).
Discussion
The results of the current analysis suggest that older adults with cancer and lower education level who have potential cognitive impairment, defined as a BOMC score of 5–10, were more likely to experience severe chemotherapy toxicity. To our knowledge, we are the first to report an association between BOMC score and risk of severe chemotherapy toxicity in patients aged ≥65 years.
Our finding is consistent with prior studies demonstrating associations between cognitive impairment and chemotherapy toxicity using another cognitive screening tool, MMSE. In the Chemotherapy Risk-Assessment Scale for High-age patients (CRASH) model, developed by Extermann and colleagues in patients age 70 or older with cancer, any abnormality on the MMSE (score <30) was associated with increased risk of grade three to four non-hematologic toxicity.(9) In a study evaluating first-line chemotherapy for metastatic colon cancer in older adults, MMSE score of ≤27 was associated with nearly a 4-fold increased risk of grade three to four chemotherapy toxicity.(10) These two studies included patients with severe CI and utilized a longer cognition screening tool. It is unclear in these studies if healthcare providers were notified of the cognition screening tool results if severe cognitive impairment was identified. This impacts interpretation of the toxicity results as providers may adjust treatment plans if notified of severe cognitive impairments. We were able to demonstrate a lower threshold score (5–10) on a shorter cognitive screening tool, the BOMC, was associated with increased risk of chemotherapy toxicity among older adults with cancer who have a lower education level.
Cognition was evaluated as a potential variable during the development of the original CARG Toxicity Risk tool and was not found to independently predict severe chemotherapy toxicity. This prior analysis included patients with a BOMC score of ≥11, which is consistent with severe cognitive impairment. During the prior analysis, multiple cut-points were evaluated. BOMC was not found to be predictive of severe chemotherapy toxicity risk during this prior analysis, likely due to the inclusion of those with a BOMC score of ≥11 with several possible explanations. First, there was a relatively small number of patients with severe cognitive impairment in the primary studies (N=45, 6%). The low rate of patients with severe cognitive impairment may be due to a selection bias of patients enrolled on the study, as patients with more severe cognitive impairment may not be offered chemotherapy and were not enrolled in the studies. Second, a potential reason is that treating oncologists were notified if enrolled patients had a BOMC score ≥11, alerting them to the issue, and thus potentially influencing the chosen treatment approach, specifically to avoid toxicity (e.g. from reduced doses or less toxic regimen). This study focuses on patients who would otherwise not be flagged as having severe cognitive impairment on the BOMC, those with a BOMC score of five to ten).
The association of potential cognitive impairment, based on a BOMC score of 5–10, with increased risk of severe chemotherapy toxicity was no longer statistically significant after adjustment for CARG Toxicity Risk group. This attenuation was not surprising as the CARG Toxicity Risk score and the BOMC score are weakly correlated. This correlation may be in part explained by the likelihood that some of the variables utilized in the CARG Toxicity Risk model are surrogate markers of cognitive impairment, such as requiring assistance to take medications.
During subgroup analysis, we found the association between potential cognitive impairment and increased risk of severe chemotherapy toxicity remained significant in those with a high school or lower education, even after adjusting for CARG Toxicity Risk group. Although a subgroup analysis, we found that among those with a high school or lower education, patients with potential cognitive impairment were almost twice as likely to have severe chemotherapy toxicity compared to those with normal cognition. Due to the screening nature of the BOMC, a moderate BOMC score may not indicate clinical diagnosis of cognitive impairment in this subgroup, but rather may be an indicator of difficulties in comprehension or memorization of complex instructions. Although a higher education level has been associated with higher cognition in older adults, cognitive screening tools are influenced by level of education attained.(34–38) A high rate of false positivity has been seen with the MMSE in patients with a lower level of education.(37) There is limited information in the literature regarding the influence of education level on the predictive value of BOMC in identifying cognitive impairment. In a study of women with breast cancer, BOMC score prior to chemotherapy was associated with education level.(38) However, we did not find an interaction between potential cognitive impairment and race/ethnicity or potential cognitive impairment and education level in this cohort.
There are some limitations in this study. The BOMC is a screening tool and patients with a positive result require a more in-depth evaluation to confirm mild cognitive impairment or dementia. Although based on a threshold identified in older adults without dementia,(28) validation of a BOMC score of 5 to 10 compared to formal neurocognitive testing is needed. The highest risk found among patients with a high school or lower education is difficult to interpret given the limitations of cognitive screening tools in patients with lower education levels and as this was found in the subgroup analysis. Further studies are needed to validate this finding. The patient population in this cohort is heterogeneous in regard to cancer type and stage as well as line of therapy, some of which has been shown to influence chemotherapy toxicity risk.(17) The exclusion of patients with BOMC score ≥11 limits expansion of these findings to older adults with severe cognitive impairments.
In summary, we have identified an association between potential cognitive impairment, identified by a moderate score on the BOMC (5–10), and increased risk of severe chemotherapy toxicity in older adults with cancer and lower education level. These results support screening for cognitive impairment in older adults with cancer and lower education level before receiving chemotherapy, given the harmful effects of chemotherapy toxicity.(13, 14) Future studies are needed to validate these findings as well as evaluate potential interventions to address cognitive deficits to reduce chemotherapy-related toxicities, and understand the interaction between cognitive deficits and cancer-treatment related outcomes such as quality of life and survival.
Supplementary Material
Acknowledgement
We would like to acknowledge the influence of Dr. Arti Hurria to the design and analysis of this study, which was performed under her guidance. She had a strong interest in investigating cognition in the setting of older adults with cancer. The authors of this manuscript have worked closely with Dr. Hurria as colleagues or mentees.
Funding Sources
The data for this sub-study was collected through studies conducted by Dr. Hurria, which were funded through the NIH/National Institute on Aging (NIA) grant K23-AG026749–01 (Paul Beeson Career Development Awards in Aging Research) (PI: A. Hurria), the American Society of Clinical Oncology Association of Specialty Professors Junior Development Award in Geriatric Oncology (PI: A. Hurria), and City of Hope’s Center for Cancer and Aging. Support also came from the National Institute of Aging (NIA) R21/R33AG059206 (PIs: A. Hurria, W. Dale, S. Mohile), the K24-AG055693–01 (PI: A. Hurria, W. Dale), and K24-AG056589–01 (PI: S. Mohile).
Footnotes
Conflict of Interest
None to disclose.
References
- 1.Singh SD, Henley SJ, Ryerson AB. Surveillance for Cancer Incidence and Mortality- United States 2013. MMWR Suveill Summ. 2017;66(4):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’sAssociation. Alzheimer’s Association report: 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2015;11:332–84. [DOI] [PubMed] [Google Scholar]
- 3.Corre R, Greillier L, Le Caër H, Audigier-Valette C, Baize N, Bérard H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. 2016;34(13):1476–83. [DOI] [PubMed] [Google Scholar]
- 4.Hshieh TT, Jung WF, Grande LJ, Chen J, Stone RM, Soiffer RJ, et al. Prevalence of Cognitive Impairment and Association With Survival Among Older Patients With Hematologic Cancers. JAMA Oncol. 2018;4(5):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Ellis LR, et al. The Feasibility of Inpatient Geriatric Assessment for Older Adults Receiving Induction Chemotherapy for Acute Myelogenous Leukemia. J Am Geriatr Soc. 2011;58:1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh KP, Pandya C, Zittel J, Kadambi S, Flannery M, Reizine N, et al. Associations of sleep disturbance with physical function and cognition in older adults with cancer. Support Care Cancer. 2017;25(10):3161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 8.Smith BD, Smith GL, Hurria A, Nhortobagyi GN, TA B. Future of Cancer Incidence int he United States: Burdens Upon and Aging, Changing Nation. J Clin Oncol. 2009;27(17):2758–65. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the Risk of Chemotherapy Toxicity in Older Patients: The Chemotherapy Risk Assessment Scale for High-Age Patients CRASH) Score. Cancer. 2012;118:3377–86. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio T, Jouve JL, Teillet L, Gargot D, Subtil F, Le Brun-Ly V, et al. Geriatric Factors Predic Chemotherpay Feasibility: Ancillary Results of FFCD 2001–02 Phase III Study in First-Line Chemotherapy for Metastatic Colorectal Cancer in Elderly Patients. J Clin Oncol. 2013;31:1464–70. [DOI] [PubMed] [Google Scholar]
- 11.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric Assessment Predicts Survival for Older Adults Receiving Induction Chemotherapy for Acute Myelogenous Leukemia. Blood. 2013;121:4287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raji MA, Kuo YF, Freeman JL, Goodwin JS. Effect of a Dementia Diagnosis on Survival of Older Patients After a Diangosis of Breast, Colon, or Prostate Cancer. Arch Intern Med. 2008;168(18):2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohile SG, Dale W, Somerfield MR, Boyd CM, Burhenn PS, Canin B, et al. Practical Assessment and Management of Vulnerailites in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panel chair, et al. , NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® Older Adult Oncology V.1.2019. © 2019 National Comprehensive Cancer Network, Inc; Available at NCCN.org. [Google Scholar]
- 15.Mohile SG, Magnuson A, Pandya C, Velardea C, Duberstein P, Hurria A, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw. 2018;16(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliffe S, Mitchley S, Gould M, Haines A. Evaluation of the use of brief screening instruments for dementia, depression and problem drinking among elderly people in general practice. Br J Gen Pract. 1994;44(388):503–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Upadhyaya AK, Rajagopal M, Gale TM. The Six Item Cognitive Impairment Test (6-CIT) as a Screening Test for Dementia: Compairison with Mini-Mental State Examination (MMSE). Current Aging Science. 2010;3:138–42. [DOI] [PubMed] [Google Scholar]
- 18.Chodosh J, Petitti DB, Elliott M, Hays RD, Crooks VC, Reuben DB, et al. Physician Recognition of Cognitive Impairment: Evaluating the Need for Improvement. J Am Geriatr Soc. 2004;52:1051–9. [DOI] [PubMed] [Google Scholar]
- 19.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of Clin Oncol. 2011;29(25):3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34:2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildes TM, Ruwe AP, Fournier C, Gao F, Carson KR, Piccirillo JF, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity and survival in older adults with cancer. J Geriatr Oncol. 2013;4(3):277–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurria A, Dale W, Mooney M, Rowland JH, Ballman KV, Cohen HJ, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conferece recommendations. J Clin Oncol. 2014;32(24):2587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33(32):3826–33. [DOI] [PubMed] [Google Scholar]
- 24.Fuld P Psychological Testing in the Differential Diagnosis of the Dementias In: Katzman R TR, and Bick KL., editor. Alzheimer’s Disease: Senile Dementia and Related Disorders. 7 New York: Raven Press; 1978. [Google Scholar]
- 25.Fillenbaum GG, Heyman A, Wilkinson WE, Haynes CS. Comparison of Two Screening Tests in Alzheimer’s Disease: The Correlation and Reliability of the Mini-Mental State Examination and the Modified Blessed Test. Arch Neurol. 1987;44:924–7. [DOI] [PubMed] [Google Scholar]
- 26.Thal LJ, Grundman M, Golden R. Alzheimer’s disease: A correlational analysis of the Blessed Information-Memory-Concentration Test and the Mini-Mental State Exam. Neurology. 1986;36:261–4. [DOI] [PubMed] [Google Scholar]
- 27.Kawas C, Karagiiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995;8:238–42. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. [DOI] [PubMed] [Google Scholar]
- 29.Davous P, Lamour Y, Debrand E, Rondot P. A Comparative Evlauation of the Short Orientation Memory Concentration Test of Cognitive Impairment. Journal of Neurol Neurosurg Psychiatry. 1987;50:1312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a Short Orientation-Memory-Concentration Test of Cognitive Impairment. Am J Psychiatry. 1983;140(6):734–9. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, Morris JC. Four Sensitive Screening Tools to Detect Cognitive Dysfunction in Geriatric Emergency Department Patients: Brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the Caregiver-completed AD8. Acad Emerg Med. 2011;18(4):374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajian-Tilaki K Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4(2):627–35. [PMC free article] [PubMed] [Google Scholar]
- 33.Hosmer D, Lemeshow S, Sturdivant R. Applied Logistic Regression. New York, NY: A Wiley-Interscience Publication. John Wiley & Sons Inc; 2000. [Google Scholar]
- 34.Stuss DT, Meiran N, Guzman A, Lafleche G, Willmer J. Do Long Tests Yield a More Accurate Diagnosis of Dementia Than Short Tests? A Comparison of 6 Neuropsychological Tests. Arch Neurol. 1996;53:1033–9. [DOI] [PubMed] [Google Scholar]
- 35.Espanio DV, Lichtenstein MJ, Palmer RF. Ethnic Differences in Mini-Mental State Examination (MMSE) Scores: Where You Live Makes a Difference. J Am Geriatr Soc. 2001;49:538–48. [DOI] [PubMed] [Google Scholar]
- 36.Albert SM, Teresi JA. Reading Ability, Education, and Cognitive Status Assessment Among Older Adults in Harlem, New York City. Am J Public Health. 1999;89:95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scazufca M, Almeida OP, Vallada HP, Tasse WA, Menezes PR. Limitations of the Mini-Mental State Examination for screening dementia in a community with low socioeconomic status: Results from the Sao Paulo Ageing & Health Study. Eur Arch Psychiatry Clin Neurosci. 2009;259:8–15. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura ZM, Deal AM, Kirsten AN, Choi SK, Wood WA, Muss HB. Associations of functional, psychosocial, medical, and socio-demographic factors with cognitive screening in chemotherapy naïve patients with breast cancer. Psychooncology. 2019;28(1):167–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.